Abstract
Research on the biology of NAD+ has been gaining momentum, providing many critical insights into the pathogenesis of age-associated functional decline and diseases. In particular, two key NAD+ intermediates, nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), have been extensively studied over the past several years. Supplementing these NAD+ intermediates has shown preventive and therapeutic effects, ameliorating age-associated pathophysiologies and disease conditions. Although the pharmacokinetics and metabolic fates of NMN and NR are still under intensive investigation, these NAD+ intermediates can exhibit distinct behavior and their fates appear to depend on the tissue distribution and expression levels of NAD+ biosynthetic enzymes, nucleotidases, and presumptive transporters for each. A comprehensive concept that connects NAD+ metabolism to the control of aging and longevity in mammals has been proposed, and the stage is now set to test whether these exciting preclinical results can be translated to improve human health.
Introduction
Recent years have witnessed a resurgence of interest in nicotinamide adenine dinucleotide (NAD+) biology. This has been driven in part by the discoveries that two intermediates of NAD+ biosynthesis, nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), effectively increase NAD+ concentration in a variety of tissues, in many cases with beneficial or therapeutic effects. The purpose of this review is to summarize recent progress in understanding NAD+ biology, with a focus on the effects of these two molecules.
NAD+ was originally discovered by Harden and Young in 1906 as a factor that could enhance the rate of fermentation in yeast extracts (Harden, 1906). Over subsequent years, NAD+ was determined to be a nucleotide by von Euler-Chelpin and to play a role in redox reactions by Warburg (Berger et al., 2004). Elvehjem demonstrated that nicotinamide is able to prevent canine pellagra (a disease of NAD+ deficiency) by contributing to NAD+ biosynthesis (Elvehjem, 1949), leading to the eventual designation of the classical NAD+ precursors nicotinamide and nicotinic acid as “vitamin B3”. Since the recognition of NAD+ (and the related NADP+) as key players in cellular metabolism, there have been two separate occasions where the number of publications including the term “nicotinamide adenine dinucleotide” doubled within a relatively short period. The first occurred just after Chambon, Weill, and Mandel published their historical 1963 paper reporting that nicotinamide mononucleotide (NMN) activated a new DNA-dependent polyadenylic acid synthesizing nuclear enzyme (Chambon et al., 1963). Their findings led to a series of phenomenal discoveries of poly-ADP-ribose and poly-ADP-ribose polymerases (PARPs). During this period of time, Hayaishi and his colleagues began to reveal the NAD+ biosynthetic pathway from tryptophan through another NAD+ intermediate, nicotinic acid mononucleotide, in mammals (Ikeda et al., 1965). Gholson also proposed the concept of an active turnover cycle of NAD+ in 1966, predicting “an important, but as yet unknown, function” of NAD+ in cellular metabolism (Gholson, 1966). In 1976, Rechsteiner and his colleagues provided convincing evidence for rapid NAD+ turnover, reaffirming that “it seems likely that in eukaryotic cells, NAD has some other major function in addition to the classical cytoplasmic role in oxidation and reduction” (Rechsteiner et al., 1976). This was an exciting era in NAD+ biology research, over a decade full of critical discoveries. The elucidation of this “other major function” of NAD+ had to be awaited until 1989 and then 2000. In 1989, Lee and his colleagues discovered a novel NAD+ metabolite, cyclic ADP-ribose, after incubation of NAD+ with sea urchin egg extracts (Lee et al., 1989). The responsible enzyme, ADP-ribosyl cyclase, was found first in Aplysia in 1991 (Lee and Aarhus, 1991) and then in mammals as CD38 (States et al., 1992). The second doubling of papers on NAD+ occurred between 2000 and the present. In 2000, Guarente and Imai made the critical discovery that yeast SIR2 (silent information regulator 2) and a mouse ortholog SIRT1 possess NAD+-dependent protein deacetylase activity, fueling new interests in NAD+ biology (Imai et al., 2000). Indeed, human nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT), an important NAD+ biosynthetic enzyme whose activity was originally reported in 1952, was finally isolated and fully characterized in 2001 (Schweiger et al., 2001). Following these discoveries, nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme that initiates NAD+ biosynthesis from nicotinamide in mammals, was also isolated and characterized (Revollo et al., 2004; Rongvaux et al., 2002). Additionally, nicotinamide riboside, another key NAD+ intermediate, was shown to be incorporated into NAD+ via nicotinamide riboside kinases (NRKs) by Bieganowski and Brenner in 2004 (Bieganowski and Brenner, 2004). In this early 21st century, almost a half century after the first wave of enthusiasm on NAD+ biology, we are now in another exciting era of this classical but newly revisited field of science.
Reflecting such rapid progress in the field, there have already been a number of excellent review articles about general NAD+ biology and its relevance to disease (Belenky et al., 2007; Canto et al., 2015; Chini et al., 2016; Fang et al., 2017; Imai and Guarente, 2014; Katsyuba and Auwerx, 2017; Verdin, 2015; Yang and Sauve, 2016). Therefore, in this review article, we will particularly focus on the biology of two key NAD+ intermediates, NMN and NR, in an in vivo context. As mentioned above, these two NAD+ intermediates have long been known, and are found in a wide variety of our daily foods, such as vegetables, fruits, and meat (Figure 1A) (Mills et al., 2016). Interestingly, recent studies have documented both at micromolar concentrations in human and cow milk (Bieganowski and Brenner, 2004; Ummarino et al., 2017). Nonetheless, it is relatively recent that the importance and the potential of these NAD+ intermediates have been extensively investigated in various rodent models. Additionally, accumulating evidence suggests that NAD+ levels decline with age at a systemic level in diverse organisms, including rodents and humans, contributing to the development of many age-associated pathophysiologies (Canto et al., 2015; Imai and Guarente, 2014; Verdin, 2015). Therefore, there has been an increasing interest in using NAD+ intermediates as effective interventions to ameliorate or even prevent certain aspects of age-associated functional decline. In this review article, we will first summarize the current knowledge on these two NAD+ intermediates including their pharmacokinetic and pharmacological features. We will also discuss the physiological importance of these NAD+ intermediates in mammalian systems and their applications for aging and disease.
Figure 1. NAD+ intermediates, biosynthetic enzymes, and downstream mediators.
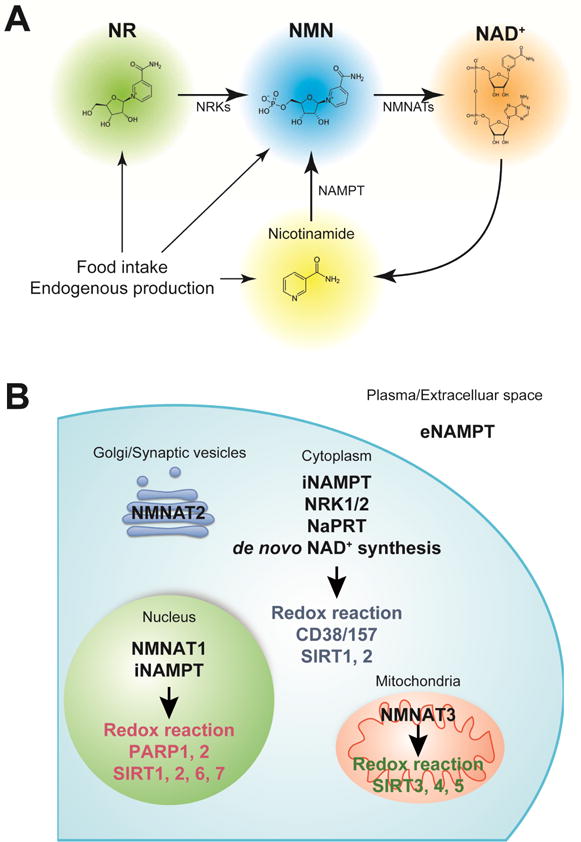
(A) Nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) are natural compounds that effectively enhance NAD+ biosynthesis and have health benefits. In mammals, NMN is synthesized from nicotinamide by the rate-limiting enzyme, nicotinamide phosphoribosyltransferase (NAMPT). NMN is also synthesized from NR via an NR kinase (NRK)-mediated phosphorylation reaction. NMN is then converted into NAD+ by NMN adenylyltransferases (NMNATs).
(B) NAMPT has two distinct forms: intracellular and extracellular NAMPT (iNAMPT and eNAMPT, respectively). iNAMPT is present in the cytoplasm and nucleus. eNAMPT is secreted by multiple cell types, including adipocytes and immune cells. eNAMPT is fully active as an NAD+ biosynthetic enzyme capable of catalyzing the generation of NMN, whereas it has also been reported to have a role as an inflammatory cytokine that are independent of catalytic activity. Nicotinic acid phosphoribosyltransferase (NaPRT) catalyzes the first step in the Preiss-Handler pathway, which converts nicotinic acid to NAD+, and de novo synthesis from tryptophan occurs through a complex series of steps ending with conversion of nicotinic acid adenine dinucleotide (NaAD+) to NAD+ by glutamine-dependent NAD+ synthase. NRK1 and NRK2 convert NR to NMN, which can then be acted on by NMNAT1–3. NMNAT1 is exclusively nuclear. NMNAT2 has been reported to localize to the Golgi complex and synaptic vesicles, and is predominantly expressed in neurons. NMNAT3 is the only NAD+ biosynthetic enzyme shown to be localized in the mitochondrial matrix, except in erythrocytes where it is expressed in the cytosol. NAD+ is consumed by NAD+-dependent enzymes, such as poly-ADP-ribose polymerases (PARPs), sirtuins, CD38/157, and other NAD+ glycohydrolases, and redox reaction.
Nicotinamide Mononucleotide (NMN)
NMN is synthesized from nicotinamide, a form of water-soluble vitamin B3, and 5′-phosphoribosyl-1-pyrophosphate (PRPP), by NAMPT, the rate-limiting NAD+ biosynthetic enzyme in mammals (Figure 1A) (Imai and Yoshino, 2013; Revollo et al., 2004; Wang et al., 2006). NMN is also synthesized from NR via an NRK-mediated phosphorylation reaction, which will be discussed further in the next section. Conversion of NMN into NAD+ is catalyzed by NMNATs (Figure 1B). Evidence accumulating from studies conducted in rodents has demonstrated that systemic NMN administration effectively enhances NAD+ biosynthesis in various peripheral tissues, including pancreas (Yoshino et al., 2011), liver (Peek et al., 2013; Yoshino et al., 2011), adipose tissue (Stromsdorfer et al., 2016; Yoshino et al., 2011), heart (Karamanlidis et al., 2013; Martin et al., 2017; North et al., 2014; Yamamoto et al., 2014), skeletal muscle (Gomes et al., 2013), kidney (Guan et al., 2017), testis (North et al., 2014), eyes (Lin et al., 2016), and blood vessel (aorta) (de Picciotto et al., 2016), under normal and pathophysiological conditions. Although it remains unclear whether NMN can cross the blood-brain-barrier (BBB), intraperitoneal NMN administration rapidly (within 15 min) increases NAD+ levels in brain regions such as hippocampus and hypothalamus (Stein and Imai, 2014; Yoon et al., 2015), suggesting that NMN could pass through the BBB and contribute to NAD+ biosynthesis in the brain. Finally, it was recently reported that long-term (1-year) oral administration of NMN (up to 300 mg/kg) is safe and well-tolerated and does not cause any obvious deleterious or toxic effects in normal wild-type C57BL/6 mice (Mills et al., 2016).
These results suggest that NMN could offer broad applications and therapeutic potential. Indeed, there is a growing body of evidence showing that NMN has beneficial effects on a diverse array of key physiological functions and therapeutic implications in various disease models (Table 1). Of particular note are the remarkable metabolic benefits from NMN administration. Pioneering studies have suggested that pancreatic β-cells are very sensitive to systemic NAD+ decline and NMN administration. A single bolus injection of NMN (500 mg/kg) enhances glucose-stimulated insulin secretion and thus improves glucose tolerance in age- and diet-induced diabetic mice (Caton et al., 2011; Yoshino et al., 2011), Nampt heterozygous knockout mice (Revollo et al., 2007), and aged wild-type and β cell-specific Sirt1-overexpressing (BESTO) mice (Mills et al., 2016; Ramsey et al., 2008; Yoshino et al., 2011). NMN enhances not only insulin secretion but also insulin action: NMN treatment ameliorates high-fat diet-induced hepatic insulin resistance by restoring NAD+ biosynthesis, SIRT1 activity, and gene expression related to inflammation, oxidative stress, and circadian rhythms (Yoshino et al., 2011). Oral NMN administration also increases adipose tissue NAD+ biosynthesis and SIRT1 activity and normalizes severe hypoadiponectinemia and multi-organ insulin resistance in adipocyte-specific Nampt knockout (ANKO) mice (Stromsdorfer et al., 2016). In addition, long-term NMN administration suppresses age-associated adipose tissue inflammation and improves whole-body insulin sensitivity independently from effects on body weight in regular chow-fed wild-type C57BL/6 mice (Mills et al., 2016). Given that adipose tissue NAD+ biosynthesis is severely impaired in obese and aged mice (Camacho-Pereira et al., 2016; Yoshino et al., 2011), these findings suggest that adipose tissue NAD+ could be a good therapeutic target for insulin resistance, which is an important risk factor of type 2 diabetes and cardiovascular disease (DeFronzo and Ferrannini, 1991; Reaven, 1988; Yamaguchi and Yoshino, 2017). NMN has also been reported to improve mitochondrial function in various metabolic organs, including skeletal muscle (Gomes et al., 2013; Mills et al., 2016), liver (Peek et al., 2013; Uddin et al., 2016), heart (Lee et al., 2016), and eyes (Lin et al., 2016). NMN-treated mice have increased mitochondrial oxidative phosphorylation in skeletal muscle (Gomes et al., 2013; Mills et al., 2016), likely contributing to weight loss by increasing whole-body energy expenditure (Mills et al., 2016). NMN increases SIRT3 activity and hepatic mitochondrial lipid oxidation in circadian mutant mice (Peek et al., 2013) and hepatic citrate synthase activity in high-fat diet fed obese mice (Uddin et al., 2016). An important aspect of the above findings is that aged animals appear to be more responsive to NMN treatment, compared to young animals. NMN improves high fat diet-induced glucose intolerance and dyslipidemia (Yoshino et al., 2011), skeletal muscle mitochondrial oxidative metabolism (Gomes et al., 2013), and endothelial function (de Picciotto et al., 2016), better in aged mice than young mice. It is conceivable that age-associated decline in NAD+ availability (Braidy et al., 2011; Camacho-Pereira et al., 2016; Frederick et al., 2016; Gomes et al., 2013; Massudi et al., 2012; Stein and Imai, 2014; Yoshino et al., 2011) could sensitize aged mice to NAD+ replenishment by NMN administration. Therefore, it will be of great interest to carefully compare the metabolic effects of NMN in aged mice to those in young mice.
Table 1.
Effects of NMN administration in vivo
| Model | Gender | Dose | Duration | Outcome | Ref |
|---|---|---|---|---|---|
| Nampt heterozygous knockout mice | female | 500 mg/kg (IP) | Single | Improved glucose tolerance and insulin secretion. | (Revollo et al., 2007) |
| β cell-specific Sirt1 overexpressing (BESTO) mice | female | 500 mg/kg (IP) | Single | Improved glucose tolerance and insulin secretion. | (Ramsey et al., 2008) |
| High fat diet (HFD)-induced obese mice | female male | 500 mg/kg (IP) | 7–10 days | Improved glucose tolerance and insulin sensitivity (female). Improved glucose tolerance and insulin secretion (male). | (Yoshino et al., 2011) |
| Age-induced diabetic mice | female male | 500 mg/kg (IP) | 11 days Single | Improved insulin sensitivity and plasma lipids profile (HFD-induced diabetic female mice). Improved glucose tolerance and insulin secretion (naturally occurring diabetic male mice). | (Yoshino et al., 2011) |
| Fructose-rich diet fed mice | male | 500 mg/kg (IP) | Single | Improved insulin secretion and inhibited inflammation. | (Caton et al., 2011) |
| C57BL/6J | unspecified | 500 mg/kg (IP) | 7 days | Enhanced skeletal muscle mitochondrial oxidative metabolism in aged mice. | (Gomes et al., 2013) |
| Bmal1 knockout mice | unspecified | 250 mg/kg (IP), 500 mg/kg (IP) | Single 10 days | Increased hepatic mitochondrial respiration. | (Peek et al., 2013) |
| Cardiac-specific Ndufs4 knockout (cKO) mice | unspecified | 500 mg/kg (IP) | 3 days | Decreased the mitochondrial protein acetylation and improved the permeability transition in mitochondria (mPTP) sensitivity. | (Karamanlidis et al., 2013) |
| Adenovirus vector overexpressing miR-34a injected mice | male | 500 mg/kg (IP) | 10 days | Improved glucose tolerance and increased expression of fatty acid β-oxidation genes. | (Choi et al., 2013) |
| C57BL/6N mice | male | 100, 300 mg/kg (drinking water) | 12 months | Maintained the neural stem/progenitor cells pool. | (Stein and Imai, 2014) |
| Ischemia/reperfusion heart injury in C57BL/6J mice | unspecified | 500 mg/kg (IP) | Once or 4 times | Reduced the infarct area after ischemia/reperfusion injury. | (Yamamoto et al., 2014) |
| Alzheimer’s disease-relevant (AD-Tg) mice | female and male | 100 mg/kg (SC) | 28 days (every other day) | Decreased amyloid precursor protein (APP) levels and increase mitochondrial function. | (Long et al., 2015) |
| Adipocyte-specific Nampt knockout (ANKO) mice | female | 500 mg/kg (IP). | Single | Restored physical activity. | (Yoon et al., 2015) |
| Cerebral ischemia in C57BL/6 mice | male | 31.25~50 0 mg/kg (IP) | Single | Reduced cell death of neuron and improved neurological outcome (noted 62.5 mg/kg induced the best outcome). | (Park et al., 2016) |
| C57BL/6 mice | male | 300 mg/kg (drinking water) | 8 weeks | Improved carotid artery endothelium-dependent dilation. | (de Picciotto et al., 2016) |
| Rod-specific Nampt knockout mice, light-induced retinal dysfunction (129S1/SvlmJ) | unspecified | 150 mg/kg (IP) 300 mg/kg (IP) | 4 weeks 10 days | Improved retinal degeneration and protected retina from light-induced injury. | (Lin et al., 2016) |
| Adipocyte-specific Nampt knockout (ANKO) mice | female | 500 mg/kg, (drinking water) | 6–8 weeks | Improved multi-organ insulin sensitivity, increased adiponectin production, and decreased free fatty acids production. | (Stromsdorfer et al., 2016) |
| High fat diet-induced obese mice | female | 500 mg/kg (IP) | ~17 days | Improved glucose tolerance, liver citrate synthase activity and triglyceride accumulation. | (Uddin et al., 2016) |
| Transverse aortic constriction (TAC)-stressed mice, cKO mice | male | 500 mg/kg (IP) | 33 days (every three days) | Improved mitochondrial function and protected mice from heart failure. | (Lee et al., 2016) |
| C57BL/6NTac wild type mice and Nrk1 KO, or Nrk1/2 double KO mice | male | 50, 500 mg/kg (IP) | Single | Failed to increase NAD+ concentrations in kidney and brown adipose tissue of KO mice. | (Ratajczak et al., 2016) |
| C57BL/6N mice | male | 100, 300 mg/kg (drinking water) | 12 months | Inhibited age-induced weight gain, improved insulin sensitivity and plasma lipids, and increased physical activity, energy expenditure, and muscle mitochondrial function. | (Mills et al., 2016) |
| Intracerebroventricular infusion of Aβ1–42 oligomer in Wister rats (Alzheimer’s disease mode) | male | 500 mg/kg (IP) | 10 days | Improved learning and memory. | (Wang et al., 2016) |
| APPswe/PS1dE9 transgenic mice (Alzheimer’s disease model) and wild-type mice | unspecified | 100 mg/kg (SC) | 28 days (every other day) | Improved cognitive function. | (Yao et al., 2017) |
| Collagen-induced intracerebral hemorrhage (cICH) in CD1 mice | male | 300 mg/kg (IV) 300 mg/kg (IV and IP) | Single 7 days | Reduced brain edema and cell death (one bolus) and promoted the recovery of body weight and neurological function (7 days). | (Wei et al., 2017a) |
| Old C57BL/6J mice and young irradiated C57BL/6J mice | unspecified | 500 mg/kg (IP) 2000 mg/kg (PO) | 7 days 8 days | Reduced DNA damage and protected against irradiation-induced alterations in white blood cell counts, lymphocytes, and hemoglobin. | (Li et al., 2017) |
| Ischemia-reperfusion or cisplatin-induced acute kidney injury in Sirt1 heterozygotes (+/−) knockout mice, C57BL/6 mice, and 129S2/Sv mice | unspecified | 500 mg/kg (IP) | 4 days | Protected renal function from cisplatin-induced injury in wild type mice but not in Sirt1 (+/−) mice. | (Guan et al., 2017) |
| Cardiac-specific Fxn knockout mice (FXN-KO) (Friedreich’s ataxia cardiomyopathy model) and Sirt3-KO/FKO-KO mice | male | 500 mg/kg (IP) | 4–5 weeks (twice a week) | Improved cardiac functions and reduced energy waste and improves energy utilization in FXN-KO mice but not in Sirt3-KO/FKO-KO mice. | (Martin et al., 2017) |
| Tissue plasminogen activator-treated cerebral ischemia CD1 mice | male | 300 mg/kg (IP) | Single | Improved mortality, brain infarction, edema, apoptosis, hemorrhage, and BBB integrity. | (Wei et al., 2017b) |
Abbreviations: IP, intraperitoneal; SC. subcutaneous; IV, intravenous; PO, per oral.
Recent studies have suggested that NMN improves numerous neuronal functions in the brain. NMN administration improves cognition and memory in mouse and rat models of Alzheimer’s disease (Long et al., 2015; Wang et al., 2016; Yao et al., 2017). NMN protect neurons from cell death after ischemia (Park et al., 2016) or intracerebral hemorrhage (Wei et al., 2017a) and ameliorates the loss of BBB integrity and tissue plasminogen activator-induced hemorrhagic transformation in brain ischemia (Wei et al., 2017b). NMN also restores severe retinal degeneration, through mitochondrial sirtuins SIRT3 and SIRT5, in rod or cone photoreceptor-specific Nampt knockout mice (Lin et al., 2016). Furthermore, long-term NMN administration prevents age-associated loss of the neural stem/progenitor pool in the dentate gyrus in wild-type C57BL/6 mice (Stein and Imai, 2014). Interestingly, data obtained from dose-response studies have indicated that lower doses of NMN may be more beneficial, as compared to larger doses for some neuronal outcomes. For example, 100 mg/kg of NMN improves physical activity better than 300 mg/kg (Mills et al., 2016). 62.5 mg/kg of NMN more effectively treats ischemia-induced brain damage than higher doses (125 to 500 mg/kg) (Park et al., 2016). Therefore, it is possible that local NMN accumulation, due to administration of high doses of NMN, has negative impacts in a brain region- or function-specific manner. Indeed, data obtained from in vitro experiments suggest that NMN promotes Wallerian degeneration, although this notion was recently challenged (Di Stefano et al., 2015; Gerdts et al., 2016). Future studies are awaited to fully elucidate the pharmacokinetics of NMN in the brain and determine the optimal dosing regimen of NMN in each key neuronal function and brain region.
In addition to its anti-diabetic and neurological effects, NMN administration inhibits acute renal injury in a SIRT1-dependent manner (Guan et al., 2017), heart failure (Karamanlidis et al., 2013; Lee et al., 2016; Martin et al., 2017; Yamamoto et al., 2014), and radiation-induced DNA damage (Li et al., 2017), further demonstrating pleiotropic benefits of NMN. Given that NMN is effective in aged mice and suppresses age-associated physiological decline (Mills et al., 2016), it will be of great importance to evaluate the effects of NMN administration on other age-associated diseases such as cancer and sarcopenia, and aging and lifespan per se in rodents.
Nicotinamide Riboside (NR)
The direct contribution of NR to NAD+ metabolism was first recognized by Bieganowski and Brenner in 2004 (Bieganowski and Brenner, 2004). This report described a class of enzymes known as NR kinases (NRKs) that can convert NR directly to NMN, bypassing the need for NAMPT in the salvage pathway (Figure 1). Since the reaction catalyzed by NAMPT is rate-limiting, requires the use of energetically costly PRPP, and is subject to feedback inhibition by NAD+ (Dietrich et al., 1968), NR also offers an interesting opportunity to boost NAD+ levels beyond what is achievable through conventional B vitamin metabolism. There is a substantial body of evidence supporting metabolic benefits for NR, as well as efficacy in a variety of disease models and a modest lifespan increase in aged mice (Table 2).
Table 2.
Effects of NR administration in vivo
| Mouse model | Gender | Dose | Duration | Outcome | Ref |
|---|---|---|---|---|---|
| Chow-fed or High fat diet-induced obese C57BL/6 mice | male | ~400 mg/kg (diet) | 12 weeks | Decreased weight gain, improved insulin sensitivity. | (Canto et al., 2012) |
| Tg2576 mice (Alzheimer’s disease model, C57BL/6 background) | unspecified | ~250 mg/kg (diet) | 3 months | Slowed cognitive decline. | (Gong et al., 2013) |
| “Deletor” mice (Twinkle mutant causing mitochondrial myopathy, C57BL/6 background) | male | ~400mg/kg (diet) | 4 months pre- or post-manifestation | Induced mitochondrial biogenesis and stimulated mitochondrial unfolded protein response | (Khan et al., 2014) |
| Sco2 knockout E129K knockin mice | unspecified | ~400mg/kg (diet | 4 weeks | Improved endurance and mitochondrial respiratory capacity in muscle | (Cerutti et al., 2014) |
| Human Unconventional RBP5 interactor knockin mice (inducible, liver-specific) | unspecifed | ~500mg/kg (diet) | 9–57 weeks | Prevented DNA damage and tumor development in livers when given early, tumor regression when given late | (Tummala et al., 2014) |
| 4 or 18 month old C57BL/6 wt and Csbm/m mice | male | 500 mg/kg (IP) | 1 week | Improved the function of mitochondria isolated from cerebellum. | (Scheibye-Knudsen et al., 2014) |
| 3 month old C57BL/6 wild-type or Xpa−/−/Csa−/− (CX) mice | unspecified | 500 mg/kg (SC) | 14 days | Lowered mitochondrial membrane potential and ROS (Also noted that CX genotype is lethal if mice are weaned to hard chow and NR in the drinking water of mothers failed to rescue.) | (Fang et al., 2014) |
| 129/SvEv mice with cardiac specific deletion of the transferrin receptor (causing heart failure) | unspecified | 750 mg/kg (IP) | P5-death (up to 10 days) | Increased survival time, decreased mitochondrial unfolded protein response, enhanced autophagy. | (Xu et al., 2015) |
| KK/HIJ mice | male | 100 mg/kg (osmotic pump) | 7 days | Improved glucose homeostasis, increased adiponectin, and lowered hepatic cholesterol. | (Lee et al., 2015) |
| C57BL/6, Balb/c, CBA mice, C57BL/6 Sirt3 KO | unspecified | 1000 mg/kg (IP) | 5 days before and/or 14 days after noise injury (twice daily injection) | Protection against transient or permanent noise-induced hearing loss in all wt strains, but not in Sirt3 KO | (Brown et al., 2014) |
| C57BL/6 wild-type and liver-specific Sirt1 KO fed high fat high sucrose diet, ApoE KO fed high fat high cholesterol diet | male | ~400 mg/kg (diet) | 2–18 weeks | Prevented or reversed fatty liver and induced the mitochondrial unfolded protein response with diminished effects in Sirt1 KO. | (Gariani et al., 2016) |
| C57BL/6 fed chow, high fat diet, or high fat diet + streptozotocin | male | 3000 ppm in diet | 8 weeks | Improved glucose tolerance, reduced weight gain, reduced hepatic steatosis, protected against neuropathy. | (Trammell et al., 2016b) |
| C57BL/6 young, aged, muscle stem cell-specific Sirt1 KO, mdx on C57BL/10SnJ background | male | 400 mg/kg (diet) | 6–8 weeks, or 2 years old death | Improved endurance, grip strength, and recovery from cardiotoxin-induced muscle injury in aged mice, induced the mitochondrial unfolded protein response and delayed senescence in stem cells, increased lifespan | (Zhang et al., 2016) |
| C57BL/6J mice fed HFD | male | 200 mg/kg (diet) | 4 weeks | Reduced lipid accumulation and fibrosis in liver | (Zhou et al., 2016) |
| C57BL/6 wt or muscle-specific Nampt KO | male and female | ~400 mg/kg (drinking water) | 6 weeks | Restored muscle mass, force generation, endurance, and mitochondrial respiratory capacity in Nampt KO. | (Frederick et al., 2016) |
| C57BL/6 mice, healthy humans | male (mice), not specified (human) | 185 mg/kg (gavage), 500 mg/kg (IP), 1000 mg (PO) | Single, 6 days (mice), single, 7 days (human) | Direct incorporation of intact NR into hepatic NAD+ (mice), no detection of NAD+ or precursor metabolites in plasma (human) | (Trammell et al., 2016a) |
| Sprague-Dawley rats | male and female | 5000 mg/kg (gavage), 750~500 0 mg/kg (gavage), 300~300 0 mg/kg (gavage) | Single, 14 days, 90 days | Toxicity profile similar to nicotinamide, affecting liver, kidneys, ovaries, and testes with no adverse effects observed below 1000 mg/kg/day. | (Conze et al., 2016) |
| Wild-type and mdx C57BL/10SnJ, mdx/utrophin KO | male | 400 mg/kg (diet) | 8–13 weeks | Improved muscle function and reduced heart pathology | (Ryu et al., 2016) |
| C57BL/6 wild-type or liver-specific Nampt KO mice | male | 400 mg/kg (drinking water) | 14 days prior to and 2 days after partial hepatectomy | Improved liver regeneration, reduced hepatic steatosis, reversal of the poor regeneration phenotype in liver specific Nampt KO mice | (Mukherjee et al., 2017) |
| C57BL/6 wild-type and Atm−/− mice | male | 12 mM (drinking water) | 14 days | Improved motor coordination, behavior, Purkinje cell number, and dramatically improved lifespan (Atm−/−) | (Fang et al., 2016) |
| Human Unconventional RBP5 interactor knockin mice (inducible, liver-specific), C57BL/6 mice fed a high fat diet | unspecified | ~500mg/ kg (diet) | 5 weeks (3–8 weeks of age) | Decreased DNA damage and inflammatory cell infiltration in liver | (Gomes et al., 2016) |
| Wistar rats | male | 300 mg/kg (gavage) | 21 days | Non-significant decrease in swimming performance | (Kourtzidis et al., 2016) |
| C57BL/6NTac wild-type and Nrk1 KO, or Nrk1/2 double KO mice | male | 500 mg/kg, 50 mg/kg (IP) | 1 hour | Some degradation of NR to NAM detectable in plasma | (Ratajczak et al., 2016) |
| C57BL/6JRcc mice fed a med/high fat diet | male | 5–900 ppm (diet) | 30 ppm for 4 weeks, then stratified to test groups for 15 weeks | Improved metabolic flexibility. | (Shi et al., 2017) |
| Sprague-Dawley rats injected with paclitaxel or vehicle | female | 200 mg/kg (gavage) | 7 days prior to and 24 days post-paclitaxel, or 21 days beginning 14 days post-paclitaxel | Prevention or reversal of paclitaxel-induced hypersensitivity to pain, no significant effect on basal locomotor activity (rotarod or open field) | (Hamity et al., 2017) |
| ICR wt mice fed a high fat diet for 18 weeks | male | 300 mg/kg (gavage) | 2 weeks | Reduced hepatic ER stress induced by the high fat diet | (Wang et al., 2017) |
Abbreviations: IP, intraperitoneal; SC. Subcutaneous; PO, per oral.
The first major study of NR in mice examined metabolic health after high-fat diet feeding. Mice receiving NR at a dose of 400 mg/kg/day in the diet were protected from weight gain, were more insulin sensitive, and had increased mitochondrial content in skeletal muscle and brown adipose tissue as compared to untreated controls (Canto et al., 2012). Accordingly, NR was found to confer increased endurance and improved cold tolerance in the high-fat diet-fed mice. Whether NR has significant benefits in lean, healthy muscle is less clear, as only a nonsignificant trend toward increased endurance was observed in regular chow-fed mice (Canto et al., 2012), and a subsequent study in rats showed a trend toward decreased endurance in a forced swim test (also not significant) (Kourtzidis et al., 2016).
NR has been shown to have therapeutic effects in a number of muscle disorders. Despite not correcting the underlying genetic defects, NR improved mitochondrial abundance and function in two different mitochondrial myopathies (Cerutti et al., 2014; Khan et al., 2014). NR also increased survival time, induced autophagy, and decreased the mitochondrial unfolded protein response in a model of heart failure induced by cardiac-specific deletion of the transferrin receptor (Cerutti et al., 2014). Mice lacking Nampt in skeletal muscle exhibit a progressive wasting syndrome that is reversed by providing NR in the drinking water, with endurance restored in as little as one week of treatment (Frederick et al., 2016). These mice histologically and transcriptionally resemble the mdx model of Duchenne’s muscular dystrophy, which has also been reported to display decreased NAD+ content in skeletal muscle (Chalkiadaki et al., 2014). Intriguingly, NR treatment has since been shown to improve stem cell function and partially ameliorate the muscle wasting phenotype in mdx mice, leading to hope for a rapidly translatable therapy for the human condition (Ryu et al., 2016; Zhang et al., 2016). Improvement in stem cell function appears to be a general phenomenon during NR treatment, and has been suggested to underlie a small, but significant extension of lifespan in mice treated beginning from 2 years of age (Zhang et al., 2016).
NR improves liver health in a variety of contexts. It potently reduces fat accumulation through a mechanism that involves induction of the mitochondrial unfolded protein response (Gariani et al., 2016). It further reduces inflammation at least in part through decreased activity of the NLRP3 inflammasome, and lessens the development of fibrosis (Lee et al., 2015). Moreover, NAD+ biosynthesis was found to be impaired in a mouse model of hepatocellular carcinoma, and both DNA damage and tumorigenesis were prevented when NR was provided in the diet to restore NAD+ (Tummala et al., 2014). In the regenerating liver (following partial hepatectomy), NR reduces lipid accumulation, promotes hepatocyte replication, increases hepatic ATP content, and leads to faster regain of liver weight (Mukherjee et al., 2017). Together, these observations suggest that enhancing NAD+ content by NR is a promising therapeutic strategy to improve liver health.
Like NMN, NR has further been reported to have a number of intriguing benefits in the nervous system. One of the first studies to examine the effects of NR in vivo revealed a striking improvement in the progression of Alzheimer’s disease pathology in the Tg2576 model of the disease (Gong et al., 2013). NR has been shown to prevent noise-induced hearing loss and neurite retraction from hair cells in the inner ear through a SIRT3-dependent mechanism (Brown et al., 2014). NR also protects against diabetic and chemotherapy-induced neuropathy in mice (Hamity et al., 2017; Trammell et al., 2016b), raising hopes that it could have a role in the management of chronic pain. It has recently been recognized that PARP-mediated NAD+ depletion plays a major role in the pathogenesis of neurodegenerative disorders that involve DNA repair defects, including Cockayne syndrome, Xeroderma pigmentosum, and Ataxia Telangiectasia. While NR is unable to correct the primary DNA repair deficiencies, it dramatically improves phenotypes in mouse models of each of these conditions, most remarkably more than tripling survival time in ataxic mice (Fang et al., 2016; Fang et al., 2014; Scheibye-Knudsen et al., 2014).
Limitations of our current understanding and possible detrimental effects of enhancing NAD+ biosynthesis
As outlined above, it is clear that both NMN and NR have beneficial effects in multiple conditions in rodents. Indeed, there are a number of pathophysiological conditions that show significant decreases in tissue NAD+ levels (Table 3). Although both compounds have been tested in some models, no side-by-side comparisons have been conducted between NMN and NR. Therefore, even though both compounds are capable of enhancing NAD+ biosynthesis, there might be certain interesting differences in their effects on these pathophysiological conditions. Additionally, in the vast majority of cases in which NMN and NR are effective, it still remains unclear what downstream mechanisms mediate their beneficial effects. NAD+ is required as a cosubstrate for PARPs, sirtuins, ADP-ribosyl cyclases, and mono-ADP ribosyltransferases, but also serves as a redox cofactor for countless enzymes (Figure 1B). In several cases, deletion of sirtuins has been shown to block key benefits of NAD+ supplementation, supporting a role for these enzymes (Brown et al., 2014; Gomes et al., 2013; Guan et al., 2017; Martin et al., 2017). In contrast, pharmacological or genetic inhibition of other key NAD+ consuming enzymes, such as PARP1/2 and CD38, is sufficient to confer beneficial effects that have been attributed to increased tissue NAD+ availability (Bai et al., 2011a; Bai et al., 2011b; Camacho-Pereira et al., 2016). Given the wide range of benefits that have been reported, and the potentially complex interactions between NAD+-dependent processes, a great deal of work will be required to define the precise mechanisms acting downstream of NAD+ supplementation to promote health.
Table 3.
Conditions in which NAD+ decline has been documented
| Disease/Condition | Tissue | NAD+ concentration (% of normal) | Ref |
|---|---|---|---|
| Aging | Muscle | ~30–35% (22 mo), ~40–45% (30 mo), | (Gomes et al., 2013) |
| ~60–65% (32 mo) | (Camacho-Pereira et al., 2016) | ||
| ~80–85% (25–31 mo) | (Yoshino et al., 2011) | ||
| ~65–70% (24 mo) | (Frederick et al., 2016) | ||
| ~50% (24 mo) | (Mouchiroud et al., 2013) | ||
| Liver | ~85–90% (25–31 mo) | (Yoshino et al., 2011) | |
| ~85–90% (12 mo), ~75–80% (20 mo), ~70–75% (<45 vs. >60 humans) | (Zhou et al., 2016) | ||
| unchanged (12 mo), ~40–50% (24 mo) | (Braidy et al., 2011) | ||
| 55–60% (32 mo) | (Camacho-Pereira et al., 2016) | ||
| ~60% (24 mo) | (Mouchiroud et al., 2013) | ||
| Adipose | 55–60% (32 mo) | (Camacho-Pereira et al., 2016) | |
| 70–75% (25–31 mo) | (Yoshino et al., 2011) | ||
| Brain | 63–66% (12 mo), ~90% (at age 70 vs. 20 based on regression) | (Stein and Imai, 2014; Zhu et al., 2015) | |
| Pancreas | ~80–85% | (Yoshino et al., 2011) | |
| Spleen | 35–40% (32 mo) | (Camacho-Pereira et al., 2016) | |
| Heart | unchanged (12 mo), ~30–40% (24 mo) | (Braidy et al., 2011) | |
| Kidney | unchanged (12 mo), ~15–20% (24 mo) | (Braidy et al., 2011) | |
| Lung | unchanged (12 mo), ~20–25% (24 mo) | (Braidy et al., 2011) | |
| CSF | ~91% (<45 vs. >=45 humans) | (Guest et al., 2014) | |
| Obesity | Liver | ~50–60% | (Wang et al., 2017) |
| unchanged | (Escande et al., 2010) | ||
| ~30–35% | (Gariani et al., 2016) | ||
| ~80–85%, | (Trammell et al., 2016b) | ||
| ~50–55% | (Yoshino et al., 2011) | ||
| Adipose | ~15–20% | (Yoshino et al., 2011) | |
| Muscle | unchanged | (Yoshino et al., 2011) | |
| ~80–85% | (Frederick et al., 2015) | ||
| mdx (Duchenne muscular dystrophy model) | Muscle | ~70–75% | (Chalkiadaki et al., 2014) |
| ~45–50% | (Ryu et al., 2016) | ||
| hURI overexpression (Oncogenic transgene that leads to spontaneous hepatocellular carcinoma) | Liver | ~25% | (Tummala et al., 2014) |
| Noise injury | Cochlea | ~40–50% | (Brown et al., 2014) |
| Liver regeneration | Liver | ~60–70% | (Mukherjee et al., 2017) |
| Atm mutant mice (Ataxia telangiectasia model) | Cerebellum | ~40% | (Fang et al., 2016) |
Values are estimated from graphs when not stated in the cited reference. In most cases, a decrease in NAD+ cannot be distinguished from a shift in the NAD+/NADH ratio, and in at least some cases, the effect of a redox shift appears to be important (e.g., in the age-related changes reported by Braidy et al. (Braidy et al., 2011) and in the NAD+ decline in human brain (Zhu et al., 2015).
It is also critical to carefully assess potentially detrimental side effects for these NAD+ intermediates. Although NAD+ appears to promote certain pathways related to DNA repair and suppression of inflammation, NAD+ depleting drugs are currently under development as cancer chemotherapeutics, and there might be a risk that boosting NAD+ could drive tumor growth (Gujar et al., 2016). Similarly, one of the downstream targets that is hypothesized to mediate many of NMN and NR’s effects, SIRT1, has been shown to have pro-carcinogenic or anti-carcinogenic effects in different contexts (Chalkiadaki and Guarente, 2015). Additionally, we cannot exclude the possibility that increased accumulation of nicotinamide, a potent inhibitor of sirtuins (Avalos et al., 2005; Bitterman et al., 2002), could have detrimental effects in NMN- and NR-treated mice. It is also clear that high dose supplementation greatly exceeds the body’s requirement for niacin equivalents, and thus will result in substantial elimination through the urine. One major route to nicotinamide elimination is methylation via nicotinamide N-methyltransferase, and there has been little investigation of whether this can lead to methyl donor depletion over time. Thus, further preclinical and clinical studies are needed to establish the long-term safety of NMN and NR as human therapeutics.
In vivo pharmacokinetics of NMN and NR
Despite the growing interest in NMN and NR, their in vivo pharmacokinetics remain poorly understood. Only a handful of published studies have carefully investigated the distribution and metabolism of NMN or NR in rodents or humans. It was reported that NMN rapidly appears in plasma, liver, white adipose tissue, and pancreas in wild type mice within 15 min after one bolus intraperitoneal injection of NMN (500 mg/kg) (Yoshino et al., 2011). Then, NMN is immediately utilized for NAD+ biosynthesis, leading to markedly increased NAD+ (2~3-fold) concentrations in liver over 60 min (Yoshino et al., 2011). Consistently, a recent study using doubly-labeled isotopic NMN (C13-D-NMN) shows that orally administered NMN is rapidly absorbed and converted to NAD+ in peripheral organs such as liver and skeletal muscle (Mills et al., 2016). Additionally, data obtained from blood and urine pharmacokinetics conducted in rats suggest that intraperitoneally administered NMN appears to be retained within the body longer than nicotinamide (Kawamura et al., 2016). Although these findings firmly demonstrate the bioavailability of NMN in vivo, the mechanism of NMN uptake into cells or tissues is currently unclear and under debate. Two mechanisms have been proposed and discussed to account for NMN uptake (Figure 2): One is the direct uptake of NMN presumably through specific transporter(s). The other is extracellular dephosphorylation of NMN into NR by ectonucleotidases (e.g. CD73) before uptake. The first possibility is supported by the fact that intraperitoneal or oral administration of NMN (300–500 mg/kg) immediately (within 5 min) increases NMN in plasma and then NAD+ content in peripheral organs (Mills et al., 2016; Yoshino et al., 2011), suggesting the presence of an active NMN uptake system in the gut and other organs. This hypothesis was challenged by a recent report using Nrk1 knockout mice. These knockout mice cannot phosphorylate NR to NMN, thus showing a lack of effective increases in NAD+ biosynthesis in kidney and brown adipose tissue after one bolus intraperitoneal injection of NMN (500 mg/kg), compared to wild-type mice (Ratajczak et al., 2016). However, in these Nrk1 knockout mice, NMN still results in a robust increase in NAD+ content in the liver (~130 % increase relative to basal values), suggesting that NMN is utilized for hepatic NAD+ biosynthesis in an NRK1-independent manner. Several in vitro studies show that chemical or genetic inhibition of 5′-nucletidase or Nrk1/2 abolishes the utilization of NMN in cultured cells (Fletcher et al., 2017; Grozio et al., 2013; Nikiforov et al., 2011; Ratajczak et al., 2016; Sociali et al., 2016). It is possible that extracellular NMN is transported into cells or tissues and utilized for NAD+ biosynthesis in a tissue- and cell type-specific manner. For example, NRK1 is barely detectable in heart and white adipose tissue (Ratajczak et al., 2016). Nonetheless, systemic NMN administration has been shown to increase NAD+ biosynthesis in both heart and white adipose tissue (Karamanlidis et al., 2013; Stromsdorfer et al., 2016; Yamamoto et al., 2014; Yoshino et al., 2011), implying a significant contribution of direct NMN uptake, or substantial breakdown to nicotinamide and resynthesis after uptake into these organs. Further studies using tissue-specific Nrk1/2 knockout mice in conjunction with isotopically-labeled NMN should clarify these models.
Figure 2. Uptake of NMN and NR in vivo.
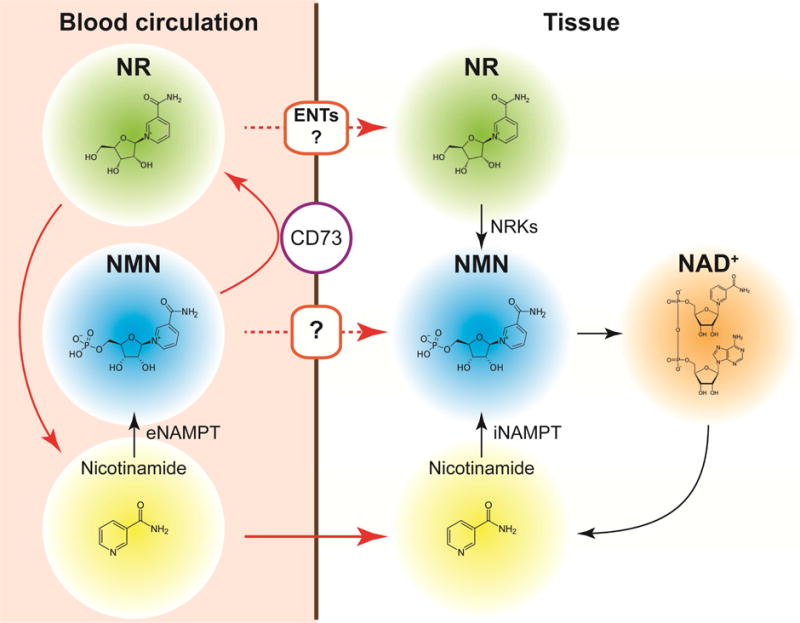
Extracellular conversion and degradation of NMN and NR and their possible uptake mechanisms are indicated. See details in the text. eNAMPT, extracellular nicotinamide phosphoribosyltransferase; iNAMPT, intracellular NAMPT; ENTs, equilibrative nucleoside transporters.
Information regarding in vivo NR pharmacokinetics is also limited. A recent study by Trammell et al. used targeted NAD+ metabolomics approaches and provided a comprehensive analysis of the effects of oral NR administration on peripheral blood mononuclear cells (PBMCs) in humans and on hepatic NAD+ metabolism in mice (Trammell et al., 2016a). In this study, NR increased the concentrations of all detected NAD+ metabolites in PBMCs except for nicotinamide. Interestingly, nicotinic acid adenine dinucleotide (NaAD+), which is not expected to be produced from NR, appeared with a slight delay relative to other metabolites but with a very large fold change owing to a low background level, making it potentially an attractive biomarker for NAD+ biosynthesis. In mice, hepatic levels of nicotinamide, NMN, and NAD+ were increased approximately 4-fold 6 hours after oral administration of NR (185 mg/kg). NR was not detected in human PBMCs or mouse liver, presumably due to rapid conversion of NR to NMN by NRKs. To fully understand the pharmacokinetics and uptake mechanisms of NR and NMN, it will be of great importance to evaluate their stability in the blood circulation. Indeed, data obtained from a recent in vivo study using doubly labeled isotopic NR (C13-D-NR) demonstrate that oral NR administration (200 mg/kg) markedly increases circulating nicotinamide concentrations without a major effect on whole blood NR concentrations (Frederick et al., 2016). Doubly labeled NR and NAD+ were detected in the liver, but not in skeletal muscle, suggesting a significant effect of first pass metabolism and/or limited half-life in the blood. Furthermore, it has recently been reported that NR, but not NMN, is unstable and quickly degrades into nicotinamide in murine plasma or FBS-containing culture medium (Ratajczak et al., 2016). Although in vitro studies demonstrate that NR can be directly transported through equilibrative nucleoside transporters (ENTs) and utilized for intracellular NAD+ biosynthesis (Nikiforov et al., 2011; Ratajczak et al., 2016), a substantial fraction of orally administered NR is likely converted into nicotinamide by first pass metabolism in the liver or by hydrolysis in the blood circulation before its uptake into other tissues in vivo (Figure 2). Indeed, Rowen and Kornberg reported the efficient degradation (the “phosphorolysis”) of NR into nicotinamide and ribose-1-phosphate by liver extracts in 1951 (Rowen and Kornberg, 1951). Similarly, NMN may be subjected to first pass metabolism or converted into NR by ectonucleotidases and then degrades into nicotinamide before uptake (Figure 2). Importantly, NMN and NR fail to increase NAD+ but nicotinamide still can in some tissues of Nrk1 knockout mice, strongly supporting the conclusion that at least part of the administered compounds reaches the target tissues before being degraded to nicotinamide.
These findings indicate that NMN and NR have distinct in vivo behavior and kinetics, leading us to the following three outstanding questions: First, how are NMN and NR metabolized in the liver and blood circulation after systemic administration? Second, are there any tissue- or cell-specific mechanisms responsible for uptake of NMN and NR? Lastly, are there any particular conditions in which one works more efficiently than another? Additional studies are needed to compare NMN and NR side-by-side by using isotopically-labeled tracers to determine the spatiotemporal pharmacokinetics of NAD+ intermediates and metabolites in blood and tissues. Elucidating in vivo pharmacokinetics of NMN and NR is critical to better understand the mechanisms of their pharmacological actions. However, what is their pathophysiological relevance in mammalian systems? In the next section, we will discuss this important issue, focusing on the function of extracellular NAMPT (eNAMPT), a key regulator of systemic NAD+ biosynthesis.
Systemic NAD+ biosynthesis
One peculiar but interesting feature related to NMN is that NAMPT, the enzyme responsible for the production of NMN, has two different forms in mammals, namely, intracellular and extracellular NAMPT (iNAMPT and eNAMPT, respectively) (Figure 1B). Considerable amounts of eNAMPT exist in blood circulation in mice (Revollo et al., 2007; Yoon et al., 2015) and humans (Friebe et al., 2011), as well as in human cerebrospinal fluid (Hallschmid et al., 2009) and seminal plasma (Riammer et al., 2016). It has also been reported that many different cell types, including adipocytes (Revollo et al., 2007; Yoon et al., 2015), hepatocytes (Schuster et al., 2014), leukocytes (Friebe et al., 2011), cardiomyocytes (Pillai et al., 2013), glia cells (Zhao et al., 2014), epithelial cells (Zhao et al., 2014), and monocytes (Yano et al., 2015), produce eNAMPT. Although it is unclear whether all these cell types have actively regulated mechanisms for eNAMPT production, both fully differentiated white and brown adipocytes actively secrete eNAMPT, which possesses a significantly higher enzymatic activity compared to iNAMPT (Revollo et al., 2007). Interestingly, iNAMPT is acetylated, and SIRT1-dependent deacetylation of iNAMPT at lysine 53 (K53) predisposes the protein to secretion in adipocytes and enhances its enzymatic activity (Yoon et al., 2015). Fasting-induced enhancement of eNAMPT secretion from adipose tissue is completely abrogated in whole-body and adipose tissue-specific Sirt1 knockout mice, demonstrating the physiological importance of this SIRT1-mediated eNAMPT secretion from adipose tissue. A surprise came when adipose tissue-specific Nampt knockout and knockin (ANKO and ANKI) mice were found to show reciprocal changes in hypothalamic NAD+, SIRT1 activity, and physical activity, as well as changes in circulating eNAMPT levels (Yoon et al., 2015). Consistently, systemic injection of a NAMPT-neutralizing antibody significantly reduces hypothalamic NAD+ levels in vivo, whereas purified eNAMPT can enhance NAD+, SIRT1 activity, and neuronal activity in ex vivo hypothalamic explants (Yoon et al., 2015). Collectively, these findings provide compelling evidence that eNAMPT plays a crucial, systemic role in remotely regulating hypothalamic NAD+ biosynthesis and function. Additionally, it has recently been shown that monocyte-derived eNAMPT plays a critical role in preserving myocardial NAD+ levels and SIRT1 activity in pressure-overloaded mouse hearts (Yano et al., 2015), providing another line of evidence for the pathophysiological importance of eNAMPT.
For NAMPT to function as an NAD+ biosynthetic enzyme, it forms a dimer. Indeed, the crystal structure of NAMPT clearly demonstrates that this protein belongs to the dimeric class of type II phosphoribosyltransferases (Kim et al., 2006; Wang et al., 2006). Based on its size, circulating eNAMPT appears to be a dimer under normal conditions (Korner et al., 2007). However, it has recently been reported that serum levels of monomeric eNAMPT are selectively elevated in high-fat diet-fed diabetic mice (Kieswich et al., 2016). It seems that monomeric eNAMPT contributes to metabolic impairment in a diabetic condition via NAD+-independent pro-inflammatory effects, whereas dimeric eNAMPT or its product NMN mediates protective effects against metabolic dysfunction. These findings likely provide an important clue to resolve a current controversy around the pathophysiological importance of eNAMPT as a systemic NAD+ biosynthetic enzyme vs. a proinflammatory cytokine (Garten et al., 2015; Hara et al., 2011) and also suggest that circulating dimeric eNAMPT contributes to NMN synthesis to maintain normal NAD+ biosynthesis at a systemic level. Indeed, NMN has been detected in mouse and human plasma (Mills et al., 2016; Ramsey et al., 2008; Revollo et al., 2007). However, additional studies are required to carefully evaluate where and how exactly circulating eNAMPT synthesizes NMN. It is not known whether NR and NRKs exist in blood circulation, although some cultured human cells might be capable of secreting NR (Kulikova et al., 2015). It will certainly be of great interest to examine the dynamics of blood and extracellular NMN and NR metabolism under various pathophysiological conditions.
Given the pathophysiological importance of eNAMPT and the pharmacokinetic features of NMN, an interesting concept, named the “NAD World”, has been proposed for the systemic regulatory mechanism of mammalian aging and longevity. The original concept of the NAD World was proposed in 2009, as a systemic regulatory network that connects NAD+ metabolism, biological rhythm, and aging and longevity control in mammals (Imai, 2009a, b, 2010). SIRT1 and NAMPT are two key components that comprise the NAD World: SIRT1 functions as a critical metabolic regulator in many different organs and tissues in response to changes in NAD+ availability, whereas NAMPT functions as a pace maker that produces circadian-oscillatory NAD+ production (Nakahata et al., 2009; Ramsey et al., 2009) and fine-tunes SIRT1 activity at a systemic level. In this concept, both eNAMPT and NMN play an important role in regulating NAD+ biosynthesis systemically. The availability of NMN is particularly important for tissues and organs that possess very low levels of iNAMPT, such as pancreatic β cells and central neurons. Recently, this concept has been reformulated with new findings over the past 8 years, now called the NAD World 2.0 (Imai, 2016). NAD World 2.0 proposes the importance of the inter-tissue communications among three key tissues, namely, the hypothalamus, adipose tissue, and skeletal muscle, for mammalian aging and longevity control. In this concept, NMN is hypothesized to function as a systemic signaling molecule that maintains biological robustness of the NAD World.
It will be of great interest to examine whether NR and NRKs play a critical role in regulating systemic NAD+ biosynthesis. Given that NAD+ levels decline with age at a systemic level (Braidy et al., 2011; Camacho-Pereira et al., 2016; Frederick et al., 2016; Gomes et al., 2013; Massudi et al., 2012; Stein and Imai, 2014; Yoshino et al., 2011) (Table 3), supplementing NMN or NR to enhance systemic NAD+ biosynthesis could be an effective anti-aging intervention to maintain physiological functions and delay the aging process in mammals. Several clinical trials are currently being conducted to evaluate the effects of NMN and NR on a wide variety of physiological outcomes, including glucose metabolism, insulin sensitivity, cardiovascular function, immune function, and cognitive function (https://clinicaltrials.gov). Therefore, it may not be so long until we know whether NMN and NR can effectively mitigate physiological decline or have therapeutic effects in humans.
Concluding remarks
In this new era of NAD+ biology, we are now revisiting many old questions and at the same time, producing many new questions as well. One such question in the former category is about the compartmentalization of NAD+ biosynthesis. For example, subcellular compartments, such as the nucleus and mitochondria, appears to possess distinct machinery to synthesize NAD+. But, are NAD+ intermediates shared among different subcellular compartments? Does NMN or NR actually go into mitochondria? If so, how? Another interesting question in the latter category is whether specific transporters exist for NMN and NR. If so, where are they expressed? And what is their physiological and pathophysiological importance in aging? Are there any tissue specificities for NMN and NR transport? Intensive investigations are currently underway to address both old and new questions. As Gholson predicted more than 50 years ago, “an important, but as yet unknown, function” for NAD+ and even for NAD+ intermediates could be discovered. With all these exciting developments in the field of NAD+ biology, the door is fully open for new breakthroughs and translation of these remarkable preclinical results to effective interventions in humans within our life time.
Acknowledgments
We apologize to those whose work is not cited due to space limitations. We thank members in the Yoshino, Baur, and Imai labs for critical discussions and suggestions. J. Y. is supported by grants from NIDDK (DK104995) and the Longer Life Foundation. J.A.B. is supported by grants from NIA (AG043483) and NIDDK (DK098656). S.I. is supported by grants from NIA (AG037457, AG047902), the American Federation for Aging Research, and the Tanaka Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interest
The authors have no competing financial interest.
NAD+ is increasingly being recognized to play an important role in the pathogenesis of age-associated functional decline and diseases. Two key NAD+ intermediates, nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), have been instrumental in driving recent discoveries. We discuss key findings and future therapeutic and translational potential for these molecules.
References
- Avalos JL, Bever KM, Wolberger C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell. 2005;17:855–868. doi: 10.1016/j.molcel.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Bai P, Canto C, Brunyanszki A, Huber A, Szanto M, Cen Y, Yamamoto H, Houten SM, Kiss B, Oudart H, et al. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 2011a;13:450–460. doi: 10.1016/j.cmet.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011b;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Berger F, Ramirez-Hernandez MH, Ziegler M. The new life of a centenarian: signalling functions of NAD(P) Trends Biochem Sci. 2004;29:111–118. doi: 10.1016/j.tibs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, Jaffrey SR. Activation of SIRT3 by the NAD(+) precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014;20:1059–1068. doi: 10.1016/j.cmet.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Pereira J, Tarrago MG, Chini CC, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016;23:1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia. 2011;54:3083–3092. doi: 10.1007/s00125-011-2288-0. [DOI] [PubMed] [Google Scholar]
- Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, Leoni V, Schon EA, Dantzer F, Auwerx J, et al. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014;19:1042–1049. doi: 10.1016/j.cmet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkiadaki A, Guarente L. The multifaceted functions of sirtuins in cancer. Nat Rev Cancer. 2015;15:608–624. doi: 10.1038/nrc3985. [DOI] [PubMed] [Google Scholar]
- Chalkiadaki A, Igarashi M, Nasamu AS, Knezevic J, Guarente L. Muscle-specific SIRT1 gain-of-function increases slow-twitch fibers and ameliorates pathophysiology in a mouse model of duchenne muscular dystrophy. PLoS Genet. 2014;10:e1004490. doi: 10.1371/journal.pgen.1004490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P, Weill JD, Mandel P. Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res Commun. 1963;11:39–43. doi: 10.1016/0006-291x(63)90024-x. [DOI] [PubMed] [Google Scholar]
- Chini CC, Tarrago MG, Chini EN. NAD and the aging process: Role in life, death and everything in between. Mol Cell Endocrinol. 2016 doi: 10.1016/j.mce.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SE, Fu T, Seok S, Kim DH, Yu E, Lee KW, Kang Y, Li X, Kemper B, Kemper JK. Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. 2013;12:1062–1072. doi: 10.1111/acel.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conze DB, Crespo-Barreto J, Kruger CL. Safety assessment of nicotinamide riboside, a form of vitamin B3. Hum Exp Toxicol. 2016 doi: 10.1177/0960327115626254. [DOI] [PubMed] [Google Scholar]
- de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- Di Stefano M, Nascimento-Ferreira I, Orsomando G, Mori V, Gilley J, Brown R, Janeckova L, Vargas ME, Worrell LA, Loreto A, et al. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ. 2015;22:731–742. doi: 10.1038/cdd.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich LS, Muniz O, Powanda M. NAD synthesis in animal tissues. J Vitaminol (Kyoto) 1968;14(Suppl):123–129. doi: 10.5925/jnsv1954.14.supplement_123. [DOI] [PubMed] [Google Scholar]
- Elvehjem CA. Pellagra, a deficiency disease. Proc Am Philos Soc. 1949;93:335–339. [PubMed] [Google Scholar]
- Escande C, Chini CC, Nin V, Dykhouse KM, Novak CM, Levine J, van Deursen J, Gores GJ, Chen J, Lou Z, et al. Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J Clin Invest. 2010;120:545–558. doi: 10.1172/JCI39319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Kassahun H, Croteau DL, Scheibye-Knudsen M, Marosi K, Lu H, Shamanna RA, Kalyanasundaram S, Bollineni RC, Wilson MA, et al. NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016;24:566–581. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, Bohr VA. NAD+ in Aging: Molecular Mechanisms and Translational Implications. Trends Mol Med. 2017;23:899–916. doi: 10.1016/j.molmed.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, Mitchell JR, Croteau DL, Bohr VA. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 2014;157:882–896. doi: 10.1016/j.cell.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RS, Ratajczak J, Doig CL, Oakey LA, Callingham R, Da Silva Xavier G, Garten A, Elhassan YS, Redpath P, Migaud ME, et al. Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol Metab. 2017;6:819–832. doi: 10.1016/j.molmet.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DW, Davis JG, Davila A, Jr, Agarwal B, Michan S, Puchowicz MA, Nakamaru-Ogiso E, Baur JA. Increasing NAD synthesis in muscle via nicotinamide phosphoribosyltransferase is not sufficient to promote oxidative metabolism. J Biol Chem. 2015;290:1546–1558. doi: 10.1074/jbc.M114.579565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DW, Loro E, Liu L, Davila A, Jr, Chellappa K, Silverman IM, Quinn WJ, 3rd, Gosai SJ, Tichy ED, Davis JG, et al. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab. 2016;24:269–282. doi: 10.1016/j.cmet.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe D, Neef M, Kratzsch J, Erbs S, Dittrich K, Garten A, Petzold-Quinque S, Bluher S, Reinehr T, Stumvoll M, et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia. 2011;54:1200–1211. doi: 10.1007/s00125-010-2042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, Moullan N, Zhang H, Perino A, Lemos V, et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190–1204. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11:535–546. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Milbrandt J, DiAntonio A. Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron. 2016;89:449–460. doi: 10.1016/j.neuron.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholson RK. The pyridine nucleotide cycle. Nature. 1966;212:933–935. doi: 10.1038/212933a0. [DOI] [PubMed] [Google Scholar]
- Gomes AL, Teijeiro A, Buren S, Tummala KS, Yilmaz M, Waisman A, Theurillat JP, Perna C, Djouder N. Metabolic Inflammation-Associated IL-17A Causes Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell. 2016;30:161–175. doi: 10.1016/j.ccell.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging. 2013;34:1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozio A, Sociali G, Sturla L, Caffa I, Soncini D, Salis A, Raffaelli N, De Flora A, Nencioni A, Bruzzone S. CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J Biol Chem. 2013;288:25938–25949. doi: 10.1074/jbc.M113.470435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Wang SR, Huang XZ, Xie QH, Xu YY, Shang D, Hao CM. Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1-Dependent Manner. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J, Grant R, Mori TA, Croft KD. Changes in oxidative damage, inflammation and [NAD(H)] with age in cerebrospinal fluid. PLoS One. 2014;9:e85335. doi: 10.1371/journal.pone.0085335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar AD, Le S, Mao DD, Dadey DY, Turski A, Sasaki Y, Aum D, Luo J, Dahiya S, Yuan L, et al. An NAD+-dependent transcriptional program governs self-renewal and radiation resistance in glioblastoma. Proc Natl Acad Sci U S A. 2016;113:E8247–E8256. doi: 10.1073/pnas.1610921114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallschmid M, Randeva H, Tan BK, Kern W, Lehnert H. Relationship between cerebrospinal fluid visfatin (PBEF/Nampt) levels and adiposity in humans. Diabetes. 2009;58:637–640. doi: 10.2337/db08-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamity MV, White SR, Walder RY, Schmidt MS, Brenner C, Hammond DL. Nicotinamide riboside, a form of vitamin B3 and NAD+ precursor, relieves the nociceptive and aversive dimensions of paclitaxel-induced peripheral neuropathy in female rats. Pain. 2017;158:962–972. doi: 10.1097/j.pain.0000000000000862. [DOI] [PubMed] [Google Scholar]
- Hara N, Yamada K, Shibata T, Osago H, Tsuchiya M. Nicotinamide phosphoribosyltransferase/visfatin does not catalyze nicotinamide mononucleotide formation in blood plasma. PLoS One. 2011;6:e22781. doi: 10.1371/journal.pone.0022781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden AYWJ. The Alcoholic Ferment of Yeast-Juice. Part II.–The Conferment of Yeast-Juice. Proc R Soc Lond. 1906;78:369–375. [Google Scholar]
- Ikeda M, Tsuji H, Nakamura S, Ichiyama A, Nishizuka Y, Hayaishi O. Studies on the Biosynthesis of Nicotinamide Adenine Dinucleotide. Ii. A Role of Picolinic Carboxylase in the Biosynthesis of Nicotinamide Adenine Dinucleotide from Tryptophan in Mammals. J Biol Chem. 1965;240:1395–1401. [PubMed] [Google Scholar]
- Imai S. From heterochromatin islands to the NAD World: a hierarchical view of aging through the functions of mammalian Sirt1 and systemic NAD biosynthesis. Biochim Biophys Acta. 2009a;1790:997–1004. doi: 10.1016/j.bbagen.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S. The NAD World: a new systemic regulatory network for metabolism and aging–Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys. 2009b;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S. “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging. Biochim Biophys Acta. 2010;1804:1584–1590. doi: 10.1016/j.bbapap.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes Metab. 2013;15(Suppl 3):26–33. doi: 10.1111/dom.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai SI. The NAD World 2.0: the importance of the inter-tissue communication mediated by NAMPT/NAD+/SIRT1 in mammalian aging and longevity control. NPJ Syst Biol Appl. 2016;2:16018. doi: 10.1038/npjsba.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC, Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18:239–250. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsyuba E, Auwerx J. Modulating NAD+ metabolism, from bench to bedside. EMBO J. 2017 doi: 10.15252/embj.201797135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Mori N, Shibata K. beta-Nicotinamide Mononucleotide, an Anti-Aging Candidate Compound, Is Retained in the Body for Longer than Nicotinamide in Rats. J Nutr Sci Vitaminol (Tokyo) 2016;62:272–276. doi: 10.3177/jnsv.62.272. [DOI] [PubMed] [Google Scholar]
- Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsstrom S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J, et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med. 2014;6:721–731. doi: 10.1002/emmm.201403943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieswich J, Sayers SR, Silvestre MF, Harwood SM, Yaqoob MM, Caton PW. Monomeric eNAMPT in the development of experimental diabetes in mice: a potential target for type 2 diabetes treatment. Diabetologia. 2016;59:2477–2486. doi: 10.1007/s00125-016-4076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MK, Lee JH, Kim H, Park SJ, Kim SH, Kang GB, Lee YS, Kim JB, Kim KK, Suh SW, et al. Crystal structure of visfatin/pre-B cell colony-enhancing factor 1/nicotinamide phosphoribosyltransferase, free and in complex with the anti-cancer agent FK-866. J Mol Biol. 2006;362:66–77. doi: 10.1016/j.jmb.2006.06.082. [DOI] [PubMed] [Google Scholar]
- Korner A, Garten A, Bluher M, Tauscher R, Kratzsch J, Kiess W. Molecular characteristics of serum visfatin and differential detection by immunoassays. J Clin Endocrinol Metab. 2007;92:4783–4791. doi: 10.1210/jc.2007-1304. [DOI] [PubMed] [Google Scholar]
- Kourtzidis IA, Stoupas AT, Gioris IS, Veskoukis AS, Margaritelis NV, Tsantarliotou M, Taitzoglou I, Vrabas IS, Paschalis V, Kyparos A, et al. The NAD(+) precursor nicotinamide riboside decreases exercise performance in rats. J Int Soc Sports Nutr. 2016;13:32. doi: 10.1186/s12970-016-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikova V, Shabalin K, Nerinovski K, Dolle C, Niere M, Yakimov A, Redpath P, Khodorkovskiy M, Migaud ME, Ziegler M, et al. Generation, Release, and Uptake of the NAD Precursor Nicotinic Acid Riboside by Human Cells. J Biol Chem. 2015;290:27124–27137. doi: 10.1074/jbc.M115.664458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CF, Chavez JD, Garcia-Menendez L, Choi Y, Roe ND, Chiao YA, Edgar JS, Goo YA, Goodlett DR, Bruce JE, et al. Normalization of NAD+ Redox Balance as a Therapy for Heart Failure. Circulation. 2016;134:883–894. doi: 10.1161/CIRCULATIONAHA.116.022495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 1991;2:203–209. doi: 10.1091/mbc.2.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Walseth TF, Bratt GT, Hayes RN, Clapper DL. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989;264:1608–1615. [PubMed] [Google Scholar]
- Lee HJ, Hong YS, Jun W, Yang SJ. Nicotinamide Riboside Ameliorates Hepatic Metaflammation by Modulating NLRP3 Inflammasome in a Rodent Model of Type 2 Diabetes. J Med Food. 2015;18:1207–1213. doi: 10.1089/jmf.2015.3439. [DOI] [PubMed] [Google Scholar]
- Li J, Bonkowski MS, Moniot S, Zhang D, Hubbard BP, Ling AJ, Rajman LA, Qin B, Lou Z, Gorbunova V, et al. A conserved NAD+ binding pocket that regulates protein-protein interactions during aging. Science. 2017;355:1312–1317. doi: 10.1126/science.aad8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JB, Kubota S, Ban N, Yoshida M, Santeford A, Sene A, Nakamura R, Zapata N, Kubota M, Tsubota K, et al. NAMPT-Mediated NAD(+) Biosynthesis Is Essential for Vision In Mice. Cell Rep. 2016;17:69–85. doi: 10.1016/j.celrep.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AN, Owens K, Schlappal AE, Kristian T, Fishman PS, Schuh RA. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol. 2015;15:19. doi: 10.1186/s12883-015-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AS, Abraham DM, Hershberger KA, Bhatt DP, Mao L, Cui H, Liu J, Liu X, Muehlbauer MJ, Grimsrud PA, et al. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Chellappa K, Moffitt A, Ndungu J, Dellinger RW, Davis JG, Agarwal B, Baur JA. Nicotinamide adenine dinucleotide biosynthesis promotes liver regeneration. Hepatology. 2017;65:616–630. doi: 10.1002/hep.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov A, Dolle C, Niere M, Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286:21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Rosenberg MA, Jeganathan KB, Hafner AV, Michan S, Dai J, Baker DJ, Cen Y, Wu LE, Sauve AA, et al. SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J. 2014;33:1438–1453. doi: 10.15252/embj.201386907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Long A, Owens K, Kristian T. Nicotinamide mononucleotide inhibits post-ischemic NAD(+) degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiol Dis. 2016;95:102–110. doi: 10.1016/j.nbd.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai VB, Sundaresan NR, Kim G, Samant S, Moreno-Vinasco L, Garcia JG, Gupta MP. Nampt secreted from cardiomyocytes promotes development of cardiac hypertrophy and adverse ventricular remodeling. Am J Physiol Heart Circ Physiol. 2013;304:H415–426. doi: 10.1152/ajpheart.00468.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Joffraud M, Trammell SA, Ras R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud ME, et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat Commun. 2016;7:13103. doi: 10.1038/ncomms13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M, Hillyard D, Olivera BM. Magnitude and significance of NAD turnover in human cell line D98/AH2. Nature. 1976;259:695–696. doi: 10.1038/259695a0. [DOI] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riammer S, Garten A, Schaab M, Grunewald S, Kiess W, Kratzsch J, Paasch U. Nicotinamide phosphoribosyltransferase production in human spermatozoa is influenced by maturation stage. Andrology. 2016;4:1045–1053. doi: 10.1111/andr.12252. [DOI] [PubMed] [Google Scholar]
- Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Rowen JW, Kornberg A. The phosphorolysis of nicotinamide riboside. J Biol Chem. 1951;193:497–507. [PubMed] [Google Scholar]
- Ryu D, Zhang H, Ropelle ER, Sorrentino V, Mazala DA, Mouchiroud L, Marshall PL, Campbell MD, Ali AS, Knowels GM, et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med. 2016;8:361ra139. doi: 10.1126/scitranslmed.aaf5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Mitchell SJ, Fang EF, Iyama T, Ward T, Wang J, Dunn CA, Singh N, Veith S, Hasan-Olive MM, et al. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 2014;20:840–855. doi: 10.1016/j.cmet.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster S, Penke M, Gorski T, Petzold-Quinque S, Damm G, Gebhardt R, Kiess W, Garten A. Resveratrol differentially regulates NAMPT and SIRT1 in Hepatocarcinoma cells and primary human hepatocytes. PLoS One. 2014;9:e91045. doi: 10.1371/journal.pone.0091045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M, Hennig K, Lerner F, Niere M, Hirsch-Kauffmann M, Specht T, Weise C, Oei SL, Ziegler M. Characterization of recombinant human nicotinamide mononucleotide adenylyl transferase (NMNAT), a nuclear enzyme essential for NAD synthesis. FEBS Lett. 2001;492:95–100. doi: 10.1016/s0014-5793(01)02180-9. [DOI] [PubMed] [Google Scholar]
- Shi W, Hegeman MA, van Dartel DAM, Tang J, Suarez M, Swarts H, van der Hee B, Arola L, Keijer J. Effects of a wide range of dietary nicotinamide riboside (NR) concentrations on metabolic flexibility and white adipose tissue (WAT) of mice fed a mildly obesogenic diet. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201600878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sociali G, Raffaghello L, Magnone M, Zamporlini F, Emionite L, Sturla L, Bianchi G, Vigliarolo T, Nahimana A, Nencioni A, et al. Antitumor effect of combined NAMPT and CD73 inhibition in an ovarian cancer model. Oncotarget. 2016;7:2968–2984. doi: 10.18632/oncotarget.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States DJ, Walseth TF, Lee HC. Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human lymphocyte antigen CD38. Trends Biochem Sci. 1992;17:495. doi: 10.1016/0968-0004(92)90337-9. [DOI] [PubMed] [Google Scholar]
- Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014;33:1321–1340. doi: 10.1002/embj.201386917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromsdorfer KL, Yamaguchi S, Yoon MJ, Moseley AC, Franczyk MP, Kelly SC, Qi N, Imai S, Yoshino J. NAMPT-Mediated NAD(+) Biosynthesis in Adipocytes Regulates Adipose Tissue Function and Multi-organ Insulin Sensitivity in Mice. Cell Rep. 2016;16:1851–1860. doi: 10.1016/j.celrep.2016.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016a;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammell SA, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA, Brenner C. Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci Rep. 2016b;6:26933. doi: 10.1038/srep26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummala KS, Gomes AL, Yilmaz M, Grana O, Bakiri L, Ruppen I, Ximenez-Embun P, Sheshappanavar V, Rodriguez-Justo M, Pisano DG, et al. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell. 2014;26:826–839. doi: 10.1016/j.ccell.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Uddin GM, Youngson NA, Sinclair DA, Morris MJ. Head to Head Comparison of Short-Term Treatment with the NAD(+) Precursor Nicotinamide Mononucleotide (NMN) and 6 Weeks of Exercise in Obese Female Mice. Front Pharmacol. 2016;7:258. doi: 10.3389/fphar.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ummarino S, Mozzon M, Zamporlini F, Amici A, Mazzola F, Orsomando G, Ruggieri S, Raffaelli N. Simultaneous quantitation of nicotinamide riboside, nicotinamide mononucleotide and nicotinamide adenine dinucleotide in milk by a novel enzyme-coupled assay. Food Chem. 2017;221:161–168. doi: 10.1016/j.foodchem.2016.10.032. [DOI] [PubMed] [Google Scholar]
- Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- Wang T, Zhang X, Bheda P, Revollo JR, Imai S, Wolberger C. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat Struct Mol Biol. 2006;13:661–662. doi: 10.1038/nsmb1114. [DOI] [PubMed] [Google Scholar]
- Wang X, Hu X, Yang Y, Takata T, Sakurai T. Nicotinamide mononucleotide protects against beta-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016;1643:1–9. doi: 10.1016/j.brainres.2016.04.060. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang ZF, Zheng GH, Wang AM, Sun CH, Qin SP, Zhuang J, Lu J, Ma DF, Zheng YL. The Inhibitory Effects of Purple Sweet Potato Color on Hepatic Inflammation Is Associated with Restoration of NAD(+) Levels and Attenuation of NLRP3 Inflammasome Activation in High-Fat-Diet-Treated Mice. Molecules. 2017;22 doi: 10.3390/molecules22081315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CC, Kong YY, Li GQ, Guan YF, Wang P, Miao CY. Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway. Sci Rep. 2017a;7:717. doi: 10.1038/s41598-017-00851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CC, Kong YY, Xia H, Li GQ, Zheng SL, Cheng MH, Wang P, Miao CY. NAD replenishment with nicotinamide mononucleotide protects blood-brain barrier integrity and attenuates delayed tPA-induced haemorrhagic transformation after cerebral ischemia. Br J Pharmacol. 2017b doi: 10.1111/bph.13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Barrientos T, Mao L, Rockman HA, Sauve AA, Andrews NC. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep. 2015;13:533–545. doi: 10.1016/j.celrep.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Yoshino J. Adipose tissue NAD+ biology in obesity and insulin resistance: From mechanism to therapy. Bioessays. 2017 doi: 10.1002/bies.201600227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9:e98972. doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sauve AA. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbapap.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Akazawa H, Oka T, Yabumoto C, Kudo-Sakamoto Y, Kamo T, Shimizu Y, Yagi H, Naito AT, Lee JK, et al. Monocyte-derived extracellular Nampt-dependent biosynthesis of NAD(+) protects the heart against pressure overload. Sci Rep. 2015;5:15857. doi: 10.1038/srep15857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Yang W, Gao Z, Jia P. Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neurosci Lett. 2017;647:133–140. doi: 10.1016/j.neulet.2017.03.027. [DOI] [PubMed] [Google Scholar]
- Yoon MJ, Yoshida M, Johnson S, Takikawa A, Usui I, Tobe K, Nakagawa T, Yoshino J, Imai S. SIRT1-Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD(+) and Function in Mice. Cell Metab. 2015;21:706–717. doi: 10.1016/j.cmet.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liu XZ, Tian WW, Guan YF, Wang P, Miao CY. Extracellular visfatin has nicotinamide phosphoribosyltransferase enzymatic activity and is neuroprotective against ischemic injury. CNS Neurosci Ther. 2014;20:539–547. doi: 10.1111/cns.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CC, Yang X, Hua X, Liu J, Fan MB, Li GQ, Song J, Xu TY, Li ZY, Guan YF, et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol. 2016;173:2352–2368. doi: 10.1111/bph.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A. 2015;112:2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]


