Introduction
Since licensure in 2006, two rotavirus vaccines have been increasingly used worldwide to prevent rotavirus diarrhea morbidity and mortality in children less than 5 years of age. RotaTeq (Merck and Co) is a three-dose pentavalent bovine-human reassortant rotavirus vaccine and Rotarix (GSK Biologics) is a two-dose monovalent human rotavirus vaccine [1, 2]. Two other three-dose vaccines, ROTAVAC (Bharat Biotech) and ROTASIIL (Serum Institute of India), were licensed recently and are under review for World Health Organization (WHO) prequalification [3, 4].
Rotavirus vaccines are licensed for use as part of the infant immunization schedule concomitantly with diphtheria-tetanus-pertussis vaccine (DTP). Randomized control trials (RCT) for Rotarix and RotaTeq assessed vaccine efficacy beyond the first year of life; in low income countries, waning immunity after 1 year of age was observed in clinical trials [5, 6] while protection was found to persist in the RCTs in high income countries [7]. Some post-licensure, “real world” effectiveness evaluations in several low and middle-income settings also indicate there may be waning protection after the first year of life, though they were not sufficiently powered to detect differential vaccine performance by age group [8–18]. Rotarix VE after 12 months of age was found to be a median of 31% lower in middle income countries, compared to 5% higher in high income countries in a recent systematic literature review [19]. These observations raise concern that waning immunity may leave vaccinated children vulnerable to rotavirus diarrhea morbidity and mortality in the second year of life and beyond. In the absence of vaccine, approximately 30% of rotavirus deaths occur during the second year of life, though this varies substantially from region to region [20]. A booster dose of rotavirus vaccine later in infancy has been proposed as an approach to address waning rotavirus vaccine immunity [21].
Measles-containing vaccines (MCV) are generally recommended by national immunization programs for administration at 9 or 12 months of age. Delivering a booster dose of rotavirus vaccine at the same healthcare visit as MCV could be a logistically feasible way to integrate a booster dose of rotavirus vaccine into the Expanded Programme on Immunization (EPI) and extend protection into the second year of life. A RTC in Bangladesh recently demonstrated non-interference of a rotavirus vaccine dose delivered concomitantly with the injected, live measles-rubella vaccine, a necessary first step in determining feasibility of such a strategy [21]. As a secondary objective, the RTC assessed immunogenicity of the booster rotavirus vaccine dose; all children in the study received 2 infant doses of rotavirus vaccine. Among children in Bangladesh who were sero-negative at 9 months, 44% seroconverted after receiving a third dose of rotavirus vaccine with MCV, compared with 6% of placebo recipients. While seroconversion is not directly correlated with clinical outcomes [22], these results are encouraging.
Before any policy recommendation for a 9- or 12-month dose of RV can be considered, the potential benefits of booster dose will need to be weighed against the additional cost and vaccine supply requirements. Additionally, a clinical efficacy or large-scale immunogenicity trials will need to be conducted, which will be resource intensive. As a critical first step in deciding whether to pursue this strategy, we aimed to evaluate the potential impact of a booster dose of rotavirus vaccine on rotavirus mortality.
Methods
Using UNICEF 2014 <5 child mortality rates, we divided countries into three strata. Low mortality countries were defined as those with rates in the lowest quartile (1.9 to 7 deaths per 1,000 live births), medium mortality countries as those in the second lowest quartile (8 to 17 deaths per 1,000 live births), and high mortality countries as those in the highest two quartiles (18 to 157 deaths per 1,000 live births) [23]. Countries categorized in the medium and high mortality strata are included in this exercise; countries with low child mortality were not, as waning protection of rotavirus vaccine has not been observed in these settings.
Model construction
We calculated national reductions in rotavirus deaths as follows:
where i refers to country and j to the week of life. In this formula, VE refers to vaccine effectiveness and Coverage refers to full series rotavirus vaccine coverage.
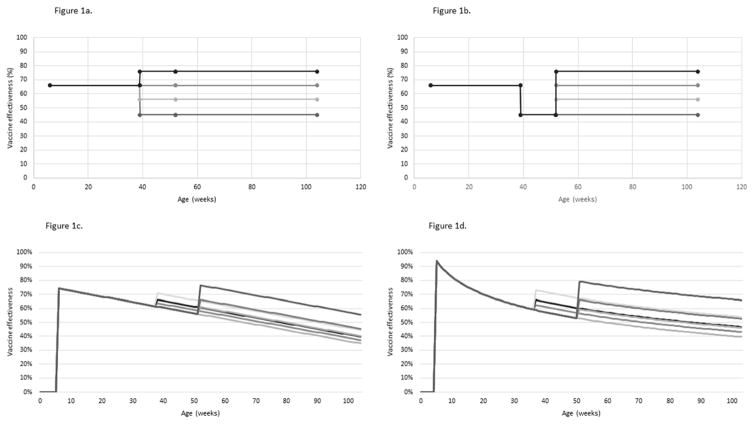
We followed the PRISMA guidelines in a systematic literature review of full-series rotavirus vaccine VE estimates from medium- and high-mortality countries published between 1 January 2006 and 2 December 2016 using the PubMed, MEDLINE, Embase, and Global Health databases. The methods are described in detail elsewhere [19]. Briefly, we included post-licensure, observational evaluations that reported Rotarix VE estimates for children <12 months of age and children ≥12 months of age in low and middle income countries against hospitalization for rotavirus disease. Additionally, we included two pre-licensure evaluations that address waning in low and middle income countries [5, 6, 8–18]. We calculated summary VE estimates with a random effects model (Table 1); this portion of analysis was performed using R v3.2.4. We considered three functional forms for VE waning. In the stepwise model, we assumed full VE is achieved at 6 weeks of age and remains constant until waning at 9 months of age; in this model, VE is constant from 9 to 24 month of age (Figures 1a and 1b). This model reflects the results of the meta-analysis, which summarize VE estimates from children in these broad age categories. We used the same <9 month and ≥9 month point estimates and 95%CIs to generate logarithmic and linear waning patterns by assigning the VE point estimates to 6 months of age and 18 months of age (Figures 1c and 1d). VE estimates by week of age generated by the three waning forms were included as model inputs.
Table 1.
Studies identified through a systematic review that present age stratified rotavirus vaccine efficacy measures from systematic review of studies in low and middle income countries and pooled age stratified estimates.
| Country | First Author | Publication Year | Type of study | Cases | Controls | <12 months | ≥12 months | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| VE | 95% CI | VE | 95% CI | |||||||
| Armenia | Sahakyan | 2016 | Observational | 75 | 826 | 68 | 24, 86 | 60 | 20, 80 | [8] |
| Bolivia | Patel | 2013 | Observational | 400 | 718 | 64 | 34, 80 | 72 | 52, 86 | [9] |
| Bolivia | Pringle | 2016 | Observational | 203 | 298 | 76 | 50, 89 | 45 | 0, 70 | [10] |
| Botswana | Gastañaduy | 2016 | Observational | 242 | 368 | 52 | 8, 75 | 67 | 8, 89 | [11] |
| Brazil | Correia | 2010 | Observational | 70 | 484 | 81 | 47, 93 | 5 | −187, 69 | [12] |
| Brazil | Justino | 2011 | Observational | 170 | 312 | 56 | 12, 78 | 32 | −4, 56 | [13] |
| Colombia | Cotes-Cantillo | 2014 | Observational | 143 | 670 | 84 | 23, 97 | −79 | −558, 51 | [14] |
| Ghana | Armah | 2016 | Observational | 207 | 448 | 78 | 2, 95 | 50 | −57, 84 | [15] |
| Malawi | Cunliff | 2012 | Clinical Trial | 39 | 938 | 49 | 11, 72 | 3 | −101, 53 | [5] |
| Malawi | BarZeev | 2016 | Observational | 241 | 692 | 71 | 34, 87 | 32 | −141, 81 | [16] |
| Moldova | Gheorghita | 2016 | Observational | 95 | 819 | 84 | 67, 92 | 46 | −16, 75 | [17] |
| South Africa | Madhi | 2012 | Clinical Trial | 9 | 750 | 72 | 40, 88 | 3 | −43, 82 | [6] |
| South Africa | Groome | 2014 | Observational | 540 | 1434 | 54 | 32, 68 | 61 | 35, 77 | [18] |
| Pooled estimates* | 66 | 57, 73 | 45 | 29, 57 | ||||||
From random effects meta-analysis
Figure 1.
Figure 1a. Stepwise rotavirus immunity waning model with 9 month recommended age of measles-containing vaccine administration
Figure 1b. Stepwise rotavirus immunity waning model with 12 month recommended age of measles-containing vaccine administration
Figure 1c. Linear rotavirus immunity waning model with 9 and 12 month recommended age of measles-containing vaccine administration
Figure 1d. Logarithmic rotavirus immunity waning model with 9 and 12 month recommended age of measles-containing vaccine administration
Full series vaccination coverage by country was obtained from the WHO/Unicef annual Joint Reporting Form (JRF), which provides a “best estimate” of national vaccine-specific coverage for children <12 months of age using administrative data, surveys, and other national estimates [24]. The JRF reports full series rotavirus vaccine coverage for countries that have introduced rotavirus vaccine in their national immunization programs. However in this exercise, we assumed full series rotavirus vaccine coverage to be equal to DTP coverage for all countries from 6 weeks of age, regardless of rotavirus vaccine introduction status or recommended schedule. As rotavirus vaccine is usually recommended for co-administration with DTP, coverage is expected to be similar for both vaccines. In countries that have introduced rotavirus vaccine, some countries have experienced an initial lag in coverage compared to DTP. We assumed coverage with a booster dose of rotavirus vaccine would be equal to first-dose measles or full-series DTP coverage, whichever was least [25].
The estimated national rotavirus deaths and distribution of deaths by week of age used in this exercise were previously published and were most recently updated in 2013 [20, 26, 27].
Booster Scenarios
We calculated the baseline number of rotavirus deaths for each functional waning form. For countries that had not introduced rotavirus vaccine before 2013 we calculated the baseline number of deaths due to rotavirus, assuming that the primary series was introduced, by subtracting the number of deaths prevented under each of the three waning scenarios from the estimated number of rotavirus deaths in 2013. Among countries that introduced rotavirus vaccine before 2013, we used the number of rotavirus deaths in 2013 as baseline under all three waning scenarios.
Given that the true effect of a booster does is unknown, we simulated three scenarios with each waning model: (a) reduced VE waning in the second year of life by 50%, (b) reestablished second year of life VE to levels from the first year of life, and (c) boosted VE by 50% of the difference between VE in the first and second years of life. In all boosting scenarios, the slope and functional form of waning is assumed to be the same before and after the booster rotavirus vaccine dose. Regardless of vaccine introduction status, the number of deaths prevented under the nine boosting scenarios were calculated the same way for all countries. Summary results are presented globally and by WHO region. Booster doses were assumed to be administered concomitantly with MCV at either 9 or 12 months, based on the current recommended age of administration by country.
Simulations
To quantify uncertainty in averted death estimates, we performed a sensitivity analysis by generating 1,000 simulations of each model. We independently sampled <12 month and ≥12 month VE from a normal distribution from the confidence intervals generated by the meta-analysis; the number of rotavirus deaths in 2013 from a normal distribution from the published confidence intervals; and coverage from +/− 5% of the JRF estimates, assuming a uniform distribution. Vaccination coverage was not allowed to exceed 100%. We then calculated the median number of deaths prevented and the point estimate at the 2.5 and 97.5 percentiles as the confidence interval from the 1,000 samples. Model calculations and simulations were performed using SAS v9.4.
Results
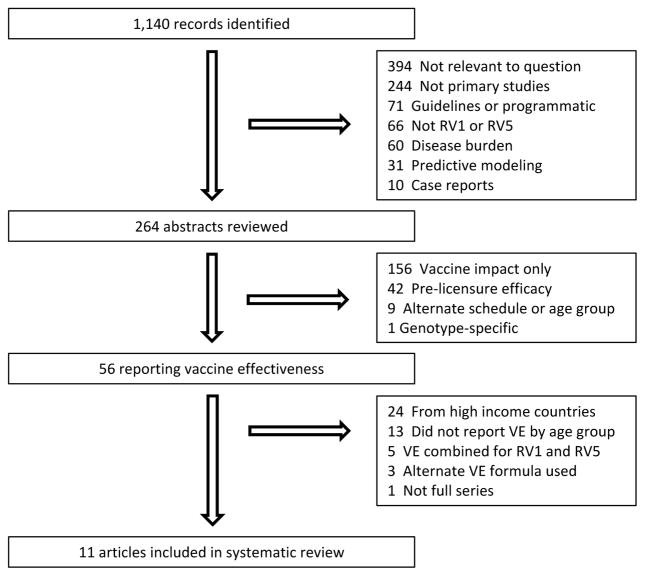
Eleven studies, 9 post-licensure and 2 pre-licensure, from 9 countries met the meta-analysis inclusion criteria (Figure 2) These articles were included in the random effects model to estimate a pooled VE (Table 1). VE for children <12 months of age was estimated to be 66% (95%CI: 57, 73); VE for children ≥12 months of age was estimated to be 45% (95%CI: 29, 57).
Figure 2.
Article review and selection process.
We assumed all countries had introduced a primary rotavirus vaccine series in calculating baseline deaths due to rotavirus disease. Across all WHO regions, we calculated an estimated 122,400 (95%CI: 119000, 126200), 113,300 (95%CI: 109900, 116700), and 114,200 (95%CI: 110700, 117700), deaths under the stepwise, logarithmic, and linear baseline waning models, respectively (Table 2).
Table 2.
Regional-level baseline rotavirus deaths and deaths averted under three rotavirus vaccine booster dose scenarios.
| WHO region (# of countries) | Stepwise waning | Linear waning | Logarithmic waning | |||
|---|---|---|---|---|---|---|
| Median | 95%CI | Median | 95%CI | Median | 95%CI | |
| Baseline | ||||||
| African (47) | 64,036 | 61971, 66025 | 62,466 | 60612, 64444 | 62,382 | 60531, 64358 |
| Americas (32) | 1,864 | 1793, 1934 | 1,724 | 1663, 1789 | 1,722 | 1660, 1786 |
| Eastern Mediterranean (21) | 19,915 | 18909, 20943 | 16,935 | 15979, 17830 | 16,918 | 15960, 17815 |
| European (27) | 881 | 818, 945 | 925 | 854, 992 | 903 | 834, 970 |
| Southeast Asia (11) | 30,768 | 28078, 33671 | 28,507 | 25883, 31133 | 27,838 | 25273, 30398 |
| Western Pacific (22) | 4,933 | 4687, 5177 | 3,591 | 3375, 3818 | 3,507 | 3297, 3728 |
| Total (160) | 122,407 | 119010, 126165 | 114,157 | 110680, 117685 | 113,268 | 109857, 116741 |
|
| ||||||
| Reduced waning of vaccine effectiveness | ||||||
| African (47) | 4,383 | 4226, 4555 | 886 | 854, 921 | 1,345 | 1297, 1398 |
| Americas (32) | 94 | 89, 100 | 19 | 18, 21 | 48 | 46, 50 |
| Eastern Mediterranean (21) | 1,249 | 1161, 1340 | 256 | 238, 275 | 400 | 372, 428 |
| European (27) | 88 | 81, 95 | 18 | 17, 19 | 54 | 50, 58 |
| Southeast Asia (11) | 3,477 | 3150, 3857 | 718 | 650, 796 | 1,090 | 987, 1209 |
| Western Pacific (22) | 504 | 472, 538 | 94 | 87, 101 | 144 | 134, 154 |
| Total (160) | 9,804 | 9399, 10219 | 1,993 | 1910, 2078 | 3,083 | 2956, 3212 |
|
| ||||||
| Reestablished vaccine effectiveness | ||||||
| African (47) | 8,765 | 8453, 9111 | 1,772 | 1709, 1841 | 2,690 | 2594, 2795 |
| Americas (32) | 189 | 178, 201 | 39 | 37, 41 | 96 | 91, 100 |
| Eastern Mediterranean (21) | 2,498 | 2322, 2681 | 512 | 476, 550 | 800 | 744, 857 |
| European (27) | 176 | 163, 190 | 36 | 33, 39 | 107 | 100, 116 |
| Southeast Asia (11) | 6,953 | 6299, 7715 | 1,435 | 1300, 1539 | 2,179 | 1974, 2418 |
| Western Pacific (22) | 1,009 | 944, 1076 | 188 | 175, 202 | 288 | 267, 308 |
| Total (160) | 19,608 | 18798, 20437 | 3,985 | 3820, 4156 | 6,165 | 5912, 6424 |
|
| ||||||
| Boosted vaccine effectiveness | ||||||
| African (47) | 13,148 | 12679, 13666 | 2,658 | 2563, 2762 | 4,035 | 3891, 4193 |
| Americas (32) | 283 | 267, 301 | 110 | 105, 115 | 144 | 137, 151 |
| Eastern Mediterranean (21) | 3,747 | 3482, 4021 | 800 | 745, 855 | 1,201 | 1117, 1285 |
| European (27) | 263 | 244, 285 | 128 | 118, 138 | 161 | 149, 174 |
| Southeast Asia (11) | 10,430 | 9449, 11572 | 2,153 | 1950, 2389 | 3,269 | 2961, 3627 |
| Western Pacific (22) | 1,513 | 1415, 1615 | 285 | 265, 305 | 432 | 401, 462 |
| Total (160) | 29,412 | 28197, 30656 | 6,138 | 5887, 6394 | 9,248 | 8868, 9636 |
Globally under the stepwise model, there were 9,800 (95%CI: 9400, 10200), 19,600 (95%CI: 18800, 20400), and 29400 (95%CI: 28200, 30700) additional rotavirus deaths averted when a booster rotavirus dose reduced VE waning, reestablished VE, and boosted VE, respectively (Table 2). This represents 8%, 16%, and 24% fewer rotavirus deaths compared with the estimated stepwise model baseline deaths. Under the logarithmic model, there were 3,100 (95%CI: 3000, 3200), 6,200 (95%CI: 5900, 6400), and 9,200 (95%CI: 8900, 9600) additional rotavirus deaths averted when a booster rotavirus dose reduced VE waning, reestablished VE, and boosted VE, respectively. This represents 3%, 5%, and 8% fewer rotavirus deaths compared with the estimated logarithmic model baseline deaths. Under the linear model, there were 1,200 (95%CI: 1900, 2100), 4,000 (95%CI: 3800, 4200), and 6,100 (95%CI: 5900, 6400) additional rotavirus deaths averted when a booster rotavirus dose reduced VE waning, reestablished VE, and boosted VE, respectively. This represents 2%, 3%, and 5% fewer deaths compared with the estimated linear model waning baseline deaths. For all scenarios the greatest number of additional deaths averted was in the AFRO region (due to the relative high mortality in that region), while the greatest proportion of additional deaths averted was in the SEARO region (due to the older age distribution of rotavirus deaths in that region).
We reevaluated some of our initial assumptions under the stepwise waning model. If all countries administered a booster dose of rotavirus vaccine at 9 months rather than 12 months, 107 (95%CI: 103, 111), 214 (95%CI: 206, 223), and 667 (95%CI: 308, 334) additional estimated rotavirus deaths would be averted under the reduced VE waning, reestablished VE, and boosted VE scenarios, respectively. Our assumption that primary series rotavirus vaccination coverage equaled DTP full series coverage in countries that have already introduced rotavirus vaccine accounted for 373 (95%CI: 333, 419), 746 (95%CI: 666, 837), and 1119 (95%CI: 999, 1265) rotavirus deaths averted under the reduced VE waning, reestablished VE, and boosted VE scenarios, respectively.
Discussion
We found from 4,000 (95%CI: 3800, 4200) to 19,600 (95%CI: 18800, 20400) additional rotavirus deaths could be averted in medium and high child mortality countries if a booster dose of rotavirus vaccine reestablished VE to the levels in the first year of life during the second year of life. This represents a 3–16% reduction in estimated deaths due to rotavirus disease as compared to estimated baseline deaths. The absolute number of rotavirus deaths averted varied significantly by region, baseline burden of disease, and age distribution of rotavirus deaths. For example, the African Region had the highest number of rotavirus deaths in 2013 and, in absolute numbers, saw the biggest decline in the three baseline models; all nine boosting scenarios showed substantial impacts, ranging from nearly 900 deaths to over 13,100 deaths averted. Though the Southeast Asia Region had about half the number of baseline deaths as the African Region, the absolute number of deaths averted was only slightly lower in the Southeast Asia compared to the African Region. This is because in Asia more deaths are in older children than in African Region countries, and thus a booster dose has greater benefits.
While there is a lot of uncertainty in the form and degree of waning for rotavirus vaccine after the first year of life, the three models we proposed as a structural uncertainty analysis were consistent in estimating the baseline number of deaths. Each region the nine models and boosting scenarios produced a wide range of deaths averted. For example, the stepwise model ranged from 51% in the European Region to 81% in the Western Pacific Region than the linear models under the boosted VE scenario. These discrepancies highlight the need for better understanding of the mechanism and patterns of waning rotavirus vaccine induced immunity. While more refined inputs would improve our estimates, much of the uncertainty in our estimates is between the three waning forms. Inferring the temporal patterns of waning may not be possible in standard vaccine trials and relatively large age groups, one year in this case, do not provide the necessary granularity to determine if waning follows a stepwise, logarithmic or linear pattern. Moreover, over time, the placebo arm in a trial becomes more similar to the vaccine group by natural infection, making it difficult to disentangle true loss of immunity and measurement bias.
This exercise had several additional limitations. Most importantly, there remains uncertainty about the relationship between seroconversion and clinical endpoints, limiting our ability to quantify the impact of a booster dose from current immunogenicity data. Trial data with a clinical endpoint will narrow the range of plausible assumptions. Our model does not account for herd immunity or catch-up vaccination for the primary rotavirus vaccine series. The meta-analysis provided VE estimates for a range of ages and we assigned these estimates to specific time points to generate the linear and logarithmic waning models. Future research may provide more age-specific VE estimates to improve our assumptions. Finally, this analysis focused on regional and global impact of introducing a booster dose, however we may be overlooking important differences at the country- or subnational-levels.
These results show the potential for an important impact on rotavirus diarrhea mortality by adding a booster dose of rotavirus vaccine administered at 9 or 12 months of age. However, the benefits of booster will have to be weighed against considerations of additional cost, vaccine supply needed, safety, and programmatic considerations. Nonetheless, these results inform consideration of booster doses of rotavirus vaccine.
Footnotes
Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the official positions of the US Centers for Disease Control and Prevention.
References
- 1.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 2.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:2136–43. doi: 10.1016/S0140-6736(13)62630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isanaka S, Guindo O, Langendorf C, Matar Seck A, Plikaytis BD, Sayinzoga-Makombe N, et al. Efficacy of a Low-Cost, Heat-Stable Oral Rotavirus Vaccine in Niger. N Engl J Med. 2017;376:1121–30. doi: 10.1056/NEJMoa1609462. [DOI] [PubMed] [Google Scholar]
- 5.Cunliffe NA, Witte D, Ngwira BM, Todd S, Bostock NJ, Turner AM, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine. 2012;30(Suppl 1):A36–43. doi: 10.1016/j.vaccine.2011.09.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madhi SA, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, et al. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine. 2012;30(Suppl 1):A44–51. doi: 10.1016/j.vaccine.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 7.Vesikari T, Karvonen A, Ferrante SA, Ciarlet M. Efficacy of the pentavalent rotavirus vaccine, RotaTeq(R), in Finnish infants up to 3 years of age: the Finnish Extension Study. Eur J Pediatr. 2010;169:1379–86. doi: 10.1007/s00431-010-1242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahakyan G, Grigoryan S, Wasley A, Mosina L, Sargsyan S, Asoyan A, et al. Impact and Effectiveness of Monovalent Rotavirus Vaccine in Armenian Children. Clin Infect Dis. 2016;62(Suppl 2):S147–54. doi: 10.1093/cid/ciw045. [DOI] [PubMed] [Google Scholar]
- 9.Patel MM, Patzi M, Pastor D, Nina A, Roca Y, Alvarez L, et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ. 2013;346:f3726. doi: 10.1136/bmj.f3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pringle KD, Patzi M, Tate JE, Iniguez Rojas V, Patel M, Inchauste Jordan L, et al. Sustained Effectiveness of Rotavirus Vaccine Against Very Severe Rotavirus Disease Through the Second Year of Life, Bolivia 2013–2014. Clin Infect Dis. 2016;62(Suppl 2):S115–20. doi: 10.1093/cid/civ1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gastanaduy PA, Steenhoff AP, Mokomane M, Esona MD, Bowen MD, Jibril H, et al. Effectiveness of Monovalent Rotavirus Vaccine After Programmatic Implementation in Botswana: A Multisite Prospective Case-Control Study. Clin Infect Dis. 2016;62(Suppl 2):S161–7. doi: 10.1093/cid/civ1207. [DOI] [PubMed] [Google Scholar]
- 12.Correia JB, Patel MM, Nakagomi O, Montenegro FM, Germano EM, Correia NB, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis. 2010;201:363–9. doi: 10.1086/649843. [DOI] [PubMed] [Google Scholar]
- 13.Justino MC, Linhares AC, Lanzieri TM, Miranda Y, Mascarenhas JD, Abreu E, et al. Effectiveness of the monovalent G1P[8] human rotavirus vaccine against hospitalization for severe G2P[4] rotavirus gastroenteritis in Belem, Brazil. Pediatr Infect Dis J. 2011;30:396–401. doi: 10.1097/INF.0b013e3182055cc2. [DOI] [PubMed] [Google Scholar]
- 14.Cotes-Cantillo K, Paternina-Caicedo A, Coronell-Rodriguez W, Alvis-Guzman N, Parashar UD, Patel M, et al. Effectiveness of the monovalent rotavirus vaccine in Colombia: a case-control study. Vaccine. 2014;32:3035–40. doi: 10.1016/j.vaccine.2014.03.064. [DOI] [PubMed] [Google Scholar]
- 15.Armah G, Pringle K, Enweronu-Laryea CC, Ansong D, Mwenda JM, Diamenu SK, et al. Impact and Effectiveness of Monovalent Rotavirus Vaccine Against Severe Rotavirus Diarrhea in Ghana. Clin Infect Dis. 2016;62(Suppl 2):S200–7. doi: 10.1093/cid/ciw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar-Zeev N, Jere KC, Bennett A, Pollock L, Tate JE, Nakagomi O, et al. Population Impact and Effectiveness of Monovalent Rotavirus Vaccination in Urban Malawian Children 3 Years After Vaccine Introduction: Ecological and Case-Control Analyses. Clin Infect Dis. 2016;62(Suppl 2):S213–9. doi: 10.1093/cid/civ1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gheorghita S, Birca L, Donos A, Wasley A, Birca I, Cojocaru R, et al. Impact of Rotavirus Vaccine Introduction and Vaccine Effectiveness in the Republic of Moldova. Clin Infect Dis. 2016;62(Suppl 2):S140–6. doi: 10.1093/cid/civ1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groome MJ, Page N, Cortese MM, Moyes J, Zar HJ, Kapongo CN, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis. 2014;14:1096–104. doi: 10.1016/S1473-3099(14)70940-5. [DOI] [PubMed] [Google Scholar]
- 19.Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of Rotavirus Vaccination: A systematic review of the first decade of global post-licensure data, 2006–2016. Clin Infect Dis. 2017 doi: 10.1093/cid/cix369. [DOI] [PubMed] [Google Scholar]
- 20.Organization WH. Child rota deaths 2008. 2009. [Google Scholar]
- 21.Zaman K, Fleming JA, Victor JC, Yunus M, Bari TI, Azim T, et al. Noninterference of Rotavirus Vaccine With Measles-Rubella Vaccine at 9 Months of Age and Improvements in Antirotavirus Immunity: A Randomized Trial. J Infect Dis. 2016;213:1686–93. doi: 10.1093/infdis/jiw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis. 2013;208:284–94. doi: 10.1093/infdis/jit166. [DOI] [PubMed] [Google Scholar]
- 23.UNICEF. Under-five mortality rate. 2016. [Google Scholar]
- 24.Organization WH. Reported estimates of rotavirus last dose coverage. 2016. [Google Scholar]
- 25.Organization WH. 2016 global summary. 2016. WHO vaccine-preventable diseases: monitoring system. [Google Scholar]
- 26.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD World Health Organization-Coordinated Global Rotavirus Surveillance N. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin Infect Dis. 2016;62(Suppl 2):S96–S105. doi: 10.1093/cid/civ1013. [DOI] [PubMed] [Google Scholar]
- 27.Organization WH. Child rotavirus deaths by country 2000–2013. 2014. [Google Scholar]