Summary
The thioredoxin domain‐containing 5 (TXNDC5) gene is associated with susceptibility to rheumatoid arthritis (RA) and exhibits increased expression in the synovial tissues. TXNDC5 is also associated strongly with diabetes, a metabolic disease characterized by interrupted insulin signalling. This study investigated whether TXNDC5 contributes to RA via the insulin signalling pathway. In this study, RA synovial fibroblast‐like cells (RASFs) transfected with an anti‐TXNDC5 small interfering RNA (siRNA) were analysed with an insulin signaling pathway RT2 profiler polymerase chain reaction (PCR) array and an insulin resistance RT2 profiler PCR array. The PCR arrays detected significantly increased expression of insulin‐like growth factor binding protein 1 (IGFBP1) in RASFs with suppressed TXNDC5 expression. The result was verified using real‐time PCR and Western blot analyses. Significantly elevated IGFBP1 expression and decreased interleukin (IL)‐6 secretion were also detected in culture medium of transfected RASFs. Furthermore, decreased IGFBP1 mRNA and protein expression levels were detected in RA synovial tissues. Additionally, significantly increased apoptosis and decreased cell proliferation and cell migration were observed in RASFs transfected with the anti‐TXNDC5 siRNA, whereas transfection with the anti‐IGFBP1 siRNA or a mixture of the anti‐IGFBP1 and anti‐TXNDC5 siRNAs restored normal cell proliferation, migration and IL‐6 level in RASFs. Insulin‐like growth factor (IGF) has potent prosurvival and anti‐apoptotic functions, and IGFBP1 can suppress IGF activity. Based on the results of the present study, we suggest that TXNDC5 contributes to abnormal RASF proliferation, migration and IL‐6 production by inhibiting IGFBP1 expression.
Keywords: diabetes, IGF‐1 (insulin‐like growth factor 1), IGFBP1 (IGF binding protein 1), rheumatoid arthritis, TXNDC5
Introduction
Thioredoxin domain‐containing 5 (TXNDC5) belongs to a family of protein disulphide isomerases and has three thioredoxin domains and an endoplasmic reticulum retention sequence at its C‐terminus, resulting primarily in expression in the endoplasmic reticulum 1, 2. TXNDC5 is a key enzyme in the rate‐limiting reaction involved in disulphide bond formation, isomerization and reduction, which is important for the anti‐oxidative injury and anti‐apoptosis responses induced by anoxia and promotes cell proliferation 1, 2, 3. Abnormal TXNDC5 expression plays an important role in the pathological processes of certain diseases, such as cancers, diabetes, neurodegenerative illnesses, vitiligo and arthritis 3, 4, 5, 6, 7.
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease characterized by joint damage, synovial hyperplasia and irregular angiogenesis. In our previous studies, we revealed an association between the TXNDC5‐encoding gene and RA risk. Additionally, TXNDC5 expression was up‐regulated in synovial tissues and blood from RA patients in response to hypoxia stimulation 7, 8, 9. However, the pathogenic role of this enzyme is unclear.
Diabetes mellitus is a metabolic disease characterized by hyperglycaemia and is caused by defective insulin secretion or an insulin function disorder. As shown in many studies, inflammatory activity and certain medications impact glucose metabolism, insulin signalling and insulin resistance and consequently drive diabetes development in RA 9, 10, 11, 12, 13, 14, 15. Epidemiological studies have also confirmed an association between RA and an increased risk of diabetes 11, 14, 16. Li et al. 17 recently sequenced the exomes of 8554 individuals and analysed the effects of predicted loss‐of‐function variants on 20 chronic disease risk factor phenotypes. Elevated fasting glucose levels were observed in individuals with heterozygous TXNDC5 loss‐of‐function variation, which encodes a biomarker for type 1 diabetes progression. According to Holmgren et al. 18, TXNDC5 not only catalyzes a reduction in insulin disulphides to decrease binding activity with its receptor, but also reduces insulin synthesis, increasing the risk of diabetes. These studies suggest that TXNDC5 is associated with genetic susceptibility to both RA and diabetes and is involved in the pathogenesis of these two diseases. Thus, we hypothesized that TXNDC5 may play an important role in RA progression by modulating insulin resistance or insulin signalling pathways, similar to its role in diabetes. The present study aimed to determine whether TXNDC5 contributes to RA pathogenesis by modulating insulin resistance or insulin signalling pathways and investigated how TXNDC5 regulates this signalling. The results may also be helpful for understanding the strong association between RA and diabetes at the molecular level.
Materials and methods
Synovial tissue sample collection
Human RA synovial tissues were collected from RA patients during knee joint replacement surgery (n = 10, nine females aged 23–68 years, mean = 49 years) for tissue culture. We collected additional synovial samples from six RA patients (four females, aged 52–62 years, mean = 52 years) for Western blot analysis of insulin‐like growth factor binding protein 1 (IGBP1) expression. All RA patients met the standards of the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria. We also collected six synovial tissues from osteoarthritis (OA) patients during knee joint replacement surgery (two females aged 50–62 years, mean = 52 years) to be used as controls. Informed consent was obtained from all patients, and the study was approved by the Ethics Committee of Shandong Provincial Qianfoshan Hospital (Jinan, China).
Cell culture
Synovial tissue samples were minced and digested with 0·4 g/l collagenase type II (Solarbio, Beijing, China) for 6 h in Dulbecco's modified Eagle's medium (DMEM; HyClone, Logan, UT, USA) at 37°C, after which they were redigested with 0·25% trypsin for 15 min. The tissue suspensions were filtered through a 70‐μm cell strainer, and the cells were then centrifuged, resuspended and cultured in DMEM containing 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) at 37°C in 5% CO2 for several days. RA synovial fibroblast‐like cells (RASFs) were passaged five times and used for all experiments in this study.
Transient small interfering RNA (siRNA) transfection
RASFs were plated in six‐well plates (2 × 105 cells per well) and transfected with an anti‐TXNDC5 small interfering RNA (siRNA) using HiPerFect transfection reagent (Qiagen, Valencia, CA, USA) at 20 nM, according to the manufacturer's instructions. The sequence of the siRNA targeting theTXNDC5 mRNA was 5′‐GGCCCTAACTAGAAGTTCTA‐3′. Following transfection and culture for 72 h, RASFs were used for cell proliferation, cell migration and cell apoptosis assays. The supernatants of the transfected RASFs were also collected and centrifuged at 1000 × g for 10 min, and then stored at −80°C until the enzyme‐linked immunosorbent assay (ELISA). Inhibition of TXNDC5 expression was confirmed by performing real‐time PCR and Western blot analyses. Parallel experiments applying the Mm/Hs‐mitogen‐activated protein kinase 1 (MAPK1) siRNA (5′‐AATGCTGACTCCAAAGCTCTG‐3′) and Allstars siRNA, which were included in the kit, were performed as positive and negative controls, respectively. The Allstars siRNA does not inhibit gene expression in any human cells.
We applied the same protocol to transfect RASFs with an anti‐IGF binding protein 1 (IGFBP1) siRNA (5′‐AGAGCACGGAGATAACTGA‐3′). In addition, we also transfected RASFs with a mixture of 10 mM anti‐TXNDC5 siRNA and 10 mM anti‐IGFBP1 siRNA.
Real‐time PCR
Real‐time PCR was performed on a ViiA7 DX Instrument (Life Technologies, Carlsbad, CA, USA) using SYBR Green (SYBR Green Real‐time PCR Master Mix; Toyobo, Osaka, Japan). The PCR reactions were performed in a total volume of 10 μl and contained 1 μl of cDNA, 5 μl of SYBR Green real‐time PCR Master Mix (Toyobo), 1 μl of each primer and 2 μl of water. The reaction conditions were set according to primer annealing temperatures. The following PCR primer sequences were used: TXNDC5 forward: 5′‐CTCTGGGCCTTGAACATT‐3′, TXNDC5 reverse: 5′‐CCCTCAGTGACTCCAAA‐3′; IGFBP1 forward: 5′‐CCCAGAGAGCACGGAGATAA‐3′, IGFBP1 reverse: 5′‐AGAGCCTTCGAGCCATCATA‐3′; IGFBP3 forward: 5′‐AGAGCACAGATACCCAGAACT‐3′, IGFBP3 reverse: 5′‐GGTGATTCAGTGTGTCTTCCATT‐3′; IGF‐1 forward: 5′‐AGGATATTGGGCTTTACAACCTG‐3′, IGF‐1 reverse: 5′‐GAGGTAACAGAGGTCAGCATTTT‐3′; IL‐1R1 forward: 5′‐ATGAAATTGATGTTCGTCCCTGT‐3′, IL‐1R1 reverse: 5′‐ACCACGCAATAGTAATGTCCTG‐3′; phosphoenolpyruvate carboxykinase 1(PCK1) forward: 5′‐ACATCTGTGACGGCTCTGAG‐3′, PCK1 reverse: 5′‐GGATGGGCACTGTGTCTCTT‐3′; solute carrier family 2, member 4 (SLC2A4) forward: 5′‐TGGGCGGCATGATTTCCTC‐3′, SLC2A4 reverse: 5′‐GCCAGGACATTGTTGACCAG‐3′; and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) forward: 5′‐CAGAACATCATCCCTGCCTCTAC‐3′, GAPDH reverse: 5′‐TTGAAGTCAGAGGAGACCACCTG‐3′.
PCR arrays
An insulin signaling pathway RT2 profiler PCR array (catalogue number: PAHS‐030ZA; Qiagen) and an insulin resistance RT2 profiler PCR array (catalogue number: PAHS‐156ZA; Qiagen) were used to examine the expression patterns of 168 genes involved in insulin‐like growth factor (IGF) signalling in RASFs following siRNA transfection. RASFs transfected with the Allstars siRNA were used as controls. Total RNA was isolated from RASFs using an RNeasy Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer's protocol. Total RNA from each sample (100 ng each) was mixed into a pool at equal concentrations, then reverse‐transcribed into cDNA with a RT2 first‐strand kit (Qiagen). RT2 SYBR Green qPCR Master Mix was used for the real‐time PCR reactions with the following universal cycling conditions: 95°C for 10 min, 95°C for 15 s and 60°C for 1 min, repeated for 45 cycles. Gene expression levels were analysed using the web‐based software RT2 profiler PCR Array Data Analysis version 3.5. For each gene in the control group and the experimental group, P‐values were calculated using Student's t‐test to compare the replicate 2−▵▵Ct values. Genes with an at least fourfold change in expression were considered biologically significant, according to the manufacturer's recommendation.
Western blot analysis
Cultured RASFs were harvested and treated with lysis buffer [radioimmunoprecipitation assay (RIPA) lysis buffer, Beyotime, Jiangsu, China] at 4°C for 30 min, then centrifuged at 12 000 g for 30 min. Total protein concentrations were determined with a bicinchoninic acid (BCA) protein assay kit (Solarbio). Cell lysates were incubated in sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) sample loading buffer (Beyotime) at 95°C for 5 min. Total proteins were separated by performing SDS‐PAGE followed by transblotting onto nitrocellulose membranes (Immobilon‐P, Burlington, MA, USA). The Western blot analysis was conducted using an anti‐IGFBP1rabbit monoclonal antibody (31025T; Cell Signaling Technology, Danvers, MA, USA) at a 1000‐fold dilution or an anti‐human TXNDC5 antibody (Abcam, Cambridge, MA, USA) at a 1000‐fold dilution. The anti‐TXNDC5 antibody was prepared by immunizing a rabbit with a recombinant human TXNDC5 fragment that corresponds to amino acids 198–432. A horseradish peroxidase (HRP)‐conjugated anti‐rabbit immunoglobulin (Ig)G antibody at a 5000‐fold dilution was used as the secondary antibody to trace the expression of target proteins. A separate membrane was prepared using the same protocol and probed with an anti‐GAPDH antibody (Proteintech, Wuhan, China) at a dilution of 1 : 10 000 to normalize sample loading. Immunoreactive signals were detected using an enhanced chemiluminescence (ECL) plus kit (Beyotime Biotech).
We applied the same protocol to examine IGFBP1 expression in RA synovial tissues.
ELISA
IGFBP1 levels in RASF culture supernatants were measured using IGFBP1 ELISA kits (Abcam; ab100539). One hundred microlitres of cell culture supernatant were added into appropriate wells and incubated for 2·5 h at room temperature. After four washes, 100 μl of biotinylated IGFBP1 antibody were added to each well, followed by incubation for 1 h at room temperature. After an additional four washes with wash solution, 100 μl of HRP–streptavidin solution were added to each well and incubated for 45 min at room temperature. Finally, 100 μl of TMB (3, 3′, 5, 5′‐tetramethyl benzidine) one‐step substrate reagent were added to each well and incubated for 30 min at room temperature. The reaction was stopped by adding 50 μl of stop solution to each well. The absorbance was read immediately at 450 nm using a spectrophotometer (Spectramax 190; Molecular Devices, Sunnyvale, CA, USA).
We applied a similar protocol to measure tumour necrosis factor (TNF)‐α, interleukin (IL)‐1α, IL‐1β and IL‐6 levels using ELISAs. RASFs were transfected with the Allstars siRNA, anti‐TXNDC5 siRNA, anti‐IGFBP1 siRNA or a mixture of anti‐IGFBP1 siRNA and anti‐TXNDC5 siRNA for 72 h. The culture medium was collected and centrifuged at 3000 g for 10 min at 4°C. Levels of TNF‐α, IL‐1α, IL‐1β and IL‐6 were measured using ELISA kits provided by R&D Systems (Minneapolis, MN, USA). The experiment was conducted according to the manufacturer's protocol. The absorbance at 405 nm was measured using a plate reader.
Cell proliferation assay
Cell proliferation levels were evaluated using the cell counting kit‐8 (CCK‐8) as a marker for cellularity after defined culture periods. RASFs (10 × 103 cells/well) were dispensed into 96‐well round‐bottomed microtitre plates in a total volume of 100 μl/well. RASFs were transfected with Allstars siRNA, anti‐TXNDC5 siRNA, anti‐IGFBP1 siRNA or a mixture of anti‐IGFBP1 siRNA and anti‐TXNDC5 siRNA. The incubation was performed for 24, 48 or 72 h at 37°C. Ten microlitres of CCK‐8 solution (Dojindo, Kumamoto, Japan) in 100 μl of complete DMEM medium were added to each well and incubated for 2 h. The plates were read on a spectrophotometer at an absorbance of 450 nm.
Transwell migration assay
Twenty‐four‐well transwell polycarbonate filters with an 8·0‐μm pore size (Corning, NY, USA) were used to detect RASF migration ability. RASFs were transfected with Allstars siRNA, anti‐TXNDC5 siRNA, anti‐IGFBP1 siRNA or a mixture of anti‐IGFBP1 siRNA and anti‐TXNDC5 siRNA. A total of 5 × 104 RASFs/200 μl of serum‐free DMEM medium was seeded into the upper chambers for 48 h following transfection, and 600 μl of DMEM medium containing 10% fetal bovine serum (Gibco‐BRL) was added to the lower chamber. After incubation at 37°C in 5% CO2 for 24 h, the cells that migrated to the lower side of the filter were immersed in methanol for 15 min at room temperature, then stained with 0·25% crystal violet for 10 min at room temperature. The cells that migrated to the underside of the filter were photographed (magnification ×4) and counted (magnification ×10) using an inverted microscope.
Cell apoptosis analysis
Apoptosis was detected using a fluorescein isothiocyanate (FITC)‐annexin V and propidium iodide (PI) kit (Biolegend, San Diego, CA, USA), according to the manufacturer's instructions. RASFs were transfected with siRNAs as described above and plated on six‐well culture plates (Costar, Cambridge, MA, USA) at a density of 50 × 104cells/well. Forty‐eight h after siRNA transfection, cells were harvested via trypsinization and washed twice with ice‐cold phosphate‐buffered saline (PBS). Next, 5–10 × 104 cells were treated with annexin V binding buffer, followed by the addition of 5 μl of FITC‐annexin V and 8 μl of PI. The cells were incubated for 15 min at room temperature in the dark. Finally, 400 μl of annexin V binding buffer were added to each tube. Apoptosis was detected with a Coulter Epics XL flow cytometer (BD FACSAria TM II; Becton Dickinson, San Jose, CA, USA).
Statistical analysis
In this study, paired and/or unpaired Student's t‐tests were used to evaluate the statistical significance of differences between two groups. One‐way analysis of variance (anova) was used to compare differences among multiple groups in the experiments. Statistical differences were considered significant at P < 0·05. The Kolmogorov–Smirnov (K‐S) test was performed to test whether the data followed a normal distribution.
Results
Detection of gene expression in anti‐TXNDC5 siRNA‐transfected RASFs
RASFs were transfected with the anti‐TXNDC5 siRNA. Real‐time PCR and Western blotting were conducted to determine the knock‐down efficiency of the anti‐TXNDC5 siRNA in RASFs (n = 10) 72 h after transfection. Real‐time PCR detected significantly decreased TXNDC5 mRNA expression in each anti‐TXNDC5 siRNA‐transfected synovial sample compared with that in RASFs transfected with the Allstars siRNA (P < 0·0001). The Western blot analysis also detected significantly decreased expression of the TXNDC5 protein in each anti‐TXNDC5 siRNA‐transfected synovial sample compared with that in RASFs transfected with the Allstars siRNA (P < 0·0001). These results are presented in Supporting information, Fig. S1. Equal concentrations of total RNA from each sample were pooled and analysed by PCR array. An insulin signaling pathway RT2 profiler PCR array and an insulin resistance RT2 profiler PCR array were used to identify any significant changes in gene expression between anti‐TXNDC5 siRNA‐transfected RASFs and negative control siRNA‐transfected RASFs. The insulin signaling pathway RT2 profiler PCR array detected a significant increase (5·56‐fold) in IGFBP1 mRNA expression in RASFs transfected with the anti‐TXNDC5 siRNA (Fig. 1a). The insulin resistance RT2 profiler PCR array detected a significant increase (4·06‐fold) in PCK1 expression and a decrease in insulin‐like growth factor 1 (IGF‐1) expression (3·85‐fold), SLC2A4 expression (7·07‐fold) and interleukin 1 receptor type 1 (IL1R1) expression (3·58‐fold) in anti‐TXNDC5 siRNA‐transfected RASFs (Fig. 1b). The PCR array results are depicted in one map (Fig. 1c).
Figure 1.
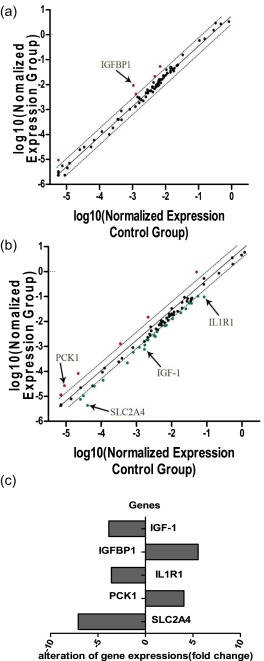
Analysis of crucial genes involved in the insulin signalling pathway in rheumatoid arthritis synovial fibroblast‐like cells (RASFs) (n = 10) transfected with the anti‐TXNDC5 siRNA using (a) insulin signalling pathway and (b) insulin resistance polymerase chain reaction (PCR) arrays. Fold‐change values greater than 1 indicate a positive change or up‐regulation. Fold‐change values less than 1 indicate a negative change or down‐regulation. Fold‐change values greater than 2 are indicated in red, and fold‐change values less than 0·5 are indicated in green. (c) The PCR array results are depicted in one map. Five genes, specifically insulin‐like growth factor binding protein 1 (IGFBP1), phosphoenolpyruvate carboxykinase 1 (PCK1), solute carrier family 2, member 4 (SLC2A4), interleukin 1 receptor type 1 (IL‐1R1) and insulin‐like growth factor 1 (IGF‐1), with significantly altered expression, were detected in RASFs transfected with the anti‐TXNDC5 siRNA.
IGF1, IGFBP1, IL1R1, PCK1 and SLC2A4 mRNA levels were analysed in RASF obtained from every patient (n = 10) by performing real‐time PCR analyses. Mean IGFBP1 mRNA levels were elevated significantly (twofold; P = 0·018) in anti‐TXNDC5 siRNA‐transfected RASFs (Fig. 2a), but no significant changes in the expression of IGF1, IL1R1, PCK1 and SLC2A4 were detected in anti‐TXNDC5 siRNA‐transfected RASFs compared with that in RASFs transfected with the Allstars siRNA (P = 0·218, P = 0·377, P = 0·701 and P = 0·367, respectively) (Fig. 2b–e). We also examined the mRNA levels of IGFBP3, the primary IGF‐1 binding protein. IGFBP3 levels were increased slightly in anti‐TXNDC5 siRNA‐transfected RASFs, but the difference was not significant (P = 0·306) (Fig. 2f). A Western blot analysis was performed next to examine the expression of the IGFBP1 protein in TXNDC5 knock‐down RASFs. Elevated IGFBP1 expression (threefold) was detected in anti‐TXNDC5 siRNA‐transfected RASFs compared with that in Allstars siRNA‐transfected RASFs (P < 0·0001) (Fig. 3).
Figure 2.
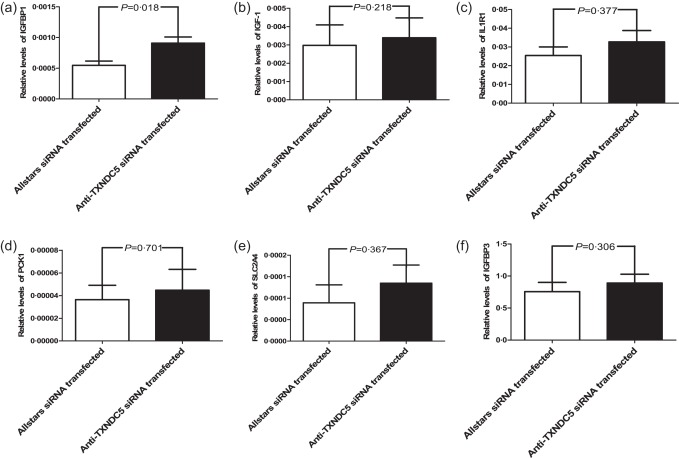
Verification of the polymerase chain reaction (PCR) array results using real‐time PCR. (a) Insulin‐like growth factor binding protein 1 (IGFBP1), (b) insulin‐like growth factor 1 (IGF‐1), (c) interleukin 1 receptor type 1 (IL‐1R1), (d) phosphoenolpyruvate carboxykinase 1 (PCK1), (e) solute carrier family 2 (SLC2A) and (f) insulin‐like growth factor binding protein 3 (IGFBP3) mRNA levels in siRNA‐transfected rheumatoid arthritis synovial fibroblast‐like cells (RASFs) (n = 10). The figure shows the standard deviation (s.d.) to indicate the variability of the data.
Figure 3.
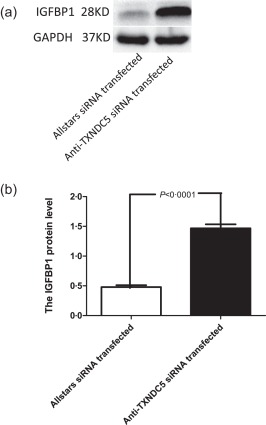
Western blot analysis of insulin‐like growth factor binding protein 1 (IGFBP1) expression in rheumatoid arthritis synovial fibroblast‐like cells (RASFs). (a) Western blotting was used to detect IGFBP1 protein expression in RASF samples (n = 10). (b) IGFBP1 protein expression was normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) expression. The figure shows the standard deviation (s.d.) to indicate the variability of the data.
ELISA was performed to detect concentrations of the IGFBP1 protein in culture supernatants of TXNDC5 knock‐down RASFs (n = 10). Elevated levels (1·75‐fold) of IGFBP1 (350 pg/ml) were detected in the medium obtained from anti‐TXNDC5 siRNA‐transfected RASFs compared to those in Allstars siRNA‐transfected RASFs (200 pg/ml) (P = 0·001). The results are presented in Fig. 4.
Figure 4.
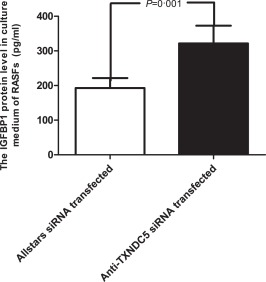
Measurement of insulin‐like growth factor binding protein 1 (IGFBP1) levels in the supernatants of cultured rheumatoid arthritis synovial fibroblast‐like cells (RASFs) using enzyme‐linked immunosorbent assay (ELISA). RASFs (n = 10) were cultured and transfected with siRNAs for 72 h. The figure shows the standard deviation (s.d.) to indicate the variability of the data.
We used paired t‐tests to compare results from cells transfected with the Allstars negative control siRNA and anti‐TXNDC5 siRNA shown in Figs 2, 3, 4 and Supporting information, Fig. S1 because the K‐S test showed that the data were distributed normally (P > 0·05).
Detection of RASF proliferation, migration and apoptosis
Cell proliferation, cell migration and apoptosis assays were performed to determine the effects of anti‐TXNDC5 siRNA on cultured RASFs (n = 10). The expression of the TXNDC5 mRNA was decreased significantly in anti‐TXNDC5 siRNA‐transfected RASFs compared with that in cells transfected with the negative control siRNA, as shown in Supporting information, Fig. S1. RASFs were also transfected with an anti‐IGFBP1 siRNA. We used paired t‐tests to compare results from cells transfected with the Allstars negative control siRNA and anti‐IGFBP1 siRNA because the K‐S test showed that the data were distributed normally (P > 0·05). The efficacy of IGFBP1 silencing after anti‐IGFBP1 siRNA transfection is shown in Supporting information, Fig. S2. IGFBP1 mRNA and protein expression were decreased significantly in anti‐IGFBP1 siRNA‐transfected RASFs compared with that in cells transfected with the negative control siRNA (P = 0·025 and P = 0·003, respectively).
RASF proliferation was measured using the CCK‐8 assay. Seventy‐two h after transfection with the anti‐TXNDC5siRNA, RASF proliferation was decreased significantly upon down‐regulation of TXNDC5 expression compared with that in Allstars siRNA‐transfected RASFs (P = 0·012). When RASFs were transfected with the anti‐IGFBP1 siRNA, cell proliferation was elevated significantly compared with that in the group transfected with the Allstars siRNA (P = 0·036). When RASFs were transfected with a mixture of the anti‐IGFBP1 siRNA and anti‐TXNDC5 siRNA, RASF proliferation was not changed considerably compared with that in the group transfected with the Allstars siRNA (P = 0·776). The results are shown in Fig. 5. These observations indicated that transfection with the anti‐TXNDC5 siRNA suppressed RASF proliferation considerably, and transfection with the anti‐IGFBP1 siRNA or a mixture of the anti‐IGFBP1 and anti‐TXNDC5 siRNAs elevated the proliferation ability significantly compared to the control.
Figure 5.
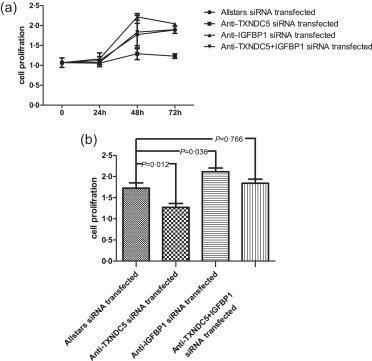
Measurement of rheumatoid arthritis synovial fibroblast‐like cell (RASFs) cell proliferation using a cell counting kit‐8 (CCK‐8) assay. RASFs (n = 10) were transfected with anti‐TXNDC5 siRNA for 72 h. The proliferation of RASFs transfected with siRNA is shown in (a) and depicted in the bar graph in (b). The figure shows the standard deviation (s.d.) to indicate the variability of the data.
The cell migration ability of RASFs was measured using a two‐compartment transwell system. Cell migration was reduced significantly in anti‐TXNDC5 siRNA‐transfected RASFs compared with that in Allstars siRNA‐transfected RASFs (P < 0·0001). When RASFs were transfected with the anti‐IGFBP1 siRNA, migration was changed significantly compared with that in the group transfected with the Allstars siRNA (P = 0·038). When RASFs were transfected with the mixture of the anti‐IGFBP1 siRNA and anti‐TXNDC5 siRNA, migration ability was not changed significantly compared with that in the group transfected with the Allstars siRNA (P = 0·766). The results are shown in Fig. 6. These observations indicated that transfection with the anti‐TXNDC5 siRNA retard RASF migration considerably and transfection with the anti‐IGFBP1 siRNA elevate the migration ability significantly, although the transfection with a mixture of the anti‐IGFBP1 and anti‐TXNDC5 siRNAs did not change the RASF migration level significantly compared to the control.
Figure 6.
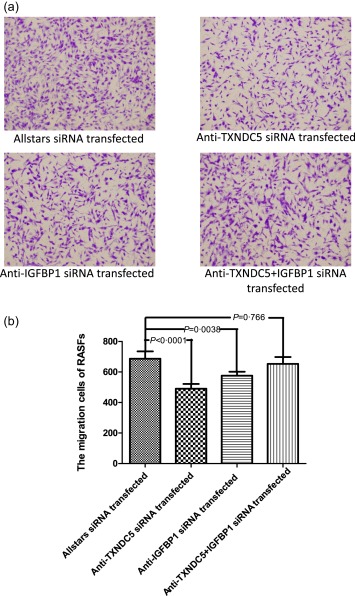
Measurement of rheumatoid arthritis synovial fibroblast‐like cell (RASF) migration using a two‐compartment transwell system. Cells that migrated to the underside of the filter were photographed (magnification ×4) and counted (magnification ×10) with an inverted microscope. (a) RASFs (n = 10) transfected with the siRNA. (b) The results of the RASF migration assay are presented in a bar graph. The figure shows the standard deviation (s.d.) to indicate the variability of the data.
RASF apoptosis was measured using flow cytometry. The apoptosis rate was increased to a certain extent in anti‐TXNDC5 siRNA‐transfected RASFs compared with that in Allstars siRNA‐transfected RASFs (P = 0·032). When RASFs were transfected with the anti‐IGFBP1 siRNA, the apoptosis rate did not show a significant change compared with that in the group transfected with the Allstars siRNA (P = 0·092). When RASFs were transfected with the anti‐IGFBP1 and anti‐TXNDC5 siRNA mixture, the apoptosis rate did not show a significant change compared with that in the group transfected with the Allstars siRNA (P = 0·855). The results are shown in Fig. 7. These observations indicated that transfection with the anti‐TXNDC5 siRNA elevated RASF apoptosis significantly, but transfection with the anti‐IGFBP1 siRNA or the mixture of the anti‐IGFBP1 did not change RASF apoptosis compared to the control.
Figure 7.
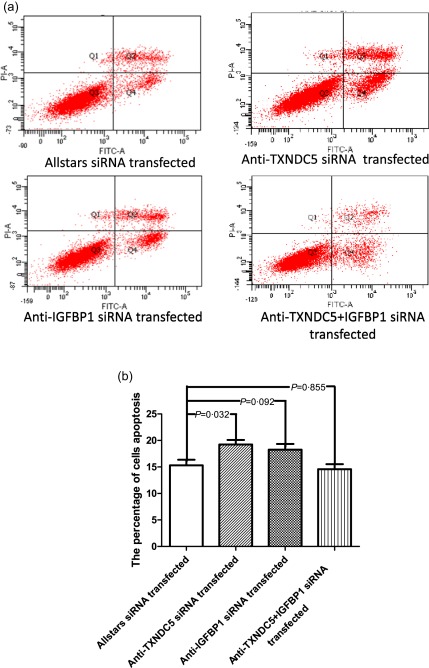
Measurement of rheumatoid arthritis synovial fibroblast‐like cell (RASF) apoptosis using flow cytometry. (a) RASFs (n = 10) transfected with the siRNA. (b) The results of the RASF apoptosis assay are presented in a bar graph. The figure shows the standard deviation (s.d.) to indicate the variability of the data.
Detection of IGFBP1 expression in RA synovial tissues
We examined IGFBP1 expression in RA synovial membranes (n = 6) and OA synovial membranes (n = 6). We used the unpaired t‐test to compare results from RA and OA samples because the K‐S test showed that the data were distributed normally (P > 0·05). Real‐time PCR detected significantly decreased IGFBP1 expression in RA synovial tissues compared with that in OA synovial tissues (P = 0·011) (Fig. 8a). A Western blot analysis was also used to detect levels of the IGFBP1 protein in synovial tissues. IGFBP1 expression was decreased significantly by an average of twofold in RA synovial tissues compared with that in OA samples (P = 0·014) (Fig. 8b,c).
Figure 8.
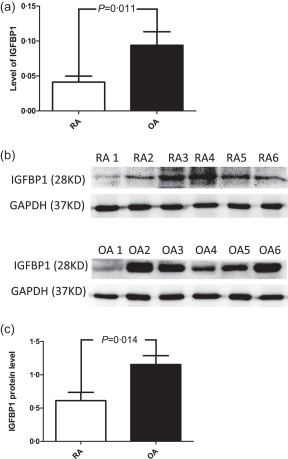
Measurement of insulin‐like growth factor binding protein (IGFBP) expression in rheumatoid arthritis (RA) synovial tissues. (a) Real‐time polymerase chain reaction (PCR) was used to detect IGFBP1 mRNA expression in RA synovial tissues (n = 6) and osteoarthritis (OA) synovial tissues (n = 6). (b) Western blotting was used to detect IGFBP1 protein expression in RA synovial tissues (n = 6) and OA synovial tissues (n = 6). (c) IGFBP1 protein expression was normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) expression. The figure shows the standard deviation (s.d.) to indicate the variability of the data.
Detection of IL‐6 levels in RASF culture supernatants
An ELISA was performed to determine the IL‐6 levels in culture supernatants of TXNDC5 knock‐down RASFs (n = 10). The IL‐6 level was decreased significantly (2·96 ng/ml) in culture medium from anti‐TXNDC5 siRNA‐transfected RASFs compared to that in medium from Allstars siRNA‐transfected RASFs (4·22 ng/ml, P = 0·048). In contrast, the IL‐6 level was increased significantly in culture medium from RASFs transfected with the anti‐IGFBP1 siRNA (8·60 ng/ml) and in culture medium from RASFs transfected with a mixture of the anti‐IGFBP1 siRNA and anti‐TXNDC5 siRNA (9·74 ng/ml) compared to that in medium from cells transfected with the Allstars siRNA (P = 0·002 and P = 0·001, respectively). The results are presented in Fig. 9. These observations indicated that transfection with the anti‐TXNDC5 siRNA suppressed IL‐6 production in RASFs significantly, and transfection with the anti‐IGFBP1 siRNA or a mixture of the anti‐IGFBP1 and anti‐TXNDC5 siRNAs elevated IL‐6 production considerably compared to the control.
Figure 9.
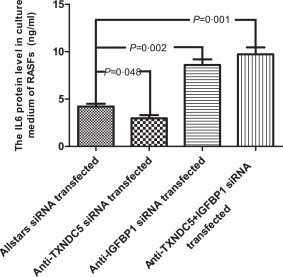
Measurement of interleukin (IL)‐6 levels in the supernatants of cultured rheumatoid arthritis synovial fibroblast‐like cells (RASFs) using enzyme‐linked immunosorbent assay (ELISA). RASFs (n = 10) were cultured and transfected with siRNAs for 72 h. The figure shows the standard deviation (s.d.) to indicate the variability of the data.
Although we detected significantly decreased levels of TNF‐α, IL‐1α and IL‐β in RASFs transfected with anti‐TXNDC5 siRNA in a previous study, the current study did not detect significant changes in TNF‐α, IL‐1α and IL‐1β levels in RASFs transfected with either the anti‐IGFBP1 siRNA or the mixture of the anti‐IGFBP1 siRNA and anti‐TXNDC5 siRNA compared with those in the Allstars siRNA‐transfected control group. These results are not shown.
We used anovas with corrections for multiple comparisons to compare the four groups of data shown in Figs 5, 6, 7 and 9 because the K‐S test showed that the data were distributed normally (P > 0·05). We compared the means of each group to the mean of the Allstars siRNA‐transfected group using Dunnett's multiple comparison test.
Discussion
In the present study, a PCR array analysis detected increased expression of IGFBP1 in RASFs in which TXNDC5 expression was inhibited using an siRNA. This result was verified at both the transcriptional and translational levels by performing real‐time PCR and Western blotting, respectively. An ELISA also detected significantly elevated levels of IGFBP1 in the supernatants of transfected RASFs. In addition, significantly decreased IGFBP1 expression was detected in RA synovial tissues. Previously, we detected significantly increased TXNDC5 expression in the synovial membranes, synovial fluids and blood of RA patients 7. These results suggest that increased TXNDC5 expression down‐regulates IGFBP1 expression in RA synovial tissues. The current study did not detect significantly altered expression of genes involved in the insulin resistance pathway when TXNDC5 expression was suppressed in the siRNA‐transfected RASFs. Thus, TXNDC5 does not appear to be associated with the insulin resistance pathway in RA, although some studies have reported the importance of this signalling pathway in its pathogenesis 19, 20.
We performed immunohistochemistry previously to localize the expression of TXNDC5 in RA synovial membranes. The immunohistochemistry analysis revealed extensive TXNDC5 expression in the fibroblast‐like cells of the synovial membranes from RA patients 8. These results indicate that TXNDC5 is expressed mainly in RASFs, but not in other types of synovial cells such as B cell clusters and infiltrating T cells. In the present study, we tried to examine the immunolocalization of TXNDC5 and IGFBP1 in RA synovial tissues. Consistent with our previous results, immunohistochemistry revealed the localization and substantial expression of TXNDC5 in RA synovial fibroblast‐like cells 8. However, significant expression of IGFBP1 was not observed in RA synovial tissues. The real‐time PCR and Western blot analyses showed that the expression of IGFBP1 was low in RA synovial tissues, and the ELISA results showed that the IGFBP1 protein was secreted in the supernatant of cultured RASFs. Based on these results, the unsuccessful immunolocalization of IGFBP1 in RA synovial membranes is due to inhibition of IGFBP1 expression in these tissues by the increased TXNDC5 levels.
IGF‐1 is a hormone with a similar molecular structure to insulin, which exerts growth‐promoting effects on almost every cell type in the body. Following binding with IGF receptors, IGF exhibits tyrosine kinase activity and activates signalling pathways such as phosphoinositide 3‐kinase/protein kinase B (PI3K/AKT) and RAS/MAPK, thus affecting cell proliferation, differentiation, survival and apoptosis 21. IGF activity is regulated finely by a family of six high‐affinity IGFBPs. IGFBPs usually inhibit IGF actions but prolong the IGF half‐life 22. Therefore, the IGF/IGFBP molar ratio is considered a better indicator of IGF bioavailability than IGF itself 22, 23. IGFBP3 is the most abundant of the IGFBPs and forms a complex with IGF‐1 to serve as a reservoir of IGF‐1 in the circulation. IGFBP3 levels are significantly higher in patients with RA and notably elevated in patients with active RA. IGFBP3 sensitizes RASFs to TNF‐α‐induced apoptosis in vitro and increases apoptosis significantly in an in‐vivo model of Matrigel implants engrafted into immunodeficient mice 24. However, the present study did not detect significant alterations in IGFBP3 expression in RASFs following transfection of the anti‐TXNDC5 siRNA. This result suggests that TXNDC5 modulates IGFBP1 rather than IGFBP3 expression in RASFs to affect IGF activity. IGFBP1 also binds IGF‐1 with high affinity, prolongs the half‐life of IGFs and mediates the interactions between IGF and its cell surface receptors to modulate the downstream signalling pathway 25, 26. The current study detected little change in IGF‐1mRNA levels in RASFs transfected with the anti‐TXNDC5 siRNA. Lee et al. 27 and Toussirot et al. 28 also detected no changes in IGF‐1 levels in RA using ELISA. Thus, we consider that the increased IGFBP1 expression observed in RASFs in which TXNDC5 expression was suppressed results in a decreased IGF‐1/IGFBP1 ratio. Additionally, we detected significantly decreased expression of IGFBP1 in RA synovial tissues. The above results suggest that TXNDC5 over‐expression leads to increased IGF activity by inhibiting IGFBP1 expression in RA synovium in vivo.
RASFs are the primary constituents of the synovial lining of the RA joint. In the current study, we observed decreased RASF proliferation and migration, as well as increased RASF apoptosis following anti‐TXNDC5 siRNA transfection, whereas IGFBP1 expression was increased. When RASFs were transfected with the anti‐IGFBP1 siRNA or a mixture of the anti‐IGFBP1 siRNA and TXNDC5 siRNA, the proliferation and migration of RASFs were restored to normal levels or to levels even higher than RASFs transfected with the Allstars siRNA, although the apoptosis rate did not change compared to RASFs transfected with the Allstars siRNA. These results suggest that increased TXNDC5 expression in RA synovial tissues contributes to abnormal RASF proliferation and migration by reducing IGFBP1 levels and subsequently increasing IGF activity in vivo. IGF‐1 is produced primarily by macrophages in the RA synovium. TNF‐α, a proinflammatory cytokine, has been shown to induce aberrant IGF‐1 production in RASFs. Inhibition of the insulin‐like growth factor system may be a potential therapy for RA 26, 29. IGF exerts potent prosurvival and anti‐apoptotic activities 30, 31. IGF‐1 plays an anti‐apoptotic role by activating PI3K/Akt‐regulated glycometabolism 32. The IGF family increases tumour cell proliferation and transformation and inhibits cell apoptosis 33, consistent with our observations in the present study.
In the present study, a significant decrease in IL‐6 levels was detected in the culture medium of RASFs following transfection with the anti‐TXNDC5 siRNA, and IL‐6 levels were elevated significantly in the supernatants of RASFs transfected with the anti‐IGFBP1 siRNA or a mixture of the anti‐IGFBP1 siRNA and anti‐TXNDC5 siRNA compared to those in the control group. This observation reveals that TXNDC5 can up‐regulate IL‐6 levels by suppressing IGFBP1 expression. IL‐6 is a proinflammatory cytokine involved in the pathogenesis of various autoimmune and chronic inflammatory diseases, and plays a key role in RA. The IL‐6 inhibitor, tocilizumab, a humanized anti‐IL‐6 receptor monoclonal antibody, shows outstanding efficacy in RA 34, 35. We have detected significant increases previously in IL‐1α, IL‐1β, IL‐17 and TNF‐α production in TXNDC5‐over‐expressing mice and decreases in the expression of these cytokines in RASFs transfected with the anti‐TXNDC5 siRNA 9. Thus, we suggest that TXNDC5 decreases IL‐6 production rather than the production of other cytokines by regulating IGFBP1 expression. IGF‐1 receptor signalling was shown recently to contribute to IL‐6 production in RA 36, which supports our findings.
Increasing evidence suggests an important role for the IGF‐IGFBP axis in the maintenance of normal glucose and lipid metabolism in diabetes. Low circulating levels of IGFBP1 are associated with insulin resistance and predict the development of type 2 diabetes 37, 38, 39, which corresponds with our observations of low IGFBP1 expression in RA synovial tissues. Additionally, some clinical studies have reported an association between low IGF‐1 levels or a genetic polymorphism and an increased risk of glucose intolerance or diabetes 40, 41. Schneider et al. suggested that both low and high IGF‐1 levels increase the risk of diabetes 42. Recent studies have revealed the importance of TXNDC5 in diabetes 17, 18. The current study provides evidence that high expression of TXNDC5 suppresses IGFBP1 expression. Based on our observations and those of others, we suggest that abnormal TXNDC5 expression and the subsequent interruption of IGFBP1 expression is involved in the pathogenesis of both RA and diabetes, which explains the strong association between these two diseases.
In recent years, several studies have shown that many of the inflammatory molecules that play a pathogenic role in RA are also involved in type 2 diabetes 43, 44, 45, 46. Both preclinical and clinical observations have reported the efficacy of IL‐1β antagonism therapy in both type 2 diabetes and RA 47, 48, 49. We observed a significant increase in IL‐1β, IL‐1α, IL‐17 and TNF‐α production in TXNDC5‐over‐expressing mice 9, which was decreased in RASFs following transfection with the anti‐TXNDC5 siRNA. Thus, the up‐regulation of the expression of inflammatory molecules, such as IL‐1α, IL‐1β, IL‐17 and TNF‐α, by TXNDC5 may also explain the strong association between RA and diabetes.
In summary, the present study found that inhibiting TXNDC5 expression in RASFs resulted in increased IGFBP1 expression but not IGFBP3 and IGF‐1 expression. IGFBP1 is expressed at low levels in RA synovial tissues. Thus, increased expression of TXNDC5 in the RA synovium may down‐regulate IGFBP1 expression and consequently increase IGF activity, which may up‐regulate cell proliferation, migration and IL‐6 production. The effects of TXNDC5 down‐regulation on IGFBP1 expression also help to explain the high occurrence of diabetes in RA patients and the molecular link between these two diseases, because reduced IGFBP1 expression and abnormal TXNDC5 activity have been reported in diabetes.
Disclosure
The authors have no conflicts of interest to disclose.
Author contributions
X. C. had full access to all the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis, and prepared the manuscript. J. L. and B. X. performed the molecular analyses and performed the statistical analyses. B. X., J. L., C. W., X. Y. and L. Z. were involved in the conception of the study and contributed to the study design and data interpretation. C. W., X. Y. and L. Z. collected and evaluated the synovial samples. All authors were involved in drafting or revising the manuscript. All authors read and approved the final manuscript.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Thioredoxin domain‐containing 5 (TXNDC5) expression in rheumatoid arthritis synovial fibroblast‐like cells (RASFs). (a) Real‐time polymerase chain reaction (PCR) was used to detect TXNDC5 mRNA levels in RASFs (n = 10) 72 h after transfection with the anti‐TXNDC5 siRNA. (b) Western blotting was used to detect TXNDC5 protein expression in RASFs (n = 10) 72 h after anti‐TXNDC5 siRNA transfection. (c) TXNDC5 protein expression was normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) expression. The figure shows the standard deviation (s.d.) to indicate the variability of the data.
Fig. S2. Insulin‐like growth factor binding protein 1 (IGFBP1) expression in rheumatoid arthritis synovial fibroblast‐like cells (RASFs). (a) Real‐time polymerase chain reaction (PCR) was used to detect IGFBP1 mRNA levels in RASFs (n = 10) 72 h after anti‐IGFBP1 siRNA transfection. (b) Western blotting was used to detect IGFBP1 protein expression in RASFs (n = 10)72 h after anti‐IGFBP1 siRNA transfection. (c) IGFBP1 protein expression was normalized to GAPDH expression. The figure shows the standard deviation (s.d.) to indicate the variability of the data.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NTFC) (81373218), the Key R&D Projects of Shandong Province (2014GSF118135, 2015GGH318019) and the Natural Science Foundation of Shandong Province (ZR2014HP018).
References
- 1. Knoblach B, Keller BO, Groenendyk J et al ERp19 and ERp46, new members of the thioredoxin family of endoplasmic reticulum proteins. Mol Cell Proteomics 2003; 2:1104–19. [DOI] [PubMed] [Google Scholar]
- 2. Sullivan DC, Huminiecki L, Moore JW et al EndoPDI, a novel protein‐disulfide isomerase‐like protein that is preferentially expressed in endothelial cells acts as a stress survival factor. J Biol Chem 2003; 278:47079–88. [DOI] [PubMed] [Google Scholar]
- 3. Horna‐Terrón E, Pradilla‐Dieste A, Sánchez‐de‐Diego C, Osada J. TXNDC5, a newly discovered disulfide isomerase with a key role in cell physiology and pathology. Int J Mol Sci 2014; 15:23501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y, Ma Y, Lu B, Xu E, Huang Q, Lai M. Differential expression of mimecan and thioredoxin domain‐containing protein 5 in colorectal adenoma and cancer: a proteomic study. Exp Biol Med (Maywood) 2007; 232:1152–9. [DOI] [PubMed] [Google Scholar]
- 5. Jeong KH, Shin MK, Uhm YK, Kim HJ, Chung JH, Lee MH. Association of TXNDC5 gene polymorphisms and susceptibility to nonsegmental vitiligo in the Korean population. Br J Dermatol 2009; 162:759–64. [DOI] [PubMed] [Google Scholar]
- 6. Chang X, Xu B, Wang L, Wang Y, Wang Y, Yan S. Investigating a pathogenic role for TXNDC5 in tumors. Int J Oncol 2013; 43:1871–84. [DOI] [PubMed] [Google Scholar]
- 7. Chang X, Cui Y, Zong M et al Identification of proteins with increased expression in rheumatoid arthritis synovial tissues. J Rheumatol 2009; 36:872–80. [DOI] [PubMed] [Google Scholar]
- 8. Chang X, Zhao Y, Yan X, Pan J, Fang K, Wang L. Investigating a pathogenic role for TXNDC5 in rheumatoid arthritis. Arthritis Res Ther 2011; 13:R124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Zheng Y, Xu H, Yan X, Chang X. Investigate pathogenic mechanism of TXNDC5 in rheumatoid arthritis. PLOS ONE 2013; 8:e53301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung CP, Oeser A, Solus JF et al Inflammation‐associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum 2008; 58:2105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Movahedi M, Beauchamp ME, Abrahamowicz M et al Risk of incident diabetes mellitus associated with the dosage and duration of oral glucocorticoid therapy in patients with rheumatoid arthritis. Arthritis Rheumatol 2016; 68:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schultz O, Oberhauser F, Saech J et al Effects of inhibition of interleukin‐6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLOS ONE 2010; 5:e14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol 2007; 26:1495–8. [DOI] [PubMed] [Google Scholar]
- 14. Antohe JL, Bili A, Sartorius JA et al Diabetes mellitus risk in rheumatoid arthritis: reduced incidence with anti‐tumor necrosis factor alpha therapy. Arthritis Care Res (Hoboken) 2012; 64:215–21. [DOI] [PubMed] [Google Scholar]
- 15. Herlitz‐Cifuentes HS, Garces PC, Fernandez LI, Guzman‐Gutierrez EA. Effect of systemic inflammation on the function of insulin and glucose metabolism in rheumatoid arthritis. Curr Diabetes Rev 2015; 12:156–62. [DOI] [PubMed] [Google Scholar]
- 16. AbouAssi H, Tune KN, Gilmore B et al Adipose depots, not disease‐related factors, account for skeletal muscle insulin sensitivity in established and treated rheumatoid arthritis. J Rheumatol 2014; 41:1974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li AH, Morrison AC, Kovar C et al Analysis of loss‐of‐function variants and 20 risk factor phenotypes in 8,554 individuals identifies loci influencing chronic disease. Nat Genet 2015; 47:640–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem 1979; 254:9627–32. [PubMed] [Google Scholar]
- 19. Nicolau J, Lequerré T, Bacquet H, Vittecoq O. Rheumatoid arthritis, insulin resistance, and diabetes. Joint Bone Spine 2016; 84:411–6. [DOI] [PubMed] [Google Scholar]
- 20. Masuko K. Angiopoietin‐like 4: a molecular link between insulin resistance and rheumatoid arthritis. J Orthop Res 2016; 35:939–43. [DOI] [PubMed] [Google Scholar]
- 21. Mathew R, Pal Bhadra M, Bhadra U. Insulin/insulin‐like growth factor‐1 signalling (IIS) based regulation of lifespan across species. Biogerontology 2017; 18:35–53. [DOI] [PubMed] [Google Scholar]
- 22. Bach LA. Insulin‐like growth factor binding proteins – an update. Pediatr Endocrinol Rev 2015; 13:521–30. [PubMed] [Google Scholar]
- 23. Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin‐like growth factor‐I (IGF‐I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst 2002; 94:972–80. [DOI] [PubMed] [Google Scholar]
- 24. Lee HS, Woo SJ, Koh HW et al Regulation of apoptosis and inflammatory responses by insulin‐like growth factor binding protein 3 in fibroblast‐like synoviocytes and experimental animal models of rheumatoid arthritis. Arthritis Rheumatol 2014; 66:863–73. [DOI] [PubMed] [Google Scholar]
- 25. Lee PD, Giudice LC, Conover CA, Powell DR. Insulin‐like growth factor binding protein‐1: recent findings and new directions. Proc Soc Exp Biol Med 1997; 216:319–57. [DOI] [PubMed] [Google Scholar]
- 26. Suzuki S, Morimoto S, Fujishiro M et al Inhibition of the insulin‐like growth factor system is a potential therapy for rheumatoid arthritis. Autoimmunity 2015; 48:251–8. [DOI] [PubMed] [Google Scholar]
- 27. Lee SD, Chen LM, Kuo WW et al Serum insulin‐like growth factor‐axis and matrix metalloproteinases in patients with rheumatic arthritis or rheumatic heart disease. Clin Chim Acta 2006; 367:62–8. [DOI] [PubMed] [Google Scholar]
- 28. Toussirot E, Nguyen NU, Dumoulin G, Aubin F, Cédoz JP, Wendling D. Relationship between growth hormone‐IGF‐I‐IGFBP‐3 axis and serum leptin levels with bone mass and body composition in patients with rheumatoid arthritis. Rheumatology (Oxf) 2005; 44:120–5. [DOI] [PubMed] [Google Scholar]
- 29. O'Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. Regulation of IGF‐I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol 2008; 252:91–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Werner H, Sarfstein R, LeRoith D, Bruchim I. Insulin‐like growth factor 1 signaling axis meets p53 genome protection pathways. Front Oncol 2016; 6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varewijck AJ, Janssen JA. Insulin and its analogues and their affinities for the IGF1 receptor. Endocr Relat Cancer 2012; 19:F63–75. [DOI] [PubMed] [Google Scholar]
- 32. Sima AAF, Li ZG. The effect of C‐peptide on cognitive dysfunction and hippocampal apoptosis in type 1 diabetes. Diabetes 2005; 5:1497–505. [DOI] [PubMed] [Google Scholar]
- 33. Kasprzak A, Kwasniewski W, Adamek A, Gozdzicka‐Jozefiak A. Insulin‐like growth factor (IGF) axis in cancerogenesis. Mutat Res 2017; 772:78–104. [DOI] [PubMed] [Google Scholar]
- 34. Narazaki M, Tanaka T, Kishimoto T. The role and therapeutic targeting of IL‐6 in rheumatoid arthritis. Expert Rev Clin Immunol 2017; 13:535–51. [DOI] [PubMed] [Google Scholar]
- 35. Ho LJ, Luo SF, Lai JH. Biological effects of interleukin‐6: clinical applications in autoimmune diseases and cancers. Biochem Pharmacol 2015; 97:16–26. [DOI] [PubMed] [Google Scholar]
- 36. Erlandsson MC, Töyrä Silfverswärd S, Nadali M et al IGF‐1R signalling contributes to IL‐6 production and T cell dependent inflammation in rheumatoid arthritis. Biochim Biophys Acta 2017; 1863:2158–70. [DOI] [PubMed] [Google Scholar]
- 37. Kim MS, Lee DY. Insulin‐like growth factor (IGF)‐I and IGF binding proteins axis in diabetes mellitus. Ann Pediatr Endocrinol Metab 2015; 20:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haywood NJ, Cordell PA, Tang KY et al Insulin‐like growth factor binding protein 1 could improve glucose regulation and insulin sensitivity through its RGD domain. Diabetes 2017; 66:287–99. [DOI] [PubMed] [Google Scholar]
- 39. Lewitt MS, Hilding A, Brismar K, Efendic S, Ostenson CG, Hall K. IGF‐binding protein 1 and abdominal obesity in the development of type 2 diabetes in women. Eur J Endocrinol 2010; 163:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin‐like growth factor‐I and development of glucose intolerance: a prospective observational study. Lancet 2002; 359:1740–5. [DOI] [PubMed] [Google Scholar]
- 41. Vaessen N, Heutink P, Janssen JA et al A polymorphism in the gene for IGF‐I: functional properties and risk for type 2 diabetes and myocardial infarction. Diabetes 2001; 50:637–42. [DOI] [PubMed] [Google Scholar]
- 42. Schneider HJ, Friedrich N, Klotsche J et al Prediction of incident diabetes mellitus by baseline IGF1 levels. Eur J Endocrinol 2011; 164:223–9. [DOI] [PubMed] [Google Scholar]
- 43. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011; 11:98–107. [DOI] [PubMed] [Google Scholar]
- 44. Ruscitti P, Cipriani P, Di Benedetto P et al Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)‐1β via the nucleotide‐binding domain and leucine‐rich repeat containing family pyrin 3(NLRP3)‐inflammasome activation: a possible implication for therapeutic decision in these patients. Clin Exp Immunol 2015; 182:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dasu MR, Devaraj S, Jialal I. High glucose induces IL‐1beta expression in human monocytes: mechanistic insights. Am J Physiol Endocrinol Metab 2007; 293:E337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 2013; 62:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Larsen CM, Faulenbach M, Vaag A et al Interleukin‐1‐receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007; 356:1517–26. [DOI] [PubMed] [Google Scholar]
- 48. Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 2014; 13:465–76. [DOI] [PubMed] [Google Scholar]
- 49. Giacomelli R, Ruscitti P, Alvaro S et al IL‐1β at the crossroad between rheumatoid arthritis and type 2 diabetes: may we kill two birds with one stone? Expert Rev Clin Immunol 2016; 12:849–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Thioredoxin domain‐containing 5 (TXNDC5) expression in rheumatoid arthritis synovial fibroblast‐like cells (RASFs). (a) Real‐time polymerase chain reaction (PCR) was used to detect TXNDC5 mRNA levels in RASFs (n = 10) 72 h after transfection with the anti‐TXNDC5 siRNA. (b) Western blotting was used to detect TXNDC5 protein expression in RASFs (n = 10) 72 h after anti‐TXNDC5 siRNA transfection. (c) TXNDC5 protein expression was normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) expression. The figure shows the standard deviation (s.d.) to indicate the variability of the data.
Fig. S2. Insulin‐like growth factor binding protein 1 (IGFBP1) expression in rheumatoid arthritis synovial fibroblast‐like cells (RASFs). (a) Real‐time polymerase chain reaction (PCR) was used to detect IGFBP1 mRNA levels in RASFs (n = 10) 72 h after anti‐IGFBP1 siRNA transfection. (b) Western blotting was used to detect IGFBP1 protein expression in RASFs (n = 10)72 h after anti‐IGFBP1 siRNA transfection. (c) IGFBP1 protein expression was normalized to GAPDH expression. The figure shows the standard deviation (s.d.) to indicate the variability of the data.


