Abstract
Most of the reported mitochondria targeting molecules are lipophilic and cationic, which may become cytotoxic with accumulation. Here we show enzymatic cleavage of branched peptides that carry negative charges for targeting mitochondria. Conjugating a well-established protein tag (i.e., FLAG-tag) to self-assembling motifs affords the precursors that form micelles. Enzymatic cleavage of the hydrophilic FLAG motif (DDDDK) by enterokinase (ENTK) turns the micelles to nanofibers. After being taken up by cells, the micelles, upon the action of intracellular ENTK, turns into nanofibers to locate mainly at mitochondria. The micelles of the precursors are able to deliver cargos (either small molecules or proteins) into cells, largely to mitochondria and within two hours. Preventing ENTK proteolysis diminishes mitochondria targeting. As the first report of using enzymatic self-assembly for targeting mitochondria and delivery cargos to mitochondria, this work illustrates a fundamentally new way to target subcellular organelles for biomedicine.
Graphical abstract
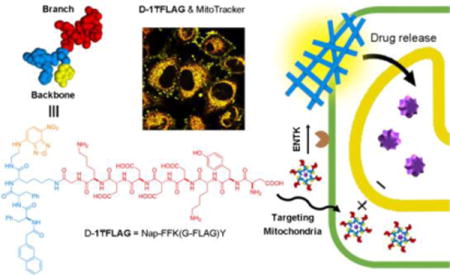
As a subcellular organelle, mitochondria plays essential roles in many cellular processes, such as apoptosis and metabolism.1 Intensive research efforts have focused on developing therapeutics that target mitochondria.2 In fact, some of the therapeutics have already entered clinical trials.3 Except gramicidin S derivatives developed by Wipf et. al.,2a most of the molecules reported for targeting mitochondria are lipophilic and cationic.4 Although those lipophilic cations are able to prevent mitochondrial damage in vitro, they have a major drawback due the toxicity resulted from the accumulation in the mitochondrial matrix.5 Thus, it is necessary to develop new molecules or processes for targeting mitochondria. We have been exploring enzyme-instructed self-assembly (EISA),5 a multistep molecular process that is finding increasing number of applications,6 including selective inhibiting cancer cells.7 During our study on enzymatic supramolecular hydrogelation of branched peptides6, we serendipitously found that EISA of branched peptides is able to target mitochondria.
Here we show enzymatic cleavage of branched peptides that carry negative charges for targeting mitochondria. Conjugating a well-established protein tag (i.e., FLAG-tag)7 to self-assembling motifs8 affords the precursors that are the substrates of enterokinase (ENTK).9 The precursors form micelles, which turn into nanofibers upon enzymatic cleavage of the hydrophilic branch that contains FLAG motif (DDDDK).7 Fluorescent microscopy of live cells reveals that, after being taken up by cells, the branched peptides and their enzyme cleaved products, mainly localize at mitochondria. Moreover, the micelles of the precursors are able to deliver cargos (either small molecules (e.g., doxorubicin (Dox)) or proteins (e.g., red phycoerythrin (RPE)10)) into cells, largely to mitochondria and within two hours. Western blot indicates ENTK on the isolated mitochondria of cancer cells. Transmission electron microscopy (TEM) of the isolated mitochondria confirms the conversion of the micelles to nanofibers at the mitochondria. Preventing ENTK proteolysis diminishes the mitochondria targeting. These results confirm EISA as the process for targeting mitochondria. As the first report of using EISA for targeting mitochondria and delivery molecular cargos to mitochondria (Scheme 1), this work illustrates a fundamentally new molecular motif and process for targeting mitochondria and exploring the applications of protease-instructed assembly for biomedicine.
Scheme 1.
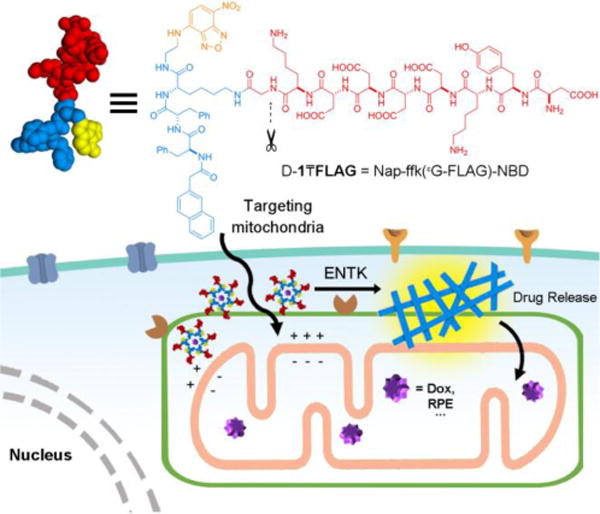
Structure of a representative branched peptide and ENTK cleaving the branch to convert micelles to nanofibers on mitochondria.
Scheme 1 shows the structure of the designed substrate of ENTK. We use the symbol “₸” to represent the branching so that the substrate is denoted as D-1₸FLAG. The branched peptides consist of (i) the FLAG-tag (DYKDDDDK)7 as the substrate of ENTK for enzymatic recognition and cleavage, (ii) a self-assembling peptide sequence D-1 composed of a 2-acetylnaphthyl group (Nap), a D-tripeptide (D-Phe-D-Phe-D-Lys (ffk)), and a nitrobenzoxadiazoly-ethylenediamino moiety (NBD-EA, for enhanced fluorescence upon self-assembly11) at the C-terminal of the D-peptide, and (iii) a glycine as the spacer for (i) and (ii). Using Fmoc-based solid-phase peptide synthesis,12 we first produce the peptide segments (i.e., FLAG-Gly and the Nap-ffk, Scheme S1). After the synthesis, we generate D-1 by conjugating NBD-EA the Nap-ffk via C-terminal activation. Subsequently, we connect the C-terminal of FLAG-Gly to the side chain of lysine in D-1 using the same activation method. Finally, the removal of all protecting groups and the purification by high pressure liquid chromatography (HPLC) produce the designed branch peptide (i.e., D-1₸FLAG).
D-1₸FLAG forms nanoparticles (46±11 nm in diameter) at 200 μM (critical micelle concentration (CMC), Fig. S1), which become nanofibers (18±3 nm in diameter) upon the addition of ENTK (Figure 1A, S2 and S3). Circular dichroism (CD) measurement indicates the cleavage of the branch results in largely β-sheet (D-1) and random-coil (FLAG-tag) like secondary structures for the case of D-1₸FLAG (Fig. S4), as well as for those of D- and L-2₸FLAGs.6 After the incubation of HeLa or U87MG cells with D-1₸FLAG for two hours, fluorescent imaging (Figure 1B) of these the cells exhibits strong fluorescence, indicating a rapid cellular uptake of D-1₸FLAG. Our prelimilary endocytosis study suggests that the uptake of the precursors by HeLa cells likely involves macropinocytosis and clathrin-dependent pathway (Fig. S5). Cell viability assay reveals that D-1₸FLAG exhibits little cytotoxicity to HeLa and U87MG cells (Fig. S6), excluding the possibility that the rapid internalization of D-1₸FLAG originates from the increased membrane permeability caused by cell death.13
Figure 1.
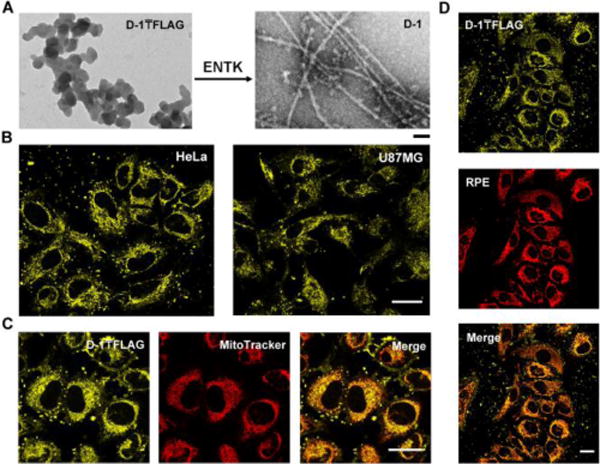
(A) TEM images of D-1₸FLAG before (left) and after (right) adding ENTK (24 h), scale bar = 100 nm. (B) Fluorescent images of HeLa and U87MG cells incubated with D-1₸FLAG for 2 h. (C) The fluorescence images of D-1₸FLAG and MitoTracker in HeLa cells. (D) HeLa cells treated by the mixture of RPE (1 μg/mL) and D-1₸FLAG for 2 h. Scale bar = 30 μm in (B, C). The concentrations of D-1₸FLAG are 200 μM for A-D.
The pattern of the distribution of D-1₸FLAG in cytosol resembles to that of mitochondria (Figures 1B and S7). Co-staining D-1₸FLAG with MitoTracker or Lyso-tracker in HeLa cells shows that the yellow fluorescence from D-1₸FLAG mostly overlaps with the red fluorescence of MitoTracker (Figure 1C), but co-localizes poorly with the fluorescence from Lyso-tracker (Fig. S8). These results suggest that D-1₸FLAG, escaping lysosomes after endocytosis, specifically localizes in mitochondria. However, HeLa cells treated by D-1 exhibit fluorescence both outside the cells (including membrane) and in cytosol nonspecifically (Fig. S9). Thus, we infer that the mitochondria-targeting ability of D-1₸FLAG likely depends on its unique branching architecture, as well as the electrostatic interaction between the FLAG-tag moiety (negative potential, due to carboxylic groups) and the intermembrane space of mitochondria (positive potential, resulted from abundant H+),14 as illustrated in Scheme 1. Notably, D-1₸FLAG appears to accumulate in the lysosomes of a normal cell line (HS-5) (Fig. S10). Such cell selectivity worth further investigation. Moreover, after mixing R-phycoerythrin (RPE), a protein, with D-1₸FLAG, we incubate them with HeLa cells for two hours. While the control cells (without micelles) show little fluorescence (Fig. S11), RPE and D-1₸FLAG localize in the mitochondria (Figure 1D). TEM of the mixture of RPE and D-1₸FLAG support the encapsulation of RPE by the micelles (Fig. S12). These results indicate that the micelles formed by D-1₸FLAG are able to deliver cargo molecules to mitochondria.
Mitochondria targeting of D-1₸FLAG implies that the micelles formed by other FLAG-tagged precursors made of branched peptides would localize and deliver cargo molecules to mitochondria. Thus, we examine whether precursors D-2₸FLAG (an analog of D-1₸FLAG without NBD, Scheme S2) and L-2₸FLAG (a diastereomer of D-2₸FLAG) are able to deliver cargo molecules to mitochondria. The similar CMC values of D-1₸FLAG and D-2₸FLAG (Fig. S1) support the interchangeability between them. After mixing RPE, a protein, or doxorubicin (Dox), a drug molecule, with L-2₸FLAG or D-2₸FLAG, we incubate them with HeLa or U87MG cells for two hours. While the control cells (without the micelles formed by the precursors) show little fluorescence (Fig. S11), the cells incubated with the micelles and the cargo molecules exhibit strong fluorescence (Figure 2A and B), indicating that the intracellular delivery of RPE and Dox by L-2₸FLAG or D-2₸FLAG. Similar to D-1₸FLAG, the patterns of the distribution of RPE (or Dox) inside the cells are akin to that of mitochondria.
Figure 2.
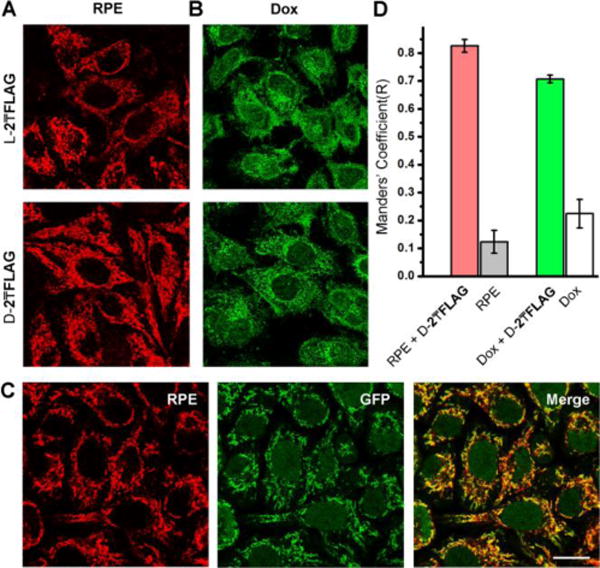
(A, B) HeLa cells incubated with (A) RPE (1 μg/mL) and (B) Dox (2 μM) mixing with D-2₸FLAG and L-2₸FLAG (200 μM) for 2 h. (C) GFP-cyt c HeLa cells treated by the mixture of RPE (1 μg/ml) and D-2₸FLAG (200 μM) for 2 h. Scale bar = 30 μm. (D) Co-localization analysis of GFP-cyt c and Omi-mcherry expressing HeLa cells treated by RPE or Dox in the presence of D-2₸FLAG. R=1 indicates perfect co-localization.
To further verify whether the FLAG-tagged precursors deliver cargo molecules to mitochondria specifically, we incubate the HeLa cells expressing GFP-Cyt c15 or Omi-mcherry16 (in mitochondria) with RPE or Dox in the presence of D-2₸FLAG. Fluorescent images confirm that the fluorescence of RPE or Dox extensively overlaps with that from GFP-cyt c and Omi-mcherry (Figures 2C and S13). Quantification of the overlap of the fluorescence17 confirm that RPE or Dox alone hardly co-localize with mitochondria, but D-2₸FLAG significantly boost the localization of RPE or Dox in mitochondria (Figure 2D). Moreover, Dox, after mixing with D-2₸FLAG or L-2₸FLAG, exhibits significantly increased cytotoxicity against HeLa cells compared to Dox only. That is, the branched peptide precursors decrease the IC50 of Dox from 3.0 μM to 400 nM (Fig. S14D). In addition, D-2₸FLAG significantly boosts the activity of Dox against Dox-resistant cancer cell line (MESA/Dx5,18 Fig. S14D). The synergistic effect (Fig. S14E) of Dox and the FLAG-tagged precursors against cancer cells likely originates from that the micelles enhance the uptake of Dox and Dox interfering with mitochondrial DNA. These results confirm that the generality of the FLAG-tagged branch peptide precursors serve as carriers for delivering cargo molecules to mitochondria.
To confirm that the nanofibers form at mitochondria after ENTK cleaves the branch of the branched peptides, we isolated mitochondria from HeLa cells incubated with D-2₸FLAG (200 μM) for different time (Figure 3 and S15). The cells treated by D-2₸FLAG for 2 h and 24 h produce mitochondria surrounded by nanoparticles and nanofibers, respectively (Figure 3), while the mitochondria from the HeLa cells incubated with only culture medium exhibit a surface without either nanoparticles or nanofibers (Fig. S15). Western blot analysis of the mitochondria isolated from HeLa and U87MG cells showed two ENTK bands (Fig. S16, the major band is the light chain19), suggesting the presence of ENTK on mitochondria. Moreover, LC/MS analysis of the isolated mitochondria confirmed that D-2 is the main component of the nanofibers (Fig. S17), indicating that D-2₸FLAG, after reaching mitochondria, is cleaved by ENTK. The in-situ formation of nanofibers results in the localization of the nanofibers at mitochondria. To verify whether the proteolysis is necessary for mitochondria targeting, we made the D-peptide control (D-1₸flag) by connecting D-1 to the D-enantiomer of FLAG tag (flag), which is resistant to ENTK. The fluorescent images of HeLa cells incubated with D-1₸flag (200 μM, 2h) failed to exhibit a mitochondria-specific distribution (Fig. S18), suggesting that the prevention of proteolysis (the conversion to nanofibers) catalyzed by ENTK abolishes the mitochondria targeting.
Figure 3.
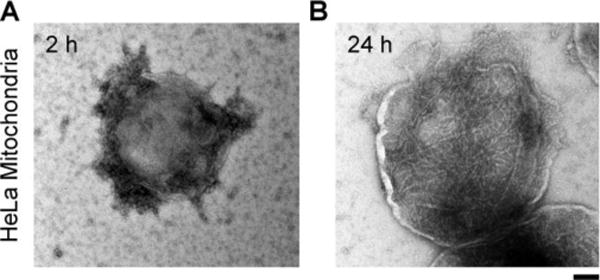
TEM images of mitochondria isolated from HeLa cells being incubated with D-2₸FLAG (200 μM) for (A) 2h and (B) 24 h. Scale bar equals 100 nm.
In conclusion, we demonstrate the branched peptides as a novel type of substrates for enzymatic self-assembly to target mitochondria. Although carrying multiple negative charges, the FLAG-tagged precursors undergo rapid endocytosis and specifically accumulate in mitochondria. The deposition of the nanofibers affect little on the cellular activity, likely due to that the nanofibers impact less than cationic molecules on the membrane potential of mitochondria. The branch architecture is uniquely important for targeting mitochondria since the linear peptide, L-2-FLAG, is unable to deliver cargo molecule to mitochondria (Fig. S19). This work highlights the importance of reactions, in addition to nanoarchitectonics,20 for controlling biological systems. Contrasting to most of the reported mitochondria targeting molecules that usually are lipophilic and cationic,7 this observation illustrates a new mechanism, the integration of enzymatic reaction and self-assembly (i.e., EISA), for targeting mitochondria, which would complement other approaches (e.g., triphenyl phosphonium (TPP)21). Although the targeting of mitochondria relies on ENTK in this work, the strategy demonstrated here should allow the development of the substrate of other enzymes on mitochondria for mitochondria targeting. Among numerous approaches for mitochondria-specific drug delivery,22 the delivery of cargo molecules to target mitochondria by the FLAG-tagged precursors is particularly promising and worth further exploration for potential applications in biomedicine because of the cell compatibility of the FLAG-tagged precursors (Fig. S9A) and the biological importance of mitochondria.23
Supplementary Material
Acknowledgments
This work was partially supported by NIH (R01CA142746) and NSF (MRSEC-1420382).
Footnotes
This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interests.
References
- 1.(a) Green DR, Kroemer G. Science. 2004;305(5684):626. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]; (b) McBride HM, Neuspiel M, Wasiak S. Curr Biol. 2006;16(14):R551. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]; (c) Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Science. 2005;309(5733):481. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]; (d) Balaban RS, Nemoto S, Finkel T. Cell. 2005;120(4):483. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.(a) Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE. Acc Chem Res. 2008;41(1):87. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]; (b) Murphy MP, Smith RAJ. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annual Review of Pharmacology and Toxicology. 2007;47:629. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]; (c) Yousif LF, Stewart KM, Kelley SO. Chembiochem. 2009;10(12):1939. doi: 10.1002/cbic.200900185. [DOI] [PubMed] [Google Scholar]
- 3.(a) Thomas DA, Stauffer C, Zhao K, Yang H, Sharma VK, Szeto HH, Suthanthiran M. J Am Soc Nephrol. 2007;18(1):213. doi: 10.1681/ASN.2006080825. [DOI] [PubMed] [Google Scholar]; (b) Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. J Am Soc Nephrol. 2013;24(8):1250. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Trachootham D, Alexandre J, Huang P. Nat Rev Drug Discov. 2009;8(7):579. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 4.(a) Yousif LF, Stewart KM, Horton KL, Kelley SO. Chembiochem. 2009;10(12):2081. doi: 10.1002/cbic.200900017. [DOI] [PubMed] [Google Scholar]; (b) Mahon KP, Potocky TB, Blair D, Roy MD, Stewart KM, Chiles TC, Kelley SO. Chem & Bio. 2007;14(8):923. doi: 10.1016/j.chembiol.2007.07.011. [DOI] [PubMed] [Google Scholar]; (c) Zhao KS, Luo GX, Giannelli S, Szeto HH. Biochem Pharmacol. 2005;70(12):1796. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]; (d) Smith RAJ, Porteous CM, Coulter CV, Murphy MP. Eur J Biochem. 1999;263(3):709. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]; (e) Murphy MP, Smith RA. Annu Rev Pharmacol Toxicol. 2007;47:629. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]; (f) Murphy MP. Trends Biotechnol. 1997;15(8):326. doi: 10.1016/S0167-7799(97)01068-8. [DOI] [PubMed] [Google Scholar]; (g) Hurt EC, Muller U, Schatz G. Embo Journal. 1985;4(13A):3509. doi: 10.1002/j.1460-2075.1985.tb04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Horton KL, Stewart KM, Fonseca SB, Guo Q, Kelley SO. Chem Biol. 2008;15(4):375. doi: 10.1016/j.chembiol.2008.03.015. [DOI] [PubMed] [Google Scholar]; (i) Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE. Acc Chem Res. 2008;41(1):87. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MP. Trends Biotechnol. 1997;15(8):326. doi: 10.1016/S0167-7799(97)01068-8. [DOI] [PubMed] [Google Scholar]
- 6.He H, Wang H, Zhou N, Yang D, Xu B. Chem Commun. 2018;54:86. doi: 10.1039/c7cc08421h. [DOI] [PubMed] [Google Scholar]
- 7.Hopp TP, Prickett KS, Price VL, Libby RT, March CJ, Cerretti DP, Urdal DL, Conlon PJ. Nat Biotechnol. 1988;6(10):1204. [Google Scholar]
- 8.(a) Reches M, Gazit E. Science. 2003;300(5619):625. doi: 10.1126/science.1082387. [DOI] [PubMed] [Google Scholar]; (b) Burattini S, Greenland BW, Merino DH, Weng W, Seppala J, Colquhoun HM, Hayes W, Mackay ME, Hamley IW, Rowan SJ. J Am Chem Soc. 2010;132(34):12051. doi: 10.1021/ja104446r. [DOI] [PubMed] [Google Scholar]; (c) Zhang Y, Kuang Y, Gao Y, Xu B. Langmuir. 2010;27(2):529. doi: 10.1021/la1020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavlov IP, Thompson WH. The work of the digestive glands. Charles Griffin; 1902. [Google Scholar]
- 10.Teale F, Dale R. Biochem J. 1970;116(2):161. doi: 10.1042/bj1160161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y, Shi J, Yuan D, Xu B. Nat Commun. 2012;3:1033. doi: 10.1038/ncomms2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan WC, White PD. Fmoc solid phase peptide synthesis. Oxford University Press; 2000. [Google Scholar]
- 13.Elmore S. Toxicol Pathol. 2007;35(4):495. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wikstrom MK. Nature. 1977;266(5599):271. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. Nat Cell Biol. 2000;2(3):156. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- 16.Tait SW, Parsons MJ, Llambi F, Bouchier-Hayes L, Connell S, Muñoz-Pinedo C, Green DR. Dev Cell. 2010;18(5):802. doi: 10.1016/j.devcel.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manders E, Verbeek F, Aten J. J Microsc. 1993;169(3):375. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 18.Harker WG, Sikic BI. Cancer Res. 1985;45(9):4091. [PubMed] [Google Scholar]
- 19.Lu D, Fütterer K, Korolev S, Zheng X, Tan K, Waksman G, Sadler JE. J Mol Biol. 1999;292(2):361. doi: 10.1006/jmbi.1999.3089. [DOI] [PubMed] [Google Scholar]
- 20.Komiyama M, Yoshimoto K, Sisido M, Ariga K. Bull Chem Soc Jpn. 2017;90(9):967. [Google Scholar]
- 21.Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez-Vivar J, Cheng G, Lopez M, Kalyanaraman B. Chem Rev. 2017;117(15):10043. doi: 10.1021/acs.chemrev.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Yamada Y, Akita H, Kogure K, Kamiya H, Harashima H. Mitochondrion. 2007;7(1–20):63. doi: 10.1016/j.mito.2006.12.003. [DOI] [PubMed] [Google Scholar]; (b) Schatz G. J Biol Chem. 1996;271(50):31763. doi: 10.1074/jbc.271.50.31763. [DOI] [PubMed] [Google Scholar]
- 23.Green DR, Galluzzi L, Kroemer G. Science. 2014;345(6203):1466. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


