Abstract
Rationale
The beneficial effects of moderate alcohol may differ in aging men versus women.
Objectives
Cognitive and functional decline and neuropathology were investigated in a cohort of aging men and women with diverse alcohol histories.
Methods
Non-demented (Clinical Dementia Rating (CDR) of ≤ 0.5 and a Mini-Mental State Examination (MMSE) score of > 24), autonomously living participants were tracked in longitudinal aging studies to examine self-report and objective tests of rates of decline in a cohort (n = 486) of octogenarians. Neurofibrillary tangles (NFTs; Braak stage) and neuritic plaques (NPs) were staged at autopsy in a subset of participants (n = 149) using current standard neuropathologic diagnostic criteria.
Results
Moderate drinking men had an attenuated rate of decline compared to rare/never drinkers and women on the Mini-Mental State Examination (MMSE) and Clinical Dementia Rating (CDR) sum of boxes. In contrast, moderate drinking women had a reduced rate of decline only in the logical memory delayed recall test (LMDR) compared to rare/never drinkers and men. Moderate alcohol consumption was associated with a reduction in the incidence of advanced (stage 5 – 6) Braak NFT stage in men (p < 0.05), with no effect in women.
Conclusions
In this cohort, men experienced a broader range of beneficial effects associated with alcohol. Alcohol’s effects may differ in men and women in important ways that suggest a narrower beneficial window.
Search terms: Alcohol, Sex differences, Cognitive aging, Alzheimer’s disease, Neuropathology, Dementia
1. Introduction
The effect of moderate alcohol consumption on the aging brain is unclear. A recent review found that moderate alcohol consumption slowed the rate of cognitive decline associated with aging (Ilomaki et al. 2015). However, there was substantial heterogeneity across studies with many of these reports consisting of participants of early retirement age, excluding those of more advanced age. Differential responses to alcohol in men and women may explain some of the variability across studies, and few studies have examined effects independently by sex. Women are more susceptible to the harmful effects of alcohol. This is most readily apparent in the context of mortality rates, where women that abuse alcohol exhibit double the mortality rate of men that abuse alcohol and nearly five times the mortality rate of women that do not abuse alcohol (John et al. 2013; Roerecke and Rehm 2013). In women, increased vulnerability to alcohol-induced damage is also apparent in alcohol-induced liver disease, cardiomyopathy, and peripheral neuropathy (Ammendola et al. 2000; Fernandez-Sola and Nicolas-Arfelis 2002; Tuyns and Pequignot 1984). Whether this vulnerability extends to the brain remains controversial, although preclinical and clinical data generally support a similar increased susceptibility to alcohol-induced brain damage in women compared to men (Hashimoto and Wiren 2008; Hommer 2003; Wilhelm et al. 2015). It is unclear if the beneficial effects of alcohol exhibit a similar sexually dimorphic pattern. This study was designed to: 1) confirm the association of moderate alcohol use with attenuated age-related cognitive and functional decline, 2) determine whether or not there are sexually dimorphic effects of alcohol use on age-related decline, and 3) examine whether moderate alcohol consumption influences the development of Alzheimer’s disease (AD) pathology. AD pathology is characterized by neuritic plaques (NPs; extracellular accumulations of amyloid beta) and neurofibrillary tangles (NFTs; deposits of hyperphosphorylated tau). Though the presence of NPs and NFTs is required for the diagnosis of AD, NPs and NFTs are often found in non-demented individuals as well (e.g. Swerdlow 2011).
2. Methods
2.1. Research participants
Participants were recruited from two ongoing, longitudinal studies: the Oregon Brain Aging Study (OBAS) and the Intelligent Systems for Assessing Aging Changes (ISAAC) study. OBAS commenced in 1989 at the NIA - Layton Aging and Alzheimer’s Disease Center. Initially, entry criteria required participants to be 55 years of age or older, functionally independent community members, and free of co-morbid illnesses. In 2004, these requirements were modified to allow participants with controlled, chronic medical conditions common in older adults to better model the general population. Participants enrolled since 2004 were 85 years of age or older. ISAAC began in 2007 and enrolled participants age 70 or older. As a requirement for entry, participants needed to be non-demented, as defined by a Clinical Dementia Rating (CDR) of ≤ 0.5 and a Mini-Mental State Examination (MMSE) score of > 24, living autonomously, and of average health or better for their age. Participants with physical limitations or medical conditions likely to result in premature death were excluded.
2.2. Procedures and analysis battery
Participants completed several neuropsychological measures of cognition and function (Online Resource 1) annually either in a clinic setting, or in the participants’ homes. A detailed description of testing format and procedures has been previously published (Kaye et al. 2011). Participants underwent a neuropsychological evaluation, a comprehensive health and life events interview, and questionnaires for updating medical history. In addition, phone interviews between annual assessments were conducted to update health histories. Collateral informants of the participants were also involved in annual assessments and phone interviews. Upon review of the assessments, a final analysis battery was compiled a priori for the evaluation of the following response outcomes: Mini-Mental State Exam (MMSE), Cumulative Dementia Rating (CDR), and CDR as a summed score (CDR Sum of Boxes), Activities of Daily Living (ADL) including the Instrumental subset (IADL), the B7 function assessment questionnaire for the National Alzheimer’s Coordinating Center (FAQ), the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List Delayed Recall, the Logical Memory test with delayed recall, semantic fluency testing, digit symbol test and Trails B test. All participants provided written consent, and all study protocols received approval from the OHSU Institutional Review Board (OBAS IRB #361; ISAAC IRB #2353).
2.3. Alcohol Consumption History and Group Classification
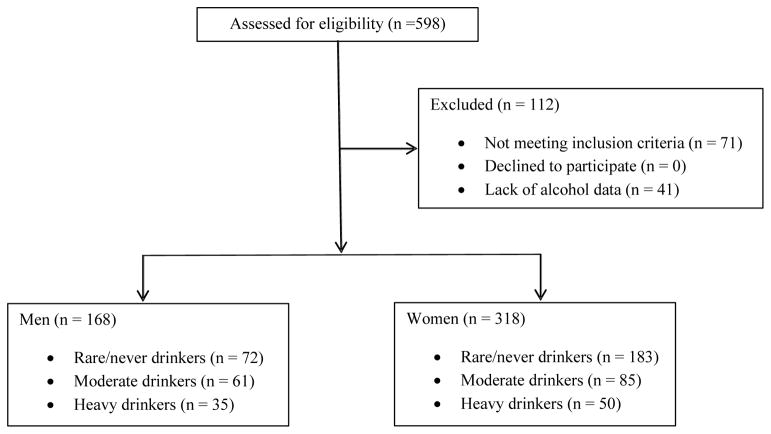
Lifetime alcohol history was determined via standard family history questionnaires (Online Resource 2) asking participants to identify the frequency (drinking days per week) of drinking and drinks per drinking day as a teen, adult and aged adult, the frequency of consuming four or more drinks on a single occasion, whether they had ever consumed one drink or more per week for more than three months, and whether they were a current drinker. Weekly alcohol consumption was calculated by multiplying the quantity of drinks per drinking day by the number of drinking days per week. One drink was defined as 12 oz of beer, 8 oz of wine, or 1.5 oz of distilled liquor. Based on their answers to these questions, the cohort was divided into six categories: rare/never drinking women, moderate drinking women, heavy drinking women, rare/never drinking men, moderate drinking men, and heavy drinking men. Rare/never drinkers were those individuals who answered “no” to the question of “have you ever consumed more than one drink per week regularly for more than three months?” Thus, individuals in the rare/never drinking group may have some experience with alcohol, but did not have a history of drinking alcohol on a consistent basis. Drinkers were those who answered “yes” to the question, “have you ever consumed more than one drink per week regularly for more than three months?” Moderate drinkers (low risk alcohol consumption as defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA)) were those women who reported consuming no more than three drinks per day and no more than seven drinks per week and men who reported consuming no more than four drinks per day and no more than 14 drinks per week during any life period (teen, adult, aged adult). Heavy drinkers were those individuals that reported drinking patterns in excess of participants in the moderate drinking group during any period of life and considered a distinct cohort from rare/never drinkers and moderate drinkers. Drinking behavior was considered stable over time unless participants completed an additional drinking assessment during a subsequent annual assessment (most participants reported drinking behavior only once and usually during their initial assessment). A total of 598 subjects were assessed for eligibility, of which 486 participants were included in the final analyses (Figure 1). Baseline characteristics for this sample as a function of sex are reported in Table I.
Figure 1.
Study Participants
Flow diagram depicting inclusion and exclusion of participants in the study. OBAS: Oregon Brain Aging Study; ISAAC: Intelligent Systems for Assessing Aging Changes, EtOH: alcohol.
Table I.
Baseline demographic and clinical characteristics of rare/never drinkers and drinkers
| MEN (n = 168) | Rare/never drinkers (n = 72) | Moderate Drinkers (n = 61) | Heavy Drinkers (n = 35) | Drinking effect p-value |
|---|---|---|---|---|
| Age, years (SD) | 83.1 (8.0) | 81.4 (8.3) | 80.6 (6.9) | 0.23 |
| Education, years (SD) | 15.2 (3.0) | 16.0 (2.3) | 16.3 (3.4) | 0.15 |
| MMSE score (SD) | 28.3 (1.5) | 28.2 (1.5) | 28.2 (1.7) | 0.96 |
| Caucasian, % (n) | 94% (68) | 98% (60) | 86% (30) | 0.0401 |
| Cumulative Illness Rating Scale score (SD) | 20.7 (3.3) | 19.9 (3.3) | 19.7 (2.75) | 0.54 |
| ApoE4, # (%) | 12 (16.7%) | 14 (23.0%) | 6 (17.1%) | 0.53 |
| BMI (kg/m2) | 26.4 (3.7) | 25.4 (2.8) | 26.9 (4.0) | 0.11 |
| Diabetic, % (n) | 12.5% (9) | 9.8% (6) | 8.5% (3) | 0.85 |
| Hypertensive, % (n) | 51.3% (37) | 57.4% (35) | 65.7% (23) | 0.39 |
| Reported # of drinks weekly | - | 5.2 (2.5) | 14.2 (7.5) | <0.001 |
| WOMEN (n = 318) | Rare/never drinkers (n = 183) | Moderate Drinkers (n = 85) | Heavy Drinkers (n = 50) | Drinking effect p-value |
|---|---|---|---|---|
| Age, years (SD) | 83.7 (6.6) | 83.9 (6.5) | 79.0 (8.4) | 0.0022 |
| Education, years (SD) | 14.2 (2.5) | 14.8 (2.4) | 15.4 (2.8) | .0113 |
| MMSE score (SD) | 28.4 (1.6) | 28.5 (1.4) | 28.9 (1.2) | 0.10 |
| Caucasian, % (n) | 92% (168) | 92% (78) | 90% (45) | 0.91 |
| Cumulative Illness Rating Scale score (SD) | 20.4 (3.4) | 20.4 (3.5) | 20.5 (3.6) | 0.99 |
| ApoE4, # (%) | 27 (14.8%) | 21 (24.7%) | 15 (30.0%) | 0.0244 |
| BMI (kg/m2) | 26.0 (4.9) | 25.7 (4.6) | 25.7 (4.9) | 0.86 |
| Diabetic, % (n) | 10.9% (20) | 9.4% (8) | 14.0% (7) | 0.70 |
| Hypertensive, % (n) | 61.7% (113) | 70.6% (60) | 68.0% (34) | 0.34 |
| Reported # of drinks weekly | - | 4.7 (1.9) | 15.7 (6.4) | <0.001 |
4 (5.6%) of the rare/never drinkers did not have ApoE4 genotype identified.
7 (11.5%) of the moderate drinkers did not have ApoE4 genotype identified.
5 (14.3%) of the heavy drinkers did not have their ApoE4 genotype identified.
significant contrast between heavy drinkers and moderate drinkers (p=0.023)
12 (6.66%) of the rare/never drinkers did not have ApoE4 genotype identified.
7 (8.3%) of the drinkers did not have ApoE4 gene identified.
2 (4.0%) of the heavy drinkers did not have ApoE4 gene identified
significant contrasts between heavy drinkers and both moderate drinkers and rare/never drinkers (p<0.001)
significant contrast between heavy drinkers and rare/never drinkers (p=0.005)
significant contrast between heavy drinkers and rare/never drinkers (p=0.028)
Data are presented as means (Standard Deviation; SD) unless otherwise indicated.
Statistical comparisons were made using one-way ANOVAs, with the exception of race/Caucasian and ApoE4, which were determined with the Fisher test. Specific contrasts between drinking groups were evaluated with pairwise t-tests and pairwise Fisher tests respectively
2.4. Neuropathological evaluations
Autopsy results were collected for a subset of individuals that died during the study (n = 149) and were examined to stage neurofibrillary tangles (NFTs) and neuritic plaques (NPs). All study participants were invited to donate their brains after death regardless of cause of death or cognitive health. The staining and analysis procedures have been described previously (Erten-Lyons et al. 2013; Green et al. 2000). In brief, brains were examined grossly and microscopically after fixation in neutral-buffered formaldehyde solution for 2 weeks as previously described (Green et al. 2000). Microscopic evaluation to determine diagnosis and staging examined bilateral cortical lobes, frontal lobe white matter, anterior cingulate gyrus, hippocampus, amygdala, bilateral striatum, thalamus, midbrain, pons, medulla, and cerebellum. Braak and Braak neurofibrillary tangle stage (Braak and Braak 1995) and neuritic plaque density were assessed in each patient based on the distribution of lesions observed with reference to current diagnostic criteria (Hyman et al. 2012).
2.5. Statistical analysis
Differences in baseline characteristics between drinking groups were assessed using one-way ANOVAs for continuous variables and chi-square tests for categorical covariates with follow-up pairwise t tests. To analyze the relationship between alcohol consumption and measures of cognition and function, linear mixed-effects regression models were used. Factors known to influence neuropsychological assessments were included as controlling covariates in the model: age at study entry, years of education, the Cumulative Illness Rating Scale (CIRS (Linn et al. 1968)) at study entry, and expression of the apolipoprotein E, isoform 4 (ApoE4) genotype, represented as a binary factor encompassing carriers against non-carriers. With an a priori interest in determining whether alcohol differentially reduced cognitive and functional decline in men vs. women over the course of study participation, all non-pathological responses were modelled as rates of change relative to study entry. Specifically, the analysis considered the influences of sex, reported drinking status and their interaction on the change in the response variables over time. Direct contrasts between moderate or heavy drinkers and rare/never drinkers were also examined. Statistical models were created using R software with lme4 (Bates et al. 2015; Bates et al. 2014) and ggplot2 (Wickham 2009) packages installed. Cognitive and functional outcomes with pre-defined minimums and maximums were log transformed to better fit normality assumptions, verified by inspection of the distributions with utility from standard normality tests such as the Shapiro-Wilk and normal Anderson-Darling tests. Non-linear associations of time with the outcome variables were evaluated using multiple fractional polynomial modeling, a common technique in identifying non-linear effects (Royston et al. 1999; Sterne et al. 2009). Visual assessment of residual plots and calculation of Cook’s distance was done to inspect the integrity of the model and to remove outliers with excessive model leverage. Autopsy results were analyzed using logistic regression with ApoE4 status used as a covariate. Assessment of statistical significance relied on the use of the Holm-Sidak correction for multiple comparisons to account for multiple outcomes of interest within a given cognitive domain while clinical effect sizes were based on the precision of the corresponding test statistics as well as the calculated rates of change for each gender-drinking subset in the cohort.
3. Results
3.1. Participant characteristics
The final cohort included 486 participants and was composed of 34.6% men (Table I). Among all participants 30.0% (146 participants) of this cohort were classified as moderate drinkers while 17.5% (85 participants) were classified as heavy drinkers. Men had similar ages, education, ApoE4 prevalence and CIRS scores across groups upon inclusion in the study, though there were fewer Caucasians in the heavy drinking group relative to the moderate drinking group (85% vs 98%; p = 0.023). Women in the heavy drinking group had similar profiles to moderate drinking and rare/never drinking women with respect to ethnicity and CIRS score. Women in the heavy drinking group were younger than women in the rare/never drinking group (p < 0.001) and moderate drinking group (p < 0.001), attained higher levels of education than rare/never drinkers (p = 0.005), and had a higher prevalence of ApoE4 carriers than women in the rare/never drinking group (p = 0.028). Comparing between men and women, moderate drinking men and women drank similar amounts of alcohol (t = 1.01; p = 0.49) as did heavy drinking men and women (t = 0.81; p = 0.42). Most participants were enrolled in the study for 6 – 8 years with assessments occurring annually. Due to missing data with respect to drinking status or study outcomes, 41 subjects were excluded. However, this did not lead to bias in the cohort as no significant differences were found after subject attrition with respect to principal covariates of age at entry (t = 0.06, p = 0.95), CIRS score (t = 0.22, p = 0.83), years of schooling (t = 0.36, p = 0.72), or ApoE4 carrier status (OR = 1.07, p = 0.069).
3.2. Influence of moderate drinking on age-related decline
Mixed-models were used to explore the effect of moderate and heavy alcohol consumption on cognition and functional ability as main effects. P values were adjusted using the Holm-Sidak method to correct for multiple comparisons and tested comparisons for all factors and interactions are presented in Online Resource 3. All measures of cognition and function worsened with time (p’s < 0.001). An effect of alcohol was observed on the logical memory delayed test (t = 2.77, p = 0.007). Alcohol did not exert a significant effect on any of the other measures examined.
3.3. Decline profiles differ for moderate drinking men and women
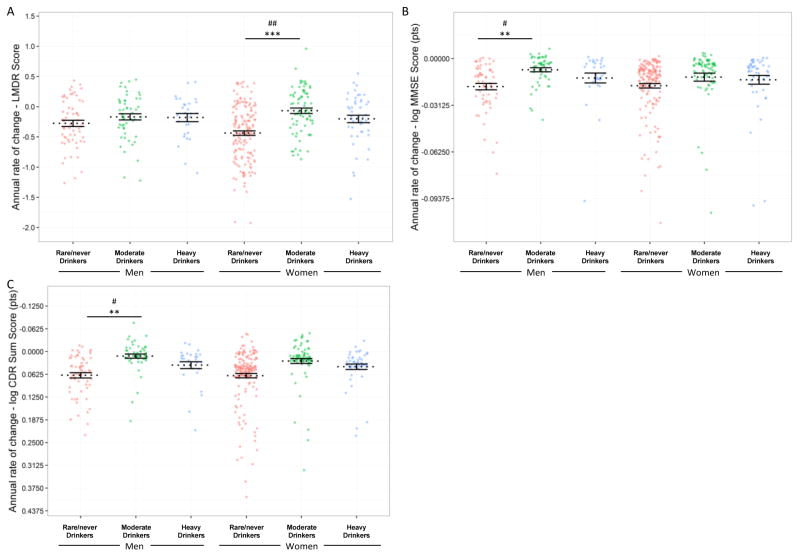
The potential differential influence of alcohol and sex on neuropsychological measures of decline was examined (Figure 2, Online Resource 4). Moderate drinking women exhibited reduced decline over time on the logical memory delayed test (Figure 2A; t = 3.37, p < 0.001) compared to rare/never drinking women. The reduced declined observed in moderate drinking women was larger than the effect observed in men (t = 2.61, p = 0.0094). No effect of moderate drinking on the logical memory delayed test was observed in men. Moderate drinking men exhibited reduced decline on the MMSE (Figure 2B; t = 2.68, p = 0.0076) and CDR sum of boxes (Figure 2C; t = −3.1, p = 0.0021) compared to rare/never drinking men. Direct contrasts indicated significant differences in the effect of moderate drinking between sexes for MMSE (t = 2.14, p = 0.033) and CDR sum of boxes: (t = 2.02, p = 0.044). Moderate drinking men also had reduced decline in ADL, IADL and categorical fluency relative to rare/never drinking men (Supplemental Figure S1A–C; ADL: t = 2.57, p = 0.011; IADL: t = 2.12, p = 0.035; categorical fluency: t = 2.45, p = 0.016). Moderate drinking women exhibited reduced decline in Trails B times relative to rare/never drinking women (Supplemental Figure S1D; t = 2.15, p = 0.033) Effects of moderate drinking were not different when comparing between the sexes on ADL, IADL, categorical fluency or Trails B times. For all outcomes, the annualized rates of change of heavy drinkers were not statistically different from rare/never drinkers or moderate drinkers although visual inspection of the plots indicated longitudinal profiles more akin to rare/never drinkers than the protective effects observed in moderate drinkers.
Figure 2.
Neuropsychological Measures
Moderate drinking groups exhibit reduced rates of decline and differ in men and women. Annual rates of decline on the (A) Logical Memory Delayed task (LMDR), (B) Mini-Mental State Examination (MMSE) and (C) Clinical Dementia Rating (CDR) sum of boxes over time for individual subjects. Annualized rates of change are taken from the mixed-effects models with subject-specific random effects included and fixed-effects rates indicated for each group. Individuals in the moderate alcohol drinking groups exhibited reduced decline in both women and men, but on different tasks. Rates of decline were reduced in the group of moderate drinking women on the LMDR and in the moderate drinking group of men slowed on the MMSE and CDR sum of boxes. ** p < 0.01, *** p < 0.001 comparing same-sex rare/never drinkers to moderate drinkers. # p < 0.05, ## p < 0.01 comparing the effect of moderate drinking between men and women.
Though initially corrected for in all models, the influence of ApoE4 on decline was also examined. ApoE4 carriers generally exhibited faster rates of decline in the cognitive outcomes compared to non-carriers: MMSE (t = 1.87, p = 0.076), CDR (t=2.61, p=0.0081), IADL (t=1.86, p=0.076), the CERAD word task (t=2.83, p=0.0059), logical memory (t=2.11, p=0.035) and Trails B (t=2.51, p=0.012). However, when contrasted between moderate drinkers and rare/never drinkers, no significant differences in the attenuated rates of decline were seen within either carriers or non-carriers with maintenance of the previously cited significant gender-based contrasts in drinking status.
3.4 Association between alcohol consumption and development of Alzheimer’s disease pathology
Autopsy and pathology results were available for 60 men (36 rare/never drinkers, 13 moderate drinkers and 11 heavy drinkers) and 89 women (61 rare/never drinkers, 16 drinkers and 12 heavy drinkers) and are summarized in Table II. Analysis of Braak stage indicated that men in the moderate drinking group had a significant reduction in severe NFT pathology (stage 5 – 6) relative to rare/never drinkers (p = 0.037, Odds ratio = 4.28, 95% CI 1.16 – 20.9). There was no difference in severe NFT pathology rates between men in the heavy drinking and rare/never drinking groups (p = 0.24, Odds ratio = 2.97, 95% CI 0.75 – 23.7). As opposed to the effects observed in men, NFT pathology stage was not influenced by a moderate drinking history in women (p = 0.56, Odds ratio = 1.36, 95% CI 0.25 – 2.01) nor heavy drinking in women (p = 0.11, Odds ratio = 4.71, 95% CI 0.85 – 36.8). Moderate alcohol drinking groups had a similar incidence of frequent neocortical NPs than the respective rare/never drinking groups (men p = 0.68, Odds ratio = 1.19, 95% CI 0.25 – 2.46; women p = 0.25, Odds ratio = 1.73, 95% CI 0.68 – 4.67).
Table II.
AD pathology
| Braak Stage | Neuritic Plaque Density | |||
|---|---|---|---|---|
| 0–4 | 5–6 | 0–2 | 3 | |
| Rare/never drinking men, n (%) | 21 (58%) | 15 (42%) | 29 (81%) | 7 (19%) |
| Moderate drinking men, n (%) | 12 (92%) | 1 (8%) | 13 (100%) | 0 (0%) |
| Heavy drinking men, n (%) | 6 (55%) | 5 (45%) | 6 (55%) | 5 (45%) |
| Rare/never drinking women, n (%) | 45 (74%) | 16 (26%) | 49 (80%) | 12 (20%) |
| Moderate drinking women, n (%) | 12 (75%) | 4 (25%) | 14 (88%) | 2 (12%) |
| Heavy drinking women, n (%) | 6 (50%) | 6 (50%) | 5 (41.7%) | 7 (58.3%) |
Autopsy results were divided into groups based on Braak stage and neuritic plaque density. Braak stage scores of 0 – 4 were grouped as limited to moderate pathology, and scores of 5 – 6 were grouped to indicate severe pathology. NP densities of 0 – 2 were grouped as modest pathology, while scores of 3 were grouped to indicate severe NP pathology. Numbers in parenthesis indicate the percentage of participants in each group with the indicated stage of pathology.
Comparison of demographics between drinking groups within the neuropathology cohort yielded a similar profile as observed in the primary study cohort. For men, no differences were observed with respect to age at study entry, CIRS score, years of education, or ApoE4 status though there were fewer non-Caucasians in the moderate drinking group compared to the heavy drinking group (1.6% vs 14.3%, p = 0.041). Meanwhile, women in the heavy drinking group were again younger than women in the rare/never drinking group (p < 0.001) and moderate drinking group (p = 0.002), attained higher levels of education than rare/never drinkers (p = 0.011), and displayed a slightly higher prevalence of ApoE4 carriers compared to women in the rare/never drinking group (p = 0.024); however, there were no differences in racial background or CIRS comorbidity. Contrasting between men and women, again no differences were observed within drinking classifications with regards to drink consumption or time of study participation.
Similar impacts of imposed bias due to subject attrition were considered between the testing cohort of 486 subjects and the 149 subjects with available neuropathology data. No significant differences were observed between the cohort with respect to CIRS score (t = 0.01, p = 0.99), years of schooling (t = 0.35, p = 0.73), or ApoE4 carrier status (Odds ratio = 1.10, p = 0.069). Although there was an observed difference in age at study entry of 2.6 years (t = 5.66, p < 0.001) between the study cohort (82.9 years) and the neuropathology cohort (85.5) this was based on largely on the disposition between the parent cohorts. Specifically, significantly more subjects from OBAS (n=146), which had a minimum age of entry 15 years higher than ISAAC (n=45), had neuropathological evaluation, stemming solely from differences between the study protocols (Odds ratio = 3.65, p < 0.001). Regardless of these differences in baseline age between the cohorts, no influence of age at study entry was found in either NFT pathology stage (p = 0.67) or neocortical NP burden (p = 0.48).
4. Discussion
Two systematic meta-reviews of longitudinal studies suggest associations between moderate alcohol consumption and reduced frequency of AD and dementia (Ilomaki et al. 2015) in cohorts of adults that tended to be younger than those in this study. Past studies of cognitive decline in aging have generally indicated a positive effect of moderate alcohol drinking (Britton et al. 2004; Downer et al. 2015; Reas et al. 2016; Solfrizzi et al. 2007; Weyerer et al. 2011; Wright et al. 2006), however, while these studies often include sex in their models, they typically did not examine them individually to determine sex-specific effects. The present findings are considered preliminary, but suggest that effects of alcohol are modest when considering mixed groups of men and women. When men and women were examined separately, however, the moderate drinking groups exhibited reduced decline on several measures of cognitive function. These findings were observed in spite of the reliance on self-reported alcohol consumption.
The heavy drinking group performed similarly to the rare/never drinking group over time on most neuropsychological tasks. It is important to note that this study was not designed to assess the negative health consequences of heavy alcohol use and the majority of individuals who engage in long-term heavy drinking would not have met criteria for inclusion in this study. Individuals in the heavy drinking group exceeded NIAAA-recommended guidelines for moderate drinking, but only by a relatively small amount. Alcohol effects are commonly associated with a j-shaped curve (Andreasson 1998), with beneficial effects seen at lower doses and with increasing consumption a transition back to no- or harmful effects in a dose-related fashion. Thus, it is likely that individuals in the heavy drinking group in the current study drank enough to exceed the beneficial effects, but not so much that they experienced the harmful effects of alcohol. Individuals in the heavy drinking group in this study averaged approximately 2 drinks per day, while in studies designed to examine heavy drinking, it is not uncommon for subjects to drink 10 or more drinks per day (Kurlawala and Vatsalya 2016). Moderate alcohol consumption in men was associated with greater reductions in decline than women in two neuropsychological measures (MMSE and CDR sum of boxes). By contrast, the group of moderate drinking women, despite having a larger sample size and therefore greater power to detect differences, exhibited a decreased rate of decline relative to men in only the logical memory delayed test. Other factors such as patterns of consumption, differences in body composition and rates of alcohol metabolism may also contribute to sex-differences in alcohol effects (Baraona et al. 2001; Eng et al. 2005). Importantly, with the exception of logical memory, the main effects of alcohol consumption were non-significant with the influence of moderate alcohol consumption only being revealed after directly contrasting between genders. The need to carefully examine sex-differences in alcohol effects has been observed by others as well, with unmasking of sexually dimorphic effects observed only after comparing males and females independently (Schuckit et al. 2012). This evidence suggests that the underlying associations with alcohol, both beneficial as seen in this study and harmful as seen previously, exhibit different physiological manifestations in men vs. women.
The current findings observed a significant reduction in development of NFTs in the moderate drinking group of men relative to rare/never drinkers. No such reduction was observed in the group of moderate drinking women. The heavy drinking group exhibited pathology similar to or generally worse than the rare/never drinking group. Though a direct link between alcohol intake and brain health has not been established, several clinical or preclinical studies suggest mechanisms by which alcohol may act to slow cognitive decline or the development of dementia (reviewed in Panza et al. 2009). Alcohol drinking has been associated with a reduction in white matter lesions and brain infarcts (Mukamal et al. 2001). Preclinical studies suggest that alcohol may also improve brain function by facilitating the release of acetylcholine (Henn et al. 1998; Stancampiano et al. 2004). Alcohol drinking was associated with a reduced risk of microvascular complications in type 1 diabetes patients (Beulens et al. 2008), though effects of alcohol on microvascular disease in aging are unclear. Alcohol also increases levels of HDL cholesterol (Gaziano et al. 1993). Cholesterol is a critical component of the brain and dysregulation of cholesterol homeostasis occurs in several neurodegenerative diseases including AD (Orth and Bellosta 2012). Alcoholic beverages (red wine in particular) are also a source of antioxidants (reviewed in Panza et al. 2009). Thus, the beneficial effects of alcohol could be mediated via several potential mechanisms.
The major limitation of this study was that participants were largely Caucasian and from middle to upper socioeconomic classes. Thus, the generalizability of these findings to more diverse populations is unclear. Other limitations of the study include the reliance on self-report measures for alcohol consumption and limited reporting of alcohol consumption throughout the study. The difference in years of education between the groups of moderate drinking women and the rare/never drinking women was unlikely to confound our interpretations because our mixed model analysis corrected for differences in this variable. An additional limitation is that the drinking groups were broadly defined and did not take into account many potential variables including each individual’s length of time regularly consuming alcohol or type of alcohol consumed. It is also possible that with repeated testing, some individuals may improve their performance on some of the tasks administered over time. Improvements with repeated testing have been observed previously including in the digit symbol substitution test (Beres and Baron 1981), however such improvements were observed with repeated testing over consecutive days, as opposed to the infrequent (typically annually) testing used in the current study. An important consideration for this study is that individuals that suffer the deleterious effects of alcohol may have been excluded due to pre-existing conditions or premature death. Previous studies suggest that if anything, this would bias the beneficial effects toward women, as women are more likely to die prematurely due to alcohol-related issues than men (Roerecke and Rehm 2013). Alternatively, healthy octogenarians may be endowed with genetic or environmental factors that result in longer and healthier lives regardless of alcohol. This is unlikely because of the relatively strict entry criteria for the study which required participants to be healthy, independent, and of similar age. Thus all groups started with similar levels of cognitive function and lack of disability, thereby allowing us to explore the association of alcohol with age-related decline, albeit in a selection of individuals that have aged favorably. The frequency of ApoE4 allele carriers was increased in women in the heavy drinking group versus the rare/never drinking group. ApoE4 status may be an important factor in determining the effect of alcohol on the risk of dementia and predementia (reviewed in Panza et al. 2009; Solfrizzi et al. 2008). ApoE4 status was very similar between the rare/never drinking and moderate drinking groups across the sexes, however, the heavy drinking women group had a higher prevalence of ApoE4 genotype than the group of heavy drinking men. The autopsy results are limited by the relatively small sample size, particularly in the moderate alcohol drinking groups. This small sample size prevented us from examining associations between neuropathology and age-related decline. It is also possible that some factors that affect aging differ between the alcohol drinking groups. Previous studies have found increases in physical activity, healthy eating, and access and use of medical care in groups of moderate drinkers compared to abstainers (Mukamal et al. 2006). It is important to note that these lifestyle differences were moderated when adjustments for race and education were included, nevertheless, we cannot rule out the possibility that other unmeasured factors contribute to our observations.
The results are strengthened by the longitudinal design of the study and the large cohort of relatively healthy participants (mean time in study 6 – 8 yrs). This design allowed for a systematic, within-subject analysis of the progression of individuals in distinct drinking groups throughout aging on brain function and pathology in aged adults. The battery of tests used incorporated a cross-section of well-validated, widely-used and available measures that broadly assess cognitive and functional decline using both self-report and objective measures. Lastly, though not particularly diverse, the participants were generally well-matched, which makes some unmeasured cultural confounds less likely to explain our observations. Drinking groups were very similar for men, with only a modest reduction in the proportion of Caucasians observed in the heavy drinking group versus the rare/never drinking group. More differences were observed among drinking groups for women, with the heavy drinking group exhibiting a lower age at study inclusion, more years of education and a higher percentage of ApoE4 carriers than the rare/never drinking group. Importantly, however, is that no group differences were observed between moderate drinkers and rare/never drinkers in men or women.
Based on these findings and given the propensity for alcohol-induced damage in women, the potential beneficial effects of moderate alcohol use in women appear modest. Given the relative insensitivity to alcohol-induced damage in men (compared to women), and reductions in neuropsychological decline and AD-related pathology in moderate drinking men, alcohol consumption may have worthwhile health benefits in men. Reducing cognitive decline may also increase longevity, as recent studies suggest cognitive impairment correlates with excess mortality (Sachs et al. 2011). Reductions in decline among moderate alcohol drinking groups were different in aging men vs. women. Moderate alcohol consumption may be an effective lifestyle factor that combats age-related decline in men and to a lesser degree in women. These findings also support the current recommendations for moderate alcohol use by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), though it is unclear if separate recommendations for men and women should persist for older adults. At present the recommendation for adults over age 65 is no more than three drinks on a given day and no more than seven drinks per week for both men and women (NIAAA). Future work should better delineate the influences of moderate alcohol consumption on brain pathology and the dose response relationship as well as the timing required for the beneficial or harmful effects of alcohol with a particular focus on sex differences.
Supplementary Material
Figure S1. Moderate alcohol groups exhibit reduced decline in different tasks in men and women. Annual rates of decline on the (A) Older American Resources and Services (OARS) Activities of Daily Living (ADL), (B) Instrumental Activities of Daily Living (IADL), (C) Category Fluency Test (Animals) and (D) Trail Making Test Part B (Trails B) over time for individual subjects. Annualized rates of change are taken from the mixed-effects models with subject-specific random effects included and fixed-effects rates indicated for each group. Men in the moderate drinking group exhibited decreased rates of decline in the ADL, IADL and Category Fluency tasks and women in the moderate drinking group had decreased rates of decline on the Trails B task. * p < 0.05 comparing same-sex rare/never drinkers to moderate drinkers.
Figure S2. Measures unaffected by alcohol. Annual rates of decline on the (A) Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List Delayed Recall, (B) Functional Activities Questionnaire (FAQ) and (C) Digit Symbol Test (DST) over time for individual subjects. Annualized rates of change are taken from the mixed-effects models with subject-specific random effects included and fixed-effects rates indicated for each group. No significant effects of alcohol were observed.
Acknowledgments
Contents do not necessarily represent the views of the U.S. Department of Veterans Affairs or the United States Government. This work was supported by VA Merit Review Award #BX002061 (JML) and Career Development Award #BX001294 (CJW) from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development and from the National Institutes of Health (P30AG024978, R01AG024059, P30AG008017) for support of the ISAAC and OBAS projects.
References
- Ammendola A, Gemini D, Iannaccone S, Argenzio F, Ciccone G, Ammendola E, Serio L, Ugolini G, Bravaccio F. Gender and peripheral neuropathy in chronic alcoholism: a clinical-electroneurographic study. Alcohol and alcoholism. 2000;35:368–71. doi: 10.1093/alcalc/35.4.368. [DOI] [PubMed] [Google Scholar]
- Andreasson S. Alcohol and J-shaped curves. Alcoholism, clinical and experimental research. 1998;22:359S–364S. doi: 10.1097/00000374-199807001-00013. [DOI] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Schaefer C, Lieber CS. Gender differences in pharmacokinetics of alcohol. Alcoholism, clinical and experimental research. 2001;25:502–7. [PubMed] [Google Scholar]
- Bates DM, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- Bates DM, Maechler M, Bolker BM, Walker SC. R Package Version 1.1–7. 2014. lme4: Linear Mixed-Effects Models Using Eigen and S4. [Google Scholar]
- Beres CA, Baron A. Improved digit symbol substitution by older women as a result of extended practice. J Gerontol. 1981;36:591–7. doi: 10.1093/geronj/36.5.591. [DOI] [PubMed] [Google Scholar]
- Beulens JW, Kruidhof JS, Grobbee DE, Chaturvedi N, Fuller JH, Soedamah-Muthu SS. Alcohol consumption and risk of microvascular complications in type 1 diabetes patients: the EURODIAB Prospective Complications Study. Diabetologia. 2008;51:1631–8. doi: 10.1007/s00125-008-1091-z. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiology of aging. 1995;16:271–8. doi: 10.1016/0197-4580(95)00021-6. discussion 278–84. [DOI] [PubMed] [Google Scholar]
- Britton A, Singh-Manoux A, Marmot M. Alcohol consumption and cognitive function in the Whitehall II Study. Am J Epidemiol. 2004;160:240–7. doi: 10.1093/aje/kwh206. [DOI] [PubMed] [Google Scholar]
- Downer B, Jiang Y, Zanjani F, Fardo D. Effects of alcohol consumption on cognition and regional brain volumes among older adults. Am J Alzheimers Dis Other Demen. 2015;30:364–74. doi: 10.1177/1533317514549411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng MY, Schuckit MA, Smith TL. The level of response to alcohol in daughters of alcoholics and controls. Drug Alcohol Depend. 2005;79:83–93. doi: 10.1016/j.drugalcdep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Erten-Lyons D, Woltjer R, Kaye J, Mattek N, Dodge HH, Green S, Tran H, Howieson DB, Wild K, Silbert LC. Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology. 2013;81:977–83. doi: 10.1212/WNL.0b013e3182a43e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Sola J, Nicolas-Arfelis JM. Gender differences in alcoholic cardiomyopathy. The journal of gender-specific medicine: JGSM: the official journal of the Partnership for Women’s Health at Columbia. 2002;5:41–7. [PubMed] [Google Scholar]
- Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, Willett W, Hennekens CH. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med. 1993;329:1829–34. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]
- Green MS, Kaye JA, Ball MJ. The Oregon brain aging study: neuropathology accompanying healthy aging in the oldest old. Neurology. 2000;54:105–13. doi: 10.1212/wnl.54.1.105. [DOI] [PubMed] [Google Scholar]
- Hashimoto JG, Wiren KM. Neurotoxic consequences of chronic alcohol withdrawal: expression profiling reveals importance of gender over withdrawal severity. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:1084–96. doi: 10.1038/sj.npp.1301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn C, Loffelholz K, Klein J. Stimulatory and inhibitory effects of ethanol on hippocampal acetylcholine release. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:640–7. doi: 10.1007/pl00005219. [DOI] [PubMed] [Google Scholar]
- Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol research & health: the journal of the National Institute on Alcohol Abuse and Alcoholism. 2003;27:181–5. [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilomaki J, Jokanovic N, Tan EC, Lonnroos E. Alcohol Consumption, Dementia and Cognitive Decline: An Overview of Systematic Reviews. Curr Clin Pharmacol. 2015;10:204–12. doi: 10.2174/157488471003150820145539. [DOI] [PubMed] [Google Scholar]
- John U, Rumpf HJ, Bischof G, Hapke U, Hanke M, Meyer C. Excess mortality of alcohol-dependent individuals after 14 years and mortality predictors based on treatment participation and severity of alcohol dependence. Alcoholism, clinical and experimental research. 2013;37:156–63. doi: 10.1111/j.1530-0277.2012.01863.x. [DOI] [PubMed] [Google Scholar]
- Kaye JA, Maxwell SA, Mattek N, Hayes TL, Dodge H, Pavel M, Jimison HB, Wild K, Boise L, Zitzelberger TA. Intelligent Systems For Assessing Aging Changes: home-based, unobtrusive, and continuous assessment of aging. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i180–90. doi: 10.1093/geronb/gbq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlawala Z, Vatsalya V. Heavy Alcohol Drinking Associated Akathisia and Management with Quetiapine XR in Alcohol Dependent Patients. J Addict. 2016;2016:6028971. doi: 10.1155/2016/6028971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–6. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Ding EL, Djousse L. Alcohol consumption, physical activity, and chronic disease risk factors: a population-based cross-sectional survey. BMC Public Health. 2006;6:118. doi: 10.1186/1471-2458-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamal KJ, Longstreth WT, Jr, Mittleman MA, Crum RM, Siscovick DS. Alcohol consumption and subclinical findings on magnetic resonance imaging of the brain in older adults: the cardiovascular health study. Stroke; a journal of cerebral circulation. 2001;32:1939–46. doi: 10.1161/hs0901.095723. [DOI] [PubMed] [Google Scholar]
- NIAAA. NIAAA Drinking Levels Defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- Orth M, Bellosta S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol. 2012;2012:292598. doi: 10.1155/2012/292598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Capurso C, D’Introno A, Colacicco AM, Frisardi V, Lorusso M, Santamato A, Seripa D, Pilotto A, Scafato E, Vendemiale G, Capurso A, Solfrizzi V. Alcohol drinking, cognitive functions in older age, predementia, and dementia syndromes. J Alzheimers Dis. 2009;17:7–31. doi: 10.3233/JAD-2009-1009. [DOI] [PubMed] [Google Scholar]
- Reas ET, Laughlin GA, Kritz-Silverstein D, Barrett-Connor E, McEvoy LK. Moderate, Regular Alcohol Consumption is Associated with Higher Cognitive Function in Older Community-Dwelling Adults. J Prev Alzheimers Dis. 2016;3:105–113. doi: 10.14283/jpad.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M, Rehm J. Alcohol use disorders and mortality: a systematic review and meta-analysis. Addiction. 2013;108:1562–78. doi: 10.1111/add.12231. [DOI] [PubMed] [Google Scholar]
- Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–74. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- Sachs GA, Carter R, Holtz LR, Smith F, Stump TE, Tu W, Callahan CM. Cognitive impairment: an independent predictor of excess mortality: a cohort study. Ann Intern Med. 2011;155:300–8. doi: 10.7326/0003-4819-155-5-201109060-00007. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Kuperman S, Kramer J, Hesselbrock V, Bucholz KK, Nurnberger JI, Jr, Hesselbrock M, Saunders G. Sex differences in how a low sensitivity to alcohol relates to later heavy drinking. Drug and alcohol review. 2012;31:871–80. doi: 10.1111/j.1465-3362.2012.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfrizzi V, Capurso C, D’Introno A, Colacicco AM, Santamato A, Ranieri M, Fiore P, Capurso A, Panza F. Lifestyle-related factors in predementia and dementia syndromes. Expert Rev Neurother. 2008;8:133–58. doi: 10.1586/14737175.8.1.133. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, D’Introno A, Colacicco AM, Capurso C, Del Parigi A, Baldassarre G, Scapicchio P, Scafato E, Amodio M, Capurso A, Panza F Italian Longitudinal Study on Aging Working G. Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology. 2007;68:1790–9. doi: 10.1212/01.wnl.0000262035.87304.89. [DOI] [PubMed] [Google Scholar]
- Stancampiano R, Carta M, Cocco S, Curreli R, Rossetti ZL, Fadda F. Biphasic effects of ethanol on acetylcholine release in the rat prefrontal cortex. Brain research. 2004;997:128–32. doi: 10.1016/j.brainres.2003.09.078. [DOI] [PubMed] [Google Scholar]
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Brain aging, Alzheimer’s disease, and mitochondria. Biochimica et biophysica acta. 2011;1812:1630–9. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyns AJ, Pequignot G. Greater risk of ascitic cirrhosis in females in relation to alcohol consumption. Int J Epidemiol. 1984;13:53–7. doi: 10.1093/ije/13.1.53. [DOI] [PubMed] [Google Scholar]
- Weyerer S, Schaufele M, Wiese B, Maier W, Tebarth F, van den Bussche H, Pentzek M, Bickel H, Luppa M, Riedel-Heller SG German AgeCoDe Study g. Current alcohol consumption and its relationship to incident dementia: results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age Ageing. 2011;40:456–63. doi: 10.1093/ageing/afr007. [DOI] [PubMed] [Google Scholar]
- Wickham H. Ggplot2: elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Beard DK, Wiren KM. Females Uniquely vulnerable to alcohol-induced neurotoxicity show altered glucocorticoid signaling. Brain research. 2015;1601:102–116. doi: 10.1016/j.brainres.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CB, Elkind MS, Luo X, Paik MC, Sacco RL. Reported alcohol consumption and cognitive decline: The northern Manhattan study. Neuroepidemiology. 2006;27:201–7. doi: 10.1159/000096300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Moderate alcohol groups exhibit reduced decline in different tasks in men and women. Annual rates of decline on the (A) Older American Resources and Services (OARS) Activities of Daily Living (ADL), (B) Instrumental Activities of Daily Living (IADL), (C) Category Fluency Test (Animals) and (D) Trail Making Test Part B (Trails B) over time for individual subjects. Annualized rates of change are taken from the mixed-effects models with subject-specific random effects included and fixed-effects rates indicated for each group. Men in the moderate drinking group exhibited decreased rates of decline in the ADL, IADL and Category Fluency tasks and women in the moderate drinking group had decreased rates of decline on the Trails B task. * p < 0.05 comparing same-sex rare/never drinkers to moderate drinkers.
Figure S2. Measures unaffected by alcohol. Annual rates of decline on the (A) Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List Delayed Recall, (B) Functional Activities Questionnaire (FAQ) and (C) Digit Symbol Test (DST) over time for individual subjects. Annualized rates of change are taken from the mixed-effects models with subject-specific random effects included and fixed-effects rates indicated for each group. No significant effects of alcohol were observed.