Key Points
Question
Does use of the pulmonary embolism rule-out criteria (PERC) in emergency department patients with low clinical probability of pulmonary embolism (PE) safely exclude the diagnosis of PE?
Findings
In this cluster-randomized crossover noninferiority trial that included 1916 patients with very low clinical probability of PE, the 3-month risk of a thromboembolic event when using a PERC strategy compared with a conventional strategy was 0.1% vs 0%, a difference that met the noninferiority criterion of 1.5%.
Meaning
In emergency department patients at very low risk of PE, the use of a PERC-based strategy did not lead to an inferior rate of subsequent thromboembolic events.
Abstract
Importance
The safety of the pulmonary embolism rule-out criteria (PERC), an 8-item block of clinical criteria aimed at ruling out pulmonary embolism (PE), has not been assessed in a randomized clinical trial.
Objective
To prospectively validate the safety of a PERC-based strategy to rule out PE.
Design, Setting, and Patients
A crossover cluster–randomized clinical noninferiority trial in 14 emergency departments in France. Patients with a low gestalt clinical probability of PE were included from August 2015 to September 2016, and followed up until December 2016.
Interventions
Each center was randomized for the sequence of intervention periods. In the PERC period, the diagnosis of PE was excluded with no further testing if all 8 items of the PERC rule were negative.
Main Outcomes and Measures
The primary end point was the occurrence of a thromboembolic event during the 3-month follow-up period that was not initially diagnosed. The noninferiority margin was set at 1.5%. Secondary end points included the rate of computed tomographic pulmonary angiography (CTPA), median length of stay in the emergency department, and rate of hospital admission.
Results
Among 1916 patients who were cluster-randomized (mean age 44 years, 980 [51%] women), 962 were assigned to the PERC group and 954 were assigned to the control group. A total of 1749 patients completed the trial. A PE was diagnosed at initial presentation in 26 patients in the control group (2.7%) vs 14 (1.5%) in the PERC group (difference, 1.3% [95% CI, −0.1% to 2.7%]; P = .052). One PE (0.1%) was diagnosed during follow-up in the PERC group vs none in the control group (difference, 0.1% [95% CI, −∞ to 0.8%]). The proportion of patients undergoing CTPA in the PERC group vs control group was 13% vs 23% (difference, −10% [95% CI, −13% to −6%]; P < .001). In the PERC group, rates were significantly reduced for the median length of emergency department stay (mean reduction, 36 minutes [95% CI, 4 to 68]) and hospital admission (difference, 3.3% [95% CI, 0.1% to 6.6%]).
Conclusions and Relevance
Among very low-risk patients with suspected PE, randomization to a PERC strategy vs conventional strategy did not result in an inferior rate of thromboembolic events over 3 months. These findings support the safety of PERC for very low-risk patients presenting to the emergency department.
Trial Registration
clinicaltrials.gov Identifier: NCT02375919
This crossover cluster–randomized clinical noninferiority trial compared the safety of using pulmonary embolism rule-out criteria (PERC) vs a conventional strategy (D-dimer and CTPA) for excluding the diagnosis of pulmonary embolism (PE) among emergency department patients with low clinical probability of PE.
Introduction
The diagnostic strategy for pulmonary embolism (PE) in the emergency department (ED) is well established, with the evaluation of the clinical probability, followed by either D-dimer testing or computed tomographic pulmonary angiography (CTPA). This pathway is endorsed by European guidelines and is associated with a very low risk of failure. However, there are growing concerns on the potential overuse of diagnostic tests (especially CTPA) and possible overdiagnosis of PE.
The pulmonary embolism rule-out criteria (PERC) rule is an 8-item set of clinical criteria that includes arterial oxygen saturation (Spo2) of 94% or less, pulse rate of at least 100/min, patient age of 50 years or older, unilateral leg swelling, hemoptysis, recent trauma or surgery, prior PE or deep venous thrombosis (DVT), and exogenous estrogen use. These criteria were derived to select from among patients with a low clinical probability of PE, that is a population (PERC-negative patients) with a very low prevalence of PE in whom the risk-benefit ratio of further testing would be unfavorable (ie, a prevalence of PE <1.8%). A meta-analysis of observational studies reported that the prevalence of PE is less than 1% in PERC-negative patients. However, to our knowledge, no prospective study has yet implemented this rule, and conflicting results from European populations have resulted in PERC-based strategies not being included in most guidelines or recommendations.
This multicenter noninferiority randomized clinical trial was conducted to test the hypothesis that the diagnosis of PE can be safely excluded among ED patients with a low clinical probability and a PERC score of zero without further diagnostic testing.
Methods
Study Design
The population and study design of the PROPER trial (PERC Rule to Exclude Pulmonary Embolism in the Emergency Deparment) is available in Supplement 1. The design for this study was a noninferiority, crossover cluster–randomized clinical trial aimed at assessing the safety of the PERC-based strategy. Fourteen EDs in France participated in the study for two 6-month periods separated by a 2-month washout period. The trial recruitment began in August 2015, ended in September 2016, and follow-up ended in December 2016. The study was approved by Comité de protection des personnes Ile de France VI–P140913. All patients provided signed informed consent before inclusion. The reporting of this study followed the Consolidated Standards of Reporting Trials (CONSORT) statement extended to cluster randomized trials.
Patients and Intervention
All patients who presented to the ED with suspicion of a PE were eligible for enrollment in the study. The inclusion criteria were new-onset presence or worsening of shortness of breath or chest pain and a low clinical probability of PE, estimated by the treating physician’s gestalt as an expectation below 15%. The physician’s gestalt evaluation consists of an unstructured impression of the treating physician as to whether the probability of PE in the patient is low, moderate, or high. This evaluation has been reported to perform at least as well as other structured methods. Patients were excluded if they had an obvious etiology to the acute presentation other than PE (eg, pneumothorax or acute coronary syndrome), an acute severe presentation (hypotension, Spo2<90%, respiratory distress), a contraindication to CTPA (impaired renal function with an estimated creatinine clearance <30 mL/min; known allergy to intravenous radio-opaque contrast), pregnancy, inability to be followed up, or if they were receiving any anticoagulant therapy.
Each center was randomized to start with a 6-month control period (usual care), followed by a 6-month intervention period (PERC-based strategy), or in reverse order (Figure). The unit of randomization was the ED. Randomization was computer generated in blocks, using 3 blocks of 4 and 1 block of 2. For each number of the list (1-14), the order of exposure to the intervention was randomly assigned (3 numbers for each order of exposure). Then this randomization list was combined with the blinded list of centers previously numbered. The 2 periods were separated by a 2-month washout period. In the intervention group, the diagnostic work-up included an initial calculation of the PERC score. If the PERC score was zero, PE was ruled out without subsequent testing. If the PERC score was positive, the usual diagnostic strategy was applied.In the control group, the diagnostic work-up for PE followed the usual diagnostic strategy—after inclusion and classification as low gestalt probability, D-dimer testing was recommended for all patients, followed (if D-dimer–positive) by a CTPA. We used the age-adjusted threshold for D-dimer interpretation. PE was excluded if one of these 2 tests was negative. A CTPA with emboli was considered positive, including isolated subsegmental PE. If the CTPA was judged inconclusive, the patients would undergo further testing (pulmonary ventilation-perfusion [V̇/Q̇] scan or lower-leg Doppler ultrasound).
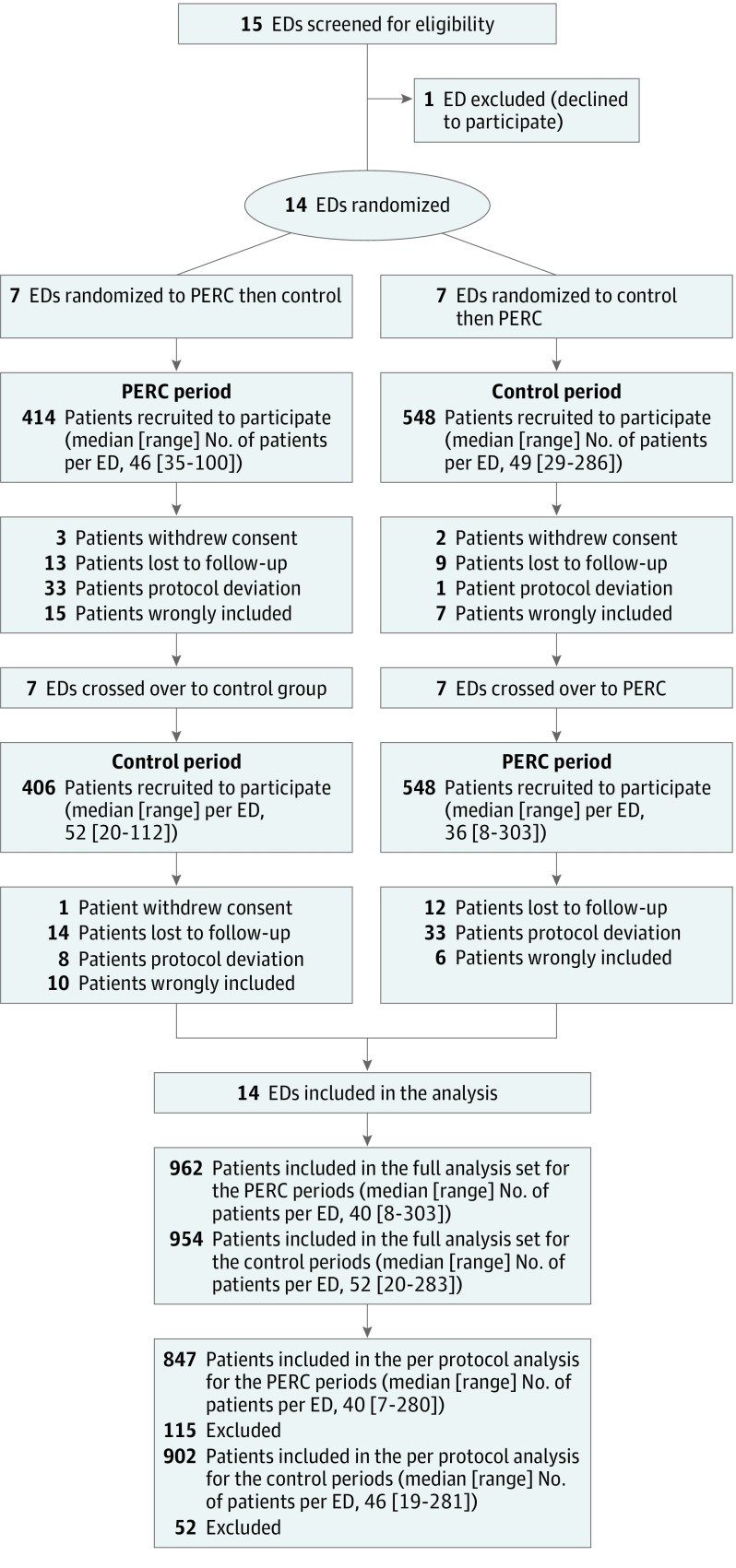
Figure. Flow of Patients With Workup for Pulmonary Embolism With the Use of Pulmonary Embolism Rule-Out Criteria vs Initial Standard Workup (Control).
The number of eligible patients screened but not included was not recorded. ED indicates emergency department; PERC indicates pulmonary embolism rule-out criteria.
Follow-up
All patients were observed for 3 months and interviewed by phone at the end of this period. They were instructed to return to the same ED or hospital in case of recurrent or worsening symptoms. A local clinical research assistant checked any return visit to the ED or admission to the hospital during the follow-up period. The phone interview was performed using a structured questionnaire that recorded consultation with any physician, hospital visit, and change in medication or imaging study. In case of inability to perform the phone interview, the patient’s general practitioner was contacted. In cases of inability to contact the patient or physician, we sought any death records from the administrative record of patient’s town of birth.
Outcomes
The primary objective of the study was to assess the percentage of failure of the diagnostic strategy. The primary end point was the occurrence of a symptomatic thromboembolic event during the 3-month follow-up period, which was not diagnosed at the time of the inclusion visit. Secondary end points included the proportion of patients investigated with CTPA, the rate of CTPA-related adverse events requiring therapeutic intervention within 24 hours, length of stay in the ED, rate of hospital admission or readmission, onset of anticoagulation regimen, severe hemorrhage in patients with anticoagulation therapy, and all-cause mortality at 3 months. An adjudication committee confirmed the occurrence of all suspected thromboembolic events during the follow-up period and adjudicated all deaths as to whether or not they were likely to have been related to a PE. The adjudication committee was composed of 3 experts with special interest in hemostasis (a professor of emergency medicine from France, a professor of pneumology from France, and a professor of emergency medicine from Switzerland). The 3 experts were independent to the study and blinded to the strategy allocation.
Sample Size Estimation and Statistical Analysis
The statistical plan and sample size calculation are reported in Supplement 2. Based on previous diagnostic studies on PE, we assumed that the primary end point rate in the control group would be 1.5%. The noninferiority margin for the difference of the primary end point between the 2 groups (delta) was set at 1.5%, which meant that the event rate had to have an upper CI limit of less than 3% in the intervention group. This 3% threshold corresponds to the failure rate observed after negative pulmonary angiography and is the threshold used in other studies. Alpha was set at 5% and power at 80%, which produced a sample size of 1624 patients. With an intraclass correlation coefficient of 0.002, an intraperiod correlation of 0.001, and a mean cluster size of 60 patients, the cluster design effect increased the sample size to 1818. Allowing for 5% nonevaluable patients, we needed to recruit 1920 patients.
Baseline characteristics of the intention-to-treat (ITT) population were expressed as number (%) for qualitative variables, and mean (SD) or median (interquartile range [IQR]) for quantitative variables, depending on their distribution. The analysis of the primary end point was performed based on the per-protocol population with follow-up. Noninferiority was assessed on the upper bound of the 1-sided 95% CI of the difference of percentage of primary end point occurrence (delta). If the upper bound of the CI of the difference was greater than 1.5%, the noninferiority hypothesis would have been rejected. Clustering effect and period and order effect were checked in a secondary analysis on the ITT population after replacing missing data by considering the worst-case scenario. Sixty primary outcomes were missing among the 1916 patients (3%; 54 patients were lost to follow-up and 6 withdrew). A generalized linear mixed model with Poisson distribution was performed, taking into account center as a random effect and period and strategy-by-period interaction as fixed effects. The logarithm of the number of patients was included as an offset term in the model. The P values reported for fixed effects were based on t tests with the denominator degrees of freedom specified using Kenward-Roger approximation. The Dunnett and Gent χ2 test was used to test noninferiority on the ITT population. The secondary end points were compared under a superiority hypothesis on the ITT population and using available data. Qualitative variables were compared using the Pearson χ2 test or the Fisher exact test, and continuous variables were compared using a Wilcoxon rank-sum test. The prevalence of PE in both groups at baseline was compared using the Pearson χ2 test.
Because the PERC group included patients with lower probability of PE compared with the control group, 2 posthoc sensitivity analyses were performed (one after the exclusion of 150 very low-risk patients from the PERC group; another after the addition of 175 simulated patients with no work-up for PE in the control group), which allowed a comparison between groups of similar PE clinical probability. All superiority tests were 2-sided and P values of less than .05 were considered significant. SAS version 9.3 software (SAS Institute) was used for statistical analyses.
Results
Fifteen EDs were invited to participate in the study (1 of which declined). The 14 participating EDs recruited 1916 patients during the study period—954 in the control period group and 962 in the PERC period group (ITT population). Details on the participating EDs are reported in eTable 1 in Supplement 3. Patients who were lost to follow-up, withdrew consent, or had protocol violations were excluded (Figure). In the control group, 9 patients (0.9%) did not undergo D-dimer testing, and in the PERC group, 46 PERC-negative patients (5%) underwent D-dimer testing. The primary end point was therefore adjudicated in 1749 patients (per-protocol with follow-up population)—902 in the control group and 847 in the PERC group. The mean (SD) age was 44 (17) years and 980 (51%) were women. The main baseline characteristics of patients are summarized in Table 1 and Table 2.
Table 1. Baseline Characteristics for Patients Receiving Initial Pulmonary Embolism Rule-Out Criteria vs Standard Treatment (Control).
| Variable | No. (%) | |
|---|---|---|
| PERC (n = 962) | Control (n = 954) | |
| Age, mean (SD), y | 44 (17) | 45 (17) |
| Women | 460 (48) | 520 (54) |
| Comorbidities | ||
| Chronic respiratory insufficiency | 28 (3) | 25 (3) |
| Chronic heart failure | 20 (2) | 19 (2) |
| Stroke | 11 (1) | 6 (1) |
| Emergency department presentation | ||
| Chest pain | 876 (91) | 863 (91) |
| Shortness of breath | 311 (32) | 405 (43) |
| Syncope | 12 (1) | 19 (2) |
| Heart rate, mean (SD), beats/min | 82 (16) | 86 (18) |
| Heart rate >100 beats/min | 128 (13.3) | 185 (19.4) |
| Respiratory rate, mean (SD), breaths/min | 18 (4)a | 18 (5)b |
| Spo2, median (IQR), % | 99 (97-100)a | 99.0 (97-100)b |
| Spo2 < 95% | 51 (5.3)a | 49 (5.2)b |
| Systolic blood pressure, mean (SD), mm Hg | 136 (19)a | 137 (21)b |
| Temperature, mean (SD), °C | 36.7 (0.5)a | 36.7 (0.5)b |
| Risk factors for PE | ||
| Estrogen use | 62 (7) | 98 (10) |
| Clinical signs of DVT | 39 (4) | 64 (7) |
| Past history of PE or DVT | 29 (3) | 41 (4) |
| Surgery or trauma requiring immobilization within 1 mo | 16 (2) | 32 (3) |
| Hemoptysis | 8 (1) | 10 (1) |
| Active malignancy | 8 (1) | 10 (1) |
Abbreviations: DVT, deep venous thrombosis; IQR, interquartile range; PE, pulmonary embolism; PERC, pulmonary embolism rule-out criteria; Spo2, oxygen saturation by pulse oximetry.
For the PERC group, the total number of patients reporting data were 848 for respiratory rate, 959 for Spo2 (mean and median), 959 for systolic blood pressure, and 955 for temperature. For other categories, number of patients was 962.
For the control group, the total number of patients reporting data were 832 for respiratory rate, 950 for Spo2 (mean and median), 951 for systolic blood pressure, and 942 for temperature. For other categories, number of patients was 954.
Table 2. Pulmonary Embolism–Related Characteristics in the Emergency Department.
| No. (%) | ||
|---|---|---|
| PERC (n = 962) | Control (n = 954) | |
| Simplified Revised Geneva scorea | ||
| 0 | 281 (29) | 195 (20) |
| 1 | 546 (57) | 577 (61) |
| 2 | 111 (12) | 148 (16) |
| 3 | 22 (2) | 28 (3) |
| 4 | 2 (<1) | 6 (<1) |
| Low risk (<2) | 827 (86) | 772 (81) |
| Intermediate risk (≥2 and <5) | 135 (14) | 182 (19) |
| Wells scoreb | ||
| <2 (Low risk) | 875 (91) | 746 (78) |
| ≥2 and ≤6 (Intermediate risk) | 79 (8) | 178 (19) |
| >6 (High risk) | 8 (1) | 30 (3) |
| PERC scorec | ||
| 0 | 459 (48)d | 364 (38) |
| >0 | 499 (52)d | 590 (62) |
| Tested with D-dimer | 526 (55) | 945 (99) |
| D-dimer <0.5 μg/mL | 183 (35) | 474 (50) |
| CTPA | 129 (13) | 220 (23) |
| PE diagnosed in the ED | 14 (1.5) | 26 (2.7) |
| Subsegmental PE | 1 (7) | 5 (19) |
Abbreviations: CTPA, computed tomography of the pulmonary artery; ED, emergency department; PE, pulmonary embolism; PERC, pulmonary embolism rule-out criteria.
SI conversion for D-dimer: to convert to nmol/L, multiply by 5.476.
Simplified Revised Geneva score ranges from 0 (lowest probability of PE) to 9 (highest probability of PE).
Wells score ranges from 0 (lowest probability of PE) to 12.5 (highest probability of PE).
PERC score ranges from 0 (lowest probability of PE) to 8 (highest probability of PE).
PERC score was not assessed in 4 patients in the PERC group.
In the PERC group, there were 459 (48%) PERC-negative patients (Table 2). A PE was diagnosed at the initial visit in 40 (2%) patients overall, 14 (1.5%) in the PERC group vs 26 (2.7%) in the control group (difference, 1.3% [95% CI, −0.1% to 2.7%]; P = .052). In these 40 patients, 39 PEs were diagnosed using CTPA in the ED and 1 with V̇/Q̇ scan. Six PEs were subsegmental (1 in the PERC group and 5 in the control group).
There were 5 deaths, which were reviewed by the adjudication committee, and none were considered likely to be linked to a PE. There was 1 thromboembolic event in the PERC group after 3-month follow-up (0.1%) and none in the control group. The difference of proportion (delta) between the 2 groups was therefore 0.1% (1-sided 95% CI, −∞% to 0.8%). The only missed pulmonary embolism or failure of the PERC rule to identify a PE that occurred in this study was that of a young male with chest pain and no previous medical history. He was PERC-negative and initially discharged but then seen again the next day with ongoing pain. When he presented the second time, a D-dimer was checked and found to be positive followed by a CTPA, interpreted as inconclusive, with radiological signs consistent with pneumonia. The patient was admitted, had lower-limb Doppler ultrasonography that showed no VTE and then a V̇/Q̇ scan showed subsegmental defects. He was treated with direct oral anticoagulation for 6 months and had a normal scan at follow up after conclusion of therapy.
Overall, a CTPA was performed in 349 cases (18%), of which 39 were positive for PE. The diagnostic yield of CTPA for the diagnostic of PE in the ED was therefore 11% across both groups. Patients in the PERC group were significantly less frequently investigated by CTPA (129 [13%]) vs 220 (23%) in the control group (difference, 9.7% [95% CI, 6.1% to 13.2%) (Table 3). In the PERC group, there was a significant reduction in median ED length of stay (4 h 36 min [IQR, 3 h 16 min to 6 h 22 min] vs 5 h 14 min [IQR, 3 h 50 min to 7 h 19 min] in the control group; P < .001). Hospital admission rates were 13% (121 patients) in the PERC group vs 16% (152 patients) in the control group (difference, 3.3% [95% CI, 0.1% to 6.6%]). There was no significant difference in the rate of all-cause mortality at 3 months (0.3% [3 patients] in the PERC group vs 0.2% [2 patients] in the control group [difference, 0.1% {95% CI, −0.5% to 0.7%}]; P > .99), in 3-month hospital readmission rates (4% [43 patients] in the PERC group vs 7% [62 patients] in the control group; P = .051), and there was no severe hemorrhage or severe adverse events subsequent to CTPA (0 in both groups). These findings for the secondary end points were also observed in the per-protocol population (Table 3). An ITT analysis with a worst-case scenario that assumed all lost to follow-up patients met the primary end point resulted in a 0.2% difference in the primary end point (1-sided 95% CI, −∞% to 1.6%; P = .12) (Table 3). In this ITT population, there was no significant period effect (P = .62) and the sequence order of the periods was not associated with a higher risk of pulmonary embolism at 3 months (P = .64). The intercluster coefficient was 0.019 and the intracluster coefficient was 0.034.
Table 3. Main Outcomes in the Study of Pulmonary Embolism Rule-Out Criteria.
| Characteristics | No. (%) | Mean Difference, % (95% CI) | Number Needed to Treat | P Value | |
|---|---|---|---|---|---|
| PERC | Control | ||||
| Intention-to-treat population, No.a | 962 | 954 | |||
| Thromboembolic event at 3 mo (primary outcome) | 32 (3) | 29 (3) | 0.2 (−∞ to 1.6)b | .12 | |
| CTPA performed | 129 (13) | 220 (23) | 9.7 (6.1 to 13.2) | 10 | <.001 |
| Length of ED stay, median (IQR), h:min | 4:36 (3:16 to 6:21) | 5:14 (3:50 to 7:18) | −00:36 (−1:08 to −0:04) | <.001 | |
| Hospital admission | 121 (13) | 152 (16) | 3.3 (0.1 to 6.6) | 30 | .04 |
| Anticoagulation therapy introduced | 21 (2) | 33 (3) | 1.3 (0.3 to 2.9) | 78 | .09 |
| Hospital readmission at 3 mo | 43 (4) | 62 (7) | 2.1 (−0.1 to 4.3) | 48 | .051 |
| All-cause death at 3 mo | 3 (0.3) | 2 (0.2) | 0.1 (−0.5 to 0.7) | >.99 | |
| Per-protocol population, No.a | 847 | 902 | |||
| Thromboembolic event at 3 mo (primary outcome) | 1 (0.1) | 0 | 0.1 (−∞ to 0.8)b | ||
| CTPA performed | 114 (14) | 211 (23) | 9.9 (6.2 to 13.6) | 10 | <.001 |
| Length of ED stay, median (IQR), h:min | 4:34 (3:12 to 6:14) | 5:12 (3:50 to 7:17) | −00:37 (−1:11 to −0:02) | <.001 | |
| Hospital admission | 101 (12) | 139 (15) | 3.5 (0.2 to 6.8) | 29 | .03 |
| Anticoagulation therapy introduced | 19 (2) | 28 (3) | 0.8 (−0.8 to 2.5) | 116 | .27 |
| Hospital readmission at 3 mo | 38 (4) | 62 (7) | 2.4 (0.1 to 4.7) | 42 | .03 |
| All-cause death at 3 mo | 1 (0.1) | 1 (0.1) | 0.01 (−0.4 to 0.4) | >.99 | |
Abbreviations: CTPA, computed tomographic pulmonary angiography; ED, emergency department; IQR, interquartile range; PERC, pulmonary embolism rule-out criteria.
Mean difference and its 95% CI. The full analysis set comprised the 1916 patients who were cluster-randomized and included in the study. After the exclusion of wrongly included patients, those lost to follow-up, those with protocol deviations, or those who withdrew consent, the per-protocol population comprised 1749 patients.
One sided 95% CI.
The posthoc sensitivity analyses are presented in the Supplement 2. The findings of these analyses are that the 2 groups had similar clinical risk of PE (eTable 2 in Supplement 3). The noninferiority hypothesis remained confirmed in the per-protocol population, and the rate of CTPA was significantly reduced in the PERC group (eTable 3 in Supplement 3).
Discussion
In this cluster-randomized trial of very low-risk patients with suspected PE, randomization to a PERC strategy vs conventional strategy did not result in an inferior rate of thromboembolic events over 3 months. In addition, the PERC strategy was associated with a benefit in terms of reduced CTPA use, ED length of stay, and likelihood of initial admission into hospital.
After initial validation on observational studies in the United States, the PERC rule has been challenged in Europe by 2 studies that reported an unacceptable rate (>5%) of PERC-negative patients with a PE diagnosis. One reason for the reported high prevalence of PE among PERC-negative patients could be the overall higher prevalence of PE in a European population. Another reason was that the authors used a structured score to evaluate the clinical probability (Wells or Geneva scores), which is made redundant when the PERC rule is used. One study reported better results when combining PERC with low gestalt clinical probability with no false negative of the PERC rule. The primary end point chosen, ie, the presence of PE after formal work up in the ED, can also explain in part the discrepancies, leading to a substantial rate of overdiagnosis, with a greater number of small PEs diagnosed that could be left untreated.
The end point chosen for the current study was a symptomatic pulmonary thromboembolism at 3 months and did not include the presence of a PE after formal work up in the ED. This could explain the difference of prevalence of PE described in the 2 groups (1.5% in the PERC group vs 2.7% in the control group; P = .052). The real PE prevalence in the PERC group may actually have been higher if all patients had undergone formal work up with D-dimer testing. It is very likely that some patients in the PERC group were discharged with a small untreated PE, which was not symptomatic even at 3-month follow-up. There was no significant difference in diagnostic yield of CTPA for PE (11% in both groups). A increased yield with the use of PERC might have been expected as has been reported with other clinical decision rules. However in this study, the small number of diagnosed PEs may have resulted in a lack of power to detect significant differences between the 2 groups. In the PERC group, there were fewer initial PEs diagnosed than in the usual care group, with little difference between the 2 groups in clinically significant thromboembolic events at 3 months. This suggests that PERC may be inferior at capturing low-risk events such as small subsegmental PE, with no clinical benefit in diagnosing the missed PEs in the PERC group. This may represent a tolerable risk to patient safety because small subsegmental PE can be left untreated. Another potential reason for the lower rate of PE in the PERC group may be that the 2 groups were not similar in their initial clinical probability of PE. In the posthoc sensitivity analyses with similar groups, the PE prevalence in the 2 groups was slightly modified (1.7% in the PERC group vs 2.7% in the control group in the first posthoc analysis and 1.5% in the PERC group vs 2.3% in the control group in the second posthoc analysis; eTable 4 in Supplement 3). However, this difference was no longer statistically significant.
Future research could evaluate the economic benefit of implementing PERC in routine practice. Furthermore, the safety and benefit of the modified PERC rule for patients younger than 35 years should be evaluated and compared to the initial PERC rule.
Limitations
This study has several limitations. First, the observed prevalence of PE in patients with a low gestalt probability is very low. Although this category should include patients with a PE prevalence below 15%, only 26 patients (2.7%) in the control group were diagnosed with a PE in the ED. This corresponds with an overestimation of the risk of PE by the clinician’s gestalt, which has been described previously. The 2.7% prevalence (95% CI, 1.8% to 4.0%) reported here is below that reported in previous studies. However, similar PE prevalences for low-risk patients have also been reported in a recent study (pooled prevalence in the ED, 3.1% [1.0% in the United States and 4.3% outside of the United States]). Furthermore, among low-risk patients with chest pain or dyspnea, the reported prevalence of PE was 0.9%. In a recent large multicenter prospective study, Penaloza et al reported a prevalence of 4.7% (95% CI, 3.5% to 6.1%). The mean age of the patients was 44 years in this study, which is younger than in other main studies. This could partially explain the low prevalence of PE in this sample. Furthermore, a CTPA was defined as positive if it showed an isolated subsegmental PE. This could be considered controversial as these PEs could be left untreated.
Second, the failure rate of the diagnosis strategy in the control group was below the estimation, and therefore, the sample size calculation was not accurate. The 3% maximal failure rate that has been used in other trials is derived from studies published more than 15 years ago. Since this percentage is very large compared to the less than 1% of events rate recently reported, this raises the question of its validity. Recently, the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis published a recommendation to decrease the maximal acceptable failure rate to 2%. With this new recommended threshold, the present results would still be valid.
Third, this was not a patient-level randomized trial, therefore a bias inherent to the cluster design cannot be excluded. This shortcoming is however partly limited by the absence of period and sequence order effects. Fourth, it is possible that an occult inclusion bias may have been introduced, in which emergency physicians were willing to discharge PERC-negative patients with no further testing and therefore did not include some of these patients in the trial during the control period. This could not be assessed because data were not recorded regarding the number of eligible patients who were not enrolled. The difference in the rate of PERC-negative patients and clinical probability of PE between the 2 groups (Table 2) suggests that physicians could have included more very low-risk patients in the PERC group, as they were willing to discharge them with no further testing. However, 2 post-hoc sensitivity analyses confirmed the primary result of noninferiority, although with smaller effect on the secondary end points.
Fifth, there were 54 patients lost to follow-up and the presence of a few events among these patients would have altered the conclusion of this study. A worst-case scenario simulation would have led to the rejection of the noninferiority hypothesis (difference, 0.2% [upper bound of the 95% CI at 1.6% for a margin set at 1.5%]). Sixth, it is possible that the use of PERC induced diversion of unnecessary testing, and therefore patients not tested for PE were tested for another acute condition such as coronary syndrome. However, these data were not collected for these patients in this study.
Conclusions
Among very low-risk patients with suspected PE, randomization to a PERC strategy vs conventional strategy did not result in an inferior rate of thromboembolic events over 3 months. These findings support safety of PERC for very low-risk patients presenting to the emergency department.
Trial Protocol
Statistical Plan
eTable 1. Characteristics of Participating Emergency Departments
eTable 2. Clinical Probability and PERC Score
eTable 3. Posthoc Sensitivity Analyses
eTable 4. Initial Diagnosis of Pulmonary Embolism in the Emergency Department
Abbreviations List
- CTPA
computed tomographic pulmonary angiography
- PERC
pulmonary embolism rule-out criteria
- PE
pulmonary embolism
References
- 1.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-3069, 3069a-3069k.. [DOI] [PubMed] [Google Scholar]
- 2.van Belle A, Büller HR, Huisman MV, et al. ; Christopher Study Investigators . Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295(2):172-179. [DOI] [PubMed] [Google Scholar]
- 3.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. BMJ. 2013;347(jul02):f3368-f3368.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kline JA, Mitchell AM, Kabrhel C, Richman PB, Courtney DM. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2(8):1247-1255. [DOI] [PubMed] [Google Scholar]
- 6.Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med. 1980;302(20):1109-1117. [DOI] [PubMed] [Google Scholar]
- 7.Singh B, Mommer SK, Erwin PJ, Mascarenhas SS, Parsaik AK. Pulmonary embolism rule-out criteria (PERC) in pulmonary embolism–revisited: a systematic review and meta-analysis. Emerg Med J. 2012;30(9):701-706.. [DOI] [PubMed] [Google Scholar]
- 8.Lapner ST, Kearon C. Diagnosis and management of pulmonary embolism. BMJ. 2013;346:f757. [DOI] [PubMed] [Google Scholar]
- 9.Campbell MK, Piaggio G, Elbourne DR, Altman DG; CONSORT Group . CONSORT 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. [DOI] [PubMed] [Google Scholar]
- 10.Penaloza A, Verschuren F, Meyer G, et al. Comparison of the unstructured clinician gestalt, the wells score, and the revised Geneva score to estimate pretest probability for suspected pulmonary embolism. Ann Emerg Med. 2013;62(2):117-124.e2. [DOI] [PubMed] [Google Scholar]
- 11.Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311(11):1117-1124. [DOI] [PubMed] [Google Scholar]
- 12.Righini M, Le Gal G, Aujesky D, et al. Diagnosis of pulmonary embolism by multidetector CT alone or combined with venous ultrasonography of the leg: a randomised non-inferiority trial. Lancet. 2008;371(9621):1343-1352. [DOI] [PubMed] [Google Scholar]
- 13.Righini M, Aujesky D, Roy PM, et al. Clinical usefulness of D-dimer depending on clinical probability and cutoff value in outpatients with suspected pulmonary embolism. Arch Intern Med. 2004;164(22):2483-2487. [DOI] [PubMed] [Google Scholar]
- 14.Perrier A, Roy P-M, Aujesky D, et al. Diagnosing pulmonary embolism in outpatients with clinical assessment, D-dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study. Am J Med. 2004;116(5):291-299. [DOI] [PubMed] [Google Scholar]
- 15.Freund Y, Rousseau A, Guyot-Rousseau F, et al. PERC rule to exclude the diagnosis of pulmonary embolism in emergency low-risk patients: study protocol for the PROPER randomized controlled study. Trials. 2015;16:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. [DOI] [PubMed] [Google Scholar]
- 17.Hugli O, Righini M, Le Gal G, et al. The pulmonary embolism rule-out criteria (PERC) rule does not safely exclude pulmonary embolism. J Thromb Haemost. 2011;9(2):300-304. [DOI] [PubMed] [Google Scholar]
- 18.Righini M, Le Gal G, Perrier A, Bounameaux H.. More on: clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2005;3(1):188-191. [DOI] [PubMed] [Google Scholar]
- 19.Penaloza A, Kline J, Verschuren F, et al. European and American suspected and confirmed pulmonary embolism populations: comparison and analysis. J Thromb Haemost. 2012;10(3):375-381. [DOI] [PubMed] [Google Scholar]
- 20.Pernod G, Caterino J, Maignan M, Tissier C, Kassis J, Lazarchick J; DIET study group . D-Dimer use and pulmonary embolism diagnosis in emergency units: why is there such a difference in pulmonary embolism prevalence between the United States of America and countries outside USA? PLoS One. 2017;12(1):e0169268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penaloza A, Verschuren F, Dambrine S, Zech F, Thys F, Roy P-M. Performance of the Pulmonary Embolism Rule-out Criteria (the PERC rule) combined with low clinical probability in high prevalence population. Thromb Res. 2012;129(5):e189-e193. [DOI] [PubMed] [Google Scholar]
- 22.Tan S, Haramati LB. Overdiagnosis versus misdiagnosis of pulmonary embolism. AJR Am J Roentgenol. 2016;206(4):W59. [DOI] [PubMed] [Google Scholar]
- 23.Sheh SH, Bellin E, Freeman KD, Haramati LB. Pulmonary embolism diagnosis and mortality with pulmonary CT angiography versus ventilation-perfusion scintigraphy: evidence of overdiagnosis with CT? AJR Am J Roentgenol. 2012;198(6):1340-1345. [DOI] [PubMed] [Google Scholar]
- 24.Raja AS, Ip IK, Prevedello LM, et al. Effect of computerized clinical decision support on the use and yield of CT pulmonary angiography in the emergency department. Radiology. 2012;262(2):468-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mongan J, Kline J, Smith-Bindman R. Age and sex-dependent trends in pulmonary embolism testing and derivation of a clinical decision rule for young patients. Emerg Med J. 2015;32(11):840-845. [DOI] [PubMed] [Google Scholar]
- 26.Kline JA, Stubblefield WB. Clinician gestalt estimate of pretest probability for acute coronary syndrome and pulmonary embolism in patients with chest pain and dyspnea. Ann Emerg Med. 2014;63(3):275-280. [DOI] [PubMed] [Google Scholar]
- 27.Penaloza A, Soulié C, Moumneh T, et al. Pulmonary embolism rule-out criteria (PERC) rule in European patients with low implicit clinical probability (PERCEPIC): a multicentre, prospective, observational study. Lancet Haematol. 2017;4(12):e615-e621. [DOI] [PubMed] [Google Scholar]
- 28.Stein PD, Goodman LR, Hull RD, Dalen JE, Matta F. Diagnosis and management of isolated subsegmental pulmonary embolism: review and assessment of the options. Clin Appl Thromb Hemost. 2012;18(1):20-26. [DOI] [PubMed] [Google Scholar]
- 29.Kruip MJHA, Leclercq MGL, van der Heul C, Prins MH, Büller HR. Diagnostic strategies for excluding pulmonary embolism in clinical outcome studies: a systematic review. Ann Intern Med. 2003;138(12):941-951. [DOI] [PubMed] [Google Scholar]
- 30.Dronkers CEA, van der Hulle T, Le Gal G, et al. ; Subcommittee on Predictive and Diagnostic Variables in Thrombotic Disease . Towards a tailored diagnostic standard for future diagnostic studies in pulmonary embolism: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15(5):1040-1043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Plan
eTable 1. Characteristics of Participating Emergency Departments
eTable 2. Clinical Probability and PERC Score
eTable 3. Posthoc Sensitivity Analyses
eTable 4. Initial Diagnosis of Pulmonary Embolism in the Emergency Department