This phase 2 randomized clinical trial examines the feasibility and safety of high-intensity treadmill exercise in patients with de novo Parkinson disease who are not taking medication and whether the effect on the motor system warrants a phase 3 trial.
Key Points
Questions
Is high- and moderate-intensity treadmill exercise safe for patients with Parkinson disease who are not yet taking medication, can they exercise at target intensity with hypothesized adherence 3 times per week, and are changes in motor symptoms sufficient to warrant further investigation?
Findings
This phase 2 randomized clinical trial of 128 participants established that 80% to 85% and 60% to 65% exercise intensities are safe and feasible. Furthermore, high-intensity treadmill exercise is nonfutile; therefore, an efficacy trial is warranted for high- but not moderate-intensity exercise.
Meaning
High-intensity treadmill exercise can be safely prescribed for patients with Parkinson disease; further investigation with a phase 3 exercise study is warranted to establish efficacy.
Abstract
Importance
Parkinson disease is a progressive neurologic disorder. Limited evidence suggests endurance exercise modifies disease severity, particularly high-intensity exercise.
Objectives
To examine the feasibility and safety of high-intensity treadmill exercise in patients with de novo Parkinson disease who are not taking medication and whether the effect on motor symptoms warrants a phase 3 trial.
Design, Setting, and Participants
The Study in Parkinson Disease of Exercise (SPARX) was a phase 2, multicenter randomized clinical trial with 3 groups and masked assessors. Individuals from outpatient and community-based clinics were enrolled from May 1, 2012, through November 30, 2015, with the primary end point at 6 months. Individuals with idiopathic Parkinson disease (Hoehn and Yahr stages 1 or 2) aged 40 to 80 years within 5 years of diagnosis who were not exercising at moderate intensity greater than 3 times per week and not expected to need dopaminergic medication within 6 months participated in this study. A total of 384 volunteers were screened by telephone; 128 were randomly assigned to 1 of 3 groups (high-intensity exercise, moderate-intensity exercise, or control).
Interventions
High-intensity treadmill exercise (4 days per week, 80%-85% maximum heart rate [n = 43]), moderate-intensity treadmill exercise (4 days per week, 60%-65% maximum heart rate [n = 45]), or wait-list control (n = 40) for 6 months.
Main Outcomes and Measures
Feasibility measures were adherence to prescribed heart rate and exercise frequency of 3 days per week and safety. The clinical outcome was 6-month change in Unified Parkinson’s Disease Rating Scale motor score.
Results
A total of 128 patients were included in the study (mean [SD] age, 64 [9] years; age range, 40-80 years; 73 [57.0%] male; and 108 [84.4%] non-Hispanic white). Exercise rates were 2.8 (95% CI, 2.4-3.2) days per week at 80.2% (95% CI, 78.8%-81.7%) maximum heart rate in the high-intensity group and 3.2 (95% CI, 2.8-3.6; P = .13) days per week at 65.9% (95% CI, 64.2%-67.7%) maximum heart rate in the moderate-intensity group (P < .001). The mean change in Unified Parkinson’s Disease Rating Scale motor score in the high-intensity group was 0.3 (95% CI, −1.7 to 2.3) compared with 3.2 (95% CI, 1.4 to 5.1) in the usual care group (P = .03). The high-intensity group, but not the moderate-intensity group, reached the predefined nonfutility threshold compared with the control group. Anticipated adverse musculoskeletal events were not severe.
Conclusions and Relevance
High-intensity treadmill exercise may be feasible and prescribed safely for patients with Parkinson disease. An efficacy trial is warranted to determine whether high-intensity treadmill exercise produces meaningful clinical benefits in de novo Parkinson disease.
Trial Registration
clinicaltrials.gov Identifier: NCT01506479
Introduction
Parkinson disease is a progressive neurodegenerative disorder characterized by rigidity, tremor, bradykinesia, and postural instability, ultimately leading to disability. Because medications have adverse effects and reduced effectiveness over time, disease-modifying nonpharmacologic interventions are needed. One intervention, endurance exercise, promotes neurogenesis and neuroprotection in animals and has had symptom-modifying and health-related benefits in patients with Parkinson disease. Retrospective studies have found that moderate to vigorous exercise in midlife can protect against Parkinson disease. Studies in humans suggest that high-intensity endurance exercise improves motor symptoms, but current evidence is insufficient to determine whether exercise intensity influences symptom modification or disease progression. To date, no study has been conducted at 80% to 85% maximum heart rate, and typically most studies were powered on fitness or functional measures but not disease severity. This study (Study in Parkinson Disease of Exercise [SPARX]) was a phase 2 randomized clinical trial to investigate the dose response of treadmill exercise performed at 2 different intensities (high and moderate). To remove the potential confounder of medication, this study included patients with de novo Parkinson disease. The study examined whether patients with de novo Parkinson disease can consistently and safely exercise on a treadmill at high intensity (80%-85% maximum heart rate) or moderate intensity (60%-65% maximum heart rate) at least 3 days per week and whether high- or moderate-intensity treadmill exercise warrants further investigation for treatment of motor symptoms.
Methods
Study Design
SPARX was a randomized clinical trial in de novo Parkinson disease comparing high- and moderate-intensity treadmill exercise with usual care (a wait-list control group). The setting was outpatient clinics and community-based exercise facilities. The institutional review board of each site approved the protocol (Colorado Multiple Institution Review Board; University of Illinois Institutional Review Board; Northwestern University Institutional Review Board Office; Institutional Review Board, Human Research Protection Office, University of Pittsburgh; and Rush University Medical Center Institutional Review Board). All participants provided written informed consent, and all data were deidentified. The trial protocol can be found in the Supplement.
Study Participants
Participants with idiopathic Parkinson disease were enrolled from May 1, 2012, through November 30, 2015, in Chicago, Illinois; Denver, Colorado; and Pittsburgh, Pennsylvania. Participants were aged 40 to 80 years, had Hoehn and Yahr stage 1 or 2 disease, were within 5 years of diagnosis, were not exercising at moderate intensity more than 3 times per week, and were not expected to need dopaminergic medication within 6 months. Race/ethnicity was by self-report according to the National Institute of Neurological Disease and Stroke Common Data Elements.
Screening, Baseline Testing, and Randomization
Prescreening occurred by telephone and in neurology clinics. Participants who met the criteria underwent baseline assessments, including a graded exercise test with measured maximum heart rate. Participants were randomly assigned to high-intensity treadmill exercise (80%-85% maximum heart rate), moderate-intensity treadmill exercise (60%-65% maximum heart rate), or usual care. The usual care group was instructed to maintain exercise habits.
Originally, permuted block randomization stratified by site was used for study assignment. The randomization lists were generated by the statistician (C.G.M.) and uploaded into a web-based data system. Assignment was concealed until a screened participant was deemed to be eligible. At approximately one-third of recruitment, a significant imbalance was detected among the 3 groups on the primary clinical outcome. With approval from the Safety Monitoring Committee, the randomization was changed to a minimal sufficient balancing strategy.
Exercise Interventions
Treadmill exercise was prescribed for 4 days per week for 26 weeks with an a priori hypothesized adherence of 3 days per week. Included were 5 to 10 minutes of warm-up, 30 minutes of treadmill exercise at the target heart rate, and 5 to 10 minutes of cool down. Exercise frequency and intensity were increased during weeks 1 to 8 to reach target levels. Thereafter, target heart rate was maintained by adjusting treadmill speed and/or incline. Rating of perceived exertion was used to monitor exercise intensity for participants who initiated use of chronotropic medications during the intervention.
Participants used a heart rate monitor to record intensity of all exercise sessions. All sessions in weeks 1 to 2 were supervised at the study site. Thereafter, participants exercised at the study site at least monthly, when heart rate data were downloaded. Protocol fidelity was ensured by monthly conference calls with study coordinators.
Outcomes
Outcome assessors were masked to group allocation. The feasibility outcome for achieving intensity targets was derived from the mean heart rate during each exercise session during weeks 9 to 26, expressed as a percentage of the measured maximum heart rate. Adherence was determined by exercise frequency. The primary clinical outcome was the 6-month change in the Unified Parkinson’s Disease Rating Scale (UPDRS) motor score, assessed by study neurologists (B.M.K., B.D.B., C.L.C., D.A.H., S.J.). If a participant began taking medication, the UPDRS was administered before initiation when possible. When participants changed medication without informing the study coordinators, the UPDRS from the last study visit without medications was used. In addition, any participant who had begun taking medication was asked to refrain from taking medication overnight for 12 hours and was tested while not taking medication. Secondary outcomes included changes in UPDRS total and subscores and the Movement Disorders Society UPDRS (MDS-UPDRS) subscores, as well as maximal aerobic power (V̇o2max). Daily step counts were assessed by accelerometry. Safety outcomes were monitored during exercise and monthly contact. We used the UPDRS for our primary outcome measure because when we designed the study (2009), data were limited on the MDS-UPDRS as an outcome measure. We present the MDS-UPDRS data because this measure is currently recommended.
Statistical Analysis
Within-group achievement of the exercise intensity and frequency (hypothesized to be at least 3 days per week) was tested using 1-sample t tests. A log-rank test was used to compare the rates of Parkinson disease medication initiation. An intention-to-treat approach was used to compare 6-month UPDRS motor change in usual care to each exercise group with a priori, planned unpaired 2-sample t tests with 1-sided α = .10 and a futility threshold of θ = 3.5. The null hypothesis being tested for each exercise arm independently was that the exercise intensity resulted in at least 3.5 points less change on the UPDRS motor score at 6 months compared with usual care and, thus, warranted further investigation. We estimated the between-group difference and compared the 90% upper confidence boundary with the 3.5-point futility threshold. Sensitivity analyses explored the effect of using off-state UPDRS scores for participants who initiated treatment and multiple imputation for missing data. Secondary outcomes were reported as differences in means with 95% CIs.
The number needed per group for good precision (±2.5%) for mean intensity was 36 (95% CI half width of 2.4%, σ = 7.0%), providing 83% power to detect 3.5% intensity difference (σ = 7, α = .05). A sample size of 36 finishers per group provided more than 84% power to reject the null hypothesis if there was no difference in the UPDRS motor score between the exercise and usual care groups (SDs, 5.5-6.5; 1-sided α = .10). We aimed for 42 participants per group, assuming 15% attrition at 6 months.
Results
Baseline Characteristics of Participants
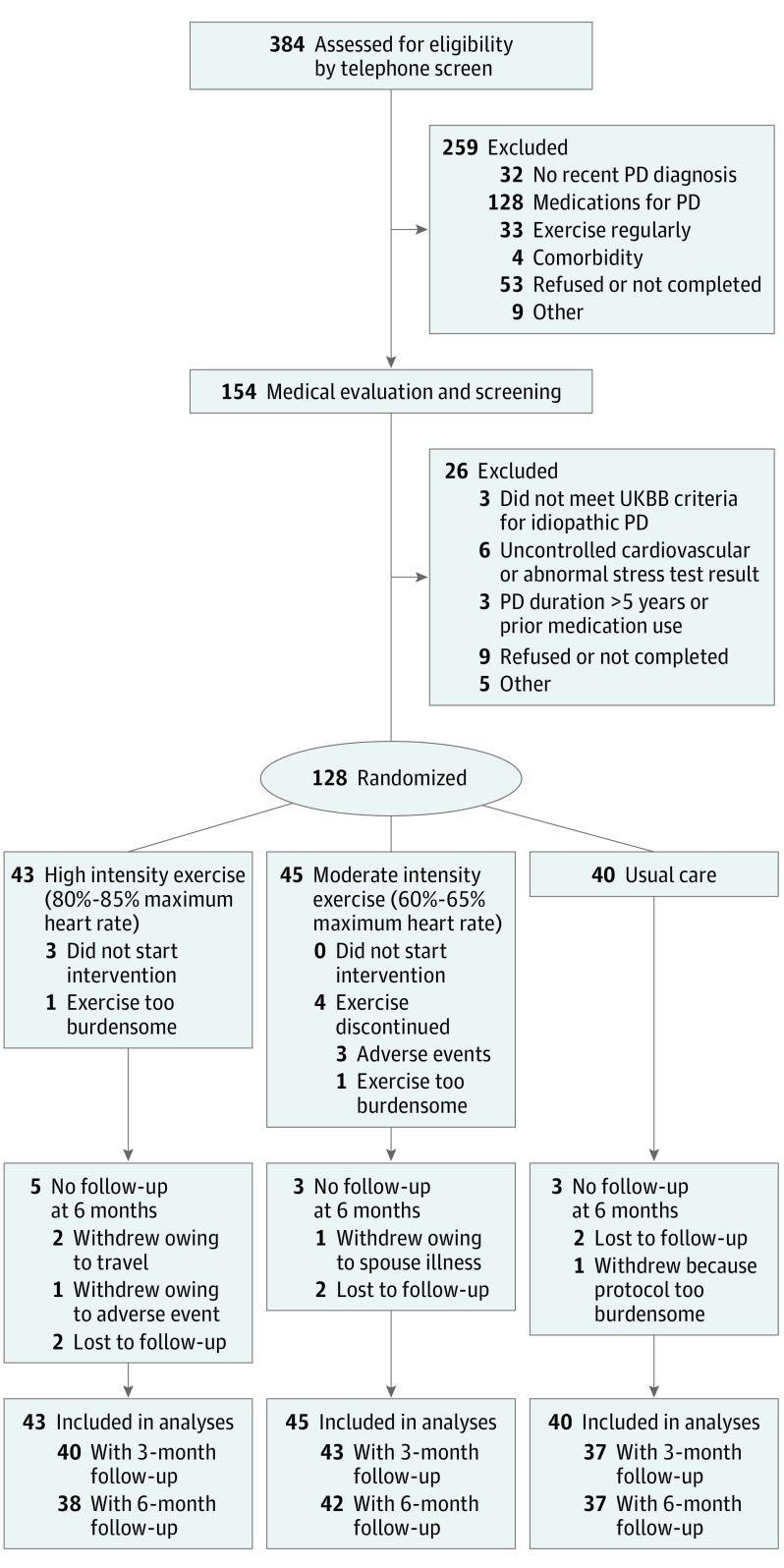
We evaluated and screened 154 volunteers for eligibility; 128 were randomized (mean [SD] age, 64 [9] years; age range, 40-80 years; 73 [57.0%] male; and 108 [84.4%] non-Hispanic white) (Figure 1). Groups were well matched across baseline characteristics (Table 1).
Figure 1. Flow Diagram of Patient Participation in the Study.
PD indicates Parkinson disease; UKBB, UK Parkinson’s Disease Society Brain Bank.
Table 1. Demographic and Clinical Characteristics of the Study Participants at Baselinea.
| Characteristic | High-Intensity Exercise (n = 43) |
Moderate-Intensity Exercise (n = 45) |
Usual Care (n = 40) |
|---|---|---|---|
| Age, y | 64 (9) | 63 (10) | 64 (10) |
| Male sex, No. (%) | 22 (51) | 27 (60) | 24 (60) |
| Body mass indexb | 26 (4) | 27 (4) | 27 (4) |
| Race, No. (%) | |||
| Asian | 2 (5) | 2 (4) | 2 (5) |
| Black | 1 (2) | 2 (4) | 2 (5) |
| White | 39 (91) | 40 (89) | 36 (90) |
| Not reported | 1 (2) | 1 (2) | 0 |
| Hispanic ethnicity, No. (%) | 3 (7) | 2 (4) | 1 (3) |
| Time since PD diagnosis, median (IQR), y | 0.3 (0.1-1.3) | 0.3 (0.2-0.8) | 0.4 (0.1-0.8) |
| Duration of symptoms, median (IQR), y | 1.5 (1.0-2.6) | 1.5 (0.9-3.1) | 1.4 (0.9-2.1) |
| Hoehn and Yahr stage, No. (%) | |||
| 1 | 12 (28) | 13 (29) | 8 (20) |
| 2 | 31 (72) | 32 (71) | 32 (80) |
| Parkinson Disease Questionnaire 39 Summary Index score | 11 (7) | 8 (6) | 9 (8) |
| Beck Depression Inventory score | 5 (4) | 4 (4) | 5 (3) |
| Montreal Cognitive Assessment score | 28 (1) | 28 (1) | 28 (1) |
| Maximum heart rate | 147 (22) | 153 (19) | 145 (16) |
| UPDRSc | |||
| Total score | 24 (10) | 23 (9) | 23 (8) |
| Part 1 score | 1.0 (1.5) | 0.8 (0.9) | 0.7 (0.9) |
| Part 2 score | 6 (3) | 6.0 (4) | 6.0 (3) |
| Part 3 score | 17 (7) | 16 (7) | 17 (7) |
| V̇o2max, mL/kg/mind | 23 (6) | 24 (7) | 24 (4) |
| Total daily stepse | 5146 (3107) | 5702 (2521) | 5005 (2987) |
Abbreviations: IQR, interquartile range; PD, Parkinson disease; UPDRS, Unified Parkinson’s Disease Rating Scale.
Data are presented as mean (percentage) unless otherwise indicated. The χ2 test was used for categorical variables and 1-way analysis of variance for continuous variables. There were no significant between-group differences in any baseline characteristics. High-intensity treadmill exercise was 4 days per week at 80%-85% maximum heart rate; moderate-intensity treadmill exercise, 4 days per week at 60%-65% maximum heart rate.
Calculated as weight in kilograms divided by the height in meters squared.
The UPDRS part 1 (mentation, behavior, and mood) is a summation of 4 items on a 5-point Likert scale scored 0 to 4; part 2 (activities of daily living), 13 items on a 5-point Likert scale scored 0 to 4; part 3 (motor), 27 items on a 5-point Likert scale scored 0 to 4; and total, 44 items on a 5-point Likert scale scored 0 to 4.
V̇o2max is the maximal aerobic power in milliliters of oxygen consumed per kilogram of body weight per minute.
Activity was measured by waist-worn ActiGraph GT3X+ and GT3X-BT accelerometers (Actigraph); because of missing data, sample sizes are 36 for 80% to 85% maximum heart rate, n = 42 for 60% to 65% maximum heart rate, and 36 for usual care.
Feasibility Outcomes
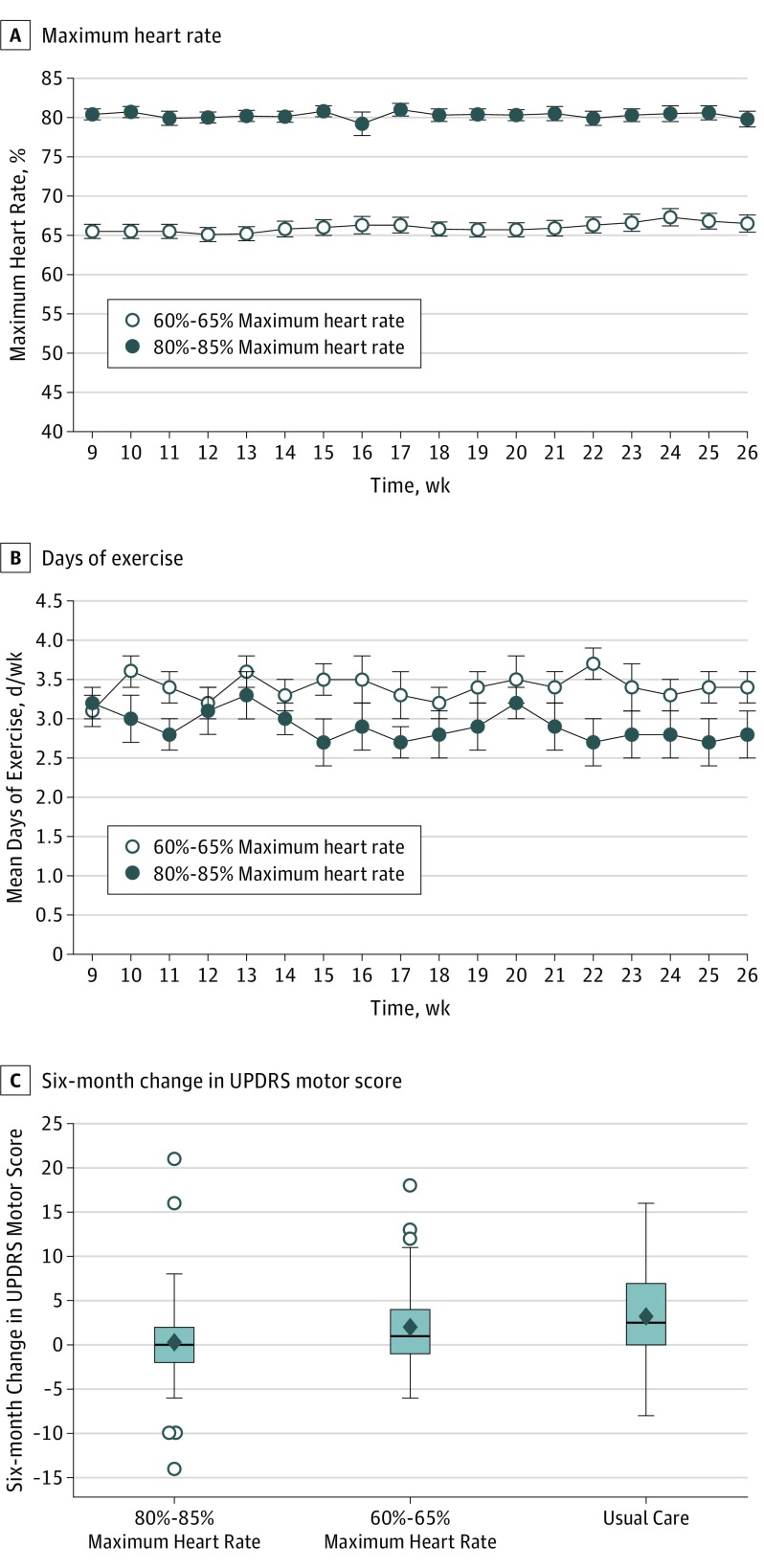
Both exercise groups met targeted treadmill exercise intensity (Figure 2A). The mean percent maximum heart rates were 80.2% (95% CI, 78.8%-81.7%) for the high-intensity group and 65.9% (95% CI, 64.2%-67.7%) for the moderate-intensity group, with no changes over time (P = .25). Mean weekly treadmill exercise frequency was 2.8 (95% CI, 2.4-3.2) days per week for the high-intensity group and 3.2 (95% CI, 2.8-3.6) days per week for the low-intensity group (P = .13) (Figure 2B), with a slight negative trend over time (−0.02 per week, P < .001).
Figure 2. Study Outcomes.
A, Percentage maximum heart rate for each intervention group at 60% to 65% maximum heart rate and 80% to 85% across weeks 9 to 26. B, Mean days exercised for each intervention group across weeks 9 to 26. In A and B, symbols represent the means and bars are the SEs. Trends over time were tested using linear mixed models. C, Distribution of the 6-month Unified Parkinson’s Disease Rating Scale (UPDRS) motor change scores (6 months minus baseline), in which a positive change indicates worsening of motor symptoms. Each boxplot contains the median (horizontal line in the box), the upper quartile (75th percentile, top of box), the lower quartile (25th percentile, bottom of box), the mean (diamonds), whiskers, and outliers that are beyond 1.5 times the interquartile range (75th percentile minus the 25th percentile) from the 25th or 75th percentiles (circles). For usual care, the whiskers extend to the observed minimum and maximum. For the 80% to 85% maximum heart rate and the 60% to 65% maximum heart rate, the whiskers extend to the maximum and minimum values observed that were not outliers.
A similar proportion of participants in each group (5 [12%] high intensity, 10 [22%] moderate intensity, and 6 [15%] usual care) initiated use of medication before 6 months (log-rank test P = .40). Overall attrition was 8.6%.
Futility Analysis Outcome
The mean change in UPDRS motor score in the high-intensity group was 0.3 (95% CI, −1.7 to 2.3) compared with 3.2 (95% CI, 1.4-5.1) in the usual care group (Figure 2C); thus, high-intensity treadmill exercise led to less motor change compared with usual care and the null hypothesis was not rejected (P = .34) (Table 2), indicating nonfutility and that high-intensity treadmill exercise warrants further investigation. Mean change in UPDRS motor score in the moderate-intensity treadmill exercise group was 2.0 (95% CI, 0.38-3.7) (Figure 2C); thus, the null hypothesis that moderate-intensity exercise was associated with less change of motor symptoms compared with usual care was rejected (P = .03) (Table 2), indicating futility of further investigating moderate-intensity exercise. Neither result (high-intensity nor moderate-intensity treadmill exercise) was affected by imputation using off-state UPDRS assessments and multiple imputation for missing data (Table 2).
Table 2. Six-Month Changes From Baseline in Study Measures and Between-Group Differences in the Change From Baselinea.
| Measure | Mean (SD) [Sample Size] | Usual Care vs High-Intensity Exercise | Usual Care vs Moderate-Intensity Exercise | ||||
|---|---|---|---|---|---|---|---|
| High-Intensity Exercise | Moderate-Intensity Exercise | Usual Care | Δ (CI)b | t Statistic (P Value)c | Δ (CI)b | t Statistic (P Value)c | |
| Primary Outcomes | |||||||
| UPDRS motor, primary analysisd | 0.3 (6.3) [39] | 2.0 (5.3) [42] | 3.2 (5.6) [38] | 2.9 (<4.7) | −0.42 (.34) | 1.2 (<2.8) | −1.9 (.03) |
| UPDRS motor, sensitivity analysis, off stated | 0.2 (6.3) [39] | 1.7 (6.0) [42] | 3.2 (5.6) [38] | 3.0 (<4.8) | −0.36 (.36) | 1.5 (<3.2) | −1.51 (.07) |
| UPDRS motor, sensitivity analysis, multiple imputationd | 0.5 (6.2) [43] | 1.9 (5.2) [45] | 3.2 (5.5) [40] | 2.7 (<4.4) | −0.62 (.27) | 1.2 (<2.8) | −1.9 (.03) |
| Secondary Outcomes | |||||||
| UPDRS Totald | 2.1 (7.2) | 3.0 (7.2) | 3.9 (6.3) | 1.8 (−1.3 to 4.9) | 1.18 (.24) | 0.9 (−2.1 to 3.9) | 0.60 (.55) |
| Part 1d | 0.3 (1.4) | −0.04 (1.2) | 0.05 (1.0) | −0.3 (−0.8 to 0.3) | −1.04 (−.30) | 0.1 (−0.4 to 0.6) | 0.40 (.69) |
| Part 2 d | 1.4 (3.4) | 1.0 (3.5) | 0.6 (2.4) | −0.8 (−2.2 to 0.5) | −1.23 (.22) | −0.4 (−1.7 to 1.0) | −0.55 (.59) |
| MDS-UPDRS motore | 0.3 (8.2) | 1.8 (7.4) | 4.2 (7.4) | 4.0 (0.4 to 7.5) | 2.21 (.03) | 2.4 (−0.9 to 5.7) | 1.46 (.15) |
| Part 1e | 1.0 (3.1) | −0.1 (3.2) | 0.7 (2.4) | −0.3 (−1.6 to 1.0) | −0.46 (.65) | 0.8 (−0.4 to 2.1) | 1.29 (.20) |
| Part 2e | 1.5 (3.3) | 0.7 (3.0) | 0.9 (2.8) | −0.6 (−2.0 to 0.8) | −0.84 (.40) | 0.2 (−101 to 1.5) | 0.27 (.79) |
| V̇o2max, mL/kg/minf | 1.9 (2.9) [35] | 0.1 (4.4) [41] | −1.3 (2.5) [36] | −3.2 (−4.5 to −1.9) | −5.03 (<.001) | −1.4 (−3.0 to 0.2) | −1.77 (.08) |
| Total step countg | 187 (3146) [31] | −334 (1929) [38] | −291 (2736) [31] | −477 (−1975 to 1021) | −0.64) (.53) | 44 (−1080 to 1167) | 0.08 (.94) |
Abbreviations: MDS, Movement Disorders Society; UPDRS, Unified Parkinson’s Disease Rating Scale.
High-intensity treadmill exercise was 4 days per week at 80%-85% maximum heart rate; moderate-intensity treadmill exercise, 4 days per week at 60%-65% maximum heart rate.
Difference between the usual care group changes and the exercise group changes. The CIs are 1-sided 90% CIs for the primary outcomes and 2-sided 95% CIs for the secondary outcomes. For the primary outcomes, a 90% upper confidence bound for Δ that is greater than 3.5 indicates that the data are consistent with the hypothesis that exercise is associated with a 3.5 lessening of motor symptom progression on the UPDRS motor score.
The t statistic represents the comparison of each exercise group’s mean change with the mean change in the usual care group relative to the variability of these changes and sample sizes in the groups. For the primary outcomes, t values and P values are null adjusted for the 3.5 futility threshold and 1-sided test (α = .10). A t value less than −1.28 (P < .10) indicates that data are not consistent with the hypothesis that exercise is associated with a 3.5 lessening of motor symptom progression on the UPDRS motor score. For the secondary outcomes, t values and P values are 2-sided tests with null equal to 0 (α = .05).
The UPDRS part 1 (mentation, behavior, and mood) is a summation of 4 items on a 5-point Likert scale scored 0 to 4; part 2 (activities of daily living), 13 items on a 5-point Likert scale scored 0 to 4; part 3 (motor), 27 items on a 5-point Likert scale scored 0 to 4; and total, 44 items on a 5-point Likert scale scored 0 to 4.
The MDS-UPDRS part 1 (nonmotor) is a summation of 13 items on a 5-point Likert scale scored 0 to 4; part 2 (daily living), 13 items on a 5-point Likert scale scored 0 to 4; and part 3 (motor), 33 items on a 5-point Likert scale scored 0 to 4.
V̇o2max is the maximal aerobic power in milliliters of oxygen consumed per kilogram of body weight per minute.
Activity was measured by waist-worn ActiGraph GT3X+ and GT3X-BT accelerometers (Actigraph).
Secondary Outcomes
The high-intensity treadmill exercise group had no difference in outcomes compared with usual care at 6 months for other UPDRS and MDS-UPDRS total scores and subscores, with the exception of MDS-UPDRS motor subscore, for which the high-intensity group had significantly less change (4.0; 95% CI, 0.4-7.5; P = .03) (Table 2). The moderate-intensity treadmill exercise group had no differences compared with usual care at 6 months for all UPDRS and MDS-UPDRS measures. V̇o2max improved for participants in the high-intensity group and decreased in the usual care group during 6 months (Table 2). Change in total step count revealed no difference between groups (Table 2).
Adverse Events
Adverse events related to endurance exercise were as expected (Table 3). Only 2 serious adverse events occurred, both in the moderate-intensity group and unrelated to exercise (Table 3).
Table 3. Adverse Events and Serious Adverse Eventsa.
| Event | Participants With Event, No. (%) | ||
|---|---|---|---|
| High-Intensity Exercise (n = 43) |
Moderate-Intensity Exercise (n = 45) |
Usual Care (n = 40) |
|
| Adverse events | |||
| Adverse events related to exercise | 13 (30.2) | 8 (17.8) | 0 |
| Adverse events related to exercise, severity greater than mild | 9 (20.9) | 4 (8.9) | 0 |
| Events >10% in a single arm | |||
| Fall | 6 (14.0) | 5 (11.1) | 9 (22.5) |
| Fall with severity greater than mild | 1 (2.3) | 1 (2.2) | 0 |
| Pain in extremity | 8 (18.6) | 3 (6.7) | 1 (2.5) |
| Pain with severity greater than mild | 4 (9.3) | 3 (6.7) | 1 (2.5) |
| Organ system >10% in a single group | |||
| Musculoskeletal and connective-tissue disorders | 15 (34.9) | 6 (13.3) | 2 (5.0) |
| Musculoskeletal and connective-tissue disorders with severity greater than mild | 10 (23.2) | 4 (8.9) | 2 (5.0) |
| Injury, poisoning, and procedural complicationsb | 7 (16.3) | 8 (17.8) | 9 (22.5) |
| Injury, poisoning, and procedural complications with severity greater than mild | 2 (4.6) | 3 (6.7) | 0 (0.0) |
| Infections and infestations | 6 (14.0) | 3 (6.7) | 2 (5.0) |
| Infections and infestations with severity greater than mild | 5 (11.6) | 3 (6.7) | 2 (5.0) |
| Serious adverse events | |||
| Gastrointestinal tract disorders | 0 | 1 (2.3) | 0 |
| Renal and urinary tract disorders | 0 | 1 (2.3) | 0 |
Adverse events were categorized using the Common Terminology Criteria for Adverse Events. High-intensity treadmill exercise was 4 days per week at 80%-85% maximum heart rate; moderate-intensity treadmill exercise, 4 days per week at 60%-65% maximum heart rate.
Includes fall events.
Discussion
Participants with Parkinson disease adhered to the prescribed exercise intensity and met the hypothesized frequency of 3 days per week during 6 months. The futility analysis indicated that high-intensity treadmill exercise warrants further investigation as an intervention for motor symptoms in de novo Parkinson disease. Adverse musculoskeletal events were expected with endurance exercise, based on previous exercise trials, and not serious, demonstrating that patients with Parkinson disease can exercise safely without direct supervision when guided by exercise specialists. The protocol was well tolerated as evidenced by the low attrition rate of 8.6%.
In a previous study, individuals with early- or middle-stage Parkinson disease had an improved UPDRS motor score after 6 months of exercise at 69.7% (95% CI, 67.1%-71.8%) of age-predicted maximum heart rate. However, to our knowledge, no previous endurance exercise studies have found differences among different intensities. To date, no studies have been conducted in patients with de novo Parkinson disease to eliminate the confounding of medication, have been powered on the UPDRS motor score, or have used 80% to 85% of maximum heart rate measured throughout each session. We found that high-intensity treadmill exercise is feasible and attenuates worsening on the UPDRS motor score consistent with the clinically meaningful threshold of 3.5. Furthermore, the between-group difference for the MDS-UPDRS motor subscores was comparable to the reported within-person minimal clinically important improvement of 3.2. In light of a recent report that low-dose, patient-centered, goal-directed physiotherapy and occupational therapy in patients in the early stages of Parkinson disease is not effective, a demonstration of the nonfutility of high-intensity treadmill exercise in patients with mild Parkinson disease is particularly important.
This trial was designed to test whether each intervention was nonfutile compared with change in the control group regardless of the direction. The CI for the observed UPDRS motor score difference between the high-intensity and usual care groups included the a priori prespecified 3.5 points for nonfutility. Lack of UPDRS motor score improvement in the high-intensity group is not surprising, given that participants were de novo with a baseline mean UPDRS score of 17. With regard to exercise session frequency, we set 3 days as the adherence criterion in the protocol for feasibility and clinical relevance. We prescribed 4 days per week, recognizing that participants might miss some sessions.
The exercise dose met the guidelines for cardiovascular benefits of endurance exercise and the recommendations of 150 minutes per week of moderate-intensity physical activity. The high-intensity treadmill exercise group had an increase in V̇o2max of 1.9 mL/min/kg (8%) compared with baseline, whereas the usual care group had a decrease of 1.3 mL/min/kg (5%). This finding agrees with the 7% to 8% change in V̇o2max in patients with Parkinson disease reported by Shulman et al for treadmill exercise at 70% to 80% heart rate reserve for 30 minutes.
No between-group differences were found in total daily step count change scores during 6 months. This finding suggests that exercisers reduced their daily steps outside their treadmill-training period such that overall step counts remained similar throughout the day. We did not provide patients with advice for what to do in the nonexercise period of the day. van Nimwegen et al reported that exercise training coupled with behavioral modification training increased total physical activity and daily energy expenditure in patients with Parkinson disease. Because sedentary behavior is an independent risk factor for all-cause mortality, understanding how structured exercise affects nonexercise physical activity behaviors in patients with Parkinson disease will provide important clinical information on how to maximize the long-term therapeutic benefits of exercise in this population.
This phase 2, multicenter futility design is of particular importance for the study of exercise in patients with Parkinson disease for several reasons. Only a few moderately sized exercise studies have been reported in this population. One of the limiting factors to moving to phase 3 trials is that the appropriate dose of exercise has yet to be established for any exercise modality. Exercise imposes a substantial participant commitment of time and effort compared with pharmacologic interventions. The futility design was used to specifically establish whether further study of specific exercise dose is warranted, proving a method to efficiently determine the appropriate dose before moving forward to the first phase 3 exercise trial in Parkinson disease. Findings of nonfutility of high-intensity treadmill exercise should move the field forward substantially.
Limitations
Our study has limitations. It was highly controlled, with only treadmill training allowed; thus, other modes of endurance exercise were not evaluated but should be in future investigations. We manipulated both treadmill speed and incline; it is not clear whether one or both can affect motor symptoms of Parkinson disease. It has been argued that cadence is the key variable to improve motor symptoms in Parkinson disease. To minimize participant burden, outcomes such as gait speed, gait endurance, and movement economy were not measured. Furthermore, other forms of exercise are important in Parkinson disease (eg, strength training, Tai chi). Investigations are needed to determine the combination of interventions that have the greatest effect on motor and other symptoms in patients with de novo Parkinson disease. Finally, the effect of this intervention on other factors (eg, cognition, sleep) should be compared for the moderate- and high-intensity groups.
Conclusions
We demonstrated feasibility and safety of high-intensity treadmill exercise in patients with de novo Parkinson disease. A larger efficacy trial is warranted to determine whether exercising at 80% to 85% maximum heart rate produces meaningful clinical benefits in de novo Parkinson disease. Meanwhile, clinicians may safely prescribe exercise at this intensity level for this population.
Trial Protocol.
References
- 1.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896-912. [DOI] [PubMed] [Google Scholar]
- 2.Shulman LM, Gruber-Baldini AL, Anderson KE, et al. . The evolution of disability in Parkinson disease. Mov Disord. 2008;23(6):790-796. [DOI] [PubMed] [Google Scholar]
- 3.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670-1683. [DOI] [PubMed] [Google Scholar]
- 4.Smith AD, Zigmond MJ. Can the brain be protected through exercise? lessons from an animal model of parkinsonism. Exp Neurol. 2003;184(1):31-39. [DOI] [PubMed] [Google Scholar]
- 5.Zigmond MJ, Smeyne RJ. Exercise: is it a neuroprotective and if so, how does it work? Parkinsonism Relat Disord. 2014;20(suppl 1):S123-S127. [DOI] [PubMed] [Google Scholar]
- 6.Tuon T, Souza PS, Santos MF, et al. . Physical training regulates mitochondrial parameters and neuroinflammatory mechanisms in an experimental model of Parkinson’s disease. Oxid Med Cell Longev. 2015;2015:261809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang Y, Koo JH, Kang EB, et al. . Neuroprotective effects of endurance exercise in MPTP-induced Parkinson’s disease mice. Brain Res. 2017;1655:186-193. [DOI] [PubMed] [Google Scholar]
- 8.Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12(7):716-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uc EY, Doerschug KC, Magnotta V, et al. . Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology. 2014;83(5):413-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenkman M, Hall DA, Barón AE, Schwartz RS, Mettler P, Kohrt WM. Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys Ther. 2012;92(11):1395-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shulman LM, Katzel LI, Ivey FM, et al. . Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol. 2013;70(2):183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair. 2009;23(6):600-608. [DOI] [PubMed] [Google Scholar]
- 13.Fisher BE, Wu AD, Salem GJ, et al. . The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil. 2008;89(7):1221-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77(3):288-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Q, Park Y, Huang X, et al. . Physical activities and future risk of Parkinson disease. Neurology. 2010;75(4):341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridgel AL, Phillips RS, Walter BL, Discenzo FM, Loparo KA. Dynamic high-cadence cycling improves motor symptoms in Parkinson’s disease. Front Neurol. 2015;6:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamotte G, Rafferty MR, Prodoehl J, et al. . Effects of endurance exercise training on the motor and non-motor features of Parkinson’s disease: a review. J Parkinsons Dis. 2015;5(1):21-41. [DOI] [PubMed] [Google Scholar]
- 18.Bergen JL, Toole T, Elliott RG III, Wallace B, Robinson K, Maitland CG. Aerobic exercise intervention improves aerobic capacity and movement initiation in Parkinson’s disease patients. NeuroRehabilitation. 2002;17(2):161-168. [PubMed] [Google Scholar]
- 19.Moore CG, Schenkman M, Kohrt WM, Delitto A, Hall DA, Corcos D. Study in Parkinson disease of exercise (SPARX): translating high-intensity exercise from animals to humans. Contemp Clin Trials. 2013;36(1):90-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study, 1992. Neurology. 2001;57(10)(suppl 3):S34-S38. [PubMed] [Google Scholar]
- 21.Goetz CG, Poewe W, Rascol O, et al. ; Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease . Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020-1028. [DOI] [PubMed] [Google Scholar]
- 22.National Institute of Health NINDS Common Data Elements. https://www.commondataelements.ninds.nih.gov/PD.aspx#tab=Data_Standards. Accessed August 1, 2017.
- 23.Zhao W, Hill MD, Palesch Y. Minimal sufficient balance-a new strategy to balance baseline covariates and preserve randomness of treatment allocation. Stat Methods Med Res. 2015;24(6):989-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao W, Weng Y. Block urn design: a new randomization algorithm for sequential trials with two or more treatments and balanced or unbalanced allocation. Contemp Clin Trials. 2011;32(6):953-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahn S, Elton RL UPDRS Program Members. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson’s Disease. Vol 2. Florham Park, NJ: Macmillan Healthcare Information; 1987:153-163, 293-304. [Google Scholar]
- 26.Langston JW, Widner H, Goetz CG, et al. . Core assessment program for intracerebral transplantations (CAPIT). Mov Disord. 1992;7(1):2-13. [DOI] [PubMed] [Google Scholar]
- 27.Goetz CG, Fahn S, Martinez-Martin P, et al. . Movement Disorder Society–sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22(1):41-47. [DOI] [PubMed] [Google Scholar]
- 28.Kohrt WM, Malley MT, Coggan AR, et al. . Effects of gender, age, and fitness level on response of VO2max to training in 60-71 yr olds. J Appl Physiol (1985). 1991;71(5):2004-2011. [DOI] [PubMed] [Google Scholar]
- 29.Christiansen C, Moore C, Schenkman M, et al. . Factors associated with ambulatory activity in de novo Parkinson’s disease. J Neurol Phys Ther. 2017;41(2):93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03. Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health, National Cancer Institute; June 2010.
- 31.Goetz CG, Tilley BC, Shaftman SR, et al. ; Movement Disorder Society UPDRS Revision Task Force . Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. [DOI] [PubMed] [Google Scholar]
- 32.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the Unified Parkinson’s Disease Rating Scale. Arch Neurol. 2010;67(1):64-70. [DOI] [PubMed] [Google Scholar]
- 33.Horváth K, Aschermann Z, Ács P, et al. . Minimal clinically important difference on the motor examination part of MDS-UPDRS. Parkinsonism Relat Disord. 2015;21(12):1421-1426. [DOI] [PubMed] [Google Scholar]
- 34.Clarke CE, Patel S, Ives N, et al. ; PD REHAB Collaborative Group . Physiotherapy and occupational therapy vs no therapy in mild to moderate Parkinson disease. JAMA Neurol. 2016;73(3):291-299. [DOI] [PubMed] [Google Scholar]
- 35.Pescatello LS, Arena R, Riebe D, Thompson PD. ACSM’s Guidelines for Exercise Testing and Prescription. 9th ed Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 36.Physical Activity and Health https://www.cdc.gov/physicalactivity/basics/pa- health/index.htm. Accessed September 12, 2016.
- 37.van Nimwegen M, Speelman AD, Overeem S, et al. ; ParkFit Study Group . Promotion of physical activity and fitness in sedentary patients with Parkinson’s disease: randomised controlled trial. BMJ. 2013;346:f576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evenson KR, Herring AH, Wen F. Accelerometry-assessed latent class patterns of physical activity and sedentary behavior with mortality. Am J Prev Med. 2017;52(2):135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corcos DM, Robichaud JA, David FJ, et al. . A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord. 2013;28(9):1230-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F, Harmer P, Fitzgerald K, et al. . Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366(6):511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridgel AL, Peacock CA, Fickes EJ, Kim CH. Active-assisted cycling improves tremor and bradykinesia in Parkinson’s disease. Arch Phys Med Rehabil. 2012;93(11):2049-2054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.