Abstract
Uridine 5′-diphosphate (UDP) plays an important role in controlling vascular tone; however, UDP-mediated response in metabolic syndromes, including obesity and type 2 diabetes in females, remains unclear. In this study, we investigated UDP-mediated response in the aorta of female obese Otsuka Long–Evans Tokushima Fatty (OLETF) rats and control Long–Evans Tokushima Otsuka (LETO) rats. In OLETF rat aortas precontracted by phenylephrine (PE) (vs. LETO), (1) UDP-induced relaxation was increased, whereas acetylcholine (ACh)-induced relaxation was decreased; (2) no UDP- or ACh-induced relaxations were observed in endothelial denudation, whereas UDP-induced small contraction was observed; and (3) NG-nitro-L-arginine [L-NNA, a nitric oxide (NO) synthase inhibitor] eliminated UDP-induced relaxation and small contraction, whereas caused contrasting responses by ACh, including slight relaxations (LETO) and contractions (OLETF). Indomethacin, a cyclooxygenase inhibitor, eliminated the difference in UDP- and ACh-induced relaxations between the groups by increased UDP-induced relaxation in the LETO group and increased ACh-induced relaxation in the OLETF group. MRS2578, a P2Y6 receptor antagonist, eliminated the difference in UDP-induced relaxations between the groups by decreasing UDP-induced relaxation in the OLETF group. MRS2578 had no effect on UDP-induced contraction in endothelium-denuded aortas. Therefore, these findings demonstrate opposite trends of relaxations by UDP and ACh in OLETF and LETO rat aortas. These differences may be attributed to the imbalance between NO and vasoconstrictor prostanoids upon stimulations. Increased UDP-induced relaxation in OLETF rat aorta may be caused by the activation of endothelial MRS2578-sensitive P2Y6 receptor.
Electronic supplementary material
The online version of this article (10.1007/s11302-017-9595-y) contains supplementary material, which is available to authorized users.
Keywords: Obese, Female, P2Y6, Relaxation, UDP
Introduction
Extracellular nucleotides (ATP, ADP, UTP, and UDP) act as intercellular signal messengers responding to various stimuli in (patho)physiological conditions in an autocrine or a paracrine manner through activation of the P2 purinergic receptors family [1, 2]. Based on molecular structure and signaling pathways, so far, the P2 purinergic receptors are subdivided into ionotropic receptors P2X (P2X1–7) and metabotropic G-protein-coupled receptors P2Y (P2Y1,2,4,6,11–14) [1, 2]. Among purinoceptors, P2Y6 receptor, a receptor for UDP, plays a pivotal role in various responses, including inflammatory and immune responses and cardiovascular physiology [1–6]. For example, UDP has been shown to cause contractile responses in mouse coronary, mesenteric, and renal arteries; rat cerebral, intrapulmonary, mesenteric, and femoral arteries and human cerebral arteries [7–13]. Moreover, Bar et al. [14] observed that UDP led to endothelium-dependent relaxation in mouse aorta, but this relaxation was abolished in P2Y6 receptor-deficient mice, and UDP-induced contraction was observed in aorta under nitric oxide (NO) synthase (NOS) inhibition, but this contraction was abolished in P2Y6 receptor-deficient mice. Therefore, UDP plays an important role in the regulation of vascular function. However, UDP-mediated vascular responses in pathophysiological states, especially, in females, remain unexplored.
Metabolic syndrome, which is characterized by obesity, dyslipidemia, high blood pressure, and hyperglycemia, has been shown to increase risk for cardiovascular disease and diabetes [15]. The Otsuka Long–Evans Tokushima Fatty (OLETF) rat is a model of obesity and is characterized by hyperphagia-induced obesity due to a spontaneous lack of cholecystokinin CCK1 receptors, and this rat is an established model of type 2 diabetes and hyperphagia-induced obesity [16–19]. In male OLETF rats, we [20–24] and others [25] demonstrated vascular dysfunction in several arteries. Conversely, in female OLETF rats, although reproductive or metabolic dysfunction were reported [19, 26, 27], we recently observed that impaired endothelium-derived, hyperpolarization-mediated relaxation was impaired in superior mesenteric arteries for this animal model [28]; no study has focused on the relationship between vascular function and nucleotides in female OLETF rats.
Therefore, we hypothesize that UDP-mediated vascular responses would be altered in aorta isolated from female OLETF rats compared to their genetic control, Long–Evans Tokushima Otsuka (LETO) rats. We investigated the effect of UDP on vascular tone in thoracic aorta isolated from these rats at 12 months of age, which is consistent with the age of male rats exhibiting vascular dysfunction [22–24].
Materials and methods
All experimental procedures were approved by the Hoshi University Animal Care and Use Committee, and all studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals adopted by the Committee on the Care and Use of Laboratory Animals of Hoshi University (accredited by the Ministry of Education, Culture, Sports, Science, and Technology, Japan).
Detailed methods, including the experimental animals, vascular functional studies, drugs and materials, and statistical analysis, are described in the Supplementary Data.
Results and discussion
At the time of the experiment, body weight and nonfasting blood glucose were greater in the OLETF group [595.1 ± 17.2 g (n = 12), P < 0.05 and 164.7 ± 7.6 mg/dL (n = 12), P < 0.05] than in the age-matched LETO rats [308.2 ± 5.6 g (n = 12) and 115.7 ± 5.6 mg/dL (n = 12)].
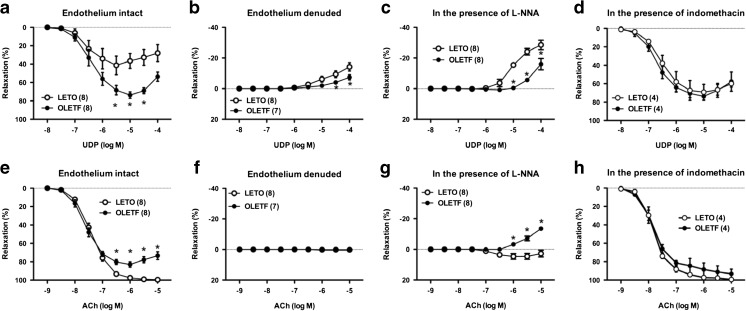
As shown in Fig. 1a, UDP elicited relaxation in endothelium-intact aorta of both OLETF and LETO rats. Unexpectedly, UDP-induced relaxation was greater in aorta from OLETF rats than from LETO rats, whereas ACh-induced relaxation was impaired in the OLETF group compared to the LETO group (Fig. 1e). SNP-induced relaxation was similar in both groups (Supplementary Fig. S1a). In endothelium-denuded aorta, no relaxation was observed and a higher UDP concentration caused contraction (Fig. 1b). This UDP-induced contraction was lower in the OLETF group than in the LETO group (Fig. 1b). ACh-induced relaxation was completely eliminated by endothelium denudation in both groups (Fig. 1f). These results suggest that UDP induces endothelium-dependent vasorelaxant responses, and there is a differing extent of relaxant responses between UDP and ACh when comparing OLETF and LETO rats.
Fig. 1.
Relaxation responses of thoracic aorta of female Otsuka Long–Evans Tokushima Fatty (OLETF) rats and Long–Evans Tokushima Otsuka (LETO) rats to uridine 5′-diphosphate (UDP) (a–d) and acetylcholine (ACh) (e–h) in intact endothelium (a, e), denuded endothelium (b, f), and in the presence of a nitric oxide synthase inhibitor (L-NNA; 10−4 M) (c, g) or cyclooxygenase inhibitor (indomethacin; 10−5 M) (d, h). Results are shown as means ± SEM; n = 4–8. Number of determinations is shown within parentheses. *P < 0.05 vs. LETO
Since NO is a major endothelium-derived relaxing factor in thoracic aorta and NO bioavailability and/or signaling are impaired in various pathophygiological states, including obesity, hypertension, and diabetes [20–22, 29], we investigated the effects of a NOS inhibitor on UDP- and ACh-induced relaxation (Fig. 1c, g, respectively). Under NOS inhibition with L-NNA (10−4 M), UDP-induced contraction was lower in the OLETF group than in the LETO group (Fig. 1c). Conversely, ACh induced a slight relaxation in the LETO group and a contraction in the OLETF group (Fig. 1g). These results suggest that UDP-induced relaxation is largely mediated by NO and the contractile component is unmasked by NOS inhibition, which was lower in OLETF rat aorta (vs. LETO).
To regulate vascular tone, endothelial cells release relaxant [such as endothelium-derived relaxing factors (EDRFs)] and contractile factors [such as endothelium-derived contracting factors (EDCFs)], and the balance between these factors is altered at various disease states [20–22, 29–33]. Moreover, purinoceptor ligands release EDCFs [30, 33]. Among these factors, vasoconstrictor prostanoids play an important role in regulating vascular tone by functioning as EDCFs [24, 25, 29, 31, 32]. In addition, in male OLETF rats, EDCF-mediated contraction has been reported to increase in superior mesenteric arteries [20–22]. Moreover, Kagota et al. have reported impaired ACh-induced endothelium-dependent relaxation in OLETF rat aorta (vs. LETO rats), and this impairment was restored by indomethacin [25]. Thus, we investigated the effect of a COX inhibitor indomethacin on UDP- (Fig. 1d and supplementary Table S1) and ACh- (Fig. 1h and supplementary Table S1) induced relaxations in rat aorta. Indomethacin (10−5 M) eliminated the difference in UDP- and ACh-induced relaxations between the groups by increasing UDP-induced relaxation in the LETO group and increasing ACh-induced relaxation in the OLETF group. These results suggest that the component of contraction by COX-derived prostanoids may be the key contributor in determining the difference in relaxant responses to each UDP and ACh between LETO and OLETF rat aortas.
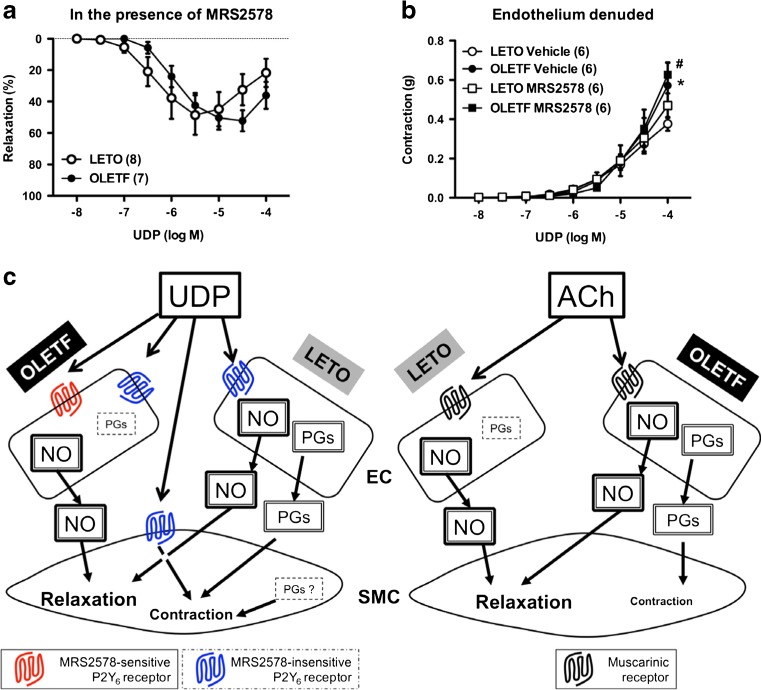
Further, we constructed concentration–relaxation curves for another specific P2Y6 agonist MRS2693 (supplementary Fig. S1b) and for UDP in the presence of P2Y6 antagonist MRS2578 (Fig. 2a) to investigate whether UDP-mediated response would activate P2Y6 receptors. Similar to UDP-induced response (Fig. 1a), MRS2693-induced relaxation was greater in OLETF rat aorta than in LETO rat aorta (Fig. S1b). Moreover, the difference in UDP-induced relaxation between the groups was eliminated in the presence of MRS2578 resulting from the decreased UDP-induced relaxation in the OLETF rat aorta (Fig. 2a and Table S1). These findings suggest that the difference in UDP-induced relaxation between the OLETF and LETO groups is caused by the relaxant component of MRS2578-sensitive P2Y6 receptor activation.
Fig. 2.
a Relaxation responses of thoracic aorta of female Otsuka Long–Evans Tokushima Fatty (OLETF) rats and Long–Evans Tokushima Otsuka (LETO) rats to uridine 5′-diphosphate (UDP) in the presence of the selective P2Y6 antagonist MRS2578 (10−5 M). b Contractile responses of the endothelium-denuded aorta of OLETF and LETO rats to UDP in the absence (vehicle, dimethyl sulfoxide) and presence of MRS2578 (10−5 M). Results are shown as means ± SEM; n = 6–8. Number of determinations is shown within parentheses. *P < 0.05, LETO vehicle vs. OLETF vehicle. # P < 0.05, LETO MRS2578 vs. OLETF MRS2578. c Summary of the present results is described in detail in the main text
Although this is the first study to investigate aortic vascular function using female OLETF rats, impaired endothelium-dependent relaxation induced by ACh was observed in previous studies using female models of metabolic diseases [34, 35]. We very recently observed that the female OLETF rat at approximately 1 year of age manifests obesity, hypertension, hyperglycemia, hyperinsulinemia, and hyperlipidemia [28]. Endothelial dysfunction is observed in animals suffering from each of the above factors [31, 32]; thus, in female OLETF rats aged 12 months, endothelial dysfunction may result from the long-term suffering of aforementioned phenomena. A novel and important finding is that UDP-induced relaxation is increased from OLETF rat aorta (vs. LETO rats) even if ACh-induced relaxation is decreased in OLETF rat aorta (vs. LETO rat aorta). There are different interactions of endothelium-derived factors with UDP and ACh in aorta isolated from female OLETF and LETO rats. Mechanisms underlying these alterations based on our results are shown in Fig. 2c. NO (relaxant effect) and prostanoids (contractile effect) participate in the UDP-induced aortic response in LETO rats, but the prostanoid component is smaller in the response of OLETF rat aorta. Because UDP-induced relaxation was similar between both the groups under COX inhibition, the NO component upon UDP stimulation was also similar between both the groups. Moreover, with regard to the vasomotor properties of endothelium-derived NO (relaxation) and prostanoids (contraction), (1) UDP-induced relaxation in endothelium-intact PE-precontracted aorta (viz., relaxation > contraction) and (2) UDP-induced contraction was greater in endothelium-denuded preparations (Fig. 2b) than in intact endothelium preparations (Fig. S1c). If prostanoids were predominant, the response would have decreased by endothelial denudation. Considering these results, NO is predominant rather than prostanoids upon UDP stimulation (Fig. 2c). In contrast, NO and prostanoids contributed to the ACh-induced aortic response in OLETF rats, whereas prostanoid contributed less to the response in LETO rat aorta. Varying contributions of endothelium-derived factors are seen in arteries of various disease models, including hypertension and diabetes [31, 32]. We speculate that the mode of generation and/or release of endothelium-derived factors (EDRFs and EDCFs) upon UDP or ACh stimulation in endothelial cells may be altered as a result of chronic diseases because both NO and COX-derived prostanoids could be generated by activation of the P2Y6 and muscarinic receptors [1, 2, 20–22]. In the present study, a UDP-induced contractile response was observed under endothelium denudation and NOS inhibition, and it was decreased in OLETF rat aorta than in LETO rat aorta. Moreover, the UDP-induced contraction was greater with NOS inhibition [OLETF, − 15.92 ± 3.69% (% relaxation of PE-induced precontraction) (n = 8); LETO, − 28.5 ± 3.10% (n = 8)] rather than in endothelium denudation [OLETF, − 7.31 ± 1.70% (n = 7); LETO, − 14.13 ± 2.64% (n = 8)]. Therefore, we cannot rule out the possibility that P2Y6 activation on smooth muscle cells may generate prostanoids. However, further investigations are required; for example, time-course alterations in both aortic expressions of P2Y6 receptors and NOS and COX activities in this model need clarification.
Notably, the present study has limitations. Some reports suggest that UDP induces vasorelaxation and vasocontraction in various arteries [7–13]. Moreover, Bar et al. [14] have demonstrated, using P2Y6 receptor knockout mice, that functional P2Y6 receptors were present in endothelial and smooth muscle cells. We found that MRS2578 only affects the elimination of the difference between UDP-induced relaxation in PE-precontracted aortas in the OLETF and LETO groups; however, relaxation still existed in the presence of MRS2578 (Fig. 2a). Moreover, Fig. 2b shows that MRS2578 did not affect UDP-induced contraction in endothelium-denuded aortas in both OLETF and LETO groups. Because another specific P2Y6 agonist MRS2693 also caused relaxation, which was increased in OLETF rat aorta than in LETO rat aorta (supplemental Fig. S1b), P2Y6 receptor stimulation definitely caused vasorelaxation in rat aorta under PE precontraction. However, at present, we can only report receptors, namely, “MRS2578-sensitive” or “MRS2578-insensitive” P2Y6 receptors (Fig. 2c). Moreover, we speculate that the activation component of MRS2578-sensitive P2Y6 receptor by UDP stimulation in endothelium may be attributable to the difference in UDP-induced relaxations between the OLETF and LETO groups. However, we cannot define which effect preferably increased NO or suppressed prostanoid productions upon MRS2578-sensitive P2Y6 receptor stimulation by UDP. Furthermore, the possible mechanisms underlying the activation of other receptors, such as GPR17 [36], or heterodimers, such as angiotensin type 1/P2Y6 receptors [37, 38], in our findings may be related to UDP-mediated responses. Because contrasting trends are observed in UDP-induced contraction in endothelium-denuded aorta between the groups, that is, between the basal and PE-precontracted aorta, the role of P2Y6 (probably MRS2578-sensitive receptor) in contraction may also be different between OLETF and LETO rat aortas. Currently, the relationship between these putative P2Y6 receptors and vascular functional properties in endothelium and smooth muscle cells remain unclear in female OLETF rat aortas. Thus, further investigations of these mechanisms are required.
Conclusions
In the present study, we observed that UDP-induced relaxation was increased in aorta from female OLETF rats compared to control LETO rats. These alterations may result from changes in the production of endothelium-derived factors on UDP stimulation. Although the precise pathophysiological significance of UDP signaling in the female obese state is not yet clear, we believe that our findings should stimulate further interest in the UDP-mediated vasomotor response as a potential therapeutic target in female metabolic syndrome-associated vascular complications.
Electronic supplementary material
(DOC 1.25 mb)
Acknowledgements
We thank T. Kamioka, S. Oshima, Y. Ohta, S. Ohashi, A. Ozeki, S. Takeno, M. Tani, and M. Tamura for technical assistance. This study was supported in part by JSPS KAKENHI Grant Numbers JP17K08318 and JP15K07975 and by the Suzuken Memorial Foundation (Japan). The authors would like to thank Enago (www.enago.jp) for the English language review.
Abbreviations
- ACh
Acetylcholine
- COX
Cyclooxygenase
- EDCF
Endothelium-derived contracting factor
- EDRF
Endothelium-derived relaxing factor
- KHS
Krebs–Henseleit Solution
- LETO
Long-Evans Tokushima Otsuka
- L-NNA
NG-nitro-L-arginine
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- OLETF
Otsuka Long-Evans Tokushima Fatty
- PE
Phenylephrine
- SNP
Sodium nitroprusside
- UDP
Uridine 5′-diphosphate
Compliance with ethical standards
Conflicts of interest
Shota Kobayashi declares that he has no conflict of interest.
Takayuki Matsumoto declares that he has no conflict of interest.
Makoto Ando declares that he has no conflict of interest.
Maika Iguchi declares that he/she has no conflict of interest.
Shun Watanabe declares that he has no conflict of interest.
Kumiko Taguchi declares that she has no conflict of interest.
Tsuneo Kobayashi declares that he has no conflict of interest.
Ethical approval
This study was approved by the Hoshi University Animal Care and Use Committee, and all studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals adopted by the Committee on the Care and Use of Laboratory Animals of Hoshi University (accredited by the Ministry of Education, Culture, Sports, Science, and Technology, Japan).
Footnotes
Shota Kobayashi and Takayuki Matsumoto contributed equally to this work and should be considered as co-first authors.
Electronic supplementary material
The online version of this article (10.1007/s11302-017-9595-y) contains supplementary material, which is available to authorized users.
Contributor Information
Takayuki Matsumoto, Phone: 81-3-5498-5726, Email: t-matsu@hoshi.ac.jp.
Tsuneo Kobayashi, Phone: +81-3-5498-5849, Email: tkoba@hoshi.ac.jp.
References
- 1.Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 2.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Prinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnstock G. Purinergic signaling in the cardiovascular system. Circ Res. 2017;120:207–228. doi: 10.1161/CIRCRESAHA.116.309726. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z, Wang Z, Ren H, Yue M, Huang K, Gu H, Liu M, Du B, Qian M. P2Y(6) agonist uridine 5′-diphosphate promotes host defense against bacterial infection via monocyte chemoattractant protein-1-mediated monocyte/macrophage recruitment. J Immunol. 2011;186:5376–5387. doi: 10.4049/jimmunol.1002946. [DOI] [PubMed] [Google Scholar]
- 5.Stachon P, Peikert A, Michel NA, Hergeth S, Marchini T, Wolf D, Dufner B, Hoppe N, Ayata CK, Grimm M, Cicko S, Schulte L, Reinohl J, von zur Muhlen C, Bode C, Idzko M, Zirlik A. P2Y6 deficiency limits vascular inflammation and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2014;34:2237–2245. doi: 10.1161/ATVBAHA.114.303585. [DOI] [PubMed] [Google Scholar]
- 6.Riegel AK, Faigle M, Zug S, Rosenberger P, Robaye B, Boeynaems JM, Idzko M, Eltzschig HK. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood. 2011;117:2548–2555. doi: 10.1182/blood-2010-10-313957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vial C, Evans RJ. P2X1 receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol. 2002;62:1438–1445. doi: 10.1124/mol.62.6.1438. [DOI] [PubMed] [Google Scholar]
- 8.Malmsjo M, Hou M, Pendergast W, Erlinge D, Edvinsson L. Potent P2Y6 receptor mediated contractions in human cerebral arteries. BMC Pharmacol. 2003;3:4. doi: 10.1186/1471-2210-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vonend O, Stegbauer J, Sojka J, Habbel S, Quack I, Robaye B, Boeynaems JM, Rumb LC. Noradrenaline and extracellular nucleotide cotransmission involves activation of vasoconstrictive P2X(1,3)- and P2Y6-like receptors in mouse perfused kidney. Br J Pharmacol. 2005;145:66–74. doi: 10.1038/sj.bjp.0706151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffenstein G, Tamareille S, Prunier F, Roy C, Ayer A, Toutain B, Billaud M, Isakson BE, Grimaud L, Loufrani L, Rousseau P, Abraham P, Procaccio V, Monyer H, de Wit C, Boeynaems JM, Robaye B, Kwak BR, Henrion D. Central role of P2Y6 UDP receptor in arteriolar myogenic tone. Arterioscler Thromb Vasc Biol. 2016;36:1598–1606. doi: 10.1161/ATVBAHA.116.307739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haanes KA, Spray S, Syberg S, Jorgensen NR, Robaye B, Boeynaems JM, Edvinsson L. New insight on pyrimidine signalling within the arterial vasculature—different roles for P2Y2 and P2Y6 receptors in large and small coronary arteries of the mouse. J Mol Coll Cardiol. 2016;93:1–11. doi: 10.1016/j.yjmcc.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell C, Syed NI, Tengah A, Gurney AM, Kennedy C. Identification of contractile P2Y1, P2Y6, and P2Y12 receptors in rat intrapulmonary artery using selective ligands. J Pharmacol Exp Ther. 2012;343:755–762. doi: 10.1124/jpet.112.198051. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto T, Tostes RC, Webb RC. Alterations in vasoconstrictor responses to the endothelium-derived contracting factor uridine adenosine tetraphosphate are region specific in DOCA-salt hypertensive rats. Pharmacol Res. 2012;65:81–90. doi: 10.1016/j.phrs.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, Bult H, Robaye B. Knockout mice reveal a role for P2Y6 receptor in macrophage, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol. 2008;74:777–784. doi: 10.1124/mol.108.046904. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne CM, Hoogeveen RC, McNeill AM, Heiss G, Schmidt MI, Duncan BB, Pankow JS. Metabolic syndrome risk for cardiovascular disease and diabetes in the ARIC study. Int J Obes. 2008;32:S21–S24. doi: 10.1038/ijo.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 17.Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract. 1994;24:S317–S320. doi: 10.1016/0168-8227(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 18.Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol. 2006;48:360–367. doi: 10.1002/dev.20149. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder M, Zagoory-Sharon O, Shbiro L, Marco A, Hyun J, Moran TH, Bi S, Weller A. Development of obesity in the Otsuka Long-Evans Tokushima Fatty rat. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1749–R1760. doi: 10.1152/ajpregu.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto T, Kakami M, Noguchi E, Kobayashi T, Kamata K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;293:H1480–H1490. doi: 10.1152/ajpheart.00229.2007. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto T, Noguchi E, Ishida K, Kobayashi T, Yamada N, Kamata K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol. 2008;295:H1165–H1176. doi: 10.1152/ajpheart.00486.2008. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto T, Nakayama N, Ishida K, Kobayashi T, Kamata K. Eicosapentaenoic acid improves imbalance between vasodilator and vasoconstrictor actions of endothelium-derived factors in mesenteric arteries from rat at chronic stage of type 2 diabetes. J Pharmacol Exp Ther. 2009;329:324–334. doi: 10.1124/jpet.108.148718. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto T, Watanabe S, Kawamura R, Taguchi K, Kobayashi T. Epigallocatechin gallate attenuates ET-1-induced contraction in carotid artery from type 2 diabetic OLETF rat at chronic stage of disease. Life Sci. 2014;118:200–205. doi: 10.1016/j.lfs.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto T, Watanabe S, Ando M, Yamada K, Iguchi M, Taguchi K, Kobayashi T. Diabetes and age-related differences in vascular function of renal artery: possible involvement of endoplasmic reticulum stress. Rejuvenation Res. 2016;19:41–52. doi: 10.1089/rej.2015.1662. [DOI] [PubMed] [Google Scholar]
- 25.Kagota S, Yamaguchi Y, Nakamura K, Kunitomo M. Altered endothelium-dependent responsiveness in the aortas and renal arteries of Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a model of non-insulin-dependent diabetes mellitus. Gen Pharmacol. 2000;34:201–209. doi: 10.1016/S0306-3623(00)00061-6. [DOI] [PubMed] [Google Scholar]
- 26.Amon L, Hazut N, Tabachinik T, Weller A, Koren L. Maternal testosterone and reproductive outcome in a rat model of obesity. Theriogenology. 2016;86:1042–1047. doi: 10.1016/j.theriogenology.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder M, Kronfeld-Schor N, Weller A. Selective leptin insensitivity and alterations in female-reproductive patterns linked to hyperleptinemia during infancy. PLoS One. 2013;8:e59937. doi: 10.1371/journal.pone.0059937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto T, Kobayashi S, Ando M, Watanabe S, Iguchi M, Taguchi K, Kobayashi T. Impaired endothelium-derived hyperpolarization-type relaxation in superior mesenteric arteries isolated from female Otsuka Long-Evans Tokushima Fatty rats. Eur J Pharmacol. 2017;807:151–158. doi: 10.1016/j.ejphar.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy CG, Wenceslau CF, Goulopoulou S, Ogbi S, Matsumoto T, Webb RC. Autoimmune therapeutic chloroquine lowers blood pressure and improves endothelial function in spontaneously hypertensive rats. Pharmacol Res. 2016;113:384–394. doi: 10.1016/j.phrs.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsaqati M, Chan SL, Ralevic V. Investigation of the functional expression of purine and pyrimidine receptors in porcine isolated pancreatic arteries. Purinergic Sig. 2014;10:241–249. doi: 10.1007/s11302-013-9403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto T, Goulopoulou S, Taguchi K, Tostes RC, Kobayashi T. Constrictor prostanoids and uridine adenosine tetraphosphate: vascular mediators and therapeutic targets in hypertension and diabetes. Br J Pharmacol. 2015;172:3980–4001. doi: 10.1111/bph.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease—a 30th anniversary update. Acta Physiol. 2017;219:22–96. doi: 10.1111/apha.12646. [DOI] [PubMed] [Google Scholar]
- 33.Ishida K, Matsumoto T, Taguchi K, Kamata K, Kobayashi T. Mechanisms underlying altered extracellular nucleotide-induced contractions in mesenteric arteries from rats in later-stage type 2 diabetes: effect of ANG II type 1 receptor antagonism. Am J Physiol Heart Circ Physiol. 2011;301:H1850–H1861. doi: 10.1152/ajpheart.00502.2011. [DOI] [PubMed] [Google Scholar]
- 34.Naderali EK, Brown MJ, Pickavance LC, Wilding JP, Doyle PJ, Williams G. Dietary obesity in the rat induces endothelial dysfunction without causing insulin resistance: a possible role for triacylglycerols. Clin Sci. 2001;101:499–506. doi: 10.1042/cs1010499. [DOI] [PubMed] [Google Scholar]
- 35.Pieper GM, Siebeneich W, Moore-Hilton G, Roza AM. Reversal by L-arginine of a dysfunctional arginine/nitric oxide pathway in the endothelium of the genetic diabetic BB rat. Diabetologia. 1997;40:910–915. doi: 10.1007/s001250050767. [DOI] [PubMed] [Google Scholar]
- 36.Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimura A, Sunggip C, Tozaki-Saitoh H, Shimauchi T, Numaga-Tomita T, Hirano K, Ide T, Boeynaems JM, Kurose H, Tsuda M, Robaye B, Inoue K, Nishida M. Purinergic P2Y6 receptor heterodimerize with angiotensin AT1 receptors to promote angiotensin II-induced hypertension. Sci Signal. 2016;9:ra7. doi: 10.1126/scisignal.aac9187. [DOI] [PubMed] [Google Scholar]
- 38.Sunggip C, Nishimura A, Shimoda K, Numaga-Tomita T, Tsuda M, Nishida M. Purinergic P2Y6 receptors: a new therapeutic target of age-dependent hypertension. Pharmacol Res. 2017;120:51–59. doi: 10.1016/j.phrs.2017.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 1.25 mb)