Abstract
Background
In adult patients with chronic urticaria (CU), the prevalence of food allergy is low compared to childhood patients. However, there are many patients who report food-related aggravation of CU, and some of them may have histamine intolerance.
Objective
The aim of this study was to evaluate the role of ingested histamine and to investigate the effect of a histamine-free diet in adult patients with CU.
Methods
Twenty-two adult patients with CU were enrolled. Foods with high amounts of histamine were prohibited to all patients for four weeks. The degree of severity of the urticaria was evaluated using the urticaria severity score (USS) and urticaria activity score (UAS). Plasma histamine levels and diamine oxidase (DAO) activity were determined and compared before (baseline) and after the histamine-free diet.
Results
Twenty-two adult patients were recruited and completed four weeks of histamine-free diet. The USS and UAS scores each showed significant differences before and after the histamine-free diet (p=0.010, p=0.006). There was a significant reduction in plasma histamine level after the histamine free-diet, compared with baseline (p=0.010). However, DAO activity did not change after the histamine-free diet (p=0.165).
Conclusion
Our study suggested that ingested histamine might be related to CU severity and that a histamine-free diet is helpful for treatment of adult patients with CU.
Keywords: Diet therapy, Food intolerance, Histamine, Urticaria
INTRODUCTION
Urticaria is a common skin condition associated with immunological and non-immunological mechanisms. It can be caused by release of many mediators, such as histamine, from skin mast cells and by an increase in the blood vessel permeability. This leads to temporary leakage and accumulation of plasma in tissues and to cutaneous symptoms, such as itchy wheals or swelling1. Chronic urticaria (CU) is defined as frequent episodes of urticaria that last more than six weeks2. However, most cases (~70% to 95%) of CU patients remain idiopathic3 and treatment options are limited. The mainstay of therapy is symptomatic control with antihistamines4. Itching is sometimes not controlled by antihistamines, so physicians may encounter challenges when treating patients with CU2,4.
So-called food allergy, despite negative food allergy test results, is common5. As several foods may contain high amounts of histamine, their ingestion may be responsible for some cases of food intolerance, which may include allergic like symptoms such as sneezing, flush, headache, diarrhea, and even shortness of breath5,6. There is an explanation for this phenomenon: disturbances in the metabolism of ingested histamine in patients with CU and increased mucosal passage of histamine6,7.
Histamine belongs to the biogenic amines, one of the important mediators for urticaria. Histamine intolerance results from disequilibrium between the accumulated histamine and the capacity for histamine degradation. Exceeding the histamine tolerance level of an individual gives rise to concentration-dependent histamine-mediated symptoms such as increasing gastric acid secretion and heart rate (1~2 ng/ml), tachycardia, headache, flush, urticaria, pruritus (3~5 ng/ml), decreased arterial pressure (6~8 ng/ml), bronchospasm (7~12 ng/ml), and cardiac arrest (<100 ng/ml). These can occur when patients take foods rich in histamine or impaired histamine degradation based on reduced diamine oxidase (DAO) activity, the main enzyme catabolizing histamine in the gut7,8. These can be described as histamine-mediated pseudo-allergic reactions or pseudo-food allergies, because the clinical manifestations mimic immunoglobulin (Ig)E-dependent immunologically-medicated allergic diseases without distinct immunologic sensitization9. There are many reports that showed lower DAO activity of CU patients compared with healthy controls7,10, even another study does not support this11. Anisakis species, roundworm parasites that have lifecycles involving fish and marine mammals, are infective to humans and cause anisakiasis. People who produce IgE in response to this parasite may subsequently have an allergic reaction, including anaphylaxis, after eating fish infected with Anisakis species. It was reported that DAO levels in CU depend on fish-eating habits, and in CU with sensitization against Anisakis, on the amount of specific IgE production11. Also, it was reported that there were no significant differences in DAO activity among CU patients without gastrointestinal (GI) symptoms, CU patients who frequently experienced GI symptoms, and healthy control subjects12. Until now, the association between excessive intake of histamine-rich food and symptoms of CU has been controversial. Siebenhaar et al.13 reported CU due to histamine intolerance appears to be rare; only a few patients with CU respond to oral histamine provocation. However, there have been many studies about the effectiveness of histamine restriction or histamine-free diet for CU5,9,10,13,14.
In a previous study, we tried to evaluate the effect of a histamine-free diet on CU patients but there was no significant improvement of symptoms or decrease in the number of antihistamine tablets taken in the restricted diet groups, compared with the control group15. However, there were limitations to the study: that many patients suffered difficulties trying to fulfill the restricted diet strictly and making out a menu by themselves. Also, in Korea, fermented foods, such as kimchi (fermented cabbage or radish), soybean paste, and red pepper paste, are frequently consumed. However, not many papers report studies on the histamine content in such fermented foods and the restriction of these fermented foods was not done in the previous study.
Therefore, we consulted with the Department of Nutrition and made a standard menu for seven days based on a histamine-free diet. We also evaluated the histamine levels of preferred Korean foods, including fermented foods, and measured changes in the histamine levels in relation to the cooking method. Based on that study16, the current prospective study was initiated to systematically examine the role of a histamine-free diet for improving the symptoms of CU with regular previous standard treatment.
MATERIALS AND METHODS
Patient selection
We enrolled 22 patients (≥18 years of age) with physician-diagnosed CU who visited between September 2014 and December 2015. Two dermatologists in the hospital diagnosed patients according to their symptoms and history. Diagnosis of CU was defined according to the criteria of Warin and Smith (1976)17 which included whealing and/or angioedema lasting longer than six weeks and occurring at least twice a week. We included the patients who could make and control their menu according to study instructions and whose symptoms were not controlled by standard therapy (steroid, H1 anti-histamine). Patients who had any dermatologic or systemic diseases (atopic dermatitis, diabetes, chronic renal failure, etc.) that could induce itching, and uncontrolled chronic diseases, were excluded. Patients with diseases (inflammatory bowel disease, squamous cell carcinoma, acute epipharyngitis) and drugs (heparin, cimetidine, aminoguanidine, chloroquine, dihydralazine, isoniazid, cefuroxim, cefotiam, chloroquine, aminophylline, verapamil, alprenolol, dihydralazine, contrast media, opiates, etc.) that could affect histamine release and DAO activity, pregnant patients, patients with any abnormalities discovered in physical examination, and patients with food allergy were also excluded. Patients who visited our clinic for the first time were asked to bring their previous prescriptions, and detailed medical records and history taking were used to confirm whether their condition meet the inclusion criteria or not.
All patients were on a free and uncontrolled diet before starting the study. We evaluated plasma histamine level and DAO activity before and after a histamine-free diet. Before entering the study, all patients were evaluated by their history and by the MAST Korean IgE Panel (MAST Immunosystem, Inc., Mountain View, CA, USA) to exclude food allergy. Informed consent was obtained from all patients and approval for the study was obtained from the university's standing committee on ethical practice (Hallym University Kangnam Sacred Heart Hospital Institution Review Board no. 2014-10-138).
During the period of study, oral antihistamine and topical steroids for CU were allowed. However, to evaluate the effect of histamine-free diet, the amount and kinds of drugs for CU treatment could be reduced from the baseline but could not be increased. For the patients who visited our clinic for the first time, the amount and kinds of drugs for CU treatment were not changed at their first visit to Kangnam Sacred Heart Hospital, Department of Dermatology. Patients were also excluded if the amount or kinds of drugs they used had to be increased.
Methods
1) Histamine-free diet
Patients restricted foods with high histamine levels for four weeks, based on previous studies16. We educated patients to restrict histamine-rich foods such as tuna, mackerel, Pacific saury (mackerel pike), pork, chicken, and spinach. This included in particular, fermented foods such as fermented cabbage or radish, soy bean paste, red pepper paste, mayonnaise, yogurt, cheese, ketchup, wine, and beer, which have large amounts of histamine. The food ranking by histamine level result from our previous study is summarized in Supplementary Table 1. Along with these, instant foods, grapes, bananas, strawberries, and citrus fruits known to release histamine (lemons, oranges, tangerines), as well as pineapples, tomatoes, nuts including peanuts, alcoholic beverages including wine, green tea, and chocolate were also restricted. The Department of Nutrition supplied a reference menu to patients and consulted with them to preventing nutritional imbalance. An example of the menu is presented in Table 1.
Table 1. Example of an experimental menu: a histamine-free diet.
| Breakfast | Lunch | Dinner | |
|---|---|---|---|
| 1st day | White rice | Rice with sorghum | White rice |
| Bean sprouts soup | Fish cake soup | Dried pollack soup | |
| Bulgogi | Grilled yellow croaker | Grilled tofu | |
| Soy sauce braised potatoes | Balloon flower root salad | Steamed egg | |
| Seasoned Chamnamul | Seasoned bean sprouts | Bracken salad | |
| 2nd day | White rice | Rice with millet | White rice |
| Egg soup with chives | Radish soup with mussel | Radish soup with beef | |
| Steamed tofu | Stir-fried mushroom | Grilled yellow croaker | |
| Stir-fried shitake | Steamed egg | Black bean boiled in soy sauce | |
| Grilled sweet pumpkin salad | Seasoned pigweed | Green pumpkin salad | |
| 3rd day | Cereal flakes | Rice with pea | White rice |
| Milk | Potato hand-pulled dough soup | Soft tofu stew | |
| Grilled beef | Grilled sole | ||
| Grilled tofu | Cucumber salad | ||
| Stir-fried fish cake and vegetables | Soy sauce braised potatoes | ||
| 4th day | White rice | White rice | White rice |
| Soft tofu stew | Tteokguk with beef | Hot bean sprouts soup | |
| Steamed egg | Grilled pomfret | Bulgogi | |
| Seasoned acorn jelly salad | Seasoned aster | Steamed egg | |
| Seasoned water parsley | Stir-fried chopped potato | Seasoned pigweed | |
| 5th day | White rice | White rice | White rice |
| Radish soup with perilla | Egg soup | Codfish soup | |
| Grilled yellow croaker grilled deodeok root | Grilled tofu | Grilled beef | |
| Seasoned bean sprouts | Seasoned green pumpkin | Grilled tofu | |
| Seasoned perilla leaf | Cucumber salad | ||
| 6th day | Rice with sorghum | White rice | White rice |
| Beef bone soup | Bean sprouts soup | Fish cake soup | |
| Grilled tofu | Bulgogi | Steamed soft soybean curd | |
| Lettuce wrap | Cucumber salad | Radish salad | |
| Broiled parsley | Seasoned pepper and lettuce | Lotusroot boiled in soy sauce | |
| 7th day | Toast | White rice | White rice |
| Soymilk or milk | Chilled cucumber soup | Bean sprouts soup | |
| Steamed soft soybean curd | Bulgogi | ||
| Black bean boiled in soy sauce | Soy sauce braised potatoes | ||
| Radish salad | Seasoned Chamnamul |
Please use as small amounts of seasonings as possible. Season with salts, vinegar and ground pepper rather than soy sauce, soybean paste and red pepper paste.
Please avoid boiling in soy sauce. Boiling is recommended than grilling.
Menu is exchangeable. For balanced diet, divide foods to rice, main dish and side dishes and menu change should be done within same division (ex. White rice can be exchanged to rice with sorghum or rice with pea, not to bulgogi which is considered as main dish).
Pan-frying and deep-frying is possible (ex. Pan-fried beef meat bal, pumpkin pancake, sweat and sour beef, deep-fried lotus root and deep fried eggplant).
2) Measurement of serum histamine level and diamine oxidase activity
Blood was drawn from each patient during urticaria skin reactions. Plasma was separated and kept at −20℃ until needed. Histamine assays were performed using enzyme-linked immunoassay kits (ELISA; IBL International GMBH, Hamburg, Germany). DAO activity was assayed using the ELISA kit (Cusabio Biotech Co., Ltd., Wuhan, China).
3) Severity evaluation of chronic urticaria
To evaluate the severity of CU before and after the histamine-free diet, the urticaria severity score (USS) and urticaria activity score (UAS) were used18,19. All patients were recommended to visit the outpatient clinic every week and to submit their self-reported note of daily UAS. UAS was composed of the number of wheals and the degree of pruritus. The score of each portion ranges from ‘0’ to ‘3,’ so the total scores ranged from ‘0’ to ‘6.’ In this study, we used UAS7. Patients recorded their daily UAS and calculated scores over seven days for total scores of the week (UAS7), and checked for changes every week. This method (UAS7) requires a week to score; so it was hard for us to get the UAS (UAS7) score at the baseline in this study. The UAS7 ranges from 0 to 42. The USS was composed of several questions such as the degree of pruritus that week, days of hives that week, average hours or hives each day, body distribution of hives, daily average, average antihistamine pills daily, average prednisone pills daily, nights of hives and sleep interference, hive interference with work, school or social life, and so on. The USS of each patient was evaluated two times, at the baseline and after four weeks of histamine-free diet. The USS ranges from 0 to 92. All assessment was done by one dermatologist.
Statistical analysis
The Wilcoxon signed rank test for variables, such as serum histamine level, DAO activity, and clinical severity using USS and UAS, were used to determine the significance of differences before and after the histamine-free diet. In all subjects with blood samples, the relationship between DAO activity and plasma histamine concentrations, the relationship between USS or UAS and plasma histamine concentrations, and the relationship between DAO activity and USS or UAS; were evaluated by Pearson, Spearman, or Kendall correlation. Significance levels for all analyses were set at p<0.05. All statistical analysis was performed using PASW Statistics ver. 18.0 for Windows (IBM Co., Armonk, NY, USA).
RESULTS
The baseline patient characteristics
Overall, 22 adult patients with CU were involved in the study and all finished four weeks of the histamine-free diet. Among patients who completed the study, 11 were male and 11 were female. The average of their ages was 41.2±16.1 (range, 18~70 years old). The average of their duration of treatment before enrollment was 22±25.2 (range, 0~73) months.
The effect of the histamine-free diet on the clinical severity of chronic urticaria
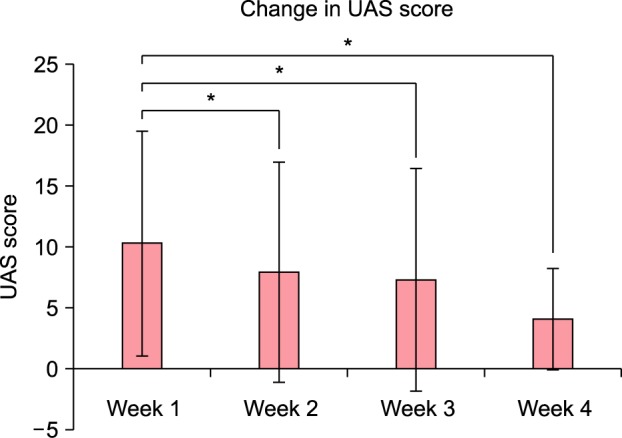
The baseline USS was 25.023±11.433 (range, 7.5~15.4). The UAS (UAS7) of the first week was 10.263±9.216 (range, 0~34). After four weeks of histamine-free diet, USS was 16.227±12.794 (range, 0.5~42; Fig. 1) and UAS was 4.056±4.143 (range, 2~21; Fig. 2). The scores of USS and UAS were quite low and reflect that antihistamines for these patients were continuously prescribed. These measures were reduced after histamine-free diet and statistically significant (p=0.010 and 0.006, respectively). In the case of UAS, the scores of week 2 and week 3 were reduced to 7.895±9.014, 7.278±9.134, respectively. They were all statistically significant (p=0.004 and 0.003) (Fig. 2).
Fig. 1. Change in the urticaria severity score (USS) after four weeks of histamine-free diet. Data are presented as mean and standard error (bars), with statistical significance denoted by lines and asterisks (p=0.010). *p<0.05.
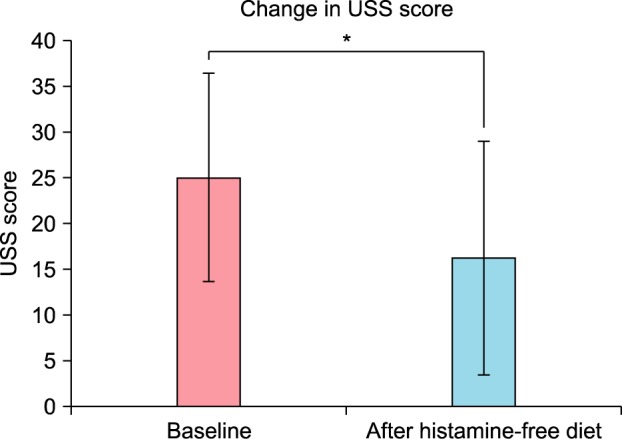
Fig. 2. Change in the urticaria activity score (UAS) with time in histamine-free diet for four weeks. Data are presented as mean and standard error (bars), with statistical significance denoted by lines and asterisks (p=0.006). *p<0.05.
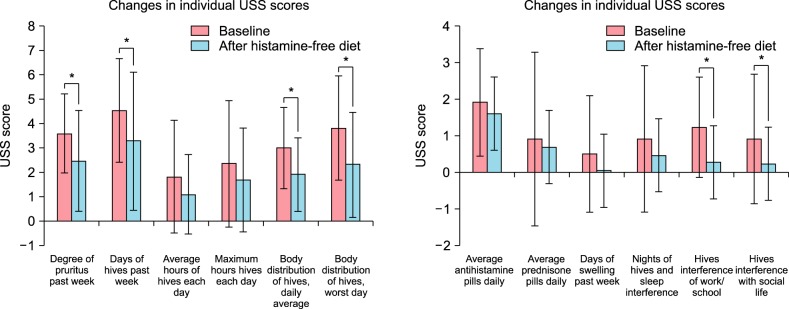
The changes of individual urticaria severity score scores and medication use
To delineate the specific symptoms or severity that were potentially driving these differences in total USS score between baseline and after the histamine-free diet, the results of the individual questions on the 12-point USS questionnaire were analyzed (Fig. 3). Interestingly, after the histamine-free diet, there were reports of decreased degree of pruritus in the past week, decreased number of days with hives, decreased body distribution of hives on an average day, and decreased body distribution of hives on the worst day (p=0.031, 0.034, 0.027, and 0.035). There were also decreased interference with work/school (p=0.005) and social life (p=0.026). There were no differences in the average hours of hives each day or maximum hours with hives each day, although these question items were generally scored low. There were also trends toward decreased number of days with swelling (p=0.197) and decreased interference with sleep (p=0.276), but these were not statistically significant. In cases of medication, there was no difference in the average number of daily antihistamine pills (p=0.105) or prednisone pills (p=0.785).
Fig. 3. Changes in the individual urticaria severity score (USS) components before (baseline) and after the 4-week histamine-free diet: Data are presented as mean and standard error (bars), with statistical significance denoted by lines and asterisks. *p<0.05.
The changes of serum histamine level and diamine oxidase activity
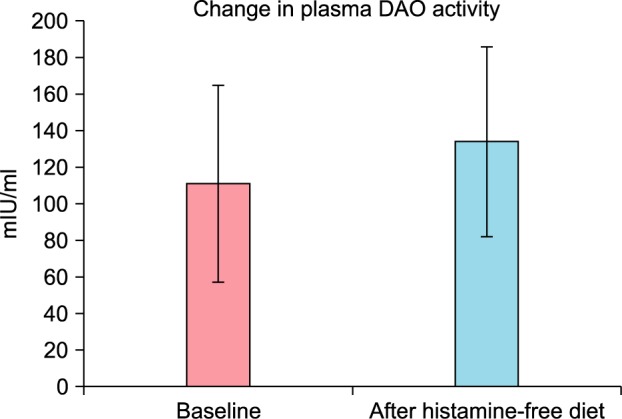
Blood sampling after consent was done for only 19 of the 22 patients. Normally, histamine was not detectable (less than 1 ng/ml) in plasma from normal subjects20. The average of baseline serum histamine level of 19 patients with CU was 44.600±3.818 ng/ml, and after four weeks of histamine-free diet, the serum histamine level was 16.650±1.623 ng/ml. This reduction was statistically significant (p=0.010) (Fig. 4). Of the 19 patients with CU in our study, the baseline plasma DAO activity was 110.888±53.910 (mIU/ml), and after four weeks on the histamine-free diet, it was 133.967±51.724 (mIU/ml). According to the literature, DAO activity lower than 3 U/ml was considered decreased21. Serum DAO activity <10 U/ml was the threshold suggested as a cutoff for probable histamine intolerance22. Compared with the known normal range of DAO activity, the baseline DAO activity of 19 patients with CU was lower, but the difference after four weeks of histamine-free diet was not statistically significant (p=0.165) (Fig. 5).
Fig. 4. Change in the plasma histamine level after a 4-week histamine-free diet: Data are presented as mean and standard error (bars), with statistical significance denoted by lines and asterisks (p=0.010). *p<0.05.
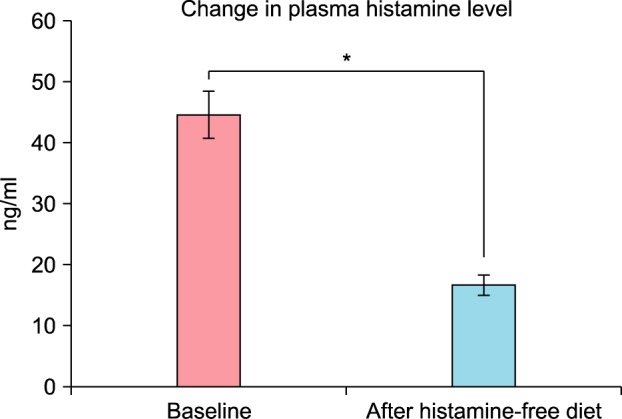
Fig. 5. Change in plasma diamine oxidase (DAO) activity after a 4-week histamine-free diet: Data are presented as mean and standard error (bars) (p=0.165).
Correlation between plasma histamine concentrations, diamine oxidase activity and symptom severity score
In 19 subjects, the relationship between changes in DAO activity and plasma histamine concentrations showed no statistical significance, when evaluated by Kendall or Spearman correlation (p=0.325 and p=0.200, respectively). In the same way, the relationships between changes in USS or UAS and plasma histamine concentrations also showed no statistical significance (p=0.927, p=0.946 and p=0.114, p=0.130, respectively). The relationships between changes in DAO activity and USS or UAS were also evaluated in the same manner, and all of them were not statistically significant (p=0.160, p=0.195 and p=0.169, p=0.228, respectively).
DISCUSSION
The aim of the present study was to evaluate the changes of plasma histamine concentration and DAO activity in CU patients before and after a histamine-free diet. In the present study, we found histamine plasma levels in CU patients were diminished after four weeks of a histamine-free diet12. This finding suggests that histamine plasma levels are dependent on diet in patients with CU12. In this study, there was no significant decrease in the number of pills of antihistamine after histamine-free diet; however, the severity of symptoms, base on questionnaires had been changed12. Compared to our previous study, we tried to make the histamine-free diet menu individually suitable for each patient and more applicable to Koreans12. CU is a mast cell-dependent disease of mostly unknown cause23. Mast cell mediator release has been shown to be the major common feature of all cutaneous whealing, and even though a number of potential mediators might be released from mast cells during this process, histamine seems to play a major role. This is because the whealing can be largely suppressed by drugs directed against the H1 type histamine receptor23.
Many people think that foods are the reason or an aggregating factor and therefore undergo inappropriate food restriction24,25. Some of them suffer nutritional imbalance and decline in quality of life due to inappropriate food restriction. However, with CU, food allergy is known to be a rare cause. It is reported in less than 2% of cases, food itself or food additives are the reason for CU26.
Many reports have suggested associations between CU and histamine, or histamine intolerance, but not many prospective studies have been performed5,9,10,13,14,27. Wantke et al.5 and Metcalfe27 reported that an ologiantigenic and histamine-free diet, respectively, induced significant clinical improvement in patients with CU. Also, additive- and preservative-free diets have been shown to improve clinical symptoms of CU9. In Guida et al.'s study10, the disappearance or reduction of symptoms after the histamine-free diet appeared to be correlated with lower histamine plasma levels. Additionally, in Wagner et al.'s study14, a total of 75% of the patients had a benefit from the low-histamine diet while DAO activity remained stable.
Increased levels of skin histamine have been reported in patients with CU28. Also, increased histamine plasma levels have been reported in CU10,12. In addition, Kanny et al.7 reported that plasma histamine levels were significantly higher after digestive histamine challenge in patients with CU, compared with controls. Especially in the kinetics of histamine, two groups showed significant difference. In the control population, the plasma histamine level was lowered as time passed, but in patients with CU, there was no significant difference between plasma histamine levels as time passed. A reduction of the intestinal barrier against chemical compounds (e.g., histamine) contained in some foods is hypothesized as a pathogenic mechanism of pseudoallergic reactions to food in CU29.
It is suggested that increase in plasma histamine corresponds either to abnormal passage of histamine across the duodenal mucosa due to intestinal hyperpermeability, or to a deficit in the enzyme systems of histamine degradation12. A defect in the activity of the enzymes (DAO) that catabolize histamine can explain the increased passage of histamine into the circulation in subjects with CU30. In the study of Manzotti et al.22, the mean value (±standard deviation) of DAO activity in the cohort of patients with histamine intolerance symptoms was 7.04±6.90 U/ml compared to 39.50±18.16 U/ml in 34 healthy controls (p=0.0031). This means that, in patients with symptoms triggered by histamine-rich food, measuring the serum DAO activity can help identify subjects who could benefit from a histamine limited diet and/or DAO supplementation22. Properly designed, controlled studies investigating histamine intolerance that include histamine provocation are indispensable for providing insights into the area of food intolerance22.
In previous studies, DAO activity lower than 3 (U/ml) was considered decreased21 and lower than 10 (U/ml) was considered probable histamine intolerance22. Therefore, the baseline DAO activity of 19 patients with CU was significantly lower in this study. It should be noted that this study might be limited in that the presence of GI symptoms, or its severity, in our patients was not investigated, even though GI symptoms are common symptoms of histamine intolerance. In addition, in our previous study about the relation between plasma histamine and DAO in CU, CU patients did not show significantly lower DAO activity12. A study by Wagner et al.14, fifty-five blood samples with CU patients were evaluated and only five patients (9%) showed DAO activity below 3 U/ml, suggesting histamine intolerance. There was also no significant change stated between DAO measurements after histamine- free diet. Large group studies will be required to elucidate the relationship between plasma histamine concentrations and DAO activity.
Inappropriate food restriction without medical evidence can lead to nutrient insufficiency. Children with food allergies were smaller for their age than controls, even when they received similar nutrient intakes. Therefore, nutritional evaluation is essential for the follow up of children with food allergies31.
The limitations of this study include the following. First, most patients had been taking antihistamines for so long that their plasma DAO activity could have been influenced by it. However, in our study, antihistamines for CU patients could not be discontinued. Also, each patient had different duration and dose of drug before the study. Second, the duration of the histamine-free diet was relatively short and the sample size was relatively small. To check the influence of diet or nutrient, the study period might need to be longer than four weeks. Study periods of oligoantigenic and histamine-free diet or pseudoallergen-free diet were different for three weeks, three months, six months or 12 months9,10,32. Third, making individual histamine-free diet menus was difficult, so help was needed from the Department of Nutrition. Also, it was difficult to offer a personalized diet for Koreans, and this required professional dietary knowledge. However, the menu itself was not so difficult to follow. In the end, future controlled study is needed to confirm the role of histamine-free diet in CU.
In this study, after being on a histamine-free diet for four weeks, patients showed significant improvement in their symptoms and decreasing plasma histamine level. Even so, the doses of drugs being used could not be reduced and there was no significant change in plasma DAO activity. In other words, there was no correlation between plasma histamine level and DAO activity, or between plasma histamine level and clinical subjective severity scores.
Although this is an open study based on the hypothesis of high plasma histamine level and a deficiency or reduced activity of DAO in CU according to the previous data, patients with CU seem to have increased sensitivity to histamine so avoidance of histamine rich food could be effective to reduce symptoms from histamines. Because there are many adult CU patients who seemed to suffer symptoms related to foods in Korea, and because a histamine free diet is a harmless treatment, this study is meaningful as evidence of the value of a therapeutic histamine-free diet. Additional further studies with longer duration of strict histamine-free diets may be needed in patients who have been using the conventional drugs with adjusted dose and duration.
We evaluated the effect of a histamine-free diet for four weeks for 22 adult patients with CU. In this study, there were significant clinical improvements in urticaria severity and the plasma histamine level was significantly reduced after the histamine-free diet. Even if the number of drugs used was not reduced and plasma DAO activity was not changed, a histamine-free diet cannot be said to be ineffective because it has resulted in a USS or UAS change that can be linked to the quality of life of the patient. The significance of this study is that it provides practical confirmation of the effectiveness of safe adjuvant therapy without major side effects. Thus, a histamine-free diet can be considered an applicable additional therapy for CU.
ACKNOWLEDGMENT
This research was supported by National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A3A04049491, 2015-M2A2A6A04044376, NRF-2017R1A2B4006252). This research was supported by HI17C0597.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
Supplementary Material
Supplementary data can be found via http://anndermatol.org/src/sm/ad-30-164-s001.pdf.
The food ranking of histamine level studied by our previous study
References
- 1.Kaplan AP. Urticaria and angioedema. In: Adkinson NF, Bochner BS, Busse WW, Holgate ST, Lemanske RF, Simons FER, editors. Middleton's allergy: principles and practice. 7th ed. St. Louis: Mosby Elsevier; 2009. p. 1063. [Google Scholar]
- 2.Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664–672. doi: 10.1067/mai.2000.105706. [DOI] [PubMed] [Google Scholar]
- 3.Champion RH. Urticaria: then and now. Br J Dermatol. 1988;119:427–436. doi: 10.1111/j.1365-2133.1988.tb03246.x. [DOI] [PubMed] [Google Scholar]
- 4.Khan DA. Chronic urticaria: diagnosis and management. Allergy Asthma Proc. 2008;29:439–446. doi: 10.2500/aap.2008.29.3151. [DOI] [PubMed] [Google Scholar]
- 5.Wantke F, Götz M, Jarisch R. Histamine-free diet: treatment of choice for histamine-induced food intolerance and supporting treatment for chronic headaches. Clin Exp Allergy. 1993;23:982–985. doi: 10.1111/j.1365-2222.1993.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 6.Kanny G, Grignon G, Dauca M, Guedenet JC, Moneret-Vautrin DA. Ultrastructural changes in the duodenal mucosa induced by ingested histamine in patients with chronic urticaria. Allergy. 1996;51:935–939. doi: 10.1111/j.1398-9995.1996.tb04497.x. [DOI] [PubMed] [Google Scholar]
- 7.Kanny G, Moneret-Vautrin DA, Schohn H, Feldman L, Mallie JP, Gueant JL. Abnormalities in histamine pharmacodynamics in chronic urticaria. Clin Exp Allergy. 1993;23:1015–1020. doi: 10.1111/j.1365-2222.1993.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 8.Biegański T, Kusche J, Lorenz W, Hesterberg R, Stahlknecht CD, Feussner KD. Distribution and properties of human intestinal diamine oxidase and its relevance for the histamine catabolism. Biochim Biophys Acta. 1983;756:196–203. doi: 10.1016/0304-4165(83)90092-2. [DOI] [PubMed] [Google Scholar]
- 9.Zuberbier T, Chantraine-Hess S, Hartmann K, Czarnetzki BM. Pseudoallergen-free diet in the treatment of chronic urticaria. A prospective study. Acta Derm Venereol. 1995;75:484–487. doi: 10.2340/0001555575484487. [DOI] [PubMed] [Google Scholar]
- 10.Guida B, De Martino CD, De Martino SD, Tritto G, Patella V, Trio R, et al. Histamine plasma levels and elimination diet in chronic idiopathic urticaria. Eur J Clin Nutr. 2000;54:155–158. doi: 10.1038/sj.ejcn.1600911. [DOI] [PubMed] [Google Scholar]
- 11.Daschner A, González-Fernández J, Valls A, de Frutos C, Rodero M, Cuéllar C. Diamine oxidase levels in different chronic urticaria phenotypes. Allergol Immunopathol (Madr) 2015;43:593–600. doi: 10.1016/j.aller.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Cho HJ, Cho SI, Kim HO, Park CW, Lee CH. Lack of association of plasma histamine with diamine oxidase in chronic idiopathic urticaria. Ann Dermatol. 2013;25:189–195. doi: 10.5021/ad.2013.25.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siebenhaar F, Melde A, Magerl M, Zuberbier T, Church MK, Maurer M. Histamine intolerance in patients with chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2016;30:1774–1777. doi: 10.1111/jdv.13778. [DOI] [PubMed] [Google Scholar]
- 14.Wagner N, Dirk D, Peveling-Oberhag A, Reese I, Rady-Pizarro U, Mitzel H, et al. A popular myth-low-histamine diet improves chronic spontaneous urticaria-fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650–655. doi: 10.1111/jdv.13966. [DOI] [PubMed] [Google Scholar]
- 15.Park CK, Choi JH, Park CW, Lee CH. A study of diet restriction in chronic idiopathic urticaria. Korean J Dermatol. 2008;46:1155–1162. [Google Scholar]
- 16.Chung BY, Park SY, Byun YS, Son JH, Choi YW, Cho YS, et al. Effect of different cooking methods on histamine levels in selected foods. Ann Dermatol. 2017;29:706–714. doi: 10.5021/ad.2017.29.6.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warin RP, Smith RJ. Challenge test battery in chronic urticaria. Br J Dermatol. 1976;94:401–406. doi: 10.1111/j.1365-2133.1976.tb06117.x. [DOI] [PubMed] [Google Scholar]
- 18.Jariwala SP, Moday H, de Asis ML, Fodeman J, Hudes G, de Vos G, et al. The Urticaria Severity Score: a sensitive questionnaire/index for monitoring response to therapy in patients with chronic urticaria. Ann Allergy Asthma Immunol. 2009;102:475–482. doi: 10.1016/S1081-1206(10)60120-2. [DOI] [PubMed] [Google Scholar]
- 19.Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau A, et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417–1426. doi: 10.1111/j.1398-9995.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- 20.Horakova Z, Keiser HR, Beaven MA. Blood and urine histamine levels in normal and pathological states as measured by a radiochemical assay. Clin Chim Acta. 1977;79:447–456. doi: 10.1016/0009-8981(77)90441-7. [DOI] [PubMed] [Google Scholar]
- 21.Maintz L, Benfadal S, Allam JP, Hagemann T, Fimmers R, Novak N. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106–1112. doi: 10.1016/j.jaci.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 22.Manzotti G, Breda D, Di Gioacchino M, Burastero SE. Serum diamine oxidase activity in patients with histamine intolerance. Int J Immunopathol Pharmacol. 2016;29:105–111. doi: 10.1177/0394632015617170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henz BM, Zuberbier T. Most chronic urticaria is food-dependent, and not idiopathic. Exp Dermatol. 1998;7:139–142. doi: 10.1111/j.1600-0625.1998.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 24.Juhlin L. Recurrent urticaria: clinical investigation of 330 patients. Br J Dermatol. 1981;104:369–381. doi: 10.1111/j.1365-2133.1981.tb15306.x. [DOI] [PubMed] [Google Scholar]
- 25.Bischoff SC, Manns MP. Intolerance reaction caused by biogenic amines. A separate disease entity? Internist (Berl) 1998;39:317–318. [PubMed] [Google Scholar]
- 26.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 27.Metcalfe DD. Food hypersensitivity. J Allergy Clin Immunol. 1984;73:749–762. doi: 10.1016/0091-6749(84)90442-1. [DOI] [PubMed] [Google Scholar]
- 28.Smith CH, Kepley C, Schwartz LB, Lee TH. Mast cell number and phenotype in chronic idiopathic urticaria. J Allergy Clin Immunol. 1995;96:360–364. doi: 10.1016/s0091-6749(95)70055-2. [DOI] [PubMed] [Google Scholar]
- 29.Lessof MH, Gant V, Hinuma K, Murphy GM, Dowling RH. Recurrent urticaria and reduced diamine oxidase activity. Clin Exp Allergy. 1990;20:373–376. doi: 10.1111/j.1365-2222.1990.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald BR, Robertson DA. Diamine oxidase, urticaria and intestinal oedema. Clin Exp Allergy. 1990;20:341–342. doi: 10.1111/j.1365-2222.1990.tb02791.x. [DOI] [PubMed] [Google Scholar]
- 31.Flammarion S, Santos C, Guimber D, Jouannic L, Thumerelle C, Gottrand F, et al. Diet and nutritional status of children with food allergies. Pediatr Allergy Immunol. 2011;22:161–165. doi: 10.1111/j.1399-3038.2010.01028.x. [DOI] [PubMed] [Google Scholar]
- 32.Mušič E, Korošec P, Šilar M, Adamič K, Košnik M, Rijavec M. Serum diamine oxidase activity as a diagnostic test for histamine intolerance. Wien Klin Wochenschr. 2013;125:239–243. doi: 10.1007/s00508-013-0354-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The food ranking of histamine level studied by our previous study