Abstract
The expression of Ccn1 (Cyr61) is essential for cardiovascular development during embryogenesis, whereas in adulthood it is associated with inflammation, wound healing, injury repair, and related pathologies including fibrosis and cancer. Recent studies have found that CCN1 plays a critical role in promoting wound healing and tissue repair. Mechanistically, CCN1 functions through direct interaction with specific integrin receptors expressed in various cell types in the wound tissue microenvironment to coordinate diverse cellular functions for repair. Here we briefly summarize the current knowledge on the functions of CCN1 in tissue injury repair and discuss pertinent unanswered questions.
Keywords: wound healing, tissue regeneration, injury repair, integrin, inflammation
Introduction
CCN1, previously named Cyr61, was identified as a cysteine-rich protein encoded by a growth factor-inducible immediate-early gene (Lau and Nathans 1987; O'Brien et al. 1990). Subsequent identification of other highly conserved proteins gave rise to the CCN family of six secreted matricellular proteins with a similar modular structure (Lau and Lam 1999; Leask and Abraham 2006; Holbourn et al. 2008; Chen and Lau 2009). Ccn1 is an essential gene required for cardiovascular development during embryogenesis (Mo et al. 2002; Mo and Lau 2006), whereas in adulthood its expression is tightly associated with inflammation and injury repair (Lau 2011). Numerous studies have found elevated expression of Ccn1 and other members of the Ccn gene family in tissue injury, chronic diseases and related pathologies, including fibrosis and cancer in animal models and human patients (Jun and Lau 2011; Kubota and Takigawa 2013). In some instances, the expression of members of the Ccn gene family is shown to promote disease pathology or cancer progression (Kubota and Takigawa 2015; Kleer 2016). For example, Ccn2 expression has been widely associated with fibrotic conditions and appears to mediate or exacerbate fibrosis (Leask 2013). By contrast, recent findings have indicated that CCN1 plays important roles in promoting injury repair in several organs and tissues.
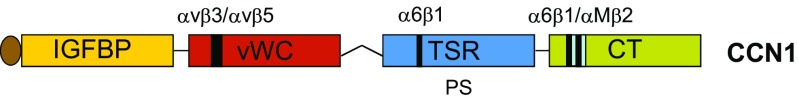
Wound healing and injury repair, irrespective of the organ or tissue, generally proceed through three broad phases that are temporally overlapping but functionally distinct (Stramer et al. 2007; Gurtner et al. 2008). Infiltration of neutrophils and macrophages comprise the initial inflammatory phase, whereby immune cells combat microbes and eliminate cell debris. This is followed by the proliferation phase when myofibroblasts produce a large amount of extracellular matrix (ECM) to promote tissue integrity and parenchymal cell proliferation. Finally, the provisional ECM is remodeled in the maturation phase to rebuild the architecture of the original uninjured tissue. Interestingly, CCN1 has been shown to regulate each of these phases in injury repair by interacting with disparate cell types through specific integrin receptors (Lau 2016). For example, CCN1 binds integrins αVβ3 and αVβ5 in epithelial cells and α6β1 in fibroblastic cells, and its specific binding sites for these receptors have been identified (Fig. 1) (Leu et al. 2003; Chen et al. 2004; Leu et al. 2004). Furthermore, site-specific CCN1 mutations targeting these binding sites that abolish integrin binding have been prepared, and knockin mice with these mutant alleles replacing the Ccn1 genomic locus have been constructed (Chen et al. 2007; Kim et al. 2013, 2015). Thus, Ccn1D125A/D125A mice express CCN1 with a single amino acid change (D125 to A) that abrogates binding to αVβ3/αVβ5, and Ccn1DM/DM mice carry an allele encoding CCN1 unable to bind α6β1. In contrast to the embryonic lethality of Ccn1 knockout mice (Mo et al. 2002; Mo and Lau 2006), these knockin mice are fertile and viable, allowing the investigation of CCN1 functions through specific integrin receptors in various mouse models of injury (Chen et al. 2007; Kim et al. 2013, 2015).
Fig. 1. Schematic diagram of CCN1.
Human CCN1 is a 381 a.a. protein with an N-terminal secretory peptide followed by 4 structural domains conserved in the CCN protein family. A binding site for integrins αVβ3 and αVβ5 is located in the von Willebrand factor C domain (VWC), and the C-terminal (CT) domain contains two α6β1 binding site that partially overlap with an αMβ2 binding site (Leu et al. 2004; Schober et al. 2002). The thrombospondin type 1 repeat (TSR) domain binds phosphatidylserine (Jun et al. 2015), and contains an α6β1 binding site that functions when CCN1 acts as an immobilized substrate (Leu et al. 2003; Leu et al. 2004)
CCN1 in cutaneous wound healing
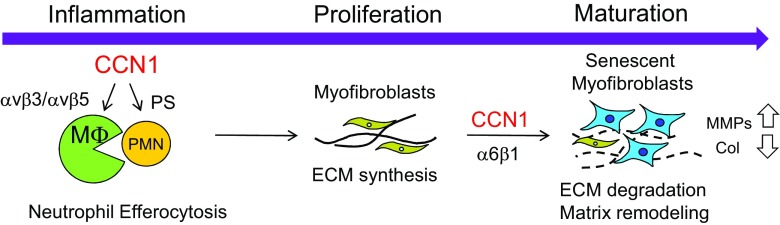
Upon skin injury, Ccn1 is upregulated in a biphasic manner during the inflammatory phase and the maturation phase (Jun et al. 2015). A remarkable activity of CCN1 in the inflammatory phase of cutaneous wound healing is the stimulation of efferocytosis, or phagocytic clearance of apoptotic cells (Jun et al. 2015). Infiltration of neutrophils is one of the first events to occur in response to wounding to defend against microbial infections. However, neutrophils are short-lived and rapidly undergo apoptosis. The apoptotic neutrophils must be removed in order for healing to proceed, since these cells can undergo secondary necrosis and secrete proteases and cytokines that cause further tissue damage (Silva 2010). Ccn1D125A/D125A knockin mice, which express a mutant CCN1 unable to bind αVβ3/αVβ5, suffer impaired wound healing with the accumulation of apoptotic neutrophils. Mechanistically, CCN1 acts as a bridging molecule and binds phosphatidylserine (PS), the “eat-me” signal on apoptotic cells, and αVβ3/αVβ5 in macrophages to trigger phagocytic removal of the apoptotic neutrophils (Fig. 2). Wounds of Ccn1D125A/D125A mice are deficient in CCN1-mediated clearance of apoptotic neutrophils and are stalled at the inflammatory phase, resulting in impaired progression to the proliferation phase (Jun et al. 2015).
Fig. 2. CCN1 functions in wound healing.
In the inflammatory phase, CCN1 stimulates the clearance of apoptotic neutrophils (Jun et al. 2015), allowing wound healing to progress to the proliferation phase in which myofibroblasts produce ECM to promote tissue integrity. During the maturation phase, CCN1 induces cellular senescence in myofibroblasts to stimulate matrix remodeling and limit fibrosis (Jun and Lau 2010a)
During the proliferation phase, resident fibroblasts, pericytes, and mesenchymal stem cell-like cells may transdifferentiate into myofibroblasts and produce ECM to promote tissue integrity and cell proliferation (Jun and Lau 2018). When healing enters the maturation phase, these fibrogenic myofibroblasts cease to produce ECM by undergoing apoptosis, senescence, dedifferentiation, or lineage reprogramming (Jun and Lau 2018). However, if the injury is chronic or wound healing is deregulated, uncontrolled stimulation of myofibroblasts can lead to fibrotic scar formation and loss of organ function (Bochaton-Piallat et al. 2016; Wynn and Vannella 2016). CCN1 level declines after the inflammatory phase in skin wounds but is elevated again in the maturation phase (Jun et al. 2015). CCN1 triggers myofibroblasts to undergo cellular senescence through engagement of integrin α6β1, whereupon senescent myofibroblasts express the senescence-associated secretory phenotype (SASP) that includes upregulation of matrix degradation enzymes and downregulation of collagen (Jun and Lau 2010a). Thus, CCN1 turns the ECM-producing, fibrogenic myofibroblasts into ECM-degrading, anti-fibrotic senescent cells in the maturation phase (Jun and Lau 2010b). As such, CCN1 accelerates matrix remodeling in the maturation phase and limits fibrosis. Consistently, Ccn1DM/DM mice that express CCN1 unable to bind α6β1 suffer exacerbated fibrosis as myofibroblasts fail to undergo senescence (Jun and Lau 2010a). Treatment of wounds with CCN1 protein reduces accumulation of collagen with a concomitant increase in senescent myofibroblasts. CCN1 triggers senescence in fibroblastic cells through α6β1-mediated activation of RAC1 and NOX1 to generate the sustained accumulation of reactive oxygen species (ROS), which trigger senescence by activating the p53 and p16INK4a/pRb pathways (Jun and Lau 2010a).
CCN1 in hepatobiliary injury repair
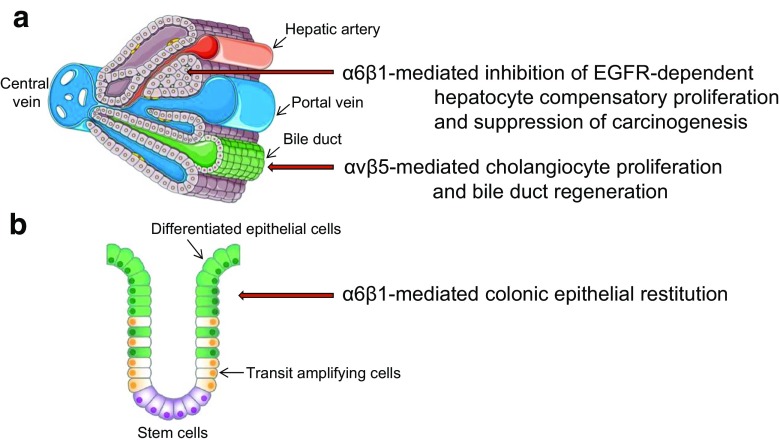
The liver is a highly regenerative organ and CCN1 plays critical roles in the proliferation and maturation phases of hepatobiliary injury repair. Hepatocytes and cholangiocytes are the two distinct epithelial cell types of the liver, and they respond to damage differently (Fig. 3a). In cholestatic injury, the major repair response during the proliferation phase is ductular reaction, which includes cholangiocyte proliferation, expansion of bile ducts, and differentiation of hepatic progenitor cells (HPCs) into cholangiocytes. CCN1 is essential for the regeneration of bile ducts after injury from either bile duct ligation (BDL) or diethoxycarbonyl dihydrocollidine (DDC) diet, experimental models that mimic human cholestatic liver disease (Kim et al. 2015). Indeed, Ccn1D125A/D125A mice perish within days after BDL, whereas wild-type mice survive during the same experimental period. The rapid mortality is because ductular reaction fails to occur, and the bile acids that cannot drain into the gallbladder and intestine leak out to the liver parenchyma and cause massive hepatic necrosis. CCN1 acts by engagement of integrins αVβ5 in cholangiocytes, thus activating NFκB-mediated Jag1 expression and Jag1/Notch signaling required for cholangiocyte proliferation (Kim et al. 2015). Consistent with the essential role of CCN1 for cholangiocyte proliferation and bile duct expansion through the αV-NFκB axis, wild-type mice phenocopy Ccn1D125A/D125A mice in response to BDL when subjected to blockade of integrins αVβ3/αVβ5 or inhibition of NFκB activation (Kim et al. 2015). Moreover, injection of Jag1 rescues the bile duct regeneration defects in Ccn1D125A/D125A mice. CCN1 also activates Jag1 expression in hepatic stellate cells, which interact with HPCs through Jag1/Notch signaling and induced the differentiation of HPCs into cholangiocytes to promote bile duct regeneration (Kim et al. 2015; Boulter et al. 2012). These observations show that CCN1 is essential for ductular reaction in bile duct regeneration, acting through αVβ5-mediated activation of NFκB and Jag1/Notch signaling.
Fig. 3. Parenchymal-specific CCN1 functions during the proliferation phase in repair.
a In the liver, CCN1 inhibits compensatory proliferation of hepatocytes when damage is induced by the hepatocarcinogen DEN, thus suppressing tumor initiation (Chen et al. 2016). However, when cholangiocytes are damaged by cholestasis, CCN1 induces the proliferation of cholangiocytes and bile duct regeneration (Kim et al. 2015). b In the bowel, CCN1 promotes intestinal epithelial restitution in response to inflammatory injury (Choi et al. 2015)
CCN1 also accelerates matrix remodeling in the maturation phase and limits fibrosis. When the liver suffers chronic injuries due to hepatotoxin intoxication (e.g., carbon tetrachloride) or cholestasis (e.g., BDL), myofibroblasts are activated to produce ECM in the injury response. In both injury models, CCN1 dampens and limits fibrosis by inducing senescence in the myofibroblasts, leading to their expression of the anti-fibrotic SASP (Kim et al. 2013). Ccn1DM/DM mice and mice with hepatocyte-specific deletion of Ccn1 (Ccn1ΔHep) suffer exacerbated fibrosis concomitant with deficiencies in myofibroblast senescence, whereas overexpression of Ccn1 in hepatocytes or adenoviral expression of Ccn1 in the liver reduces liver fibrosis with enhanced senescence (Kim et al. 2013; Borkham-Kamphorst et al. 2014). Moreover, administration of purified CCN1 protein results in accelerated resolution of pre-established fibrosis (Kim et al. 2013). Together, these results show that CCN1 is required for bile duct regeneration after cholestasis and for limiting fibrosis following hepatobiliary injuries.
CCN1 in intestinal mucosal healing
As the intestinal epithelium is constantly exposed to microbes and ingested toxins, the intestinal epithelial cells are prone to damage. Therefore, differentiated intestinal epithelial cells are removed continuously and are replaced by new cells generated from stem cells located at the base of the intestinal crypt. Typically, epithelial cells of the small intestine are replaced every 4–5 days (Blander 2016). Inflammatory bowel diseases, including Crohn’s disease and ulcerative colitis, are associated with inflammatory injury of the bowel and impaired mucosal healing (Abraham and Cho 2009). A common murine model for inflammatory injury to the bowel is exposure to dextran sodium sulfate (DSS), which causes intestinal epithelial cell death and inflammation, followed by mucosal healing and repair. Remarkably, administration of CCN1 protein in wild-type mice greatly accelerates mucosal healing following exposure to DSS, and enhances intestinal epithelial restitution (Choi et al. 2015). Furthermore, Ccn1DM/DM mice suffer high mortality, impaired mucosal healing, and diminished interleukin-6 (IL-6) expression during the repair phase of DSS-induced colitis. Moreover, CCN1 induces IL-6 expression in macrophages through integrin αMβ2 and in fibroblasts through α6β1, and IL-6 promotes intestinal epithelial cell proliferation (Choi et al. 2015). Administration of CCN1 protein or IL-6 resulted in full or partial rescue of the mucosal healing defects and mortality of Ccn1DM/DM mice, respectively (Choi et al. 2015). These findings show that CCN1 plays a critical role in intestinal mucosal healing after colitis in part through integrin-mediated activation of IL-6 expression, and suggest a therapeutic potential for CCN1 for treating inflammatory bowel disease (Fig. 3b).
CCN1 in injury repair of other tissues
Tissue repair often requires angiogenesis to support new cell growth and for vascularization of the new tissue. CCN1 can induce angiogenesis through direct binding to integrin αVβ3 in endothelial cells (Babic et al. 1998; Leu et al. 2002). Endothelial cells of the angiogenic sprouts are organized into the tip cell at the leading edge that migrates directionally toward the angiogenic signal gradient, followed by stalk cells that form the endothelial lumen of the vessel (Blanco and Gerhardt 2013). The tip cell expresses the Notch ligand Dll4 and interacts with stalk cells to activate Notch signaling, thereby establishing a hierarchy that maintains organization of the angiogenic sprout (Blanco and Gerhardt 2013). Interestingly, CCN1 regulates Dll4 expression and Dll4-Notch signaling between tip and stalk cells as well as VEGFR2 signaling in the angiogenic sprout, thus regulating the interaction between tip and stalk cells (Hasan et al. 2011; Chintala et al. 2015). Consequently, endothelial cell-specific deletion of Ccn1 leads to endothelial hyperplasia and loss of the distinctive hierarchical organization, resulting in blood vessels that form a flat hyperplastic sinus (Chintala et al. 2015). Thus, CCN1 may contribute to wound repair through its angiogenic function. For example, Ccn1 is highly expressed during bone fracture healing in rat and sheep (Hadjiargyrou et al. 2000; Lienau et al. 2006), potentially enhancing fracture healing through its angiogenic activity (Athanasopoulos et al. 2007). Indeed, application of CCN1 protein in a collagen matrix to the site of osteotomy improves fracture repair by increasing callus diameter, bone volume, and torsional strength in a rabbit trauma model (Frey et al. 2012).
In the lung, both positive and negative effects of CCN1 have been reported in injury repair, although differences in the injury models and experimental protocols may underlie the disparate observations. Treatment with recombinant CCN1 protein curtails hyperoxia-induced injury in neonatal rats, in part by reducing macrophage and neutrophil infiltration and inflammasome activation (Vaidya et al. 2017). CCN1 also protects against hyperoxia-induced cell death in lung epithelial cells via activation of the Akt (Jin et al. 2005). Furthermore, recombinant CCN1 accelerated repair whereas anti-CCN1 antibodies impaired repair from inflammatory injury to the lung epithelium in an in vitro assay (Zemans et al. 2013). In contrast to these repair-promoting functions, it has also been reported that adenoviral overexpression of CCN1 in the lung induces injury with weight loss, increased neutrophil counts in bronchoalveolar lavage, and increased mortality (Grazioli et al. 2015). However, it is not clear which cell types are overexpressing CCN1, and the mechanistic basis for this injury is unknown. It has also been reported that siRNA knockdown of Ccn1 downregulates profibrotic gene expression and TGF-β1-induced SMAD3 signaling in lung fibroblasts, thus attenuating bleomycin-induced lung injury (Kurundkar et al. 2016). These findings suggest that CCN1 may promote TGF-β1 actions in specific contexts.
CCN1 inhibits injury-induced hepatocellular carcinogenesis
Elevated expression of CCN1 has been observed in many cancers (Lau 2011), and overexpression of Ccn1 in xenografts of established cancer cell lines generally promotes tumor growth, potentially through stimulation of angiogenesis, cell migration, epithelial-mesenchymal transition, and cancer stemness (Babic et al. 1998; Haque et al. 2011; Huang et al. 2017). However, CCN1 plays an inhibitory role in cancer initiation induced by injury. Hepatocellular carcinoma (HCC) is often driven by chronic inflammatory damage occurring over an extended period of time. A commonly used mouse model of HCC is the exposure to diethylnitrosamine (DEN), which causes DNA damage and apoptosis in hepatocytes. This leads to the phagocytic elimination of cell debris by Kupffer cells, which produce hepatomitogens that trigger hepatocyte proliferation to compensate for the cell loss. However, this compensatory proliferation may stimulate the expansion of damaged cells that are prone to oncogenic transformation and is a critical driver of hepatocarcinogenesis resulting from hepatocyte damage (Kuraishy et al. 2011). In this context, the epidermal growth factor receptor (EGFR) plays a critical role, and inhibition of EGFR signaling by erlotinib abolishes compensatory hepatocyte proliferation and abrogates development of HCC (Chen et al. 2016). CCN1 suppresses tumorigenesis in DEN-induced HCC by inhibiting EGFR-dependent hepatocyte compensatory proliferation through α6β1-mediated generation of ROS and p53 activation, leading to a cell cycle block (Chen et al. 2016). As a result, Ccn1ΔHep and Ccn1DM/DM mice suffer increased compensatory proliferation and tumorigenicity after exposure to DEN (Chen et al. 2016).
Summary and perspectives
As summarized above, CCN1 interacts with distinct cell types through specific integrin receptors to coordinate diverse and critical functions in wound healing and injury repair (Table 1). In the inflammatory phase, CCN1 stimulates macrophage efferocytosis of apoptotic neutrophils, thereby facilitating the progression to the proliferation phase (Jun et al. 2015). In cholestatic injury to the liver, CCN1 is essential for inducing Jag1/Notch signaling and cholangiocyte proliferation (Kim et al. 2015). However, when hepatocytes are damaged due to hepatocarcinogens such as DEN, CCN1 restricts compensatory proliferation and therefore suppresses cancer initiation (Chen et al. 2016). In the intestinal epithelium, CCN1 greatly promotes mucosal restitution following inflammatory injury, in part through the induction of IL-6 (Choi et al. 2015). Finally, in the maturation phase, CCN1 accelerates matrix remodeling and dampens fibrosis by inducing myofibroblast senescence (Kim et al. 2013; Jun and Lau 2010a). Moreover, CCN1 may contribute to tissue repair in wound healing through its induction of angiogenesis (Babic et al. 1998; Chintala et al. 2015). These findings illustrate the broad range of biological functions that CCN1 can coordinate through various cell types in tissue repair and portend additional functions yet to be uncovered. Explorations using disparate injury models will provide additional insights. Since CCN1 induces diverse functions in distinct cell types at various stages of wound healing and tissue repair, inhibition or overexpression of CCN1 may have different consequences depending on the tissue, nature of the damage, and timing of perturbation. These diverse effects may be important considerations in contemplating CCN1 as a therapeutic target.
Table 1.
CCN1-induced functions mediated through specific integrins in tissue injury repair
| Cell type | αVβ3/αVβ5 | α6β1 | αMβ2 | References |
|---|---|---|---|---|
| Endothelial cells | Angiogenesis | (Babic et al. 1998; Leu et al. 2002) | ||
| Hepatocytes | Inhibition of EGFR-dependent proliferation | (Chen et al. 2016) | ||
| Cholangiocytes | Jag1/Notch signaling and cell proliferation | (Kim et al. 2015; Kim and Lau 2015) | ||
| Macrophages | Phagocytic clearance of apoptotic cells | Expression of cytokines | (Jun et al. 2015; Choi et al. 2015; Bai et al. 2010) | |
| Myofibroblasts | Senescence | (Jun and Lau 2010a) |
Currently much is unknown about how Ccn1 expression is regulated in diverse cell types in the context of injury repair. Furthermore, since the specific functions of CCN1 are mediated through its binding to specific integrins and their associated co-receptors, it will be important to understand how the molecular interaction between CCN1-integrin complexes with specific co-receptors affects signaling mechanisms (Lau 2016; Juric et al. 2012). The functional significance and regulation of post-translational modification of CCN1, including fucosylation and proteolysis, are also not well understood (Niwa et al. 2015; Pendurthi et al. 2005; Choi et al. 2013). The large number of conserved cysteine residues in CCN proteins suggests that specific tertiary structure is important for their function, although little structural information is currently available. Future determination of the 3-dimensional structure of CCN1 will greatly enhance our understanding of its mechanism of action.
Acknowledgements
This work was supported by NIH grants R01AR061791, R01DK108994, and R01GM078492 to L.F.L.
References
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasopoulos AN, Schneider D, Keiper T, Alt V, Pendurthi UR, Liegibel UM, Sommer U, Nawroth PP, Kasperk C, Chavakis T. Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J Biol Chem. 2007;282:26746–26753. doi: 10.1074/jbc.M705200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, product of a growth factor-inducible immediate-early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T, Chen C-C, Lau LF. The matricellular protein CCN1 activates a pro-inflammatory genetic program in murine macrophages. J Immunol. 2010;184:3223–3232. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco R, Gerhardt H. VEGF and notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM. Death in the intestinal epithelium-basic biology and implications for inflammatory bowel disease. FEBS J. 2016;283:2720–2730. doi: 10.1111/febs.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochaton-Piallat, M. L., Gabbiani, G. & Hinz, B. (2016) The myofibroblast in wound healing and fibrosis: answered and unanswered questions, F1000Res. 5:752.10.12688/f1000research.8190.1 [DOI] [PMC free article] [PubMed]
- Borkham-Kamphorst E, Schaffrath C, Van de Leur E, Haas U, Tihaa L, Meurer SK, Nevzorova YA, Liedtke C, Weiskirchen R. The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-beta signaling. Biochim Biophys Acta. 2014;1843:902–914. doi: 10.1016/j.bbamcr.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, van RN, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ. Macrophage-derived Wnt opposes notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Lau LF. Functions and mechanisms of action of CCN Matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Leu S-J, Todorovic V, Lam SC-T, Lau LF. Identification of a novel integrin αvβ3 binding site in CCN1 (CYR61) critical for pro-angiogenic activities in vascular endothelial cells. J Biol Chem. 2004;279:44166–44176. doi: 10.1074/jbc.M406813200. [DOI] [PubMed] [Google Scholar]
- Chen CC, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF. Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling. EMBO J. 2007;26:1257–1267. doi: 10.1038/sj.emboj.7601596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Kim KH, Lau LF. The matricellular protein CCN1 suppresses hepatocarcinogenesis by inhibiting compensatory proliferation. Oncogene. 2016;35:1314–1323. doi: 10.1038/onc.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintala H, Krupska I, Yan L, Lau LF, Grant M, Chaqour B. The matricellular protein CCN1 controls retinal angiogenesis by targeting VEGF, Src homology 2 domain phosphatase-1 and notch signaling. Development. 2015;142:2364–2374. doi: 10.1242/dev.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Lin A, Shrier E, Lau LF, Grant MB, Chaqour B. Degradome products of the matricellular protein CCN1 as modulators of pathological angiogenesis in the retina. J Biol Chem. 2013;288:23075–23089. doi: 10.1074/jbc.M113.475418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Kim KH, Lau LF. The matricellular protein CCN1 promotes mucosal healing in murine colitis through IL-6. Mucosal Immunol. 2015;8:1285–1296. doi: 10.1038/mi.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SP, Doht S, Eden L, Dannigkeit S, Schuetze N, Meffert RH, Jansen H. Cysteine-rich matricellular protein improves callus regenerate in a rabbit trauma model. Int Orthop. 2012;36:2387–2393. doi: 10.1007/s00264-012-1659-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazioli S, Gil S, An D, Kajikawa O, Farnand AW, Hanson JF, Birkland T, Chen P, Duffield J, Schnapp LM, Altemeier WA, Matute-Bello G. CYR61 (CCN1) overexpression induces lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2015;308:L759–L765. doi: 10.1152/ajplung.00190.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Hadjiargyrou M, Ahrens W, Rubin CT. Temporal expression of the chondrogenic and angiogenic growth factor CYR61 during fracture repair. J Bone Miner Res. 2000;15:1014–1023. doi: 10.1359/jbmr.2000.15.6.1014. [DOI] [PubMed] [Google Scholar]
- Haque I, Mehta S, Majumder M, Dhar K, De A, McGregor D, Vanveldhuizen PJ, Banerjee SK, Banerjee S. Cyr61/CCN1 signaling is critical for epithelial-mesenchymal transition and stemness and promotes pancreatic carcinogenesis. Mol Cancer. 2011;10:8. doi: 10.1186/1476-4598-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan, A., Pokeza, N., Shaw, L., Lee, H. S., Lazzaro, D., Chintala, H., Rosenbaum, D., Grant, M. B. & Chaqour, B. (2011) The matricellular protein cysteine-rich protein 61 (CCN1/ Cyr61) enhances physiological adaptation of retinal vessels and reduces pathological neovascularization associated with ischemic retinopathy. J Biol Chem 286:9542-9554 [DOI] [PMC free article] [PubMed]
- Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33:461–473. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YT, Lan Q, Lorusso G, Duffey N, Ruegg C. The matricellular protein CYR61 promotes breast cancer lung metastasis by facilitating tumor cell extravasation and suppressing anoikis. Oncotarget. 2017;8:9200–9215. doi: 10.18632/oncotarget.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Kim HP, Ifedigbo E, Lau LF, Choi AM. Cyr61 protects against hyperoxia-induced cell death via Akt pathway in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2005;33:297–302. doi: 10.1165/rcmb.2005-0144OC. [DOI] [PubMed] [Google Scholar]
- Jun JI, Lau LF. The Matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Cellular senescence controls fibrosis in wound healing. Aging (Albany NY) 2010;2:627–631. doi: 10.18632/aging.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Resolution of organ fibrosis. J Clin Invest. 2018;128:97–107. doi: 10.1172/JCI93563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Kim KH, Lau LF. The Matricellular protein CCN1 mediates neutrophil Efferocytosis in cutaneous wound healing. Nat Commun. 2015;6:7386. doi: 10.1038/ncomms8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juric V, Chen CC, Lau LF. TNFalpha-induced apoptosis enabled by CCN1/CYR61: pathways of reactive oxygen species generation and cytochrome c release. PLoS One. 2012;7:e31303. doi: 10.1371/journal.pone.0031303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Lau LF. CCN1 in hepatobiliary injury repair. Oncotarget. 2015;6:34053–34054. doi: 10.18632/oncotarget.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Chen CC, Monzon RI, Lau LF. The Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic Myofibroblasts. Mol Cell Biol. 2013;33:2078–2090. doi: 10.1128/MCB.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Chen CC, Alpini G, Lau LF. CCN1 induces hepatic ductular reaction through integrin αvβ5-mediated activation of NF-κB. J Clin Invest. 2015;125:1886–1900. doi: 10.1172/JCI79327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleer CG. Dual roles of CCN proteins in breast cancer progression. J Cell Commun Signal. 2016;10:217–222. doi: 10.1007/s12079-016-0345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. The CCN family acting throughout the body: recent research developments. Biomol Concepts. 2013;4:477–494. doi: 10.1515/bmc-2013-0018. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. Cellular and molecular actions of CCN2/CTGF and its role under physiological and pathological conditions. Clin Sci (Lond) 2015;128:181–196. doi: 10.1042/CS20140264. [DOI] [PubMed] [Google Scholar]
- Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35:467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurundkar AR, Kurundkar D, Rangarajan S, Locy ML, Zhou Y, Liu RM, Zmijewski J, Thannickal VJ. The matricellular protein CCN1 enhances TGF-beta1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury. FASEB J. 2016;30:2135–2150. doi: 10.1096/fj.201500173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci. 2011;68:3149–3163. doi: 10.1007/s00018-011-0778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF. Cell surface receptors for CCN proteins. J Cell Commun Signal. 2016;10:121–127. doi: 10.1007/s12079-016-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- Lau LF, Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987;84:1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. CCN2: a novel, specific and valid target for anti-fibrotic drug intervention. Expert Opin Ther Targets. 2013;17:1067–1071. doi: 10.1517/14728222.2013.812074. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Leu S-J, Lam SC-T, Lau LF. Proangiogenic activities of CYR61 (CCN1) mediated through integrins αvβ3 and α6β1 in human umbilical vein endothelial cells. J Biol Chem. 2002;277:46248–46255. doi: 10.1074/jbc.M209288200. [DOI] [PubMed] [Google Scholar]
- Leu S-J, Liu Y, Chen N, Chen CC, Lam SC, Lau LF. Identification of a novel integrin α6β1 binding site in the angiogenic inducer CCN1 (CYR61) J Biol Chem. 2003;278:33801–33808. doi: 10.1074/jbc.M305862200. [DOI] [PubMed] [Google Scholar]
- Leu S-J, Chen N, Chen C-C, Todorovic V, Bai T, Juric V, Liu Y, Yan G, Lam SC-T, Lau LF. Targeted mutagenesis of the matricellular protein CCN1 (CYR61): selective inactivation of integrin α6β1-heparan sulfate proteoglycan coreceptor-mediated cellular activities. J Biol Chem. 2004;279:44177–44187. doi: 10.1074/jbc.M407850200. [DOI] [PubMed] [Google Scholar]
- Lienau J, Schell H, Epari DR, Schutze N, Jakob F, Duda GN, Bail HJ. CYR61 (CCN1) protein expression during fracture healing in an ovine tibial model and its relation to the mechanical fixation stability. J Orthop Res. 2006;24:254–262. doi: 10.1002/jor.20035. [DOI] [PubMed] [Google Scholar]
- Mo F-E, Lau LF. The matricellular protein CCN1 is essential for cardiac development. Circ Res. 2006;99:961–969. doi: 10.1161/01.RES.0000248426.35019.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y, Suzuki T, Dohmae N, Simizu S. O-Fucosylation of CCN1 is required for its secretion. FEBS Lett. 2015;589:3287–3293. doi: 10.1016/j.febslet.2015.09.012. [DOI] [PubMed] [Google Scholar]
- O'Brien TP, Yang GP, Sanders L, Lau LF. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990;10:3569–3577. doi: 10.1128/MCB.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendurthi UR, Tran TT, Post M, Rao LV. Proteolysis of CCN1 by plasmin: functional implications. Cancer Res. 2005;65:9705–9711. doi: 10.1158/0008-5472.CAN-05-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober JM, Chen N, Grzeszkiewicz TM, Emeson EE, Ugarova TP, Ye RD, Lau LF, Lam SC-T. Identification of integrin αMβ2 as an adhesion receptor on peripheral blood moncytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2), immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–4465. doi: 10.1182/blood.V99.12.4457. [DOI] [PubMed] [Google Scholar]
- Silva MT. Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett. 2010;584:4491–4499. doi: 10.1016/j.febslet.2010.10.046. [DOI] [PubMed] [Google Scholar]
- Stramer BM, Mori R, Martin P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol. 2007;127:1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- Vaidya R, Zambrano R, Hummler JK, Luo S, Duncan MR, Young K, Lau LF, Wu S. Recombinant CCN1 prevents hyperoxia-induced lung injury in neonatal rats. Pediatr Res. 2017;82:863–871. doi: 10.1038/pr.2017.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemans RL, McClendon J, Aschner Y, Briones N, Young SK, Lau LF, Kahn M, Downey GP. Role of beta-catenin-regulated CCN matricellular proteins in epithelial repair after inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2013;304:L415–L427. doi: 10.1152/ajplung.00180.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]