Abstract
GNAS is a complex imprinted gene encoding the alpha-subunit of the stimulatory heterotrimeric G protein (Gsα). GNAS gives rise to additional gene products that exhibit exclusively maternal or paternal expression, such as XLαs, a large variant of Gsα that shows exclusively paternal expression and is partly identical to the latter. Gsα itself is expressed biallelicaly in most tissues, although the expression occurs predominantly from the maternal allele in a small set of tissues, such as renal proximal tubules. Inactivating mutations in Gsα-coding GNAS exons are responsible for Albright’s hereditary osteodystrophy (AHO), which refers to a constellation of physical and developmental disorders including obesity, short stature, brachydactyly, cognitive impairment, and heterotopic ossification. Patients with Gsα mutations can present with AHO in the presence or absence of end-organ resistance to multiple hormones including parathyroid hormone. Maternal Gsα mutations lead to AHO with hormone resistance (i.e. pseudohypoparathyroidism type-Ia), whereas paternal mutations cause AHO alone (i.e. pseudo-pseudohypoparathyroidism). Heterotopic ossification associated with AHO develops through intramembranous bone formation and is limited to dermis and subcutis. In rare cases carrying Gsα mutations, however, ossifications progress into deep connective tissue and skeletal muscle, a disorder termed progressive osseous heteroplasia (POH). Here I briefly review the genetic, clinical, and molecular aspects of these disorders caused by inactivating GNAS mutations, with particular emphasis on heterotopic ossification.
The GNAS complex locus
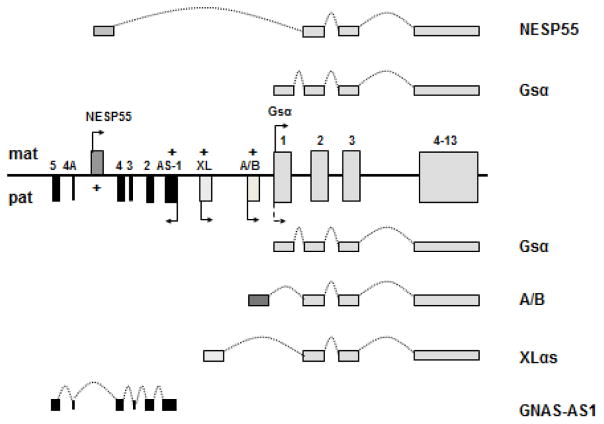
GNAS encodes the alpha-subunit of the stimulatory G protein, a signaling protein necessary for the actions of numerous hormones, neurotransmitters, and autocrine/paracrine factors [1–3]. Upon activation by one of many cell surface G protein-coupled receptors, Gsα stimulates membrane-bound adenylyl cylases and, thereby, leads to the generation of second messenger cAMP. Gsα also stimulates other “effectors”, such as Src tryrosine kinase, but the stimulation of adenylyl cyclase by Gsα takes place in a wide range of tissues, is the most extensively studied Gsα action, and could well be the most important cellular role of this ubiquitously expressed protein. In addition to Gsα, GNAS gives rise to several coding and non-coding transcripts that show parent-of-origin specific expression (Fig. 1). The maternal GNAS allele leads to transcripts encoding the neuroendocrine secretory protein-55 (NESP55), which belongs to the chromogranin family of proteins. NESP55 uses a unique promoter and a first exon that splices onto exon 2–13 that encode Gsα. From the paternal GNAS allele originates a large Gsα variant termed extra-large Gα (XLαs) and the so-called A/B transcript (also known as 1A or 1′), both of which also use unique promoters and first exons that splice onto Gsα exons 2–13. In the NESP55 transcript, sequences derived from exons 2–13 are located within the 3′-untranslated region, whereas in the XLαs transcript these sequences are within the coding region. Thus, the XLαs protein is partly identical to the Gsα protein. The A/B transcript is considered to be non-coding and regulate imprinting within GNAS; however, it has been suggested that the translational product of A/B mRNA is an amino-terminally truncated form of Gsα that antagonize the latter [4]. A non-coding antisense transcript (GNAS-AS1), which acts as a negative regulator, in cis, of the NESP55 expression, is also derived from the paternal GNAS allele.
Figure 1. The GNAS complex locus.
Located on chromosome 20q13.32 (GRCh38/hg38 chr20:58818918-58911196), GNAS encodes the ubiquitous signaling protein Gsα through the use of exons 1–13. Although Gsα is expressed biallelically in most tissues, paternal Gsα allele is silenced in some tissues (dotted arrow). Additional transcripts include the maternally expressed NESP55 and the paternally expressed XLαs and A/B (also referred to as 1A or 1′). These additional transcripts use individual promoters and first exons that splice onto exons 2–13 of GNAS. GNAS-AS1 transcript is a non-coding transcript derived from the paternal GNAS allele. Boxes and connecting lines depict exons and introns, respectively. Maternal (mat) and paternal (pat) GNAS products are illustrated above and below the gene structure, respectively, and the splicing patterns indicated by dotted curves. “+” indicates the regions of CpG methylation. Arrows indicate direction of transcription.
Diseases caused by inactivating mutations within Gsα-coding GNAS exons
Albright’s hereditary osteodystrophy and pseudohypoparathyroidism type-Ia
Consistent with the importance of Gsα in development and many physiological processes, genetic alterations that disrupt Gsα expression or activity result in a constellation of physical features including obesity, short stature, brachydactyly, cognitive impairment, and heterotopic ossification. Described originally by Albright et al [5], these features are now collectively termed Albright’s hereditary osteodystrophy (AHO). Heterozygous inactivating mutations within one of 13 GNAS exons encoding Gsα are responsible for AHO [6, 7]. These physical features are often found in patients who also present with hypocalcemia unresponsive to exogenously administered parathyroid hormone [5]. These patients are resistant to the actions of PTH, particularly in the renal proximal tubule, and therefore, develop not only hypocalcemia, which is a consequence of reduced 1,25 dihydroxyvitamin D levels, but also hyperphosphatemia. Indicating PTH resistance, serum PTH levels are elevated in these patients despite these biochemical alterations [8]. Most AHO patients also display resistance to some other hormones whose actions occur through Gsα, including thyroid stimulating hormone, gonadotropins, and growth hormone releasing hormone [1–3, 9, 10]. Therefore, hypothyroidism, hypogonadism, and growth hormone deficiency are common hormonal abnormalities in these patients. This disorder of AHO with multihormone resistance is referred to as pseudohypoparathyroidism type-Ia (PHP-Ia). By definition, patients with PHP type-I display blunted excretion of urinary cAMP and phosphate in response to exogenously administered biologically active PTH. This is in contrast to PHP type-II, in which PTH-induced phosphate but not cAMP excretion is blunted [11]. Another subtype of PHP type-I is PHP-Ib, which describes patients who have PTH resistance without AHO features [12, 13]. PHP-Ib is caused by epigenetic defects within the GNAS complex locus [14–16]. This PHP-I subtype has recently been reviewed elsewhere [10, 17, 18].
Pseudo-pseudohypoparathyroidism
Certain patients with AHO do not show any evidence of hormone resistance. This disorder has been termed “pseudo-pseudohypoparathyroidism” (PPHP) [19]. Like PHP-Ia, PPHP is caused by heterozygous inactivating mutations within Gsα-coding GNAS exons. Patients with PHP-Ia and PPHP can be found in the same kindreds, but it is the parent-of-origin of the mutation that determines the outcome in the offspring [20]. When the mutation is inherited from a female obligate carrier (i.e. maternal), the individual develops PHP-Ia, i.e. AHO plus hormone resistance. In contrast, when a male obligate carrier transmits the same mutation (i.e. paternal), then the offspring develops PPHP, i.e. AHO alone. The parent-of-origin specific inheritance of the hormone resistance reflects the finding that Gsα expression is biallelic in most tissues but predominantly maternal in a small number of hormone-responsive tissues, including renal proximal tubule, thyroid, gonads, and anterior pituitary [21–25]. Due to their manifestation upon both maternal and paternal GNAS mutations, AHO features are considered to occur as a result of Gsα haploinsufficiency in various tissues, except for obesity and cognitive impairment, which develop predominantly upon maternal transmission of Gsα mutations [26, 27]. Investigations using mouse models suggest that the obesity related to GNAS mutations results from Gsα deficiency in the dorsomedial hypothalamus [28]. Recent studies have shown that both PHP-Ia and PPHP patients can present with intrauterine growth retardation; however, it appears that this finding is significantly more severe in those carrying paternal (PPHP) than maternal mutations (PHP-Ia) [29, 30], implicating a paternally expressed GNAS product in fetal growth. This product is likely XLαs, given that mice with XLαs ablation [31], as well as patients with either maternal uniparental disomy involving the chromosomal region including GNAS (20q13.32) or large paternal GNAS deletions, also have perinatal growth retardation [32–36].
Heterotopic ossifications in AHO
Heterotopic ossification found in the context of AHO is limited to dermis and subcutis and cause painful lesions that may require surgery [37–49]. Patients can show heterotopic ossification after minor trauma, but the lesions often appear to arise spontaneously. Heterotopic ossification could be the presenting feature of AHO during childhood or adulthood. In some cases, the lesion appears as a bony plate under the skin, hence termed plate-like osteoma cutis. Histological analysis of biopsy specimens clearly indicates the presence of mineralized bone tissue with marrow elements. No cartilage is detected, indicating that GNAS-related heterotopic ossification arises primarily from intramembranous bone formation. Unlike hormone resistance, as mentioned above, cutaneous or subcutaneous ectopic ossification can develop both after maternal and paternal transmission of the Gsα mutation [20]. Therefore, it is thought that these osseous lesions reflect Gsα haploinsufficiency and are not related to the abnormalities in serum calcium and phosphate levels.
The pathogenesis of GNAS-related heterotopic ossification involves aberrant differentiation of mesenchymal stem cells or early progenitors located in the dermis or subcutaneous fat. Role of Gsα has been studied in mesenchymal stem cells with respect to osteogenic differentiation. In human mesenchymal stem cells, reduction of Gsα protein levels has been shown to cause osteogenic differentiation, while inhibiting the formation of adipocytes [50, 51]. In addition, Runx2, a key regulator of osteoblast-specific gene expression, appears to suppress Gsα expression [52], suggesting that Gsα actions oppose osteogenic differentiation. In addition, ablation of Gsα in progenitor cells that express osterix, a marker of early osteoblasts, leads to reduced number of osteoprogenitors but enhanced osteoblast differentiation [53]. Accordingly, activating Gsα mutations are found in fibrous dysplasia of bone, a disorder characterized by intramedullary accumulation of fibrous tissue containing poorly differentiated early osteoblasts [54]. Demonstrating a direct role of Gsα deficiency in the development of heterotopic ossification, Huso et al have found ectopic bone formation in dermis and subcutaneous tissue of adult mice in which Gnas exon 1 is ablated [55]. Histological analyses revealed mineral deposits and bone marrow elements located around hair follicles, and these findings were confirmed by further imaging studies. As in patients with PHP-Ia and PPHP, the subcutaneous lesions were observed regardless of the parental origin of the genetic manipulation. Cheeseman et al obtained similar findings independently, revealing heterotopic ossification in another mouse model in which the Gnas locus has a loss-of-function missense mutation in exon 6 [56]. Additional investigations using mice with paternal ablation of Gnas exon 1 have demonstrated that loss of one Gsα allele enhances osteogenic differentiation of stromal cells derived from adipose tissue while inhibiting their adipogenic differentiation [57, 58].
Progressive Osseous Heteroplasia
Progressive osseous heteroplasia (POH) is a disorder in which patients display severe extraskeletal ossifications that involve deep connective tissue and skeletal muscle [59–61]. The lesions mostly start during infancy as cutaneous or subcutaneous ossification and subsequently become invasive. Patients often develop severe ankylosis of affected joints, which restrict growth. Few patients with POH demonstrate AHO features and, consistent with the occasional co-existence of these two sets of clinical defects, heterozygous inactivating Gsα mutations have been identified as a cause of POH [62–64]. Several of the identified GNAS mutations in POH are identical to those found in PHP-Ia/PPHP kindreds in whom no progressive and invasive heterotopic ossification is present [63, 64]. The severity of the heterotopic ossification in these patients can be comparable to that of fibrodysplasia ossificans progressiva (FOP) [65, 66]. Unlike in the latter, however, the heterotopic bone in POH is formed primarily through intramembranous ossifications, i.e. similar to ossifications seen in AHO. Nevertheless, chondrocyte clusters have been documented in the histopathological analysis of a POH patient in whom existence of a GNAS mutation was confirmed [67].
Disease mechanisms leading to POH
Since the same GNAS mutations can cause either AHO or POH, it appears that genetic background, epigenetic events, or environmental factors contribute to the severity of heterotopic ossifications. It is important to note that in most POH patients, the heterotopic bone formation appears to be the isolated clinical finding, i.e. other typical AHO features are seemingly absent [64, 68]. Furthermore, most, but not all, GNAS mutations that lead to POH are inherited from male obligate carriers or occur de novo on the paternal allele [30, 64, 67, 69]. In fact, as shown previously, the same GNAS mutation in a three-generation kindred led to POH upon paternal transmission but typical AHO features upon maternal transmission [64]. These observations suggest that important distinctions exist between the molecular and genetic mechanisms governing POH and AHO.
Based on the finding that most GNAS mutations causing POH are paternal, it appears plausible that the deficiency of a GNAS product showing exclusive paternal expression contributes to the molecular pathogenesis of POH. A paternal GNAS product that is partly identical to Gsα is XLαs [70], which, when overexpressed, can mimic the action of Gsα with respect to cAMP signaling [71–73]. XLαs is expressed in various tissues, with abundant expression in central nervous system, neuroendocrine tissues, and muscle [74, 75]. XLαs expression has also been detected in bone and osteoprogenitor cells [57, 76, 77]. Strikingly, Pignolo et al have determined that XLαs and Gsα mRNA levels are comparable in mouse soft tissue stromal cells differentiated toward the osteogenic lineage [57]. Upon reviewing a large cohort of patients with POH, Adegbite et al revealed that, with the exception of one mutation, all Gsα mutations are located within exons shared by Gsα and XLαs [68]. It has also been shown that, despite the knowledge that exon 1 is specific for Gsα, expression level of XLαs mRNA is significantly lower in differentiated soft tissue stromal cells from mice heterozygous for paternal ablation of Gnas exon 1 than wild-type mice [57]. Based on these findings, it appears likely that, when the Gsα mutation is on the paternal allele, a more severe deficiency of cAMP signaling is encountered than that is observed upon Gsα haploinsufficiency alone, thus resulting in a POH-like phenotype. Considering that POH develops only in a small subset of patients carrying paternal GNAS mutations, the effect of XLαs deficiency in this regard may vary among individuals, perhaps due to variation in XLαs expression levels or activity.
Nevertheless, studies using mouse models have largely failed to confirm that XLαs has a physiological role as a stimulator of cAMP generation; in fact, agonist-induced cAMP response appears more exuberant in certain XLαs knockout tissues, such as brown adipose tissue and renal proximal tubules [31, 78]. Moreover, our group has recently shown, by studying mouse renal proximal tubules during early development, that XLαs knockout mice demonstrate deficiency of PLC/PKC signaling rather than cAMP signaling [78]. In addition, POH is also observed in few cases carrying maternal Gsα mutations [79, 80]), indicating that XLαs deficiency is not necessary for the development of invasive heterotopic ossification. Thus, it remains to be determined whether XLαs deficiency contributes, at least in some cases, to POH development, and if so, whether this effect occurs due to diminished PLC/PKC and/or cAMP signaling.
Recently, several patients have been analyzed systematically regarding their clinical phenotypes, revealing that the POH lesions predominantly show a dermomytomal distribution and a significant lateralization bias [81]. This study also showed that a severe loss of Gsα activity (via injection of dominant negative Gsα) in chick somitic cells is sufficient to cause POH-like formations. These findings led to a novel hypothesis that the pathogenesis of this disorder entails aberrant differentiation of progenitor cells of somitic origin, which may sustain severe Gsα deficiency due to a possible loss of heterozygosity at the GNAS locus, i.e. inactivation of the intact GNAS allele [81]. Accordingly, homozygous ablation of Gsα in different mouse models leads to invasive heterotopic ossification [82], unlike the subcutaneous heterotopic ossification observed in mice with heterozygous Gsα ablation [55, 56]. Recently, Happle has presented a strong argument for this hypothesis, proposing that POH should be accepted as a disorder of type-2 segmental mosaicism rather than a mendelian disorder [83]. Type-2 segmental mosaicism refers to a genetic phenomenon in which loss of heterozygosity is acquired during early development on top of a germ-line inactivating mutation. This is a plausible hypothesis that accommodates most of the clinical and genetic observations in POH; however, the significant tendency of the GNAS mutations to be paternal and the existence of few familial cases are difficult to explain. POH might also arise from a similar but not identical genetic mechanism, in which, instead of severe Gsα deficiency caused by loss of heterozygosity, a second mutational hit takes place in another essential gene in the same molecular pathway. Genetic analysis of the affected tissues in POH patients will be required to dissect out these hypotheses.
Cellular and molecular mechanisms underlying GNAS-related heterotopic ossification
Extensive extraskeletal ossifications that resemble POH lesions have been obtained in mice upon deletion of both Gnas alleles through the use of various Cre lines that are active in mesenchymal tissues, including Prx1-Cre (limb bud development), Dermo1-Cre (mid-gestational mesodermal tissues including mesenchyme-derived chondrocytes and osteoblasts), or Ap2α-Cre (embryonic face and limb mesenchyme) [82]. In addition, a POH-like severe phenotype was also observed upon the use of a renin-Cre transgenic mouse model; however, this outcome resulted from an unexpected activation of Cre in an as-yet-unidentified progenitor cell in soft tissues, including skin and developing limb [84]. For a better understanding of the pathophysiology and effective therapies, it is important to identify the specific lineage of mesenchymal stem cells critical for GNAS-related heterotopic ossification.
The activation of the hedgehog signaling pathway has recently been documented in osteoblasts and progenitor cells in POH lesions [82]. It was also shown that Gsα ablation causes severe heterotopic ossification through activation of hedgehog signaling [82]. Furthermore, in a limb-bud culture model, small molecule inhibitors of Gli transcription factors– which mediate hedgehog signaling – suppress the expression of hedgehog target genes induced by Gsα deficiency [82]. Hence, this signaling pathway, which is a key player in skeletal development and osteogenic differentiation [85], may serve as a potential therapeutic target for POH. It is uncertain whether hedgehog signaling also plays a role in the dermal and subcutaneous ossification seen in patients with AHO. Liu et al have found that various early and mature osteoblast markers, such as Msx2, are expressed at modestly higher levels in adipose-derived stromal cells from mice heterozygous for paternal Gnas exon 1 ablation than in those from wild-type mice [58]. Under in vitro adipogenic conditions, the mutant cells showed impaired differentiation into adipocytes and retained higher expression of the osteoblast markers. While these findings indicate a shift in the commitment of the mutant stromal cells toward the osteoblast lineage, the hedgehog pathway has not been scrutinized in this setting. It is conceivable that hyperactive hedgehog signaling is unique to POH and presents a precondition for the lesions to become progressive. Further investigations are needed to elucidate the underlying molecular mechanisms.
Acknowledgments
Funding information
The author’s lab has received research funding from NIH/NIDDK (RO1DK73911, as well as P01DK11794 to J.T. Potts/H.M. Kronenberg, the Endocrine Unit, Massachusetts General Hospital), the March of Dimes Foundation, the Milton Fund, and Sanofi-Genzyme iAwards.
Footnotes
The author has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinstein LS, Yu S, Warner DR, Liu J. Endocrine Manifestations of Stimulatory G Protein alpha-Subunit Mutations and the Role of Genomic Imprinting. Endocr Rev. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- 2.Bastepe M. The GNAS Locus: Quintessential Complex Gene Encoding Gsalpha, XLalphas, and other Imprinted Transcripts. Curr Genomics. 2007;8:398–414. doi: 10.2174/138920207783406488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plagge A, Kelsey G, Germain-Lee EL. Physiological functions of the imprinted Gnas locus and its protein variants Galpha(s) and XLalpha(s) in human and mouse. J Endocrinol. 2008;196:193–214. doi: 10.1677/JOE-07-0544. [DOI] [PubMed] [Google Scholar]
- 4.Puzhko S, Goodyer CG, Mohammad AK, Canaff L, Misra M, Jüppner H, Bastepe M, Hendy GN. Parathyroid hormone signaling via Galphas is selectively inhibited by an NH(2) -terminally truncated Galphas: Implications for pseudohypoparathyroidism. J Bone Miner Res. 2011;26:2473–85. doi: 10.1002/jbmr.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albright F, Burnett CH, Smith PH, Parson W. Pseudohypoparathyroidism - an example of “Seabright-Bantam syndrome”. Endocrinology. 1942;30:922–932. [Google Scholar]
- 6.Weinstein LS, Gejman PV, Friedman E, Kadowaki T, Collins RM, Gershon ES, Spiegel AM. Mutations of the Gs alpha-subunit gene in Albright hereditary osteodystrophy detected by denaturing gradient gel electrophoresis. Proc Natl Acad Sci U S A. 1990;87:8287–90. doi: 10.1073/pnas.87.21.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patten JL, Johns DR, Valle D, Eil C, Gruppuso PA, Steele G, Smallwood PM, Levine MA. Mutation in the gene encoding the stimulatory G protein of adenylate cyclase in Albright’s hereditary osteodystrophy. New Engl J Med. 1990;322:1412–1419. doi: 10.1056/NEJM199005173222002. [DOI] [PubMed] [Google Scholar]
- 8.Tashjian AH, Jr, Frantz AG, Lee JB. Pseudohypoparathyroidism: assays of parathyroid hormone and thyrocalcitonin. Proc Natl Acad Sci U S A. 1966;56:1138–42. doi: 10.1073/pnas.56.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein LS, Liu J, Sakamoto A, Xie T, Chen M. Minireview: GNAS: normal and abnormal functions. Endocrinology. 2004;145:5459–64. doi: 10.1210/en.2004-0865. [DOI] [PubMed] [Google Scholar]
- 10.Turan S, Bastepe M. GNAS Spectrum of Disorders. Curr Osteoporos Rep. 2015;13:146–58. doi: 10.1007/s11914-015-0268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drezner M, Neelon FA, Lebovitz HE. Pseudohypoparathyroidism type II: a possible defect in the reception of the cyclic AMP signal. N Engl J Med. 1973;289:1056–60. doi: 10.1056/NEJM197311152892003. [DOI] [PubMed] [Google Scholar]
- 12.Peterman MG, Garvey JL. Pseudohypoparathyroidism; case report. Pediatrics. 1949;4:790. [PubMed] [Google Scholar]
- 13.Reynolds TB, Jacobson G, Edmondson HA, Martin HE, Nelson CH. Pseudohypoparathyroidism: report of a case showing bony demineralization. J Clin Endocrinol Metab. 1952;12:560. doi: 10.1210/jcem-12-5-560. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Litman D, Rosenberg M, Yu S, Biesecker L, Weinstein L. A GNAS1 imprinting defect in pseudohypoparathyroidism type IB. J Clin Invest. 2000;106:1167–1174. doi: 10.1172/JCI10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastepe M, Lane AH, Jüppner H. Paternal uniparental isodisomy of chromosome 20q (patUPD20q) - and the resulting changes in GNAS1 methylation - as a plausible cause of pseudohypoparathyroidism. Am J Hum Genet. 2001;68:1283–1289. doi: 10.1086/320117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastepe M, Pincus JE, Sugimoto T, Tojo K, Kanatani M, Azuma Y, Kruse K, Rosenbloom AL, Koshiyama H, Jüppner H. Positional dissociation between the genetic mutation responsible for pseudohypoparathyroidism type Ib and the associated methylation defect at exon A/B: evidence for a long-range regulatory element within the imprinted GNAS1 locus. Hum. Mol. Genet. 2001;10:1231–1241. doi: 10.1093/hmg/10.12.1231. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani G, Spada A, Elli FM. Pseudohypoparathyroidism and Gsalpha-cAMP-linked disorders: current view and open issues. Nat Rev Endocrinol. 2016;12:347–56. doi: 10.1038/nrendo.2016.52. [DOI] [PubMed] [Google Scholar]
- 18.Tafaj O, Juppner H. Pseudohypoparathyroidism: one gene, several syndromes. J Endocrinol Invest. 2017;40:347–356. doi: 10.1007/s40618-016-0588-4. [DOI] [PubMed] [Google Scholar]
- 19.Albright F, Forbes AP, Henneman PH. Pseudo-pseudohypoparathyroidism. Trans Assoc Am Physicians. 1952;65:337–350. [PubMed] [Google Scholar]
- 20.Davies AJ, Hughes HE. Imprinting in Albright’s hereditary osteodystrophy. J Med Genet. 1993;30:101–103. doi: 10.1136/jmg.30.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu S, Yu D, Lee E, Eckhaus M, Lee R, Corria Z, Accili D, Westphal H, Weinstein LS. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein α-subunit (Gsα) knockout mice is due to tissue-specific imprinting of the Gsα gene. Proc. Natl. Acad. Sci USA. 1998;95:8715–8720. doi: 10.1073/pnas.95.15.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Germain-Lee EL, Ding CL, Deng Z, Crane JL, Saji M, Ringel MD, Levine MA. Paternal imprinting of Galpha(s) in the human thyroid as the basis of TSH resistance in pseudohypoparathyroidism type 1a. Biochem Biophys Res Commun. 2002;296:67–72. doi: 10.1016/s0006-291x(02)00833-1. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani G, Ballare E, Giammona E, Beck-Peccoz P, Spada A. The Gsalpha Gene: Predominant Maternal Origin of Transcription in Human Thyroid Gland and Gonads. J Clin Endocrinol Metab. 2002;87:4736–4740. doi: 10.1210/jc.2002-020183. [DOI] [PubMed] [Google Scholar]
- 24.Hayward BE, Barlier A, Korbonits M, Grossman AB, Jacquet P, Enjalbert A, Bonthron DT. Imprinting of the G(s)alpha gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest. 2001;107:R31–6. doi: 10.1172/JCI11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Erlichman B, Weinstein LS. The stimulatory G protein α-subunit Gsα is imprinted in human thyroid glands: implications for thyroid function in pseudohypoparathyroidism types 1A and 1B. J Clin Endocrinol Metabol. 2003;88:4336–41. doi: 10.1210/jc.2003-030393. [DOI] [PubMed] [Google Scholar]
- 26.Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL. Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Galpha(s) in the development of human obesity. J Clin Endocrinol Metab. 2007;92:1073–9. doi: 10.1210/jc.2006-1497. [DOI] [PubMed] [Google Scholar]
- 27.Mouallem M, Shaharabany M, Weintrob N, Shalitin S, Nagelberg N, Shapira H, Zadik Z, Farfel Z. Cognitive impairment is prevalent in pseudohypoparathyroidism type Ia, but not in pseudopseudohypoparathyroidism: possible cerebral imprinting of Gsalpha. Clin Endocrinol (Oxf) 2008;68:233–9. doi: 10.1111/j.1365-2265.2007.03025.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, Shrestha YB, Podyma B, Cui Z, Naglieri B, Sun H, Ho T, Wilson EA, Li YQ, Gavrilova O, Weinstein LS. Gsalpha deficiency in the dorsomedial hypothalamus underlies obesity associated with Gsalpha mutations. J Clin Invest. 2017;127:500–510. doi: 10.1172/JCI88622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richard N, Molin A, Coudray N, Rault-Guillaume P, Jüppner H, Kottler ML. Paternal GNAS Mutations Lead to Severe Intrauterine Growth Retardation (IUGR) and Provide Evidence for a Role of XLalphas in Fetal Development. J Clin Endocrinol Metab. 2013;98:E1549–56. doi: 10.1210/jc.2013-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebrun M, Richard N, Abeguile G, David A, Coeslier Dieux A, Journel H, Lacombe D, Pinto G, Odent S, Salles JP, Taieb A, Gandon-Laloum S, Kottler ML. Progressive osseous heteroplasia: a model for the imprinting effects of GNAS inactivating mutations in humans. J Clin Endocrinol Metab. 2010;95:3028–38. doi: 10.1210/jc.2009-1451. [DOI] [PubMed] [Google Scholar]
- 31.Plagge A, Gordon E, Dean W, Boiani R, Cinti S, Peters J, Kelsey G. The imprinted signaling protein XLalphas is required for postnatal adaptation to feeding. Nat Genet. 2004;36:818–26. doi: 10.1038/ng1397. [DOI] [PubMed] [Google Scholar]
- 32.Chudoba I, Franke Y, Senger G, Sauerbrei G, Demuth S, Beensen V, Neumann A, Hansmann I, Claussen U. Maternal UPD 20 in a hyperactive child with severe growth retardation. Eur J Hum Genet. 1999;7:533–40. doi: 10.1038/sj.ejhg.5200287. [DOI] [PubMed] [Google Scholar]
- 33.Eggermann T, Mergenthaler S, Eggermann K, Albers A, Linnemann K, Fusch C, Ranke MB, Wollmann HA. Identification of interstitial maternal uniparental disomy (UPD) (14) and complete maternal UPD(20) in a cohort of growth retarded patients. J Med Genet. 2001;38:86–9. doi: 10.1136/jmg.38.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulchandani S, Bhoj EJ, Luo M, Powell-Hamilton N, Jenny K, Gripp KW, Elbracht M, Eggermann T, Turner CL, Temple IK, Mackay DJ, Dubbs H, Stevenson DA, Slattery L, Zackai EH, Spinner NB, Krantz ID, Conlin LK. Maternal uniparental disomy of chromosome 20: a novel imprinting disorder of growth failure. Genet Med. 2016;18:309–15. doi: 10.1038/gim.2015.103. [DOI] [PubMed] [Google Scholar]
- 35.Aldred MA, Aftimos S, Hall C, Waters KS, Thakker RV, Trembath RC, Brueton L. Constitutional deletion of chromosome 20q in two patients affected with albright hereditary osteodystrophy. Am J Med Genet. 2002;113:167–72. doi: 10.1002/ajmg.10751. [DOI] [PubMed] [Google Scholar]
- 36.Genevieve D, Sanlaville D, Faivre L, Kottler ML, Jambou M, Gosset P, Boustani-Samara D, Pinto G, Ozilou C, Abeguile G, Munnich A, Romana S, Raoul O, Cormier-Daire V, Vekemans M. Paternal deletion of the GNAS imprinted locus (including Gnasxl) in two girls presenting with severe pre- and post-natal growth retardation and intractable feeding difficulties. Eur J Hum Genet. 2005;13:1033–9. doi: 10.1038/sj.ejhg.5201448. [DOI] [PubMed] [Google Scholar]
- 37.Piesowicz AT. Pseudo-Pseudo-Hypoparathyroidism with Osteoma Cutis. Proc R Soc Med. 1965;58:126–8. [PMC free article] [PubMed] [Google Scholar]
- 38.Barranco VP. Cutaneous ossification in pseudohypoparathyroidism. Arch Dermatol. 1971;104:643–7. [PubMed] [Google Scholar]
- 39.Eyre WG, Reed WB. Albright’s hereditary osteodystrophy with cutaneous bone formation. Arch Dermatol. 1971;104:634–42. [PubMed] [Google Scholar]
- 40.Brook CG, Valman HB. Osteoma cutis and Albright’s hereditary osteodystrophy. Br J Dermatol. 1971;85:471–5. doi: 10.1111/j.1365-2133.1971.tb14056.x. [DOI] [PubMed] [Google Scholar]
- 41.Trueb RM, Panizzon RG, Burg G. Cutaneous ossification in Albright’s hereditary osteodystrophy. Dermatology. 1993;186:205–9. doi: 10.1159/000247347. [DOI] [PubMed] [Google Scholar]
- 42.Izraeli S, Metzker A, Horev G, Karmi D, Merlob P, Farfel Z. Albright hereditary osteodystrophy with hypothyroidism, normocalcemia, and normal Gs protein activity: a family presenting with congenital osteoma cutis. Am J Med Genet. 1992;43:764–7. doi: 10.1002/ajmg.1320430424. [DOI] [PubMed] [Google Scholar]
- 43.Prendiville JS, Lucky AW, Mallory SB, Mughal Z, Mimouni F, Langman CB. Osteoma cutis as a presenting sign of pseudohypoparathyroidism. Pediatr Dermatol. 1992;9:11–8. doi: 10.1111/j.1525-1470.1992.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 44.Goeteyn V, De Potter CR, Naeyaert JM. Osteoma cutis in pseudohypoparathyroidism. Dermatology. 1999;198:209–11. doi: 10.1159/000018115. [DOI] [PubMed] [Google Scholar]
- 45.Schimmel RJ, Pasmans SG, Xu M, Stadhouders-Keet SA, Shore EM, Kaplan FS, Wulffraat NM. GNAS-associated disorders of cutaneous ossification: two different clinical presentations. Bone. 2010;46:868–72. doi: 10.1016/j.bone.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward S, Sugo E, Verge CF, Wargon O. Three cases of osteoma cutis occurring in infancy. A brief overview of osteoma cutis and its association with pseudo-pseudohypoparathyroidism. Australas J Dermatol. 2011;52:127–31. doi: 10.1111/j.1440-0960.2010.00722.x. [DOI] [PubMed] [Google Scholar]
- 47.Martin J, Tucker M, Browning JC. Infantile osteoma cutis as a presentation of a GNAS mutation. Pediatr Dermatol. 2012;29:483–4. doi: 10.1111/j.1525-1470.2011.01469.x. [DOI] [PubMed] [Google Scholar]
- 48.Falsey RR, Ackerman L. Eruptive, hard cutaneous nodules in a 61-year-old woman. Osteoma cutis in a patient with Albright hereditary osteodystrophy (AHO) JAMA Dermatol. 2013;149:975–6. doi: 10.1001/jamadermatol.2013.149a. [DOI] [PubMed] [Google Scholar]
- 49.Hon KL, Chow CM, Choi PC, Wong GW. A useful skin biopsy. Int J Dermatol. 2014;53:238–40. doi: 10.1111/j.1365-4632.2012.05487.x. [DOI] [PubMed] [Google Scholar]
- 50.Lietman SA, Ding C, Cooke DW, Levine MA. Reduction in Gsalpha induces osteogenic differentiation in human mesenchymal stem cells. Clin Orthop Relat Res. 2005:231–8. doi: 10.1097/01.blo.0000153279.90512.38. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, Ding S. A high-throughput siRNA library screen identifies osteogenic suppressors in human mesenchymal stem cells. Proc Natl Acad Sci U S A. 2007;104:9673–8. doi: 10.1073/pnas.0703407104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertaux K, Broux O, Chauveau C, Hardouin P, Jeanfils J, Devedjian JC. Runx2 regulates the expression of GNAS on SaOs-2 cells. Bone. 2006;38:943–50. doi: 10.1016/j.bone.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Wu JY, Aarnisalo P, Bastepe M, Sinha P, Fulzele K, Selig MK, Chen M, Poulton IJ, Purton LE, Sims NA, Weinstein LS, Kronenberg HM. Gsalpha enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 2011;121:3492–504. doi: 10.1172/JCI46406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinstein LS. G(s)alpha mutations in fibrous dysplasia and McCune-Albright syndrome. J Bone Miner Res. 2006;21(Suppl 2):P120–4. doi: 10.1359/jbmr.06s223. [DOI] [PubMed] [Google Scholar]
- 55.Huso DL, Edie S, Levine MA, Schwindinger W, Wang Y, Jüppner H, Germain-Lee EL. Heterotopic ossifications in a mouse model of albright hereditary osteodystrophy. PLoS One. 2011;6:e21755. doi: 10.1371/journal.pone.0021755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheeseman MT, Vowell K, Hough TA, Jones L, Pathak P, Tyrer HE, Kelly M, Cox R, Warren MV, Peters J. A mouse model for osseous heteroplasia. PLoS One. 2012;7:e51835. doi: 10.1371/journal.pone.0051835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pignolo RJ, Xu M, Russell E, Richardson A, Kaplan J, Billings PC, Kaplan FS, Shore EM. Heterozygous inactivation of Gnas in adipose-derived mesenchymal progenitor cells enhances osteoblast differentiation and promotes heterotopic ossification. J Bone Miner Res. 2011;26:2647–55. doi: 10.1002/jbmr.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu JJ, Russell E, Zhang D, Kaplan FS, Pignolo RJ, Shore EM. Paternally inherited gsalpha mutation impairs adipogenesis and potentiates a lean phenotype in vivo. Stem Cells. 2012;30:1477–85. doi: 10.1002/stem.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaplan FS, Craver R, MacEwen GD, Gannon FH, Finkel G, Hahn G, Tabas J, Gardner RJ, Zasloff MA. Progressive osseous heteroplasia: a distinct developmental disorder of heterotopic ossification. Two new case reports and follow-up of three previously reported cases. J Bone Joint Surg Am. 1994;76:425–36. [PubMed] [Google Scholar]
- 60.Kaplan FS, Shore EM. Progressive osseous heteroplasia. J Bone Miner Res. 2000;15:2084–94. doi: 10.1359/jbmr.2000.15.11.2084. [DOI] [PubMed] [Google Scholar]
- 61.Pignolo RJ, Ramaswamy G, Fong JT, Shore EM, Kaplan FS. Progressive osseous heteroplasia: diagnosis, treatment, and prognosis. Appl Clin Genet. 2015;8:37–48. doi: 10.2147/TACG.S51064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eddy MC, De Beur SM, Yandow SM, McAlister WH, Shore EM, Kaplan FS, Whyte MP, Levine MA. Deficiency of the alpha-subunit of the stimulatory G protein and severe extraskeletal ossification. J Bone Miner Res. 2000;15:2074–83. doi: 10.1359/jbmr.2000.15.11.2074. [DOI] [PubMed] [Google Scholar]
- 63.Yeh GL, Mathur S, Wivel A, Li M, Gannon FH, Ulied A, Audi L, Olmstead EA, Kaplan FS, Shore EM. GNAS1 mutation and Cbfa1 misexpression in a child with severe congenital platelike osteoma cutis. J Bone Miner Res. 2000;15:2063–73. doi: 10.1359/jbmr.2000.15.11.2063. [DOI] [PubMed] [Google Scholar]
- 64.Shore EM, Ahn J, Jan de Beur S, Li M, Xu M, Gardner RJ, Zasloff MA, Whyte MP, Levine MA, Kaplan FS. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346:99–106. doi: 10.1056/NEJMoa011262. [DOI] [PubMed] [Google Scholar]
- 65.Buyse G, Silberstein J, Goemans N, Casaer P. Fibrodysplasia ossificans progressiva: still turning into wood after 300 years? Eur J Pediatr. 1995;154:694–9. doi: 10.1007/BF02276711. [DOI] [PubMed] [Google Scholar]
- 66.Shore EM, Kaplan FS. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP) Bone. 2008;43:427–33. doi: 10.1016/j.bone.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schrander DE, Welting TJ, Caron MM, Schrander JJ, van Rhijn LW, Korver-Keularts I, Schrander-Stumpel CT. Endochondral ossification in a case of progressive osseous heteroplasia in a young female child. J Pediatr Orthop B. 2014;23:477–84. doi: 10.1097/BPB.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 68.Adegbite NS, Xu M, Kaplan FS, Shore EM, Pignolo RJ. Diagnostic and mutational spectrum of progressive osseous heteroplasia (POH) and other forms of GNAS-based heterotopic ossification. Am J Med Genet A. 2008;146A:1788–96. doi: 10.1002/ajmg.a.32346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goto M, Mabe H, Nishimura G, Katsumata N. Progressive osseous heteroplasia caused by a novel nonsense mutation in the GNAS1 gene. J Pediatr Endocrinol Metab. 2010;23:303–9. doi: 10.1515/jpem.2010.23.3.303. [DOI] [PubMed] [Google Scholar]
- 70.Kehlenbach RH, Matthey J, Huttner WB. XLαs is a new type of G protein (Erratum in Nature 1995 375:253) Nature. 1994;372:804–809. doi: 10.1038/372804a0. [DOI] [PubMed] [Google Scholar]
- 71.Klemke M, Pasolli H, Kehlenbach R, Offermanns S, Schultz G, Huttner W. Characterization of the extra-large G protein alpha-subunit XLalphas. II. Signal transduction properties. J Biol Chem. 2000;275:33633–40. doi: 10.1074/jbc.M006594200. [DOI] [PubMed] [Google Scholar]
- 72.Bastepe M, Gunes Y, Perez-Villamil B, Hunzelman J, Weinstein LS, Jüppner H. Receptor-Mediated Adenylyl Cyclase Activation Through XLalphas, the Extra-Large Variant of the Stimulatory G Protein alpha-Subunit. Mol Endocrinol. 2002;16:1912–9. doi: 10.1210/me.2002-0054. [DOI] [PubMed] [Google Scholar]
- 73.Liu Z, Segawa H, Aydin C, Reyes M, Erben RG, Weinstein LS, Chen M, Marshansky V, Frohlich LF, Bastepe M. Transgenic Overexpression of the Extra-Large Gs{alpha} Variant XL{alpha}s Enhances Gs{alpha}-Mediated Responses in the Mouse Renal Proximal Tubule in Vivo. Endocrinology. 2011;152:1222–33. doi: 10.1210/en.2010-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasolli H, Klemke M, Kehlenbach R, Wang Y, Huttner W. Characterization of the extra-large G protein alpha-subunit XLalphas. I. Tissue distribution and subcellular localization. J Biol Chem. 2000;275:33622–32. doi: 10.1074/jbc.M001335200. [DOI] [PubMed] [Google Scholar]
- 75.Krechowec SO, Burton KL, Newlaczyl AU, Nunn N, Vlatkovic N, Plagge A. Postnatal Changes in the Expression Pattern of the Imprinted Signalling Protein XLalphas Underlie the Changing Phenotype of Deficient Mice. PLoS One. 2012;7:e29753. doi: 10.1371/journal.pone.0029753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Michienzi S, Cherman N, Holmbeck K, Funari A, Collins MT, Bianco P, Robey PG, Riminucci M. GNAS transcripts in skeletal progenitors: evidence for random asymmetric allelic expression of Gs{alpha} Hum Mol Genet. 2007;16:1921–30. doi: 10.1093/hmg/ddm139. [DOI] [PubMed] [Google Scholar]
- 77.Mariot V, Wu JY, Aydin C, Mantovani G, Mahon MJ, Linglart A, Bastepe M. Potent constitutive cyclic AMP-generating activity of XLalphas implicates this imprinted GNAS product in the pathogenesis of McCune-Albright Syndrome and fibrous dysplasia of bone. Bone. 2011;48:312–20. doi: 10.1016/j.bone.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He Q, Zhu Y, Corbin BA, Plagge A, Bastepe M. The G protein alpha subunit variant XLalphas promotes inositol 1,4,5-trisphosphate signaling and mediates the renal actions of parathyroid hormone in vivo. Sci Signal. 2015;8:ra84. doi: 10.1126/scisignal.aaa9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahmed SF, Barr DG, Bonthron DT. GNAS1 mutations and progressive osseous heteroplasia. N Engl J Med. 2002;346:1669–71. doi: 10.1056/NEJM200205233462115. [DOI] [PubMed] [Google Scholar]
- 80.Gelfand IM, Hub RS, Shore EM, Kaplan FS, Dimeglio LA. Progressive osseous heteroplasia-like heterotopic ossification in a male infant with pseudohypoparathyroidism type Ia: a case report. Bone. 2007;40:1425–8. doi: 10.1016/j.bone.2006.12.058. [DOI] [PubMed] [Google Scholar]
- 81.Cairns DM, Pignolo RJ, Uchimura T, Brennan TA, Lindborg CM, Xu M, Kaplan FS, Shore EM, Zeng L. Somitic disruption of GNAS in chick embryos mimics progressive osseous heteroplasia. J Clin Invest. 2013;123:3624–33. doi: 10.1172/JCI69746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Regard JB, Malhotra D, Gvozdenovic-Jeremic J, Josey M, Chen M, Weinstein LS, Lu J, Shore EM, Kaplan FS, Yang Y. Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med. 2013;19:1505–12. doi: 10.1038/nm.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Happle R. Progressive osseous heteroplasia is not a Mendelian trait but a type 2 segmental manifestation of GNAS inactivation disorders: A hypothesis. Eur J Med Genet. 2016;59:290–4. doi: 10.1016/j.ejmg.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Castrop H, Oppermann M, Mizel D, Huang Y, Faulhaber-Walter R, Weiss Y, Weinstein LS, Chen M, Germain S, Lu H, Ragland D, Schimel DM, Schnermann J. Skeletal abnormalities and extra-skeletal ossification in mice with restricted Gsalpha deletion caused by a renin promoter-Cre transgene. Cell Tissue Res. 2007;330:487–501. doi: 10.1007/s00441-007-0491-6. [DOI] [PubMed] [Google Scholar]
- 85.Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. 2008;90(Suppl 1):19–24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]