Key Points
Question
What is the association of bone mineral density with intracranial aneurysms?
Findings
In this cross-sectional study that included 12 785 participants, lower bone mineral density tertiles were associated with increased risk of intracranial aneurysm and larger aneurysm size. Those in the lowest tertile of bone mineral density had approximately 30% higher risk of harboring an intracranial aneurysm compared with those in the highest tertile, and patients with bone mineral density lower than normal based on T score also had a higher risk of intracranial aneurysm and large and multiple aneurysms.
Meaning
Bone mineral density may be associated with the presence, size, and multiplicity of intracranial aneurysm.
Abstract
Importance
Disruption of extracellular matrix integrity is critically involved in both intracranial aneurysm and bone fragility. Furthermore, both intracranial aneurysm and osteoporosis have a female predominance, and sex hormones are considered to affect this discrepancy.
Objective
To evaluate the association between bone mineral density and intracranial aneurysm.
Design, Setting, and Participants
A cross-sectional study conducted with 14 328 patients who underwent brain magnetic resonance angiography and bone mineral densitometry as a part of a health examination at a specialized center for comprehensive health examination in Seoul, the largest metropolitan area in the Republic of Korea, between December 2004 and November 2015. After excluding patients with insufficient clinical information (n = 1102) and with ambiguous intracranial arterial lesion (n = 441), 12 785 were included in the analysis.
Exposures
Bone mineral density was measured at the lumbar vertebrae (L1 to L4), femur neck, and total hip using dual-energy x-ray absorptiometry.
Main Outcomes and Measures
Multiple logistic regression or linear regression was used to examine the association between tertiles of bone mineral density and the presence, size, and multiplicity of intracranial aneurysms. In secondary analyses, we analyzed postmenopausal women and men 50 years and older (n = 8722) because they are particularly at risk of decreased bone mineral density.
Results
Among 12 785 patients in the study (7242 women [56.6%]; mean [SD] age, 54.8 [10.1] years) intracranial aneurysms were found in 472 patients (3.7%). Lower bone mineral density was associated with an increased risk of harboring intracranial aneurysm. In multivariable logistic regression analyses, odds ratios for the highest compared with the lowest bone mineral density tertile were 1.30 (95% CI, 1.03-1.64) in the lumbar spine, 1.30 (95% CI, 1.03-1.64) in the femoral neck, and 1.27 (95% CI, 1.01-1.60) in the total hip after adjusting for age, sex, and vascular risk factors. In a linear regression model adjusted for age, sex, and vascular risk factors, the lowest tertile of bone mineral density in the lumbar spine was associated with an increased log-transformed size of aneurysm (β, 0.196; SE, 0.047). In secondary analyses, these associations were more definite and a low T score (<−1 SD) was additionally associated with multiple aneurysms (OR, 1.84; 95% CI, 1.05-3.30) after adjusting for age, sex, and vascular risk factors.
Conclusions and Relevance
Bone mineral density may be associated with the presence, size, and multiplicity of intracranial aneurysm. The study findings provide evidence for shared pathophysiology between intracranial aneurysm and bone fragility.
This study evaluates the relationship between bone mineral density and intracranial aneurysm.
Introduction
Intracranial aneurysm (IA) is an outpouching of a weakened portion of an intracranial vessel wall that has a prevalence of 2% to 5% in the adult population.1,2 Most IAs are asymptomatic, but the rupture of the aneurysm results in devastating consequences. Previous studies have aimed to identify the risk factors for the formation, growth, and rupture of IA, but these investigations have been limited to the association between IA and age, sex, family history, and vascular risk factors.3
Constituents of the extracellular matrix (ECM), such as collagens, are widely distributed along the intracranial arterial wall and are also the main components of the organic part of bone.4,5 Disruption of ECM integrity and pathological matrix remodeling are critically associated with both the development of IA and bone fragility.4,6,7 Genetic disorders that impair connective tissue structure, such as Marfan syndrome and Ehler-Danlos syndrome, manifest both vascular and skeletal system abnormalities.8,9,10 In addition, both IA and osteoporosis show a female predominance, and this predominance may be attributed to the sex hormones.11,12 Because women experience differing degrees of estrogen exposure over their lifetime owing to different stages, such as menarche, menopause, oophorectomy, and hormone therapy, they may also have differing susceptibilities to IA and osteoporosis. Bone mineral density (BMD) is an indicator of bone health and has been suggested as a good surrogate marker to evaluate cumulative estrogen exposure.13,14 In this regard, we hypothesized that associations between IA and BMD may exist and evaluated the associations between BMD and the risk of IA and its characteristics, using a large cross-sectional health examination data set.
Methods
Study Population
We initially enrolled 14 328 participants who underwent intracranial magnetic resonance angiography and bone mineral densitometry as a part of a comprehensive health checkup program at Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Republic of Korea, between December 2004 and November 2015. The primary purpose of the health checkup program is to find medical problems before overt disease manifestation, and the patients voluntarily underwent a series of tests to screen for cancer, vascular disease, and other disorders and were mostly free of symptoms. We excluded 1102 patients who had no available medical history taken from the preliminary consultation or prespecified systematic questionnaire to assess vascular risk factors. The study was approved by the institutional review board of Seoul National University Hospital with a waiver of consent because this study did not involve contact with participants or any intervention.
Clinical, Laboratory, and Imaging Data Collection
Clinical information was collected from the health checkup program records. Demographic and vascular risk factors (age, sex, body mass index, blood pressure, hypertension, diabetes, hyperlipidemia, history of cardiovascular disease, smoking status, and alcohol use), laboratory findings (serum glucose, hemoglobin A1C, total cholesterol, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels), magnetic resonance angiography, and dual-energy x-ray absorptiometry results were obtained and analyzed. For patients who had undergone more than 1 health checkup, data from the earliest examination were used. Initial screening for IA was done using formal reading reports of magnetic resonance angiography by radiologists, and finally, the presence of IA was confirmed by 2 neurologists (Y.-W.S. and K.-H.J.) who reviewed the raw images. The neurologists were blinded to the dual-energy x-ray absorptiometry results when reviewing the images. Patients suspected of having dissecting or mycotic aneurysms and aneurysm-like lesions indistinguishable from infundibulum, fenestration, or atherosclerotic remodeling were excluded from final analysis (n = 441). The size of the aneurysm was defined as the maximum measurement among largest diameter, neck width, and maximum height from the neck to the tip of the dome of the aneurysm.
Bone Mineral Density Measurement and Interpretation
Bone mineral density was assessed at the lumbar vertebrae (L1 to L4), femur neck, and total hip using dual-energy x-ray absorptiometry (Lunar Prodigy; GE Medical) as recommended by the International Society for Clinical Densitometry for the diagnosis of osteoporosis in clinical practice.15 All measurements were conducted by experienced operators following standardized procedures. Bone mineral loss is marked after age 50 years or menopause,16,17 and clinical interest in BMD is mostly focused on these populations to screen for a decrease in BMD and assess the risk of osteoporotic fracture. Thus, postmenopausal women and men 50 years or older were classified as an at-risk population and subjected to the secondary analysis. For this population, BMD results were categorized into 3 groups based on the lowest T score as defined by the World Health Organization: normal (T score, ≥−1 SD), osteopenia (T score between −1 and −2.5 SD), or osteoporosis (T score, ≤−2.5 SD).18 A low T score was defined as having the lowest T score less than −1 SD (ie, within the range of osteopenia or osteoporosis).
Statistical Analysis
All statistical analyses were performed using R, version 3.3.1 (R Programming). Continuous variables are expressed as mean (SD), and categorical data are expressed as count (percentage). Age is a strong determinant of both BMD and IA. Thus, patients were stratified into 5-year age range groups for each sex, and measured BMD values were assigned to 1 of 3 BMD tertiles in each stratum. Separate models were created for each of the 3 skeletal sites. Three binary logistic regression models (unadjusted, adjusted for age and sex alone, and adjusted for age, sex, and vascular risk factors such as hypertension, diabetes, hyperlipidemia, history of cardiovascular disease, ever smoker, and alcohol use) were used to determine the risk of harboring IA for each age- and sex-specific BMD tertile from the 3 skeletal sites. The analyses were primarily conducted for the total population, and then secondary analyses were performed for the at-risk population who were beyond the stage of life when peak bone mass occurs and within the stage of life when bone mineral loss occurs. For the at-risk population, an assessment of the relationship between low T score and the presence of IA was additionally conducted to test the robustness and the clinical applicability of the relationship. The relationships between BMD and size of the aneurysm or multiplicity were further analyzed in patients with IA. The size of the largest aneurysm was used if the patient had multiple aneurysms. The size of the aneurysm was analyzed both as continuous (natural log-transformed size) and as categorical variables (size larger than 3 mm). Seven patients (6 women and 1 man) who had undergone endovascular coiling or surgical clipping of IA without any record to determine the size of the aneurysm were excluded from any analysis involving the size of the aneurysm. Pearson correlation was used to analyze the correlation between the log size of the aneurysm and BMD. Linear or logistic regression models were used as appropriate to evaluate the relationship between BMD and size variables or multiplicity. A P value less than .05 (2-tailed) was considered statistically significant.
Results
Patient Characteristics
After excluding 1543 patients, a total of 12 785 patients were included in the analysis (eFigure in the Supplement). The mean (SD) age of the population was 54.8 (10.1) years, and 7242 patients (56.6%) were women. Intracranial aneurysms were found in 472 patients (3.7%). In the at-risk population, the mean (SD) age was 59.8 (7.5) years, and 5020 patients (57.6%) were women. Intracranial aneurysms were found in 398 patients (4.6%). The baseline characteristics of the study population and the at-risk population are summarized in Table 1.
Table 1. Baseline Characteristics of the Study Population.
| Characteristic | Mean (SD) | |||
|---|---|---|---|---|
| Total | At-Risk Population | |||
| Without
IA (n = 12 313) |
With IA (n = 472) |
Without
IA (n = 8324) |
With IA (n = 398) |
|
| Age, y | 54.7 (10.1) | 58.9 (9.3) | 59.7 (7.5) | 61.5 (7.5) |
| Female, No. (%) | 6948 (56.4) | 294 (62.3) | 4770 (57.3) | 250 (62.8) |
| BMI | 23.6 (3.1) | 23.6 (3.1) | 23.8 (2.9) | 23.6 (3.0) |
| SBP, mm Hg | 118.2 (15.8) | 119.4 (17.3) | 120.1 (15.9) | 120.3 (17.3) |
| DBP, mm Hg | 76.1 (11.5) | 76.1 (11.8) | 76.6 (11.1) | 76.3 (11.7) |
| Vascular risk factors, No. (%) | ||||
| Hypertension | 4581 (37.2) | 212 (44.9) | 3704 (44.5) | 190 (47.7) |
| Diabetes | 1737 (14.1) | 89 (18.9) | 1468 (17.6) | 82 (20.6) |
| Hyperlipidemia | 4354 (35.4) | 194 (41.1) | 3293 (39.6) | 171 (43) |
| Coronary artery disease | 554 (4.5) | 24 (5.1) | 476 (5.7) | 24 (6.0) |
| Smoking, current | 2751 (22.3) | 108 (22.9) | 2000 (24.0) | 87 (21.9) |
| Smoking, past | 1923 (15.6) | 62 (13.1) | 989 (11.9) | 42 (10.6) |
| Alcohol use | 6072 (49.3) | 229 (48.5) | 3702 (44.5) | 184 (46.2) |
| Laboratory findings | ||||
| Glucose, mg/dL | 99.8 (21.4) | 102 (20.7) | 101.8 (22.0) | 103.2 (21.4) |
| HbA1C, % | 5.8 (0.7) | 5.9 (0.7) | 5.9 (0.7) | 6.0 (0.7) |
| Cholesterol, mg/dL | 199.1 (36.2) | 197.1 (35.1) | 200.6 (36.9) | 198.2 (36.1) |
| Triglyceride, mg/dL | 112.2 (71.0) | 110.1 (59.6) | 112.0 (66.0) | 110.3 (59.4) |
| HDL cholesterol, mg/dL | 54.6 (13.6) | 54.9 (13.8) | 54.3 (13.5) | 54.8 (14.1) |
| LDL cholesterol, mg/dL | 122 (33.6) | 120.2 (32.0) | 123.9 (34.2) | 121.4 (32.4) |
| BMD measurements | ||||
| Lumbar spine | 1.145 (0.184) | 1.104 (0.183) | 1.117 (0.192) | 1.089 (0.184) |
| Femoral neck | 0.901 (0.130) | 0.867 (0.134) | 0.878 (0.129) | 0.852 (0.131) |
| Total hip | 0.966 (0.137) | 0.935 (0.144) | 0.948 (0.139) | 0.922 (0.143) |
| T score | ||||
| Lumbar spine | NA | NA | −0.3 (1.5) | −0.5 (1.4) |
| Femoral neck | NA | NA | −0.4 (1.0) | −0.5 (1.0) |
| Total hip | NA | NA | 0.1 (1.1) | −0.1 (1.1) |
| BMD category, No. (%) | ||||
| Normal | NA | NA | 4927 (59.2) | 200 (50.3) |
| Osteopenia | NA | NA | 2977 (35.8) | 177 (44.5) |
| Osteoporosis | NA | NA | 420 (5.0) | 21 (5.3) |
| Low T score, No. (%) | NA | NA | 3397 (40.8) | 198 (49.7) |
| IA characteristics | ||||
| Size, mm | NA | 3.2 (2.1) | NA | 3.3 (2.1) |
| Size >3 mm, No. (%) | NA | 171 (36.8) | NA | 155 (39.4) |
| Multiple aneurysms, No. (%) | NA | 68 (14.4) | NA | 61 (15.3) |
Abbreviations: BMD, bone mineral density; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DBP, diastolic blood pressure; HbA1C, hemoglobin A1C; HDL, high-density lipoprotein; IA, intracranial aneurysm; LDL, low-density lipoprotein; NA, not applicable; SBP, systolic blood pressure.
SI conversion factor: To convert cholesterol levels to millimoles per liter, muliply by 0.0259; hemoglobin A1C to proportion of total hemoglobin, multiply by 0.01; glucose levels to millimoles per liter, multiply by 0.0555; triglyceride levels to millimoles per liter, multiply by 0.0113.
Association Between BMD and the Risk of IA
The risk of harboring IA was generally increased in patients with age- and sex-stratified BMD in tertiles 2 and 3 (middle and lower tertiles) compared with patients with BMD in tertile 1 (higher tertile) in all skeletal sites. The difference in the risk of harboring IA reached the level of statistical significance between patients with BMD in tertile 1 and tertile 3 of the lumbar spine and femoral neck in an unadjusted model and a model adjusted for age and sex. In a multivariable analysis adjusted for all covariates, the difference was significant for all skeletal sites. The odds ratio (OR) ranged between 1.26 (95% CI, 1.00-1.58) and 1.30 (95% CI, 1.03-1.64) in all comparisons with statistically significant differences (Table 2). Secondary analyses showed whether the associations were valid in the at-risk population. Apart from the lumbar spine BMD, the BMD of the other 2 skeletal sites showed similar associations, with the risk of IA and the strength of the associations increased slightly, with an OR ranging between 1.31 (95% CI, 1.02-1.69) and 1.35 (95% CI, 1.05-1.74) (Table 2).
Table 2. Multivariable Logistic Regression Models Examining the Relationship Between BMD and the Risk of IAa.
| BMD Measurement Site | Unadjusted | Adjusted for Age and Sex | Adjusted for All Covariatesb | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Total population | ||||||
| Lumbar spine | ||||||
| Tertile 1 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Tertile 2 | 1.16 (0.92-1.46) | .21 | 1.16 (0.92-1.46) | .22 | 1.18 (0.94-1.49) | .16 |
| Tertile 3 | 1.26 (1.01-1.59) | .04 | 1.26 (1.00-1.58) | .05 | 1.30 (1.03-1.64) | .03 |
| Femoral neck | ||||||
| Tertile 1 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Tertile 2 | 1.23 (0.97-1.55) | .08 | 1.22 (0.97-1.54) | .09 | 1.24 (0.98-1.57) | .07 |
| Tertile 3 | 1.28 (1.02-1.61) | .04 | 1.27 (1.01-1.60) | .04 | 1.30 (1.03-1.64) | .03 |
| Total femur | ||||||
| Tertile 1 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Tertile 2 | 1.19 (0.95-1.50) | .14 | 1.19 (0.94-1.50) | .14 | 1.21 (0.96-1.52) | .12 |
| Tertile 3 | 1.24 (0.98-1.56) | .07 | 1.23 (0.98-1.55) | .08 | 1.27 (1.01-1.60) | .04 |
| At-risk population | ||||||
| Lumbar spine | ||||||
| Tertile 1 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Tertile 2 | 1.14 (0.89-1.47) | .30 | 1.14 (0.89-1.47) | .30 | 1.16 (0.90-1.49) | .26 |
| Tertile 3 | 1.23 (0.96-1.58) | .10 | 1.22 (0.95-1.57) | .11 | 1.27 (0.99-1.63) | .07 |
| Femoral neck | ||||||
| Tertile 1 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Tertile 2 | 1.18 (0.91-1.52) | .21 | 1.18 (0.91-1.52) | .21 | 1.20 (0.93-1.55) | .17 |
| Tertile 3 | 1.32 (1.03-1.70) | .03 | 1.31 (1.02-1.69) | .03 | 1.35 (1.05-1.74) | .02 |
| Total femur | ||||||
| Tertile 1 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Tertile 2 | 1.23 (0.95-1.58) | .12 | 1.22 (0.95-1.58) | .12 | 1.24 (0.96–1.59) | .10 |
| Tertile 3 | 1.28 (1.00-1.65) | .06 | 1.27 (0.99-1.64) | .06 | 1.31 (1.02-1.69) | .04 |
Abbreviations: BMD, bone mineral density; IA, intracranial aneurysm; NA, not applicable; OR, odds ratio.
BMD measurements are assigned to age- and sex-specific tertiles from tertile 1 (higher) to tertile 3 (lower).
Covariates for adjustment in the analyses include age, sex, hypertension, diabetes, hyperlipidemia, history of cardiovascular disease, ever smoker, and alcohol use.
Size and Multiplicity of IA and BMD
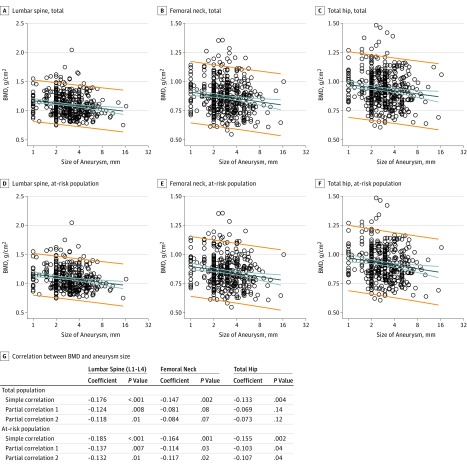
We further investigated whether a lower BMD tertile was associated with a larger IA or an increased risk of multiple aneurysms. Scatterplot and Pearson correlation coefficients showed a negative correlation between BMD and the log size of the aneurysm in both the total population and the at-risk population for all skeletal sites (strongest in lumbar spine: r = −0.176; P = <.001 in the total population; r = −0.185; P = <.001 in the at-risk population; Figure). In partial correlations controlling for age and sex and for all covariates, lumbar spine BMD was negatively correlated with the log size of the aneurysm in the total population (partial correlation for age and sex, r = −0.124; P = .008; partial correlation for all covariates, r = −0.118; P = .01). The BMD measured in all skeletal sites showed a negative correlation with the log size of the aneurysm in the at-risk population (Figure). Analyses conducted with BMD tertile showed that patients with BMD in a lower tertile had larger IA log sizes and increased risk of having an IA larger than 3 mm compared with patients with BMD in higher tertiles, with variations across the population group and skeletal site (Table 3; eTable 1 in the Supplement). The associations between a lower BMD tertile and the increased risk of having an aneurysm larger than 3 mm was stronger in the at-risk population (strongest in lumbar spine, OR, 2.69; 95% CI, 1.74-4.19; P < .001 after adjustment for age, sex, and vascular risk factors; eTable 1 in the Supplement). Multiplicity of IAs did not show any significant association with BMD in both the total and at-risk populations (eTable 2 in the Supplement).
Figure. Scatterplot and Pearson Correlation for Bone Mineral Density (BMD) and Size of Aneurysm.
Bone mineral density of lumbar spine, femoral neck, and total hip in total population (A, B, and C, respectively) and at-risk population (D, E, and F, respectively) showing a decreasing trend along with increasing size of aneurysm. Size of aneurysm is represented in logarithmic scale along the x-axis and BMD along the y-axis. Regression lines are shown with 95% CIs (blue lines) and 95% prediction interval (orange lines). Pearson correlation and partial correlations between BMD and log size of aneurysm are summarized (G).
Table 3. Linear Regression Analyses of the Relationship Between BMD and Log Size of IA (Tertile 3 vs Tertiles 1 and 2).
| BMD Measurement Site | Unadjusted | Adjusted for Age and Sex | Adjusted for All Covariates | |||
|---|---|---|---|---|---|---|
| β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | |
| Total population | ||||||
| Lumbar spine | 0.206 (0.047) | <.001 | 0.206 (0.046) | <.001 | 0.196 (0.047) | <.001 |
| Femoral neck | 0.083 (0.048) | .08 | 0.082 (0.047) | .08 | 0.088 (0.048) | .07 |
| Total hip | 0.068 (0.048) | .16 | 0.068 (0.047) | .15 | 0.074 (0.048) | .12 |
| At-risk population | ||||||
| Lumbar spine | 0.220 (0.052) | <.001 | 0.220 (0.051) | <.001 | 0.214 (0.051) | <.001 |
| Femoral neck | 0.135 (0.052) | .01 | 0.134 (0.052) | .01 | 0.138 (0.052) | .008 |
| Total hip | 0.101 (0.053) | .06 | 0.100 (0.052) | .06 | 0.104 (0.052) | .05 |
Abbreviations: BMD, bone mineral density; IA, intracranial aneurysm; β, nonstandardized regression coefficient.
Low T Score and IA in the At-Risk Population
To facilitate a clinical value in the at-risk population, we applied the T score, which may also represent summarized BMD measurements at 3 skeletal sites. A low T score was associated with an increased risk of harboring IA (OR, 1.28; 95% CI, 1.04-1.59; P = .02 after adjustment for age, sex, and vascular risk factors; Table 4). Larger aneurysms and multiple aneurysms were also associated with a low T score (Table 4; eTable 3 in the Supplement). Sex-specific analysis showed that a low T score was associated with an increased risk of harboring IA only in men but not in women; however, the size of the aneurysm was associated with a low T score in women but not in men (Table 4; eTable 3 in the Supplement). A sex-specific analysis for multiplicity of IA was not undertaken because of the small number of patients with multiple aneurysms.
Table 4. Logistic Regression Analyses of the Relationship Between Low T Score and IA Characteristics in At-Risk Population.
| Characteristic | Unadjusted | Adjusted for Age and Sex | Adjusted for All Covariates | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Harboring IA | ||||||
| Total | 1.44 (1.17-1.76) | <.001 | 1.26 (1.02-1.56) | .04 | 1.28 (1.04-1.59) | .02 |
| Women | 1.31 (1.02-1.70) | .04 | 1.13 (0.86-1.48) | .38 | 1.13 (0.87-1.49) | .36 |
| Men | 1.53 (1.08-2.14) | .02 | 1.47 (1.03-2.07) | .03 | 1.54 (1.08-2.17) | .02 |
| Size >3 mm | ||||||
| Total | 2.05 (1.36-3.11) | .001 | 2.07 (1.35-3.18) | .001 | 2.04 (1.32-3.16) | .001 |
| Women | 2.42 (1.43-4.17) | .001 | 2.63 (1.53-4.63) | .001 | 2.67 (1.53-4.75) | .001 |
| Men | 1.40 (0.69-2.82) | .35 | 1.41 (0.69-2.84) | .34 | 1.36 (0.65-2.83) | .41 |
| Multiplicity | 1.98 (1.14-3.54) | .02 | 1.80 (1.01-3.26) | .05 | 1.84 (1.05-3.30)a | .04 |
Abbreviations: IA, intracranial aneurysm; OR, odds ratio.
There were a small number of patients with multiple aneurysms (n = 61), and stepwise regression analysis was conducted to prevent overfitting.
Discussion
Our study found that lower BMD tertile was generally associated with an increased risk of harboring IA and larger aneurysms, but the associations differed according to skeletal site and population group. In the at-risk population, a low T score was also associated with the presence of IA, the size of the aneurysm, and multiplicity. Sex-specific analysis showed that a low T score was associated with a risk of harboring IA in men and with a larger aneurysm in women.
The associations between BMD and IA may be attributed to the biological sharing of connective tissue components and related factors, hormonal effects, and other mechanisms. Bone health and IA development share a common pathogenetic link. Local hemodynamic stress and a mounting inflammatory response on the vessel wall as well as pathologic ECM remodeling contribute to the development of IA.19 The ECM is an active and dynamic structure that interacts with sets of vascular cells and continuously undergoes a remodeling process to maintain the integrity of the vessel wall. Genetic association and gene expression studies have suggested that the integrity of ECM is critically involved in the pathogenesis of IA.19,20 The components of ECM, such as collagens and noncollagenous proteins, also constitute the organic part of the bone matrix.5 Type I collagen is the most abundant protein, composing approximately 95% of the total bone collagen and about 80% of the whole bone proteins, and alteration of collagen properties can affect bone quality and strength.7
Lysyl oxidase (LOX), a copper-dependent enzyme catalyzing collagen and elastin cross-linking, is a key molecule in the formation and repair of the ECM21 and is a potential common denominator in the pathophysiology of IA and bone fragility. Downregulation of LOX activity is associated with the alteration of ECM structure, impairment of vascular endothelial function, and vascular smooth muscle cell migration and proliferation.22 Development of IA under dysregulated LOX activity was demonstrated in our previous study involving a low-copper diet animal model.23 However, the role of LOX activity or copper deficiency in IA pathogenesis in humans is currently unclear and remains to be elucidated. The importance of LOX activity in bone structure and function has also been acknowledged.24 Studies have shown that copper deficiency is associated with skeletal integrity25,26 and that LOX knockout mice show deficient bone formation.27 An investigation into the factors influencing pathophysiological sharing would potentially elucidate the pathogenesis of both disorders.
Both osteoporosis and IA are female-dominant diseases; therefore, it is plausible that sex hormones are associated with the pathophysiological process of the 2 diseases. Estrogen receptors are expressed on endothelial cells and vascular smooth muscle cells. Experimental studies have shown that estrogen exerts a protective effect via various mechanisms including proliferation of endothelial cells, modulation of inflammatory mediators, and a reduction in vascular tone and oxidative stress.28,29,30,31,32 Reports have shown that bilateral oophorectomy increases the susceptibility of IA formation in female rats33 and the administration of 17-β estradiol continuous-release pellets decreases the frequency of IA formation.34 Similarly, ovariectomized female mice had a significantly higher incidence of IA, which was reversed by treatment with estrogen receptor-β agonist.35 Administration of 17-β estradiol or estrogen receptor-β agonist also reduced the incidence of aneurysm rupture in ovariectomized female mice.36
In clinical studies, female sex is associated with an increased risk of IA formation and growth.37,38 Women are more likely to have multiple aneurysms,39,40 and the postmenopausal state has also been shown to be an independent risk factor for the formation of multiple aneurysms.40 However, there is conflicting evidence about the increased rate of aneurysm rupture in women and the protective effect of hormone replacement therapy.11 In our study, there were stronger associations between decreased BMD and increased aneurysm size in women compared with men and in postmenopausal women compared with women in general. However, given the lack of association of low T scores with the presence of IA in women, the estrogen exposure hypothesis should be cautiously interperted. Female sex itself is a risk factor for IA formation, and perhaps a variance in estrogen exposure plays less of a role in determining the variations in IA formation susceptibility in the female population. Alternatively, other factors specific to the female sex may offset the effect of sex hormones or other mediators of bone mineral loss. Our results suggest that IA growth still seems to be sensitive to the factors that affect bone mineral loss in women, while other effects might outweigh these factors in men. More detailed evaluation of sex-specific factors is necessary to identify the causes of sex differences and to clarify the role of sex hormones in IA pathophysiology.
To our knowledge, this is the first study to evaluate the relationship between BMD and IA. The strengths of our study include the large number of patients, the detailed analysis of IA including the presence, size, and multiplicity of aneurysms, and the use of age- and sex-stratified BMD tertiles. The application of multiple statistical models with different populations and different types of variables of BMD and aneurysm size to evaluate the robustness of the relationship between BMD and IA are additional strengths of this study. However, our analyses yielded a few inconsistent findings that should be cautiously interpreted. Bone mineral density was associated with the size of IA but not with the presence of IA in women, as discussed previously. The presence of multiple aneurysms was not associated with BMD tertiles in any of the skeletal sites but was associated with low T scores. An additional inconsistency was that while low BMD was associated with the presence of IA, the difference in the rates of osteopenia was greater than the rates of osteoporosis between patients with and without IA (Table 1), which may have weakened the strength of overall associations between BMD and IA. The population structure with only a small proportion of patients with osteoporosis may explain this inconsistent trend. Further studies with different population would add insight to this issue.
Limitations
There were several limitations in this study. First, the data were collected retrospectively, and selection bias may have been introduced. Second, the study population may not represent the general population. The prevalence of vascular risk factors in the study population was higher than in the general population, implying the possibility that the decision to seek a medical checkup was affected by the underlying comorbidities of the patients. Racial/ethnic homogeneity in the study population also limit generalizability of the results. However, the prevalence of IAs was similar to that of the general population.1,2 Additionally, the health checkup program is designed for every adult who wishes to comprehensively evaluate his or her health status regardless of active symptoms. Therefore, this study may have higher generalizability than studies conducted with patients in tertiary care hospitals. Third, aneurysms smaller than 3 mm made up a large proportion of the study population. Small aneurysms are often not visible, especially in low-quality magnetic resonance angiographies, and even if found, most rarely undergo rupture with a low clinical significance. Although larger aneurysms are relatively less representative in this study population, our subgroup analysis indicated that BMD is more closely associated with increased size of aneurysms. Therefore, it is conceivable that future investigations with a different cohort, including a larger IA population, would demonstrate strengthened associations. Fourth, this study was cross-sectional and only analyzed the association between BMD and IA characteristics at the time of evaluation. A follow-up study that evaluates the relationship between BMD and the growth and rupture of aneurysms could provide additional information on the association between the 2 disorders.
Conclusions
In this large, cross-sectional study of health examination patients, we found that lower BMD tertiles were associated with increased risk of harboring intracranial aneurysm and increased aneurysm size, particularly in the at-risk population. Patients with a low T score were significantly associated with the presence of IA and large and multiple aneurysms. Our findings may help to enhance knowledge of IA pathophysiology, and the associations between low T score and IA are also clinically informative. However, the results are exploratory and resulted in a few internal inconsistencies in the associations. Additional studies are necessary to better characterize the relationship between BMD and IA.
eFigure. Flowchart Detailing the Inclusion and Exclusion Criteria and Numbers of the Study Subjects
eTable 1. Logistic Regression Analyses of the Relationship Between BMD and IA Size Larger Than 3 mm in At-Risk Population (Tertile 3 vs Tertile 1-2)
eTable 2. Logistic Regression Analyses of the Relationship Between BMD and Multiplicity of IA (Tertile 3 vs Tertile 1-2)
eTable 3. Linear Regression Analyses of the Relationship Between Low T-score and Log Size of IA in At-risk Population
References
- 1.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821-1828. [DOI] [PubMed] [Google Scholar]
- 2.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10(7):626-636. [DOI] [PubMed] [Google Scholar]
- 3.Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. 2016;12(12):699-713. [DOI] [PubMed] [Google Scholar]
- 4.Mimata C, Kitaoka M, Nagahiro S, et al. Differential distribution and expressions of collagens in the cerebral aneurysmal wall. Acta Neuropathol. 1997;94(3):197-206. [DOI] [PubMed] [Google Scholar]
- 5.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin G, Fisher S, Dickson D, Anderson D, Richardson S. The significance of the extracellular matrix in intracranial aneurysms. Ann Clin Lab Sci. 1993;23(2):97-105. [PubMed] [Google Scholar]
- 7.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17(3):319-336. [DOI] [PubMed] [Google Scholar]
- 8.Giampietro PF, Raggio C, Davis JG. Marfan syndrome: orthopedic and genetic review. Curr Opin Pediatr. 2002;14(1):35-41. [DOI] [PubMed] [Google Scholar]
- 9.Eller-Vainicher C, Bassotti A, Imeraj A, et al. Bone involvement in adult patients affected with Ehlers-Danlos syndrome. Osteoporos Int. 2016;27(8):2525-2531. [DOI] [PubMed] [Google Scholar]
- 10.Kim ST, Brinjikji W, Kallmes DF. Prevalence of intracranial aneurysms in patients with connective tissue diseases: a retrospective study. AJNR Am J Neuroradiol. 2016;37(8):1422-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turan N, Heider RA, Zaharieva D, Ahmad FU, Barrow DL, Pradilla G. Sex differences in the formation of intracranial aneurysms and incidence and outcome of subarachnoid hemorrhage: review of experimental and human studies. Transl Stroke Res. 2016;7(1):12-19. [DOI] [PubMed] [Google Scholar]
- 12.Almeida M, Laurent MR, Dubois V, et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97(1):135-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiss CJ, Sanborn CF, Nichols DL, Bonnick SL, Alford BB. Associations of body fat distribution, circulating sex hormones, and bone density in postmenopausal women. J Clin Endocrinol Metab. 1995;80(5):1591-1596. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TV, Jones G, Sambrook PN, White CP, Kelly PJ, Eisman JA. Effects of estrogen exposure and reproductive factors on bone mineral density and osteoporotic fractures. J Clin Endocrinol Metab. 1995;80(9):2709-2714. [DOI] [PubMed] [Google Scholar]
- 15.Lewiecki EM, Binkley N, Morgan SL, et al. ; International Society for Clinical Densitometry . Best practices for dual-energy x-ray absorptiometry measurement and reporting: International Society for Clinical Densitometry guidance. J Clin Densitom. 2016;19(2):127-140. [DOI] [PubMed] [Google Scholar]
- 16.Wishart JM, Need AG, Horowitz M, Morris HA, Nordin BE. Effect of age on bone density and bone turnover in men. Clin Endocrinol (Oxf). 1995;42(2):141-146. [DOI] [PubMed] [Google Scholar]
- 17.Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res. 2012;27(1):111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group. World Health Organ Tech Rep Ser. 1994;843:1-129. [PubMed] [Google Scholar]
- 19.Chalouhi N, Ali MS, Jabbour PM, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32(9):1659-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alg VS, Sofat R, Houlden H, Werring DJ. Genetic risk factors for intracranial aneurysms: a meta-analysis in more than 116,000 individuals. Neurology. 2013;80(23):2154-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88(4):660-672. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez C, Martínez-González J, Raposo B, Alcudia JF, Guadall A, Badimon L. Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008;79(1):7-13. [DOI] [PubMed] [Google Scholar]
- 23.Jung KH, Chu K, Lee ST, et al. Experimental induction of cerebral aneurysms by developmental low copper diet. J Neuropathol Exp Neurol. 2016;75(5):455-463. [DOI] [PubMed] [Google Scholar]
- 24.Trackman PC. Enzymatic and non-enzymatic functions of the lysyl oxidase family in bone. Matrix Biol. 2016;52-54:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uriu-Adams JY, Scherr RE, Lanoue L, Keen CL. Influence of copper on early development: prenatal and postnatal considerations. Biofactors. 2010;36(2):136-152. [DOI] [PubMed] [Google Scholar]
- 26.Medeiros DM. Copper, iron, and selenium dietary deficiencies negatively impact skeletal integrity: a review. Exp Biol Med (Maywood). 2016;241(12):1316-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pischon N, Mäki JM, Weisshaupt P, et al. Lysyl oxidase (lox) gene deficiency affects osteoblastic phenotype. Calcif Tissue Int. 2009;85(2):119-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gargett CE, Zaitseva M, Bucak K, Chu S, Fuller PJ, Rogers PA. 17Beta-estradiol up-regulates vascular endothelial growth factor receptor-2 expression in human myometrial microvascular endothelial cells: role of estrogen receptor-alpha and -beta. J Clin Endocrinol Metab. 2002;87(9):4341-4349. [DOI] [PubMed] [Google Scholar]
- 29.Galea E, Santizo R, Feinstein DL, et al. Estrogen inhibits NF kappa B-dependent inflammation in brain endothelium without interfering with I kappa B degradation. Neuroreport. 2002;13(11):1469-1472. [DOI] [PubMed] [Google Scholar]
- 30.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23(5):665-686. [DOI] [PubMed] [Google Scholar]
- 31.Wagner AH, Schroeter MR, Hecker M. 17beta-estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J. 2001;15(12):2121-2130. [DOI] [PubMed] [Google Scholar]
- 32.Strehlow K, Rotter S, Wassmann S, et al. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93(2):170-177. [DOI] [PubMed] [Google Scholar]
- 33.Jamous MA, Nagahiro S, Kitazato KT, Satomi J, Satoh K. Role of estrogen deficiency in the formation and progression of cerebral aneurysms: part I: experimental study of the effect of oophorectomy in rats. J Neurosurg. 2005;103(6):1046-1051. [DOI] [PubMed] [Google Scholar]
- 34.Jamous MA, Nagahiro S, Kitazato KT, Tamura T, Kuwayama K, Satoh K. Role of estrogen deficiency in the formation and progression of cerebral aneurysms: part II: experimental study of the effects of hormone replacement therapy in rats. J Neurosurg. 2005;103(6):1052-1057. [DOI] [PubMed] [Google Scholar]
- 35.Tada Y, Makino H, Furukawa H, et al. Roles of estrogen in the formation of intracranial aneurysms in ovariectomized female mice. Neurosurgery. 2014;75(6):690-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tada Y, Wada K, Shimada K, et al. Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension. 2014;63(6):1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke. 2001;32(2):485-491. [DOI] [PubMed] [Google Scholar]
- 38.Kubo Y, Koji T, Kashimura H, Otawara Y, Ogawa A, Ogasawara K. Female sex as a risk factor for the growth of asymptomatic unruptured cerebral saccular aneurysms in elderly patients. J Neurosurg. 2014;121(3):599-604. [DOI] [PubMed] [Google Scholar]
- 39.Ostergaard JR, Høg E. Incidence of multiple intracranial aneurysms: influence of arterial hypertension and gender. J Neurosurg. 1985;63(1):49-55. [DOI] [PubMed] [Google Scholar]
- 40.Ellamushi HE, Grieve JP, Jäger HR, Kitchen ND. Risk factors for the formation of multiple intracranial aneurysms. J Neurosurg. 2001;94(5):728-732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart Detailing the Inclusion and Exclusion Criteria and Numbers of the Study Subjects
eTable 1. Logistic Regression Analyses of the Relationship Between BMD and IA Size Larger Than 3 mm in At-Risk Population (Tertile 3 vs Tertile 1-2)
eTable 2. Logistic Regression Analyses of the Relationship Between BMD and Multiplicity of IA (Tertile 3 vs Tertile 1-2)
eTable 3. Linear Regression Analyses of the Relationship Between Low T-score and Log Size of IA in At-risk Population