Abstract
Brain injury in premature infants, especially periventricular leukomalacia, is an important cause of neurologic disabilities. Inflammation contributes to perinatal brain injury development, but the essential mediators that lead to early-life brain injury remain largely unknown. Neonates have reduced capacity for mounting conventional αβT-cell responses. However, γδT cells are already functionally competent during early development and are important in early-life immunity. We investigated the potential contribution of γδT cells to preterm brain injury using postmortem brains from human preterm infants with periventricular leukomalacia and two animal models of preterm brain injury—the hypoxic-ischemic mouse model and a fetal sheep asphyxia model. Large numbers of γδT cells were observed in the brains of mice, sheep, and postmortem preterm infants after injury, and depletion of γδT cells provided protection in the mouse model. The common γδT-cell–associated cytokines interferon-γ and IL-17A were not detectable in the brain. Although there were increased mRNA levels of Il17f and Il22 in the mouse brains after injury, neither IL-17F nor IL-22 cytokines contributed to preterm brain injury. These findings highlight unique features of injury in the developing brain, where, unlike injury in the mature brain, γδT cells function as initiators of injury independently of common γδT-cell–associated cytokines. This finding will help to identify therapeutic targets for preventing or treating preterm infants with brain injury.
Periventricular leukomalacia (PVL) is a distinctive form of brain injury in premature infants that affects cerebral white and gray matter and is characterized by the arrest of oligodendrocyte development at the preoligodendrocyte stage.1, 2 PVL is one of the leading causes of neurologic disabilities, such as cerebral palsy. Inflammation and hypoxia-ischemia (HI) secondary to prenatal infection and/or the developmental immaturity of the cerebral vasculature at the middle to late gestational age have been proposed to be critical in preterm brain injury.3, 4, 5 However, the specific immune cell types and associated mediators involved in preterm brain injury remain poorly defined.
Accumulating evidence suggests that lymphocytes, especially T cells, play important roles in ischemic brain injury, such as adult stroke. Most T cells are αβT cells, but there is a small population of T cells called γδT cells. Compared with αβT cells, γδT cells are the first T-lymphocyte subset to arise during ontogeny,6, 7, 8 and they are the first population of T cells to display functional responsiveness. Thus, γδT cells can contribute to immune regulation and immune responses early in life by compensating for the relative immaturity of αβT cells in the neonatal period.9, 10 γδT cells recognize qualitatively distinct antigens compared with αβT cells, and they are not restricted to antigen processing and antigen presentation on major histocompatibility complex molecules by professional antigen-presenting cells, such as αβT cells.11, 12, 13 γδT cells can also recognize stress-induced cell-surface markers, and γδT cells not only respond to infection and sterile-induced inflammation but also sense the lipid ligands that are often enriched on central nervous system (CNS) injury.14 Indeed, γδT cells are found in demyelinating lesions in the CNS of patients with multiple sclerosis15 and are detected in the mouse brain during disease onset in the mouse model of multiple sclerosis.16 These γδT cells are characterized as the IL-17–producing subtype, and they further promote IL-17 production by the ensuing CD4+ αβT cells to exacerbate the CNS pathologic features.16 γδT cells also aggravate ischemic brain injury and stroke in adult mice via IL-17A production and the recruitment of neutrophils.17, 18 Importantly, γδT cells rapidly produce IL-17 in response to the activation of innate receptors and cytokine stimulation even without triggering the T-cell receptor (TCR).19 The cytokines IL-17A and IL-17F are proinflammatory cytokines, and the CD4+ Th17-secreting IL-17–mediated inflammatory response appears to play critical roles in a rodent model of inflammation-sensitized perinatal brain injury.20 The IL-10 family cytokine IL-22 has also been implicated in CNS injury, such as multiple sclerosis,21, 22 and it represents an important effector molecule of activated Th22, Th1, and Th17 cells as well as γδT cells.
The above evidence clearly shows that the γδT-cell/IL-17/IL-22 axis represents an important signaling pathway in adult brain injury. However, its role in newborn brain injury remains largely unknown. We took advantage of both experimental preclinical models and human subjects to investigate the potential contribution of the γδT-cell/IL-17/IL-22 cascade to the development of preterm brain injury.
Materials and Methods
Mouse Model of Hypoxia-Ischemia Preterm Brain Injury
All animal experiments were approved by the Gothenburg Ethical Committee on Animal Research. TCRδ chain–targeted mutant mice (Tcrd−/−, B6.129P2-Tcrdtm1Mom/J), Rag1 knockout mice (Rag1−/−, B6.129S7-Rag1tm1Mom/J) that lack mature T cells (ie, αβT cells or γδT cells) and B cells, and C57BL/6-Trdctm1Mal/J EGFP reporter mice were purchased from Jackson Laboratories (Bar Harbor, ME), and IL-22 knockout mice (Il22−/−, B6;129S5-Il22tm1.1Lex/Mmucd) were purchased from the Mouse Biology Program, University of California, Davis. All mice were bred in the animal facility at the University of Gothenburg.
At postnatal day (PND) 4 (the day of birth was defined as PND0), different genotypes (wild type, Tcrd−/−, and Il22−/−) of mice of both sexes were subjected to HI insult to induce preterm brain injury according to a method described previously.23
For brain injury evaluation, wild-type, Tcrd−/−, and Il22−/− mice were used. There are no commercially available IL-17F–deficient mice, but intracerebroventricular injection of antibodies is an efficient way of neutralizing cytokines after brain injury.24 For the IL-17 depletion experiment, a total of 1 μg (in 2 μL of phosphate-buffered saline) of anti–IL-17 antibody (0.5 μg/μL in phosphate-buffered saline, monoclonal rat IgG2A, clone 50104, MAB421, R&D Systems, Minneapolis, MN) or 1 μg (in 2 μL) of monoclonal rat IgG2a isotype control antibody for IL-17 (IL-17 isotypes, 0.5 μg/μL in phosphate-buffered saline, monoclonal rat IgG2A, clone 54447, MAB006, R&D Systems) was injected into the left lateral ventricle immediately before HI in PND4 mice by randomization grouping. At 24, 48, and 72 hours after HI, pups were further intraperitoneally injected with the anti–IL-17 antibody or IL-17 isotype controls (0.5 μg/μL in phosphate-buffered saline).
Fetal Sheep Model of Asphyxia-Induced Preterm Brain Injury
These studies were approved by the Gothenburg Ethical Committee on Animal Research. Time-mated pregnant sheep were anesthetized, and the fetal sheep underwent aseptic surgery at 95 days' gestation as previously reported.25 Briefly, catheters were implanted into the fetal brachial artery and vein, and a silastic umbilical cord cuff was placed around the umbilical cord. In case of twins, only one fetus was instrumented, and the second uncatheterized fetus served as a control. To induce fetal asphyxia, the cord cuff was inflated and the umbilical cord was transiently occluded for 25 minutes at 99 to 100 days gestation (full term was approximately 147 days). At postmortem, coronal brain sections (10 μm) at the level of the lateral ventricle were collected and stained with thionin/acid fuchsin staining or were stained for γδT cells using the mouse anti-bovine WC1 primary antibody (catalog number MCA838G; AbD Serotec, Täby, Sweden) or antibodies against glial fibrillary acidic protein (mouse monoclonal IgG; catalog number G3893; Sigma-Aldrich, Stockholm, Sweden) and Iba-1 (rabbit polyclonal IgG; catalog number 019-19741; Wako, Stockholm, Sweden).
Human Preterm Infant Postmortem Brain Analysis
A written informed parental consent form was obtained according to the Declaration of Helsinki and the guidelines of the National Health Service UK. Ethics approval was obtained from the National Research Ethics Service, West London, United Kingdom. Six postmortem brains of preterm neonates (<36 weeks' gestational age) with and without PVL were obtained from the Perinatal Pathology Department, Imperial Health Care Trust, London, United Kingdom, and used in this study. The primary cause of death of each case was assessed by a pathologist. The details of each case are summarized in Supplemental Table S1.
The processing of the human postmortem brains and the immunohistochemical (IHC) staining were performed as described previously.26, 27 The primary antibodies were anti-human TCR (catalog number 331202, clone MG1-45; BioLegend, San Diego, CA), anti-TCRVδ1 (catalog number TCR1730; Thermo Fisher Scientific, Rockford, IL), and anti-TCRVδ2 (catalog number TCR1732; Thermo Fisher Scientific). Staining using the isotype control antibody for TCRγδ (Mouse IgG1 Isotype Control; catalog number 02-6100; Thermo Fisher Scientific) served as the negative control, and no specific staining was identified in this preparation. Human tonsil tissue was used as the positive control, and strong TCRγδ-positive staining was observed. The sections were examined by bright-field microscopy using a light microscope (DM6000 B; Leica Microsystems Ltd., Bucks, United Kingdom). Immunopositive cells for TCRVδ2 were counted per high-power field at ×400 magnification (0.0426 mm2). A mean of seven counts was made per region of interest. The high-power fields were selected on the basis of highest density of immunopositive cells identified within each region of interest.
IHC Staining of Mouse Brain Sections and Assessment of Mouse Brain Damage
At 7 days after HI, the brains were dissected, paraffin embedded, and cut into 10-μm coronal sections throughout the whole brain, and staining was performed on every 50th section. The following primary antibodies were used: mouse anti–MAP-2 (clone HM-2; Sigma-Aldrich), mouse anti–maltose-binding protein (MBP) (SMI94; Covance, Princeton, NJ), anti-TCRγδ (catalog number ab112222; Abcam, Cambridge, England), and anti-TCRγ (catalog number sc-1778; Santa Cruz Biotechnology, Dallas, TX).
The extent of white matter and gray matter injury was analyzed by quantitative measurements of microtubule-associated protein (MAP) 2–positive and MBP-positive areas in both hemispheres using a widely accepted method as previously described28, 29 with Micro Image version 4.0 (Micro-Macro AB, Gothenburg, Sweden). The investigators (A.-M.A. and X.Z.) measuring were blinded to treatment group. The tissue loss was calculated as follows: [(contralateral hemisphere − ipsilateral hemisphere)/contralateral hemisphere × 100%].
Coronal sections at the hippocampal level were used for all other staining. Thionin/acid fuchsin staining was performed as described previously.30
Quantitative RT-PCR Analysis
The mRNA expression of cytokines related to the IL-17/IL-22 pathway in the brain at different time points after HI in PND4 wild-type, Rag1−/−, and Tcrd−/− mice was analyzed by quantitative RT-PCR (RT-qPCR). A brief intracardiac perfusion was conducted before analysis of the brain to ensure that there were no blood contaminants in the brain. cDNA was prepared from 1 μg of RNA in a 20-μL reaction using the QuantiTect Reverse Transcription Kit (Qiagen, Sollentuna, Sweden). Each PCR reaction (20 μL) contained 2 μL of cDNA diluted 10 times, 10 μL of Quanti Fast SYBR Green PCR Master Mix (Qiagen), and 2 μL of PCR primer (QuantiTech Primer Assay; Qiagen) and was run on a LightCycler 480 (Roche, Bromma, Sweden). Primers are listed in Tables 1 and 2. Melting curve analysis was performed to ensure that only one PCR product was obtained. For quantification and estimation of amplification efficiency, a standard curve was generated using increasing concentrations of cDNA. The amplified transcripts were quantified with the relative standard curve and normalized against the reference gene Rna18s1 or Gapdh.
Table 1.
Primers Used for Quantitative RT-PCR
| Gene | Catalog number | Gene | Catalog number |
|---|---|---|---|
| Traf3ip2 | QT00107422 | Il22ra1 | QT00151277 |
| Ahr | QT00174251 | Il23r | QT00138719 |
| Maf | QT01063846 | Irf4 | QT00109984 |
| Hcst | QT00323162 | Jak1 | QT00158438 |
| Tyrobp | QT00129514 | Gapdh | QT01658692 |
| Ifng | QT00145250 | Rna18s1 | QT01036875 |
| Il1b | QT01048355 | Runx1 | QT00100380 |
| Il1r1 | QT00095256 | Stat1 | QT00162183 |
| Il2rg | QT00106337 | Stat3 | QT00148750 |
| Il10ra | QT00112742 | Sox13 | QT00199570 |
| Il17a | QT00103278 | Map3k7 | QT00150402 |
| Il17f | QT00144347 | Trg | QT00307692 |
| Il17ra | QT00112063 | Traf6 | QT00166663 |
| Il17rc | QT01076733 | Tyk2 | QT00114667 |
| Il22 | QT00128324 |
All primers were from Qiagen.
Table 2.
Custom-Made Primers for Quantitative RT-PCR
| Gene | Forward | Reverse |
|---|---|---|
| Trgv1 | 5′-CACTGGTACCGGCAAAAAGC-3′ | 5′-TCCCTCCTAAGGGTCGTTGA-3′ |
| Trgv2 | 5′-TCGGTCACCAGAGAGACAGAT-3′ | 5′-TGCCGGTACCAATGTATAGCTG-3′ |
| Trgv4 | 5′-AGAGCAAGAGATGAGACTGCAC-3′ | 5′-GCCGGTACCAGTGTATGGTT-3′ |
| Trgv5 | 5′-CCACTCCCGCTTGGAAATTG-3′ | 5′-GGCACAGTAGTACGTGGCTT-3′ |
| Trgv6 | 5′-TATGAGGCAAGGACATGGCAG-3′ | 5′-AGTTCCCGTGTCCTCTTCTGT-3′ |
| Trgv7 | 5′-AAGGCCCGGACAAGAGGTAT-3′ | 5′-GCCCAGGAGGCACAGTAGTA-3′ |
All primers were from Qiagen.
Statistical Analysis
The sample size required for detection of significant differences with 80% power and a significance level at α = 0.05 was determined in pilot studies using wild-type animals, and this was estimated to be a minimum of 15 animals for injury evaluation in the mouse brain. SPSS software version 19.0 (SPSS Inc., Chicago, IL) was used for all analyses. Comparisons between groups were performed with the t-test, and data with unequal variance were compared with the Mann-Whitney U test. Analysis of variance followed by the least significant difference post-hoc test was used for comparison of data from more than two groups; P < 0.05 was considered statistically significant.
Results
γδT Cells Are Found in the Postmortem Brains of Preterm Infants with Brain Injury
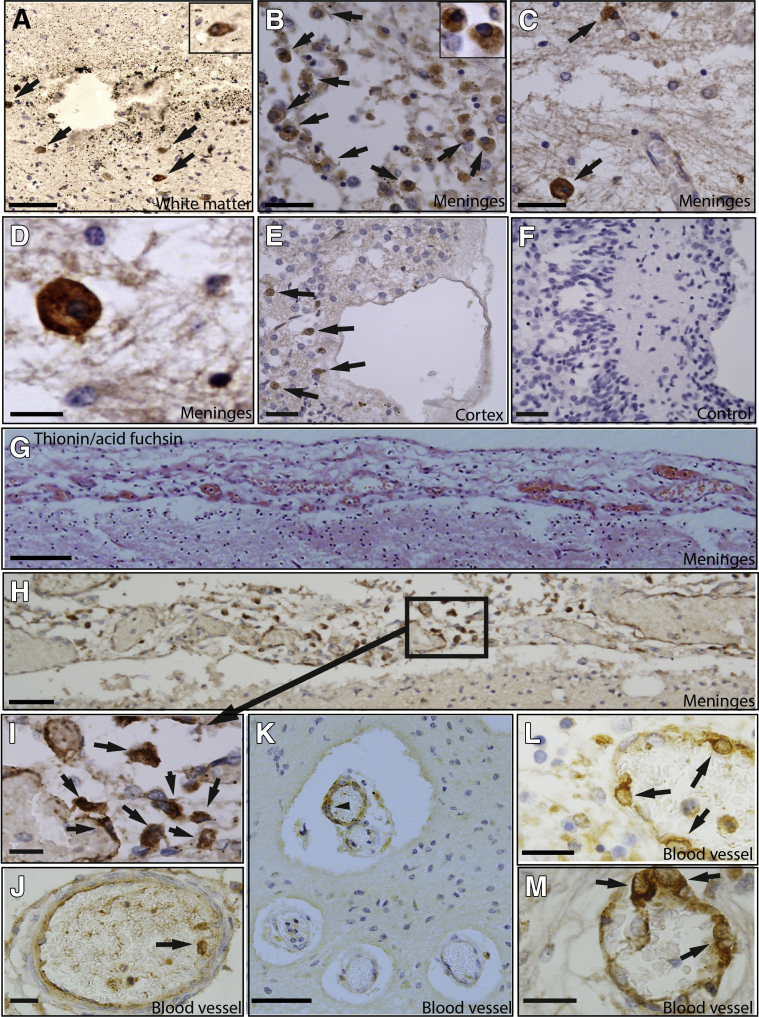
The potential presence of γδT cells in the brains of preterm human infants with brain injury was first examined, and IHC staining was performed on postmortem brain sections from preterm infants with and without PVL (Supplemental Table S1). Vδ1+ and Vδ2+ γδT cells are the major subsets in preterm and term newborn infants,10 and IHC staining showed that γδT cells were detected in the border zone of the periventricular white matter injury areas in infants with PVL and that these were Vδ2+ but not Vδ1+ cells (Figure 1, A–F). Such cells were either few or completely absent in the brains of preterm infants without evidence of brain injury (Supplemental Table S1). The mean ± SEM number of γδT cells in the PVL cases was 13.5 ± 5.5 cells/mm2, and such cells were absent in the control cases. The greatest number of γδT cells was found in the brain meninges of the PVL cases, both outside and inside the inner layer of the blood vessels (Figure 1, G–L). These meninges had evidence of cellular proliferation and were much thicker than the normally thin layer of the meninges membrane (Figure 1G). The γδT cells were found to have three distinct morphologic structures: round and large with a rich cytosol (Figure 1, A–E), irregular and slightly elongated morphologic structure (Figure 1, H and I), or round and large with a large nucleus and less rich cytosol (Figure 1, J–M). The observation of large numbers of γδT cells in the postmortem brains of preterm infants with PVL suggests a possible correlation of γδT cells with preterm brain injury.
Figure 1.
Vδ2+ γδT cells in the postmortem brains of preterm infants with brain injury. Paraffin-embedded cerebral hemispheres and frontal lobe sections at the level of Ammon's horn, including the lateral ventricle, were used for immunohistochemical (IHC) analysis. A–E and H–M: IHC staining of Vδ2+ γδT cells (arrows) in the injured brains in the periventricular white matter region adjacent to the injured area (A), the meninges tissue (B–D), and the tissue close to the cortex (E) of postmortem neonatal cases. Insets in A and B show higher magnification of Vδ2+ γδT cells. D is a higher magnification image of C. A–E are from case 1. F: Negative control stained with an isotype control antibody against TCRγδ. G: Thionin/acid fuchsin staining of the brain meninges area. H–M: γδT-positive staining in the meninges area of the postmortem brain outside the blood vessels, in the inner side of the blood vessels in the meninges (J), and in the cells of the blood vessel wall (K–M). I is a higher magnification image of the boxed area in H. G–L are from case 2. Arrowhead in K indicates IHC-positive staining of Vδ2 γδT cells. Scale bars: 50 μm (A, B, E, and F); 20 μm (C, I, J, L, and M); 10 µm (D); 100 μm (G, H, and K). Original magnification, ×40 (A and B, insets).
γδT Cells Are Detected in the Brains of Neonatal Mice and Sheep with Preterm Brain Injury
To explore the contribution of γδT cells to perinatal brain injury, a well-established preclinical mouse model of preterm brain injury was used.23 This HI brain injury model is generated using neonatal mice at PND4 because PND2 through PND5 is a developmental age when preoligodendrocytes, the most vulnerable cell types to preterm brain injury and subsequent neurodevelopmental impairments, are abundant in the rodent CNS,31 whereas at PND7 immature oligodendrocytes predominate in the rodent, which overlaps with the period between 30 weeks and term birth (36 to 40 weeks) when human immature oligodendrocytes peak in number.
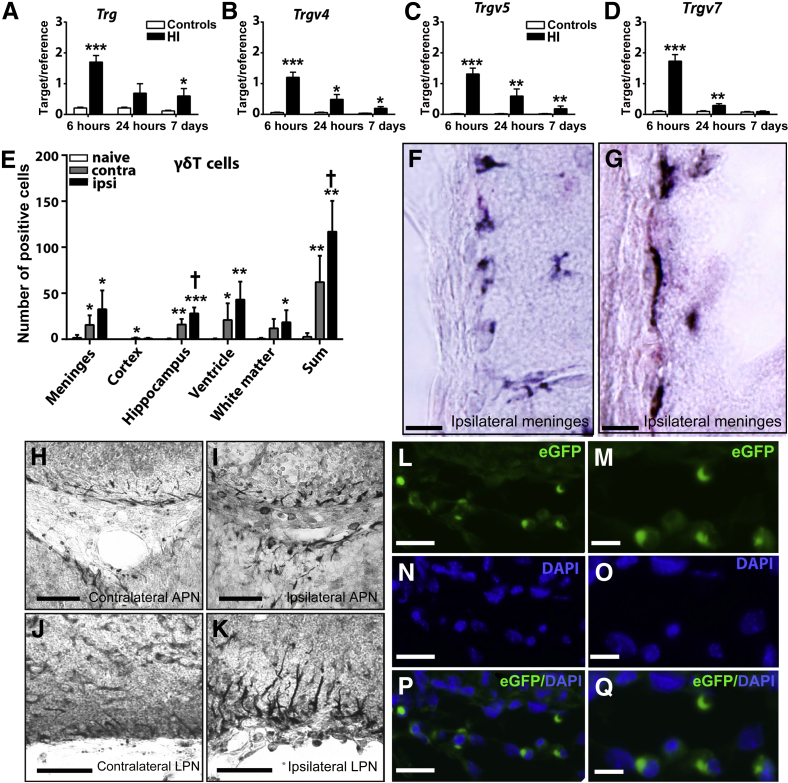
The mRNA level of the Trg gene (encoding TCRγ) was increased as early as 6 hours after HI in the ipsilateral hemisphere of the mouse brain in the wild-type mice (Figure 2A). Moreover, analysis of the γδT-cell subtypes in the neonatal mouse brain after HI-induced injury showed that they were mainly Trg-V4, 5, and 7 (Figure 2, B–D), the same subtypes as γδT cells found in the blood, spleen, lymph node, lung, skin, and gut in mice.32 Expression of the other mouse TCRγδ subtypes Trg-V1, 2, and 6 was undetectable in the newborn mouse brain.
Figure 2.
γδT cells are found in the mouse brain after hypoxia-ischemia (HI)–induced preterm brain injury. A–D: The mRNA expression of Trg (A), Trgv4 (B), Trgv5 (C), and Trgv7 (D) in the hemisphere ipsilateral to the injury compared with the mRNA level in the normal control mice. E–Q: Presence of γδT cells in the mouse brain after HI injury. E: γδT-cell counts from immunohistochemical staining at 6 hours after HI. F and G: Representative immunohistochemical staining of γδT cells at 6 hours after HI in the mouse brain in the meninges of the ventral part of the retrosplenial area (F) and in the meninges of the cortical amygdala area (G) in the ipsilateral hemisphere. H–K: γδT cells in the contralateral (H) and ipsilateral (I) periventricular area by the anterior pretectal nucleus (APN) and lateral posterior nucleus (LPN) of the thalamus and in the contralateral (J) and ipsilateral (K) meninges by the cortical amygdala area (COA). L–Q: In eGFP γδT-cell reporter mice, eGFP+ γδT-cells are shown in the meninges by the cortical amygdala area in the ipsilateral hemisphere at 6 hours after HI. L: Enhanced green fluorescent protein–positive (eGFP+) γδT cells. N: Nuclear staining with DAPI. P: Overlay of eGFP+ γδT cells and DAPI. M, O, and Q: Higher-magnification images of L, N, and P, respectively. Data are expressed as means ± SEM of the ratio of the target gene to the reference gene Rna18s1 (A–D) and means ± SEM γδT-cell counts (E). n = 6 to 8 (A–D, HI mice); n = 5 to 6 (A–D, controls); n = 8 (E, HI mice). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus control mice (n = 8); †P < 0.05 between the contralateral and the ipsilateral hemispheres. Scale bars = 10 μm (F and G); 50 μm (H–L, N, and P); 20 μm (M, O, and Q).
The accumulation of γδT cells in the mouse brain was confirmed at 6 hours after HI using IHC staining. Quantification of γδT-cell numbers (Figure 2E) showed that γδT cells were mostly found in the border zone of the injury area in the hippocampus, meninges, ventricle area, and the subcortical white matter (Figure 2, E–K). Interestingly, γδT cells in the corpus callosum white matter close to the injury area, in both the periventricular area and the meninges, displayed an irregular and elongated morphologic structure with process-like features (Figure 2, F, G, I, and K).
Tcrd-H2BEGFP reporter mice, in which γδT cells are labeled with fluorescent enhanced green fluorescent protein (eGFP),33 were used to corroborate the above observation of γδT cells in HI-injured brains. Consistent with the IHC analysis (Figure 2, E–K), eGFP-positive γδT cells were mostly found in the border zone of the injury areas in the ipsilateral hippocampus and in the meninges of the ipsilateral hemisphere of mouse brains at 6 hours after HI (Figure 2, L–Q).
The sheep model of asphyxia-induced preterm brain injury, a well-established preclinical model for preterm brain injury,25 was used to further confirm the findings of γδT cells in the injured brains. Similar to cats, cows, and chickens, sheep have a much greater percentage of γδT cells in the circulation compared with humans and mice,34 making them an ideal model to investigate the importance of γδT cells in preterm brain injury. In sheep brains with evidence of white matter lesion, gliosis, and microglia activation after asphyxia-induced preterm brain injury (Supplemental Figure S1, A–F), large numbers of γδT cells were detected that were mostly distributed in the meninges (Supplemental Figure S1G), in the inner layer of the blood vessels in the meninges (Supplemental Figure S1, H and J), and in the choroid plexus epithelial layer (Supplemental Figure S1, I and K). However, few γδT cells were observed in the periventricular white matter injured area. The morphologic structure of γδT cells in the sheep brains differed, depending on their location. In the blood vessels in the meninges (Supplemental Figure S1G), γδT cells were smooth and elongated along the inner layer of the blood vessel, whereas γδT cells in the meninges were elongated and somewhat irregular (Supplemental Figure S1H). In the choroid plexus epithelial layer, γδT cells had an irregular morphologic structure, and some of the cells also showed process-like features (Supplemental Figure S1I). Taking all the results together, γδT cells were substantially increased in the injured brains of experimental preclinical models.
Regulation of IL-17/IL-22 Signaling Pathways in HI–Induced Preterm Brain Injury
To explore the contribution of the IL-17/IL-22 signaling pathways to perinatal brain injury, the expression of transcription factors, cytokines and chemokines, and other molecules that have been implicated in the regulation of the IL-17/IL-22 signaling pathways was analyzed.35
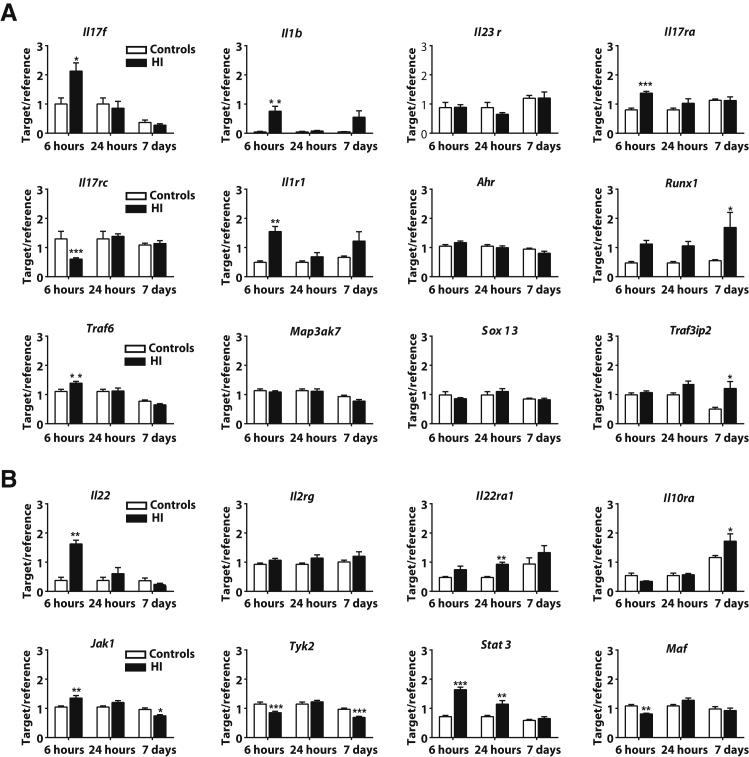
Some, but not all, of the IL-17/IL-22 pathway–related genes35 encoding cytokines (Il1b and Il2rg), receptors (Il1r1, Il23r, Il17ra, Il17rc, Il22ra1, and Il10ra), signaling adaptors (Map3k7, Tyk2, Jak1, Traf6, and Traf3ip2), and transcription factors (Stat3, Sox13, Maf, Runx1, and Ahr) were regulated at either early (6 hours) or late (24 hours or 7 days) time points after HI (Figure 3). The expression of the cytokine-encoding genes, Il17a and Ifng, was not detected in the mouse brain at any of the time points examined despite the observation that both cytokine genes were highly expressed in the spleen and thymus. The mRNA level of Il17f was up-regulated in the injured ipsilateral brain hemisphere at 6 hours, but not at 24 hours or 7 days, after HI (Figure 3A), and the same pattern was observed for the mRNA of the Il22 gene (Figure 3B).
Figure 3.
The activation of the IL-17/IL-22 signaling pathways in a hypoxia-ischemia (HI)–induced preterm brain injury mouse model. A and B: The gene expression profile of transcription factors, receptors, signaling adaptors, and cytokines for the IL-17 (A) and IL-22 (B) pathways in the mouse brain after HI-induced preterm brain injury. Data are expressed as means ± SEM of the ratio of the target gene to the reference gene Rna18s1. n = 5 to 6 for uninjured controls; n = 6 to 8 for HI groups. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Depletion of γδT Cells, but Not IL-17A/F or IL-22, Protect Mice from Preterm Brain Injury
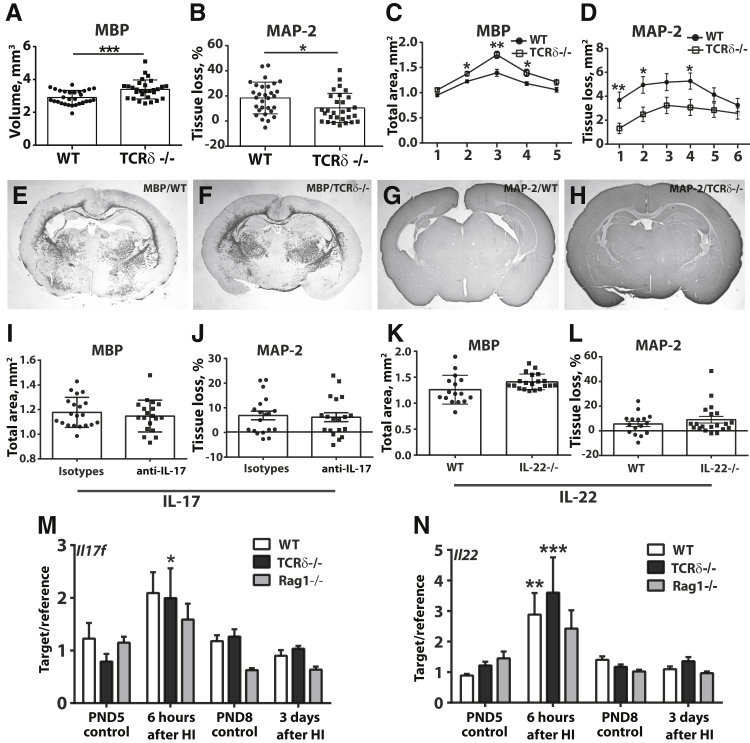
Finally, to understand the contribution of γδT cells to preterm brain injury, mice deficient in mature γδT cells subjected to HI-induced brain injury at PND4 were analyzed. Compared with wild-type mice, γδT-deficient mice had significantly reduced brain injury after HI (Figure 4, A–H). The neuroprotective effect was found in both white matter (decreased white matter volume in wild-type mice compared with γδT-deficient mice after HI, P = 0.0005) (Figure 4, A, C, E, and F) and gray matter (reduced tissue loss in γδT-deficient mice compared with wild-type mice after HI, P = 0.0154) (Figure 4, B, D, G, and H), with the most noticeable difference observed in the middle part [white matter, levels 2 (P = 0.01), 3 (P = 0.001), and 4 (P = 0.001)] (Figure 4C) and frontal-middle part of the mouse brain [gray matter, levels 1 (P = 0.005), 2 (P = 0.01), and 4 (P = 0.024)] (Figure 4D). In addition, the degree of HI-induced brain injury in males was similar to females (data not shown), even though sex effects on neonatal HI brain injury have been frequently reported.36
Figure 4.
Depletion of γδT cells but not IL-17A/F or IL-22 protects the mouse brain from hypoxia-ischemia (HI)–induced preterm brain injury. A–H: γδT cell depletion as seen in the subcortical white matter volume by measuring the maltose-binding protein (MBP) immunohistochemical (IHC)–positive staining area (A, C, E, and F) and in the total tissue loss using microtubule associated protein (MAP)-2 IHC staining (B, D, G, and H) in Tcrd−/− and wild-type (WT) mice at 7 days after HI. A: Total volume of the subcortical white matter. B: Total brain tissue loss in the WT versus Tcrd−/− mouse brain. C and D: The total subcortical white matter area (C) and total brain tissue loss at the different brain levels (D). The x axis of C and B indicates the brain levels analyzed, where level 1 refers to the frontal part of the mouse brain and level 6 refers to the posterior part of the mouse brain. E–H: Representative images of the MBP (E and F) and MAP-2 (G and H) staining from the WT (E and G) and Tcrd−/− (F and H) mouse brains at 7 days after HI. I–L: Brain injury after blocking IL-17A/F or in Il22−/− mice at 7 days after HI. M and N: The Il17f and Il22 mRNA levels in WT, Tcrd−/−, and Rag1−/− mouse brains at 6 hours and 3 days after HI and in age-matched naïve control mice at postnatal day 5 (PND5) and postnatal day 8 (PND8), respectively. Data are expressed as means ± SEM (A–D, I–L) and as means ± SEM of the ratio of the target gene to the reference gene Gapdh (M and N). n = 29 Tcrd−/− and 29 WT mice at 7 days after HI (A–H); n = 20 IL-17, 19 anti–IL-17, 15 WT, and 20 Il22−/− mice (I–L); n = 7 to 10 control mice and 8 to 11 HI mice (M and N). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. TCR, T-cell receptor.
The contribution of γδT cells to preterm brain injury appeared to be independent of the cytokines IL-17 and IL-22 because antibody-mediated depletion of IL-17 or gene deficiency of Il22 did not confer any beneficial effect in either the white matter or gray matter of the mouse brains subjected to HI (Figure 4, I–L). Moreover, analysis of the mRNA levels of Il17f (Il17a was not detectable in the brain) and Il22 in the mouse brains of wild-type, Tcrd−/−, and Rag1−/− mice (which are devoid of any mature T or B lymphocytes) showed that the increase in Il17f and Il22 mRNA expression in the wild-type mouse brains compared with the Tcrd−/− and Rag1−/− mice was similar (Figure 4, M and N), indicating that neither γδT cells nor αβT cells are the major sources of IL-17F and IL-22 in the neonatal mouse brain after HI. These findings suggest that γδT cells are critical cellular subsets that promote preterm brain injury, and they appear to be functionally different from γδT cells in adults with CNS injury because they do not act through IL-17 or IL-22 as is seen in the adult mouse brain with ischemic injury.17
Discussion
This study provides the first evidence that γδT cells contribute to preterm brain injury and that similar subtypes of γδT cells (Vγ4, Vγ5, and Vγ7) as in the mouse skin (Vγ5), intestinal mucosa (Vγ7), lung mucosa (Vγ4), and blood (Vγ4)32, 37 are present in the neonatal mouse brain after HI-induced injury. However, γδT cells in the neonatal mice with brain injury functioned independently of the IL-17/IL-22 signaling pathways, which is markedly different from the mechanism of action of Vγ4+ γδT cells in the other barrier tissues38, 39 and in adult CNS injury.17 We also provide the first evidence of the existence of large numbers of γδT cells in the sheep brain in a preclinical model of preterm brain injury and in the brain of human postmortem preterm infants with brain injury.
In the mouse and sheep brain injury models, γδT cells were observed in multiple locations of the injured brain. γδT cells in the injured mouse brain were enriched in the white matter of the corpus callosum, the meninges of the ipsilateral hemisphere, the choroid plexus in the third ventricle, and the lateral ventricle areas around the blood vessels (Supplemental Figure S2), whereas most γδT cells in the sheep brain were detected in the meninges, the choroid plexus, and the blood vessels within the meninges but not in the injured white matter. In the human preterm infant brain, most γδT cells were detected in the meninges. The differential distribution pattern possibly reflects their different morphologic structures and subtypes associated with distinct functions.
Recent studies have indicated that the meninges are one of the sites involved in the inflammatory response after CNS injury. For example, meningeal inflammation and immune cell infiltration appear to play a prominent role in the development of cerebral cortical gray matter pathologic features and an accelerated clinical course of multiple sclerosis.40 Notably, recent evidence also argues that γδT cells are a normal component of the meninges in C57bl/6 mice.41 Furthermore, the rapid increases in γδT-cell mRNA levels and γδT-cell–positive IHC stains in the mouse brain at 6 hours after HI suggest that γδT cells in the neonatal mouse brain are innate subsets and perhaps some of the γδT cells are already resident in the brain or near the brain. However, the possibility of trafficking of peripheral γδT cells into the brain in response to injury cannot be completely ruled out. Indeed, recent analysis of γδT cells in preterm and term infant cord blood has shown that Vδ2 γδT cells are mostly present in preterm infants and that Vδ1 γδT cells become the major cell population in term infants.10 The discovery of the Vδ2 γδT cells in the human postmortem brain suggests that some of the Vδ2 γδT cells in the brain might arise from the peripheral blood.
Activation of the IL-17 and IL-22 signaling pathways in γδT cells is the major contributor of γδT-mediated pathologic features in the intestinal mucosa and the skin,38, 39 as well as in adult CNS injury models.17, 18 The activation of IL-17 has also been implicated in lipopolysaccharide-sensitized perinatal brain injury using PND7 rats.20 However, the results presented here clearly show that IL-17A was not detectable in the brain of mice at this early age (ie, within 1 week after birth). Furthermore, although Il17f and Il22 expression was increased at the mRNA level in the mouse brain soon after HI, they were not produced by the γδT cells or the CD4 cells because the levels of Il17f and Il22 mRNA were similar in the brains of wild-type mice as they were in the Tcrd−/− and Rag1−/− mice (Figure 4, M and N). In addition, they are unlikely to contribute to preterm brain injury under the current experimental conditions because depletion of IL-17 or IL-22 did not confer any neuroprotection in the mouse model of preterm brain injury. Consistent with our findings (Figure 3B), the study by Yang et al,20 which reports that IL-17 is important in lipopolysaccharide-sensitized perinatal brain injury, found that increased IL-17 is only observed in the brain after dual insults (lipopolysaccharide/HI) but not in the HI-alone group.20 Therefore, any role for the IL-17/IL-22 signaling pathways in CNS injury is likely to be context and age dependent. Interestingly, a report from the Seventh International γδT-Cell Conference42 showed that γδT cells are the major source of IL-17 in the meninges in the C57Bl/6 mouse but that these cells do not produce IL-17 until 1 week of age, at which time meningeal γδT cells differentiate into IL-17 producers. This finding strongly supports our finding that γδT cells exist in the brain at an early age (PND4) and provide fast responses to injury, but unlike their counterparts in the peripheral organs (eg, mucosa, skin, peripheral circulation),43, 44 they do not produce IL-17 at this early age, and the IL-17 pathway is not the major contributor to preterm brain injury in the mouse model used in these experiments.
The Vγ4, Vγ5, and Vγ7 subtypes of γδT cells are able to produce the proinflammatory cytokine interferon-γ19; however, this cytokine does not appear to be important in this circumstance because interferon-γ is not detectable in the brain of the PND4 mouse model of preterm brain injury.23 Nevertheless, our analysis of γδT-deficient mice clearly supports the essential role of this immune subset in mediating the HI-induced pathologic findings in the developing brain at an early age, which might cooperate with the later-emerging αβT cells to exacerbate the CNS pathologic findings and induce consequent behavior alterations, as reported for other γδT-cell subsets implicated in autoimmunity.16 This view warrants further investigation of the molecular mechanisms underlying γδT-mediated CNS pathologic findings.
Our findings have a bearing on efforts to seek targets for therapeutic interventions of preterm brain injury. Targeting γδT cells has shown promising results in the immunotherapy of other inflammatory diseases and cancer, and our observations of a role for γδT cells in promoting preterm brain injury provide new insights into the pathogenesis of preterm brain injury and its associated sequelae, such as cerebral palsy, and suggest new therapeutic avenues to ameliorate these neurologic disorders in childhood. Most importantly, the findings shed light on the fundamentally different pathogenesis of brain injury in preterm infants compared with that in term infants and adults, which has important implications for the choice of therapeutic targets to ameliorate the neurologic disabilities in this vulnerable population.
Acknowledgments
We thank the patients, their families, and the referring physicians for their contributions to our continuing work on these disorders; Anna-Lena Leverin for technical assistance with RT-qPCR on the mouse brain; Pernilla Svedin for assistance in processing and IHC staining of the sheep brain; Josephine Wyatt-Ashmead for assessing the primary cause of death in each case for the postmortem preterm infant brain cases; Matthew Hogg for help with the illustrations; and David Vermijlen, Immo Prinz, and Jacqueline Lai for critical discussions of the data.
Footnotes
Supported by Swedish Research Council grants VR 2013-2475 and VR 2015-06276 (X.W.) and VR 2015-02493 (H.H.), Swedish governmental grants ALFGBG-429801 (X.W.) and ALF-GBG:426401 (H.H.) to researchers in the public health service, VINNMER–Marie Curie international qualification grant 2011–03458 (X.W.), Gothenburg Medical Society grant 011/14 (X.W.), the Frimurare Barnhus Foundation (A.N.), Wellcome Trust grant WT094823 (H.H.), the Wilhelm & Martina Lundgren Foundation (A.-M.A., X.Z., A.N., and X.W.), Chinese Scholarship Council grant 201407040032 (X.Z.), National Natural Science Foundation of China grants 81771418 (X.W.) and U1704281 (C.Z.), Department of Science and Technology of Henan Province grant 134200510023 (C.Z.), and Science and Technology Bureau of Zhengzhou grant 131PCXTD621 (C.Z.).
Disclosures: None declared.
A guest editor acted as the Editor-in-Chief for this manuscript. No one at University of Alabama at Birmingham was involved in the peer review process or final disposition of this article.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ajpath.2017.11.012.
Contributor Information
Jianmei W. Leavenworth, Email: jleavenworth@uabmc.edu.
Xiaoyang Wang, Email: xiaoyang.wang@fysiologi.gu.se.
Supplemental Data
γδT cells are found in the meninges of fetal sheep brains with asphyxia-induced preterm brain injury. A–K: Thionin/acid fuchsin staining (A and D) and immunohistochemical staining (B, C, E–K) show small lesions and diffuse injury in periventricular white matter area with inflammatory cell infiltration (asterisk in A, thionin/acid fuchsin staining), astrocytes [asterisk in B, glial fibrillary acidic protein (GFAP) staining], and microglia [asterisk in C, ionized calcium-binding adapter molecule (Iba)-1 staining] activation at 14 days after the asphyxia induction. D–F: Higher-magnification images of boxed areas in A–C, respectively. G and H: γδT-positive cells in the meninges (G) and the choroid plexus (H). I–K: Higher magnification images of γδT-positive cells (arrows) in the meninges (I), the blood vessel wall of the meninges (J), and the choroid plexus epithelial layer (K) in the sheep brain after the injury. Representative γδT-positive cells are indicated by arrows. n = 4 (asphyxia); n = 3 (control). Scale bars: 200 μm (A–C); 40 μm (D–F); 50 μm (G and H); 20 μm (I–K).
An illustration of the region of γδT-cell response within the neonatal mouse brain after hypoxia-ischemia (HI). The γδT cells can infiltrate from the ventricle choroid plexus (CP) and cerebrospinal fluid in the injured corpus callosum (CC) white matter, from the ventricle choroid plexus and cerebrospinal fluid in the injured brain parenchyma (bilateral), and from the ipsilateral meninges in the injured brain parenchyma. BV, blood vessel; HP, hippocampus; WMI, white matter injury.
References
- 1.Salmaso N., Jablonska B., Scafidi J., Vaccarino F.M., Gallo V. Neurobiology of premature brain injury. Nat Neurosci. 2014;17:341–346. doi: 10.1038/nn.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Back S.A., Rosenberg P.A. Pathophysiology of glia in perinatal white matter injury. Glia. 2014;62:1790–1815. doi: 10.1002/glia.22658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dammann O., Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y.W., Colford J.M., Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 5.Hagberg H., Mallard C., Ferriero D.M., Vannucci S.J., Levison S.W., Vexler Z.S., Gressens P. The role of inflammation in perinatal brain injury. Nat Rev Neurol. 2015;11:192–208. doi: 10.1038/nrneurol.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havran W.L., Allison J.P. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 7.De Rosa S.C., Andrus J.P., Perfetto S.P., Mantovani J.J., Herzenberg L.A., Herzenberg L.A., Roederer M. Ontogeny of gamma delta T cells in humans. J Immunol. 2004;172:1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 8.Born W.K., Yin Z., Hahn Y.S., Sun D., O'Brien R.L. Analysis of gamma delta T cell functions in the mouse. J Immunol. 2010;184:4055–4061. doi: 10.4049/jimmunol.0903679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbons D.L., Haque S.F., Silberzahn T., Hamilton K., Langford C., Ellis P., Carr R., Hayday A.C. Neonates harbour highly active gammadelta T cells with selective impairments in preterm infants. Eur J Immunol. 2009;39:1794–1806. doi: 10.1002/eji.200939222. [DOI] [PubMed] [Google Scholar]
- 10.Dimova T., Brouwer M., Gosselin F., Tassignon J., Leo O., Donner C., Marchant A., Vermijlen D. Effector Vgamma9Vdelta2 T cells dominate the human fetal gammadelta T-cell repertoire. Proc Natl Acad Sci U S A. 2015;112:E556–E565. doi: 10.1073/pnas.1412058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibata K., Yamada H., Nakamura R., Sun X., Itsumi M., Yoshikai Y. Identification of CD25+ gamma delta T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 12.Vantourout P., Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyan S., Kabelitz D. Defining the nature of human gammadelta T cells: a biographical sketch of the highly empathetic. Cell Mol Immunol. 2013;10:21–29. doi: 10.1038/cmi.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godfrey D.I., Uldrich A.P., McCluskey J., Rossjohn J., Moody D.B. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 15.Wucherpfennig K.W., Newcombe J., Li H., Keddy C., Cuzner M.L., Hafler D.A. Gamma delta T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci U S A. 1992;89:4588–4592. doi: 10.1073/pnas.89.10.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton C.E., Lalor S.J., Sweeney C.M., Brereton C.F., Lavelle E.C., Mills K.H. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Shichita T., Sugiyama Y., Ooboshi H., Sugimori H., Nakagawa R., Takada I., Iwaki T., Okada Y., Iida M., Cua D.J., Iwakura Y., Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 18.Gelderblom M., Weymar A., Bernreuther C., Velden J., Arunachalam P., Steinbach K., Orthey E., Arumugam T.V., Leypoldt F., Simova O., Thom V., Friese M.A., Prinz I., Holscher C., Glatzel M., Korn T., Gerloff C., Tolosa E., Magnus T. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood. 2012;120:3793–3802. doi: 10.1182/blood-2012-02-412726. [DOI] [PubMed] [Google Scholar]
- 19.Martin B., Hirota K., Cua D.J., Stockinger B., Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Yang D., Sun Y.Y., Bhaumik S.K., Li Y., Baumann J.M., Lin X., Zhang Y., Lin S.H., Dunn R.S., Liu C.Y., Shie F.S., Lee Y.H., Wills-Karp M., Chougnet C.A., Kallapur S.G., Lewkowich I.P., Lindquist D.M., Murali-Krishna K., Kuan C.Y. Blocking lymphocyte trafficking with FTY720 prevents inflammation-sensitized hypoxic-ischemic brain injury in newborns. J Neurosci. 2014;34:16467–16481. doi: 10.1523/JNEUROSCI.2582-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kebir H., Kreymborg K., Ifergan I., Dodelet-Devillers A., Cayrol R., Bernard M., Giuliani F., Arbour N., Becher B., Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arvin A.M., Oliver S., Reichelt M., Moffat J.F., Sommer M., Zerboni L., Berarducci B. Analysis of the functions of glycoproteins E and I and their promoters during VZV replication in vitro and in skin and T-cell xenografts in the SCID mouse model of VZV pathogenesis. Curr Top Microbiol Immunol. 2010;342:129–146. doi: 10.1007/82_2009_1. [DOI] [PubMed] [Google Scholar]
- 23.Albertsson A.M., Bi D., Duan L., Zhang X., Leavenworth J.W., Qiao L., Zhu C., Cardell S., Cantor H., Hagberg H., Mallard C., Wang X. The immune response after hypoxia-ischemia in a mouse model of preterm brain injury. J Neuroinflammation. 2014;11:153. doi: 10.1186/s12974-014-0153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liesz A., Suri-Payer E., Veltkamp C., Doerr H., Sommer C., Rivest S., Giese T., Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 25.Welin A.K., Svedin P., Lapatto R., Sultan B., Hagberg H., Gressens P., Kjellmer I., Mallard C. Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr Res. 2007;61:153–158. doi: 10.1203/01.pdr.0000252546.20451.1a. [DOI] [PubMed] [Google Scholar]
- 26.Vontell R., Supramaniam V., Thornton C., Wyatt-Ashmead J., Mallard C., Gressens P., Rutherford M., Hagberg H. Toll-like receptor 3 expression in glia and neurons alters in response to white matter injury in preterm infants. Dev Neurosci. 2013;35:130–139. doi: 10.1159/000346158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Supramaniam V., Vontell R., Srinivasan L., Wyatt-Ashmead J., Hagberg H., Rutherford M. Microglia activation in the extremely preterm human brain. Pediatr Res. 2013;73:301–309. doi: 10.1038/pr.2012.186. [DOI] [PubMed] [Google Scholar]
- 28.Wang X., Stridh L., Li W., Dean J., Elmgren A., Gan L., Eriksson K., Hagberg H., Mallard C. Lipopolysaccharide sensitizes neonatal hypoxic-ischemic brain injury in a MyD88-dependent manner. J Immunol. 2009;183:7471–7477. doi: 10.4049/jimmunol.0900762. [DOI] [PubMed] [Google Scholar]
- 29.Wang X., Hagberg H., Zhu C., Jacobsson B., Mallard C. Effects of intrauterine inflammation on the developing mouse brain. Brain Res. 2007;1144:180–185. doi: 10.1016/j.brainres.2007.01.083. [DOI] [PubMed] [Google Scholar]
- 30.Du X., Fleiss B., Li H., D'Angelo B., Sun Y., Zhu C., Hagberg H., Levy O., Mallard C., Wang X. Systemic stimulation of TLR2 impairs neonatal mouse brain development. PLoS One. 2011;6:e19583. doi: 10.1371/journal.pone.0019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craig A., Ling Luo N., Beardsley D.J., Wingate-Pearse N., Walker D.W., Hohimer A.R., Back S.A. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol. 2003;181:231–240. doi: 10.1016/s0014-4886(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 32.Carding S.R., Egan P.J. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 33.Prinz I., Sansoni A., Kissenpfennig A., Ardouin L., Malissen M., Malissen B. Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nat Immunol. 2006;7:995–1003. doi: 10.1038/ni1371. [DOI] [PubMed] [Google Scholar]
- 34.Hein W.R., Mackay C.R. Prominence of gamma delta T cells in the ruminant immune system. Immunol Today. 1991;12:30–34. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- 35.Eyerich S., Eyerich K., Cavani A., Schmidt-Weber C. IL-17 and IL-22: siblings, not twins. Trends Immunol. 2010;31:354–361. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Netto C.A., Sanches E., Odorcyk F.K., Duran-Carabali L.E., Weis S.N. Sex-dependent consequences of neonatal brain hypoxia-ischemia in the rat. J Neurosci Res. 2017;95:409–421. doi: 10.1002/jnr.23828. [DOI] [PubMed] [Google Scholar]
- 37.Hayday A.C. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 38.Cai Y., Xue F., Fleming C., Yang J., Ding C., Ma Y., Liu M., Zhang H.G., Zheng J., Xiong N., Yan J. Differential developmental requirement and peripheral regulation for dermal Vgamma4 and Vgamma6T17 cells in health and inflammation. Nat Commun. 2014;5:3986. doi: 10.1038/ncomms4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramirez-Valle F., Gray E.E., Cyster J.G. Inflammation induces dermal Vgamma4+ gammadeltaT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci U S A. 2015;112:8046–8051. doi: 10.1073/pnas.1508990112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serafini B., Rosicarelli B., Magliozzi R., Stigliano E., Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatfield J.K., Brown M.A. Group 3 innate lymphoid cells accumulate and exhibit disease-induced activation in the meninges in EAE. Cell Immunol. 2015;297:69–79. doi: 10.1016/j.cellimm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro M, Coelho J, Temido-Ferreira M, Ferreira D, Lopes L, Silva-Santos B, Ribot J: gammadelta T cells are the major source of IL-17 in the meninges and control brain cognitive functions. 7th International gamma/delta T Cell Conference [abstract B0-5]. London, UK, 2016
- 43.Blink S.E., Caldis M.W., Goings G.E., Harp C.T., Malissen B., Prinz I., Xu D., Miller S.D. gammadelta T cell subsets play opposing roles in regulating experimental autoimmune encephalomyelitis. Cell Immunol. 2014;290:39–51. doi: 10.1016/j.cellimm.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai Y., Shen X., Ding C., Qi C., Li K., Li X., Jala V.R., Zhang H.G., Wang T., Zheng J., Yan J. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
γδT cells are found in the meninges of fetal sheep brains with asphyxia-induced preterm brain injury. A–K: Thionin/acid fuchsin staining (A and D) and immunohistochemical staining (B, C, E–K) show small lesions and diffuse injury in periventricular white matter area with inflammatory cell infiltration (asterisk in A, thionin/acid fuchsin staining), astrocytes [asterisk in B, glial fibrillary acidic protein (GFAP) staining], and microglia [asterisk in C, ionized calcium-binding adapter molecule (Iba)-1 staining] activation at 14 days after the asphyxia induction. D–F: Higher-magnification images of boxed areas in A–C, respectively. G and H: γδT-positive cells in the meninges (G) and the choroid plexus (H). I–K: Higher magnification images of γδT-positive cells (arrows) in the meninges (I), the blood vessel wall of the meninges (J), and the choroid plexus epithelial layer (K) in the sheep brain after the injury. Representative γδT-positive cells are indicated by arrows. n = 4 (asphyxia); n = 3 (control). Scale bars: 200 μm (A–C); 40 μm (D–F); 50 μm (G and H); 20 μm (I–K).
An illustration of the region of γδT-cell response within the neonatal mouse brain after hypoxia-ischemia (HI). The γδT cells can infiltrate from the ventricle choroid plexus (CP) and cerebrospinal fluid in the injured corpus callosum (CC) white matter, from the ventricle choroid plexus and cerebrospinal fluid in the injured brain parenchyma (bilateral), and from the ipsilateral meninges in the injured brain parenchyma. BV, blood vessel; HP, hippocampus; WMI, white matter injury.