Synopsis
Increased age is associated with normative declines in both sleep and cognitive functioning. While there are some inconsistencies in the literature, negative sleep changes are associated with worse cognitive functioning. This negative relationship holds true across normal sleeping older adults, older adults with insomnia, older adults with sleep disordered breathing, cognitive healthy older adults, and older adults with dementia. There are mixed results regarding potential benefits of sleep treatments on cognitive functions; however, this line of research deserves added attention as the potential mechanisms of action are likely distinct from other interventions to improve cognition.
Keywords: Sleep, Insomnia, Sleep Apnea, Cognition, Cognitive Function, Age, Aging
Introduction
This paper will review the growing literature examining sleep and cognitive functioning in older adults. The main focus will be on normal, age-related cognitive changes, as opposed to neurodegenerative disease processes. Age-related cognitive changes are the result of developmental maturation. These cumulative, long-term processes are universal or nearly universal, and are resistant to efforts to reverse the change.1,2 Investigation into cognitive aging has found a general cognitive decline experienced with increasing age,3–5 which has been demonstrated to be pervasive, affecting many sub-domains of cognition including:
reaction time
sensory processing
attention
memory
reasoning
executive functioning
While much is known regarding developmental changes in cognitive functioning, comparatively little is known regarding sleep’s relationship to late-life cognitive functioning.
Sleep represents an intriguing individual difference variable as it may relate to late-life cognitive functioning. Sleep has demonstrated consistent age-related changes as a result of developmental maturation. Interestingly, many of these developmental changes parallel the age-related changes observed in cognitive functioning. For example, slow wave sleep (SWS; Stage N3) and rapid eye movement (REM) sleep both decrease with advanced age.6 In addition to these normal, developmentally appropriate changes in sleep, older adults also experience an increased prevalence of both insomnia and sleep disordered breathing.7–9 Figure 1 depicts general age-related changes in cognitive functioning and general age-related changes in sleep. The parallel changes in sleep and cognition with age, coupled with anecdotal reports of disturbed cognitive abilities following poor sleep, have resulted in research efforts focused on examining sleep and cognition in older adults. This paper summaries the literature for normal sleeping older adults, older adults with insomnia, and older adults with sleep disordered breathing.
Figure 1.
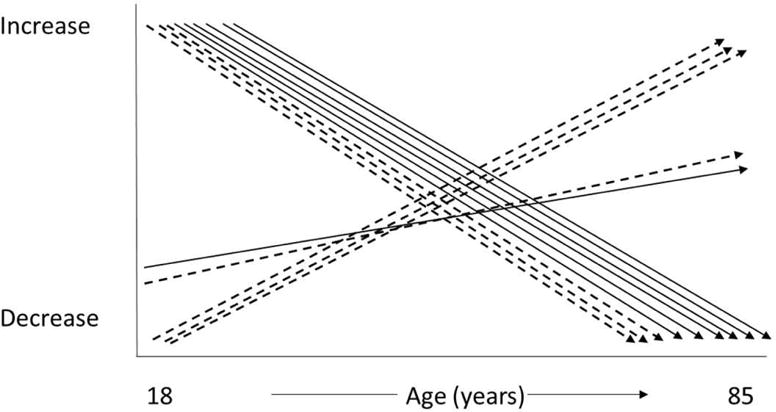
Normative changes with age in both cognitive functioning and sleep. Solid arrows represent general cognitive changes. Dashed arrows represent general sleep changes. Cognitive abilities that decline with age include: processing speed, working memory, long-term memory, attention, reasoning, and executive control. Sleep characteristics that decline with age include: total sleep time, slow wave sleep, and REM sleep. Sleep characteristics that increase with age include: waketime after sleep onset, and light sleep (Stages N1 and N2). Crystalized intelligence and sleep onset latency both demonstrated slight increased with advancing age.
Sleep and Cognition in the ‘Normal Sleeping’ Older Adult
Self-Reported Sleep Duration and Cognition
Several large-scale epidemiological studies have garnered information regarding habitual sleep duration and/or difficulty and cognitive functioning in older adults. In a study of over 3000 older adults, long sleep duration was associated with worse overall/global cognitive functioning, while no association with short sleep duration and cognitive functioning was observed.10 In a similar study of over 5000 adults, sleep duration was associated with verbal fluency and list memory, such that both long and short sleep duration were associated with poorer performance.11 In a sample of community-dwelling older women, sleeping less than 5 hours per night was associated with poorer global cognition and poorer performance across many of the individual indicators of cognitive functioning (i.e., verbal memory, verbal fluency, working memory) when compared to women sleeping 7 hours or more per night.12 The authors of these studies suggest that sleep duration may be related to cognitive functioning through changes in sleep architecture, fragmentation, quality, and neurological conditions.10,11 The relationship between self-reported retrospective recall of habitual sleep duration and cognitive functioning has been confirmed through meta-analysis demonstrating deleterious effects of both long and short sleepers on multiple domains of cognitive functioning in older adults.13
Polysomnography (PSG)-Measured Sleep and Cognition
Investigation into the relationship between PSG-assessed sleep and waking cognitive functioning has provided mixed results. It has been reported that longer sleep onset latency (SOL) is related to poorer verbal memory and executive functioning, while higher total wake time (TWT) is related to lower psychomotor speed and memory in normal sleeping older adults.13 However, in another investigation, SWS was unrelated to performance on a Simple reaction time (RT) task, Continuous Performance task, and attention test in good sleeping older adults14. As such, it appears additional research is need to further explicate the relationship between PSG-measured sleep and cognitive functioning in older adults without a sleep complaint.
Actigraphy-Measured Sleep and Cognition
It appears that there is a relationship between objective, naturally occurring sleep measured with actigraphy and cognitive performance. In a study of nearly 3000 older community-dwelling women, actigraphy-measured sleep [sleep efficiency(SE), SOL, wake after sleep onset (WASO), and napping] was associated with an increased risk of poorer general cognition and executive performance.15 However, total sleep time (TST) was not related to cognitive functioning, which led the authors to conclude that “it is disturbance of sleep rather than quantity that affects cognition.”15 In a different, but complementary vein, 7 nights of actigraphy were used to compute sleep/wake patterns in 144 community-dwelling older adults. It was reported that older adults who displayed many shifts from rest to activity performed worse on composites of executive functioning, memory, and speed than elderly with more consistent rest-activity patterns.16 The scarcity of research examining actigraphy-measured sleep and cognition in older adults preclude any definitive conclusions from being drawn.
Sleep Deprivation and Cognition
Generally speaking, short-term total sleep deprivation has a “significant deleterious effect across most cognitive domains”, including attention, working memory, processing speed, short-term memory, and reasoning – with smaller effects being observed for tasks of greater complexity.17 Webb and Levy18 and Webb19 conducted experiments to examine potential age differences in cognitive response to sleep deprivation. In both experiments, older adults’ and younger adults’ performance on a variety of cognitive tasks were compared following 2 nights of sleep deprivation. It was reported that older adults showed greater deterioration following sleep deprivation than did the younger adults in vigilance, visual search, reaction times, word detection, addition, anagrams, and objects uses.18,19 Jones and Harrison20 summarized the extant sleep deprivation work nicely by stating “neurocognitve studies present many inconsistencies, task classification is often ambiguous and, in the absence of any unifying explanation at the level of cognitive mechanisms, the overall picture is one of a disparate range of impairment following sleep loss.”20
Sleep Restriction and Cognition
Bliese and colleagues21 examined age-related changes in reaction time/attention after modest sleep restriction. Interestingly, older adults displayed less pronounced effects of sleep restriction on their reaction times than younger adults. However, 62 was the oldest adult included in the study, so aging effects must be interpreted cautiously. Nevertheless, the authors suggest that older adults may have “expended more effort across days” which could have resulted in blunted differences.21 The experimental evidence regarding the sleep-cognition relationship gained through studies employing sleep restriction methodology have consistently yielded results indicating an impact on vigilance, which may be blunted in older adults.21 Regarding potential mechanisms underlying the relationship between sleep restriction and cognitive functioning, Banks and Dinges22 summarized the evidence by suggesting there is no “definitive evidence of what is accumulating and destabilizing cognitive functions over time when sleep is regularly restricted.”22
Sleep and Learning
As opposed to examination of the negative consequences of poor sleep (or sleep loss), some researchers have investigated the potential benefits of sleep gained. In this line of research, participants are allowed to sleep while manipulating the timing of cognitive testing/training to either allow sleep to occur following testing or not in order to examine any effects of post training sleep on subsequent testing. The majority of this research has been conducted with younger adults, and with findings indicating optimized performance following sleep. Tucker and colleagues trained 16 healthy older adults in a finger tapping sequencing task and found that older adults performed significantly better following sleep than 12 hours of not sleeping, suggesting sleep-dependent motor skill performance in the elderly.23 Interestingly, older adults showed similar rates of improvement as was found in younger samples; however, specific sleep characteristics did not correlate with next day’s performance (in contrast, Stage 2 sleep and sleep spindle activity does correlate in younger adults). The authors concluded that sleep in the elderly does optimize motor skill learning; however, it may do so differently than in younger adults.23
Sleep and Cognition in the Older Adult with Insomnia
Older adults are at an increased risk for both insomnia and experiencing negative changes in cognitive functioning. Given the comorbidity of insomnia and cognitive dysfunction in older adults, researchers have attempted to understand the role of insomnia in predicting cognitive functioning in a variety of samples (e.g., cognitively intact older adults and older adults experiencing cognitive decline or dementia). Furthermore, a small number of studies have examined the effect of behavioral interventions for insomnia on cognitive outcomes. A sample of representative studies is presented below that illustrates these different approaches.
Insomnia Status and ‘Normal’ Cognitive Aging
The association between insomnia and cognitive performance in younger and middle-aged adults is well-established, with impairments in working memory, episodic memory, and some aspects of executive functioning.24 However, less is known about the association between insomnia diagnosis and cognitive performance in cognitively healthy older adults who are not experiencing cognitive decline or dementia. To date, insomnia and cognitive functioning has been examined in older adults with insomnia through cross-sectional designs with matched-healthy controls25,26 or using comparisons across insomnia sub-types.27
Overall, in contrast to healthy controls, insomnia status was associated with worse performance on a subset of cognitive tasks. Specifically, participants with insomnia performed significantly worse on memory span, integration of visual and semantic dimensions, and executive functioning tasks.26 Interestingly, the insomnia groups performed better than healthy controls on the simple attending task.25 The better attentional performance on simple attending did not persist for complex attending, perhaps reflecting the higher arousal that is characteristic of individuals diagnosed with insomnia.28 This higher arousal may be beneficial for unambiguous stimuli but a hindrance for complex tasks requiring more cognitive resources.25 In addition to differences in cognitive performance dependent on the presence or absence of insomnia, performance also appears to differ depending on the type of insomnia complaint. Specifically, Ling et al. found only early morning awakening was associated with significantly worse cognitive performance in the executive functioning domain. No association was found between difficulty initiating sleep or difficulty maintaining sleep across the following cognitive domains: attention, verbal memory, visuospatial ability, and executive functioning.27 Refer to Table 1 for differences in cognitive functioning between older adults with and without insomnia.
Table 1.
Differences in Performance Across Cognitive Tasks Group by Insomnia Status
| Cognitive Task | Better performance by Insomnia Group | Better performance by Healthy Control Group | No Group Difference |
|---|---|---|---|
| Sustained attention | X (simple tasks)25 |
X (simple tasks,26 complex tasks25) |
X (complex tasks)26 |
| Naming | X26 | ||
| Psychomotor skills | X26 | ||
| Memory span | X26 | ||
| Integration of two dimensions (visual and semantic) | X26 | ||
| Time estimation | X26 | ||
| Executive functioning | X26 |
Overall, across the reviewed studies examining cognitive performance in cognitively-intact older adults with insomnia, insomnia status and specific characteristics of insomnia (early morning awakening) appears to be associated with worse cognitive performance. However, the poorer performance of the individuals with insomnia is not consistent across all cognitive domains or across all tasks within cognitive domains. Consequently, despite a trend towards worse cognitive performance associated with insomnia status, the existing body of evidence is too small and inconsistent to arrive at a broad conclusion.
Insomnia and Cognitive Decline/Dementia Diagnoses
In comparison to research that has examined insomnia and cognitive performance in cognitively healthy older adults, more work has been done to explore the role of insomnia in predicting cognitive decline or dementia status. Despite the greater breadth of research with cognitively impaired older adults, the association between insomnia and cognitive decline and/or dementia status remains unclear.
A minority of studies identified a negative association between insomnia and cognitive decline or dementia.29,30 Specifically, older adults with long-term insomnia and long-term use of hypnotics had a two-fold risk of developing dementia during a three-year follow-up period compared to healthy controls.29 Controlling for hypnotic use, similar results were found with long-term insomnia predicting an increased risk for cognitive decline at a three-year follow-up for older adults with insomnia compared to healthy controls.30 These results point to the importance of considering the duration of the insomnia complaint as a predictor of cognitive function, as long-term, rather than concurrent sleep complaint, was associated with cognitive decline.30
Conversely, another sub-section of studies found a positive association between insomnia symptoms and cognitive performance.31,32 Using a longitudinal design, Jaussent et al. (2012) found that complaints of awakenings during the night and total number of insomnia complaints at baseline predicted a decreased risk for cognitive decline during a 8-year follow-up period.31 Additionally, a cross-sectional approach with older adults with dementia residing in assisted living facilities showed that individuals with insomnia symptoms performed significantly better on the MMSE compared to those without insomnia symptoms.32 Of note, neither of these studies assessed the duration of the insomnia complaint.
Despite a sub-set of studies showing negative and positive associations between insomnia and cognitive decline or dementia, a greater number of studies have found no association. Employing longitudinal and cross-sectional designs, with and without comparison groups, and various approaches to insomnia and cognitive evaluations, insomnia was not associated with cognitive decline33–35 or dementia status in many studies.36 Given these discrepant findings, one could conclude that insomnia status does not predict cognitive decline in older adults. However, it is more likely that the equivocal findings result from methodological differences in research approaches. Please see Table 2 for a summary of the relationship between insomnia and cognitive decline/dementia status in older adults.
Table 2.
Insomnia Status and Cognitive Decline or Dementia Diagnosis
| Negative Associations | |||
|---|---|---|---|
| Insomnia Measure | Cognitive Measure | Results | Study |
| ICD-9 codes | ICD-9 codes | Patients with insomnia diagnosis and prescribed hypnotics > risk for dementia during the 3-year follow-up compared to controls. | 29 |
| Interview indicating either problem most of the time: trouble falling asleep or waking up too early and not falling asleep again. | Pfeiffer’s Short Portable Mental Status Questionnaire (SPMSQ), ≥ 2 errors on the SPMSQ classified as cognitive decline. | Insomnia associated with increased risk of cognitive decline for men, independent of depression and comorbid with depression. Insomnia associated with increased risk of cognitive decline for women when insomnia was comorbid with depression. | 30 |
| Positive Associations | |||
| Interview and questionnaire: difficulty initiating sleep, awakenings during the night, early morning awakening, insomnia severity. | Incident cognitive impairment defined as 4-point reduction in Mini-Mental State Examination (MMSE), 4-point reduction in Benton Visual Retention Test (BVRT), and 14-point reduction in the Isaacs Set Test (IST) scores. | Number of insomnia complaints and difficulty with awakenings during the night were negatively associated with MMSE cognitive decline during follow-up. No associations found for BVRT or IST. | 31 |
| Johns Hopkins Alzheimer’s Disease Research Center questionnaire. | Severity of dementia was classified based on MMSE scores (mild = MMSE > 20; moderate = MMSE 11–19; severe = MMSE < 10). | Participants diagnosed with insomnia performed the best on the MMSE compared to those without sleep disturbance or those with insomnia and daytime sleepiness. | 32 |
| No Associations | |||
| Self-report questionnaire assessing “usually having trouble falling asleep or waking up far too early and not going back to sleep.” | Cognitive Abilities Screening Instrument (CASI); cognitive decline was defined as a ≥ 9-point drop in the CASI. | Insomnia not associated with greater risk for dementia diagnosis or cognitive decline. | 33 |
| Interview assessing difficulty falling asleep, staying asleep, or both. | Incident cognitive impairment defined as an Mini-Mental State Examination (MMSE) ≤ 21. | No sleep problems associated with increased risk for incident cognitive impairment 2/10-year follow-ups. | 34 |
| Clinical interview: at least 1 symptom (difficulty falling asleep, staying asleep, early morning awakening) occurring at least 3 times/week. | Mini-Mental State Examination (MMSE); Global Deterioration Scale (GDS). Participants with MMSE < 24.0 were evaluated for dementia by expert panel | Insomnia diagnosis was not associated with cognitive impairment | 35 |
| Insomnia Interview Schedule and Sleep Impairment Index: onset, maintenance, termination insomnia. | Alzheimer’s Disease diagnosis was made according to NINCDS/ADRDA and DSM-IV criteria. | Individuals with Alzheimer’s Disease did not differ from the healthy comparison group in terms of the frequency of onset, maintenance, termination insomnia symptoms. | 36 |
Insomnia Treatment and Cognitive Functioning
In addition to examining cross-sectional and longitudinal associations between insomnia and cognitive outcomes, a small number of studies have investigated whether the treatment of insomnia leads to an improvement in cognitive performance (see Table 3).25,37 In one such study, an insomnia intervention was employed with community-dwelling older adults with insomnia.25 The insomnia intervention consisted of sleep restriction, cognitive restructuring, sleep hygiene, bright light exposure, body temperature manipulations, and structured physical activity. Following treatment, sleep onset latency and sleep efficiency were significantly improved in the treatment group compared to the waitlist group. Treatment was also associated with improved performance on complex vigilance tasks and worsened performance on simple vigilance tasks compared to the waitlist control. A possible explanation for the performance differences on simple versus complex tasks following treatment, is that improved sleep results in a reduction of arousal levels to normal,25 resulting in slower performance on simpler tasks.
Table 3.
Insomnia Treatment and Cognitive Performance.
| Insomnia Measure | Cognitive Measure | Results | Study |
|---|---|---|---|
| DSM-IV criteria, Pittsburgh Sleep Quality Index, Sleep Disorders Questionnaire. Polysomnography and sleep diaries were also employed. | Simple and complex sustained attention assessed via computer. Outcomes: lapses, false-positive responses, and reaction times. | Compared to the waitlist control, insomnia intervention group showed increased reaction time for simple vigilance task and reduced reaction time for complex vigilance task. | 23 |
| DSM-IV-TR, ICSD-2 verified by self-report questionnaires and clinical interview. Polysomnography and the Pittsburgh Sleep Quality Index were also employed. | Neuropsychological tests assessed three cognitive domains: episodic memory, working memory, abstract reasoning | Insomnia intervention was not associated with greater improved cognitive performance compared to information-only control. | 37 |
An additional study employed a Brief Behavioral Treatment for Insomnia (BBTI) approach with a sample of community-dwelling older adults who were cognitively intact and diagnosed with insomnia.37 Individuals in the treatment group showed a significantly greater decrease in wake time after sleep onset following treatment compared to the information-only control group. However, the treatment group did not show significantly better improvement in cognitive performance across three cognitive domains (episodic memory, working memory, abstract reasoning) compared to the control group. The authors posited that the null findings might be due to the short follow-up period – 4 weeks after the start of the intervention. Allowing more time post-intervention for the sleep treatment to take effect might have enabled detection of effects on cognitive performance.
Sleep and Cognition in the Older Adult with Sleep Disordered Breathing (SDB)
The estimated prevalence of both SDB and cognitive impairment increases with age.38,39 Moreover, individuals with SDB show cognitive changes similar to those associated with aging.40 Recent research has posited that SBD and advanced age act independently to impair cognitive functioning, with the combination of both SBD and advanced age leading to cognitive impairments above and beyond either factor alone.40
While some studies have found an association between SDB and impairments in global cognitive functioning,41,42 not all cognitive domains appear to be equally affected. Instead, the domains of vigilance, executive function, and memory are particularly implicated.43 The impact of SDB on all three of these cognitive domains has been observed among both community and clinic based populations with different neuropsychological tests (Table 4). SDB in older adults has been found to impair:
Table 4.
Cognitive Performance in Older Adults with Sleep Disordered Breathing
| Cognitive Domain | Measures | Outcomes | Representative References |
|---|---|---|---|
| Global Functioning |
|
|
41,42 |
| Vigilance |
|
|
44,45,49,50 |
| Executive Function |
|
|
47, 49, 51, 52 |
| Memory |
|
|
53, 54, 55 |
Notes: SDB = Sleep Disordered Breathing, EDS = Excessive Daytime Sleepiness, AHI = Apnea-Hypopnea Index, RDI = Respiratory Disturbance Index
Although relatively few studies have examined the longitudinal impact of SDB on cognitive performance in order adults, preliminary evidence suggests that SDB can contribute to relevant long-term changes. For example, one study found that SDB was associated with a decline in global cognitive functioning over three years.41 Similarly, another study of community dwelling adults found that SDB was associated with a decline in attention abilities over eight years.56
The long-term impact of SDB may also influence the onset and course of certain neurological disorders. Older individuals with SDB have earlier rates of both mild cognitive impairment and dementia.57,58 Evidence also suggests that older adults with neurological disorders might be more vulnerable to the negative cognitive effects of SDB.45 For example, SDB can exacerbate cognitive impairments in older adults with dementia.59 The possible role of SDB in the development of neurological disorders has led researchers to examine the possible pathways contributing to impairments in cognitive functioning.
Several mechanisms through which SDB contributes to cognitive decline in older adults have been proposed. Evidence suggests that high levels of hypoxemia, an abnormally low level of oxygen in the blood, may play a prominent role. Yaffe and colleagues found that hypoxemia was associated with an increased risk of future mild cognitive impairment and dementia.58 In addition, increased hypoxemia is associated with greater impairment in global cognitive functioning, as well as declines in specific cognitive domains.44,60 However, other studies have found no association between hypoxemia and impaired cognition.41,55 As a result, both sleep fragmentation and excessive daytime sleepiness have been proposed as alternative mechanisms through which SDB may lead to cognitive dysfunction.61 For example, excessive daytime sleepiness caused by SDB, was associated with a decline in global cognitive functioning.41 Despite these findings, the relative contribution of both sleep fragmentation and excessive daytime sleepiness to cognitive impairment remains poorly understood.
While relatively strong evidence supports an association between SBD and cognitive decline in older adults, not all findings support this association. One study found that although both SDB and aging are independently associated with cognitive deficits, age did not interact with SDB to make those cognitive deficits worse.62 Other studies report similar findings. Boland and colleagues found no evidence for an association between either mild or moderate forms of SBD and cognitive functioning in verbal learning and short-term recall, psychomotor efficiency, or verbal fluency.63 Similarly, other research found no association between SDB and performance on broad standardized cognitive tests.64,65 Studies that found no association between SBD and cognitive impairment in older adults contain several methodological differences. First, they lacked comprehensive measures of cognitive functioning or relied on single measures of global functioning.63 Cognitive impairments in older adults with SDB may be subtle and may not be readily identified by general/global cognitive measures. Secondly, several of the studies that found no association between SDB and cognition used home polysomnography,65 which has been shown to differ from lab polysomnography, particularly for adults with severe SDB.66 Finally, several studies finding no associations between SDB and cognitive performance only included participants with mild to moderate SDB,63,64 suggestive of a dose-response relationship between SDB severity and cognitive performance.
While the findings are somewhat inconsistent, overall, current research suggests that SDB adversely impacts cognitive performance in older adults. Cognitive impairments are particularly pronounced in the domains of attention, executive function, and memory. These findings are largely consistent with research examining the influence of SDB in young and middle aged adults.67,68
SDB Severity and Cognitive Performance
Research has explored whether the severity of SDB [as indexed by the apnea-hypopnea index (AHI) or respiratory disturbance index (RDI)] is related to the level of cognitive impairment in older adults. Studies have found that greater AHI and RDI were associated with poorer global cognitive functioning,42 as well as adverse outcomes on specific cognitive domains such as:
However, one study examining older adults with mostly mild to moderate SDB, found no association between SDB severity and cognitive functioning,63 suggesting there may be a threshold at which SDB exerts an increasingly negative impact on cognition. Further research investigating SDB severity and cognitive impairment is warranted.
SDB Treatment and Cognitive Performance
Given the nature of cognitive dysfunction in older adults with SDB, as well as the association between SDB and neurological disorders, the influence of SDB treatment on cognitive functioning in older adults has been the subject of recent interest. The most common treatment for SBD is positive airway pressure (PAP) therapy. While PAP has been shown to decrease some of the primary sequelae of SDB, such as sleep fragmentation and nocturnal oxygen saturation, several studies suggest possible benefits of PAP treatment on cognitive performance.
Two studies report improvements in many areas of cognitive functioning following 3 months of PAP treatment.54,71 Improvements were demonstrated in the following domains:
episodic memory
short-term memory
executive functioning
attention
psychomotor speed
non-verbal delayed recall
Despite optimism about the potential benefits of PAP on cognition, some studies have shown more limited and uneven benefits of PAP treatment. For example, Kang and colleagues found that short-term PAP treatment was associated with gains in executive functioning but no other cognitive domains.72
Preliminary evidence suggests that cognitive gains observed with short-term PAP treatment might be maintained over time. PAP treatment over the course of 10 years is associated with better memory, attention, and executive functioning.51 The long-term benefits of PAP treatment may also extend to older adults with neurological disorders. Preliminary examination of long-term PAP use among individuals with Alzheimer’s disease found that treatment can slow cognitive deterioration.73 Another recent study reported that PAP treatment may delay the age of onset of mild cognitive impairment.57
Although the influence of PAP treatment on cognitive outcomes seem promising, noncompliance with the treatment remains prevalent in the general population, as well as among older adults.74 Limited compliance may severely limit the benefits of PAP treatment on cognitive abilities. Older adults who complied with treatment (average use of 8.5 h/night) displayed greater cognitive abilities compared to individuals who were non-compliant (3.9 h/night).54 One hypothesis about PAP compliance is that older adults who notice cognitive gains in readily observable domains such as attention and memory, may be more likely to comply with PAP treatment.54
Although preliminary evidence demonstrates improved cognitive outcomes for older adults treated with PAP, further research is needed to confirm these findings and better understand which cognitive domains are impacted. In addition, future research should examine whether other forms of SDB treatment, such as weight loss, oral appliance therapy, positional therapy, and surgical treatments might lead to similar cognitive gains.
Unifying Theories and Mechanisms
There are several informative hypotheses concerning sleep and cognitive functioning. These hypotheses include: the controlled attention hypothesis,75 neuropsychological hypothesis,17,20,76 vigilance/arousal hypothesis,77,78 and wake state instability hypothesis.79–81 These various theories about the role of sleep processes in regulating and maintaining cognitive functioning may not be mutually exclusive.17 The controlled attention and vigilance/arousal hypotheses are essentially parallel descriptions of the same phenomena. Many higher order cognitive functions may rely on the appropriate levels of attention and arousal. It has been suggested that impairment of the prefrontal cortex may cause decrements in attention and vigilance.82 In sum, sleep appears to impede arousal/vigilance/attention and prefrontal functioning, potentially through instability of the neurobiological systems responsible for attentional and sleep drives. Please refer to Table 5 for a description of each sleep-cognition theory. In addition to the above referenced sleep-cognition hypotheses, there are many suspected mechanisms involved in the sleep-cognition relationship in late-life. These mechanisms are graphically depicted in Figure 2.
Table 5.
Theories on the Link Between Sleep and Cognition.
| Name | Description | Reference |
|---|---|---|
| Controlled attention | Monotonous tasks are most affected by sleep loss due to the amount of top-down control needed to sustain attention while more complex/difficult tasks are intrinsically motivating (i.e., bottom-up control). | 31 |
| Neuropsychological | Sleep loss results in focal impairment in functions subserved by the prefrontal cortex (i.e., executive functions), beyond any impairment in attention or vigilance. | 17,20,76 |
| Vigilance/arousal | Attention, which is needed for the performance of many other cognitive tasks, is mediated by arousal – a common correlated feature of disturbed sleep. | 77,78 |
| Wake-state instability | Cognitive deficits observed as a result of sleep loss occur due to the interaction of the drive to maintain alertness and the homeostatic drive to initiate sleep. | 79–81 |
Please note that the above theories are not mutually exclusive, but rather attempt to explain similar phenomena in different ways.
Figure 2.
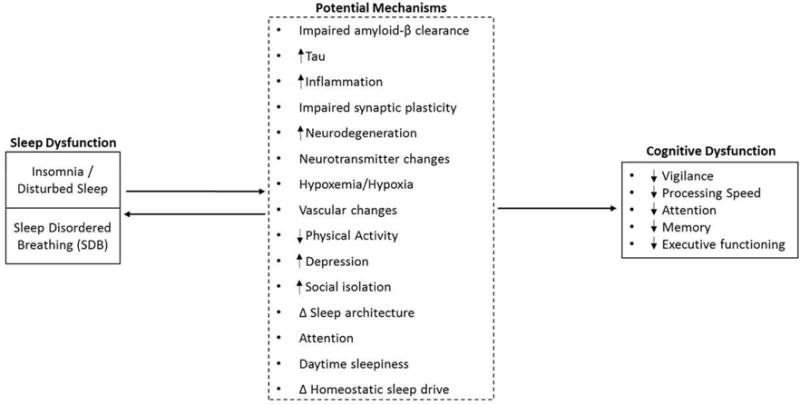
Model depicting the relationships between (1) insomnia/disturbed sleep, (2) sleep disordered breathing and cognitive functioning in older adults. Factors listed in dashed box represent potential mechanisms through which sleep may impact cognitive functioning. Adapted from.61
Conclusions and Future Directions
Sleep and cognitive functioning appear to be related in late-life; however, the exact nature of this relationship has yet to be discerned. Future investigations should continue to investigate the gamut of sleep-cognition relationships. Important questions remain concerning: (1) The role of normal sleep changes in normal cognitive aging, (2) The role of pathological sleep changes on the development of dementias, and (3) The utility of treating sleep disorders on improving cognitive functioning, warding off unwanted cognitive decline, and slowing the course of neurodegenerative diseases. An intriguing prospect for future study is to examine the additive impact of treating sleep disorders in conjunction with focused cognitive interventions. Perhaps the combination of interventions that focus on different pathways of change may have a synergistic effect and result in more pronounced cognitive improvements. Increasing our knowledge of the ways in which sleep may impact late-life cognitive functioning could have far reaching benefits.
Key Points.
Sleep and cognitive functioning both demonstrate negative changes with advanced age.
Though the relationships are not without uncertainties, sleep appears to be related to cognitive functioning within good sleeping older adults, older adults with insomnia, and older adults with sleep disordered breathing.
Both insomnia and sleep apnea may be associated with cognitive decline and dementia.
Treatment of sleep disorders may provide cognitive benefits in late-life. Additional research is warranted.
Acknowledgments
Dr. Dzierzewski was supported by a grant from the National Institute on Aging (K23AG049955). Dr. Dautovich serves as a sleep consultant for the National Sleep Foundation and Merck Sharp & Dohme Corp.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement:
No other authors report commercial or financial conflicts of interest.
Contributor Information
Joseph M. Dzierzewski, Department of Psychology, Virginia Commonwealth University, 806 West Franklin St., Room 306, PO Box 842018, Richmond, VA 23284-2018.
Natalie Dautovich, Department of Psychology, Virginia Commonwealth University, 800 West Franklin St., Room 203, PO Box 842018, Richmond, VA 23284-2018.
Scott Ravyts, Department of Psychology, Virginia Commonwealth University, PO Box 842018, Richmond, VA 23284-2018.
References
- 1.Li S-C, Huxhold O, Schmiedek F. Aging and Attenuated Processing Robustness. Gerontology. 2003;50(1):28–34. doi: 10.1159/000074386. [DOI] [PubMed] [Google Scholar]
- 2.Nesselroade JR. The warp and the woof of the developmental fabric. Vis Aesthet Environ Dev Leg Joachim F Wohlwill. 1991:213–240. [Google Scholar]
- 3.Park DC, Smith AD, Lautenschlager G, et al. Mediators of long-term memory performance across the life span. Psychol Aging. 1996;11(4):621. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- 4.Schaie KW, Willis SL, Caskie GI. The Seattle longitudinal study: Relationship between personality and cognition. Aging Neuropsychol Cogn. 2004;11(2–3):304–324. doi: 10.1080/13825580490511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salthouse TA. What and when of cognitive aging. Curr Dir Psychol Sci. 2004;13(4):140–144. [Google Scholar]
- 6.Morgan K. Sleep and Aging. In: Lichstein K, Morin C, editors. Treatment of Late-Life Insomnia. Thousand Oaks, CA: Sage Publications; 2000. [Google Scholar]
- 7.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons – an epidemiological study of 3 communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Enright PL, Manolio TA, Haponik EF, Wahl PW. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 1997;45(1):1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 9.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 10.Faubel R, López-GarcÍa E, Guallar-castillÓn P, Graciani A, Banegas JR, RodrÍguez-artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res. 2009;18(4):427–435. doi: 10.1111/j.1365-2869.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 11.Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18(4):436–446. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 12.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20(1):41–48. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 13.Bastien CH, Fortier-Brochu É, Rioux I, LeBlanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia: relationship between objective and subjective measures. J Psychosom Res. 2003;54(1):39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- 14.Crenshaw MC, Edinger JD. Slow-Wave Sleep and Waking Cognitive Performance Among Older Adults With and Without Insomnia Complaints. Physiol Behav. 1999;66(3):485–492. doi: 10.1016/S0031-9384(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 15.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61(4):405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 16.Oosterman JM, van Someren EJ, Vogels RL, van Harten B, Scherder EJ. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res. 2009;18(1):129–135. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 17.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb WB, Levy CM. Age, Sleep Deprivation, and Performance. Psychophysiology. 1982;19(3):272–276. doi: 10.1111/j.1469-8986.1982.tb02561.x. [DOI] [PubMed] [Google Scholar]
- 19.Webb WB. A Further Analysis of Age and Sleep Deprivation Effects. Psychophysiology. 1985;22(2):156–161. doi: 10.1111/j.1469-8986.1985.tb01579.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5(6):463–475. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 21.Bliese PD, Wesensten NJ, Balkin TJ. Age and individual variability in performance during sleep restriction. J Sleep Res. 2006;15(4):376–385. doi: 10.1111/j.1365-2869.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 22.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker M, McKinley S, Stickgold R. Sleep optimizes motor skill in older adults. J Am Geriatr Soc. 2011;59(4):603–609. doi: 10.1111/j.1532-5415.2011.03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16(1):83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Altena E, Van Der Werf YD, Strijers RLM, Van Someren EJW. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. J Sleep Res. 2008;17(3):335–343. doi: 10.1111/j.1365-2869.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 26.Haimov I, Hanuka E, Horowitz Y. Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6(1):32–54. doi: 10.1080/15402000701796080. [DOI] [PubMed] [Google Scholar]
- 27.Ling A, Lim ML, Gwee X, Ho RCM, Collinson SL, Ng T-P. Insomnia and daytime neuropsychological test performance in older adults. Sleep Med. 2016;17:7–12. doi: 10.1016/j.sleep.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 28.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11):2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 29.Chen P-L, Lee W-J, Sun W-Z, Oyang Y-J, Fuh J-L. Risk of dementia in patients with insomnia and long-term use of hypnotics: A population-based retrospective cohort study. In: Forloni G, editor. PLoS ONE. 11. Vol. 7. 2012. p. e49113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cricco M, Simonsick EM, Foley DJ. The Impact of Insomnia on Cognitive Functioning in Older Adults. J Am Geriatr Soc. 2001;49(9):1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 31.Jaussent I, Bouyer J, Ancelin M-L, et al. Excessive sleepiness is predictive of cognitive decline in the elderly. SLEEP. 2012 Sep; doi: 10.5665/sleep.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao V, Spiro J, Samus QM, et al. Insomnia and daytime sleepiness in people with dementia residing in assisted living: findings from the Maryland Assisted Living Study. Int J Geriatr Psychiatry. 2008;23(2):199–206. doi: 10.1002/gps.1863. [DOI] [PubMed] [Google Scholar]
- 33.Foley D, Monjan A, Masaki K, Havlik R, White L, Launer L. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49(12):1628–1632. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- 34.Keage HAD, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13(7):886–892. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Merlino G, Piani A, Gigli GL, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: A population-based study. Sleep Med. 2010;11(4):372–377. doi: 10.1016/j.sleep.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Ohadinia S, Noroozian M, Shahsavand S, Saghafi S. Evaluation of insomnia and daytime napping in Iranian Alzheimer disease patients: relationship with severity of dementia and comparison with normal adults. Am J Geriatr Psychiatry. 2004;12(5):517–522. doi: 10.1176/appi.ajgp.12.5.517. [DOI] [PubMed] [Google Scholar]
- 37.Wilckens KA, Hall MH, Nebes RD, Monk TH, Buysse DJ. Changes in Cognitive Performance Are Associated with Changes in Sleep in Older Adults With Insomnia. Behav Sleep Med. 2016;14(3):295–310. doi: 10.1080/15402002.2014.1002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 40.Ayalon L, Ancoli-Israel S, Drummond SP. Obstructive sleep apnea and age: a double insult to brain function? Am J Respir Crit Care Med. 2010;182(3):413–419. doi: 10.1164/rccm.200912-1805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen-Zion M, Stepnowsky C, Marler, Shochat T, Kripke DF, Ancoli-Israel S. Changes in Cognitive Function Associated with Sleep Disordered Breathing in Older People. J Am Geriatr Soc. 2001;49(12):1622–1627. doi: 10.1111/j.1532-5415.2001.49270.x. [DOI] [PubMed] [Google Scholar]
- 42.Spira AP, Blackwell T, Stone KL, et al. Sleep-Disordered Breathing and Cognition in Older Women. J Am Geriatr Soc. 2008;56(1):45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman ME, Aloia MS. Sleep-Disordered Breathing and Cognition in Older Adults. Curr Neurol Neurosci Rep. 2012;12(5):537–546. doi: 10.1007/s11910-012-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Associations Between Sleep Architecture and Sleep-Disordered Breathing and Cognition in Older Community-Dwelling Men: The Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2011;59(12):2217–2225. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30(10):1309–1316. doi: 10.1093/sleep/30.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dealberto MJ, Pajot N, Courbon D, Alpérovitch A. Breathing disorders during sleep and cognitive performance in an older community sample: the EVA Study. J Am Geriatr Soc. 1996;44(11):1287–1294. doi: 10.1111/j.1532-5415.1996.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 47.Hayward L, Mant A, Eyland A, et al. Sleep disordered breathing and cognitive function in a retirement village population. Age Ageing. 1992;21(2):121–128. doi: 10.1093/ageing/21.2.121. [DOI] [PubMed] [Google Scholar]
- 48.Saint Martin M, Sforza E, Roche F, Barthélémy JC, Thomas-Anterion C. Sleep Breathing Disorders and Cognitive Function in the Elderly: An 8-Year Follow-up Study. The Proof-Synapse Cohort. Sleep. 2015;38(2):179–187. doi: 10.5665/sleep.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yesavage J, Bliwise D, Guilleminault C, Carskadon M, Dement W. Preliminary communication: intellectual deficit and sleep-related respiratory disturbance in the elderly. Sleep. 1985;8(1):30–33. doi: 10.1093/sleep/8.1.30. [DOI] [PubMed] [Google Scholar]
- 50.Alchanatis M, Zias N, Deligiorgis N, et al. Comparison of cognitive performance among different age groups in patients with obstructive sleep apnea. Sleep Breath. 2008;12(1):17–24. doi: 10.1007/s11325-007-0133-y. [DOI] [PubMed] [Google Scholar]
- 51.Crawford-Achour E, Dauphinot V, Saint Martin M, et al. Protective Effect of Long-Term CPAP Therapy on Cognitive Performance in Elderly Patients with Severe OSA: The PROOF Study. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2015;11(5):519–524. doi: 10.5664/jcsm.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ju G, Yoon I-Y, Lee SD, Kim TH, Choe JY, Kim KW. Effects of Sleep Apnea Syndrome on Delayed Memory and Executive Function in Elderly Adults. J Am Geriatr Soc. 2012;60(6):1099–1103. doi: 10.1111/j.1532-5415.2012.03961.x. [DOI] [PubMed] [Google Scholar]
- 53.Berry DT, Phillips BA, Cook YR, et al. Geriatric sleep apnea syndrome: a preliminary description. J Gerontol. 1990;45(5):M169–174. doi: 10.1093/geronj/45.5.m169. [DOI] [PubMed] [Google Scholar]
- 54.Aloia MS, Ilniczky N, Di Dio P, Perlis ML, Greenblatt DW, Giles DE. Neuropsychological changes and treatment compliance in older adults with sleep apnea. J Psychosom Res. 2003;54(1):71–76. doi: 10.1016/S0022-3999(02)00548-2. [DOI] [PubMed] [Google Scholar]
- 55.O’Hara R, Schröder CM, Kraemer HC, et al. Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE ε4 carriers. Neurology. 2005;65(4):642–644. doi: 10.1212/01.wnl.0000173055.75950.bf. [DOI] [PubMed] [Google Scholar]
- 56.Martin MS, Sforza E, Roche F, Barthélémy JC, Thomas-Anterion C, PROOF study group Sleep breathing disorders and cognitive function in the elderly: an 8-year follow-up study. the proof-synapse cohort. Sleep. 2015;38(2):179–187. doi: 10.5665/sleep.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ancoli-Israel S, Ayalon L, Salzman C. Sleep in the elderly: normal variations and common sleep disorders. Harv Rev Psychiatry. 2008;16(5):279–286. doi: 10.1080/10673220802432210. [DOI] [PubMed] [Google Scholar]
- 60.Blackwell T, Yaffe K, Laffan A, et al. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2015;63(3):453–461. doi: 10.1111/jgs.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 62.Mathieu A, Mazza S, Décary A, et al. Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patients. Sleep Med. 2008;9(2):112–120. doi: 10.1016/j.sleep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Boland LL, Shahar E, Iber C, et al. Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: the Sleep Heart Health Study. J Sleep Res. 2002;11(3):265–272. doi: 10.1046/j.1365-2869.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- 64.Sforza E, Roche F, Thomas-Anterion C, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33(4):515–521. doi: 10.1093/sleep/33.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foley DJ, Masaki K, White L, Larkin EK, Monjan A, Redline S. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep. 2003;26(5):596–599. doi: 10.1093/sleep/26.5.596. [DOI] [PubMed] [Google Scholar]
- 66.Portier F, Portmann A, Czernichow P, et al. Evaluation of Home versus Laboratory Polysomnography in the Diagnosis of Sleep Apnea Syndrome. Am J Respir Crit Care Med. 2000;162(3):814–818. doi: 10.1164/ajrccm.162.3.9908002. [DOI] [PubMed] [Google Scholar]
- 67.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. SLEEP-N Y THEN Westchest. 2003;26(3):298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 68.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: A meta-review. Respirology. 2013;18(1):61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 69.Ingram F, Henke KG, Levin HS, Ingram PT, Kuna ST. Sleep apnea and vigilance performance in a community-dwelling older sample. Sleep. 1994;17(3):248–252. doi: 10.1093/sleep/17.3.248. [DOI] [PubMed] [Google Scholar]
- 70.Kim SJ, Lee JH, Lee DY, Jhoo JH, Woo JI. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2011;19(4):374–381. doi: 10.1097/JGP.0b013e3181e9b976. [DOI] [PubMed] [Google Scholar]
- 71.Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on Cognition, Brain Function, and Structure Among Elderly Patients With OSA: A Randomized Pilot Study. Chest. 2015;148(5):1214–1223. doi: 10.1378/chest.15-0171. [DOI] [PubMed] [Google Scholar]
- 72.Kang S-H, Yoon I-Y, Lee SD, Kim T. Effects of Continuous Positive Airway Pressure Treatment on Cognitive Functions in the Korean Elderly with Obstructive Sleep Apnea. Sleep Med Res. 2016;7(1):10–15. doi: 10.17241/smr.2016.00010. [DOI] [Google Scholar]
- 73.Cooke JR, Ancoli-Israel S, Liu L, et al. Continuous positive airway pressure deepens sleep in patients with Alzheimer’s disease and obstructive sleep apnea. Sleep Med. 2009;10(10):1101–1106. doi: 10.1016/j.sleep.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russo-Magno P, O’Brien A, Panciera T, Rounds S, et al. Compliance with CPAP therapy in older men with obstructive sleep apnea. J Am Geriatr Soc. 2001;49(9):1205–1211. doi: 10.1046/j.1532-5415.2001.49238.x. [DOI] [PubMed] [Google Scholar]
- 75.Pilcher JJ, Band D, Odle-Dusseau HN, Muth ER. Human performance under sustained operations and acute sleep deprivation conditions: toward a model of controlled attention. Aviat Space Environ Med. 2007;78(Supplement 1):B15–B24. [PubMed] [Google Scholar]
- 76.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6(3):236. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 77.Bonnet MH, Arand DL. 24-Hour Metabolic-Rate in Insomniacs and Matched Normal Sleepers. Sleep. 1995;18:581–588. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 78.Richardson GS. Human physiological models of insomnia. Sleep Med. 2007;8(Supplement 4):S9–S14. doi: 10.1016/S1389-9457(08)70003-0. [DOI] [PubMed] [Google Scholar]
- 79.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance and psychomotor vigilance performance decrements during aweek of sleep restricted to 4–5 hours per night. Sleep J Sleep Res Sleep Med. 1997 http://psycnet.apa.org/psycinfo/1997-06077-003. Accessed September 27, 2016. [PubMed]
- 80.Durmer JS, Dinges DF. Seminars in Neurology. Vol. 25. Copyright\copyright 2005 by Thieme Medical Publishers, Inc; 333 Seventh Avenue, New York, NY 10001, USA: 2005. Neurocognitive consequences of sleep deprivation; pp. 117–129. https://www.thieme-connect.com/products/ejournals/html/10.1055/s-2005-867080. Accessed September 27, 2016. [DOI] [PubMed] [Google Scholar]
- 81.Goel N, Rao H, Durmer JS, Dinges DF. Seminars in Neurology. Vol. 29. copyright Thieme Medical Publishers; 2009. Neurocognitive consequences of sleep deprivation; pp. 320–339. https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0029-1237117. Accessed October 3, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boonstra TW, Stins JF, Daffertshofer A, Beek PJ. Effects of sleep deprivation on neural functioning: an integrative review. Cell Mol Life Sci. 2007;64(7–8):934–946. doi: 10.1007/s00018-007-6457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


