Abstract
Background: Diarrhoea and acute lower respiratory infections are leading causes of childhood morbidity and mortality, which can be prevented by simple low-cost interventions. Integrated strategies can provide additional benefits by addressing multiple health burdens simultaneously.
Methods: We conducted a community-randomized–controlled trial in 51 rural communities in Peru to evaluate whether an environmental home-based intervention package, consisting of improved solid-fuel stoves, kitchen sinks, solar disinfection of drinking water and hygiene promotion, reduces lower respiratory infections, diarrhoeal disease and improves growth in children younger than 36 months. The attention control group received an early child stimulation programme.
Results: We recorded 24 647 child-days of observation from 250 households in the intervention and 253 in the attention control group during 12-month follow-up. Mean diarrhoea incidence was 2.8 episodes per child-year in the intervention compared with 3.1 episodes in the control arm. This corresponds to a relative rate of 0.78 [95% confidence interval (CI): 0.58–1.05] for diarrhoea incidence and an odds ratio of 0.71 (95% CI: 0.47–1.06) for diarrhoea prevalence. No effects on acute lower respiratory infections or children’s growth rates were observed.
Conclusions: Combined home-based environmental interventions slightly reduced childhood diarrhoea, but the confidence interval included unity. Effects on growth and respiratory outcomes were not observed, despite high user compliance of the interventions. The absent effect on respiratory health might be due to insufficient household air quality improvements of the improved stoves and additional time needed to achieve attitudinal and behaviour change when providing composite interventions.
Keywords: Community-randomised trial, integrated interventions, household air pollution, household water treatment, improved cook stove, kitchen hygiene, hand-washing
Key Messages
Combined kitchen–environmental interventions, including an improved solid-fuel stove, a kitchen sink, solar treatment of drinking water and hygiene promotion, are successfully implemented at the household level. Convenience gains from improved cooking stoves and kitchen sinks are highly valued by the beneficiaries.
Integrated home-based interventions might have reduced childhood diarrhoea, but failed to impact respiratory infections and child growth.
Reasons for the lack of an effect on respiratory health might be due to insufficient reduction of household air pollution of the improved stoves and duration of follow-up.
Introduction
Diarrhoea and acute lower respiratory infections (ALRI) remain leading causes of childhood morbidity and mortality.1 Unsafe drinking water, poor sanitation, lack of personal hygiene and poor household air quality are considered amongst the most important risk factors for those diseases.2,3
Interventions to improve drinking water, sanitation and hygiene have been shown consistently to reduce diarrhoeal disease.4–7 Similarly, the odds for acute respiratory infections (ARI) were 3.5 times and for pneumonia 78% higher in children exposed to biomass fuels compared with non-exposed children.8,9 A randomized–controlled trial providing improved solid-fuel stoves to rural households in Guatemala found a rate ratio of 0.84 [95% confidence interval (CI): 0.63, 1.13] for physician-diagnosed childhood pneumonia comparing households in the intervention to those in the control group which reduced to 0.67 (95% CI: 0.45, 0.98) after multiple imputation and limited to severe pneumonia.10
Combining potentially synergistic interventions has been advocated before in the drinking-water and sanitation sector.11,12 In the presented trial, we combine interventions to tackle various household-related risks simultaneously. The interventions for this study were developed using a participatory approach during a six-month pilot phase.13,14 We identified and convened main stakeholders and beneficiaries to develop an intervention package that generates healthy household environments, addresses local beliefs and cultural views, and has potentially synergistic effects on household health and livelihoods. Additionally, the attention control group received an early child stimulation intervention to reduce bias from the open, i.e. non-blinded, trial design, which was judged to be especially important in home-based interventions.4,15
The main objective was to reduce respiratory infections and diarrhoea and to improve child growth in children less than 36 months, through an integrated environmental home-based intervention package (IHIP), comprising improved solid-fuel stoves, kitchen sinks, solar disinfection of drinking water and hygiene promotion.
Methods
Ethics
The study was approved by the ethical review board of the Nutritional Research Institute (Instituto de Investigación Nutricional, IIN), the cantonal ethical review board of Basel, Switzerland, Switzerland (Ethikkommission beider Basel, EKBB), the Cajamarca Regional Health Authority and the Peruvian National Institute of Health (Instituto Nacional de Salud, INS: 2-05-70-08-012). It was registered at a national (INS) and an international trial registry (ISRCTN: ‘ISRCTN28191222’). Community leaders and local authorities signed an agreement with the IIN and Swiss Tropical and Public Health Institute (Swiss TPH) after screening for eligibility and before randomization. The principal caregiver of each study child gave written informed consent before study implementation. Sick study children were evaluated by the study physician or referred for treatment.
Site and population
The study was conducted from September 2008 to January 2010 in the San Marcos province, located 60 kilometres south-east of Cajamarca city, in northern Peru. We chose this area because of its well-separated and accessible communities and because, to our knowledge, no major health promotion programmes were currently implemented. The province is located between 2200 and 3900 metres above sea level. Most of the population are small-scale farmers. At the time of the study, most people were using an unventilated traditional stove or open fire for cooking and heating within their homes. About 80% of the population had a piped-water system with a faucet available in the household’s yard.
Study design
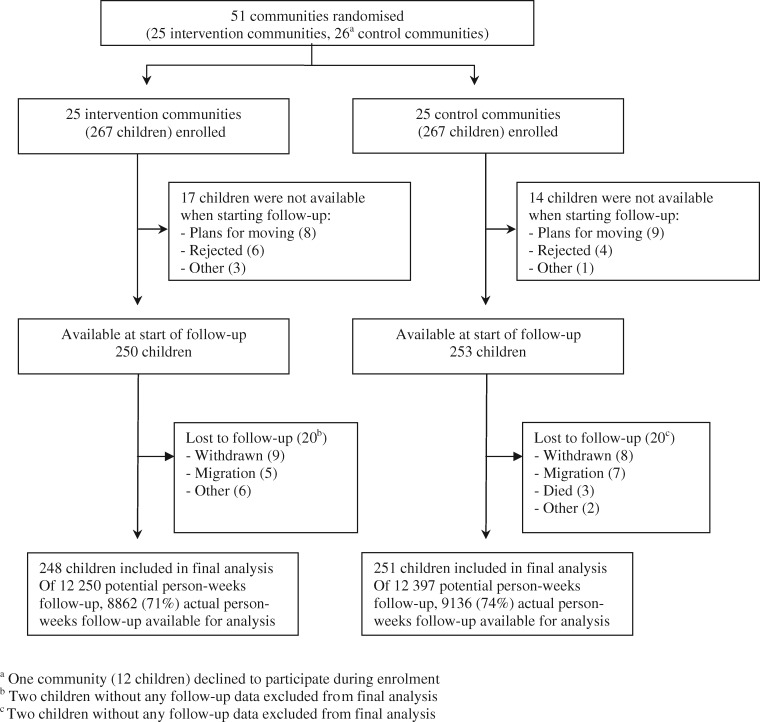
We implemented a community-randomized–controlled field trial to evaluate the IHIP interventions on reducing acute diarrhoeal illness and ARI, and improving child growth over a 12-month surveillance period. Our primary sampling units were the communities. Sample size was calculated for cluster-randomized trials using the approach of Hayes and Bennett.14 The trial was powered to detect an incidence rate (IR) reduction of 22% with 80% power at a 5% level of significance, assuming five episodes of ARI and five episodes of diarrhoea per child-year of observation and a coefficient of variation of k = 0.2. Fifty-six communities were identified by a house-to-house screening. We included only 51 communities, as five communities were very small, with fewer than four children. Three of the five communities were joined to adjacent communities and the other two were excluded because of remoteness. Within the included communities, one child aged 6–35 months was randomly selected from each eligible household willing to participate. Eligibility criteria included use of solid fuels, no public sewage connection and no intention to move during the study period. Randomization was performed at the village level. The 51 communities were randomized using covariate-based constrained randomization—a procedure that can balance individual- and group-level covariates in the experimental units, here the communities, in a group-randomized study.14,15 Randomization, enrolment and baseline data collection took place between September 2008 and January 2009 (Figure 1). Blinding of the interventions was not possible. To counteract potential unbalance of dropouts between study arms and non-blinding bias, an early child stimulation intervention, which seemed unlikely to have an impact on child diarrhoea and respiratory infections, was implemented as attention control. More details on study design can be found elsewhere.16
Figure 1.
Flow of participants.
Development of interventions
The components of the IHIP interventions were developed with a participatory approach during a six-month pilot phase in neighbouring communities not enrolled in the trial.15,16 We identified and convened main stakeholders and beneficiaries to develop an intervention package that generates healthy household environments, addresses local beliefs and cultural views, and has potentially synergistic effects on household health and livelihoods. We investigated efficacy and acceptability of the interventions, i.e. providing the stoves, kitchen sinks and plastic bottles for solar water treatment, and hygiene education. With the community members’ involvement, an improved solid-fuel stove called the ‘OPTIMA-improved stove’ and a kitchen sink providing piped water within the household’s kitchen were developed.17 The stoves were built with local materials to enable self-maintenance and repair. Nine months after installation, all stoves were revisited and repaired as needed by the original stove builders. Mothers/caretakers were also trained in solar drinking-water disinfection (SODIS) according to standard procedures.18 Mothers were instructed to wash their own and children’s hands with soap or detergent after defecation, after changing diapers, before food preparation and before eating. Additionally, mothers were instructed to separate animals and their excreta from the kitchen environment. The IHIP highlights include:
components: an improved ventilated solid-fuel stove, a kitchen sink with in-kitchen water connection, a point-of-use water-quality intervention applying solar disinfection to drinking water and a hygiene intervention focusing on hand-washing with soap and kitchen hygiene;
aims: to reduce childhood respiratory infections and diarrhoea via reduced household air pollution, increased quality and quantity of drinking water and water used for hygiene purposes, and improved personal and kitchen hygiene;
development: community engagement in the design and development of the interventions (namely involvement of local and regional stakeholders to assure development-, health- and education-sector engagement in the design and post-intervention scale-up phases).
More information on stove performance, the microbiological efficacy of SODIS and the qualitative assessment of perceptions are described elsewhere.16,17,19,20 The intervention in the control communities was based on the National WawaWasi early child development (ECD) programme, which provided psychomotor and cognitive stimulation in children under four years of age at day-care centres.21 Together with WawaWasi experts, we adapted the intervention to be applied at the household level and trained field staff. Mothers were trained in the use of the ECD toys and materials and instructed to play with their children for at least 30 minutes every day.
Training of field staff
Four teams, which received extensive specific training, were responsible for data collection. The field research team collected morbidity data and was trained in interviewing techniques, data recording, identification of signs and symptoms of child diarrhoea, and ALRI severity symptoms, as well as measuring respiratory rates. Additionally, the team collected spot-check observations using a checklist on household hygiene and environmental health conditions (e.g. presence of SODIS bottles on the roof or kitchen). The health promoters locally hired elementary school teachers, implemented and promoted the interventions and collected monthly compliance data. The anthropometric team was trained in measuring child weight and height in a standardized way. The environmental team collected environmental samples to test for faecal contamination of mothers’ hands, drinking water and kitchen cloths.
Implementation
The IHIP interventions (improved solid-fuel stoves and kitchen sinks) were installed between October 2008 and January 2009. Households without connection to piped water were connected during sink installation. SODIS and personal, child and kitchen hygiene were reinforced monthly during 12-month follow-up. Each child in the control group received six sets of toys approximately every two months, depending on the child’s progress and age.16,17 The promotion of the interventions was done with the same intensity in both groups.
Data collection
Follow-up took place from February 2009 to January 2010. Field workers visited each household weekly and collected morbidity data from the mother/caretaker on daily signs and symptoms of child diarrhoea and respiratory illness. If diarrhoea was observed, additional information on severity was collected (sunken eyes, dry mouth, tongue and mucous membranes and thirstiness). If a child had cough or fever on the day of the household visit or the previous day, we looked for danger signs22 to assess the severity of the respiratory illness by recording noisy and/or fast breathing, rhonchus/wheezing, lower chest in-draw, malaise and lack of appetite. If any of the severity signs were present, the child was examined and treated by our study physician on the same day or referred to local healthcare services. Specific questions determined the child’s health at the moment of the weekly home visit, including seeking outpatient care, hospitalization and type of medical treatment.
Height and weight were collected every two months. In deviation to the original protocol, height and weight measurements were done only once per visit instead of repeating the measurements three times. Environmental samples from the mother’s hands, kitchen cloths and drinking water were collected at baseline, mid-term and end of the surveillance period.23 However, we did not collect data on breastfeeding or child-feeding practices as potential confounders of diarrhoea and anthropometric outcomes.
Outcome measurements
Diarrhoea was defined as three or more liquid or semi-liquid stools in a 24-hour period or one stool with blood and/or mucus.24 An episode was defined to begin on the first day of diarrhoea and ended the last day of diarrhoea, followed with at least two consecutive non-diarrhoeal days.
ARI was defined as a child presenting cough and/or difficulty breathing. ALRI was defined as a child presenting cough or difficulty breathing, with a raised respiratory rate (>50 per min in children aged 6–11 months and >40 per min in children aged ≥12 months) on two consecutive measurements.22,25 An episode was defined to begin on the first day of cough or difficulty breathing and ended with the last day of the same combination, followed by at least seven days without those symptoms.25
Stunting, wasting and underweight as defined by the World Health Organization (WHO) were used to evaluate child nutritional status.26
Statistical analysis
We applied an intention-to-treat analysis comparing incidence rate of diarrhoea and respiratory infection per child-year in intervention vs control communities. Longitudinal prevalence (LP) was calculated as the number of illness days per days under observation. All children with at least one day of follow-up were included in the analysis. Generalized estimating equation (GEE) models were fitted to adjust for correlation within villages.27 The unadjusted model included only the design factors and the intervention effect. Further models adjusted for child’s age and sex. No imputation of missing data has been performed.
The statistical models included the log link function for negative binomial (relative rate RR) and logit for binomial distributed data (odds ratio OR). The logarithm of days under observation was included as offset variable in the count models. The statistical analyses were performed using SAS software v9.3 (PROC GENMOD, SAS Institute Inc.). Data management, cleaning and descriptive analysis were done using R V3.0.0 (R development core team). The coefficient of variation (k) and the 95% credible interval were estimated via Bayesian generalized random effects models using WinBUGS 1.4.
Results
Of the 51 communities, 25 communities (267 households) were randomized to the intervention and 26 (267 households) to the control arm (Figure 1). One community in the control group declined to participate. Further details on participant flow before start of follow-up are described elsewhere.16 The final analysis included 248 children from intervention and 251 children from control communities. Information on morbidity was collected for about 18 000 person-weeks, representing 71% and 74% of the total possible observation time in intervention and control arms.
Baseline characteristics were balanced between study arms with the exception of access to piped water (Table 1). Both study groups were ‘poor’ according to national standards (Table 1).28 Despite the high coverage of piped-water supply (80%), about 65% of drinking-water samples were contaminated with Escherichia coli and 10% of these were faecally contaminated with diarrhoeagenic E. coli.23 Further socio-demographic, household and environmental baseline context is also described elsewhere.16
Table 1.
Demographics and socio-economic characteristics of 503 households in rural Peru
| Characteristics | Intervention arm |
Control arm |
||
|---|---|---|---|---|
| Number | Mean (SD) or % | Number | Mean (SD) or % | |
| Demography | ||||
| Number of household members | 226 | 5.0 (1.6) | 234 | 4.6 (1.5) |
| Age in years of enrolled children | 250 | 2.1 (0.7) | 253 | 2.1 (0.7) |
| Female children | 250 | 50% | 253 | 50% |
| National poverty indicatorsa | ||||
| 1 unsatisfied basic need | 224 | 17% | 231 | 23% |
| 2 unsatisfied basic need | 224 | 25% | 231 | 28% |
| 3 unsatisfied basic need | 224 | 40% | 231 | 35% |
| 4 unsatisfied basic need | 224 | 14% | 231 | 10% |
| Household characteristics | ||||
| Household with latrines | 245 | 80% | 239 | 84% |
| Piped-water supply | 245 | 74% | 239 | 82% |
| Microbiological indicatorsb | ||||
| Drinking water | 88 | 68% | 94 | 64% |
| Kitchen wipes | 56 | 34% | 35 | 25% |
| Mother’s hands | 95 | 27% | 109 | 22% |
| Anthropometrics | ||||
| Height-for-age Z-scores [median (IQR)] | 196 | –2.2 (–2.7, –1.4) | 194 | –2.0 (–2.5, –1.4) |
| Weight-for-age Z-scores [median (IQR)] | 201 | –0.8 (–1.2, –0.2) | 202 | –0.7 (–1.2, –0.1) |
The National Poverty Indicators comprise five basic parameters: (i) inappropriate infrastructure; (ii) crowding; (iii) lack of access to basic sanitation; (iv) having at least one child of school age not attending school; and (v) family head with at least three dependents with incomplete primary-level education. A household is considered ‘poor’ if they have one unsatisfied basic need.27
E. coli-positive samples.
Diarrhoea morbidity
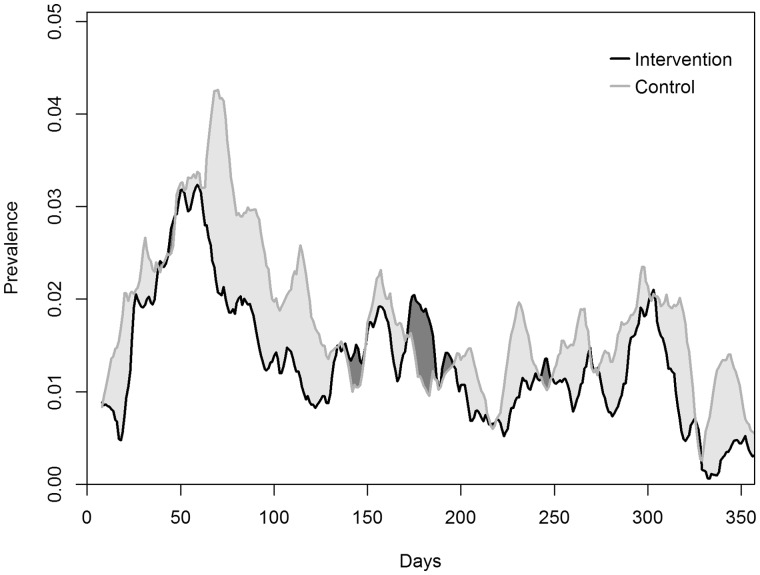
Children in the intervention arm reported a total of 301 diarrhoea episodes, which corresponds to a mean of 1.8 episodes per child-year. In the control arm, 375 episodes and a mean of 2.2 episodes per child-year occurred. The mean episode length of 2.8 days was shorter in the intervention arm compared with 3.1 days in the control arm (Table 2). The statistical analysis estimated that children in the intervention communities had 22% fewer diarrhoea episodes per year compared with children in control communities [RR: 0.78, 95% CI: 0.58–1.05, p = 0.10]. A similar result was found for the LP of diarrhoea, with an OR of 0.71 (95% CI: 0.47–1.06, p = 0.09) (Table 3). The clustering coefficient k was 0.39 (95% confidence interval: 0.25–0.57). The prevalence of child diarrhoea indicated no evident temporal effect throughout the follow-up period (Figure 2). To confirm that findings were not sensitive to the choice of covariates, we reanalysed including piped water and/or latrine ownership in the model. None of the models yielded major changes in the point estimates or confidence intervals.
Table 2.
Descriptive statistics of main diarrhoeal and respiratory health outcomes and anthropometric measurements
| Health conditions | Class or parameter | Intervention (N = 248) | Control (N = 251) |
|---|---|---|---|
| Days under observation | Median (IQR) | 265 (225–293) | 276 (235–297) |
| Days under observation | Total | 62 031 | 63 952 |
| Diarrhoeal illness | |||
| Number of episodes | Median (IQR) | 1 (0–2) | 1 (0–2) |
| Days with diarrhoea | Median (IQR) | 2 (0–4) | 2 (0–6) |
| Total number of days with diarrhoea | Total | 827 | 1125 |
| Total number of episodes | Total | 301 | 375 |
| Total number of persistent episodes (>14 days’ duration) | Total | 0 | 4 |
| Mean length of episode (days) | 2.8 | 3.1 | |
| Diarrhoea incidence (number of episodes/child-year) | Mean | 1.8 | 2.2 |
| Diarrhoea prevalence (number of diarrhoeal days/child-year) | Mean | 4.9 | 6.6 |
| Number of diarrhoeal episodes with blood | Total | 17 | 24 |
| Number of diarrhoeal episodes with vomiting | Total | 51 | 54 |
| Respiratory infections | |||
| Days with cough or difficulties breathing | Median (IQR) | 17 (8–25) | 14 (8–26) |
| Total number of days with cough or difficulties breathing | Total | 4534 | 4635 |
| Total number of days with cough or difficulties breathing and fever | Total | 951 | 1034 |
| Total number of ARI episodes | Total | 831 | 877 |
| Percentage of ARI episodes seen with respiratory rate measurements | % | 68% (554) | 63% (563) |
| Total number of ALRI episodes | Total | 25/554a | 10/563b |
| Number of children with at least one ALRI episode | Total | 17 | 10 |
| Anthropometrics | |||
| Height-for-age Z-scores [median (IQR)] | Median (IQR) | –2.1 (–2.7/–1.3) | –1.9 (–2.5/–1.4) |
| Weight-for-age Z-scores [median (IQR)] | Median (IQR) | –0.6 (–1.1/–0.2) | –0.7 (–1.2/–0.2) |
ARI, acute respiratory infections; ALRI, acute lower respiratory infections. aIn 255/554 episodes, the mother started medical treatment before the field worker assessed the respiratory rate. bIn 218/563 episodes, the mother started medical treatment before the field worker assessed the respiratory rate.
Table 3.
Effect of the intervention on diarrhoea and acute respiratory infections
| Outcome | Crude modela |
Age sex modelb |
||||
|---|---|---|---|---|---|---|
| (n = 499) | RR/OR | 95% CI | p-value | RR/OR | 95% CI | p-value |
| Number of diarrhoea episodesc (RR) | 0.78 | 0.58, 1.05 | 0.10 | 0.79 | 0.60, 1.03 | 0.09 |
| Diarrhoea prevalence (OR) | 0.71 | 0.47, 1.06 | 0.09 | 0.72 | 0.49, 1.05 | 0.09 |
| Episodes with blood (OR) | 0.80 | 0.39, 1.65 | 0.55 | 0.80 | 0.39, 1.65 | 0.54 |
| Number of ARI episodes (RR) | 0.95 | 0.82, 1.10 | 0.53 | 0.95 | 0.82, 1.10 | 0.51 |
| Number of ALRI episodes (RR) | 2.45 | 0.82, 7.39 | 0.11 | 2.47 | 0.84, 7.29 | 0.10 |
| Cough or difficulty breathing prevalence (OR) | 0.97 | 0.79, 1.19 | 0.80 | 0.97 | 0.79, 1.19 | 0.79 |
| Cough or difficulty breathing and fever prevalence (OR) | 0.89 | 0.71, 1.12 | 0.33 | 0.89 | 0.71, 1.12 | 0.33 |
Number of episodes: number of episodes per child-year; prevalence: number of days ill per days under observation; ARI, acute respiratory infections; ALRI, acute lower respiratory infections.
Adjusted for design factor (intra-village correlation).
Adjusted for child’s age and sex and design factor (intra-village correlation).
Clustering coefficient k = 0.39.
Figure 2.
Diarrhoea prevalence over time. Presented are unweighted moving averages using a bandwidth of two weeks.
Respiratory infections
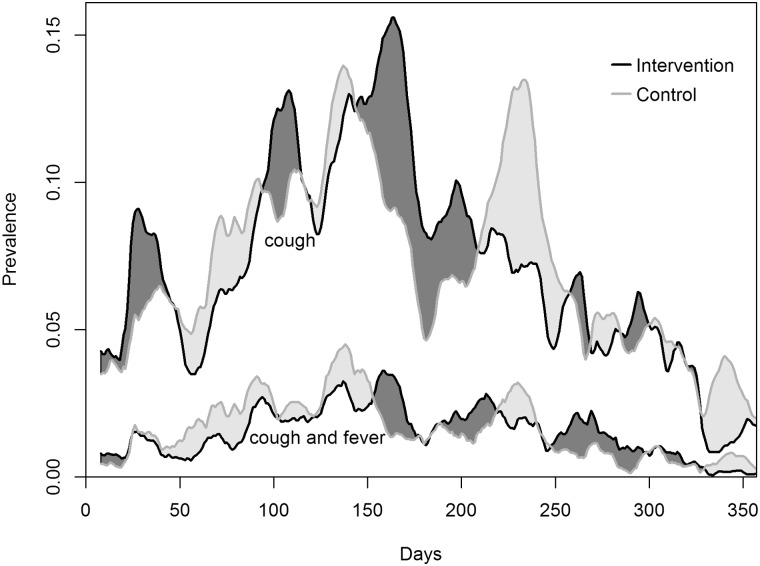
The total number of ARI episodes was 831 in the intervention group and 877 in the control group (Table 2). Out of these, we achieved 68% and 63% of respiratory rate measurements in the intervention and control groups, respectively, corresponding to 554 and 563 ARI episodes with respiratory rate assessment. In about 50% of ARI episodes, the child had already received medical treatment before respiratory rate assessment. The total numbers of ALRI episodes were 25 in the intervention and 10 in the control group (Table 2). The RR for ARI episodes was 0.95 (0.39, 1.65; p-value 0.53) and 2.45 (95% CI: 0.82 to 7.39; p-value 0.11) for ALRI. The ORs associated with cough or difficulty breathing prevalence, and cough or difficulty breathing and fever prevalence were close to 1 (Table 3). Prevalences over time are illustrated in Figure 3.
Figure 3.
Cough or difficulty breathing prevalence and cough or difficulty breathing and fever prevalence over time. Presented are unweighted moving averages using a bandwidth of two weeks.
Anthropometric measurements
At baseline, children of both study arms had similar frequencies of stunting (median of –2.2 and –2.0 z-scores below average WHO growth standards in intervention and control arm) and underweight (median –0.8 and –0.7). At the end of follow-up, no difference was observed between intervention and control children for height-for-age (–2.1 and –1.9 z-score, respectively) or weight-for-age (–0.6 and –0.7, respectively).
Microbiological samples
A total of 1994 samples of drinking water, kitchen cloths and mothers’ hands were collected throughout the study. We observed an E. coli geometric mean of CFU/100 ml of 9 (CI 95% 3.6–22.4) for drinking-water samples at baseline, 6.1 (CI 95% 0.7–48.2) at mid-study and 2.9 (CI 95% 1.9–4.5) at end-of-study evaluations in the intervention households. A similar decline in the E. coli geometric mean was observed for control households.
Compliance
Indicators and methods of measuring compliance in this trial are detailed in the Supplementary data, available at IJE online. Field workers that carried out weekly spot-check observations of compliance observed an initial prevalence of SODIS use of 60% with a steady decline throughout follow-up. At study end, SODIS was only practised by 10% of the IHIP intervention group. Self-reported use by mothers was around 90%, with a slight decrease at study end. Compliance of the improved-stove and kitchen-sink use is based on monthly maternal reporting. Ninety per cent of all mothers reported using the improved stove daily and two-thirds reported using the kitchen sink for washing utensils and children’s hands daily. Lack of continuous water flow (based on seasonal water availability) and interrupted water supply were two limitations for use.
Discussion
Our community-randomized–controlled trial in 51 rural Peruvian communities consisting of improved solid-fuel stoves, kitchen sinks, hygiene promotion and SODIS treatment might have reduced child diarrhoea episodes by 22% (RR 0.78, 95% CI: 0.49–1.05) and diarrhoea prevalence by 29% (OR 0.71, 95%, CI: 0.47, 1.06). Although the confidence intervals included unity, the observed effect is consistent with lower numbers of persistent diarrhoea, bloody stool episodes, shorter duration of illness (Table 2) and episodes requiring treatment in the intervention arm (data not shown). Objective environmental indicators such as faecal contamination of drinking water also corroborate the observed diarrhoea reduction. No effects on children’s ARI, ALRI and growth were found.
We combined different interventions that individually impact childhood diarrhoea: piped water delivered to the household’s kitchen, household drinking-water treatment and hygiene education. A recent systematic review of drinking-water and sanitation improvements found diarrhoea risk reductions when basic piped water to the household or premise was introduced on a formerly improved community water source.4 Supplying reliable drinking water directly to the household’s kitchen increases water availability and is thereby a prerequisite for hygienic practices.29 Water availability and distance to the water source were shown to be associated with reduced diarrhoea risk.30–32
The mentioned review found additional diarrhoea reductions for SODIS treatment on top of piped water to the household but there was no effect of this intervention on any baseline water source when results were adjusted for non-blinding.4 Different blinded household-level drinking-water quality studies showed no effect on diarrhoeal disease reduction.33–35
Also, the effect of hygiene promotion was thought to be susceptible to bias from unblinded designs.5 Non-blinding in intervention studies with subjective outcomes, like caregiver’s report of diarrhoea, was associated with significant overestimation of the intervention effect.35,36 To counteract this bias, we implemented a different intervention in the control group. The baseline water source might further explain the different findings of previously published SODIS intervention studies that showed larger impacts of SODIS on diarrhoeal disease—they were all conducted on unimproved or improved community water sources37–44 whereas, in our intervention, 80% of study participants already received piped water within their premises or yards. Additionally, at the end of follow-up, only 10% of study households were using SODIS. Low compliance in SODIS interventions has been described before despite extensive promotion campaigns.45 Our interventions did not lead to the provision of high-quality drinking water that has been associated with larger diarrhoea reductions.4 Additionally, having focused even more on babies, hand-washing at key times and the creation of clean playing and feeding environments could have led to increased diarrhoea reduction.46 Furthermore, the ECD intervention that we implemented in the attention control group is likely to have positively influenced playing and feeding environments and might therefore have attenuated the intervention effect. We nevertheless judged a control intervention to be highly important to prevent increased drop-out in the control group and reporting bias from the non-blinded design. Additionally, the area had received hand-washing promotion through local health centres before; therefore, there was a general understanding of appropriate hand-washing practices in both the intervention and the control groups. Finally, our study was sufficiently powered to detect a 22% reduction in diarrhoea episodes assuming five episodes per person-year of observation. However, we observed a mean of two diarrhoea episodes.
We did not observe a reduction in ARI and ALRI episodes. Potential reasons are: (i) insufficient power to detect reduction in ARI and especially ALRI, of which only a few cases were observed; (ii) the improved stove substantially lowered air pollution,18,19,47 but not to levels recommended by the WHO (indoor air quality guidelines were not available at the time of the study)48; (c) limitations of timely respiratory rate assessment, as we examined children only once per week; and (d) limitations to clinically diagnose ALRI.
The RESPIRE study, the first randomized–controlled trial on improved solid-fuel stoves, suggested a mean CO exposure reduction of 50% to achieve impact on physician-diagnosed pneumonia.10 In our study, we found only small reductions of CO and PM2.5 pollutants that were more pronounced in better-maintained stoves. We measured exposure data only once and seven months after stove implementation.18,19,47 The best-functioning stoves achieved a 45% and 27% mean reduction of PM2.5 and CO, respectively, in mothers’ personal exposure.19 It is possible that, after the introduction of the stoves, study participants spent more time in the then less-smoky kitchens, which led to increased total exposure to air pollutants. Project-initiated repairs were carried out nine months after the stoves had been installed. At this point, 35% of our stoves needed minor repairs, e.g. re-plastering, and 1% needed major repairs, e.g. a broken chimney valve. Two years after the end of the study, an evaluation showed that around 85% of the Optima-improved cooking stoves were still in use (defined as at least five times a week, twice a day).
A further limitation was the monitoring frequency for ARI and ALRI. Respiratory rate measurements were only available for about two-thirds of all reported ARI episodes. In addition, in 40% of the remaining ARI episodes, the child had already attended a health centre and/or received treatment at the time of the household visit. Therefore, the true ALRI incidence is likely higher, but this should be balanced between intervention and control communities. Hence, the observed 25 and 10 ALRI episodes in the intervention and control arm should be interpreted with caution considering also that, of the 25 observed episodes in the intervention arm, almost one-third were recorded in a single very sick child. Additionally, a more objective way of defining ALRI, e.g. through chest x-rays, could have produced more correct estimates24 but would have added substantially to costs and training requirements, which was not feasible for this study.
We could not blind the application of our interventions. Open trial designs can, however, benefit from and harness the community dynamics generating interest and motivation for a demand-driven replication. Furthermore, we believe that the selection of a highly valued intervention in the control arm (early child stimulation) reduced non-blinding/reporting bias and drop-out rates. Additionally, we used standardized data-collection tools and independent morbidity data-collection teams to minimize social desirability bias.
In conclusion, our intervention is one of the first studies to focus on addressing several household burdens simultaneously. Improved drinking-water quality and quantity, personal and kitchen hygiene and indoor air quality provide a healthy household environment that can translate into many aspects of life, including better health and poverty reduction. Even though we found no strong evidence for health impacts, the IHIP could be successfully delivered and was highly accepted.
Funding
This work was supported by the UBS Optimus Foundation, Freiwillige Akademische Gesellschaft, Basel, Stiftung Emilia-Guggenheim-Schnurr, Basel. The funders of the study had no involvement in study design, data collection, analysis or interpretation of the data, nor were they involved in the writing of this paper or in the decision to submit the paper for publication.
Supplementary Material
Acknowledgements
The authors wish to express their appreciation and thank the study families for their kind participation and local authorities for their continuous support throughout the study. We would also like to express our gratitude to the field teams, especially to Mrs Selene Flores, who was instrumental in logistic support provided for the study. Author contributions—the principal investigators: Mäusezahl, Lanata; study concept and design: Mäusezahl, Lanata; obtained funding: Mäusezahl, Lanata; acquisition of data: Hartinger, Lanata, Gil; data entry: Hartinger, Gil, analysis and interpretation of data: Hattendorf, Hartinger, Mäusezahl, Lanata; drafting of the manuscript: Hartinger, Hattendorf, Mäusezahl, Wolf; statistical analysis: Hattendorf, Hartinger; critical revision of the manuscript: Hattendorf, Lanata, Gil, Mäusezahl, Wolf. Trial registry: http://www.controlled-trials.com/ISRCTN28191222.
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1. Walker CLF, Rudan I, Liu L. et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381:1405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prüss-Ustün A, Bartram J, Clasen T. et al. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: a retrospective analysis of data from 145 countries. Trop Med Int Health 2014;19:894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. IHME. GBD 2013. , GBD Compare http://vizhub.healthdata.org/gbd-compare/ (13 January 2016, date last accessed).
- 4. Wolf J, Prüss-Ustün A, Cumming O. et al. Assessing the impact of drinking-water and sanitation on diarrhoeal disease in low-and middle-income settings: a systematic review and meta-regression. Trop Med Int Health 2014;19:928–42. [DOI] [PubMed] [Google Scholar]
- 5. Freeman MC, Stocks ME, Cumming O. et al. Hygiene and health: systematic review of handwashing practices worldwide and update of health effects. Trop Med Int Health 2014;19: 906–16. [DOI] [PubMed] [Google Scholar]
- 6. Clasen TF, Bostoen K, Schmidt WP. et al. Interventions to improve disposal of human excreta for preventing diarrhoea. Cochrane Database Syst Rev http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007180.pub2/pdf/standard (30 January 2013, date last accessed). [DOI] [PMC free article] [PubMed]
- 7. Cairncross S, Hunt C, Boisson S. et al. Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol 2010;39(Suppl 1):i193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dherani M, Pope D, Mascarenhas M. et al. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ 2008;86:390–8C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Po JY, FitzGerald JM, Carlsten C.. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax 2011;66: 232–9. [DOI] [PubMed] [Google Scholar]
- 10. Smith KR, McCracken JP, Weber MW. et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet 2011;378:1717–26. [DOI] [PubMed] [Google Scholar]
- 11. Eisenberg JNS, Scott JC, Porco T.. Integrating disease control strategies: balancing water sanitation and hygiene interventions to reduce diarrheal disease burden. Am J Public Health 2007; 97:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. VanDerslice J, Briscoe J.. Environmental interventions in developing countries: interactions and their implications. Am J Epidemiol 1995;141:135–44. [DOI] [PubMed] [Google Scholar]
- 13. Hayes RJ, Bennett S.. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol 1999;28:319–26. [DOI] [PubMed] [Google Scholar]
- 14. Moulton LH. Covariate-based constrained randomization of group-randomized trials. Clin Trials 2004;1:297–305. [DOI] [PubMed] [Google Scholar]
- 15. Hartinger SM, Lanata CF, Hattendorf J. et al. A community randomised controlled trial evaluating a home-based environmental intervention package of improved stoves, solar water disinfection and kitchen sinks in rural Peru: rationale, trial design and baseline findings. Contemp Clin Trials 2011;32:864–73. [DOI] [PubMed] [Google Scholar]
- 16. Hartinger SM, Lanata CF, Gil AI. et al. Combining interventions: improved chimney stoves, kitchen sinks and solar disinfection of drinking water and kitchen clothes to improve home hygiene in rural Peru. Field Actions Science Reports: The Journal of Field Actions, Special Issue 6 http://factsreports.revues.org/1627 (3 May 2013, date last accessed).
- 17.SODIS: Willkommen bei SODIS http://www.sodis.ch/index (1 April 2015, date last accessed).
- 18. Commodore AA, Hartinger SM, Lanata CF. et al. Carbon monoxide exposures and kitchen concentrations from cookstove-related woodsmoke in San Marcos, Peru. Int J Occup Environ Health 2013;19:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hartinger SM, Commodore AA, Hattendorf J. et al. Chimney stoves modestly improved Indoor Air Quality measurements compared with traditional open fire stoves: results from a small-scale intervention study in rural Peru. Indoor Air 2013;23: 342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cueto S, Guerrero G, Leon J. et al. Promoting early childhood development through a public programme: Wawa Wasi in Peru Report No. 51, University of Oxford, Department of International Development, Young Lives, 2009. http://www.grade.org.pe/en/publicaciones/859-promoting-early-childhood-development-through-a-public-programme-wawa-wasi-in-peru/ (9 September 2016, date last accessed).
- 21. World Health Organization. Integrated Management of Childhood Illness, Chart Booklet March 2014. http://whqlibdoc.who.int/publications/2008/9789241597289_eng.pdf (9 September 2016, date last accessed).
- 22. Gil AI, Lanata CF, Hartinger SM. et al. Fecal contamination of food, water, hands, and kitchen utensils at the household level in rural areas of Peru. J Environ Health 2014;76:102–8. [PubMed] [Google Scholar]
- 23. World Health Organization. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers, 4th edn. WHO, 2005. http://www.who.int/maternal_child_adolescent/documents/9241593180/en/ (9 September 2016, date last accessed).
- 24. Lanata CF, Rudan I, Boschi-Pinto C. et al. Methodological and quality issues in epidemiological studies of acute lower respiratory infections in children in developing countries. Int J Epidemiol 2004;33:1362–72. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization. The WHO Child Growth Standards http://www.who.int/childgrowth/en/ (1 April 2015, date last accessed).
- 26. Durán Pacheco G, Hattendorf J, Colford JM. et al. Performance of analytical methods for overdispersed counts in cluster randomized trials: sample size, degree of clustering and imbalance. Stat Med 2009;28:2989–3011. [DOI] [PubMed] [Google Scholar]
- 27. Instituto Nacional de Estadística e Informática (INEI) 2007. https://www.inei.gob.pe/ (19 January 2016, date last accessed).
- 28. Evans BE, Bartram J, Hunter P. et al. Public Health and Social Benefits of At-House Water Supplies University of Leeds, 2013. http://r4d.dfid.gov.uk/pdf/outputs/water/61005-DFID_HH_water_supplies_final_report.pdf (1 April 2014, date last accessed).
- 29. Wang X, Hunter PR.. A systematic review and meta-analysis of the association between self-reported diarrheal disease and distance from home to water source. Am J Trop Med Hyg 2010;83:582–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pickering AJ, Davis J.. Freshwater availability and water fetching distance affect child health in sub-Saharan Africa. Environ Sci Technol 2012;46:2391–7. [DOI] [PubMed] [Google Scholar]
- 31. Royal Scientific Society Amman-Jordan (RSS). Review Evidence on Minimum Household Water Security Requirements for Health Protection Research Division, Royal Scientific Society Amman-Jordon, 2011. http://www.mdgfund.org/sites/default/files/ENV_STUDY_Jordan_Minimum%20hosehole%20water%20security.pdf (9 September 2016, date last accessed).
- 32. Boisson S, Stevenson M, Shapiro L. et al. Effect of household-based drinking water chlorination on diarrhoea among children under five in Orissa, India: a double-blind randomised placebo-controlled trial. PLoS Med 2013;10:e1001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirchhoff LV, McClelland KE, Pinho MDC. et al. Feasibility and efficacy of in-home water chlorination in rural North-eastern Brazil. Journal of Hygiene 1985;94:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jain S, Sahanoon OK, Blanton E, Schmitz A. et al. Sodium dichloroisocyanurate tablets for routine treatment of household drinking water in Periurban Ghana: a randomized controlled trial. Am J Trop Med Hyg 2010;82:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Savović J, Jones HE, Altman DG. et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 2012;157: 429–38. [DOI] [PubMed] [Google Scholar]
- 36. Wood L, Egger M, Gluud LL. et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Conroy RM, Elmore-Meegan M, Joyce T. et al. Solar disinfection of drinking water and diarrhoea in Maasai children: a controlled field trial. Lancet 1996;348:1695–7. [DOI] [PubMed] [Google Scholar]
- 38. Conroy RM, Meegan ME, Joyce T. et al. Solar disinfection of water reduces diarrhoeal disease: an update. Arch Dis Child 1999;81:337–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Conroy RM, Meegan ME, Joyce T. et al. Solar disinfection of drinking water protects against cholera in children under 6 years of age. Arch Dis Child 2001;85:293–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. du Preez M, Conroy RM, Ligondo S. et al. Randomized intervention study of solar disinfection of drinking water in the prevention of dysentery in Kenyan children aged under 5 years. Environ Sci Technol 2011;45:9315–23. [DOI] [PubMed] [Google Scholar]
- 41. McGuigan KG, Samaiyar P, du Preez M. et al. High compliance randomized controlled field trial of solar disinfection of drinking water and its impact on childhood diarrhea in rural Cambodia. Environ Sci Technol 2011;45:7862–7. [DOI] [PubMed] [Google Scholar]
- 42. Rai BB, Pal R, Kar S. et al. Solar disinfection improves drinking water quality to prevent diarrhea in under-five children in Sikkim, India. J Glob Infect Dis 2010;2:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rose A, Roy S, Abraham V. et al. Solar disinfection of water for diarrhoeal prevention in southern India. Arch Dis Child 2006;91:139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Graf J, Togouet SZ, Kemka N. et al. Health gains from solar water disinfection (SODIS): evaluation of a water quality intervention in Yaounde, Cameroon. J Water Health 2010;8:779–96. [DOI] [PubMed] [Google Scholar]
- 45. Mäusezahl D, Christen A, Pacheco GD. et al. Solar drinking water disinfection (SODIS) to reduce childhood diarrhoea in rural Bolivia: a cluster-randomized, controlled trial. PLoS Med 2009;6:e1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ngure FM, Reid BM, Humphrey JH. et al. Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: making the links. Ann NY Acad Sci 2014;1308:118–28. [DOI] [PubMed] [Google Scholar]
- 47. Commodore AA, Zhang JJ, Chang Y. et al. Concentrations of urinary 8-hydroxy-2′-deoxyguanosine and 8-isoprostane in women exposed to woodsmoke in a cookstove intervention study in San Marcos, Peru. Environ Int 2013;60:112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. World Health Organization. Indoor Air Quality Guidelines: Household Fuel Combustion World Health Organization, 2014. http://www.who.int/indoorair/publications/household-fuel-combustion/en/ (30 November 2015, date last accessed). [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.