Key Points
Question
What cortical structures in the human brain are associated with blood pressure control?
Findings
In this case series of 12 patients with intractable epilepsy who underwent deep brain electrical stimulation, 4 patients had electrodes placed in Brodmann area 25 (rostral subcallosal neocortex). Stimulation of 9 such electrodes (7 left and 2 right) in this area in these patients induced significant, consistent decreases in systolic blood pressure.
Meaning
Brodmann area 25 may have a role in lowering systolic blood pressure in humans.
Abstract
Importance
A better understanding of the role of cortical structures in blood pressure control may help us understand cardiovascular collapse that may lead to sudden unexpected death in epilepsy (SUDEP).
Objective
To identify cortical control sites for human blood pressure regulation.
Design, Setting, and Participants
Patients with intractable epilepsy undergoing intracranial electrode implantation as a prelude to epilepsy surgery in the Epilepsy Monitoring Unit at University Hospitals Cleveland Medical Center were potential candidates for this study. Inclusion criteria were patients 18 years or older who had electrodes implanted in one or more of the regions of interest and in whom deep brain electrical stimulation was indicated for mapping of ictal onset or eloquent cortex as a part of the presurgical evaluation. Twelve consecutive patients were included in this prospective case series from June 1, 2015, to February 28, 2017.
Main Outcomes and Measures
Changes in continuous, noninvasive, beat-by-beat blood pressure parameter responses from amygdala, hippocampal, insular, orbitofrontal, temporal, cingulate, and subcallosal stimulation. Electrocardiogram, arterial oxygen saturation, end-tidal carbon dioxide, nasal airflow, and abdominal and thoracic plethysmography were monitored.
Results
Among 12 patients (7 female; mean [SD] age, 44.25 [12.55] years), 9 electrodes (7 left and 2 right) all in Brodmann area 25 (subcallosal neocortex) in 4 patients produced striking systolic hypotensive changes. Well-maintained diastolic arterial blood pressure and narrowed pulse pressure indicated stimulation-induced reduction in sympathetic drive and consequent probable reduction in cardiac output rather than bradycardia or peripheral vasodilation–induced hypotension. Frequency-domain analysis of heart rate and blood pressure variability showed a mixed picture. No other stimulated structure produced significant blood pressure changes.
Conclusions and Relevance
These findings suggest that Brodmann area 25 has a role in lowering systolic blood pressure in humans. It is a potential symptomatogenic zone for peri-ictal hypotension in patients with epilepsy.
This case series identifies cortical control sites for human blood pressure regulation in humans.
Introduction
Peri-ictal autonomic dysregulation can occur during and after epileptic seizures, including significant blood pressure changes. Hypotension has been described after seizures and has been suggested as a potential sudden unexpected death in epilepsy (SUDEP) biomarker. The role of cortical blood pressure control sites, through seizure-induced, disordered, or inhibited function, may be key. Although some animal studies describe cortical blood pressure control structures, only downstream, mainly brainstem regions have been convincingly identified in humans. Invasive investigations of brains of patients with refractory epilepsy undergoing assessment for epilepsy surgery provide unique opportunities for mapping such potential cortical sites. Their identification and electroclinical seizure characterization may yield clues to SUDEP pathomechanisms, as well as provide therapeutic targets in intractable essential hypertension.
Methods
Rationale
Several suprapontine brain structures governing blood pressure function have been identified, albeit inconsistently, in animals (orbitofrontal, cingulate, subcallosal, insular, hippocampal, amygdalar, temporal, and motor cortices) and in humans (orbitofrontal, insula, and anterior cingulate cortices). Therefore, these were our regions of interest for this study. Using modern stereotactic techniques in patients undergoing invasive electroencephalogram (EEG) studies as a prelude to epilepsy surgery, we aimed to identify the role of these structures in human blood pressure control. All patients provided written informed consent as participants in a University Hospitals Cleveland Medical Center Institutional Review Board–approved research project evaluating the role of cortical structures in human respiratory and autonomic function.
Patients and Clinical Setting
From June 1, 2015, to February 28, 2017, we prospectively studied 12 consecutive patients with medically intractable epilepsy undergoing stereotactic EEG evaluations for epilepsy surgery in the Epilepsy Monitoring Unit at University Hospitals Cleveland Medical Center. Inclusion criteria were patients 18 years or older who had electrodes implanted in one or more of the above-mentioned brain regions of interest and in whom direct cortical electrical stimulation was indicated for mapping of ictal onset or eloquent cortex regions. Electrodes surrounded by radiologically visible hemorrhage were excluded from study. The number and locations of depth electrodes were tailored according to the suspected location of the epileptogenic zone in each patient based on clinical history, semiology, neuroimaging, and noninvasive EEG.
Procedure
Platinum-iridium depth electrodes measuring 1.1 mm in diameter and 2.5 mm in length, evenly spaced at 5-mm intervals, were implanted stereotactically using general anesthesia. Implantation trajectories were simulated using a software package (iPlan-Stereotaxy, version 2.6; Brainlab) based on recent 3-T magnetic resonance imaging (MRI) of the brain. Cranial computed tomography was performed within 24 hours after surgery. Using the iPlan-Stereotaxy software, postsurgical cranial computed tomography and presurgical brain MRI scans were superimposed for precise localization of single-electrode contacts within the patient’s presurgical MRI.
Stimulation
Bedside cortical electrical stimulation was carried out using a stimulator (Ojemann; Integra Life Sciences) (bipolar and monopolar stimulation, 50 Hz and 0.2 milliseconds pulse width, with train durations of up to 30 seconds). Current intensity started at 1 mA to a maximum of 10 mA. These parameters were chosen for safety reasons because they are identical to those used for brain mapping for clinical purposes. If a seizure was induced, stimulation was discontinued. Resuscitation equipment was always kept in intimate proximity to the patient in case of need.
Blood Pressure, Cardiac, Respiration, and EEG Monitoring
Beat-to-beat systolic (SAP), diastolic (DAP), and mean arterial pressure (MAP) were recorded using a continuous noninvasive arterial pressure monitor (Monitor 500; CNSystems Medizintechnik AG). Nasal airflow was recorded using a nasal thermistor (Thermocouple Airflow Sensor; Pro-Tech). Arterial oxygen saturation and heart rate were monitored using pulse oximetry (Nellcor OxiMax N-600x; Covidien) and end-tidal carbon dioxide using a capnograph (Model 7900; Philips). Electroencephalogram and electrocardiogram were acquired using a diagnostic system (EEG-1200; Nihon Kohden) with a 256-channel amplifier. We arbitrarily defined significant blood pressure response as a decrease or increase by more than 5 mm Hg from the baseline mean during the stimulation period. The blood pressure response was only considered positive if there was a subsequent tendency to recover when stimulation was discontinued and when this response was consistently reproduced (during ≥5 sessions). Stimulation was initiated when SAP was within normal limits (100-125 mm Hg) and was immediately discontinued if it dropped either below 90 mm Hg or by more than 25 mm Hg from baseline. The EEG was closely scrutinized for stimulation-induced seizures and after-discharges during stimulation; if after-discharges were induced, that stimulation period was excluded from analysis.
Data Analysis
A custom-developed graphical user interface (Matlab; MathWorks Inc) that included both signal processing and computational tools was used to automatically detect electrocardiogram R-wave, SAP, and DAP values as the maximum and minimum points between 2 consecutive R peaks. A series of four 5-minute consecutive epochs of artifact-free awake-state rest recordings were identified as baseline. Twenty minutes of frequency-domain baroreflex sensitivity (BRS), blood pressure variability (BPV), and heart rate variability (HRV) values were averaged to calculate baseline values. Stimulation values were calculated from initiation of stimulus until heart rate and blood pressure returned to baseline levels.
Frequency-domain BRS was calculated as the average of the magnitude of the transfer function between oscillations of SAP and RR interval. Low-frequency (LF) range was defined between 0.04 and 0.15 Hz, and high-frequency (HF) range was defined between 0.15 and 0.40 Hz. The ratio of LF to HF was used as a measure of sympathovagal balance. Total power (TP) for BRS and HRV was calculated as the sum of the LF and HF bands (TP = LF + HF) and was used to normalize frequency-domain values to correct for overall drops in total autonomic power. These normalized values were calculated by dividing the LF or HF band by TP and are reported as a percentage using the following equation: Normalized Unit Value for HF = [HF / (LF + HF)] × 100.
The evaluation of BRS is an established tool to assess autonomic control of the cardiovascular system. Changes in the characteristics of baroreflex function reflect alterations in autonomic control of the cardiovascular system. The quantitative measure of BRS is provided by the slope of the fitted line, and it is commonly expressed as the change in RR interval in ms per mm Hg change in SAP (ms/mm Hg). Calculated slope in sleep and awake states in healthy individuals is 9 to 12 ms/mm Hg. An increase in SAP accompanied by a limited change in RR interval with a calculated slope lower than 3 ms/mm Hg identifies a pathological weak autonomic response.
Statistical Analysis
All values are expressed as the mean (SEM). A paired-samples t test was conducted to compare stimulation averages with baseline values. Significance was set at 2-sided P < .05. The strength of the linear association (correlation) between spontaneous SAP and RR intervals was assessed by Pearson product moment correlation coefficient r, and only those data sequences with r exceeding 0.7 were analyzed further.
Results
Demographics and characteristics of the 12 patients (7 female; mean [SD] age, 44.25 [12.55] years) are summarized in the Table. Patient 12 had essential hypertension treated with amlodipine besylate (5 mg/d) that was continued during the evaluation. The rest of the patients did not have any cardiorespiratory comorbidity and were not taking any cardiovascular medications. At the time of stimulation, patient 1 was taking his regular dosage of lacosamide (200 mg/d) and topiramate (400 mg/d), patient 5 was taking clonazepam (0.25 mg/d), and patient 12 was taking levetiracetam (4500 mg/d), vimpat (600 mg/d), and clobazam (30 mg/d). The remaining patients were off antiepileptic medications as a part of the clinical protocol aimed at capturing seizures to localize the epileptogenic zone. In total, 1084 electrode contacts were implanted based on the surgical hypothesis in each case. Of these, 544 electrodes were implanted in our regions of interest, including 43 amygdala, 87 hippocampus head and body, 16 insular, 31 orbitofrontal, 31 temporopolar, 296 lateral temporal, 4 basal temporal, 13 anterior cingulate, 9 subcallosal (Brodmann area 25), and 14 posterior cingulate neocortex. Of 544 electrodes, 126 were stimulated according to the study protocol, including 23 amygdala, 17 hippocampus head, 8 anterior insula, 16 orbitofrontal, 12 temporopolar, 24 lateral temporal, 2 basal temporal, 13 anterior cingulate, 9 subcallosal neocortex (Brodmann area 25), and 2 posterior cingulate (eTable in the Supplement). The rest of the electrodes (540 of 1084) were placed either in white matter or in gray matter outside our regions of interest.
Table. Patient and Epilepsy Characteristics and Blood Pressure Responses.
| Patient No./Sex/Age, y | Seizure Duration, y | Handedness | Epileptogenic Zone | Seizure Semiology | Seizure Frequency | GTCS Frequency | Etiology | Brain MRI | Hypotensive Responses |
|---|---|---|---|---|---|---|---|---|---|
| 1/F/43 | 2 | Right | Left temporal | Aura→dialeptic seizure→GTCS | 4/wk | 1/y | Cryptogenic | Normal | No |
| 2/F/36 | 4 | Right | Left temporal | Automotor seizure→right versive seizure→GTCS | 1/wk | 1/y | Cryptogenic | Normal | No |
| 3/M/48 | 3 | Right | Left temporal | Aura→dialeptic seizure | 2/d | None | Cryptogenic | Normal | No |
| 4/M/39 | 10 | Right | Left temporal | Automotor seizure→GTCS | 3/wk | 2/mo | Cryptogenic | Normal | No |
| 5/M/40 | 5 | Right | Left temporal | Dialeptic seizure→GTCS | 1-2/mo | 1/y | Cryptogenic | Normal | No |
| 6/M/66 | 30 | Right | Left temporal | Automotor seizure→GTCS | 2-3/wk | 1/y | Cryptogenic | Normal | No |
| 7/F/20 | 14 | Right | Left hemisphere | Apnea seizure→right versive seizure→GTCS | 1/d | 1/d | Cryptogenic | Normal | Yes |
| 8/F/32 | 25 | Right | Left hemisphere | Hypnopompic seizure→right versive seizure→GTCS | 1/mo | 1/mo | Heterotopia | Right frontal heterotopia | Yes |
| 9/M/26 | 8 | Right | Right temporal | Automotor seizure | 1/mo | None | Cryptogenic | Normal | Yes |
| 10/F/49 | 3 | Left | Left temporal | Automotor seizure | 2/d | 1/y | Cryptogenic | Normal | No |
| 11/F/69 | 44 | Right | Right temporal | Abdominal aura→automotor seizure→GTCS | 1/mo | Once | Cryptogenic | Normal | No |
| 12/F/63 | 50 | Right | Left frontal | Asymmetric tonic seizure→GTCS | 1/d | Twice | Cryptogenic | Normal | Yes |
Abbreviations: GTCS, generalized tonic-clonic seizure; MRI, magnetic resonance imaging.
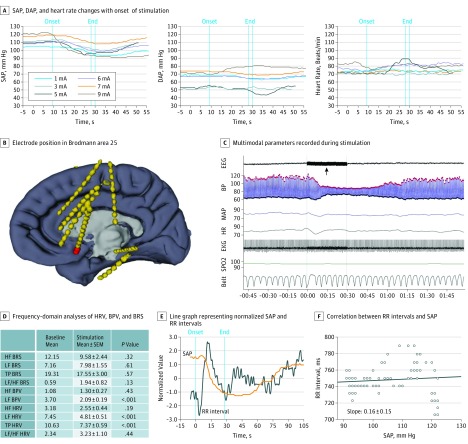
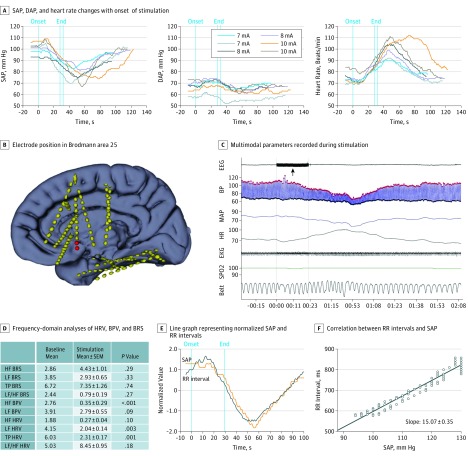
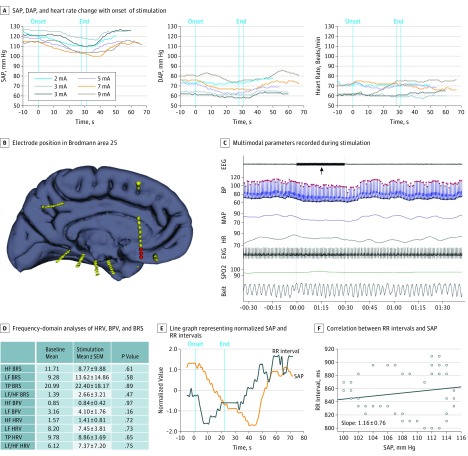
Stimulation in all electrodes placed in Brodmann area 25 in patients 7, 8, 9, and 12 (in whom 9 electrodes [7 left and 2 right] produced striking systolic hypotensive changes) resulted in rapid and consistently reproducible decreases in SAP, with a mean (SEM) drop of 15 (10-42) mm Hg (Figures 1, 2, 3, and 4 and Video 1 and Video 2). The SAP decreases appeared after a mean (SEM) latency of 8.5 (1-14) seconds. At times, the fall in SAP was preceded by a slight rise (Figure 2). Once stimulation was discontinued, SAP began to increase within a mean (SEM) of 12 (1-47) seconds (Figures 1, 2, and 3 and the eTable in the Supplement). The DAP did not change concurrently with SAP, resulting in a consistent narrowing of pulse pressure in all patients (Figures 1, 2, and 3 and the eFigure in the Supplement). Heart rate responses differed. In patient 8, heart rate increased accordingly with SAP. On the other hand, in patients 7, 9, and 12, heart rate did not significantly change (Figures 1, 2, and 3 and the eFigure in the Supplement). Arterial oxygen saturation and end-tidal carbon dioxide did not change at any time during or after stimulation. Frequency-domain analysis of BRS, BPV, and HRV comparing baseline with the stimulation period and baroreflex slope was performed in patients 7, 8, and 9 and showed a mixed picture (Figures 1, 2, and 3).
Figure 1. Stimulation-Induced Cardiovascular Changes in Patient 7.
Stimulation parameters used were 50 Hz, 0.2 milliseconds, and 1 to 9 mA. A, Systolic (SAP), diastolic (DAP), and heart rate (HR) changes with onset of stimulation. B, Electrode position in Brodmann area 25 (in red). C, Multimodal parameters recorded during stimulation (black arrow) demonstrating a clear reduction in SAP and a narrowing of pulse pressure, with no changes in oxygenation or breathing. Red dots in the blood pressure (BP) tracing represent SAP, and black dots represent DAP. D, Frequency-domain analyses of heart rate variability (HRV), blood pressure variability (BPV), and baroreflex sensitivity (BRS) showing significant decrease of low-frequency (LF) BPV and LF HRV. E, Line graph representing normalized SAP and RR intervals. F, Correlation between RR intervals and SAP, and the corresponding RR milliseconds/mm Hg slope of 0.2, indicating reduced BRS. EEG indicates electroencephalogram; EKG, electrocardiogram; HF, high frequency; MAP, mean arterial pressure; SPo2, arterial oxygen saturation; and TP, total power.
Figure 2. Stimulation-Induced Cardiovascular Changes in Patient 8.
Stimulating parameters used were 50 Hz, 0.2 milliseconds, and 1 to 10 mA. A, Systolic (SAP), diastolic (DAP), and heart rate (HR) changes with onset of stimulation. Red dots represent SAP, and black dots represent DAP. B, Electrode position in Brodmann area 25 (in red). C, Multimodal parameters recorded during stimulation (black arrow) demonstrating a clear reduction in SAP and a narrowing of pulse pressure, with no changes in oxygenation or breathing. D, Frequency-domain analyses of heart rate variability (HRV), blood pressure variability (BPV), and baroreflex sensitivity (BRS). E, Line graph representing normalized SAP and RR intervals. F, Correlation between RR intervals and SAP, and the corresponding RR milliseconds/mm Hg slope of 15, indicating intact BRS. EEG indicates electroencephalogram; EKG, electrocardiogram; HF, high frequency; MAP, mean arterial pressure; SPo2, arterial oxygen saturation; and TP, total power.
Figure 3. Stimulation-Induced Cardiovascular Changes in Patient 9 With Impaired Autonomic Responses.
Stimulating parameters used were 50 Hz, 0.2 milliseconds, and 1 to 9 mA. A, Systolic (SAP), diastolic (DAP), and heart rate (HR) changes with onset of stimulation. B, Electrode position in Brodmann area 25 (in red). C, Multimodal parameters recorded during stimulation (black arrow). Red dots represent SAP, and black dots represent DAP. D, Frequency-domain analyses of heart rate variability (HRV), blood pressure variability (BPV), and baroreflex sensitivity (BRS). E, Line graph representing normalized SAP and RR intervals. F, Correlation between RR intervals and SAP, and the corresponding RR milliseconds/mm Hg slope. EEG indicates electroencephalogram; EKG, electrocardiogram; HF, high frequency; MAP, mean arterial pressure; SPo2, arterial oxygen saturation; and TP, total power.
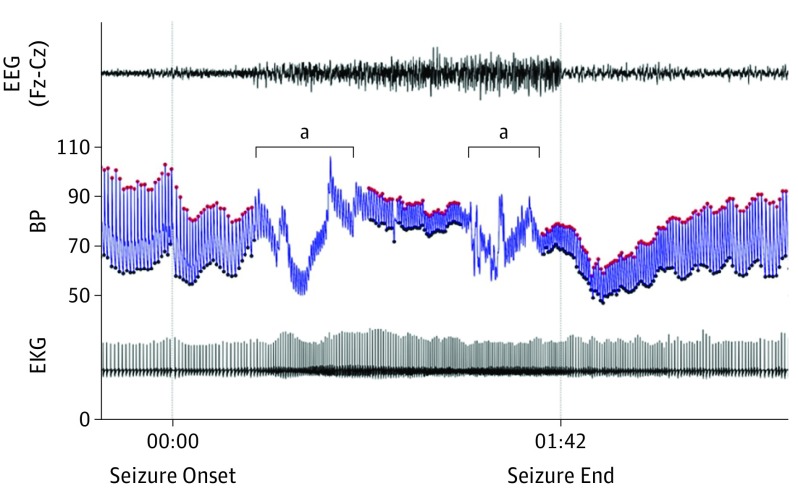
Figure 4. Hypotension During and After a Seizure.
Shown is ictal and postictal hypotension during a complex partial seizure with oral automatisms in patient 9 recorded with surface electroencephalogram (EEG) and continuous blood pressure (BP) monitoring. Red dots represent systolic arterial pressure, and black dots represent diastolic arterial pressure. EKG indicates electrocardiogram. aTwo periods during ictus when movement artifact of the blood pressure–cuffed limb renders acquisition unreliable.
Video 1. Stimulation Session in Patient 7 Showing Hypotensive Responses Induced by Brodmann Area 25 Stimulation.
The patient has no cardiovascular comorbidities. Shown is bipolar stimulation of Brodmann area 25 (electrodes FF1-FF2) in patient 7 with simultaneous recording of (from top of machine display to bottom) systolic, diastolic, and mean arterial pressure and heart rate. Stimulation periods are clearly seen in the electroencephalogram record, as evidenced by electrical artifact. Systolic arterial pressure before stimulation onset was 120 to 122 mm Hg. Eight seconds after stimulation begins, systolic arterial pressure starts rapidly decreasing to 92 mm Hg. Twenty seconds after stimulation ends, systolic arterial pressure rapidly increases and then remains stable at 116 to 117 mm Hg, slightly lower than the baseline.
Video 2. Stimulation Session in Patient 12 Showing Hypotensive Responses Induced by Brodmann Area 25 Stimulation.
The patient has essential hypertension and is receiving treatment. Shown is monopolar stimulation of Brodmann area 25 (electrode FA1 [reference electrode AI2]) in patient 12 with simultaneous recording of (from top of machine display to bottom) systolic, diastolic, and mean arterial pressure and heart rate. Stimulation periods are clearly seen in the electroencephalogram record, as evidenced by electrical artifact. Stimulation 1, Before stimulation begins, systolic arterial pressure is stable (148-150 beats/min). Seven seconds after stimulation begins at current intensity of 5 mA, systolic arterial pressure decreases progressively during stimulation and continues for 10 seconds after stimulation is discontinued, from 148 to 128 mm Hg. Then, systolic arterial pressure progressively increases and remains stable at 144 to 145 mm Hg. Stimulation 2, Stimulation at 6 mA of intensity. Systolic arterial pressure progressively decreases after 8 seconds for the entire stimulation period and for 20 seconds after stimulation is discontinued. Stimulation 3, Once systolic arterial pressure stabilizes, stimulation starts at 7 mA. After 7 seconds, systolic arterial pressure progressively decreases from 145 to 116 mm Hg and for 12 seconds after stimulation is discontinued. Systolic arterial pressure then increases back up to 138 mm Hg.
During some of the Brodmann area 25 stimulation sessions, brief after-discharges were induced, although there were no differences in blood pressure responses when the after-discharges were induced or not. However, we excluded stimulations with after-discharges to ensure that blood pressure responses were being produced exclusively by Brodmann area 25 stimulation and not by after-discharges in other brain areas.
We analyzed recorded seizures in those patients in whom we found hypotensive responses to specifically look for spontaneous peri-ictal hypotensive changes and for correlation of seizure discharges in Brodmann area 25 with hypotension. Patient 7 did not have hypotensive changes with the single seizure that was recorded; the Brodmann area 25 electrode was involved in the seizure, although the seizure discharge was widespread at that point. Patient 8 had no blood pressure recordings during seizures. Patient 9 had no seizures recorded during intracranial EEG monitoring with blood pressure recordings. However, he previously had 3 complex partial seizures with oral automatisms recorded with surface EEG and continuous blood pressure monitoring. Two of these had ictal and post-ictal hypotension (Figure 4). Patient 12 had asymmetric tonic seizures lasting for less than 10 seconds in which blood pressure did not change and where the seizure did not involve Brodmann area 25.
No significant blood pressure responses were noted after stimulation of amygdala, hippocampus, and insular, orbitofrontal, temporopolar, lateral temporal, basal temporal, anterior cingulate, and posterior cingulate neocortex. Central apnea induced by stimulation was observed in temporal lobe structures and reported separately. Apnea was not associated with blood pressure responses.
Discussion
The results of this study suggest that Brodmann area 25 has a role in lowering systolic blood pressure in humans and is a likely symptomatogenic site for peri-ictal hypotension. However, these data need to be reproduced in a larger sample of patients. This region is infrequently studied as a part of orbitofrontal and anterior cingulate invasive EEG explorations in refractory focal epilepsy, hence the small sample size in our study for which implantations were driven by the surgical rather than study hypothesis. Brodmann area 25 is also a site that has been reported to produce hypotensive changes in animals. In humans, although the role of cortical structures in blood pressure control is inferred, this has hitherto not been conclusively established, and no single brain region has been universally accepted as a control site. Anterior limbic region stimulation in dogs and monkeys has produced marked falls in arterial blood pressure, as well as occasional rises. Such falls usually occurred without significant alteration in heart rate.
Similar responses were seen after subcallosal, postorbital, anterior insular, cingulate gyrus, hippocampal, amygdalar, temporal, and motor cortices stimulation. In humans, for whom opportunities to conduct similar experiments are limited, few studies of cortical stimulation targeting blood pressure control structures exist. In one study, stimulation of bilateral rostrocaudal cingulate gyrus (Brodmann areas 9 and 10) was carried out among patients with psychosis before ablation in 12 cases. Blood pressure changes of SAP and DAP elevation in 8 patients and a drop in 1 patient were noted. Unilateral stimulation produced no responses at all. In another study, orbitofrontal cortical stimulation in 9 patients undergoing frontal lobotomies for psychiatric disease produced inconsistent elevation of SAP in 6 of them. In a third study, only subtle DAP and heart rate changes were reported after stimulation of insular cortex in 5 patients with epilepsy undergoing surgery for control of intractable seizures. Therefore, the present investigation is the first report to date of a dramatic, consistently reproducible blood pressure influence in all patients who had the same, restricted, cortical site stimulated, namely, the subcallosal region of Brodmann area 25.
Our use of stereotactic EEG provides unprecedented depth electrode placement precision in deep cortical structures. This not only localizes putative epileptogenic zones but also allows unique anatomical resolution for direct electrical stimulation and subsequent, confident identification of eloquent cortical structures. Stimulation parameters identical to those used in routine clinical cortical mapping suffice for these purposes. Repeated stimulation of 7 electrodes in the immediately adjacent anterior cingulate cortex of patients 10 and 11 herein produced no such responses. In patient 9, blood pressure responses were definitely present but were less impressive, most likely due to their significantly more anterior location close to the border between Brodmann areas 24 and 25 (Figures 1, 2, and 3). This suggests that blood pressure influences are limited to Brodmann area 25. It is also consistent with previous observations of no blood pressure responses with unilateral anterior cingulate stimulation. Similarly, albeit with the limited number of patients in our study, orbitofrontal, insula (anterior part), amygdalar, hippocampal head, posterior cingulate, temporopolar, and temporal neocortex stimulations did not produce blood pressure responses; therefore, we could not confirm the role of these structures in human autonomic control of blood pressure. Several reasons are possible. Human studies that have reported blood pressure changes with stimulation of the cingulate and insula regions have used stimulation parameters with substantially greater stimulus intensity than in our study. For example, Pool and Ransohoff used up to 120-Hz frequency and 60-second train durations in their study, possibly accounting for their positive findings in these brain structures. Patients 7, 8, and 12 had insula stimulation in our study, both of whom only had anterior insular stimulation.
The mechanism of such striking falls in SAP without concurrent falls in DAP and heart rate is likely due to a cardioinhibitory reduction in myocardial contractility and a reduction in left ventricular stroke volume, as indicated by the narrowing of pulse pressure in all of our patients. The lack of significant changes in DAP (a product of resting transmural force blood volume exerted against vascular walls) during the stimulation period excludes peripheral vasodilatation as a cause. Stimulation of Brodmann area 25 likely produces downstream influences in or distal to the lateral hypothalamic nuclei or ventral periventricular or periaqueductal gray areas, brainstem regions known to produce blood pressure effects. Rich connections with Brodmann area 25 are found in these regions. Downstream candidate brainstem structures include the nucleus of the rostral and ventrolateral medulla, medullary raphe, and the A5 noradrenergic group of the pons. The ultimate influence is likely to be a reduction in sympathetic outflow in the efferent arm of the baroreflex emanating from the rostral ventrolateral medulla.
The LF component of systolic BPV and HRV represents a good marker of sympathetic activity. Increase in HF HRV is thought to reflect an increase in vagal tone. The baroreflex response represents the autonomic response to changes in blood pressure and can be quantified by a slope (9-12 ms/mm Hg in healthy individuals). In patient 7 herein, LF BPV and LF HRV were both significantly lower (−43.51% and −35.44%) during stimulation compared with baseline values (P < .001) without significant changes in HF HRV. This finding suggests a decrease in sympathetic tone. In patient 8, frequency-domain analyses showed mixed results. Both LF and HF BPV and HRV were decreased, suggesting a combination of sympathetic and parasympathetic influences. That could reflect the combination of stimulation cardiovascular influences and a subsequent baroreflex response. The calculated baroreflex slope was 15 ms/mm Hg in this patient, pointing to an excellent baroreceptor response characterized by a decrease in vagal efferent neural traffic, allowing a compensatory increase in heart rate. On the other hand, in patient 7, the calculated baroreflex slope was 0.2 ms/mm Hg, suggesting a poor baroreflex response. In patient 9, frequency-domain BRS analysis showed no significant changes before and during stimulation, although the responses were significantly weaker (Figure 3).
The differences in BRS between patients suggest that patients 7 and 9 have impaired compensatory responses to SAP decreases. The reasons for this were not explained by any phenotypic features, including seizure type, presence of generalized tonic-clonic seizures, seizure frequency, epileptogenic zone, or duration of epilepsy, and much larger study samples may be required to discern their relevance.
Whether these findings can be extrapolated to the population with epilepsy or to healthy individuals at large is questionable given our sample size. However, autonomic dysregulation is well described in epilepsy. Impaired baroreflex function has been observed in temporal lobe epilepsy. Whereas such autonomic dysregulation may have a number of implications, a major unknown in the understanding of SUDEP pathomechanisms is the role of profound peri-ictal hypotension. None of the observed SUDEP and near-SUDEP cases reported in literature has had blood pressures recorded. It is possible that some persons with epilepsy accrue greater tendencies to autonomic dysfunction than others and thus become prone to peri-ictal, potentially fatal, hypotension due to impaired homeostatic mechanisms. Certainly, patient 9 herein demonstrated hypotension during and immediately after partial seizures, although its significance with regard to the patient’s SUDEP risk is unknown. We could not use the SUDEP-7 risk inventory score for our study because there was no nonhypotensive group with which to make SUDEP-7 score comparisons. However, this is planned as an important aspect of a larger study as we continue to accrue patients.
Our findings may have therapeutic implications. Stimulation of Brodmann area 25 in patient 12, with chronic hypertension, induced similar reduction in systolic blood pressure (Figure 4 and Video 2). Stimulation of the ventral periventricular and periaqueductal gray areas, used as a treatment for chronic pain, has been proposed as sites for deep brain stimulation for the treatment of intractable hypertension. Variability in blood pressure responses at the same locations and the potential for complications in the brainstem render the periaqueductal and periventricular gray regions as unattractive targets for that purpose. On the other hand, Brodmann area 25 is an easily accessible and safe target used in deep brain stimulation for the treatment of refractory depression. Investigations of Brodmann area 25 have not reported symptomatic hypotension and may reflect differences in stimulation parameters (typically 3.5 mA) used or brain adaptation to continuous stimulation. Further studies are warranted to determine potential effective parameters of long-term electrical stimulation of Brodmann area 25 in intractable essential hypertension.
Limitations
Some limitations of our study need to be considered. First of all, the sample size was small, and we included only patients with medically refractory focal epilepsy. These data need to be reproduced in a larger cohort of patients to elucidate if our findings can be extrapolated to the general epilepsy population or to individuals without epilepsy.
Conclusions
The findings in this study suggest that Brodmann area 25 has a role in lowering systolic blood pressure in humans. It is a potential symptomatogenic zone for peri-ictal hypotension in patients with epilepsy.
eTable. Electrode Contacts Location in Each Patient
eFigure. Stimulation of Brodmann Area 25 in Patient 12 Induced Decrease of Systolic Blood Pressure and Pulse Pressure
References
- 1.Hampel KG, Jahanbekam A, Elger CE, Surges R. Seizure-related modulation of systemic arterial blood pressure in focal epilepsy. Epilepsia. 2016;57(10):1709-1718. [DOI] [PubMed] [Google Scholar]
- 2.Magnaes B, Nornes H. Circulatory and respiratory changes in spontaneous epileptic seizures in man. Eur Neurol. 1974;12(2):104-115. [DOI] [PubMed] [Google Scholar]
- 3.Bozorgi A, Chung S, Kaffashi F, et al. Significant postictal hypotension: expanding the spectrum of seizure-induced autonomic dysregulation. Epilepsia. 2013;54(9):e127-e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nei M, Mintzer S, Skidmore C, Sperling MR, Ho RT. Heart rate and blood pressure in sudden unexpected death in epilepsy (SUDEP). Epilepsy Res. 2016;122:44-46. [DOI] [PubMed] [Google Scholar]
- 5.Kaada BR, Pribram KH, Epstein JA. Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus: a preliminary report. J Neurophysiol. 1949;12(5):347-356. [DOI] [PubMed] [Google Scholar]
- 6.Kremer WF. Autonomic and somatic reactions induced by stimulation of the cingular gyrus in dogs. J Neurophysiol. 1947;10(5):371-379. [DOI] [PubMed] [Google Scholar]
- 7.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trebuchon A, Chauvel P. Electrical stimulation for seizure induction and functional mapping in steroelectroencephalography. J Clin Neurophysiol. 2016;33(6):511-521. [DOI] [PubMed] [Google Scholar]
- 9.Smith WK. The functional significance of the rostral cingular cortex as revealed by its responses to electrical excitation. J Neurophysiol. 1945;8:241-255. [Google Scholar]
- 10.Kremer WF. Blood pressure changes in response to electrical and chemical stimulation of the cerebral cortex. Fed Proc. 1947;6(1, pt 2):145. [PubMed] [Google Scholar]
- 11.Hoff EC, Green HD. Cardiovascular reactions induced by electrical stimulation of the cerebral cortex. Am J Physiol. 1936;117:411-422. [Google Scholar]
- 12.Hoffman BL, Rasmussen T. Stimulation studies of insular cortex of Macaca mulatta. J Neurophysiol. 1953;16(4):343-351. [DOI] [PubMed] [Google Scholar]
- 13.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17(3):354-381. [PubMed] [Google Scholar]
- 14.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989;491(1):156-162. [DOI] [PubMed] [Google Scholar]
- 15.Dütsch M, Hilz MJ, Devinsky O. Impaired baroreflex function in temporal lobe epilepsy. J Neurol. 2006;253(10):1300-1308. [DOI] [PubMed] [Google Scholar]
- 16.Stephani C, Luders HO. Electrical stimulation of invasive electrodes in extratemporal lobe epilepsy In: Koubeissi MZ, Maciunas RJ, eds. Extratemporal Lobe Epilepsy Surgery. Esther, Surrey: John Libbey Eurotext; 2011. [Google Scholar]
- 17.Kahane P. Invasive EEG in the definition of the seizure onset zone: depth electrodes In: Rosenow F, Luders HO, eds. Presurgical Assessment of the Epilepsies With Clinical Neurophysiology and Functional Imaging. Amsterdam, the Netherlands: Elsevier; 2004. [Google Scholar]
- 18.La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008;13(2):191-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckberg DL, Sleight P. Human Baroreflexes in Health and Disease Oxford, England: Clarendon Press; 1992. [Google Scholar]
- 20.Parati G, Di Rienzo M, Bertinieri G, et al. Evaluation of the baroreceptor–heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension. 1988;12(2):214-222. [DOI] [PubMed] [Google Scholar]
- 21.Lacuey N, Zonjy B, Londono L, Lhatoo SD. Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology. 2017;88(7):701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaada BR. Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat, and dog: a study of responses from the limbic, subcallosal, orbito-insular, piriform and temporal cortex, hippocampus-fornix and amygdala. Acta Physiol Scand Suppl. 1951;24(83):1-262. [PubMed] [Google Scholar]
- 23.Pool JL, Ransohoff J. Autonomic effects on stimulating rostral portion of cingulate gyri in man. J Neurophysiol. 1949;12(6):385-392. [DOI] [PubMed] [Google Scholar]
- 24.Chapman WP, Livingston RB, Livingston KE. Frontal lobotomy and electrical stimulation of orbital surface of frontal lobes: effect on respiration and on blood pressure in man. Arch Neurol Psychiatry. 1949;62(6):701-716. [DOI] [PubMed] [Google Scholar]
- 25.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9):1727-1732. [DOI] [PubMed] [Google Scholar]
- 26.Green AL, Wang S, Owen SL, et al. Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport. 2005;16(16):1741-1745. [DOI] [PubMed] [Google Scholar]
- 27.Patel NK, Javed S, Khan S, et al. Deep brain stimulation relieves refractory hypertension. Neurology. 2011;76(4):405-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter HH, Dawson EA, Cable NT, et al. Deep brain stimulation of the periaqueductal grey induces vasodilation in humans. Hypertension. 2011;57(5):e24-e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421(2):172-188. [PubMed] [Google Scholar]
- 30.Loewy AD. Descending pathways to the sympathetic preganglionic neurons. Prog Brain Res. 1982;57:267-277. [DOI] [PubMed] [Google Scholar]
- 31.Surges R, Thijs RD, Tan HL, Sander JW. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol. 2009;5(9):492-504. [DOI] [PubMed] [Google Scholar]
- 32.Sevcencu C, Struijk JJ. Autonomic alterations and cardiac changes in epilepsy. Epilepsia. 2010;51(5):725-737. [DOI] [PubMed] [Google Scholar]
- 33.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966-977. [DOI] [PubMed] [Google Scholar]
- 34.Novak JL, Miller PR, Markovic D, Meymandi SK, DeGiorgio CM. Risk assessment for sudden death in epilepsy: the SUDEP-7 inventory. Front Neurol. 2015;6:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green AL, Wang S, Bittar RG, et al. Deep brain stimulation: a new treatment for hypertension? J Clin Neurosci. 2007;14(6):592-595. [DOI] [PubMed] [Google Scholar]
- 36.O’Callaghan EL, McBryde FD, Burchell AE, et al. Deep brain stimulation for the treatment of resistant hypertension. Curr Hypertens Rep. 2014;16(11):493. [DOI] [PubMed] [Google Scholar]
- 37.Green AL, Hyam JA, Williams C, et al. Intra-operative deep brain stimulation of the periaqueductal grey matter modulates blood pressure and heart rate variability in humans. Neuromodulation. 2010;13(3):174-181. [DOI] [PubMed] [Google Scholar]
- 38.Choi KS, Riva-Posse P, Gross RE, Mayberg HS. Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 2015;72(11):1252-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64(6):461-467. [DOI] [PubMed] [Google Scholar]
- 40.Puigdemont D, Portella M, Pérez-Egea R, et al. A randomized double-blind crossover trial of deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: a pilot study of relapse prevention. J Psychiatry Neurosci. 2015;40(4):224-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Electrode Contacts Location in Each Patient
eFigure. Stimulation of Brodmann Area 25 in Patient 12 Induced Decrease of Systolic Blood Pressure and Pulse Pressure