Abstract
Neural progenitor cells hold significant promise in a variety of clinical settings. While both the brain and spinal cord harbor endogenous neural progenitor or stem cells, they typically are not capable of repopulating neural populations in case of injury or degenerative disease. In vitro systems for the culture of neural progenitors has come a long ways due to advances in the method development. Recently, many groups have shown that manipulation of the oxygen-sensing pathway leading to activation of hypoxia inducible factors (HIFs) that can influence the proliferation, differentiation or maturation of neural progenitors. Moreover, different oxygen concentrations appear to affect lineage specification of neural progenitors upon their differentiation in vitro. Here we summarize some of these studies in an attempt to direct effort towards implementation of best methods to advance the use of neural progenitors from basic development towards clinical application.
Neural Progenitor Cells
Neural progenitor cells (NPCs) can be derived from a variety of sources. Neural stem cells (NSCs) reside in the subventricular and subgranular zones of mice [1–3], and can be isolated and expanded in well-established procedures that take advantage of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) to promote their proliferation. Cultured neural stem cells retain tri-lineage potential to differentiate into neurons, astrocytes, and oligodendrocytes and can be maintained for many generations in either neurosphere or adherent culture conditions [4, 5]. To isolate human neural progenitors, it is possible to derive them from human fetal brain or spinal cord tissue, or specify them from human embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) [6, 7]. Human neural progenitors are also thought to have tri-lineage potential and are also maintained in by addition of EGF and bFGF. Both murine and human neural progenitors share transcriptional profiles as well as epigenetic and metabolic features, despite being isolated from different contexts. This is not to say that they are identical, there are important differences outlined elsewhere. Here, we will describe how manipulation of HIF signaling affects the proliferation, specification or differentiation of neural progenitors, which we suspect will prove to be useful for both regulating the basic development of these cells as well as someday their clinical application.
Oxygen tension and embryonic development
Oxygen is a key environmental factor that controls developmental process, tissue homeostasis, and cellular metabolism. Majority of in vitro cell based studies are performed under atmospheric oxygen, with 95% air (contains 78% nitrogen and 21% oxygen) supplemented with 5% of carbon dioxide, resulting a final oxygen concentration to be 20%. However, the oxygen concentration in human tissues and organs is thought to be much lower than the atmospheric oxygen level. In fact, the oxygen level within adult human is heterogeneous, with 14.5% O2 in alveoli, 12% O2 in arterial blood, 5.3% O2 in venous blood, and 1.1% to 9.5% O2 in various other tissues [8]. Multiple studies suggested that the oxygen level in the human brain ranges from 2% to 4.4%, depending on the brain region and sample depth [8, 9].
In fact “Hypoxia” (less than 5% oxygen) is physiologically normoxia for the developing embryos. Before the establishment of utero–placental circulation in the second trimester, the oxygen level in uterine surface is around 2.36% O2 [10]. As a result, embryogenesis before 10 weeks of gestation occurs under low oxygen [11]. The importance of low oxygen in mammalian nervous system development was first demonstrated by Morriss and New using ex utero rat embryos. Cultured E9.5 rat embryos underwent normal cranial neurulation at 5% O2whereas high oxygen condition (20% O2 or 40% O2) resulted in abnormal morphogenesis of neural folds and failure of neural tube closure [12].
The oxygen-sensing pathway
Hypoxia-inducible factors (HIFs) belong to bHLH-PAS (basic Helix-Loop-Helix-per-Arnt-Sim) family of transcription factors that regulate cellular response to low oxygen. Besides oxygen sensing, they play a crucial role in regulating oxygen consumption, glucose uptake, metabolism, and development [13–16]. HIFs are heterodimeric transcription factors that are composed of an alpha-subunit (HIF1α, HIF2α, and HIF3α), and a constitutively expressed beta-subunit (HIF1β, also called aryl hydrocarbon receptor nuclear translocator, ARNT) [17–20]. The protein stability of alpha-subunit is regulated by prolyl hydroxylase domain proteins (PHD1–3, also known as EGLN1–3) in an oxygen-dependent manner. As a result, HIF-α subunits direct oxygen sensing in a linear range from 0.1 to 21% O2 [21]. Specifically, when oxygen is abundant, HIF1α and HIF2α are hydroxylated by PhDs in the presence of Fe2+. Hydroxylated HIF-α subunit is recognized by von Hippel-Lindau (VHL) tumor suppressor protein, a recognition component of E3 ubiquitin ligase complex. Upon VHL binding, the HIF-α subunit is targeted for ubiquitination and rapid proteasomal degradation [22]. When O2 concentration is less than 5%, decreased O2 diminishes the enzymatic activity of PHDs. As a result, HIF1α and HIF2α proteins are stabilized, translocate into nucleus and dimerize with HIF1β subunit. The heterodimer then recruits the coactivators of P300/CBP, and bind to hypoxia response element (HRE) to transactivate specific target genes [23–25].
Besides activation by hypoxia, the PHD-HIF signaling pathway can be regulated by other micro-environmental factors, such as iron and TCA cycle intermediates. The hydroxylation of HIF-α subunit by PHD requires iron and ascorbate as cofactors. Thus, PHD enzymatic activity is affected by iron availability. Because oxygen and α-ketoglutarate (α-KG, also known as 2-oxoglutarate, or 2OG) are substrates, and succinate is the product of the hydroxylation reaction, the PHD activity can be inhibited by intracellular accumulation of succinate, or TCA cycle intermediates such as fumarate and malate, which serve as competitive inhibitors of α-KG [26, 27].
HIF in embryonic development
The importance of HIF pathway in development was demonstrated by the early embryonic lethality (E9.5–10.5) in both Hif1α and Hif1β deficient mice (see review [11, 16]). In fact, abrogation of HIF activity impaired vascularization of the placental [28–30], cardiovascular morphogenesis and angiogenesis [31], heart development [32–34], and endochondrial bone formation during early embryogenesis [16, 35, 36]. In terms of nervous system development, HIFs are required for neural crest cell migration [37]. Hif1α and Hif1β knockout mice had abnormalities such as forebrain hypoplasia and neural fold closure defects [29, 38]. Furthermore, neural-specific Hif1a-deficient mice exhibited hydrocephalus, reduction in neuronal cells, and impaired spatial memory [39]. Those studies demonstrated the indispensible role of HIF in embryonic brain development.
Methods to stabilize HIF
Manipulating Oxygen concentration
Manipulating oxygen concentration is the most straightforward and physiologically relevant way to stabilize HIF in neural stem cells. One way to establish a hypoxic cell culture environment is to use a multigas incubator with an oxygen sensor. Because atmospheric oxygen concentration is 21%, nitrogen is needed to decrease the oxygen level. One limitation of this method is that once the incubator door is opened, air containing high-level of oxygen flows in. In that case, the hypoxic environment is temporarily disturbed and has to be re-established. The fluctuation of oxygen concentration during cell culture and sample collection could cause inconsistency in experiments, and even false negative results. Moreover, HIF1α protein can be degraded within 5 minutes at atmospheric oxygen due to its inherent instability. Thus, hypoxic sample collection needs to be performed promptly. Another practical limitation is that the oxygen level can be set at just one concentration for an entire incubator. Thus, it requires multiple incubators to test multiple oxygen concentrations simultaneously.
Commercially available “sub-chamber” systems and “hermetical hypoxic workstations” can solve the problem of oxygen fluctuation. The “sub-chamber” includes a sealed secondary container that can transfer the cell culture plate, and an oxygen controlled hood that allow sample collection and cell feeding under low oxygen environment. An updated version is hermetical hypoxic workstation, which is a fully sealed, oxygen-controlled environment that contains an incubator for cell culture, and a glove box for experimental procedures. Thus, this system allows for complete manipulation of cells in hypoxic environment. Nuclear translocation of HIF1α by immunostaining can be used as an indicator for successful establishment of hypoxia. In our experience, HIF1α nuclear translocation can be seen in NPCs after 6 hours in 5% O2. Lowering the oxygen to 2% resulted in a an even more robust HIF1α nuclear translocation [40].
Small molecules
Small molecules that activate HIF provide a cost-effective way to mimic hypoxia without having to physically establish a low oxygen environment. Small molecules can stabilize HIF by targeting the PHD-mediated HIF degradation pathway.
Deferoxamine (DFO)
DFO stabilizes HIF through inhibition of PHD by chelating the cofactor Fe2+ [41, 42]. DFO was the first FDA approved iron-chelating compound for the treatment of iron overload. Thus, the long-term outcome and side effects for DFO are well-characterized in patients. Significant side effects include local skin irritation, ophthalmological and auditory problems, and neurological symptoms at high doses [43]. In human NPCs, HIF1α nuclear translocation can be seen after just 6 hours of DFO treatment by immunostaining [40]. The effective dose of DFO ranges from 50 uM to 150 uM and the best result can be seen 24 hours after treatment. However, extended DFO treatment inhibited cell proliferation in NPCs. After treatment with 50 uM, 100 uM or 200 uM of DFO for 72 hours, the viability of NPCs were decreased by 40% to 70% in a dose-dependent manner [44]. Interestingly, DFO has been shown to have neuroprotective properties in several disease models. In an ischemia study using mouse model, pretreatment of DFO protected hippocampal neurons from cell death by activating HIF-1α signaling [45]. In another study, DFO administration after cerebral ischemia decreased brain damage and promoted functional recovery in rats [46]. Moreover, DFO was shown to ameliorate pathological symptoms and improve behavioral outcomes in mice with Parkinson’s disease (PD). Accumulation of iron in the Substantia Nigra (SN) is one of the defining characteristics of PD. Intranasal DFO treatment decreased iron-positive cells in the SN, prevented the degeneration of dopaminergic neurons, and efficiently alleviated behavioral deficits in the PD mouse [47].
Dimethyloxalylglycine (DMOG)
DMOG is an analogue of α-KG, the substrate of prolyl-4-hydroxylase. Thus, DMOG stabilizes HIF by inhibition of PHD by competition with its substrate [48, 49]. In our NPC cultures, 200 uM to 250 uM of DMOG was sufficient to induce nuclear translocation of HIF1α. In addition, the growth inhibition of DMOG at 250 uM is less than that of 100 uM DFO in human ESC or iPSC-derived NPCs. Milosevic et al reported that the viability of midbrain-derived human NPCs was decreased by 20% in 250 uM DMOG compared with untreated cells [44]. Similar to DFO, DMOG has shown neuroprotective function after traumatic brain injury through HIF activation and subsequent angiogenesis [50].
Cobalt (II) Chloride (CoCl2)
CoCl2 inhibits PHD enzymes by replacement of Fe2+ from the catalytic core [42, 51]. In NPCs, 50 uM of CoCl2 is sufficient to stabilize HIF1α protein under atmospheric oxygen and viability is not affected by up to 100 uM CoCl2 [44]. Interestingly, CoCl2 treated NPCs led to Increased numbers of TH+ dopaminergic neurons upon differentiation for 1 week [44].
Other PHD inhibitors
FG-4497 and Ciclopiroxolamine (CPX, or FG-2229) stabilize HIF1α by blocking the active site of PHDs as well as chelating Fe2+ [52]. 10 uM of CPX can stabilize HIF1α protein but CPX is toxic to NPCs even at 1 uM, resulting in a 70% reduction of cell number in 72 hours [44]. On the other hand, 5 uM to 10 uM of FG-4497 is sufficient to stabilize HIF1a in 10–14 week fetal-derived mesencephalic NPCs [44]. FG-4497 is not cytotoxic and was shown to increase the number of NPCs by 40% after 72 hours. This treatment also increased dopaminergic differentiation of NPCs and protected dopaminergic neurons from MPP+ induced degradation [44].
Genetic activation of HIF
The recognition of HIF by pVHL depends on hydroxylation of several conserved proline residues in the HIF1α and HIF2α protein [53–55]. Mutating those residues to alanine, such as P564A [54], P577A/P402A [56], P577A/P402A/N813A [57], P402A/P564A in HIF1α, and P530A/P405A [56], P405A/P531A [58] in HIF2α, disables the VHL targeted degradation of HIF protein, and thus stabilizes HIF protein under atmospheric oxygen. Specifically, cells can be transfected (or infected) with the plasmids that express non-degradable HIF protein under control of a tetracycline-regulated promoter. Upon doxycycline induction, a rapid expression of HIF mRNA can be detected 4 hours after induction. Maximal mRNA levels can be reached at 20 hours post doxycycline-induction and the high levels of HIF mRNA can be sustained for at least 48 hours [56]. One limitation of this method is the low transfection/infection efficiency in human NPCs, making it difficult to get homogeneous HIF-overexpression. Moreover, one should keep in mind that the endogenous HIF1α mRNA expression level is not affected by doxycycline induction.
Whole animal hypoxia
While in vitro tissue culture provides the flexibility for manipulation, whole animal hypoxia model could be a more physiologically relevant method to study development and diseases. For example, chronic hypoxemia caused by chronic obstructive pulmonary disease (COPD) or chronic altitude sickness can lead to serious neurological complications. Wistar rats exposed to 10% O2 for 10 days develop a syndrome similar to the blood oxygen tension in COPD patients, and thus can be used as an animal model to study COPD caused neurological disorders [59]. A hermetically sealed chamber with O2 control can be used to establish the hypoxic environment for the whole animal hypoxia study. Most animals cannot survive over 15 minutes in hypoxic environment (5% O2 or less). However, a recent study by Park et al reported that the African naked mole-rat can tolerate 5% O2 for 5 hours. It even survived 18 minutes of anoxia (0% O2) without apparent injury [60]. The authors discovered that those naked mole-rat switched to anaerobic metabolism that used fructose to fuel vital organs like brain and heart during anaerobic periods [60]. Therefore, the naked mole-rat is an important model to study the whole animal’s response to extreme low oxygen going forward.
Effects of oxygen tension on NSC proliferation and differentiation
Oxygen tension in control of NSC proliferation
The influence of low oxygen on proliferation and self-renewal of neural stem cells has been studied by various groups over the past decade. Different oxygen levels have diverse effects on NPC proliferation (Table 1 and 2). Specially, mild hypoxia (3%-6% O2) increased the proliferation of both rodent NSCs [61–64] and human NSCs [65–68] compared with the cells cultured in atmospheric (20%) oxygen. Whereas severe hypoxia (1–2% O2) and anoxia (<1% O2) decreased the proliferation of primary fetal tissue derived cortical progenitors [66], MYC-immortalized fetal NPCs [68], and human ESC or iPSC-derived NPCs [40]. Interestingly, the NPCs originated from different brain regions responded differently to different oxygen conditions. Storch et al reported that fetal mesencephalic precursors grew well under 2% O2but failed to proliferate at 20% O2. The forebrain precursors derived from the same samples, however, expanded equally well under 2% and 20% O2 [65] (Figure 1).
Table 1.
Effects of lowered oxygen on proliferation, survival, and differentiation of human neural progenitor cells compared to atmospheric oxygen
| References | Human cells | Oxygen (%) |
Method of expansion |
Days of expansion |
Effect on proliferation |
Effect on apoptosis |
Method of differentiation |
Days of differentiation |
Effect on lineage specification | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurons | Astrocytes | Oligodendrocytes | |||||||||
| Storch et al. [65] | Fetal 6–9 week mesencephalic precursors | 3 | Neurosphere cultured in DMEM/F12 media with bFGF (20 ng/ml) EGF (20 ng/ml). Passaged every 18–27 day. | 90–240 | Significant Increase | Not tested | EGF/bFGF withdrawal with IL1-b, IL11, LIF, and GDNF | 10 | Converted to dopaminergic neurons (TH+, DAT+) | Detected (GFAP+) | Not tested |
| Fetal 6–9 week forebrain precursors | Modest increase | Not tested | Not inducible to dopaminergic neurons | Not tested | Not tested | ||||||
| Liu et al. [71] | Fetal 11–15 week mesencephalic progenitors | 3 | Neurospheres cultured in DMEM/F12 media with bFGF (20 ng/ml), EGF (10 ng/ml), and LIF (10ng/ml). Passaged weekly. | 35–42 | Not tested | Not tested | EGF/bFGF withdrawal | 28 | Increased (Tuj1+, TH+, DA+) | Not tested | Not tested |
| Ortega et al. [66] | Fetal 14–19 week cortical progenitor cells | <1 | Fetal cortical cells were dissected and cultured in DMEM/F12, B27 supplement, bFGF (10 ng/ml), EGF (10 ng/ml). | 7 | Decrease | No differences | EGF/bFGF withdrawal, with 1day in <1% oxygen and 6 days in 20% oxygen | 7 | Decreased (Tuj1+, DCX+, GABA+) | Increased (S100β, VIM+) | Decreased (O4+, Olig2+) |
| 1 | Decrease | Increase | EGF/bFGF withdrawal | 7 | No differences (DCX+, GABA+) | Increased (S100β, VIM+) | No differences (Olig2+) | ||||
| 3 | Increase | Decrease | EGF/bFGF withdrawal | 21 | Increased (Tuj1+, GABA+) | No differences (VIM, S100β, GFAP+) | No differences (Olig2+) | ||||
| Pistollato et al. [67] | Postnatal brain subventricular zone CD133+ Nestin+ precursors | 5 | Cells cultured in DMEM/F12 media with bFGF (20 ng/ml), EGF (10ng/ml), BMP2(10ng/ml), 1% BSA, 10 µg/ml rh insulin, 200 µg/ml transferrin. Passaged weekly. | 7 | Increase | No differences | EGF/bFGF withdrawal | 21 | No differences (Tuj1+) | No differences (GFAP+) | Significantly increased(GalC+) |
| Giese et al. [72] | MYC-immortalized fetal ventral mesencephalic progenitor cell line (ReNcell VM, Millipore Inc) | 3 | Cells cultured in DMEM/F12 media with bFGF (10ng/ml), EGF(20ng/ml), Glutamax, B27 supplement, heparin sodium salt. | 3 | No differences | Decrease | EGF/bFGF withdrawal | 4 | Increase (Tuj1+) | Not tested | Not tested |
| Santilli et al. [68] | v-MYC immortalized 10.5-week fetal diencephalic and telencephalic precursors | 1 | Neurospheres cultured in growth media with bFGF (20ng/ml) and EGF. Passaged every 7–10 days. | 7 to 10 | Decrease | Increase | EGF/bFGF withdrawal | 10 | No differences (Tuj1+, MAP2+, GABA+) | Increased (GFAP+) | No differences (GalC+) |
| 2.5 | Increase | No differences | EGF/bFGF withdrawal | 10 or 17 | Increased (Tuj1+, MAP2, GABA+) | No differences (GFAP+) | Increased (GalC+) | ||||
| 5 | Increase | No differences | EGF/bFGF withdrawal | 10 or 17 | Increased (Tuj1+, GABA+) | No differences (GFAP+) | Increased (GalC+) | ||||
| Xie et al. [40] | Human ESC or iPSC derived neural progenitors | 2 | Cells cultured in DMEM/F12, N2 and B27 supplement, FGFb (20ng/ml) and EGF (50ng/ml). | 7 | Decrease | No differences | EGF/bFGF withdrawal | 21, 28, 35 or 42 | No differences (Tuj1+, MAP2+) | Increased (GFAP+) | Not tested |
Table 2.
Effects of lowered oxygen on proliferation, survival, and differentiation of murine neural progenitor cells compared to atmospheric oxygen
| Referenc es |
Rodent cells |
Oxyge n (%) |
Method of expansion |
Days of expansi on |
Effect on proliferati on |
Effect on apoptosi s |
Method of differentiatio n |
Days of differentiati on |
Effect on lineage specification | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurons | Astrocytes | Oligodendrocytes | |||||||||
| Studer et al. [61] | Rat E12 mesencepha lic precursors | 3 | Cells dissected from rat embryonic day 12 (E12) mesencephalon were mechanically dissociated, plated on DMEM/F12 medium with N2 supplement and bFGF (10 ng/ml). | 4 to 6 | Increase | Decrease | bFGF withdrawal | 5 | Increased (TH+); Decreased (GABA+, Glu+) | Not detected (GFAP+) | Not tested |
| Morrison et al. [62] | Rat E14.5 neural crest stem cell (in peripheral nervous system) | 3 to 6 | DMEM-low glucose medium with 15% chick embryo extract, bFGF (20 ng/ml), N2 and B27 supplement, 2-mercaptoethanol (50 M), retinoic acid (35 ng/ml). | 6 | Increase | No differences | Reduced bFGF (10ng/ml) and chick embryo extract (1%), with or without BMP2 | 5 to 6 | Increased neurons | Increased (GFAP+) | Not applicable |
| Reduced bFGF (10ng/ml) and chick embryo extract (1%), with BMP2, forskolin, NGF, NT-3 | 12 | Increased (TH+, DBH+, SA1+); increased dopamine and norepinephrine release | Not tested | Not applicable | |||||||
| Stacpoole et al. [74] | Rat postnatal P0-P2 cortical NPCs | 3 | Neurosphares cultured in DMEM/F12, N2 and B27 supplement, FGFb (20 ng/ml), EGF (20 ng/ml), and Heparin (5µg/ml). | 7 | Increase | Not tested | Not applicable | 0 | No differences (Tuj1+) | No differences (GFAP+) | No differences (O4+) |
| 3 | 7 | Reduced bFGF (10ng/ml) with PDGF | 5 | No differences(Tuj 1+) | Slight decrease (GFAP+) | increased (O4+) | |||||
| 3 | 28 | Reduced bFGF (10ng/ml) and PDGF | 2 | No differences(Tuj 1+) | Decreased (GFAP+) | increased (O4+, MBP+, Olig2+) | |||||
| 3 | 28 | Transplantation into rat brain | 2 | Not tested | Increased (GFAP+) | Increased oligodendrocyte progenitor (NG2+) | |||||
| Mutoh et al. [75] | Mouse E11.5 telencephalic NPCs | 2 | Cells cultured in DMEM/F12, N2 supplement, FGFb (10 ng/ml). | 4 | Not tested | Not tested | 4-day bFGF withdrawal plus 4-day with LIF | 8 | Decreased (Map2+) | Increased (GFAP+) | Not tested |
| Chen et al. [73] | Mouse E13.5 cortical NPCs | 5 | Cells cultured in N2 medium with FGFb (20 ng/ml). | 5 | Increase | Decrease | bFGF withdrawal with NT-3 | 6 | Slightly decreased (Tuj1+, MAP2+) | Increased (GFAP+) | Increased (O4+, PDGFRa+) |
| 2 | Increase | Not tested | bFGF withdrawal with NT-3 | 6 | Decreased (Tuj1+, MAP2+) | Significant increased (GFAP+) | Significant increased (O4+, PDGFa+) | ||||
| Horie et al. [64] | Mouse E15.5 ganglionic eminence-derived NPCs | 0 | Neurosphares cultured in N-2 medium (DMEM/F12 with 36.4mM glucose, 23ug/ml insulin, 92ug/ml transferrin, 55u< putrescine, 27.5nM sodium selenite, 20nM progesterone, and 50U/ml penicillin-streptomycin) with EGF (20ng/ml). | 5 | No differences | Increase (highest) | EGF withdrawal with 1% FBS | 2 | Decreased (GABA+) | Decreased (GFAP+) | Not tested |
| 1 | Increase | Increase | Increased (Tuj1+, Glu+); Decreased (GABA+) | No differences (GFAP+) | Not tested | ||||||
| 2 | Increase (highest) | Increase | Increased (Tuj1+, Glu+); Decreased (GABA+) | No differences (GFAP+) | Not tested | ||||||
| 3 | Increase | Increase | Increased (Tuj1+, Glu+); Decreased (GABA+) | No differences (GFAP+) | Not tested | ||||||
| 4 | Increase | No differences | Decreased (GABA+) | No differences (GFAP+) | Not tested | ||||||
| 10 | Increase | No differences | Decreased (GABA+) | No differences (GFAP+) | Not tested | ||||||
| Kim et al. [92] | Mouse ESC-derived NPCs | 3.5 | Cells cultured in DMEM/F12 with N2 supplement, FGFb (20 ng/ml). | 6 | Not tested | Not tested | bFGF withdrawal with ascorbic acid (200 µM), IL1-b, IL11, LIF, and GDNF | 6 | Increased (TH+, Tuj1+) | No differences (S100β+) | No differences (Olig2+) |
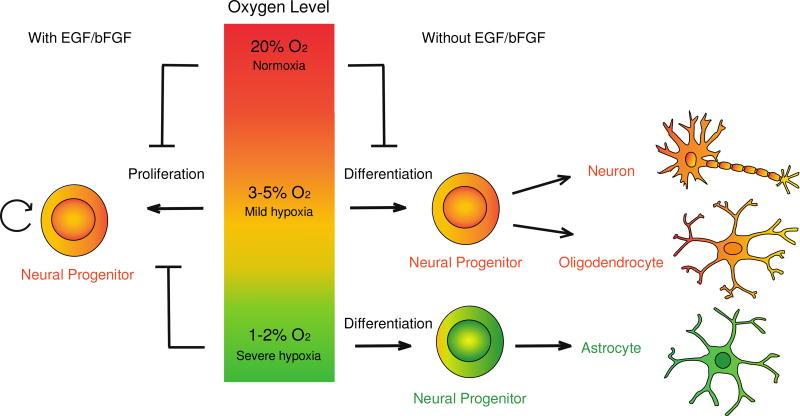
Figure 1. The model describing the influence of oxygen level on the proliferation and lineage specification of NPCs.
In the presence of growth factors (EGF and bFGF), the proliferation capacity of NPCs is higher in mild hypoxia (3–5% O2) than in 20% O2. Severe hypoxia (1–2% O2), however, slows down the proliferation of NPCs. In the absence of growth factors, the differentiation potential of NPCs is affected by different level of oxygen. Mild hypoxia promotes neurogenesis and oligodendrogenesis, whereas severe hypoxia favors the generation of astroglial lineage.
Oxygen tension in control of NSC differentiation
Mitogens, such as EGF and bFGF, are key factors to maintain viability and pluripotency of NSCs. In the absence of the mitogens, in vitro-cultured neural progenitors spontaneously differentiate into neurons, astrocytes and oligodendrocytes. The phenomenon that neurogenesis proceeds gliogenesis in vitro is consistent with the established temporal NPC specification in mammalian neocortical development [69, 70]. Mitogen withdrawal initiates NSC differentiation. Oxygen plays an important role in this process to direct NPC specification towards specific lineages as discussed below (Figure 1).
Mild hypoxia to promote neurogenesis and oligodendrogenesis
Upon NPCs differentiation, mild hypoxia (2.5–6% O2) induces the number of neuronal and oligodendrocyte lineage compared with atmospheric oxygen (Tables 1 and 2). The role of mild hypoxia in promoting neurogenesis was first identified in rat neural progenitors. Studer et al demonstrated that 3% O2 increased the number of tyrosine-hydroxylase positive (TH+) dopaminergic neurons in in vitro cultured rat mesencephalic precursors upon differentiation [61]. In a separate study, Morrison et al demonstrated that mild hypoxia promoted the generation and survival of TH+ and sympatoadrendal-1 (SA-1) positive neurons derived from rat neural crest stem cells in peripheral nervous system [62]. Later studies further demonstrated the pro-neurogenesis effect of mild hypoxia in progenitors derived from embryonic brain tissues of different developmental stages in human [66, 68, 71, 72] and mouse [64, 73].
Mild hypoxia also promotes oligodendrogenesis. When human post-natal subventricular zone neural precursors were induced to differentiation, precursors expanded in 5% O2 generated 17-fold more galactocerebroside-C positive (GalC+) oligodendrocytes than those in 20% O2. When precursors were expanded at 5% oxygen and then differentiated at 20% oxygen, oligodendrocyte maturation was further enhanced 2.5-fold [67]. A similar pro-oligodendrogenesis effect was demonstrated in 2.5% and 5% oxygen using immortalized human fetal cortical precursors [68]. Moreover, transplantation of hypoxia-cultured rat NSCs into the adult rat hippocampus showed a survival advantage and increased specification towards the oligodendrocyte lineage compared with those cultured in 20% O2 [63]. Stacpoole et al further demonstrated that 3% O2 is a suitable culture condition for oligodendrocyte differentiation from human ESCs, and they identified the pathways involved in forebrain oligodendrocyte specification [74].
Severe hypoxia promotes astrogliogenesis
While mild hypoxia enhanced NSC proliferation and promoted the generation of neurons and oligodendrocytes, severe hypoxia (1–2% O2) induced the differentiation of human NSCs towards astrocytes lineage upon growth factor withdrawal [40, 66, 68]. We reported that the ratio of astrocytes to neurons can be strongly induced by 2% O2 compared with 20% O2 in human-ESC or human-iPSC derived NPCs [40]. In a another study, 1% or less oxygen promoted astrogliogenesis in human cortical progenitors at the expense of GABAergic neurons and O4+ oligodendrocytes [66], whereas 3% O2 promoted neurogenesis and oligodendrocyte in those cortical NSCs [66, 68]. The observation that lowered oxygen promotes astrogliogenesis also holds true in mouse neural progenitors. Two separate studies using mouse E11.5 and E13.5 NPCs demonstrated more GFAP+ astrocytes were generated under 2% O2 compared with 20% O2 [73, 75]. Furthermore, the astrogliogenesis effect was more significant under 2% oxygen than 5% oxygen [73], supporting the pro-astrogliogenic effect in lower oxygen. It is important to note that the time required to differentiate into astrocytes in vitro is typically much longer than that of neurons. Depending on the maturity of NPCs, it typically takes 1 week to generate astrocytes in mouse [73, 75], versus 2–4 weeks to get astrocytes with human NPCs [40, 66, 67]. Thus, in studies with short term of differentiation, GFAP+ astrocytes are not detected [61] (Tables 1 and 2).
It is worth noting that that neural progenitors behave similarly both in vitro and in vivo. Using whole animal hypoxia model, XA de Tassigny et al demonstrated that prolonged exposure to severe hypoxia (1% O2) inhibited the survival of newly generated Tuj1+ neurons and O4+ oligodendrocytes in rat subventricular zone, whereas the number of GFAP+ astrocytes was not affected in vivo [59]. This finding is consistent with the in vitro studies discussed above that severe hypoxia inhibits neurogenesis and oligodendrogenesis, but promotes astrogliogenesis.
The mechanism of HIF signaling in NSC under hypoxia
How does HIF activation lead to NSC proliferation and self-renewal?
In pluripotent stem cells, under mild hypoxia, HIF signaling can induce expression of OCT4 and Wnt/beta-catenin signaling, which are positive regulators of stem cell self-renewal and proliferation. Binding directly to OCT4 promoter, HIF2α can transactivate OCT4 and regulates mouse ESC proliferation and maintenance [76]. Silencing of HIF2α resulted in a significant decrease in hESC proliferation and down-regulation of pluripotency genes, such as OCT4, SOX2 and NANOG [77]. In neural stem cells, the hypoxia-mediated proliferation is mediated by nuclear orphan receptor TLX. Under low oxygen tension, TLX is recruited to OCT4 promoter to augment its transcription, and thus promotes the proliferation of adult hippocampal neural progenitors [78].
Multiple studies suggested that mild hypoxia enhanced the proliferation and neurogenesis of NSCs through Wnt/β-catenin signaling [66, 79–81]. Activation of Wnt/β-catenin signaling in NSCs promote neurogenesis, whereas inhibition of Wnt signaling by Dkk1 promotes astrogliogenesis [82]. Under low oxygen, HIF-1α enhanced the activation of Wnt/β-catenin pathway and downstream effectors LEF-1 and TCF-1, which led to increased proliferation in NSCs [80]. Ortega et al further demonstrated that mild hypoxia (3% O2) induced WNT7A expression in radial glia cells (RGCs), resulting in increased proliferation and neurogenesis, whereas severe hypoxia (1% or less oxygen) reduced WNT7A level and diminished RGCs proliferation and neuronal differentiation [66].
HIF signaling in NSC differentiation
Hypoxia mediated neural progenitor survival and neuronal differentiation can also be attributed to the HIF induced soluble factors, such as erythropoietin (EPO) and vascular endothelial growth factor (VEGF). EPO increased dopaminergic neuron differentiation and survival in hypoxic conditions. Addition of EPO in 20% O2 partially recapitulated the pro-neurogenesis effect under mild hypoxia [61]. VEGF acts as a ‘neurogenic factor’ that significantly augmented the proliferation and neuronal specification of NPCs during embryonic development and adult neurogenesis (see review [83]). Moreover, VEGF and EPO are beneficial to the neuronal survival, migration, and functional recovery in pathological conditions [84, 85]. EPO increases neurogenesis and oligodendrogenesis of subventricular zone precursor cells after neonatal stroke [85]. A separate study showed that transplantation of NPCs into ischemic mouse brain attenuated apoptosis of surrounding neurons by VEGF-mediated neuronal protection [86].
HIF promotes the astrocyte lineage differentiation through diverse mechanisms. Low oxygen induced demethylation of GFAP promoter in a HIF-dependent manner, presumably as part of a program to facilitate the differentiation into astrocytes in both human [87] and mouse NPCs [75]. Notch activation was required for the hypoxia-mediated acquisition of astrogliogenic potential by NPCs [75]. Xie et al demonstrated that 2% O2 promoted astrogliogenesis through HIF mediated regulating of the evolutionarily conserved LIN28/let-7 axis. Specifically, Lin28 blocks the maturation of let-7 miRNAs, and let-7 suppresses Lin28 translation [88]. When NPCs mature, the level of LIN28 drops leading to an increase of let-7 miRNAs. Increased let-7 miRNAs promoted neural progenitors maturation as measured by increased gliogenic potential [90, 91]. Under low oxygen, HIF inhibits LIN28 transcription through the competitive inhibition of MYC in LIN28 promoter [40]. In previous work, it was also shown that decreased LIN28 enables the generation of let-7 microRNAs, and results in maturation of NPC with increased astrocytes upon differentiation [91]. Furthermore, inhibition of let-7 blocked the low oxygen mediated astrogliogenesis in NPC stage, further confirming the relationship of HIF and let-7 in promoting NSC specification towards the astroglial lineage under severe hypoxia [40] [91]. Others have also shown that let-7 is known to promote NPC differentiation through the TLX/microRNA-9 circuit [89].
Conclusions
Clearly, oxygen tension and HIF activity can play important diverse roles in the biology of neural stem and progenitor cells. These roles depend strongly on the context in which HIF activity is modulated and the degree of manipulation. In addition, because some of these manipulations are compatible with clinical-grade production of cells, the use of DFO, DMOG, or oxygen regulation will allow for Good Manufacturing Process (GMP)-compatible promotion of proliferation, specification or differentiation as outlined here.
Glossary
Neuronal Marker
- DA
Dopamine
- DAT
Dopamine transporter
- DBH
Dopamine beta-hydroxylase
- DCX
Doublecortin
- GABA
gamma-Aminobutyric acid
- Glu
Glutamate
- MAP2
Microtubule-associated protein 2
- SA1
Sympathoadrenal lineage-specific marker
- TH
Tyrosine hydroxylase
- Tuj1
Neuron-specific Class III β-tubulin
Astrocyte marker
- GFAP
Glial fibrillary acidic protein
- S100β
S100 calcium-binding protein B
- Vim
Vimentin
Oligodendrocyte marker
- GalC
Galactocerebroside
- MBP
Myelin basic protein
- NG2
Neuron-glial antigen 2
- O4
Oligodendrocyte marker O4
- Olig2
Oligodendrocyte transcription factor 2
- PDGFRa
Platelet-derived growth factor receptor alpha
Growth Factors
- bFGF
Basic fibroblast growth factor
- BMP2
Bone morphogenetic protein 2
- EGF
Epidermal growth factor
- FBS
Fetal bovine serum
- GDNF
Glial cell line-derived neurotrophic factor
- IL1-b
Interleukin 1 beta
- IL11
Interleukin 11
- LIF
Leukemia inhibitory factor
- NGF
Nerve growth factor
- NT-3
Neurotrophin-3
- PDGF
Platelet-derived growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Doetsch F, Cailleâ I, Lim DA, Garcõâa-verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 2.Levison SW, Goldman JE. Multipotential and lineage restricted precursors coexist in the mammalian perinatal subventricular zone. J. Neurosci. Res. 1997;48:83–94. doi: 10.1002/(SICI)1097-4547(19970415)48:2<83::AIDJNR1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proceedings of the National Academy of Sciences. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Åkesson E, Piao J-H, Samuelsson E-B, Holmberg L, Kjældgaard A, Falci S, et al. Long-term culture and neuronal survival after intraspinal transplantation of human spinal cord-derived neurospheres. Physiology & Behavior. 2007;92:60–66. doi: 10.1016/j.physbeh.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 5.Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proceedings of the National Academy of Sciences. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falk A, Koch P, Kesavan J, Takashima Y, Ladewig J, Alexander M, et al. Capture of Neuroepithelial-Like Stem Cells from Pluripotent Stem Cells Provides a Versatile System for In Vitro Production of Human Neurons. PLoS ONE. 2012;7:e29597. doi: 10.1371/journal.pone.0029597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S-C, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nature Biotechnology. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 8.Carreau A, Hafny Rahbi BE, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. Journal of Cellular and Molecular Medicine. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dings J, Meixensberger J, Jäger A, Roosen K. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery. 1998;43:1082–1095. doi: 10.1097/00006123-199811000-00045. [DOI] [PubMed] [Google Scholar]
- 10.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 11.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morriss GM, New DAT. Effect of oxygen concentration on morphogenesis of cranial neural folds and neural crest in cultured rat embryos. Development. 1979;54:17–35. [PubMed] [Google Scholar]
- 13.Lendahl U, Lee K, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nature Reviews Genetics. 2009 doi: 10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- 14.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nature Reviews| Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 16.Dunwoodie S. The Role of Hypoxia in Development of the Mammalian Embryo. Developmental Cell. 2009 doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proceedings of the National Academy of Sciences. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes & Development. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 20.Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- 21.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J. Cell. Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 22.Ruas JL, Poellinger L. Hypoxia-dependent activation of HIF into a transcriptional regulator. Seminars in Cell & Developmental Biology. 2005;16:514–522. doi: 10.1016/j.semcdb.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J. Clin. Invest. 2007;117:862–865. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravenna L, Salvatori L, Russo MA. HIF3α: the little we know. Febs J. 2015 doi: 10.1111/febs.13572. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 25.Webb JD, Coleman ML, Pugh CW. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell. Mol. Life Sci. 2009;66:3539–3554. doi: 10.1007/s00018-009-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Y, Mansfield KD, Bertozzi CC, Rudenko V, Chan DA, Giaccia AJ, et al. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Molecular and Cellular Biology. 2007;27:912–925. doi: 10.1128/MCB.01223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–180. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes & Development. 2000;14:3191–3203. doi: 10.1101/gad.853700a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozak KR, Abbott B, Hankinson O. ARNT-deficient mice and placental differentiation. Dev. Biol. 1997;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- 30.Cowden Dahl KD, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM, et al. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Molecular and Cellular Biology. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 32.Ryan HE, Lo J, Johnson RS. HIF-1α is required for solid tumor formation and embryonic vascularization. The EMBO Journal. 1998 doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnan J, Ahuja P, Bodenmann S, Knapik D, Perriard E, Krek W, et al. Essential role of developmentally activated hypoxia-inducible factor 1alpha for cardiac morphogenesis and function. Circulation Research. 2008;103:1139–1146. doi: 10.1161/01.RES.0000338613.89841.c1. [DOI] [PubMed] [Google Scholar]
- 34.Licht AH, Müller-Holtkamp F, Flamme I, Breier G. Inhibition of hypoxia-inducible factor activity in endothelial cells disrupts embryonic cardiovascular development. Blood. 2006;107:584–590. doi: 10.1182/blood-2005-07-3033. [DOI] [PubMed] [Google Scholar]
- 35.Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E. HIF1α regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 36.Provot S, Zinyk D, Gunes Y, Kathri R, Le Q, Kronenberg HM, et al. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol. 2007;177:451–464. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Compernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, Collen D, et al. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1α. Cardiovascular Research. 2003;60:569–579. doi: 10.1016/j.cardiores.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Lyer NV, Kotch LE, Agani F, Leung SW, Laughner E. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1a. Genes Dev. 1998 doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomita S, Ueno M, Sakamoto M, Kitahama Y, Ueki M, Maekawa N, et al. Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Molecular and Cellular Biology. 2003;23:6739–6749. doi: 10.1128/MCB.23.19.6739-6749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Y, Zhang J, Lin Y, Gaeta X, Meng X, Wisidagama DRR, et al. Defining the role of oxygen tension in human neural progenitor fate. Stem Cell Reports. 2014;3:743–757. doi: 10.1016/j.stemcr.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–3615. [PubMed] [Google Scholar]
- 42.Gleadle JM, Ebert BL, Firth JD, Ratcliffe PJ. Regulation of angiogenic growth factor expression by hypoxia, transition metals, and chelating agents. Am. J. Physiol. 1995;268:C1362–8. doi: 10.1152/ajpcell.1995.268.6.C1362. [DOI] [PubMed] [Google Scholar]
- 43.Poggiali E, Cassinerio E, Zanaboni L, Cappellini MD. An update on iron chelation therapy. Blood Transfus. 2012;10:411–422. doi: 10.2450/2012.0008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milosevic J, Adler I, Manaenko A, Schwarz SC, Walkinshaw G, Arend M, et al. Non-hypoxic Stabilization of Hypoxia-Inducible Factor Alpha (HIF-α): Relevance in Neural Progenitor/Stem Cells. Neurotox Res. 2009;15:367–380. doi: 10.1007/s12640-009-9043-z. [DOI] [PubMed] [Google Scholar]
- 45.Hamrick SEG, McQuillen PS, Jiang X, Mu D, Madan A, Ferriero DM. A role for hypoxia-inducible factor-1α in desferoxamine neuroprotection. Neuroscience Letters. 2005;379:96–100. doi: 10.1016/j.neulet.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 46.Freret T, Valable S, Chazalviel L, Saulnier R, Mackenzie ET, Petit E, et al. Delayed administration of deferoxamine reduces brain damage and promotes functional recovery after transient focal cerebral ischemia in the rat. European Journal of Neuroscience. 2006;23:1757–1765. doi: 10.1111/j.1460-9568.2006.04699.x. [DOI] [PubMed] [Google Scholar]
- 47.Guo C, Hao L-J, Yang Z-H, Chai R, Zhang S, Gu Y, et al. Deferoxaminemediated up-regulation of HIF-1α prevents dopaminergic neuronal death via the activation of MAPK family proteins in MPTP-treated mice. Exp. Neurol. 2016;280:13–23. doi: 10.1016/j.expneurol.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Baader E, Tschank G, Baringhaus KH, Burghard H, Günzler V. Inhibition of prolyl 4-hydroxylase by oxalyl amino acid derivatives in vitro, in isolated microsomes and in embryonic chicken tissues. Biochem. J. 1994;300(Pt 2):525–530. doi: 10.1042/bj3000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivan M, Haberberger T, Gervasi DC, Michelson KS, Gunzler V, Kondo K, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proceedings of the National Academy of Sciences. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sen T, Sen N. Treatment with an activator of hypoxia-inducible factor 1, DMOG provides neuroprotection after traumatic brain injury. Neuropharmacology. 2016;107:79–88. doi: 10.1016/j.neuropharm.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 52.Fraisl P, Aragonés J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nature Reviews Drug Discovery. 2009;8:139–152. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 53.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 54.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFαTargeted for VHL-Mediated Destruction by Proline Hydroxylation: Implications for O2 Sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 55.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proceedings of the National Academy of Sciences. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu C-J, Wang L-Y, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Molecular and Cellular Biology. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu C-J, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol. Biol. Cell. 2007;18:4528–4542. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan Q, Bartz S, Mao M, Li L, Kaelin WG. The hypoxia-inducible factor 2alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Molecular and Cellular Biology. 2007;27:2092–2102. doi: 10.1128/MCB.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.d'Anglemont de Tassigny X, Sirerol-Piquer MS, Gómez-Pinedo U, Pardal R, Bonilla S, Capilla-Gonzalez V, et al. Resistance of subventricular neural stem cells to chronic hypoxemia despite structural disorganization of the germinal center and impairment of neuronal and oligodendrocyte survival. Hypoxia (Auckl) 2015;3:15–33. doi: 10.2147/HP.S78248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park TJ, Reznick J, Peterson BL, Blass G, Omerbašić D, Bennett NC, et al. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science. 2017;356:307–311. doi: 10.1126/science.aab3896. [DOI] [PubMed] [Google Scholar]
- 61.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J. Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stacpoole SRL, Webber DJ, Bilican B, Compston A, Chandran S, Franklin RJM. Neural Precursor Cells Cultured at Physiologically Relevant Oxygen Tensions Have a Survival Advantage Following Transplantation. STEM CELLS Translational Medicine. 2013;2:464–472. doi: 10.5966/sctm.2012-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horie N, So K, Moriya T, Kitagawa N, Tsutsumi K, Nagata I, et al. Effects of Oxygen Concentration on the Proliferation and Differentiation of Mouse Neural Stem Cells In Vitro. Cell Mol Neurobiol. 2008;28:833–845. doi: 10.1007/s10571-007-9237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Storch A, Paul G, Csete M, Boehm BO, Carvey PM, Kupsch A, et al. Longterm proliferation and dopaminergic differentiation of human mesencephalic neural precursor cells. Exp. Neurol. 2001;170:317–325. doi: 10.1006/exnr.2001.7706. [DOI] [PubMed] [Google Scholar]
- 66.Ortega JA, Sirois CL, Memi F, Glidden N, Zecevic N. Oxygen Levels Regulate the Development of Human Cortical Radial Glia Cells. Cerebral Cortex. 2016 doi: 10.1093/cercor/bhw194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pistollato F, Chen H-L, Schwartz PH, Basso G, Panchision DM. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol. Cell. Neurosci. 2007;35:424–435. doi: 10.1016/j.mcn.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota Nodari L, Binda E, et al. Mild Hypoxia Enhances Proliferation and Multipotency of Human Neural Stem Cells. PLoS ONE. 2010;5:e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyata T, Kawaguchi D, Kawaguchi A, Gotoh Y. Mechanisms that regulate the number of neurons during mouse neocortical development. Current Opinion in Neurobiology. 2010;20:22–28. doi: 10.1016/j.conb.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Sauvageot C. Molecular mechanisms controlling cortical gliogenesis. Current Opinion in Neurobiology. 2002;12:244–249. doi: 10.1016/S0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 71.Liu S, Tian Z, Yin F, Zhao Q, Fan M. Generation of dopaminergic neurons from human fetal mesencephalic progenitors after co-culture with striatal-conditioned media and exposure to lowered oxygen. Brain Res. Bull. 2009;80:62–68. doi: 10.1016/j.brainresbull.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Giese A-K, Frahm J, Hübner R, Luo J, Wree A, Frech MJ, et al. Erythropoietin and the effect of oxygen during proliferation and differentiation of human neural progenitor cells. BMC Cell Biol. 2010;11:94. doi: 10.1186/1471-2121-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen H-L, Pistollato F, Hoeppner DJ, Ni H-T, McKay RDG, Panchision DM, et al. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007;25:2291–2301. doi: 10.1634/stemcells.2006-0609. [DOI] [PubMed] [Google Scholar]
- 74.Stacpoole SRL, Spitzer S, Bilican B, Compston A, Karadottir R, Chandran S, et al. High Yields of Oligodendrocyte Lineage Cells from Human Embryonic Stem Cells at Physiological Oxygen Tensions for Evaluation of Translational Biology. Stem Cell Reports. 2013;1:437–450. doi: 10.1016/j.stemcr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mutoh T, Sanosaka T, Ito K, Nakashima K. Oxygen levels epigenetically regulate fate switching of neural precursor cells via hypoxia-inducible factor 1α-notch signal interaction in the developing brain. Stem Cells. 2012;30:561–569. doi: 10.1002/stem.1019. [DOI] [PubMed] [Google Scholar]
- 76.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu C-J, et al. HIF- 2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes & Development. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forristal CE, Wright KL, Hanley NA, Oreffo ROC, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chavali PL, Saini RKR, Matsumoto Y, Ågren H, Funa K. Nuclear orphan receptor TLX induces Oct-3/4 for the survival and maintenance of adult hippocampal progenitors upon hypoxia. Journal of Biological Chemistry. 2011;286:9393–9404. doi: 10.1074/jbc.M110.167445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cui X-P, Xing Y, Chen J-M, Dong S-W, Ying D-J, Yew DT. Wnt/betacatenin is involved in the proliferation of hippocampal neural stem cells induced by hypoxia. Ir J Med Sci. 2011;180:387–393. doi: 10.1007/s11845-010-0566-3. [DOI] [PubMed] [Google Scholar]
- 80.Mazumdar J, O'Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, et al. O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol. 2010;12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Varela-Nallar L, Rojas-Abalos M, Abbott AC, Moya EA, Iturriaga R, Inestrosa NC. Chronic hypoxia induces the activation of the Wnt/β-catenin signaling pathway and stimulates hippocampal neurogenesis in wild-type and APPswe-PS1ΔE9 transgenic mice in vivo. Frontiers in Cellular Neuroscience. 2014;817 doi: 10.3389/fncel.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kriska J, Honsa P, Dzamba D, Butenko O, Kolenicova D, Janeckova L, et al. Manipulating Wnt signaling at different subcellular levels affects the fate of neonatal neural stem/progenitor cells. Brain Research. 2016;1651:73–87. doi: 10.1016/j.brainres.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 83.Licht T, Keshet E. Delineating multiple functions of VEGF-A in the adult brain. Cell. Mol. Life Sci. 2013;70:1727–1737. doi: 10.1007/s00018-013-1280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, et al. VEGFoverexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J. Neurosci. Res. 2007;85:740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- 85.Gonzalez FF, Larpthaveesarp A, McQuillen P, Derugin N, Wendland M, Spadafora R, et al. Erythropoietin Increases Neurogenesis and Oligodendrogliosis of Subventricular Zone Precursor Cells After Neonatal Stroke. Stroke. 2013;44:753–758. doi: 10.1161/STROKEAHA.111.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harms KM, Li L, Cunningham LA. Murine Neural Stem/Progenitor Cells Protect Neurons against Ischemia by HIF-1α–Regulated VEGF Signaling. PLoS ONE. 2010;5:e9767. doi: 10.1371/journal.pone.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yasui T, Uezono N, Nakashima H, Noguchi H, Matsuda T, Noda-Andoh T, et al. Hypoxia Epigenetically Confers Astrocytic Differentiation Potential on Human Pluripotent Cell-Derived Neural Precursor Cells. Stem Cell Reports. 2017;8:1743–1756. doi: 10.1016/j.stemcr.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thornton JE, Gregory RI. How does Lin28 let-7 control development and disease? Trends in Cell Biology. 2012;22:474–482. doi: 10.1016/j.tcb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao C, Sun G, Ye P, Li S, Shi Y. MicroRNA let-7d regulates the TLX/microRNA-9 cascade to control neural cell fate and neurogenesis. Sci Rep. 2013;3:1329. doi: 10.1038/srep01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patterson M, Chan DN, Ha I, Case D, Cui Y, Handel BV, et al. Defining the nature of human pluripotent stem cell progeny. Cell Res. 2011;22:178–193. doi: 10.1038/cr.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patterson M, Gaeta X, Loo K, Edwards M, Smale S, Cinkornpumin J, et al. let-7 miRNAs Can Act through Notch to Regulate Human Gliogenesis. Stem Cell Reports. 2014;3:758–773. doi: 10.1016/j.stemcr.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim TS, Misumi S, Jung CG, Masuda T, Isobe Y, Furuyama F, et al. Increase in dopaminergic neurons from mouse embryonic stem cell-derived neural progenitor/stem cells is mediated by hypoxia inducible factor-1α. J. Neurosci. Res. 2008;86:2353–2362. doi: 10.1002/jnr.21687. [DOI] [PubMed] [Google Scholar]