Abstract
G protein-coupled receptor 119 (GPR119) is expressed in the pancreas and gastrointestinal tract, and its activation promotes insulin secretion in the beta cells of the pancreatic islets as well as the secretion of glucagon-like peptide-1 (GLP-1) in intestinal L cells, consequently improving glucose-stimulated insulin secretion. Due to this dual mechanism of action, the development of small-molecule GPR119 agonists has received significant interest for the treatment of type 2 diabetes. We newly synthesized 1,2,4-triazolone derivatives of GPR119 agonists, which demonstrated excellent outcomes in a cyclic adenosine monophosphate (cAMP) assay. Among the synthesized derivatives, YH18968 showed cAMP=2.8 nM; in GLUTag cell, GLP-1secretion=2.3 fold; in the HIT-T15 cell, and insulin secretion=1.9 fold. Single oral administration of YH18968 improved glucose tolerance and combined treatment with a dipeptidyl peptidase 4 (DPP-4) inhibitor augmented the glucose lowering effect as well as the plasma level of active GLP-1 in normal mice. Single oral administration of YH18968 improved glucose tolerance in a diet induced obese mice model. This effect was maintained after repeated dosing for 4 weeks. The results indicate that YH18968 combined with a DPP-4 inhibitor may be an effective therapeutic candidate for the treatment of type 2 diabetes.
Keywords: Type 2 diabetes, GPR119 agonist, GLP-1, Insulin, YH18968
INTRODUCTION
Diabetes is a pathological condition characterized by hyperglycemia, and is caused by a disorder in the secretion of insulin or by abnormal glucose metabolism due to decreased sensitivity to insulin in peripheral tissues (Taylor et al., 1994; DeFronzo et al., 1999). Hyperglycemia can lead to the development of various symptoms, culminating in the discharge of excess glucose from the body system through urine (DeFronzo, 2009; Cornall et al., 2015). Insulin secretion promoters (sulfonylurea derivatives, meglitinide derivatives, etc.), insulin resistance improving agents (biguanide derivatives, thiazolidinedione derivatives, etc.), carbohydrate digestion inhibitors (alpha-glucosidase inhibitors, etc.), and incretin-based drugs such as glucagon-like peptide-1 (GLP-1) analogs and dipeptidyl peptidase 4 (DPP-4) inhibitors are used for the treatment of diabetes and its associated complications (Koro et al., 2004).
However, the most commonly used insulin secretion promoters have side effects including hypoglycemia and weight gain as demonstrated in the United Kingdom Prospective Diabetes Study (UK Prospective Diabetes Study (UKPDS) Group, 1998). Biguanide derivatives have been reported to have side effects such as lactic acidosis (Bailey, 2008), while thiazolidinedione derivatives have been reported to have side effects such as hepatotoxicity and heart failure (Nesto et al., 2003). In addition to these side effects, existing treatments require strong medicinal efficacy capable of substantially reducing glycosylated hemoglobin (HbA1c) to become effective treatments for type 2 diabetes (Cusi et al., 1996). However, the UKPDS confirmed that the administration of a single treatment gradually lost the ability to control HbA1C level. Therefore, if existing diabetic medicines are administered for a long time, the drug efficacy is gradually reduced and the dosage must be increased or the medicines administered in combination with other drugs. Existing diabetes drugs also have other disadvantages including inconvenient administration (insulin drugs, GLP-1 analog injection), drug resistance (sulfonylurea), and lack of efficacy (DPP-4 inhibitors) (Morales, 2011).
GPR119 is predominantly located in the pancreatic islet and gastrointestinal tract, and its activation acts directly on the beta cells of the pancreas to promote insulin secretion. It also stimulates GLP-1 secretion in intestinal L cells, ultimately improving glucose-stimulated insulin secretion (GSIS) (Sakamoto et al., 2006; Chu et al., 2007, 2008). Drugs that can activate GPR119 increase glucose homeostasis and thus may be effective diabetes treatment drugs with the potential to reduce food intake and body weight (Overton et al., 2008). In fact, fat reduction and body weight loss effects have already been reported based on various obesity animal model tests using GPR119 activators (Blad et al., 2012). Insulin secretion accelerated by GPR119 activation stimulates the production of cAMP (cyclic adenosine monophosphate) in beta cells, enabling the secretion of glucose-dependent insulin and reducing the risks of hypoglycemia. In addition, GLP-1 secretion due to continuous activation of GPR119 as well as the activation of cAMP increases islet beta cell mass and thereby improves autologous insulin production for the subsequent treatment of insulin-dependent diabetes (Drucker, 1998; Yip and Wolfe, 2000; Overton et al., 2006). GPR119 activators may be useful as diabetic therapeutic agents that can be administered for a long period of time as they have minimal side effects (e.g., hypoglycemia, weight gain) compared to existing hypoglycemic and anti-diabetic agents (Katz et al., 2011, 2012; Nunez et al., 2014; Ritter et al., 2106).
The development of GPR119 agonists has been led by GSK (GSK1292263, phase II), Astellas (PSN821, phase II), Metabolex (MBX2982, phase II), Arena (APD597 / ADP668, phase I), and BMS (BMS903452, phase I) with drugs in clinical trial phases 1 and 2. However, development of some GPR119 agonists was discontinued because the initial clinical pipeline had weak efficacy or the drugs decreased in efficacy after repeated administration, a phenomenon known as tachyphylaxis. The results of clinical trials with GPR119 agonists have shown no indications of specific side effects to date, but suggested that tachyphylaxis is caused by receptor desensitization due to GPCR agonism. Therefore, overcoming this problem is essential. New diabetic medicines need to have strong drug efficacy persistence, improved safety, and other cardiovascular features and advantages. To satisfy these requirements, it is necessary to develop drugs that act through a different mechanism.
We designed and synthesized novel 1,2,4-triazolone derivatives and screened them using cell based assays to evaluate their ability to induce GPR119 activation, GLP-1 stimulation, and insulin secretion. We identified a novel GPR119 agonist, YH18968, and evaluated its ability to elicit significant and sustained glycemic control in rodent models.
In this report, we describe the feasibility of YH18968 as a novel GPR119 agonist for the treatment of type 2 diabetes based on the in vitro potency in cAMP, insulin, and GLP-1 assays, the in vivo pharmacokinetics, and the efficacy profiles after oral administration of YH18968 in normal and diet induced obese (DIO) mice.
MATERIALS AND METHODS
Chemicals
In this study, YH18968, a novel 1,2,4-triazolone derivative, and MBX2982 were synthesized in-house at the Yuhan R&D Institute (Yongin, Korea) by the method described in patent (Han et al., 2014). YH18968 is composed of a piperazine scaffold containing (S)-5-(3-fluoro-4-(5-oxo-1-(tetrahydrofuran-3-yl)-1,5-dihydro-4H-1,2,4-triazol-4-yl)phenyl)-2-(4-(5-isopropyl-1,2,4-oxadiazol-3-yl)piperazin-1-yl)nicotinonitrile. Compounds were dissolved in dimethyl sulfoxide (DMSO) and diluted with cell culture media for in vitro assays.
Cell culture
A Chinese hamster ovarian (CHO-K1) cell line stably expressing human GPR119 was purchased from Invitrogen (Carlsbad, CA, USA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum, 0.1 mM non-essential amino acids (NEAA), 25 mM HEPES (pH 7.2), 100 U/mL penicillin, 100 μg/mL streptomycin, 100 ug/mL zeocin, and 600 ug/mL hygromycin (Invitrogen). The hamster insulinoma cell line HIT-T15 was purchased from ATCC (Manassas, VA, USA) and was cultured in Ham’s F12K medium with 10% horse serum, 2.5% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). The murine L cell line GLUTag (a gift from University, Incheon, Korea) was cultured in DMEM with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). All cell lines were incubated in 5% CO2 in a humidified atmosphere at 37°C.
cAMP accumulation assay
The CHO-K1 cells contained human GPR119 under the control of a doxycycline inducible system and a beta-lactamase reporter gene under the control of a cAMP response element (CRE). Addition of doxycycline to these cells allowed for GPR119 expression and a subsequent assay for GPR119 specific activity. Cells were seeded in 384-well plates at a density of 104 cells/well and doxycycline (1 ug/mL) was added to induce GPR119 expression. Cells were treated with compounds at varying concentrations. After 5 h incubation, cellular cAMP levels were measured following the manufacturer’s instructions (Invitrogen). The assay technology is based on fluorescent detection of beta-lactamase reporter gene activity using a fluorescence resonance energy transfer (FRET)-enabled substrate that generates a ratiometric cAMP response. Three replicates for each concentration of compounds were used.
GLP-1 release assay
GLUTag cells were seeded in 48-well plates at a density of 105 cells/well and then incubated for 48 h. On the day of the experiment, the cells were washed twice with serum-free DMEM media and were exposed to 0.1 uM concentrations of the test compounds in the same media at 37°C for 120 min. GLP-1 released into the culture supernatant was then collected and clarified by centrifugation for 5 min. GLP-1 concentration in the supernatant was determined using a GLP-1 ELISA kit (Shibukawa, Gunma, Japan) following the manufacturer’s instructions. Three replicates for each concentration of compounds were used.
Insulin release assay
HIT-T15 cells were seeded in 48-well plates at a density of 2×105 cells/well and incubated for 48 h. On the day of the experiment, the cells were washed twice with Krebs Ringer bicarbonate buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM KH-2PO4, 1.16 mM MgCl2, 10 mM HEPES, 2.5 mM CaCl2, 25.5 mM NaHCO3, 0.2% BSA, pH 7.4) and preincubated for 60 min at 37°C with the Krebs Ringer bicarbonate buffer containing 5 mM glucose. After preincubation, the cells were stimulated with 0.1 uM concentrations of compounds in Krebs Ringer bicarbonate buffer containing 15 mM glucose at 37°C for 60 min. Insulin released into the culture supernatant was then collected and clarified by centrifugation for 5 min. Insulin content in the supernatant was determined using an insulin ELISA kit (Mercodia, Uppsala, Sweden) following the manufacturer’s instructions.
Cytotoxicity assay
Cytotoxicity assays were performed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Hamster beta cells (HIT-T15), mouse L cells (GLUTag), and human hepatocyte (HepG2) were used for the MTT assay. The cells were harvested (2∼3×104 cells/well) and inoculated into 96-well plates for 24 h. The cultured cells were then incubated for 48 h with the test sample at a final concentration of 0.1–100 μM. Afterward, 100 μL of MTT solution (5 mg MTT in PBS, pH 7.2) were added to each well and the plates were incubated for 4 h at 37°C. After incubation, 100 μL of DMSO were added to each well, the absorbance of each well was measured at 540 nm using a microplate reader, and the surviving cell fraction was calculated. The inhibition of cell viability was calculated using% viability=[absorbance of treated cells/absorbance of untreated cells]×100.
Animals
The entire in vivo animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC No. 14216) of Yuhan Corporation (Yongin, Korea). Mice were housed under a 12 h/12 h light/dark cycle in free feeding conditions, in temperature and humidity controlled rooms. Normal C57BL/6J male mice were obtained from Orient BIO Inc (Seongnam, Korea).
Pharmacokinetics of YH18968 in normal mice
Eight-week-old male C57BL/6J mice were fasted overnight with free access to water. YH18968 was intravenously injected at 1 mg/kg via the tail vein or orally administered as a suspension in 0.5% methyl cellulose at 1, 10, and 100 mg/ kg. Blood samples were collected via the inferior vena cava under anesthesia at 0.083 (iv only), 0.25, 0.5, 1, 2, 4, and 7 h post-dose (n=2/time point). Plasma samples were taken from blood samples after centrifugation and proteins were precipitated to extract YH18968. The plasma concentrations of YH18968 were determined by LC-MS/MS analysis. Pharmacokinetic analysis was performed non-compartmentally using WinNonlin® version 6.3 (Pharsight Corporation, St. Louis, MO, USA). The pharmacokinetic parameters of YH18968 are as follows: maximum observed plasma concentration (Cmax), time to reach maximum observed plasma concentration (tmax), area under the plasma concentration-time curve from time zero to the time of the last measurable concentration (AUClast), area under the plasma concentration-time curve extrapolated to infinity (AUCinf), apparent terminal half-life (T1/2,app), total clearance (CL), volume of distribution at steady state (Vss), mean residence time from 0 to infinity (MRTinf), and bioavailability (F).
Efficacy of single administration of compounds in normal mice
To evaluate the acute glucose lowering effect of the test compounds in normal mice, oral glucose tolerance tests (OGTTs) were performed using overnight-fasted 8-week-old male C57BL/6J mice. Test compounds or 0.5% methyl cellulose (vehicle) were orally administered followed by administration of 2 g/kg of glucose after 30 min. Blood samples were collected from the tail veins at 0, 15, 30, 60, 90, and 120 min after the glucose bolus and the blood glucose levels were immediately determined using the Glucose sensor & strip system (GlucoDr+, Allmedicus, Anyang, Korea). The area under the curve (AUC) for blood glucose was calculated for statistical analysis.
Efficacy of single administration of YH18968 in DIO mice
To evaluate the acute glucose lowering effect of the compounds in a disease model, OGTTs were performed using overnight-fasted 17-week-old male DIO mice. YH18968 or 0.5% methyl cellulose (vehicle) were orally administered followed by the administration of 2 g/kg of glucose after 30 min. Blood samples were collected from the tail veins at 0, 15, 30, 60, 90, and 120 min after the glucose bolus and the blood glucose levels were immediately determined using the Glucose sensor & strip system (GlucoDr+, Allmedicus). The AUC of the blood glucose level was calculated for statistical analysis.
Efficacy of repeat administrations of compounds in DIO mice
To evaluate the efficacy of the compounds on chronic glycemic control, 17-week-old male DIO mice were orally administered with 0.5% methyl cellulose (vehicle), YH18968, MBX2982, or linagliptin once daily for 4 weeks. On days 1 and 29, the efficacy of the compounds was examined using OGTT with overnight-fasted DIO mice. Thirty min after the administration of YH18968, a glucose bolus was given orally at a dose of 2 g/kg, and blood samples were collected from the tail veins at 0, 15, 30, 60, 90, and 120 min. Blood glucose levels were immediately determined using the Glucose sensor & strip system (GlucoDr+, Allmedicus). The AUC of the blood glucose level was calculated for statistical analysis.
Statistical analysis
Values were expressed as the mean ± SEM. Student’s t-test was used to compare two groups, and multiple group comparisons were performed using one-way ANOVA with a post hoc analysis (Tukey or Dunnett’s multiple comparison test). Statistical analyses were performed using Prism software (Graphpad, La Jolla, CA, USA), and a p-value of <0.05 was considered to be statistically significant.
RESULTS
YH18968 increases GPR119-induced intracellular cAMP accumulation
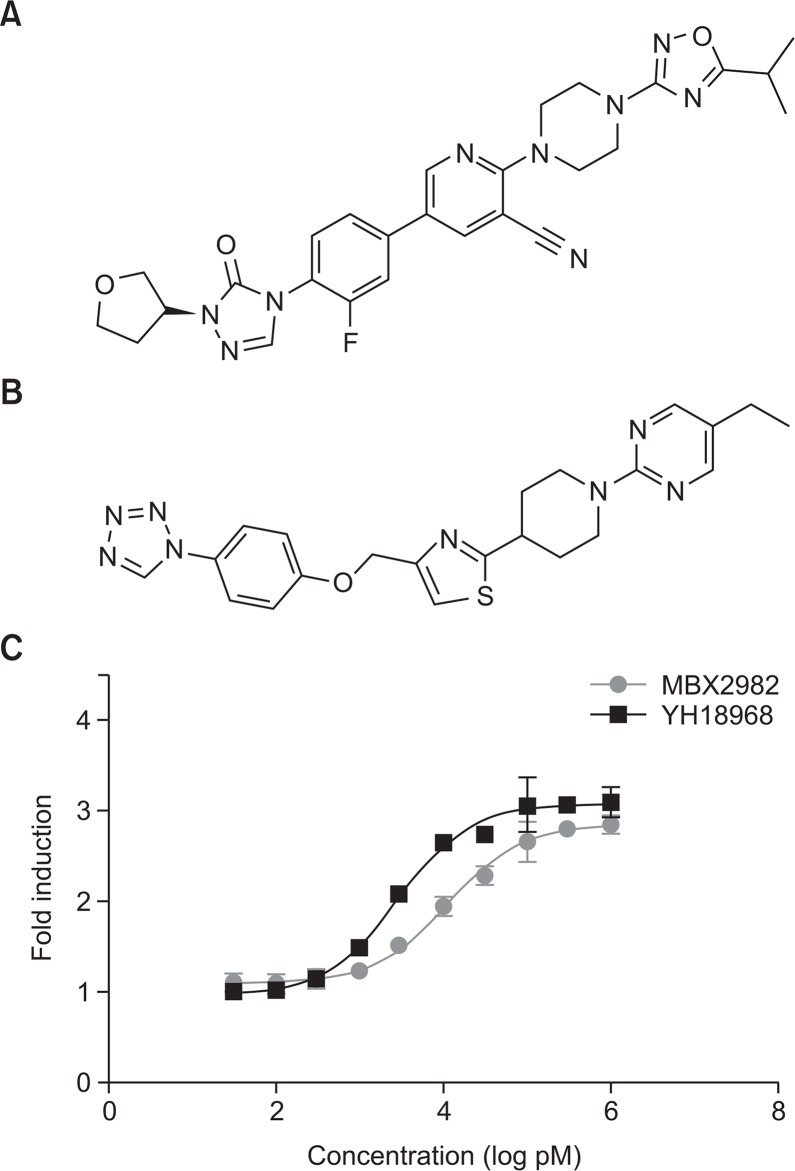
The GPR119 receptor is coupled to the signal transducer Gαs protein and its activation leads to increased adenylate cyclase activity and a rise in intracellular cAMP production. Thus, the GPR119 agonistic activity of YH18968 was measured in CHO-K1 cells stably expressing human GPR119. YH18968 potently induced intracellular cAMP accumulation in a dose-dependent manner with a half maximal effective concentration (EC50) of 2.8 nM. The potency of YH18968 was compared with that of MBX2982, a clinical GPR119 agonist. MBX2982 significantly stimulated intracellular cAMP accumulation in a dose-dependent manner, with an EC50 value of 11.2 nM for the human GPR119-CHO stable cell line. YH18968 activated human GPR119 approximately 4-fold more potently than MBX2982 (Fig. 1).
Fig. 1.
Chemical structure of YH18968, MBX2982 and stimulated GPR119-induced cAMP accumulation. (A) Structure of YH18968 (CAS number 1632498-56-6). (B) Structure of MBX2982 (CAS number 1037792-44-1). (C) Addition of doxycycline to these cells allowed for GPR119 expression and a subsequent assay to evaluate GPR119 specific activity. The cells were treated with the test drugs at 0.03, 0.1, 0.3, 1, 3, 10, 30, 100, 300, and 1,000 nM concentrations for 90 min. After 5 h incubation, intracellular cAMP levels were measured with the CRE-beta-lactamase reporter gene using fluorescence resonance energy transfer (FRET). All data represent the means ± SEM from at least three independent experiments.
YH18968 stimulates L cell GLP-1 and β cell insulin secretion
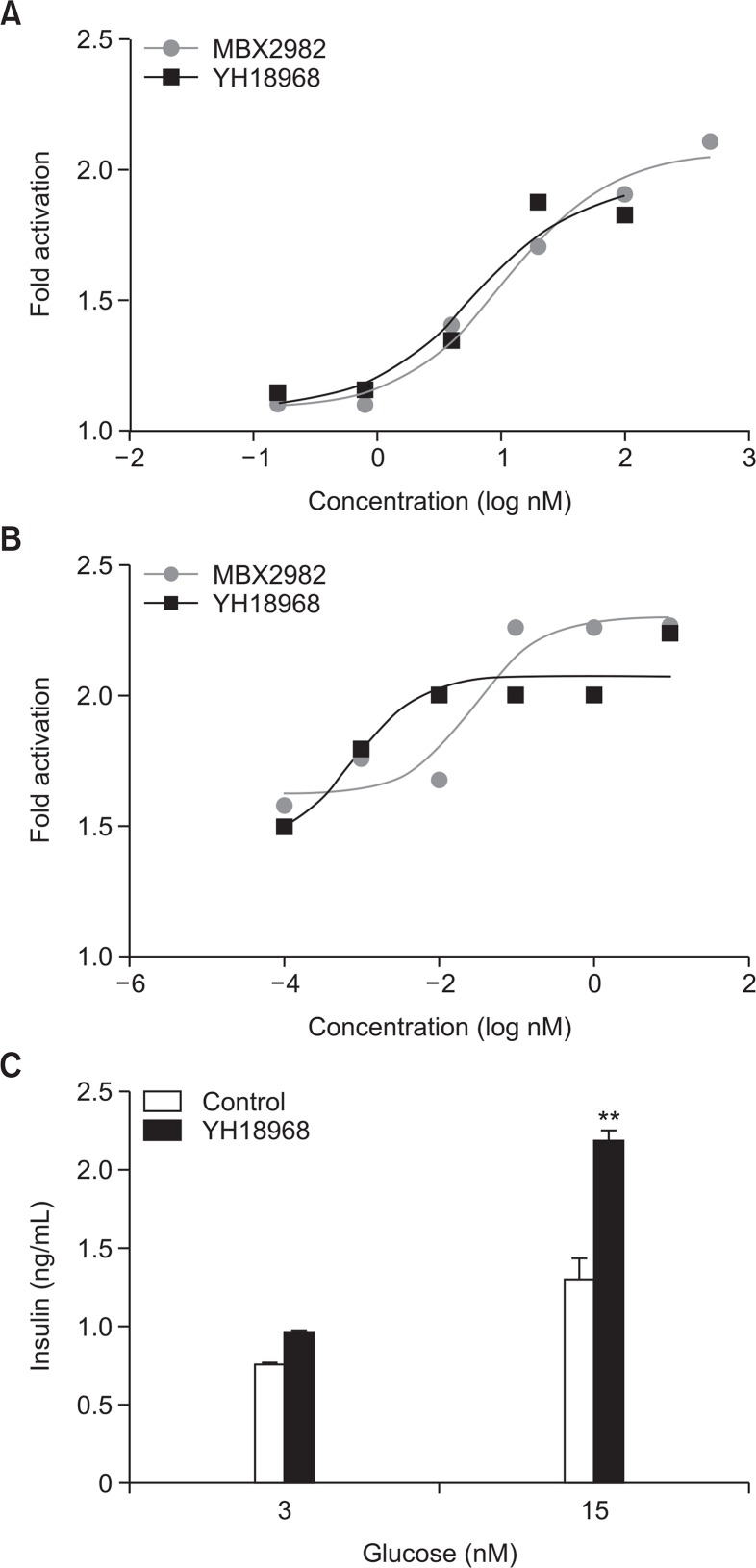
To examine the activation of GPR119 by YH18968, GPR119-induced cAMP accumulation assay was carried out in CHO-K1 cells that stably harbored human GPR119. To confirm whether the activation of GPR119 by YH18968 leads to functional stimulation of insulin and GLP-1 secretion, we measured insulin and GLP-1 release in HIT-T15 hamster insulinoma cells and GLUTag murine L cells, respectively. YH18968 elicited dose-dependent increases in insulin release from HIT-T15 cells with an EC50 value of 3.5 nM (Fig. 2A). Additionally, GLP-1 secretion from GLUTag cells was enhanced by YH18968 with an EC50 value of 0.1 nM (Fig. 2B). Next, we examined the effect of YH18968 on GSIS at 3 and 15 mM glucose concentrations. Upon the application of 100 nM YH18968 to the HIT-T15 cells, insulin release was enhanced in a glucose-dependent manner, but the compound had no effect on cells incubated without glucose (Fig. 2C). These results confirm that YH18968 mediates the dual functional activity of GLP-1 secretion and GSIS at the cellular level.
Fig. 2.
Stimulation of GLP-1 and insulin secretion by YH18968, MBX2982 was measured in GLUTag and HIT-T15 cells. (A) Measurement of insulin secretion from HIT-T15 cells. Cells were incubated with 0.1 μM of YH18968 and MBX2982 and insulin in the supernatant was measured with ELISA. (B) Measurement of GLP-1 secretion from GLUTag cells. Cells were incubated with 0.1 μM of YH18968 and MBX2982 and GLP-1 in the supernatant was measured with ELISA. (C) Measurement of GSIS from HIT-T15 cells. HIT-T15 cells were incubated with 100 nM YH18968 at 0, 3, and 15 mM glucose. After 1 h incubation, insulin in the supernatant was measured with ELISA. Triplicates for each concentration were used and the results are presented as the means ± SEM. **p<0.01 versus control.
Cytotoxic effects of YH18968 on β cells (HIT-T15), L cells (GLUTag), and hepatocytes (HepG2)
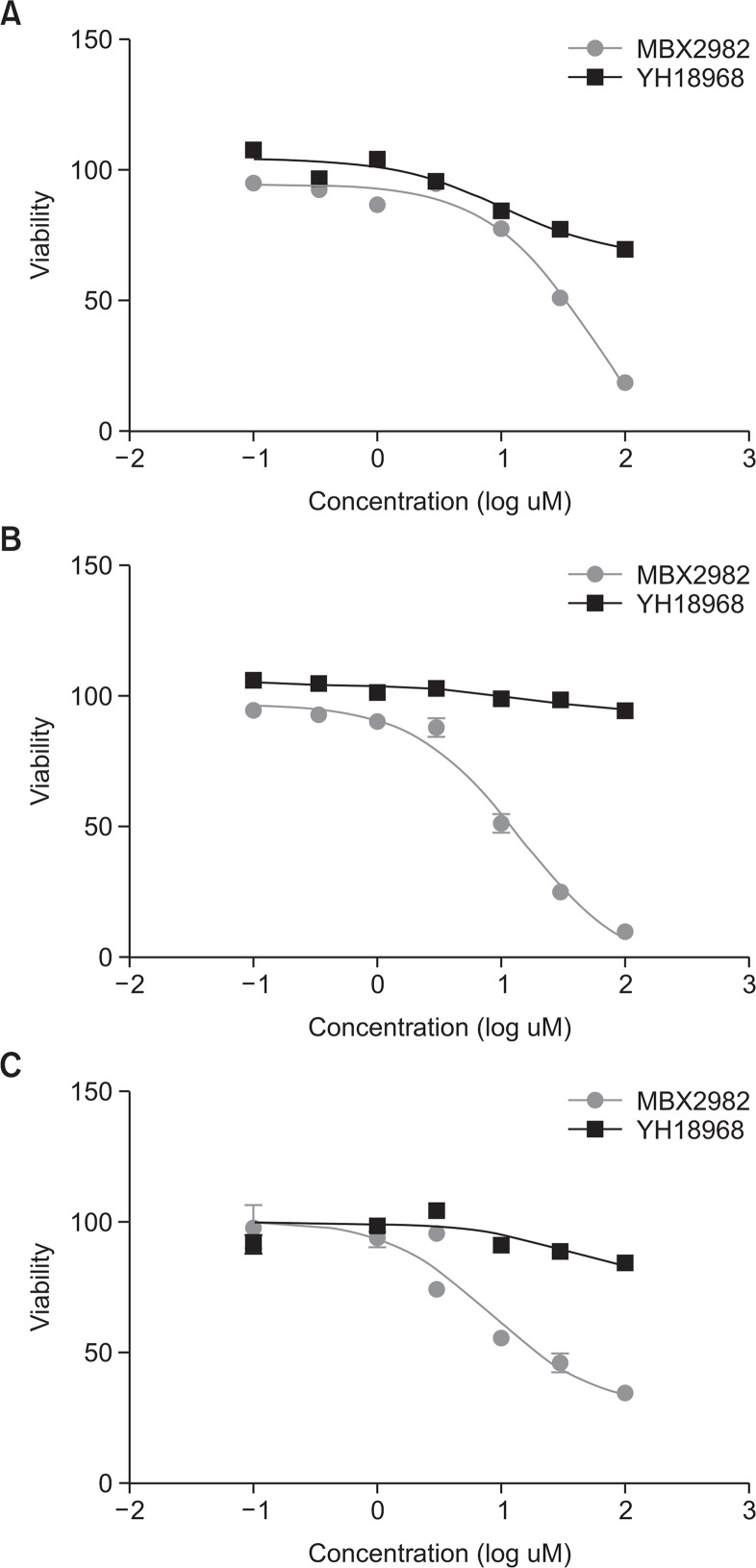
To investigate the cytotoxic effect of YH18968 and MBX2982, a cytotoxicity assay was performed in β cells (HIT-T15), L cells (GLUTag), and hepatocytes (HepG2). The 50% lethal concentration (LC50) of YH18968 was >100 μM in HIT-T15, GLUTag, and HepG2, respectively. In contrast, the LC50 of MBX2982 was 34.0 μM, 12.2 μM, and 15.9 μM in HIT-T15, GLUTag, and HepG2, respectively (Fig. 1). Cytotoxicity by YH18968 and MBX2982 was not observed up to 5 μM and 1 μM, respectively. These results indicated that YH18968 induced cytotoxic effects were less severe than those of MBX2982 on the cell lines evaluated (Fig. 3).
Fig. 3.
Cytotoxic effects of YH18968 on β cells (HIT-T15), L cells (GLUTag), and hepatocytes (HepG2). (A) Measurement of cytotoxic effect in β cells. Hamster beta cells were used for the MTT assay. The cultured cells were incubated for 48 h with YH18968 or MBX2982 at a concentration of 0.1–100 μM. Values are means ± SEM (n=3). (B) Measurement of cytotoxic effect in GLUTag L cells. Mouse L cells were used for the MTT assay. The cultured cells were incubated for 48 h with YH18968 or MBX2982 at a concentration of 0.1–100 μM. Values are means ± SEM (n=3). (C) Measurement of cytotoxic effect in HepG2 cells. Human hepatocytes were used for the MTT assay. The cultured cells were incubated for 48 h with YH18968 or MBX2982 at a concentration of 0.1–100 μM. Values are means ± SEM (n=3).
Single oral administration of YH18968 to normal C57BL/6 mice
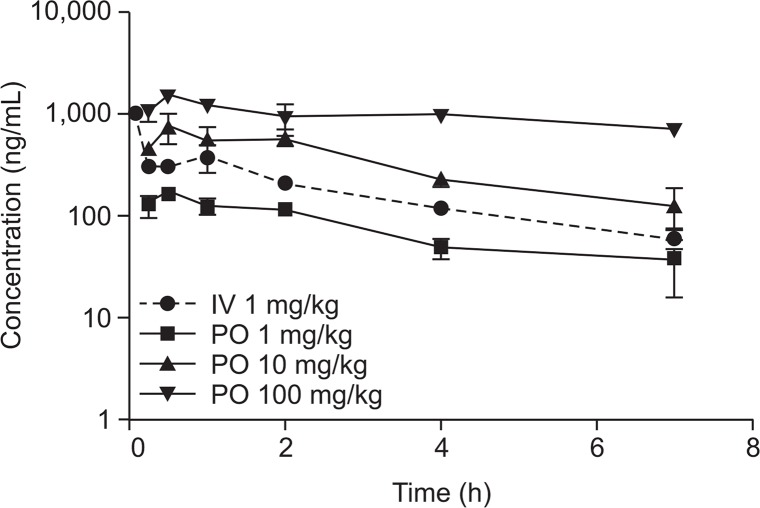
The mean pharmacokinetic parameters and time-concentration profiles of YH18968 following single intravenous and oral administration in normal C57BL/6J mice are presented in Fig. 4 and Table 1. The plasma concentration of YH18968 decreased in a biphasic manner following single intravenous administration in normal C57BL/6J mice although the plasma concentration of YH18968 was slightly elevated after 2 h. YH18968 was rapidly absorbed with a Tmax value of 0.5 h from 1 to 100 mg/kg following single oral administration in normal C57BL/6J mice. The Cmax and AUClast values for YH18968 increased as the dose increased, but the increases were not dose proportional. Specifically, as the dose increased at a ratio of 1.0:10.0:100.0, the Cmax increased at a ratio of 1.0:4.5:8.7 while the AUClast increased at a ratio of 1.0:4.5: 11.9 (Fig. 4). Oral bioavailability dramatically decreased as the dose increased, with values of 4.8 to 40.0% in a dose range of 1 to 100 mg/kg (Table 1).
Fig. 4.
Plasma concentration-time profiles of YH18968 following single intravenous and oral administrations to normal C57BL/6 mice (n=2/time point). YH18968 was intravenously injected at 1 mg/kg via the tail vein and orally administered as a suspension in 0.5% methyl cellulose at 1, 10, and 100 mg/kg, respectively. Blood samples were collected via the inferior vena cava under anesthesia at 0.083 (iv only), 0.25, 0.5, 1, 2, 4, and 7 h post-dose (n=2/ time point).
Table 1.
The pharmacokinetic parameters of YH18968 following single oral administration of YH18968 to normal C57BL/6 mice (n=2/time point)
| Pharmacokinetic parameters | YH18968 | |||
|---|---|---|---|---|
|
| ||||
| IV | PO | PO | PO | |
|
| ||||
| 1 mg/kg | 1 mg/kg | 10 mg/kg | 100 mg/kg | |
| Cmax (ng/mL) | -* | 168.6 | 759.7 | 1472.2 |
| Tmax (h) | - | 0.5 | 0.5 | 0.5 |
| AUClast (ng·h/mL) | 1375.1 | 549.7 | 2471.7 | 6538.0 |
| AUCinf (ng·h/mL) | 1619.9 | 719.8 | 2958.2 | 15773.9 |
| CL (mL/h/kg) | 617.3 | - | - | - |
| Vss (mL/kg) | 2112.2 | - | - | - |
| MRT (h) | 3.4 | - | - | - |
| T1/2,app (h) | - | 3.1 | 2.6 | 9.2 |
| F (%) | - | 40.0 | 18.0 | 4.8 |
Not applicable.
Single administration of YH18968 improves glucose tolerance in normal C57BL/6J mice
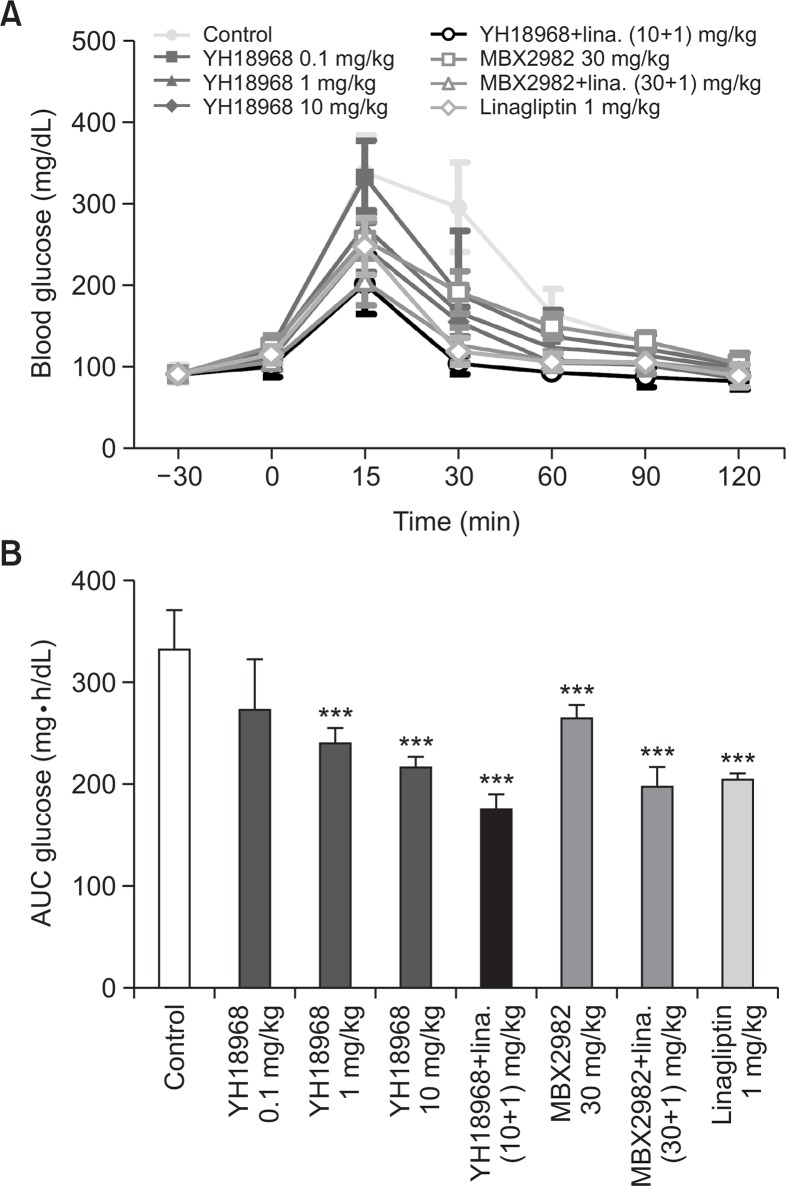
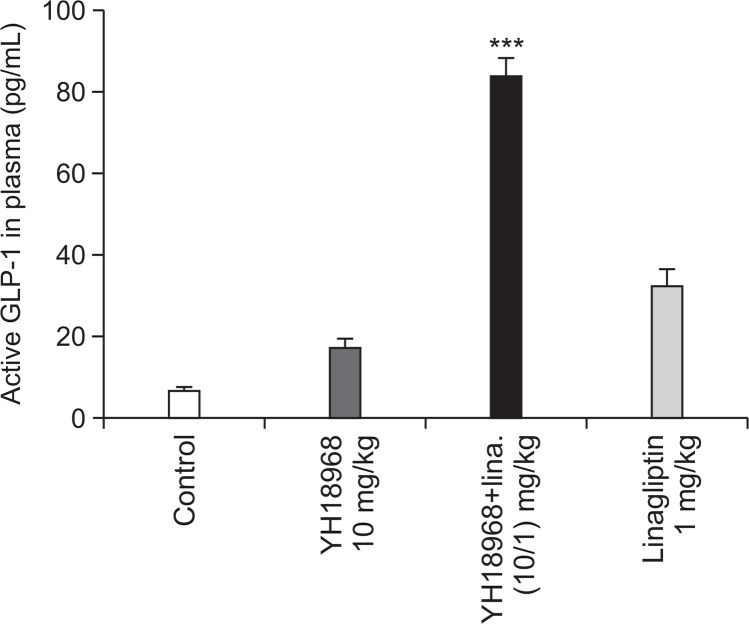
To evaluate the effects of YH18968 on acute glucose homeostasis, an OGTT using normal mice was conducted. Single oral administration of 0.1, 1, and 10 mg/kg of YH18968 significantly lowered the AUC for blood glucose in a dose-dependent manner compared to the vehicle control (Fig. 5). Additionally, 1 and 10 mg/kg of YH18968 exhibited more potent glucose lowering effects than 30 mg/kg of MBX2982. When 10 mg/kg of YH18968 was applied in combination with 1 mg/ kg of linagliptin, a DPP-4 inhibitor, a significant additive glucose lowering effect was observed compared to the application of YH18968 alone, linagliptin alone, or the combination of MBX2982 with linagliptin. When we examined the effects of YH18968 on blood GLP-1 levels using overnight-fasted mice following single oral administration, 10 mg/kg YH18968 increased active GLP-1 levels by 2.7-fold compared to the vehicle control at 30 minutes after administration. Combination treatment of YH18968 with linagliptin significantly increased the active GLP-1 level compared to YH18968 or linagliptin alone, implying synergistic effects for the combination of a GPR119 agonist and a DPP-4 inhibitor (Fig. 6).
Fig. 5.
Single YH18968 treatment improved oral glucose tolerance in normal mice (n=8). (A) OGTT in C57BL/6J mice treated with vehicle (0.5% MC, closed circle), YH18968 (0.1 mg/kg, closed square; 1 mg/kg, closed triangle; 10 mg/kg, closed diamond), YH18968+linagliptin (10+1 mg/kg, open circle), MBX2982 (30 mg/ kg, open square), MBX2982+linagliptin (30+1 mg/kg, open triangle) or linagliptin (1 mg/kg, open diamond). Animals were treated with vehicle or compounds 30 min before glucose bolus (2 g/kg). (B) The area under the plasma glucose concentration-time curve for 2 h (AUC0-2 h) in an OGTT. Results are presented as the mean ± SEM. ***p<0.001 versus control.
Fig. 6.
YH18968 enhances blood GLP-1 release in normal mice (n=6). Blood GLP-1 levels. C57BL/6J mice treated with vehicle (0.5% MC), YH18968 (10 mg/kg), linagliptin (1 mg/kg) or YH18968+linagliptin (10+1 mg/kg). Blood samples were collected 30 min after YH18968 administration and active GLP-1 levels were measured. Results are presented as the mean ± SEM. ***p<0.001 versus control.
Single administration of YH18968 improves glucose tolerance in DIO mice
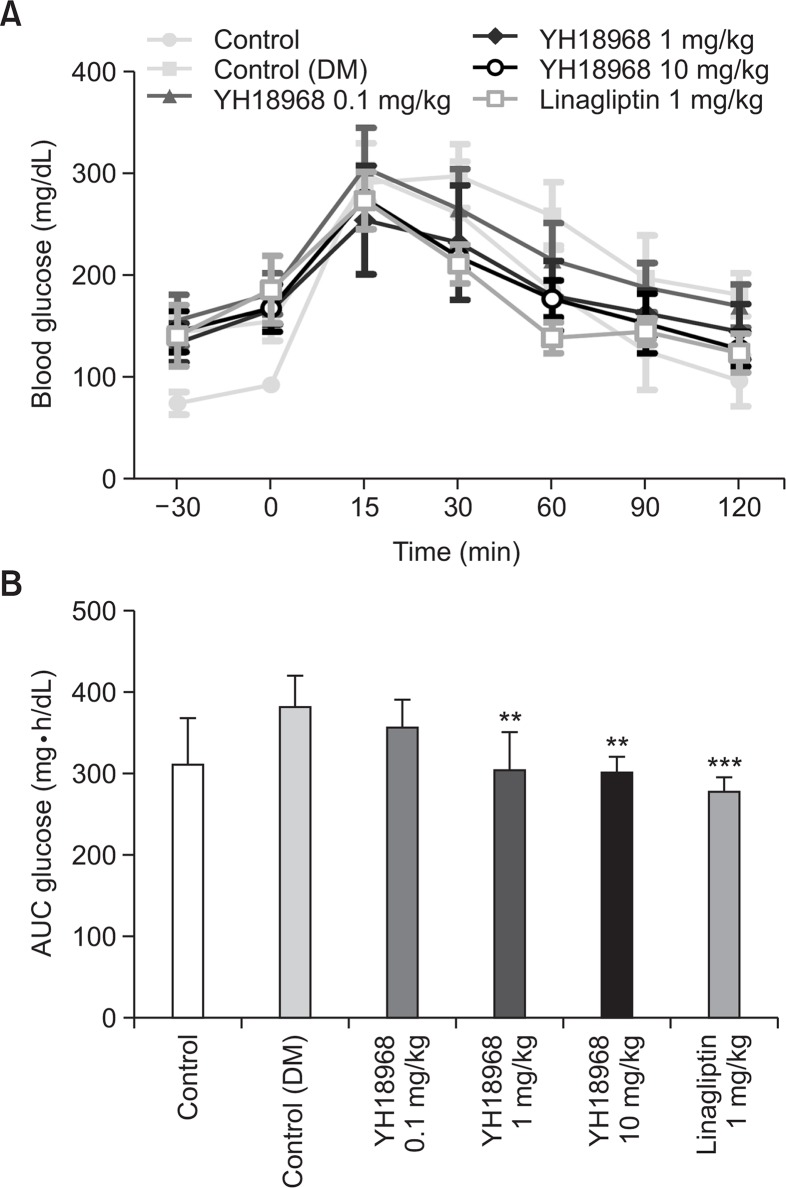
The effects of YH18968 on acute glucose homeostasis in a disease model were investigated using OGTT in a DIO mice model. Single oral administration of 0.1, 1, and 10 mg/kg of YH18968 lowered the AUC for blood glucose levels in a dose-dependent manner and reached statistical significance at 1 and 10 mg/kg compared to the vehicle control (Fig. 7). Additionally, 1 and 10 mg/kg elicited similar levels of inhibition, indicating a saturation of the glucose lowering effect. Based on these results, a repeat dose study was conducted with 1 mg/kg of YH18968 in the same animal model.
Fig. 7.
Single YH18968 treatment improved oral glucose tolerance in DIO mice (n=8). (A) OGTT in DIO mice treated with vehicle (control (DM)) (0.5% MC, closed square), YH18968 (0.1 mg/kg, closed triangle; 1 mg/kg, closed diamond; 10 mg/kg, open circle) or linagliptin (1 mg/kg, open square). Animals were treated with vehicle or compounds 30 min before glucose bolus (2 g/kg). (B) The area under the plasma glucose concentration-time curve for 2 h (AUC0-2 h) in an OGTT. Results are presented as the mean ± SEM. **p<0.01, ***p<0.001 versus control (DM).
Repeat administration of YH18968 improves glucose tolerance in DIO mice
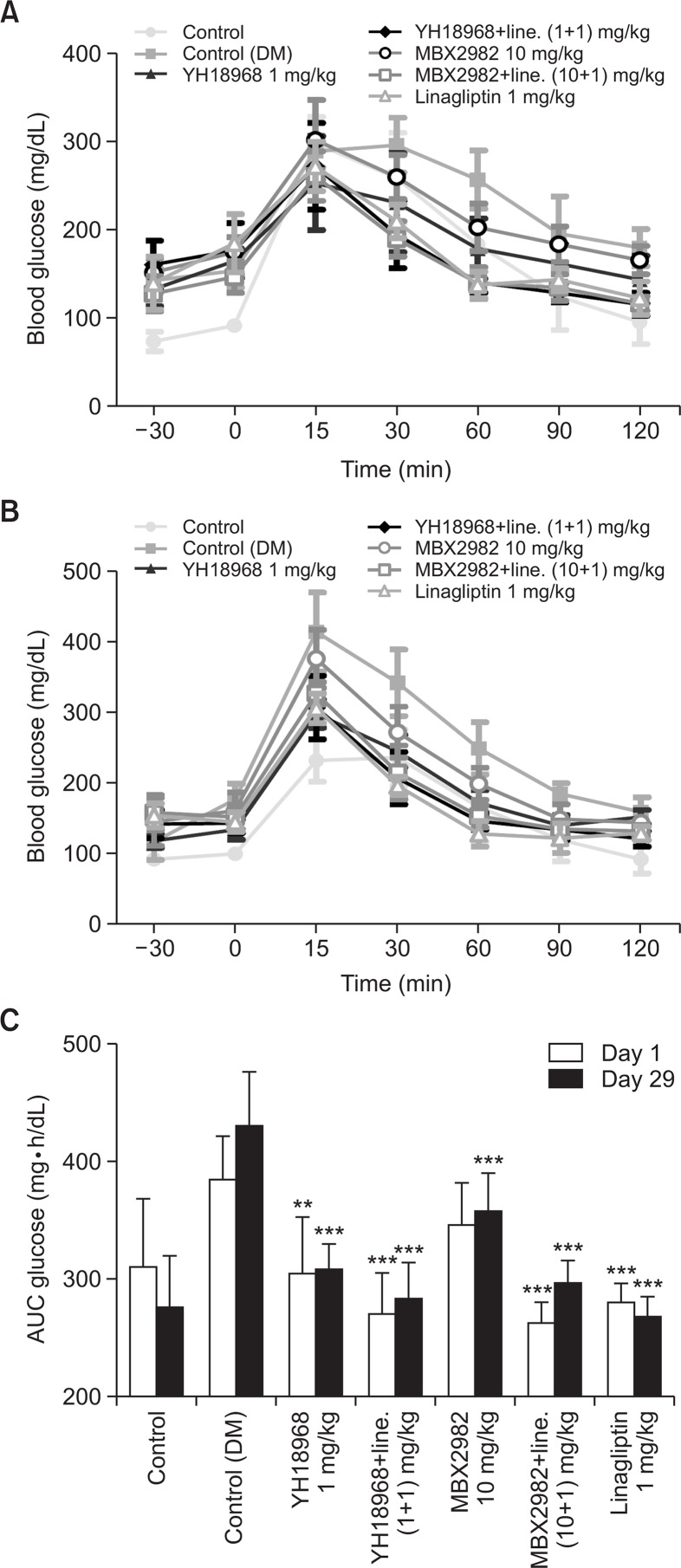
To investigate the effects of repeated administration of YH18968, DIO mice were orally administered once daily with 1 mg/kg of YH18968, 10 mg/kg of MBX2982, and 1 mg/kg of linagliptin for 4 weeks. One mg/kg of YH18968 or 10 mg/kg of MBX2982 was also administered in combination with 1 mg/kg linagliptin. An OGTT was performed on days 1 and 29 following the administration of the compounds. On day 1, YH18968 significantly lowered the AUC for blood glucose compared to the vehicle control and exhibited a more potent glucose lowering effect than 10 mg/kg of MBX2982. When YH18968 was applied in combination with 1 mg/kg of linagliptin, an additive glucose lowering effect was observed compared to the application of YH18968 or linagliptin alone. On day 29, an OGTT was performed to examine whether the efficacy of YH18968 was sustained after repeated administration for 4 weeks. YH18968 alone or in combination with linagliptin effectively improved glucose tolerance on day 29, confirming the efficacy of YH18968 was well maintained after repeated administration for 4 weeks (Fig. 8).
Fig. 8.
Repeated YH18968 treatment improved oral glucose tole rance in DIO mice (n=8). (A) OGTT in DIO mice treated with vehicle (control (DM)) (0.5% MC, closed square), YH18968 (1 mg/ kg, closed triangle), YH18968+linagliptin (1+1 mg/kg, closed diamond), MBX2982 (10 mg/kg, open circle), MBX2982+linagliptin (10+1 mg/kg, open square) or linagliptin (1 mg/kg, open triangle) at day 1. Animals were treated with vehicle or compounds 30 min before glucose bolus (2 g/kg). (B) OGTT in DIO mice treated with vehicle (control (DM)) (0.5% MC, closed square), YH18968 (1 mg/ kg, closed triangle), YH18968+linagliptin (1+1 mg/kg, closed diamond), MBX2982 (10 mg/kg, open circle), MBX2982+linagliptin (10+1 mg/kg, open square) or linagliptin (1 mg/kg, open triangle) at day 28. Animals were treated with vehicle or compounds 30 min before glucose bolus (2 g/kg). (C) The area under the plasma glucose concentration-time curve for 2 h (AUC0-2 h) in an OGTT at day1 and day 28. Results are presented as the mean ± SEM. **p<0.01, ***p<0.001 versus control (DM).
DISCUSSION
GPR119 agonists stimulate glucose-dependent insulin secretion in vitro and lower elevated blood glucose levels in vivo. Furthermore, they have been demonstrated to stimulate the release of GLP-1 (Overton et al., 2006). Consequently, GPR119 agonists have emerged as promising targets for the treatment of type 2 diabetes (Jones et al., 2009). MBX2982 was reported to enhance cAMP accumulation in GPR119-transfected HEK293 cells, with an EC50 value of 8.33 nM (Hothersall et al., 2015). YH18968 has an EC50 value of 2.8 nM for human GPR119 activation, which is approximately 3-fold more potent than the effect of MBX2982. Increased cAMP accumulation by YH18968 resulted in GLP-1 release in GLUTag L cells and insulin release in HIT-T15 β cells, both of which express GPR119 endogenously (Lauffer et al., 2009). YH18968 showed strong efficacy in the insulin secretion assay, with an EC50 value of 3.5 nM. The YH18968 effect was slightly stronger than that of MBX2982, which had an EC50 value of 10.7 nM. In the GLP-1 secretion assay, YH18968 had an EC50 value of 0.1 nM. These results are consistent with reports that GPR119 agonists play a dual role in glycemic control by enhancing both GLP-1 and insulin secretion (Chu et al., 2007).
Fig. 3 shows the superiority of YH18968 in terms of the cytotoxic effects on the HIT-T15, GLUTag, and HepG2 cell lines compared with MBX2982. Although differences exist in the cytotoxic effects of YH18968 and MBX2982, similar effects were observed on GLP-1 secretion from L cells and GSIS from HIT-T15 cells (Fig. 3). However, the EC50 values of YH18968 and MBX2982 were 2.8 nM and 11.3 nM, which were below their LC50 values, and the 12 h required for the induction of GLP-1 or GSIS by YH18968 and MBX2982 was much shorter than the duration of the cytotoxicity assay (72 h). Therefore, the secretion of GLP-1 and GSIS may not be affected by the cytotoxic effects of YH18968 or MBX2982. YH18968 may have more beneficial effects on the safety or secretion of GLP-1 and insulin in animal models or in patients with type 2 diabetes compared with MBX2982.
A pharmacokinetic study was performed to evaluate the dose proportionality and absolute bioavailability of YH18968 in normal C57BL/6J mice following single oral administration at 1 to 100 mg/kg in 0.5% MC1500 suspension and intravenous injection at 1 mg/kg, respectively. The data demonstrated that YH18968 systemic exposure increased in a less than dose proportional manner, indicating that the absolute bioavailability of YH18968 decreased as the dose increased up to 100 mg/kg. Various factors contribute to saturable absorption in the gastrointestinal tract such as solubility issues in biological fluid and the saturation of active transporters. YH18968 exhibited poor aqueous solubility in the buffer and its chemical properties likely contributed to the low systemic exposure and bioavailability in mice. Further studies to overcome the low exposure need to be conducted to increase the fraction absorption and drug effect in C57BL/6J mice.
When we compared the efficacy of YH18968 with the known efficacious dose of MBX2982 in normal and DIO mice, a lower YH18968 dose elicited more potent glucose lowering effects compared to MBX2982 in both animal models.
GLP-1 secretion can be enhanced by activating GPR119, which is highly expressed in both pancreatic beta cells and intestinal enteroendocirne L cells. Additionally, DPP-4 inhibitors can slow GLP-1 degradation, thus increasing endogenous active GLP-1 levels (Lauffer et al., 2008). GPR119 agonists and DPP-4 inhibitors are mechanistically complementary and could be an ideal therapeutic option for treating type 2 diabetes. In our study, combination treatment with YH18986 and linagliptin exhibited an additive effect compared to YH18968 or linagliptin alone in the OGTT using normal mice. This effect was apparent due to the significant increase of active GLP-1 level in the blood after co-treatment with YH18968 and linagliptin compared to treatment with the individual drugs.
YH18968 was effective in the DIO mice model. Single oral administration of YH18968 induced a dose-dependent decrease in the AUC of blood glucose at 0.1 and 1 mg/kg, but there was no difference in efficacy between 1 mg/kg and 10 mg/kg of YH18968. Due to the saturation of efficacy, 1 mg/kg of YH18968 was evaluated in a 4-week repeat dose study in DIO mice. Since the systemic exposure of 10 mg/ kg of YH18968 was higher than that of 1 mg/kg YH18968 in the pharmacokinetic study even though it was less than dose proportional, the saturation of efficacy may be attributable to factors other than the absorption of the compound.
Several early GPR119 agonists have been evaluated in clinical studies. However, tachyphylaxis has been observed for some GPR119 agonists such as GSK1292263 and JNJ-38431055 (Katz et al., 2012; Nilsson et al., 2012). Single-dose administration of these agonists decreased glucose excursion during OGTT, but multiple-dose administration for 4 weeks did not reduce the mean glucose levels. We therefore evaluated the efficacy of repeated administration of YH18968 in DIO mice. One mg/kg of YH18968 resulted in a significant decrease in the glucose AUC in OGTT on day 1 and the effect was sustained on day 29 after daily administration for 4 weeks. Sustained glucose lowering effects without tachyphylaxis can be expected in future clinical studies. In this study, the glucose lowering effect of YH18968 was not significantly different from the effect of combination treatment with linagliptin. Since 1 mg/kg of linagliptin reduces the AUC blood glucose level to a near normal range, it might be difficult to observe a significant additive reduction by combination treatment compared to linagliptin alone in this study condition.
In conclusion, we identified and evaluated a novel small-molecule GPR119 agonist, YH18968, which showed significant and sustained blood glucose control in normal and DIO mice. YH18968 also exhibited a more potent glucose lowering effect in combined treatment with a DPP-4 inhibitor, indicated by the significant increase of active plasma GLP-1 levels following combination treatment. Therefore, we report that YH18968 in combined with a DPP-4 inhibitor may be an effective therapeutic candidate for the treatment of type 2 diabetes.
Acknowledgments
This study was supported by a grant of Korean Health Technology R&D project, Ministry of Health & Welfare, Republic of Korea (HI14C1007). This work was supported by the GRRC program of Gyeonggi province (GRRCkyunghee 2017-B01).
REFERENCES
- Bailey CJ. Metformin: effects on micro and macrovascular complications in type 2 diabetes. Cardiovasc Drugs Ther. 2008;22:215–224. doi: 10.1007/s10557-008-6092-0. [DOI] [PubMed] [Google Scholar]
- Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov. 2012;11:603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, Moloney M, Gao H, Mondala H, Bagnol D, Unett D, Liang Y, Demarest K, Semple G, Behan DP, Leonard J. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007;148:2601–2609. doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- Cornall LM, Hryciw DH, Mathai ML, McAinch AJ. Direct activation of the proposed anti-diabetic receptor, GPR119 in cardiomyoblasts decreases markers of muscle metabolic activity. Mol Cell Endocrinol. 2015;402:72–85. doi: 10.1016/j.mce.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4059–4067. doi: 10.1210/jcem.81.11.8923861. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1999;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. Glucagon-like peptide. Diabetes. 1998;47:159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- Han TD, Jung EH, Yi CH, Lee BM, Park YH, Lee DH, Kang JH, Yang NY, Kim DH, Hyun KH, Park KJ, Lee CH, Nam SH. Novel triazolone derivatives or salts thereof and pharmaceutical composition comprising the same. 2014. PCT International applications WO2014175621 A1. 2014 Apr 22.
- Hothersall JD, Bussey CE, Brown AJ, Scott JS, Dale I, Rawlins P. Sustained wash-resistant receptor activation response of GPR119 agonist. Eur J Pharmacol. 2015;762:430–442. doi: 10.1016/j.ejphar.2015.06.031. [DOI] [PubMed] [Google Scholar]
- Jones RM, Leonard JN, Buzard DJ, Lehmann J. GPR119 agonists for the treatment of type 2 diabetes. Expert Opin Ther Pat. 2009;19:1339–1359. doi: 10.1517/13543770903153878. [DOI] [PubMed] [Google Scholar]
- Katz LB, Gambale JJ, Rothenberg PL, Vanapalli SR, Vaccaro N, Xi L, Polidori DC, Vets E, Sarich TC, Stein PP. Pharmacokinetics, pharmacodynamics, safety, and tolerability of JNJ-38431055, a novel GPR119 receptor agonist and potential antidiabetes agent, in healthy male subjects. Clin Pharmacol Ther. 2011;90:685–692. doi: 10.1038/clpt.2011.169. [DOI] [PubMed] [Google Scholar]
- Katz LB, Gambale JJ, Rothenberg PL, Vanapalli SR, Vaccaro N, Xi L, Sarich TC, Stein PP. Effects of JNJ-38431055, a novel GPR119 receptor agonist, in randomized, double-blind, placebo-controlled studies in subjects with type 2 diabetes. Diabetes Obes Metab. 2012;14:709–716. doi: 10.1111/j.1463-1326.2012.01587.x. [DOI] [PubMed] [Google Scholar]
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes. Diabetes Care. 2004;27:17–20. doi: 10.2337/diacare.27.1.17. [DOI] [PubMed] [Google Scholar]
- Lauffer L, Iakoubov R, Brubaker PL. GPR119: “double-dipping” for better glycemic control. Endocrinology. 2008;149:2035–2037. doi: 10.1210/en.2008-0182. [DOI] [PubMed] [Google Scholar]
- Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58:1058–1066. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J. The pharmacologic basis for clinical differences among GLP-1 receptor agonists and DPP-4 inhibitors. Postgrad Med. 2011;123:189–201. doi: 10.3810/pgm.2011.11.2508. [DOI] [PubMed] [Google Scholar]
- Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Circulation. 2003;108:2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Raun K, Yan FF, Larsen MO, Tang-Christensen M. Laboratory animals as surrogate models of human obesity. Acta Pharmacol Sin. 2012;33:173–181. doi: 10.1038/aps.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez DJ, Bush MA, Collins DA, McMullen SL, Gillmor D, Apseloff G, Atiee G, Corsino L, Morrow L, Feldman PL. Gut hormone pharmacology of a novel GPR119 agonist (GSK1292263), metformin, and sitagliptin in type 2 diabetes mellitus: results from two randomized studies. PLoS ONE. 2014;9:e92494. doi: 10.1371/journal.pone.0092494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, Reynet C. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Overton HA, Fyfe MC, Reynet C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br. J. Pharmacol. 2008;153(Suppl 1):S76–S81. doi: 10.1038/sj.bjp.0707529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter K, Buning C, Halland N, Pöverlein C, Schwink L. G protein-coupled receptor 119 (GPR119) agonists for the treatment of diabetes: Recent progress and prevailing challenges. J Med Chem. 2016;59:3579–3592. doi: 10.1021/acs.jmedchem.5b01198. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Inoue H, Kawakami S, Miyawaki K, Miyamoto T, Mizuta K, Itakura M. Expression and distribution of Gpr119 in the pancreatic islets of mice and rats: predominant localization in pancreatic polypeptide-secreting PP-cells. Biochem Biophys Res Commun. 2006;351:474–480. doi: 10.1016/j.bbrc.2006.10.076. [DOI] [PubMed] [Google Scholar]
- Taylor SI, Accili D, Imai Y. Insulin resistance or insulin deficiency. Which is the primary cause of NIDDM. Diabetes. 1994;43:735–740. doi: 10.2337/diab.43.6.735. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- Yip RG, Wolfe MM. GIP biology and fat metabolism. Life Sci. 2000;66:91–103. doi: 10.1016/S0024-3205(99)00314-8. [DOI] [PubMed] [Google Scholar]