Abstract
Background
Two decades of human neuroimaging research have associated volume reductions in the hippocampus with posttraumatic stress disorder. However, little is known about the distribution of volume loss across hippocampal subfields. Recent advances in neuroimaging methods have made it possible to accurately delineate 10 gray matter hippocampal subfields. Here, we apply a volumetric analysis of hippocampal subfields to data from a group of combat-exposed Veterans.
Method
Veterans (total, n = 68, posttraumatic stress disorder, n = 36; combat control, n = 32) completed high-resolution structural magnetic resonance imaging. Based on previously validated methods, hippocampal subfield volume measurements were conducted using FreeSurfer 6.0. The Clinician-Administered PTSD Scale assessed posttraumatic stress disorder symptom severity; Beck Depression Inventory assessed depressive symptom severity. Controlling for age and intracranial volume, partial correlation analysis examined the relationship between hippocampal subfields and symptom severity. Correction for multiple comparisons was performed using false discovery rate. Gender, intelligence, combat severity, comorbid anxiety, alcohol/substance use disorder, and medication status were investigated as potential confounds.
Results
In the whole sample, total hippocampal volume negatively correlated with Clinician-Administered PTSD Scale and Beck Depression Inventory scores. Of the 10 hippocampal subfields, Clinician-Administered PTSD Scale symptom severity negatively correlated with the hippocampus–amygdala transition area (HATA). Beck Depression Inventory scores negatively correlated with dentate gyrus, cornu ammonis 4 (CA4), HATA, CA2/3, molecular layer, and CA1. Follow-up analysis limited to the posttraumatic stress disorder group showed a negative correlation between Clinician-Administered PTSD Scale symptom severity and each of HATA, CA2/3, molecular layer, and CA4.
Conclusion
This study provides the first evidence relating posttraumatic stress disorder and depression symptoms to abnormalities in the HATA, an anterior hippocampal region highly connected to prefrontal-amygdala circuitry. Notably, dentate gyrus abnormalities were associated with depression severity but not posttraumatic stress disorder symptoms. Future confirmatory studies should determine the extent to which dentate gyrus volume can differentiate between posttraumatic stress disorder- and depression-related pathophysiology.
Keywords: posttraumatic stress disorder, depression, hippocampus, hippocampal subfield, hippocampal volume, hippocampus–amygdala-transition-area, cornu ammonis, dentate gyrus, long axis, neuroimaging, Veteran, magnetic resonance imaging
Introduction
Reductions in hippocampal volume have been associated with posttraumatic stress disorder (PTSD). Although not without inconsistencies,1 an initial description of hippocampal reduction in combat-related PTSD2 led to many magnetic resonance imaging (MRI) replications in civilians and Veterans.1,3–6 However, the studies of structural hippocampus alterations in PTSD predominantly considered the hippocampus complex as a single structure (i.e., volume of the entire right or left hippocampus)7,8 with little investigation of the relationship between psychopathology and subfields of the hippocampus.7 A growing body of clinical and preclinical literature has, however, suggested a number of possible models for specialization across the long axis of the hippocampus. Two recent papers provide an in-depth discussion and synthesis.9,10 Briefly, anterior and posterior aspects of the hippocampus may serve different functions in terms of pattern separation and pattern completion and do not share reciprocal connections with the same cortical or subcortical areas of the brain.9 Identification of specific subfield abnormalities in PTSD may have particular pathophysiological and treatment implications and add to our understanding of both the role of the hippocampal subfields and the way those roles may shape (or be shaped by) a specialization gradient along the hippocampus.
The hippocampus can be segmented into 10 gray matter subfields7,11 (described further in Table 1; see Video 1 for animated 3D visualization http://journals.sagepub.com/doi/suppl/10.1177/2470547017744538), including the cornu ammonis (CA) regions 1–4, subiculum (SUB), presubiculum (PrSUB), parasubiculum (PaSUB), granule cell and molecular layers (MLs) of the dentate gyrus (DG), hippocampal–amygdala transition area (HATA), ML of the hippocampus, and the hippocampal tail. These hippocampal subfields can be defined by their unique cellular architectures and distinct developmental and functional properties.
Table 1.
Anatomy and putative function of the gray matter hippocampal subfields.
| Subfield | Clinical and preclinical notes |
|---|---|
| HATA | Part of the anterior HPC, bounded by the HPC folds, lateral ventricle, and alveus.11 Carries afferent and efferent signals between the rest of the HPC and the caudal amygdala and may send axonal collaterals to the PFC and hypothalamus.12 Plausible role in acquiring traumatic memories, behavioral and neuroendocrine response to threat, fear conditioning,13 and consolidation of contextual fear learning and memory.14 Projections to the HTH and PFC may relate to aggressive or defensive sexual behavior.12 |
| ML | Sits above the SUB directly beneath the HPC fissure; traces the HPC folds along the CA fields and SUB.11 Interneuronal synaptic connections, serving probable role of transmission and integration of information across the HPC.15 Volume reduction has been observed in BP.16 |
| DG | Tri-layered structure beginning near the middle of the HPC head.11 Supports neurogenesis, memory formation, and neuroplasticity; rapid acquisition in spatial memory involving pattern separation (young granule cells) or pattern completion (mature granule cells).17,18 Volumetric atrophy may be associated with chronic stress,19 BD,16 depression,20 PTSD,21,22 and schizophrenia.23,24 |
| CA4 | Hilus of the DG.11 Receives excitatory inputs from the cerebral cortex and interneurons of the DG; contains mRNA related to neurogenesis, complexins which are important for neurotransmission,25 and BDNF which is important for survival and maturation of neurons.26 Various reductions in mRNA concentrations are associated with BD, possibly dysregulating neuronal transmission.25 |
| CA2/3 | Superior to DG from posterior half of HPC head to the HT.11 Extensive interconnections among principal cells forming an autoassociative network, supports formation of arbitrary spatial association, temporary maintenance of spatial working memory, and spatial pattern completion.27–30 Supports mnemonic processes in the formation of accurate spatial memory.31 Implicated in spatial pattern separation via interaction of mossy fibers/DG29 and acquisition of context-dependent fear extinction but not context-dependent fear memories.32 Influences discrete gene expression.10 Volume alterations found in PTSD.21 |
| CA1 | Extends from SUB, ending near first HPC fold.11 Trisynaptic loop created by CA1 projections through SUB to entorhinal cortex relates to acquisition of memory and spatial learning.33 Context-dependent fear extinction and retrieval of contextual memory.24,32 Insult to CA1 impairs spatial navigation and working memory, but not reference memory.33,34 Reduction in volume seen with untreated schizophrenia.35 |
| SUB | Three-layered allocortex, lateral to PrSUB and CA1.11 Part of limbic memory system, responsible for mnemonic processing,36 short-term memory retrieval, and spatial encoding.15 Stress response and inhibitory control over the HPA37 and implications in fear conditioning.38 Modulates epileptic discharges from the HPC.15 Atrophy implicated in early and advanced AD.39 |
| PrSUB | BA27; periallocortex; heavily myelinated ML; sits along HPC fissure, anterior to retrosplenial region.11 Connection with excitatory synapses supports bursting behaviors.40 Short-term memory and processing of spatial location information.41 Implicated in and possible marker for AD.39 |
| PaSUB | BA49; periallocortex; smaller than PrSUB and SUB; sits along HPC fissure.11 Continuous recognition memory for spatial navigation41,42 and integration of head direction.42 Inputs from GABAergic medial septal neurons and expression of cholinergic activity markers.43 Projecting neurons in thalamus, SUB, and PrSUB.44 Aids in determining spike timing relative to theta oscillations.45 |
| HT | Not well studied, so difficult to make reliable annotations. Volume reductions reported with increasing duration/severity of BD.46 FMRI evidence shows that HT may activate with abnormal ease in PTSD.47 |
HPC: hippocampus; HATA: hippocampal–amygdala transition area; ML: molecular layer of the HPC; DG: granule cell and ML of the dentate gyrus; CA: cornu ammonis; SUB: subiculum; PrSUB: presubiculum; PaSUB: parasubiculum; HT: HPC tail; AD: Alzheimer’s disease; BA: Broadmann area; BDNF: brain derived neurotrophic factor; BP: bipolar disorder; HPA: hypothalamus–pituitary–adrenal axis; HTH: Hypothalamus; PFC: prefrontal cortex.
Methods for delineating hippocampal subfields in human subjects have evolved.7,8,48,49 They are now included in software packages like FreeSurfer (https://surfer.nmr.mgh.harvard.edu). The use of different software packages, probabilistic atlases, or even hand-drawn segmentation of the hippocampus may explain some contradictory results reported in the literature.7,48 Recently, a new atlas11 derived from .13 mm resolution ex vivo MRI and 1 mm in vivo MRI was included in the release of FreeSurfer Version 6.0, providing more reliable and specific segmentation11 than was available previously. While preclinical evidence suggests hippocampal subfields are specialized in function,7 the historical challenge of in vivo segmentation has limited the exploration of subfields in humans, leading to volumetric investigations of less refined segmentation such as head, body, tail;50–53 or SUB, CA1-3, DG, and entorhinal cortex.21,54–57
Studies of hippocampal subfields in major depression and stress-related psychopathology found significant negative correlations between depression and DG,58–60 CA,59,60 and SUB,60 and between stress sequelae and CA1,61 CA3,61–63 and DG.61–63 However, less is known about hippocampal subfield abnormalities in PTSD. One previous study (17 PTSD; 19 non-PTSD) reported lower DG/CA3 volume in PTSD, but no correlation between hippocampal subfields and PTSD symptom severity.21 Secondary analysis in the combined sample found a negative correlation between DG/CA3 volume and insomnia severity.57 Although this pioneering pilot study had many strengths, it was also limited by its relatively small sample, with some participants unexposed to trauma, and the fact that their DG/CA3 region included the DG, CA3, CA4, and a large part of the ML. Another, more recent study reported a negative correlation between combined DG/CA4 subfield volume and PTSD symptom severity among 97 military Veterans.22 However, this study did not investigate a more comprehensive segmentation of the hippocampal subfields. To advance this line of research, we employed a state-of-the-art segmentation of 10 gray matter hippocampal subfields in a large sample of all combat-exposed US Veterans. In addition, to capture the association between a continuum of PTSD symptom severity and biological abnormalities,64,65 our primary analysis investigated the correlation between clinical severity and hippocampal subfields regardless of PTSD diagnosis.
Better understanding of the role that hippocampal subfields play in trauma- and stress-related psychopathology can provide further insight into the neural mechanisms underlying PTSD and depression and may prove to be relevant to drug development and treatment strategies. To our knowledge, the present study is among the first to evaluate the 10 gray matter hippocampal subfield volumes and is the first study to do so within the context of PTSD. Yet, considering prior literature,21,58–63 we predicted that the severity of both PTSD and depression symptoms would be associated with reduced volumes in hippocampal subfields.
Methods
Participants
This study includes 68 combat-exposed US Veterans between the age of 21 and 60 years who provided informed consent to participate in a study approved by the Human Studies Subcommittee (Institutional Review Board) at the VA Connecticut Healthcare System. The present sample has also been investigated in previous publications reporting cortical thickness reductions,65 morphometric abnormalities,66 and functional dysconnectivity64 associated with PTSD; however, the hippocampal subfields in this cohort are being investigated here for the first time. Participants were excluded if presenting with a psychotic disorder, bipolar depression, learning disorder or attention deficit disorder/attention deficit hyperactivity disorder, or moderate-to-severe traumatic brain injury. Epilepsy, brain tumors, and other gross neurological disorders with anatomical consequence were also excluded. Participants taking benzodiazepines or with standard MRI contraindications were not included in the study. Benzodiazepines were excluded because of their potential effects on the functional MRI scans, which were acquired as part of the parent study. In order to ensure generalizability of any findings, subjects with PTSD and highly co-occurring comorbidities such as unipolar depression, anxiety disorders, substance or alcohol use disorders, and those who were taking a stable dose of antidepressants were allowed to participate. These factors were evaluated as potential confounds in post hoc analyses but were largely not found to correlate with the outcomes presented below.
Clinical Measurement
PTSD diagnosis and symptom severity were determined using the Clinician-Administered PTSD Scale (CAPS) for the DSM-IV.67,68 Depressive symptoms were evaluated with the Beck Depression Inventory (BDI) Second Edition.69 The Structured Clinical Interview for the DSM-IV70 was used to evaluate psychiatric comorbidities. Combat exposure was measured with the Combat Exposure Scale (CES).71 Premorbid IQ was estimated using the Wechsler Test of Adult Reading (WTAR).72
Neuroimaging
Structural MRI (sMRI) acquisitions were conducted in a 3 Tesla magnetic field using a Siemens TIM Trio scanner and a 32-channel head coil. Following a three-plane localization, two MPRAGE scans providing T1-weighted contrast (TR = 2530 ms; TE = 2.71 ms; TI = 1200 ms; Flip = 7°) were acquired. All sMRIs were acquired with the same 256 mm FOV and 1 × 1 × 1 mm isotropic voxels. Volumetric hippocampal subfield estimates were conducted using the fully automated process provided by FreeSurfer73 Version 6.0 (https://surfer.nmr.mgh.harvard.edu) as described in previous studies74–76 and in the Supplementary Material. After completing the FreeSurfer “recon-all” pipeline, the following command was used to segment bilateral hippocampi and their 10 gray matter subfields (Code 1). Due to the lack of distinguishing contrast and the small size of the CA2 subfield, the CA2 and CA3 were combined, which are discussed below as the CA2/3.
# Code 1:
recon-all -s $subject –hippocampal-subfields-T1
Following data processing, data quality was assessed by checking recon-all segmentation quality and checking subfields and fissure images visually for discorrelation between segmentation and full region, outliers in each subfield after correcting for total intracranial volume (TIV), strong bilateral asymmetries, and unexpected subfield volumes. No manual intervention was necessary upon completion of quality evaluation, and these steps were undertaken while blinded to the demographic and clinical characteristics related to each scan.
Statistical Analysis
Participant demographics and psychiatric variables are presented in Supplemental Table S1. To examine the relationship between the hippocampus and severity of either PTSD symptoms (primary analysis) or depressive symptoms (secondary analysis), two-tailed partial correlations of each hippocampal subfield and the specific dimensional outcome were conducted after testing for normality (and log transforming as required). Age and TIV were included as covariates in all analyses. We included TIV as a covariate in our analyses, consistent with much of the literature;51–53,55,56,58–60,63 however, other methods are also commonly employed to adjust each volume of interest based on TIV. The reader should be aware that the “residual” method21,54 may yield different results than the covariate method employed here. Additional discussion of TIV correction can be found in the literature.77,78 To investigate for potential confounds, we repeated the partial correlation analyses controlling for each of the following variables: gender, WTAR, CES, comorbid anxiety, alcohol/substance use disorder, and medication status. For the full sample primary analyses, correction for multiple comparisons was performed using false discovery rate.79 Our secondary analyses in PTSD-only or medication-free subgroups were conducted with exploratory intent to inform future research on any significant or trending areas of interest. For this reason, we did not correct these follow-up analyses for multiple comparisons. For additional information about the effects of age and PTSD severity, see the Supplementary Material.
Results
Primary Analysis: PTSD Severity and Hippocampal Subfields
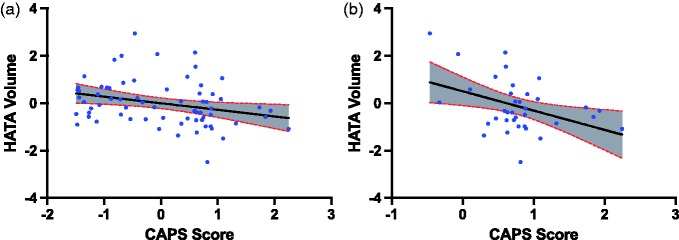
In the full sample, CAPS symptom severity negatively correlated with total hippocampal volume (r = −0.32, p = 0.008, df = 64). Among the 10 hippocampal subfields, the HATA volume significantly correlated with CAPS severity (r = −0.34, pfdr = 0.05, df = 64; Figure 1, Table 2). This correlation remained significant after controlling for each of gender, intelligence, combat severity, comorbid anxiety, alcohol/substance use disorder, and medication status. CAPS score correlations with CA1 and CA2/3 showed statistically nonsignificant trend before correction for multiple comparisons. See “CAPS (full group)” columns in Table 2 for additional results. See Supplemental Table S4 for raw subfield volumes.
Figure 1.
PTSD severity (CAPS score) is associated with HATA volume. (a) Primary analysis in full group. (b) Exploratory analysis in PTSD subgroup. CAPS score and HATA volume are residuals after controlling for age and total intracranial volume. CAPS: Clinician-Administered PTSD Scale.
Table 2.
Partial correlation of hippocampal subfields and PTSD severity.
| Hippocampal subfield | CAPS (full group) |
CAPS exploratory (PTSD
group) |
|||||
|---|---|---|---|---|---|---|---|
| r | p | FDR | df | r | p | df | |
| Total hippocampus | −.32 | .01* | — | 64 | −.42 | .02* | 32 |
| Parasubiculum | .11 | .36 | .41 | 64 | −.16 | .37 | 32 |
| Presubiculum | .05 | .67 | .67 | 64 | −.06 | .73 | 32 |
| Subiculum | −.11 | .37 | .41 | 64 | −.23 | .20 | 32 |
| CA1 | −.22 | .08 | .23 | 64 | −.33 | .06 | 32 |
| CA2/3 | −.22 | .07 | .23 | 64 | −.40 | .02* | 32 |
| CA4 | −.17 | .17 | .28 | 64 | −.40 | .05* | 32 |
| Dentate gyrus | −.16 | .20 | .28 | 64 | −.33 | .06 | 32 |
| HATA | −.34 | .01* | .05* | 64 | −.41 | .02* | 32 |
| ML of hippocampus | −.20 | .11 | .23 | 64 | −.35 | .04* | 32 |
| Hippocampal tail | −.20 | .12 | .23 | 64 | −.21 | .23 | 32 |
r: partial correlation controlling for age and total intracranial volume; FDR: significance after correction for multiple comparison; CA: cornu ammonis; ML: molecular layer; DG: granule cell and molecular layers of the dentate gyrus; HATA: hippocampal–amygdala transition area; CAPS: Clinician-Administered PTSD Scale for DSM-IV.
p ≤ 0.05. Exploratory analysis did not correct for multiple comparisons.
To further characterize the primary findings, we conducted a follow-up analysis limited to the PTSD subjects. In this exploratory analysis, CAPS score was correlated with total hippocampal volume (r = −0.42, p = 0.02, df = 32), CA2/3 volume (r = −0.40, p = 0.02, df = 32), CA4 (r = −0.34, p = 0.05, df = 32), HATA (r = −0.41, p = 0.02, df = 32), and ML (r = −0.35, p = 0.04, df = 32). No significant correlations were observed between CAPS symptom severity and the other gray matter subfields. See “CAPS Exploratory (PTSD group)” columns in Table 2 for additional results.
Secondary Analysis: Depression Severity and Hippocampal Subfields
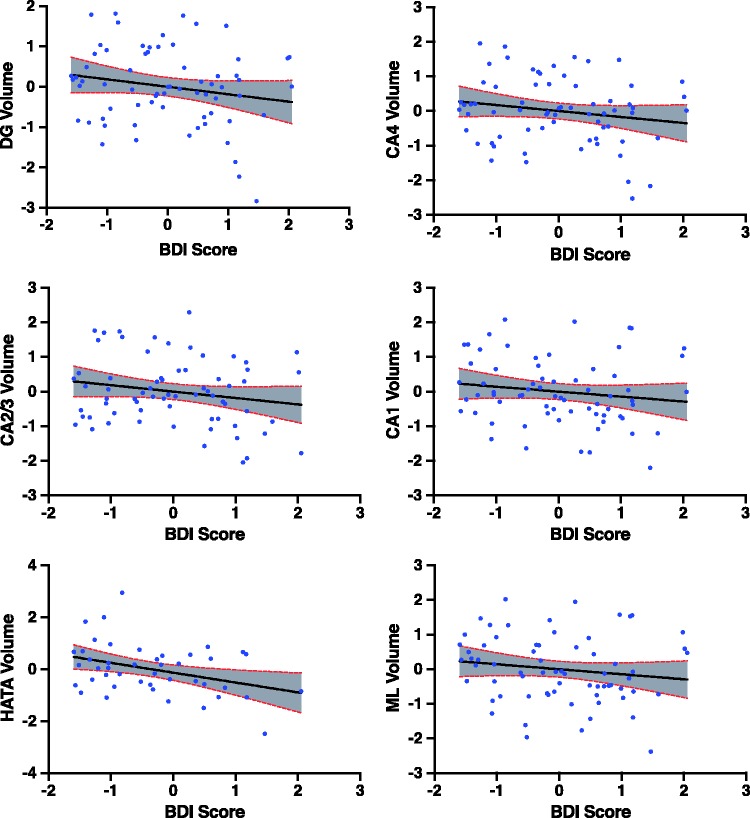
In the full sample (Table 3), BDI score negatively correlated with total hippocampal volume (r = −0.32, p = 0.01, df = 64). Among the 10 hippocampal subfields (Figure 2, Table 3), BDI negatively correlated with the DG (r = −0.33, pfdr = 0.04, df = 64), HATA (r = −0.30, pfdr = 0.04, df = 64), CA1 (r = −0.27, pfdr = 0.05, df = 64), CA2/3 (r = −0.30, pfdr = 0.04, df = 64), CA4 (r = −0.32, pfdr = 0.04, df = 64), and the ML of the hippocampus (r = −0.29, pfdr = 0.04, df = 64). These correlations remained significant after controlling for each of gender, intelligence, combat severity, comorbid anxiety, and alcohol/substance use disorder. Controlling for medication status, only the correlation between BDI and HATA remained significant. Thus, to further explore the putative confounding effects of medications, we performed a follow-up analysis in which the partial correlations between BDI and hippocampal subfields were repeated in a restricted sample including only those participants who were medication-free (n = 44). In this unmedicated subgroup, BDI showed a comparable pattern of negative correlation coefficient, but only the correlation with total hippocampal volume (r = −0.30, p = 0.05, df = 40) and HATA (r = −0.37, p = 0.02, df = 40) were statistically significant in this smaller sample. Nonsignificant trends were seen with CA1 and CA2/3. See “BDI Exploratory (med-free only)” columns in Table 3 for additional results.
Table 3.
Partial correlation of hippocampal subfields and depression severity.
| Hippocampal subfield | BDI (full group) |
BDI exploratory (med-free
only) |
|||||
|---|---|---|---|---|---|---|---|
| r | p | FDR | df | r | p | df | |
| Total hippocampus | −.32 | .01* | — | 64 | −.30 | .05* | 40 |
| Parasubiculum | .05 | .70 | .78 | 64 | .06 | .69 | 40 |
| Presubiculum | −.04 | .78 | .78 | 64 | .07 | .67 | 40 |
| Subiculum | −.12 | .36 | .45 | 64 | −.01 | .97 | 40 |
| CA1 | −.27 | .03* | .05* | 64 | −.27 | .09 | 40 |
| CA2/3 | −.30 | .02* | .04* | 64 | −.28 | .07 | 40 |
| CA4 | −.32 | .01* | .04* | 64 | −.21 | .19 | 40 |
| Dentate gyrus | −.33 | .01* | .04* | 64 | −.25 | .11 | 40 |
| HATA | −.30 | .02* | .04* | 64 | −.37 | .02* | 40 |
| ML of hippocampus | −.29 | .02* | .04* | 64 | −.26 | .10 | 40 |
| Hippocampal tail | −.13 | .29 | .41 | 64 | −.07 | .65 | 40 |
r: partial correlation controlling for age and total intracranial volume; FDR: significance after correction for multiple comparison; CA: cornu ammonis; ML: molecular layer; DG: granule cell and MLs of the dentate gyrus; HATA: hippocampal–amygdala transition area; BDI: Beck Depression Inventory, Second Edition.
p ≤ 0.05. Exploratory analysis did not correct for multiple comparisons.
Figure 2.
Depression severity (BDI score) is associated with multiple hippocampal subfield volumes. BDI score and subfield volumes in the full group analysis are residuals after controlling for age and total intracranial volume. BDI: Beck Depression Inventory; CA: cornu ammonis; ML: molecular layer.
Discussion
Although not without inconsistencies, replicated evidence supports the presence of volumetric alterations in the hippocampus among those with PTSD.1,3,4,6,51,53,80–83 The current study results provide further evidence of selective volumetric variability within the hippocampal subfields related to PTSD and depressive symptoms. The severity of PTSD and depression symptoms were associated with reduced volumes in certain hippocampal subfields. Our primary analysis revealed a novel finding of negative association between PTSD symptom severity and the volume of the HATA subfield, an area critically positioned between the amygdala and anterior hippocampus. In the full sample, PTSD severity did not correlate with either the DG or the CA2/3, subfields previous implicated in PTSD pathophysiology.21 However, a follow-up exploratory analysis showed a negative correlation between symptom severity and CA2/3 in Veterans meeting diagnostic criteria for PTSD. The ML and CA4 subfields were also negatively associated with clinical severity within the PTSD group. Consistent with the hypothesized role of the DG in depression pathology,84 our findings confirmed a negative correlation between depression symptom severity and volume of the DG subfield. HATA and other core hippocampal subfields (CA1, CA2/3, CA4, and ML) were also negatively associated with depression severity. However, our data could not rule out the possibility that the correlations between depression severity and hippocampal subfields are confounded by medication status, considering the failure to maintain statistical significance in the medication-free subgroup—although this could be due to the lack of statistical power in a relatively smaller sample.
In both primary and secondary analyses, the HATA volume was significantly associated with symptoms severity. Specifically, HATA volume was negatively correlated (and to nearly the same magnitude) with severity of both PTSD and depressive symptoms, regardless of medication or diagnostic status. The HATA is directly connected to the amygdala and also communicates with the prefrontal cortex and hypothalamus. Given the structure of this circuitry, alterations in the HATA could potentially affect a number of factors relevant to emotional perception and experiences including the acquisition, processing, and recall of traumatic memories, as well as contextual fear learning/conditioning14 and behavioral and neuroendocrine responses to traumatic stress.85,86 It is also thought that hippocampal and amygdala communication at a cellular level may influence cellular plasticity and further underlie contextual emotional learning and memory processing.14 Although these circuits are strongly implicated in PTSD,64,87–91 these results suggest that these memory and endocrine processing circuits may also be implicated in depression. Rather than observing the outcome of independent pathologies, it is possible that HATA subfield volume is particularly sensitive to chronic stress, a construct that overlaps with PTSD and depression psychopathology.
Depression and chronic stress are believed to increase glutamate excitotoxicity92,93 and glucocorticoid (GC) levels94 in the hippocampus, triggering inflammatory response, inhibiting neurogenesis,95 and lowering synaptic density.96–98 The present findings of reduced DG volumes are of interest, given that the DG is one of the only adult brain structures wherein neurogenesis occurs.99 The DG is particularly sensitive to diminished neuronal plasticity, insufficient levels of brain-derived neurotrophic factor, reduction of dendritic branching, and suppression of neurogenesis in the presence of neurotoxic GC levels.21,96–98 New granule cells in the DG are produced during active neurogenesis, and these cells are believed to play a key role in memory formation, pattern separation, and resolving interference between ambiguous and uncertain threat situations.100 In addition, the ratio of DG and CA varies as you traverse the long axis of the hippocampus, with a greater distribution of DG compared to CA subfields in the posterior aspect, which indicates that neurogenesis may be particularly relevant when discussing more posteriorly focused alterations.101 Given the implication of these fields in specialization across the hippocampus, it is notable that in our data set, the DG did not correlate with PTSD symptom severity, but uncorrected post hoc analyses revealed a negative association between CA2/3 and PTSD symptom severity, consistent with previous findings.21
Multimodal analysis of the hippocampus including, possibly, the use of histological and “brain bank” data, higher field strength MRI, and other methods may be of use in identifying what these volumetric reductions in the hippocampal subfields mean neurobiologically (i.e., reduction in cell count, projections, myelination, synaptic density, etc). More work is also necessary to understand the neurotoxic effects of stress and familial risk of PTSD102 and their relationship to these structural alterations; such work will be critical to properly interpret the clinical and etiological relevance of subfield volume reductions in PTSD and will help to shed additional light on the relationship between the specialization gradient across the long axis of the hippocampus9,10 and psychopathology.
Among the study limitations, we were unable to control for precombat childhood adversity history.103 Our sample, being mostly comprising male participants, cannot fully investigate potential sex differences related to PTSD or anatomy of the hippocampus, and thus may not be generalizable to female Veterans with PTSD. Given the heterogeneous nature of PTSD- and stress-related psychopathology generally, it is possible that individuals may have a slightly different mapping of structural subfield alterations. In addition, hippocampal subfields, and the HATA in particular, are relatively small regions according to the segmentation method discussed above. We have reported significant results between the HATA and symptom severity of both PTSD and depression, but it is well known that these symptoms can be highly correlated, and so multicollinearity is a concern. The reader should keep in mind that these measures are not totally independent—CAPS score explains 38% of the variance in BDI scores (r2 = 0.384); however, the variance inflation factor for these measures does not suggest a strong degree of multicollinearity (VIF = 1.647). Finally, as a cross-sectional study, we did not have information about premorbid hippocampal subfield volumes. Future research should strive to elucidate whether these alterations may be a risk factor for the development of PTSD, or if they are a consequence of exposure to trauma-related pathology.
Conclusion
In summary, this study benefits from a reasonable sample size and a dimensional approach, evaluating severity of PTSD and related symptoms, which may allow stronger inference to be made in translating volumetric findings to clinical phenotypes, including subthreshold presentations. The use of validated hippocampal subfield processing algorithms and atlases also lends strength to this study by providing a robust investigation of the hippocampal subfields based on a segmentation routine that is more reliable across subjects and equipment than previously available.11,104 This study makes a unique contribution to the literature by demonstrating various volumetric alterations associated with PTSD and depressive symptoms. Moreover, the results highlight the relevance of the HATA, a subfield intricately linking two regions of the brain (hippocampus and amygdala) long shown to be relevant to the study of PTSD and depression. Identification of focused relationships between symptomology and hippocampal subfields could advance our understanding of the psychopathology of trauma and chronic stress.
Supplementary Material
Acknowledgments
The authors would like to thank the Veterans who participated in this study for their invaluable contribution. The US Department of Veterans Affairs, the National Center for PTSD, the NIMH, The Brain and Behavior Foundation, and the NY Women’s Committee, who named Dr Averill the Woman of the Year Breaking the Silence Against Mental Illness.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Abdallah has served as a consultant or on advisory boards for Genentech and Janssen. He also serves as editor for the journal Chronic Stress published by SAGE Publications, Inc. Dr Krystal is a consultant for AbbVie, Inc, Amgen, Astellas Pharma Global Development, Inc, AstraZeneca Pharmaceuticals, Biomedisyn Corporation, Bristol-Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Inc, Neurovance, Inc, FORUM Pharmaceuticals, Janssen Research & Development, Lundbeck Research USA, Novartis Pharma AG, Otsuka America Pharmaceutical, Inc, Sage Therapeutics, Inc, Sunovion Pharmaceuticals, Inc, and Takeda Industries; is on the Scientific Advisory Board for Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc, Naurex, Inc, and Pfizer; serves as the Associate Editor for the journal Chronic Stress; is a stockholder in Biohaven Medical Sciences; holds stock options in Mnemosyne Pharmaceuticals, Inc; holds patents for Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia, U.S. Patent No. 5,447,948 (issued 5 September 1995), and Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued 15 July 2014); and filed a patent for Intranasal Administration of Ketamine to Treat Depression. US Application No. 14/197,767 (filed on 5 March 2014); US application or Patent Cooperation Treaty international application No. 14/306,382 (filed on 17 June 2014). Dr Southwick serves on the Editorial Board of Chronic Stress. Dr L. A. Averill serves as the Managing Editor for Chronic Stress. Mr C. L. Averill serves as Assistant Managing Editor for Chronic Stress. All other authors report that there is no conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the US Department of Veterans Affairs (DVA) National Center for PTSD; NIMH [MH-101498]; Brain and Behavior Foundation Young Investigator Awards [NARSAD]; and a DVA Career Development Award [IK2CX000772]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Supplemental Material
The 3D hippocampal subfield video and supplemental materials are available at http://journals.sagepub.com/doi/suppl/10.1177/2470547017744538
References
- 1.Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 1181–1188. [DOI] [PubMed] [Google Scholar]
- 2.Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152: 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord 2005; 88: 79–86. [DOI] [PubMed] [Google Scholar]
- 4.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus 2005; 15: 798–807. [DOI] [PubMed] [Google Scholar]
- 5.Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 2006; 30: 1004–1031. [DOI] [PubMed] [Google Scholar]
- 6.O’Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res 2015; 232: 1–33. [DOI] [PubMed] [Google Scholar]
- 7.Wisse LE, Daugherty AM, Olsen RK, et al. A harmonized segmentation protocol for hippocampal and parahippocampal subregions: why do we need one and what are the key goals? Hippocampus. 2017. 27: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Leemput K, Bakkour A, Benner T, et al. Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus 2009; 19: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci 2013; 17: 230–240. [DOI] [PubMed] [Google Scholar]
- 10.Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 2014; 15: 655–669. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias JE, Augustinack JC, Nguyen K, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 2015; 115: 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiba T. Collateral projection from the amygdalo—hippocampal transition area and CA1 to the hypothalamus and medial prefrontal cortex in the rat. Neurosci Res 2000; 38: 373–383. [DOI] [PubMed] [Google Scholar]
- 13.Fujisaki M, Hashimoto K, Iyo M, Chiba T. Role of the amygdalo-hippocampal transition area in the fear expression: evaluation by behavior and immediate early gene expression. Neuroscience 2004; 124: 247–260. [DOI] [PubMed] [Google Scholar]
- 14.Fudge JL, deCampo DM, Becoats KT. Revisiting the hippocampal-amygdala pathway in primates: association with immature-appearing neurons. Neuroscience 2012; 212: 104–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat 2005; 207: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao B, Passos IC, Mwangi B, et al. Hippocampal subfield volumes in mood disorders. Mol Psychiatry 2017; 22: 1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashiba T, Cushman JD, Pelkey KA, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 2012; 149: 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saab BJ, Georgiou J, Nath A, et al. NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory. Neuron 2009; 63: 643–656. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Zhu X, Ju S, et al. Detection of volume alterations in hippocampal subfields of rats under chronic unpredictable mild stress using 7T MRI: a follow-up study. J Magn Reson Imaging 2017; 46: 1456–1463. [DOI] [PubMed] [Google Scholar]
- 20.Treadway MT, Waskom ML, Dillon DG, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry 2015; 77: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Neylan TC, Mueller SG, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry 2010; 67: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes JP, Hayes S, Miller DR, Lafleche G, Logue MW, Verfaellie M. Automated measurement of hippocampal subfields in PTSD: evidence for smaller dentate gyrus volume. J Psychiatr Res 2017; 95: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elvsashagen T, Zuzarte P, Westlye LT, et al. Dentate gyrus-cornu ammonis (CA) 4 volume is decreased and associated with depressive episodes and lipid peroxidation in bipolar II disorder: longitudinal and cross-sectional analyses. Bipolar Disord 2016; 18: 657–668. [DOI] [PubMed] [Google Scholar]
- 24.Ota M, Sato N, Hidese S, et al. Structural differences in hippocampal subfields among schizophrenia patients, major depressive disorder patients, and healthy subjects. Psychiatry Res 2017; 259: 54–59. [DOI] [PubMed] [Google Scholar]
- 25.Eastwood SL, Harrison PJ. Hippocampal synaptic pathology in schizophrenia, bipolar disorder and major depression: a study of complexin mRNAs. Mol Psychiatry 2000; 5: 425–432. [DOI] [PubMed] [Google Scholar]
- 26.Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci 2011; 36: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amaral DG, Witter MP. The hippocampal formation. In: Paxinos G. (ed). The Rat Nervous System, San Diego, CA: Academic Press, 1995, pp. 443–494. [Google Scholar]
- 28.Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behav Brain Res 2006; 169: 142–149. [DOI] [PubMed] [Google Scholar]
- 29.Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus 2005; 15: 808–814. [DOI] [PubMed] [Google Scholar]
- 30.Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus 2004; 14: 301–310. [DOI] [PubMed] [Google Scholar]
- 31.Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learning & Memory (Cold Spring Harbor, NY) 2008; 15: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis HP, Tribuna J, Pulsinelli WA, Volpe BT. Reference and working memory of rats following hippocampal damage induced by transient forebrain ischemia. Physiol Behav 1986; 37: 387–392. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert PE, Brushfield AM. The role of the CA3 hippocampal subregion in spatial memory: a process oriented behavioral assessment. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akbari E, Naghdi N, Motamedi F. Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav Brain Res 2006; 173: 47–52. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert PE, Kesner RP. Role of the rodent hippocampus in paired-associate learning involving associations between a stimulus and a spatial location. Behav Neurosci 2002; 116: 63–71. [DOI] [PubMed] [Google Scholar]
- 36.Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron 2004; 42: 465–476. [DOI] [PubMed] [Google Scholar]
- 37.O’Mara S. Controlling hippocampal output: the central role of subiculum in hippocampal information processing. Behav Brain Res 2006; 174: 304–312. [DOI] [PubMed] [Google Scholar]
- 38.Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci 1999; 113: 283–290. [DOI] [PubMed] [Google Scholar]
- 39.Stafstrom CE. The role of the subiculum in epilepsy and epileptogenesis. Epilepsy Curr 2005; 5: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlesimo GA, Piras F, Orfei MD, Iorio M, Caltagirone C, Spalletta G. Atrophy of presubiculum and subiculum is the earliest hippocampal anatomical marker of Alzheimer’s disease. Alzheimers Dement (Amst) 2015; 1: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Funahashi M, Stewart M. Presubicular and parasubicular cortical neurons of the rat: functional separation of deep and superficial neurons in vitro. J Physiol 1997; 501 (Pt 2): 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glasgow SD, Chapman CA. Muscarinic depolarization of layer II neurons of the parasubiculum. PLoS One 2013; 8: e58901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honda Y, Ishizuka N. Organization of connectivity of the rat presubiculum: I. Efferent projections to the medial entorhinal cortex. J Comp Neurol 2004; 473: 463–484. [DOI] [PubMed] [Google Scholar]
- 44.Tang Q, Burgalossi A, Ebbesen CL, et al. Functional architecture of the rat parasubiculum. J Neurosci 2016; 36: 2289–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kesner RP, Giles R. Neural circuit analysis of spatial working memory: role of pre- and parasubiculum, medial and lateral entorhinal cortex. Hippocampus 1998; 8: 416–423. [DOI] [PubMed] [Google Scholar]
- 46.Bearden CE, Soares JC, Klunder AD, et al. Three-dimensional mapping of hippocampal anatomy in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2008; 47: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakamoto H, Fukuda R, Okuaki T, et al. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage 2005; 26: 813–821. [DOI] [PubMed] [Google Scholar]
- 48.Yushkevich PA, Amaral RS, Augustinack JC, et al. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. Neuroimage 2015; 111: 526–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giuliano A, Donatelli G, Cosottini M, Tosetti M, Retico A, Fantacci ME. Hippocampal subfields at ultra high field MRI: an overview of segmentation and measurement methods. Hippocampus 2017; 27: 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonne O, Vythilingam M, Inagaki M, et al. Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry 2008; 69: 1087–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golier JA, Yehuda R, De Santi S, Segal S, Dolan S, de Leon MJ. Absence of hippocampal volume differences in survivors of the Nazi Holocaust with and without posttraumatic stress disorder. Psychiatry Res 2005; 139: 53–64. [DOI] [PubMed] [Google Scholar]
- 52.Vythilingam M, Luckenbaugh DA, Lam T, et al. Smaller head of the hippocampus in Gulf War-related posttraumatic stress disorder. Psychiatry Res 2005; 139: 89–99. [DOI] [PubMed] [Google Scholar]
- 53.Yehuda R, Golier JA, Tischler L, et al. Hippocampal volume in aging combat veterans with and without post-traumatic stress disorder: relation to risk and resilience factors. J Psychiatr Res 2007; 41: 435–445. [DOI] [PubMed] [Google Scholar]
- 54.Mueller SG, Ng P, Neylan T, et al. Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatry Res 2015; 234: 194–201. [DOI] [PubMed] [Google Scholar]
- 55.Chalavi S, Vissia EM, Giesen ME, et al. Abnormal hippocampal morphology in dissociative identity disorder and post-traumatic stress disorder correlates with childhood trauma and dissociative symptoms. Hum Brain Mapp 2015; 36: 1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boen E, Westlye LT, Elvsashagen T, et al. Smaller stress-sensitive hippocampal subfields in women with borderline personality disorder without posttraumatic stress disorder. J Psychiatry Neurosci 2014; 39: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neylan TC, Mueller SG, Wang Z, et al. Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol Psychiatry 2010; 68: 494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Travis S, Coupland NJ, Silversone PH, et al. Dentate gyrus volume and memory performance in major depressive disorder. J Affect Disord 2015; 172: 159–164. [DOI] [PubMed] [Google Scholar]
- 59.Huang Y, Coupland NJ, Lebel RM, et al. Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol Psychiatry 2013; 74: 62–68. [DOI] [PubMed] [Google Scholar]
- 60.Han KM, Won E, Sim Y, Tae WS. Hippocampal subfield analysis in medication-naive female patients with major depressive disorder. J Affect Disord 2016; 194: 21–29. [DOI] [PubMed] [Google Scholar]
- 61.Lindqvist D, Mueller S, Mellon SH, et al. Peripheral antioxidant markers are associated with total hippocampal and CA3/dentate gyrus volume in MDD and healthy controls-preliminary findings. Psychiatry Res 2014; 224: 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol 2006; 498: 363–374. [DOI] [PubMed] [Google Scholar]
- 63.Zimmerman ME, Ezzati A, Katz MJ, et al. Perceived stress is differentially related to hippocampal subfield volumes among older adults. PLoS One 2016; 11: e0154530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdallah CG, Wrocklage KM, Averill CL, et al. Anterior hippocampal dysconnectivity in posttraumatic stress disorder: a dimensional and multimodal approach. Transl Psychiatry 2017; 7: e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wrocklage KM, Averill LA, Cobb Scott J, et al. Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. Eur Neuropsychopharmacol 2017; 27: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akiki TJ, Averill CL, Wrocklage KM, et al. The association of PTSD symptom severity with localized hippocampus and amygdala abnormalities. Chronic Stress 2017. doi: 10.1177/2470547017724069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress 1995; 8: 75–90. [DOI] [PubMed] [Google Scholar]
- 68.Weathers FW, Keane TM, Davidson JR. Clinician-Administered PTSD scale: a review of the first ten years of research. Depress Anxiety 2001; 13: 132–156. [DOI] [PubMed] [Google Scholar]
- 69.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of beck depression inventories-IA and -II in psychiatric outpatients. J Pers Assess 1996; 67: 588–597. [DOI] [PubMed] [Google Scholar]
- 70.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV-TR Axis I Disorders, Research Version, Patient Edition, New York, NY: Biometrics Research, New York State Psychiatric Institute, 2002. [Google Scholar]
- 71.Keane TM, Fairbank JA, Caddell JM, Mora CA. Clinical evaluation of a measure to assess combat exposure. Psychol Assess 1989; 1: 53–55. [Google Scholar]
- 72.Holdnack HA. Weschler Test of Adult Reading: WTAR, San Antonio, TX: Psychological Corporation, 2001. [Google Scholar]
- 73.Fischl B. FreeSurfer. Neuroimage 2012; 62: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anticevic A, Brumbaugh MS, Winkler AM, et al. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry 2013; 73: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Araujo Filho GM, Abdallah C, Sato JR, et al. Morphometric hemispheric asymmetry of orbitofrontal cortex in women with borderline personality disorder: a multi-parameter approach. Psychiatry Res 2014; 223: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 2017; 42: 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arndt S, Cohen G, Alliger RJ, Swayze VW, Andreasen NC. Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Res 1991; 40: 79–89. [DOI] [PubMed] [Google Scholar]
- 78.Sanfilipo MP, Benedict RH, Zivadinov R, Bakshi R. Correction for intracranial volume in analysis of whole brain atrophy in multiple sclerosis: the proportion vs. residual method. Neuroimage 2004; 22: 1732–1743. [DOI] [PubMed] [Google Scholar]
- 79.Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 1995; 57: 289–300. [Google Scholar]
- 80.Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 2006; 30: 1004–1031. [DOI] [PubMed] [Google Scholar]
- 81.Woodward SH, Kaloupek DG, Grande LJ, et al. Hippocampal volume and declarative memory function in combat-related PTSD. J Int Neuropsychol Soc 2009; 15: 830–839. [DOI] [PubMed] [Google Scholar]
- 82.Woodward SH, Kaloupek DG, Streeter CC, et al. Hippocampal volume, PTSD, and alcoholism in combat veterans. Am J Psychiatry 2006; 163: 674–681. [DOI] [PubMed] [Google Scholar]
- 83.Jatzko A, Rothenhofer S, Schmitt A, et al. Hippocampal volume in chronic posttraumatic stress disorder (PTSD): MRI study using two different evaluation methods. J Affect Disord 2006; 94: 121–126. [DOI] [PubMed] [Google Scholar]
- 84.Coplan JD, Gopinath S, Abdallah CG, Berry BR. A neurobiological hypothesis of treatment-resistant depression—mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front Behav Neurosci 2014; 8: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krystal JH, Neumeister A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res 2009; 1293: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: a critical review. Learning & memory (Cold Spring Harbor, NY) 2015; 22: 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuo JR, Kaloupek DG, Woodward SH. Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Arch Gen Psychiatry 2012; 69: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 88.Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron 2016; 92: 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 2012; 13: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sheynin J, Liberzon I. Circuit dysregulation and circuit-based treatments in posttraumatic stress disorder. Neurosci Lett 2017; 649: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry 2006; 59: 582–587. [DOI] [PubMed] [Google Scholar]
- 92.Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 2015; 66: 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Averill LA, Purohit P, Averill CL, Boesl MA, Krystal JH, Abdallah CG. Glutamate dysregulation and glutamatergic therapeutics for PTSD: evidence from human studies. Neurosci Lett 2017; 649: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic Stress 2017; 1: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol 2008; 18: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000; 57: 925–935. [DOI] [PubMed] [Google Scholar]
- 97.Sheline YI. Hippocampal atrophy in major depression: a result of depression-induced neurotoxicity? Mol Psychiatry 1996; 1: 298–299. [PubMed] [Google Scholar]
- 98.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry 2003; 54: 338–352. [DOI] [PubMed] [Google Scholar]
- 99.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev 2005; 85: 523–569. [DOI] [PubMed] [Google Scholar]
- 100.Besnard A, Sahay A. Adult hippocampal neurogenesis, fear generalization, and stress. Neuropsychopharmacology 2016; 41: 24–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Malykhin NV, Lebel RM, Coupland NJ, Wilman AH, Carter R. In vivo quantification of hippocampal subfields using 4.7 T fast spin echo imaging. Neuroimage 2010; 49: 1224–1230. [DOI] [PubMed] [Google Scholar]
- 102.Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002; 5: 1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Averill LA, Abdallah CG, Pietrzak RH, et al. Combat exposure severity is associated with reduced cortical thickness in combat veterans. Chronic Stress 2017. doi: 10.1177/2470547017724714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whelan CD, Hibar DP, van Velzen LS, et al. Heritability and reliability of automatically segmented human hippocampal formation subregions. Neuroimage 2016; 128: 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.