Abstract
Background:
Fine-needle aspiration cytology (FNAC) of thyroid is the initial screening test for thyroid nodules. The Bethesda system classifies thyroid FNAC into six categories. Each category is linked to a malignancy risk and has a recommended clinical management. The aim of this study is to analyze the thyroid cytology smears by Bethesda system and to correlate the diagnosis of cytopathology with histopathology, whenever surgery was done.
Materials and Methods:
This study presents our experience with the Bethesda system in 681 thyroid FNAs from 632 patients in the period between January 2013 and December 2016.
Results:
Categories were as follows: 10.1% were Category I (nondiagnostic), 68.8% Category II (benign), 12.4% were Category III (atypia of undetermined significance), 2.9% were Category IV (suspicious for follicular neoplasm), 2.6% were Category V (suspicious for malignancy), and 4.1% were Category VI (malignant). Surgery was done on 126 nodules from 119 patients with an overall rate of malignancy of 27.8% (35/126 nodules).
Conclusion:
The Bethesda System for Reporting Thyroid Cytopathology proved to be an excellent reporting system.
Keywords: Bethesda, fine-needle aspiration cytology, thyroid
BACKGROUND
Thyroid nodules are common and may be found in up to 60% of the population. Fine-needle aspiration cytology (FNAC) of thyroid nodules has higher sensitivity and predictive value for diagnosis than any other single diagnostic method. It is a rapid, cost-effective, and very useful method in classifying thyroid nodules as either benign nodules, reducing unnecessary surgery, or malignant nodules requiring surgery.[1,2,3,4,5,6] The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) was introduced in 2007 to standardize terminology used in reporting thyroid cytology. The Bethesda system used six categories for thyroid cytology reporting, and each category is supplemented by a list of criteria.[2] These categories are nondiagnostic, benign, atypia of undetermined significance (AUS)/follicular lesion of undetermined significance (FLUS), follicular neoplasm/suspicious for follicular neoplasm (SFN), suspicious for malignancy, and malignant.[3] Each diagnostic category is linked to a certain risk of malignancy and recommended clinical management that are summarized in Table 1. This is the first study to be published on the implementation of the TBSRTC in Bahrain and its diagnostic correlation with histopathology diagnosis. We have also compared our experience in using TBSRTC to other countries in the Middle East and worldwide.
Table 1.
The Bethesda System for Reporting Thyroid Cytopathology with implied risk of malignancy and recommended clinical management

MATERIALS AND METHODS
From January 2013, the Department of Pathology in Salmaniya Medical Complex, Ministry of Health in Bahrain, has reported all thyroid FNAs using the Bethesda system and followed the guidelines in the diagnostic manual “The Bethesda System for Reporting Thyroid Cytopathology “ This pathology department receives around 150 cases of thyroid FNAs per year referred from specialist endocrinologists and general surgeons. Most of the FNAs were performed under ultrasound guidance by a consultant radiologist, usually with on-site adequacy assessment. Smears made were both fixed in alcohol and stained by Papanicolaou stain or air dried and stained with Giemsa stain. Rapid assessment of adequacy was done by Diff-Quick staining. All slides were interpreted by one of five qualified cytopathologists.
The cytopathology files were searched for all thyroid FNAs performed between 1 January 2013 and 31 December 2016. Having identified all patients who underwent thyroid FNAs during this period, histopathological and clinical follow-up was obtained from all available resources. These include medical records and the surgical pathology files. We did not review FNA cases again, but we relied on the original interpretation given by the five different cytopathologists that were working in Salmaniya Medical Complex in the period studied. All of our cytopathologists were aware of TBSRTC, and adhered to its criteria and terminologies. In addition, whenever difficult cases were encountered, a second opinion from expert colleagues was sought.
However, whenever malignancy was identified in pathology reports, it was difficult to determine whether the carcinoma was the targeted nodule which had FNA or was just an incidental finding. That is because of the lack of documentation of the radiologic findings and targeted nodules at the time of ultrasound-guided FNAs.
RESULTS
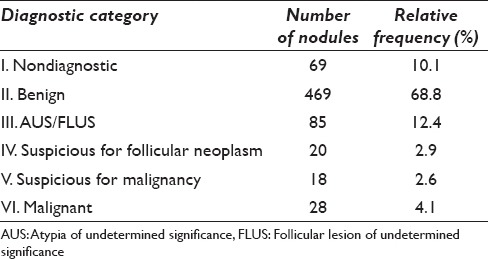
A total of 632 patients underwent 681 FNAs during the study period. The incidence of each Bethesda category is summarized in Table 2. Briefly, 69 (10.1%) were Category I/Bethesda I (nondiagnostic), 469 (68.8%) were Category II/Bethesda II (benign), 85 (12.4%) were Category III/Bethesda III (AUS), 20 (2.9%) were Category IV/Bethesda IV (SFN, 18 (2.6%) were Category V/Bethesda V (suspicious for malignancy), and 28 (4.1%) were Category VI/Bethesda VI (malignant).
Table 2.
Summary of 681 thyroid fine needle aspiration cytologys by The Bethesda System for Reporting Thyroid Cytopathology categories
Of the 681 thyroid nodules from 632 patients which underwent FNAC, surgery was done for 126 (18.5%) nodules for 119 patients, all of whom had histopathology available for review. This comprised 16/69 (23.1%) Category I cases, 60/469 (12.7%) Category II cases, 25/85 (29.4%) Category III cases, 9/20 (45%) Category IV cases, 11/18 (61.1%) Category V cases, and 12/28 (42.8%) Category VI cases. The final histopathological diagnoses of cases in each category are summarized in Table 3.
Table 3.
Histopathology of 126 thyroid fine needle aspiration cytology nodules by The Bethesda System for Reporting Thyroid Cytopathology categories
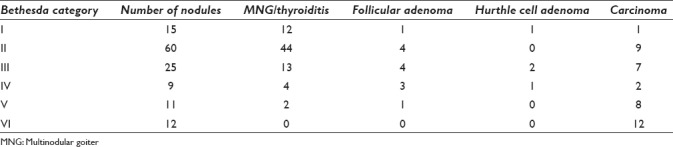
Of the 126 nodules with histopathology follow-up, carcinoma was identified in 35 cases yielding an overall rate of malignancy of 27.8% (35/126 nodules and 34/119 patients). There were 15 Bethesda I nodules (nondiagnosis) with follow-up histopathology. Of these, malignancy was found in only one case, which was a papillary thyroid microcarcinoma (6.67%, 1/15). The rest were all benign thyroid lesions including one Hurthle cell neoplasm and one follicular adenoma.
Of the 60 nodules diagnosed as Bethesda II (benign) on preoperative FNAC, nine nodules found to be malignant, yielding a malignancy rate of 15% (9/60) for those undergoing surgery, which represented around 2% of the total number of Category II nodules.
Of the 85 nodules diagnosed as Bethesda III (AUS/FLUS), 25 were followed up with surgery and malignancy identified in 7 cases (all were papillary thyroid carcinomas) with an estimated risk of malignancy of 28%.
There were 20 Bethesda IV nodules (Follicular neoplasm/SFN), with follow-up histopathology available in 9 cases and malignancy identified in 2 cases only (22% of those undergoing surgery, and 10% of the entire cohort), one of which turn out to be a poorly differentiated carcinoma. Of the remaining cases, there were one Hurthle cell neoplasm, two follicular adenomas, and four adenomatoid nodules in multinodular goiter.
There were 18 Bethesda V nodules (suspicious for malignancy), of which 11 underwent surgery and 8 (72.7%) were confirmed to be carcinoma, all are papillary carcinomas including three cases with follicular variant papillary carcinomas.
There were 28 Bethesda VI nodules (malignant), of which 12 underwent surgery and all (100%) were confirmed to be carcinoma, all papillary carcinomas including one case of follicular variant papillary carcinoma.
DISCUSSION
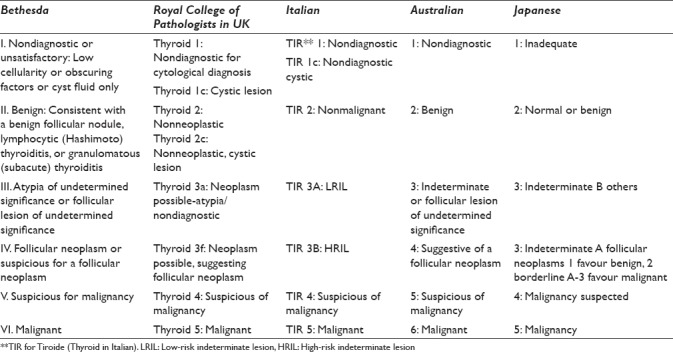
Thyroid cytopathology reporting requires clear communication between pathologists, endocrinologists, radiologists, and surgeons. Hence, consistent and reproducible diagnostic terminology is of utmost importance. Before the adoption of the TBSRTC in 2007, many classification schemes have been suggested by various professional organizations in Australia, Japan, United States (Papanicolaou Society of Cytopathology and American Thyroid Association), United Kingdom (British Thyroid Association-Royal College of Physicians (BTA-RCP), and Italy (Italian Society for Anatomic Pathology and Cytopathology-International Academy of Pathology (SIAPEC-IAP). Most of these classification schemes are 5-tiered in contrast to the 6-tiered scheme in the Bethesda system. Nevertheless, none of these schemes have been implemented internationally.[7,8] The differences in terminologies used in these schemes are summarized in Table 4.
Table 4.
Terminology of thyroid cytology reporting classifications
Based on the available scientific literature, a tiered diagnostic classification system, such as TBSRTC or even the other schemes, proved to be an excellent tool for the diagnosis and management of thyroid lesions. The main advantage of TBSRTC is the standardization of the terminologies used for reporting thyroid cytology. The 6 diagnostic categories of TBSRTC were established from the probability of showing evidence of malignancy if a thyroid lesion placed into a specific category. The advantage of this approach is that each of these diagnostic categories is associated with a sequentially increasing implied risk of malignancy that converts into a recommendation for clinical and surgical management. In contrast, the other systems do not clearly specify a risk of malignancy for each category, although their diagnostic categories are related to a management algorithm.[8,9]
In Bahrain, pathologists were using the currently used system in the United Kingdom, which was first described in 2002 by the British Thyroid Association/Royal College of Physicians, modified and restated in 2007 by the Royal College of Pathologists in association with the British Thyroid Association. The Royal College of Pathologists system uses the Thy1-5 originally suggested categories but with expanded specifications for each category.[7,8] Unfortunately, it was difficult to compile data before 2013 to compare our experience with TBSRTC to the British system.
While it is easy to diagnose most benign and straightforward malignant lesions, diagnostic challenges arise when aspirates are quantitatively or qualitatively inadequate to exclude a neoplastic process. Similar to TBSRTC, all other reporting systems also provide categories for nondiagnostic cytology samples, benign lesions, and malignant lesions. However, they differ in terminologies used in reporting borderline lesions. TBSRTC uses two distinct categories for borderline lesions: “AUS/FLUS” and “follicular neoplasm or SFN”, while the British system uses a single ThyIII category for all the borderline cases but with using “ThyIIIa” for possible neoplasm with atypia and “ThyIIIf” for possible neoplasm suggesting follicular neoplasm.[9,10] The new category of AUS/FLUS in TBSRTC includes a subset of lesions not easily classified as benign, suspicious or malignant, while SFN category is reserved for specimens suspicious of follicular carcinoma. Based on TBSRTC and supported by many studies, AUS/FLUS cases are found to have a lower malignancy risk on surgical follow-up than patients with an initial diagnosis of SFN on cytology, highlighting the importance of such distinction. In addition, they differ in the clinical management, where patients with AUS/FLUS should be followed up with repeated FNA or observation, and patients with SFN should undergo at least thyroid lobectomy to determine the type of the follicular lesion and rule out follicular carcinoma.[7,9]
On the other hand, the Italian system uses TIR 3 for follicular proliferation (Indeterminate low risk) and TIR 4 for suspicious of malignancy (Indeterminate high risk). Bongiovanni et al. have compared between the 6-tiered Bethesda system and the 5-tiered Italian system and concluded that both systems show similar negative predictive values for the benign categories (Category II in TBSRTC and TIR 2) and positive predictive values for both the follicular neoplasm categories (Category IV and TIR 3) and the malignant categories (Category VI and TIR 5). The most significant difference between the 5-tiered and 6-tiered systems was the decrease in percentage of cases classified as benign (83.9% vs. 55.4%), mainly due to the introduction of AUS/FLUS category. It is clear that cases of AUS/FLUS were downgraded in the 5-tiered systems to the benign category resulting in fewer patients referred for surgery compared to the 6-tiered one (9.1% vs. 36.5%).[8] Kiernan et al. reported also an increase in the number of preoperative thyroid FNACs after the adoption of TBSRTC and an increase in patients undergoing thyroid surgery for indeterminate FNAC results. In conclusion, the 6-tiered system (TBSRTC) appears to be associated with more aggressive surgical management approach compared to the other systems.[11,12]
The frequency of diagnosis of each Bethesda category reported in our institution is within the ranges reported in other cohorts, even the high frequency of diagnosis of Bethesda Category III (AUS/FLUS).[13] The higher percentage of this category may reflect our pathologists’ carefulness in avoiding both false positive and false negative results. According to TBSRTC, the incidence of Category III diagnosis should be <7%, while in our study it is around 12%. This diagnostic category in TBSRTC is usually reserved for specimens that meet one of the following criteria: prominent population of microfollicles in an aspirate that does not fulfill the criteria for “follicular neoplasm/SFN,” predominance of Hurthle cells in a sparsely cellular aspirate with scant colloid, interpretation of follicular cell atypia hindered by sample preparation artifacts, a moderately or markedly cellular sample composed of virtually exclusive population of Hurthle cells in the clinical setting suggestive of lymphocytic (Hashimoto) thyroiditis [Figure 1] and multinodular goiter, focal features suggestive of papillary carcinoma, atypical cyst lining cells, minor population of follicular cells showing nuclear enlargement with prominent nucleoli, or atypical lymphoid infiltrate.[12,14] However, AUS/FLUS is a heterogeneous subjective category and will continue to show wide interobserver variability. According to the management guidelines for Bethesda Category III nodules, repeat biopsy after 3 months should be done. In our study, interestingly, repeated FNA had been done in 7 cases only out of 85 cases, while surgical resection was done in 25 cases. The low number of follow-up FNAs and high number of surgical management for AUS/FLUS cases, most likely reflects treatment decisions and preferences by both clinicians and patients in our hospital and community.
Figure 1.
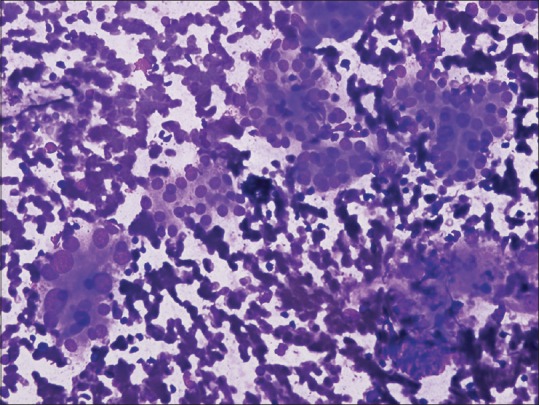
Atypia of undetermined significance (patient with history of Hashimoto's thyroiditis). Oncocytic follicular cells show nuclear enlargement (Smear, May-Grünwald Giemsa stain, ×40)
The Bethesda system assigns a risk of malignancy for each category. In comparison to the TBSRTC, the frequency of malignancy in our study was comparable for Category I (6.7% compared to 1%–4%), slightly higher in both Category II (15% compared to 0%–3%) and Category III (28% compared to 5%–15%), and close to TBSRTC in Category IV (22.2% compared to 15%–30%), Category V (72.8% compared to 60%–75%, and Category VI (100% compared to 97%–99%). The high risk of malignancy (15%) noticed in Category II (benign) which should not exceed 3% needs further evaluation including both radiological and pathological correlation to exclude cases with incidental malignancy. The same fact has been emphasized on by the 2015 American Thyroid Association Management Guidelines, which reported 1%–10% risk of malignancy associated with benign cytological category.[15] Regarding Category III, although the incidence of malignancy is slightly high, it is still within the range reported in the literature which is 6%–50%.[12,13,16,17]
A comparison between the percentage of cases in each Bethesda system category and the risk of malignancy in each category was done between our study and studies from the Middle East and worldwide, and it is summarized in Table 5. The comparison shows variable results in both the distribution of cases and the associated risk of malignancy with each category. Many factors played a role in such variability including the institutions’ experience in using TBSRTC, the cohort size, and the number of cases followed by surgery in addition to other causes such as sampling errors, particularly for cystic lesions and thyroid glands with multiple nodules, or technical reasons, including slide preparation, number of FNA passes, and use of image-guided methods. In addition, there is an overlap of cytological features between benign and malignant nodules such as the nuclear grooves and even the nuclear pseudoinclusions which are not necessarily pathognomonic of papillary thyroid carcinoma.[9,10] However, all studies have shown consistent results in term of percentage of cases suspicious of malignancy and malignant cases, and same risks of malignancy in both categories.
Table 5.
Comparison of percentage of cases in each Bethesda category and risk of malignancy on histopathology specimens between the present study and other studies from the Middle East and other countries
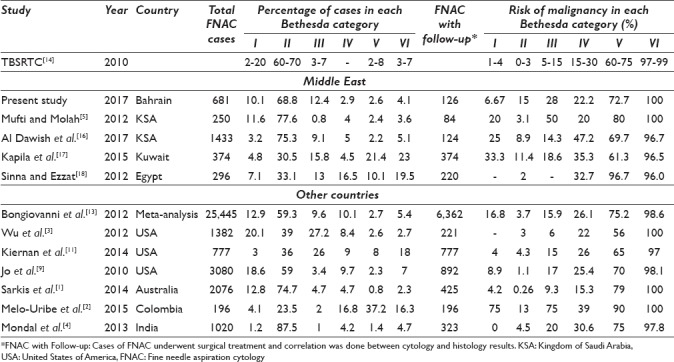
With regard to the distribution of cases, we noticed that studies with large cohorts have shown higher percentage of Category I cases (inadequate/nondiagnostic) compared to small cohorts, but it was within the ranges expected by TBSRTC.[1,3,9,13] Moreover, studies that used the same number of cases with follow-up histopathology to study the distribution of the Bethesda system categories have shown less percentage of Category II cases (Benign) and higher percentage of Category V and VI cases (Suspicious for malignancy and Malignant).[2,11,17,18] That is biased by the fact that most of the cases referred for surgery are the high-risk cases, i. e. Category V and VI. Furthermore, studies from the Middle East, including our study, have shown high percentage of Category III cases (AUS/FLUS), but interestingly it was lower than studies done in the United States.[3,11,17,18,19] That tells us that there is no much of difference between how pathologists in the Middle East use TBSRTC compared to their peers in more developed countries, i.e., the United States. All pathologists can face the same difficulties when applying a new system and they have to get used to it with time.
With regard to the risk of malignancy associated with each Bethesda system category on follow-up, higher risks of malignancy in Category I, II, and III have been noted in studies with low number of follow-up cases including our study.[2,5,16,17] Surprisingly, our study shows the highest risk of malignancy in the benign category compared to the other studies. Some of these studies show even a higher risk of malignancy in Category III compared to Category IV, which is not consistent with TBSRTC proposal of a sequentially increasing implied risk of malignancy.[2,5] That might reflect the difficulty that faces pathologists in classifying lesions into either Category III or IV. It might also be related to the increased number of indeterminate FNACs undergoing surgery.[12]
At the end, our study is still limited by being a retrospective observational study likewise most of the other published studies. That may account for some of the differences when comparing diagnostic category frequencies and malignancy risks. Prospective studies using the Bethesda System will give a better insight into the usefulness of the proposed nomenclature. In addition, clinicians should always be aware of the malignancy rate in the Bethesda categories in their respective hospitals to improve the management decisions taken regarding patients with thyroid nodules.[20]
CONCLUSION
The TBSRTC is a very useful standardized system that enhances the diagnostic accuracy of thyroid FNAC and improves the quality of reporting by decreasing the diagnostic discrepancies, facilitates the diagnostic correlation with the histopathological excisions, and leads to more consistency in management plans.[21,22,23]
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors state that there are no conflicts of interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declared that they qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. Dr. Safa Alshaikh had participated in design of the study, data collection, and drafting the manuscript. Dr. Zainab Harb participated in the design of the study and writing the manuscript, and performed the statistical analysis. Dr. Eman Aljufairi conceived the study and participated in its design and coordination and helped draft the manuscript. Dr. S. Ali Almahari helped in data collection and drafting the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from the Research and Ethics Committee in the Salmaniya Medical Complex in the Ministry of Health in Bahrain.
LIST OF ABBREVIATIONS (In alphabetic Order)
AUS - Atypia of Undetermined Significance
FLUS - Follicular Lesion of Undetermined Significance
FNA - Fine Needle Aspiration
FNAC - Fine Needle Aspiration Cytology
HRIL - High Risk Indeterminate Lesion
KSA - Kingdom of Saudi Arabia
LRIL - Low Risk Indeterminate Lesion
MNG - Multinodular Goitre
SFN - Suspicious for Follicular Neoplasm
TBSRTC - The Bethesda System for Reporting Thyroid Cytopathology
Thy - Thyroid
TIR - Tiroide (Thyroid in Italian)
USA - United States of America.
EDITORIAL/PEER REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Contributor Information
Safa Alshaikh, Email: dr_salshaikh@hotmail.com.
Zainab Harb, Email: zfh040016@gmail.com.
Eman Aljufairi, Email: eman.aljufairi@gmail.com.
S. Ali Almahari, Email: sayed.almahari@gmail.com.
REFERENCES
- 1.Sarkis LM, Norlen O, Aniss A, Watson N, Delbridge LW, Sidhu SB, et al. The Australian experience with the Bethesda classification system for thyroid fine needle aspiration biopsies. Pathology. 2014;46:592–5. doi: 10.1097/PAT.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 2.Melo-Uribe MA, Sanabria Á, Romero-Rojas A, Pérez G, Vargas EJ, Abaúnza MC, et al. The Bethesda system for reporting thyroid cytopathology in Colombia: Correlation with histopathological diagnoses in oncology and non-oncology institutions. J Cytol. 2015;32:12–6. doi: 10.4103/0970-9371.155224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu HH, Rose C, Elsheikh TM. The Bethesda system for reporting thyroid cytopathology: An experience of 1,382 cases in a community practice setting with the implication for risk of neoplasm and risk of malignancy. Diagn Cytopathol. 2012;40:399–403. doi: 10.1002/dc.21754. [DOI] [PubMed] [Google Scholar]
- 4.Mondal SK, Sinha S, Basak B, Roy DN, Sinha SK. The Bethesda system for reporting thyroid fine needle aspirates: A cytologic study with histologic follow-up. J Cytol. 2013;30:94–9. doi: 10.4103/0970-9371.112650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mufti ST, Molah R. The Bethesda system for reporting thyroid cytopathology: A five-year retrospective review of one center experience. Int J Health Sci (Qassim) 2012;6:159–73. doi: 10.12816/0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen GK, Lee MW, Ginsberg J, Wragg T, Bilodeau D. Fine-needle aspiration of the thyroid: An overview. Cytojournal. 2005;2:12. doi: 10.1186/1742-6413-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross P, Chandra A, Giles T, Johnson S, Kocjan G, Poller D, et al. 2nd ed. London: The Royal College of Pathologists; 2016. Guidance on the Reporting of Thyroid Cytology Specimens. [Google Scholar]
- 8.Borderud SP, Li Y, Burkhalter JE, Sheffer CE, Ostroff JS. Electronic cigarette use among patients with cancer: Characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer. 2014;120:3527–35. doi: 10.1002/cncr.28811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jo VY, Stelow EB, Dustin SM, Hanley KZ. Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2010;134:450–6. doi: 10.1309/AJCP5N4MTHPAFXFB. [DOI] [PubMed] [Google Scholar]
- 10.Crippa S, Mazzucchelli L, Cibas ES, Ali SZ. The Bethesda system for reporting thyroid fine-needle aspiration specimens. Am J Clin Pathol. 2010;134:343–4. doi: 10.1309/AJCPXM9WIRQ8JZBJ. [DOI] [PubMed] [Google Scholar]
- 11.Kiernan CM, Broome JT, Solórzano CC. The Bethesda system for reporting thyroid cytopathology: A single-center experience over 5 years. Ann Surg Oncol. 2014;21:3522–7. doi: 10.1245/s10434-014-3743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong LQ, LiVolsi VA, Baloch ZW. Diagnosis of atypia/follicular lesion of undetermined significance: An institutional experience. Cytojournal. 2014;11:23. doi: 10.4103/1742-6413.139725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda system for reporting thyroid cytopathology: A meta-analysis. Acta Cytol. 2012;56:333–9. doi: 10.1159/000339959. [DOI] [PubMed] [Google Scholar]
- 14.Cibas ES, Ali SZ. NCI Thyroid FNA State of the Science Conference. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658–65. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 15.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–33. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Dawish MA, Robert AA, Muna A, Eyad A, Al Ghamdi A, Al Hajeri K, et al. Bethesda system for reporting thyroid cytopathology: A three-year study at a tertiary care referral center in Saudi Arabia. World J Clin Oncol. 2017;8:151–7. doi: 10.5306/wjco.v8.i2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapila K, Qadan L, Ali RH, Jaragh M, George SS, Haji BE, et al. The Bethesda system for reporting thyroid fine-needle aspiration cytology: A Kuwaiti experience – A cytohistopathological study of 374 cases. Acta Cytol. 2015;59:133–8. doi: 10.1159/000371538. [DOI] [PubMed] [Google Scholar]
- 18.Sinna EA, Ezzat N. Diagnostic accuracy of fine needle aspiration cytology in thyroid lesions. J Egypt Natl Canc Inst. 2012;24:63–70. doi: 10.1016/j.jnci.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Archuletta PA, Gidwani R, Husain M, Johnson T, Shidham V, Alzohaili O, et al. The Bethesda system thyroid diagnostic categories in the African-American population in conjunction with surgical pathology follow-up. Cytojournal. 2012;9:7. doi: 10.4103/1742-6413.94274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams BA, Bullock MJ, Trites JR, Taylor SM, Hart RD. Rates of thyroid malignancy by FNA diagnostic category. J Otolaryngol Head Neck Surg. 2013;42:61. doi: 10.1186/1916-0216-42-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theoharis CG, Schofield KM, Hammers L, Udelsman R, Chhieng DC. The Bethesda thyroid fine-needle aspiration classification system: Year 1 at an academic institution. Thyroid. 2009;19:1215–23. doi: 10.1089/thy.2009.0155. [DOI] [PubMed] [Google Scholar]
- 22.Lew JI, Snyder RA, Sanchez YM, Solorzano CC. Fine needle aspiration of the thyroid: Correlation with final histopathology in a surgical series of 797 patients. J Am Coll Surg. 2011;213:188–94. doi: 10.1016/j.jamcollsurg.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Prathima S, Suresh TN, Harendra KM, Bhhaskaran A. Impact of the Bethesda system in reporting thyroid cytopathology. Thyroid Res Pract. 2016;13:9–14. [Google Scholar]


