Abstract
Background
We described reasons for switching to second-line antiretroviral treatment (ART) and time to switch in HIV-infected children failing first-line ART in West Africa.
Methods
We included all children aged 15 years or less, starting ART (at least three drugs) in the paediatric IeDEA clinical centres in five West-African countries. We estimated the incidence of switch (at least one a drug class change) within 24 months of ART and associated factors were identified in a multinomial logistic regression. Among children with clinical-immunological failure, we estimated the 24-month probability of switching to a second-line and associated factors, using competing risks. Children who switched to second-line ART following the withdrawal of nelfinavir in 2007 were excluded.
Results
Overall, 2820 children initiated ART at a median age of 5 years; 144 (5%) were on nelfinavir. At 24-month post-ART initiation, 188 (7%) had switched to second-line. The most frequent reasons were drug stock outs (20%), toxicity (18%), treatment failure (16%) and poor adherence (8%). Over the 24-month follow-up period, 322 (12%) children failed first-line ART after a median time of 7 months. Of these children, 21 (7%) switched to second-line after a median time of 21 weeks in failure. This was associated with older age [subdistribution hazard ratio (sHR) 1.21, 95% confidence interval (95% CI) 1.10–1.33] and longer time on ART (sHR 1.16, 95% CI 1.07–1.25).
Conclusion
Switches for clinical failure were rare and switches after an immunological failure were insufficient. These gaps reveal that it is crucial to advocate for both sustainable access to first-line and alternative regimens to provide adequate roll-out of paediatric ART programmes.
Keywords: Africa, first-line antiretroviral therapy, HIV, paediatrics, switch to second-line antiretroviral therapy
Introduction
According to the WHO, in 2012, more than 3 million children were living with HIV worldwide, 90% of whom live in sub-Saharan Africa [1]. Several studies have shown that early initiation of antiretroviral therapy (ART) irrespective of clinical, immunological or virological criteria leads to significant reductions in mortality and decreases disease progression [2]. International guidelines have been adjusted accordingly, and in 2010, early ART initiation was recommended to all HIV-infected children aged less than 2 years [3]. In 2013, the WHO consolidated guidelines recommended that ART should be started in all children in low and middle-income countries under 5 years of age [4].
However, ART is a life-long therapy and children starting ART in early life will need to continue receiving treatment into adulthood. The efficacy of first-line ART has been reported in an earlier meta-analysis, in which the pooled estimate for viral suppression was 70% by 12 months [5]. Despite this relative success, children are failing treatment and needing second-line regimens [6,7]. Paediatric treatment options are limited for many reasons, including the limited drug approval for children, lack of affordable paediatric formulations and lack of appropriate fixed-dose combination formulations [8], often resulting in children remaining on first-line ART, despite failure [9].
Data on the durability of first-line ART are limited and reasons for switching ART regimen are not always documented. In this context of limited treatment options, it is important to understand the reasons for switching to second-line treatment and to document the switch to second-line ART in children in failure. The main objective of this study was first to describe the reasons for switching to second-line ART within 24 months post-ART initiation in a large paediatric cohort in West Africa, pWADA. Second, among those in ART-failure, we described the time to and predictors for switching to second-line ART.
Materials and methods
Study population and design
The IeDEA paediatric (pWADA) West African Database to evaluate AIDS is aimed to address evolving research questions in the field of HIV/AIDS care and treatment using data from multicentric HIV/AIDS adult and child cohorts in West Africa [10,11]. This collaboration, initiated in July 2006, currently involves 11 paediatric HIV/AIDS clinics across seven West African countries. HIV-infected children are typically seen in these clinics at least every 3 months. For this study, five countries contributed: Burkina Faso, Côte d’Ivoire, Ghana, Mali and Senegal. All children aged 15 years or less, initiating ART (at least three drugs) were included in the study. ART initiation was based on the international guidelines at that time [3,12]. Patient data were collected retrospectively using a standardized data collection instrument. The following data were recorded: date of birth, sex, weight, height, WHO clinical staging, CD4+ cell counts, date of ART initiation, ART regimen, date and reason of ART switch and date of treatment failure.
Switching to second-line ART was defined as any switch from three nucleoside reverse transcriptase inhibitors (NRTIs) to two NRTIs and one protease inhibitor or two NRTIs and one nonnucleoside reverse transcriptase inhibitor (NNRTI) or from two NRTIs and one protease inhibitor to two NRTIs and one NNRTI or three NRTIs or from two NRTIs and one NNRTI to three NRTIs or two NRTIs and one protease inhibitor.
Treatment failure was clinical, immunological or both: clinical failure was defined as the appearance or reappearance of a WHO clinical stage 3 or 4 event after at least 24 weeks on ART in a treatment-adherent child (or the appearance or reappearance of CDC clinical stage 3 if WHO clinical stage was unknown). Immunological failure was defined as reaching or returning to a CD4+ percentage below less than 20% in children aged less than 12–35 months and CD4+ percentage less than 15% in children aged 36–59 months, or reaching or returning to a CD4+ cell count below 100 cells/μl for observant children aged more than 5 years, after the first 24 weeks of therapy. Date of treatment failure was considered as the date of visit (or CD4+ measurement if immunological failure). Severe anaemia was defined as haemoglobin concentration of less than 7 g/dl at ART initiation. Children with severe anaemia who had switched to second-line with no other documented reason were considered to have switched because of toxicity.
Children were followed-up from ART initiation until date of first-switch or censorship. Follow-up was censored at the earliest of the following: last clinical contact or transfer out or death. A patient was lost to follow-up if he was not known to have died or to be transferred out and he had at least 6 months of additional potential follow-up between his last visit registered in the database and the closing date of the database.
Statistical analysis
Baseline characteristics were described according to switching to second-line treatment or not, and whether the switch was linked to the withdrawal of nelfinavir (NFV). Indeed, during 2007, this commonly used protease inhibitor was taken off the market and many children switched to an NNRTI-based ART regimen as a consequence. Continuous variables were compared using the Wilcoxon rank-sum test, and comparisons between two categorical variables were performed using the chi-square test and Fisher’s exact test where appropriate.
In the following analyses, we excluded all switches linked to the withdrawal of NFV. Factors associated with different reasons for switching to second-line treatment were explored in multinomial logistic regression; the reference reason was no switch at all.
Among children failing treatment, time to switch and associated factors were investigated using competing risk survival analyses. Children failing treatment are more likely to die before having the opportunity to initiate second-line ART. We used cumulative incidence functions to estimate both the probability for death and switch in these children. To identify associated factors with each of the outcomes (switch and death), we ran a multivariate competing risks analysis, performed by the Fine and Gray proportional subdistribution hazard regression model.
Results
Baseline characteristics and follow-up
Baseline characteristics according to switch are described in Table 1. Between June 2000 and December 2009, 2820 children initiated ART at a median age of 5 years [interquartile range (IQR) 2–9]. Fifty-five percent were boys and the median CD4+ percentage at ART initiation was 13% (IQR 7–19) and 4.4% with severe anaemia. The most frequently prescribed first-line regimen was two NRTIs and one NNRTI (70.9%). Overall after 24 months on ART, the rate of switch to second-line ART was 12%; this was reduced to 7% when excluding those who switch because of NFV withdrawal.
Table 1.
Baseline characteristics of antiretroviral therapy initiation and switch during the follow-up according to the withdrawal of nelfinavir, n = 2820.
| Switch linked to NFV withdrawal | Switch not linked to NFV withdrawal | No switch | Total | P | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| n (%) | 144 (5.1) | 188 (6.7) | 2488 (88.2) | 2820 (100.0) | 0.05 |
| Boy, n (%) | 67 (46.5) | 97 (51.6) | 1394 (56.0) | 1558 (55.2) | |
| Median age, (IQR) | 3 (2–6) | 4 (2–9) | 5 (3–9) | 5 (2–9) | <0.01 |
| First-line regimens, n (%) | <0.01 | ||||
| 2 NRTIs and 1 NNRTI | 0 (0.0) | 84 (44.7) | 1914 (76.9) | 1998 (70.9) | |
| 2 NRTIs and one PI | 144 (100.0) | 100 (53.2) | 542 (21.8) | 786 (27.9) | |
| 3 NRTIs | 0 (0.0) | 4 (2.1) | 32 (1.3) | 36 (1.3) | |
| Median CD4+ cell count (cells/μl), (IQR) | 496 (315–867) | 310 (71–578) | 393 (150–710) | 388 (148–710) | <0.01 |
| Median CD4+%, (IQR) | 15 (10–22) | 11 (4–16) | 13 (7–19) | 13 (7–19) | <0.01 |
| Immunodeficiency according to agea | <0.01 | ||||
| Severe | 71 (49.3) | 88 (46.8) | 736 (29.6) | 895 (31.7) | |
| Moderate | 21 (14.6) | 30 (16.0) | 408 (16.4) | 459 (16.3) | |
| No immunodeficiency | 15 (10.4) | 7 (3.7) | 234 (9.4) | 256 (9.1) | |
| Missing | 37 (25.7) | 63 (33.5) | 1110 (44.6) | 1210 (42.9) | |
| Severe anaemia, n (%) | <0.01 | ||||
| No | 117 (81.3) | 129 (68.6) | 1653 (66.4) | 1899 (67.3) | |
| Yes | 5 (3.5) | 12 (6.4) | 108 (4.3) | 125 (4.4) | |
| Missing | 22 (15.3) | 47 (25.0) | 727 (29.2) | 796 (28.2) | |
| Failureb during ART initiation | |||||
| Response to treatment, n (%) | <0.01 | ||||
| Clinical failure alone | 0 (0.0) | 2 (1.1) | 203 (8.2) | 205 (7.3) | |
| Immunological failure alone | 4 (2.8) | 19 (10.1) | 77 (3.1) | 100 (3.5) | |
| Clinical failure and immunological failure | 0 (0.0) | 0 (0.0) | 21 (0.8) | 21 (0.7) | |
| No clinical failure and no immunological failure | 135 (93.8) | 160 (85.1) | 1621 (65.2) | 1916 (67.9) | |
| Missing data | 5 (3.5) | 7 (3.7) | 566 (22.7) | 578 (20.5) |
ART, antiretroviral therapy; NFV, nelfinavir; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Using the 2006 WHO definition.
According to definitions in Materials and methods section.
Children who initiated an NFV-based ART regimen were younger (median age: 3 years, IQR 2–6 years, P < 0.01) and sicker at ART initiation. Indeed, 50% of these children were severely immunodeficient for age at ART initiation, compared with 47% in those who switched for other reasons than NFV withdrawal and 29.6% in those who did not switch at all (P < 0.01). Within the first 24 months on ART, 179 (6.3%) children died, 16 (0.6%) were transferred out and 736 (26.1%) were lost to follow-up (Table 2).
Table 2.
Outcomes at 24-month postantiretroviral therapy initiation according to first-line antiretroviral therapy regimen, n = 2820.
| Switch linked to the NFV withdrawal | Switch no linked to the NFV withdrawal | No switch | Total | |
|---|---|---|---|---|
| n (%) | 144 (5.1) | 188 (6.7) | 2488 (88.2) | 2820 (100.0) |
| Death during 24-month post-ART, n (%) | 2 (1.4) | 12 (6.4) | 165 (6.6) | 179 (6.3) |
| Transfer out during 24-month post-ART | 3 (2.1) | 1 (0.5) | 12 (0.5) | 16 (0.6) |
| LTFU during 24-month post-ARTa | 4 (2.8) | 30 (16.0) | 702 (28.2) | 736 (26.1) |
ART, antiretroviral therapy; LTFU, lost to follow-up; NFV, nelfinavir.
At least 6 months of additional potential follow-up between his last visit registered in the database and the closing date of the database.
Switching to second-line antiretroviral therapy
Overall, 332 children switched to second-line ART within 24 months on ART at after a median time of 9 months (IQR 3 – 17): 144 had initiated an NFV-based ART regimen of whom 142 (99%) switched to two NRTIs and one NNRTI following the withdrawal of the drug. The two remaining children switched to three NRTI-based ART.
The 188 remaining children who switched to second-line ART for reasons other than NFV withdrawal switched after a median time on ART of 7 months (IQR 2–14). Eighty-four of these children (44%) were on two NRTIs and one NNRTI of whom 74 (88%) switched to protease inhibitor-based ART and 10 (12%) to three NRTIs. One hundred children (52%) had initiated protease inhibitor-based first-line ART other than NFV of whom 96% switched to two NRTIs and one NNRTI and 4% to three NRTIs. Four children were initially on three NRTIs, one switched to an NNRTI-based second-line ART regimen and three to a protease inhibitor-based ART regimen.
According to the survey, of the 188 children who switched to second-line ART independently of the NFV withdrawal, 30 children (16%) switched because of first-line treatment failure. Other reasons for switching to second-line ART included toxicity (21%), lack of drug availability (20%), poor compliance (7%) and comorbidities (4%); the remaining 32% remained unspecified.
Table 3 presents the reported causes of switch to second-line ART not linked to NFV withdrawal. Adjusted for immunodeficiency at ART initiation, year of ART initiation, type of facility and cost of first and second-line ART regimen borne by patient families, children who initiated a protease inhibitor-based first-line ART regimen or who were on three NRTIs were more likely to switch to second-line for comorbidity and toxicity than remaining on first-line ART than those on two NRTIs and one 1 NNRTI [odds ratio (OR) 4.82, 95% confidence interval (95% CI) 2.47–9.42]. These children were also 10 times more likely to switch because of lack of ART availability than remaining on first-line ART than those on two NRTIs and one NNRTI (OR 10.61, 95% CI 4.81–23.41). Switching to second-line ART because of treatment failure or poor compliance was not significantly associated with first-line ART regimen. However, children who initiated ART after 2007 were less likely to switch to second-line treatment following failure or poor compliance (versus staying on first-line ART) compared with those who initiated ART earlier (OR 0.20, 95% CI 0.04–0.95), adjusted for other variables. Children who were severely immunodeficient at ART initiation were more than twice as likely to switch to second line because of treatment failure/poor compliance or lack of ART availability rather than remaining on first-line ART than those who showed no signs of immunodeficiency (OR 2.51, 95% CI 1.21–5.21 and OR 2.34, 95% CI 1.13–4.82, respectively).
Table 3.
Multinomial logistic regression, multivariate analyses: correlates of causes of switch from first-line antiretroviral therapy to second-line antiretroviral therapy (excluding switches linked to the withdrawal of nelfinavir, n = 188), in children (n = 2676).
| Multivariate analyses – OR (95% CI)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Comorbidity and toxicity/intolerance to antiretroviral | Failure and poor compliance | Stock-out | Unspecified | P | ||||
| ART regimen | <0.01 | ||||||||
| With NNRTI | Ref. | – | Ref. | – | Ref. | – | Ref. | – | |
| With PI or 3 NRTIs | 4.82 | (2.47–9.42) | 1.33 | (0.68–2.60) | 10.61 | (4.81–23.41) | 3.85 | (2.12–6.98) | |
| Year of ART initiation | 0.01 | ||||||||
| ≤2004 | Ref. | – | Ref. | – | Ref. | – | Ref. | – | |
| 2005–2006 | 2.75 | (1.26–6.04) | 1.48 | (0.73–3.02) | 1.46 | (0.70–3.05) | 1.04 | (0.56–1.93) | |
| ≥2007 | 1.57 | (0.53–4.68) | 0.20 | (0.04–0.95) | 0.51 | (0.13–2.02) | 0.58 | (0.23–1.43) | |
| Immunodeficiency according to agea at ART initiation | 0.03 | ||||||||
| No/Missing | Ref. | – | Ref. | – | Ref. | – | Ref. | – | |
| Severe | 1.23 | (0.61–2.46 | 2.50 | (1.21–5.19) | 2.34 | (1.13–4.82) | 1.97 | (1.10–3.53) | |
| Moderate | 1.35 | (0.59–3.14) | 1.33 | (0.51–3.43) | 1.40 | (0.49–3.98) | 1.18 | (0.53–2.60) | |
| Type of facility | <0.01 | ||||||||
| Public | Ref. | – | Ref. | – | Ref. | – | Ref. | – | |
| Private | 2.36 | (1.17–4.77) | 1.55 | (0.69–3.50) | 0.31 | (0.13–0.76) | 0.85 | (0.42–1.72) | |
| Pay first-line ART | 0.03 | ||||||||
| Free | Ref. | – | Ref. | – | Ref. | – | Ref. | – | |
| Partial pay | 0.78 | (0.20–3.05) | 0.08 | (0.01–0.66) | 5.14 | (1.14–23.07) | 1.21 | (0.48–3.04) | |
| Pay second-line ART | <0.01 | ||||||||
| Free | Ref. | – | Ref. | – | Ref. | – | Ref. | – | |
| Partial pay/Unknown | 0.50 | (0.18–1.34) | 1.46 | (0.68–3.10) | 0.13 | (0.04–0.39) | 1.06 | (0.55–2.04) | |
Reference class: no ART switch. CI, confidence interval; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; OR, odds ratio; PI, protease inhibitor.
Using the 2006 WHO definitions.
We also observed associations with the type of facility and whether ART was free or only subsidized. Indeed, switching to second-line ART because of comorbidity (versus no switch) was more frequent in private facilities than public facilities (OR 2.36, 95% CI 1.17–4.77). On the contrary, switching to second line in the case of lack of ART availability (versus no switch) was less likely in those private facilities compared with public facilities (OR 0.31, 95% CI 0.13–0.76) and was also less likely if the costs of second-line ART were only subsidized rather than being free (OR 0.13, 95% CI 0.04–0.39). Furthermore, children who switch to second-line ART (versus no switch) because of failure or poor compliance were less likely to switch if their families were paying part of first line ART compared with free roll-out (OR 0.08, 95% CI 0.01–0.66).
Time to and predictors to switch to second-line antiretroviral therapy after treatment failure
Of the 2676 who initiated a first-line ART regimen that was not based on NFV, 322 (12%) were in treatment failure within the first 24 months of treatment according to our definitions. The median time to ART failure was 7 months (IQR 6–10).
Among the children in treatment failure, 205 (64%) had clinical failure alone, 96 (30%) had immunological failure alone and 21 (6%) had both clinical and immunological failure.
Twenty-one children (6%) switched to second-line ART after a median time of 21 weeks in failure. The probability of switching to second-line ART was low, with an estimated cumulative incidence of 7% (95% CI 4.4–10.7) by 96 weeks in failure. Twelve children died before switching to second-line ART, after a median time of 44 weeks. The overall cumulative mortality reached 4% (95% CI 2.1–7.2) by 96 weeks in failure (Fig. 1). Associations between baseline characteristics and switching to second-line treatment or death are described in Table 4. Switching to second-line ART was associated with older age [subdistribution hazard ratio (sHR) 1.21, 95% CI 1.09–1.33] and longer time on ART before failure (sHR 1.11, 95% CI 1.01–1.22). In this analysis, no factors were identified among age, sex, ART regimen, baseline clinical staging, type of failure and duration on ART until failure as being associated with the competing event, death.
Fig. 1.
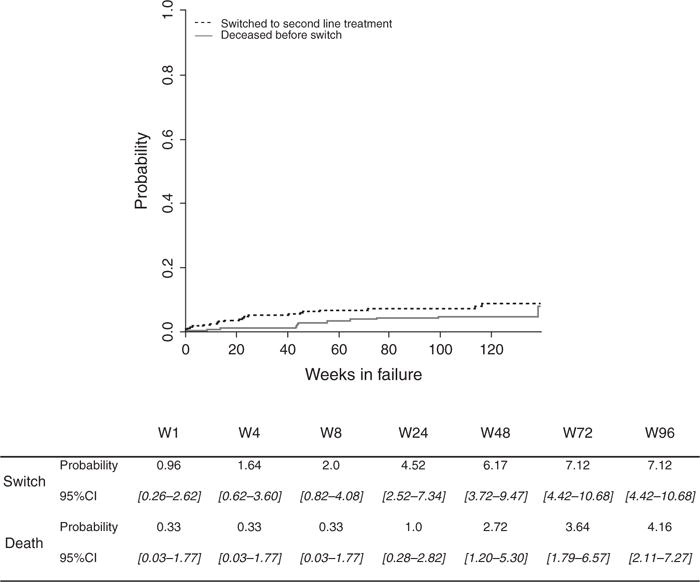
Cumulative incidence functions for switch and death before switch in the 322 HIV-infected children on first-line antiretroviral therapy in failure within the IeDEA West Africa cohort.
Table 4.
Characteristics associated with switching to second-line treatment or death in the 322 HIV-infected children on antiretroviral therapy and in failure in the IeDEApWADA cohort, Multivariate analyses – Fine and Gray model.
| Switch to second-line treatment
|
Death
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | # patients | # events | sHR | 95% CI | P | # events | sHR | 95% CI | P |
| Age (years) | 322 | 21 | 1.21 | [1.09–1.33] | <0.01 | 12 | 1.09 | [0.96–1.29] | 0.17 |
| Sex (Female/Male) | 322 | 21 | 0.94 | [0.38–2.34] | 0.87 | 12 | 0.59 | [0.17–2.07] | 0.41 |
| ART regimen | <0.01 | 0.52 | |||||||
| 2 NRTIs and 1 NNRTI | 267 | 13 | 1 | – | 9 | 1 | – | ||
| 2 NRTIs and 1 PI or three NRTIs | 55 | 8 | 2.97 | [1.20–7.41] | 2 | 1.54 | [0.42–5.73] | ||
| Baseline clinical staging | 0.11 | 0.73 | |||||||
| WHO 1, 2 or 3 (A,B) | 163 | 12 | 1 | – | 6 | 1 | – | ||
| WHO4 (AIDS) | 98 | 2 | 0.29 | [0.06–1.31] | 5 | 1.52 | [0.50–4.60] | ||
| Unknown | 61 | 7 | 1.73 | [0.62–4.86] | 1 | 0.84 | [0.06–12.41] | ||
| Type of failure | a | 0.76 | |||||||
| Clinical only | 205 | 2 | a | a | 8 | 1 | – | ||
| Immunological only | 96 | 19 | a | a | 2 | 0.54 | [0.07–4.07] | ||
| Both | 21 | 0 | a | a | 2 | 1.54 | [0.27–8.41] | ||
| Time on ART until failure (months) | 322 | 21 | 1.11 | [1.01–1.22] | 0.03 | 12 | 0.96 | [0.82–1.14] | 0.70 |
CI, confidence interval; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; sHR, subdistribution hazard ratio.
Did not converge.
Discussion
In this large collaboration of prospective cohorts of HIV-infected children initiating ART in West Africa, we make several important observations. First, 7% of the children were on second-line treatment regimen. Second, reasons for switch were rarely due to treatment failure, and many children were on second-line treatment because of ART availability issues and toxicity. Third, 12% of ART-treated HIV-infected children were failing first-line ART after a median time of 7 months; among these children, the probability for switching to second-line ART reached only 7% by 96 weeks. This was associated with older age and longer duration on ART.
The percentage of children on second-line ART in the IeDEA West Africa cohort was estimated at 7%, which is comparable to that of other cohort studies in resource-limited settings. In a health clinic in KwaZulu Natal, South Africa, 9% of the active file had switched to second-line ART by a median time of 95 weeks of follow-up [13]. A cross-sectional survey conducted in Asia and Southern Africa reported a rate of switch to second-line treatment to reach 10 and 3%, respectively, using virological outcomes [9]. However, these switching rates remain low compared with the proportion of children failing first-line ART; viral suppression is estimated to reach 70% by 12 months on ART [5].
Our study is one of the first to attempt to document reasons for switching to second-line ART. Unexpectedly, treatment failure represented only 16% of the switches. Main reasons included toxicity, stock-outs and adverse events. Seventeen percent of children switched to second-line ART because of toxicity and this was strongly associated with initiating protease inhibitor-based ART. This could be explained by the fact that as the withdrawal of NFV, there is only one protease inhibitor formulation available in combination for small children in resource-limited settings, lopinavir/ritonavir [14]. Despite the proven effectiveness of protease inhibitors [15], they are problematic for many reasons, including toxicity, poor palatability and difficulties in dosages. Although we are unable to confirm that all toxicity was caused by this class of drugs, it is likely that to avoid inadequate dual therapy that would increase the risk of resistance, children are switched to a different regimen. Poor adherence was also a frequent reason for switching to second-line ART. Both the difficulties in administrating medication in the form of unpalatable liquids or large tablets and the dependence on caregivers leads to poor adherence, which, if not addressed, results in treatment failure. However, before switching to second-line ART, healthcare workers must ensure that children are taking their medication properly and re-enforce educational therapy. In a context wherein few second-line options are available, it is essential to lead interventions to improve treatment adherence in children. The main cause of switch remained drug stock outs. In 20% of the cases, the documented cause of switch was unavailability of the drug in 2009. As more and more children are accessing ART, systems for pharmaceutical supply in many parts of Africa are overwhelmed [16]. A WHO survey in 2009 revealed that 36 (38%) out of 94 reporting countries had documented at least one stock out of antiretroviral drugs in health facilities [17]. The supply chain of paediatric regimens is particularly vulnerable to breakdown because of storage requirements. In a survey of decentralized HIV service provision in Malawi, Uganda and Zimbabwe, 24% of clinics experienced stockouts of paediatric ART, whereas only 7% experienced stock outs of adult ART [18]. These findings underline one of many operational difficulties in sustainable roll-out of paediatric ART.
The second main finding of this study is a 12% (N = 322) treatment failure rate in children initiating first-line ART, according to WHO definitions [4]. Clinical failure was most common followed by immunological failure with only a small proportion having both. Among these children, 21 (0.8%) switched to second-line ART. However, according to the survey filled-in by the clinicians, 30 children switched because of treatment failure. This result implies that at least nine children were misclassified as failing ART. Furthermore, among the 21 children in treatment failure who switched to second-line ART, only less than 1% of those in clinical failure switched to second-line ART. These findings underline the difficulties and missed opportunities for identifying treatment failure in children in a context in which virological monitoring is not yet available. The WHO recommends viral load monitoring to measure treatment effectiveness and identify those in virological failure at the earliest convenience before the emergence of resistance; in the light of these results, it is crucial to improve both universal access to viral load monitoring and second-line regimens for children on ART in West Africa. Furthermore, the cumulative risk for mortality among children in failure reached 4% by 96 weeks. Children who are failing ART must be identified as early as possible. In the absence of virological testing, effective routine monitoring strategies must be identified to prevent clinical failure. This question has been investigated in the Anti-Retroviral Research for Watoto (ARROW) trial in Uganda and Zimbabwe, which showed that children can be safely monitored without the need for expensive routine laboratory tests [19]. However, this trial did raise concerns about using clinically driven monitoring without routine testing for effectiveness: children with low CD4+ cell counts who should be switched to second-line ART will be missed (and then be at risk of getting very sick) or children with high CD4+ cell counts will be switched unnecessarily. In the absence of laboratory monitoring, it is essential to identify more specific and sensitive indicators of first-line ART failure. Many studies have reported the predicted value of CD4+ cell count on ART, but few have described weight gain, although this could be a sensitive indicator for treatment failure, as reported in the ARROW trial [19]. Monitoring height and weight is routinely performed in the follow-up of ART-treated children and Yotebieng et al. [20] have recently constructed age-specific normative curves developed for HIV-infected children. These could be used as a sustainable tool to identify those children at a higher risk of death. Further research is still needed in order to develop an improved clinical algorithm in the absence of viral load monitoring as in the West-African context.
We found that among children in failure, older children and those who had been on ART for a longer period of time were more likely to switch to second-line ART. Similarly, studies have shown this reluctance to switch a young child failing first-line ART elsewhere [21]. Indeed, in the context of few second-line treatment options and no third-line therapy, it is reasonable to first re-enforce adherence and educational therapy before switching to second-line ART.
Children who had initiated first-line protease inhibitor-based ART (or three NRTIs) and who were failing were more likely to switch to second-line ART. This result has not been reported elsewhere. However, we hypothesize that because of the difficulties in administrating protease inhibitors and the higher pill burden discussed above, these failing children were probably nonadherent and this issue could be addressed by switching drug class. However, as the recently published results reporting the superiority of ritonavir-boosted lopinavir-based ART in HIV-infected children [15], WHO recommendations include the use of protease inhibitors as first-line ART [4]. In the light of our results, it is urgent to implement new interventions in order to improve adherence in children initiating first-line ART.
Weaknesses of our prospective study include first the retrospective documentation of reasons of ART switches of our data collection. These were collected in the context of routine busy clinics resulting in missing data for 35% of the switches. Furthermore, a high proportion of children are lost to follow-up (LTFU). In the context of paediatric HIV care in West Africa where very few children were transferred out to decentralized centres, there is reason to believe that LTFU is associated with disease progression and that a significant proportion of those children are actually in failure and/or deceased [22]. Consequently, the validity of our results could be adversely affected: first, the number of children in failure is underestimated, thus overestimating the probability of switching in case of failure; second, these LTFU children are censored in our model and therefore the risks of death are underestimated. But our outcomes provide conservative estimates of adverse outcomes.
Second, the size of the cohort resulted in a relatively large absolute number of failures; however, the number of switches was limited, which may impact on inference, as we lack statistical power. We advise caution in the interpretation of our results, as we have grouped together different reasons for switch. Third, the IeDEA clinics are urban and relatively well resourced compared with those outside of a research context, which may not be representative of all sites across West Africa. Finally, we were unable to document some potential determinants for failing first-line ART and switching to second-line ART, which are often discussed in the literature such as exposure to PMTCT or failure to thrive. Nevertheless, this study reflects well the clinical practice in the management and roll-out of ART in a context in which routine viral load monitoring is not available and is the first to document reasons for switching.
Conclusion
The rate of switch to second-line therapy in children living in West-African resource-limited settings appears to be low. Key challenges include identifying children who are failing first-line ART in a context in which virological monitoring is not available and improving the availability of adequate paediatric ART formulations and most importantly their continuous provision. Switch practices were most often observed in children who were not in failure, partly due to the lack of paediatric formulations. Switches after clinical or immunological failure were insufficient. A large proportion of children who switched to second-line ART appeared to be nonadherent. Hence, educational therapy and interventions to improve adherence are essential in which few treatment options are available. It is essential to regularly follow-up ART-treated children and advocate for both sustainable access to first-line and second-line regimens to provide adequate roll-out of paediatric ART.
Acknowledgments
The IeDEAWest Africa Collaboration Study Group (as of June 2014): Participating sites (*members of the Steering Committee, members of the Executive Committee): Benin, Cotonou.
Adults: Djimon Marcel Zannou*, Carin Ahouada, Jocelyn Akakpo, Christelle Ahomadegbé, Jules Bashi, Alice Gougounon-Houéto, Angèle Azon-Kouanou, Fabien Houngbé, Jean Sehonou (CNHU Hubert Maga).
Pediatrics: Sikiratou Koumakpaï*§, Florence Alihonou, Marcelline d’Almeida, Irvine Hodonou, Ghislaine Hounhoui, Gracien Sagbo, Leı¨la Tossa-Bagnan, Herman Adjide (CNHU Hubert Maga).
Burkina Faso: Adults: Joseph Drabo*, René Bognounou, Arnaud Dienderé, Eliezer Traore, Lassane Zoungrana, Béatrice Zerbo (CHU Yalgado, Ouagadougou), Adrien Bruno Sawadogo*§, Jacques Zoungrana, Arsène Héma, Ibrahim Soré, Guillaume Bado, Achille Tapsoba (CHU Souro Sanou, Bobo Dioulasso).
Paediatrics: Diarra Yé, Fla Kouéta, Sylvie Ouedraogo, Rasmata Ouédraogo, William Hiembo, Mady Gansonré (CH Charles de Gaulle, Ouagadougou).
Côte d’Ivoire, Abidjan: Adults: Eugène Messou*, Joachim Charles Gnokoro, Mamadou Koné, Guillaume Martial Kouakou, (ACONDA-CePReF); Clarisse Amani Bosse*, Kouakou Brou, Achi Isidore Assi (ACONDA-MTCT-Plus); Henri Chenal*, Denise Hawerlander, Franck Soppi (CIRBA); Albert Minga*, Yao Abo, Jean-Michel Yoboue (CMSDS/CNTS); Serge Paul Eholié*§, Mensah Deborah Noelly Amego, Viviane Andavi, Zelica Diallo, Frédéric Ello, Aristophane Koffi Tanon (SMIT, CHU de Treichville), Serge Olivier Koule*, Koffi Charles Anzan, Calixte Guehi (USAC, CHU de Treichville).
Paediatrics: Edmond Addi Aka*, Koffi Ladji Issouf, Jean-Claude Kouakou, Marie-Sylvie N’Gbeche, (ACONDA-CePReF); Touré Pety*, Divine Avit-Edi (ACONDA-MTCT-Plus); Kouadio Kouakou*, Magloire Moh, Valérie Andoblé Yao (CIRBA); Madeleine Amorissani Folquet*, Marie-Evelyne Dainguy, Cyrille Kouakou, Véronique Tanoh Méa-Assande, Gladys Oka-Berete, Nathalie Zobo, Patrick Acquah, Marie-Berthe Kokora (CHU Cocody); Tanoh François Eboua*, Marguerite Timité-Konan, Lucrèce Diecket Ahoussou, Julie Kebé Assouan, Mabéa Flora Sami, Clémence Kouadio (CHU Yopougon).
Ghana, Accra:
Paediatrics: Lorna Renner*§, Bamenla Goka, Jennifer Welbeck, Adziri Sackey, Seth Ntiri Owiafe (Korle Bu TH).
Guinea-Bissau: Adults: Christian Wejse*§, Zacarias José Da Silva*, Joao Paulo (Bandim Health Project), The Bissau HIV cohort study group: Amabelia Rodrigues (Bandim Health Project), David da Silva (National HIV programme Bissau), Candida Medina (Hospital National Simao Mendes, Bissau), Ines Oliviera-Souto (Bandim Health Project), Lars Østergaard (Dept of Infectious Diseases, Aarhus University Hospital), Alex Laursen (Department of Infectious Diseases, Aarhus University Hospital), Morten Sodemann (Department of Infectious Diseases, Odense University Hospital), Peter Aaby (Bandim Health Project), Anders Fomsgaard (Department of Virology, Statens Serum Institut, Copenhagen), Christian Erikstrup (Department of Clinical Immunology), Jesper Eugen-Olsen (Department of Infectious Diseases, Hvidovre Hospital, Copenhagen).
Guinea: Adults: David Leuenberger*, Jean Hebelamou§ (Centre Medical Macenta)
Mali, Bamako: Adults: Moussa Y Maïga*§, Fatoumata Fofana Diakité, Abdoulaye Kalle, Drissa Katile (CH Gabriel Toure), Hamar Alassane Traore*, Daouda Minta*, Tidiani Cissé, Mamadou Dembelé, Mohammed Doumbia, Mahamadou Fomba, Assétou Soukho Kaya, Abdoulaye M Traoré, Hamady Traoré, Amadou Abathina Toure (CH Point G).
Paediatrics: Fatoumata Dicko*, Mariam Sylla, Alima Berthé, Hadizatou Coulibaly Traoré, Anta Koïta, Niaboula Koné, Clémentine N’Diaye, Safiatou Touré Coulibaly, Mamadou Traoré, Naı¨chata Traoré (CH Gabriel Toure).
Nigeria: Adults: Man Charurat* (UMB/IHV), Vivian Kwaghe*§, Samuel Ajayi, Georgina Alim, Stephen Dapiap, Otu (UATH, Abuja), Festus Igbinoba (National Hospital Abuja), Okwara Benson*, Clément Adebamowo*, Jesse James, Obaseki, Philip Osakede (UBTH, Benin City), John Olasode (OATH, Ile-Ife).
Senegal, Dakar: Adults: Moussa Seydi*§, Papa Salif Sow, Bernard Diop, Noël Magloire Manga, Judicael Malick Tine§, Coumba Cissé Bassabi (SMIT, CHU Fann).
Paediatrics: Haby Signate Sy*, Abou Ba, Aida Diagne, Hélène Dior, Malick Faye, Ramatoulaye Diagne Gueye, Aminata Diack Mbaye (CH Albert Royer).
Togo, Lomé: Adults: Akessiwe Patassi*§, Awèrou Kotosso, Benjamin Goilibe Kariyare, Gafarou Gbada-massi, Agbo Komi, Kankoé Edem Mensah-Zukong, Pinuwe Pakpame (CHU Tokoin/Sylvanus Olympio).
Paediatrics: Koko Lawson-Evi*§, Yawo Atakouma, Elom Takassi, Améyo Djeha, Ayoko Ephoévi-gah, Sherifa El-Hadj Djibril (CHU Tokoin/Sylvanus Olympio).
Executive Committee*: François Dabis (Principal Investigator, Bordeaux, France), Emmanuel Bissagnene (Co-Principal Investigator, Abidjan, Côte d’Ivoire), Elise Arrivé (Bordeaux, France), Patrick Coffie (Abidjan, Côte d’Ivoire), Didier Ekouevi (Abidjan, Côte d’Ivoire), Antoine Jaquet (Bordeaux, France), Valériane Leroy (Bordeaux, France), Charlotte Lewden (Bordeaux, France), Annie J Sasco (Bordeaux, France).
Operational and Statistical Team: Jean-Claude Azani (Abidjan, Côte d’Ivoire), Eric Balestre (Bordeaux, France), Serge Bessekon (Abidjan, Côte d’Ivoire), Sophie Karcher (Bordeaux, France), Jules Mahan Gonsan (Abidjan, Côte d’Ivoire), Jérôme Le Carrou (Bordeaux, France), Séverin Lenaud (Abidjan, Côte d’Ivoire), Célestin Nchot (Abidjan, Côte d’Ivoire), Karen Malateste (Bordeaux, France), Amon Roseamonde Yao (Abidjan, Côte d’Ivoire).
Administrative Team: Abdoulaye Cissé (Abidjan, Côte d’Ivoire), Alexandra Doring§ (Bordeaux, France), Adrienne Kouakou (Abidjan, Côte d’Ivoire), Guy Gneppa (Abidjan, Côte d’Ivoire), Elodie Rabourdin (Bordeaux, France), Jean Rivenc (Pessac, France).
Consultants/Working Groups: Xavier Anglaret (Bordeaux, France), Boubacar Ba (Bamako, Mali), Renaud Becquet (Bordeaux, France), Juan Burgos Soto (Bordeaux, France), Jean Bosco Essanin (Abidjan), Andrea Ciaranello (Boston, USA), Sébastien Datté (Abidjan, Côte d’Ivoire), Sophie Desmonde (Bordeaux, France), Jean-Serge Elvis Diby (Abidjan, Côte d’Ivoire), Geoffrey S.Gottlieb (Seattle, USA), Apollinaire Gninlgninrin Horo (Abidjan, Côte d’Ivoire), Julie Jesson (Bordeaux, France), Serge N’zoré Kangah (Abidjan, Côte d’Ivoire), David Meless (Abidjan, Côte d’Ivoire), Aida Mounkaila-Harouna (Bordeaux, France), Camille Ndondoki (Bor-deaux, France), Caroline Shiboski (San Francisco, USA), Boris Tchounga (Abidjan, Côte d’Ivoire), Rodolphe Thiébaut (Bordeaux, France), Gilles Wandeler (Dakar, Senegal).
Funding for this study was provided by The National Cancer Institute (NCI), the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) and the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health (NIH), as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) under Award Number U01AI069919. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Coordinating centre: ISPED, Univ Bordeaux Segalen, Bordeaux, France
Regional Office: PAC-CI, Abidjan, Côte d’Ivoire
Methodologic Support: MEREVA, Bordeaux, France
Website: http://www.mereva.net/iedea
Footnotes
Conflicts of interest
There are no conflicts of interest to declare.
References
- 1.World Health Organisation. UNAIDS report on the global AIDS epidemic. 2013 http://www.unaids.org/en/media/unaids/con-tentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Accessed 15 December 2014]
- 2.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation. Antiretroviral therapy for HIV infection in infants and children: towards universal access. Recommendations for a public health approach. 2010 Revision 2010. http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. [Accessed 15 December 2014] [PubMed]
- 4.World Health Organisation. The use of antiretroviral drugs for treating and preventing HIV infection. 2013 http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [Accessed 15 December 2014]
- 5.Ciaranello AL, Chang Y, Margulis AV, Bernstein A, Bassett IV, Losina E, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009;49:1915–1927. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sebunya R, Musiime V, Kitaka SB, Ndeezi G. Incidence and risk factors for first line anti retroviral treatment failure among Ugandan children attending an urban HIV clinic. AIDS Res Ther. 2013;10:25. doi: 10.1186/1742-6405-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacha T, Tilahun B, Worku A. Predictors of treatment failure and time to detection and switching in HIV-infected Ethiopian children receiving first line antiretroviral therapy. BMC Infect Dis. 2012;12:197. doi: 10.1186/1471-2334-12-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renaud-Thery F, Nguimfack BD, Vitoria M, Lee E, Graaff P, Samb B, et al. Use of antiretroviral therapy in resource-limited countries in 2006: distribution and uptake of first- and second-line regimens. AIDS. 2007;21(Suppl 4):S89–S95. doi: 10.1097/01.aids.0000279711.54922.f0. [DOI] [PubMed] [Google Scholar]
- 9.TREAT Asia Pediatric HIV Observational Database (TApHOD) and The International Epidemiologic Databases to Evaluate AIDS (IeDEA) Southern Africa Paediatric Group. A biregional survey and review of first-line treatment failure and second-line paediatric antiretroviral access and use in Asia and southern Africa. J Int AIDS Soc. 2011;14:7. doi: 10.1186/1758-2652-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anaky MF, Duvignac J, Wemin L, Kouakoussui A, Karcher S, Touré S, et al. Scaling up antiretroviral therapy for HIV-infected children in Côte d’Ivoire: determinants of survival and loss to programme. Bull World Health Organ. 2010;88:490–499. doi: 10.2471/BLT.09.068015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekouevi DK, Azondekon A, Dicko F, Malateste K, Touré P, Eboua FT, et al. 12-month mortality and loss-to-program in antiretroviral-treated children: the IeDEA pediatric West African Database to evaluate AIDS (pWADA), 2000-2008. BMC Public Health. 2011;11:519. doi: 10.1186/1471-2458-11-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organisation. Antiretroviral therapy of HIV infection in infants and children: towards universal access. Recommendations for a public health approach. 2006 http://www.who.int/hiv/pub/mtct/arv_guidelines_mtct.pdf. [Accessed 15 December 2014] [PubMed]
- 13.Zanoni BC, Sunpath H, Feeney ME. Pediatric response to second-line antiretroviral therapy in South Africa. PloS One. 2012;7:e49591. doi: 10.1371/journal.pone.0049591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald F, Penazzato M, Gibb D. Development of antiretroviral resistance in children with HIV in low- and middle-income countries. J Infect Dis. 2013;207(Suppl 2):S85–S92. doi: 10.1093/infdis/jit115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366:2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quick JD, Boohene NA, Rankin J, Mbwasi RJ. Medicines supply in Africa. BMJ. 2005;331:709–710. doi: 10.1136/bmj.331.7519.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organisation. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2010. Geneva, Switzerland: WHO; 2010. [Google Scholar]
- 18.Chan AK, Ford D, Namata H, Muzambi M, Nkhata MJ, Abongomera G, et al. The Lablite project: a cross-sectional mapping survey of decentralized HIV service provision in Malawi, Uganda and Zimbabwe. BMC Health Serv Res. 2014;14:352. doi: 10.1186/1472-6963-14-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organisation. Towards Universal access: Scaling up priority HIV/AIDS interventions in the health sector. Progress Report Geneva 2010 [updated 2010] Available from: http://www.who.int/hiv/pub/2010progressreport/summary_en.pdf. [Accessed 15 December 2014]
- 20.Yotebieng M, Van Rie A, Moultrie H, Meyers T. Six-month gains in weight, height, and CD4 predict subsequent antiretroviral treatment responses in HIV-infected South African children. AIDS. 2011;24:139–146. doi: 10.1097/QAD.0b013e328332d5ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies MA, Moultrie H, Eley B, Rabie H, Van Cutsem G, Giddy J, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa – the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr. 2011;56:270–278. doi: 10.1097/QAI.0b013e3182060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leroy V, Malateste K, Rabie H, Lumbiganon P, Ayaya S, Dicko F, et al. Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multi-regional collaboration. J Acquir Immune Defic Syndr. 2013;62:208–219. doi: 10.1097/QAI.0b013e31827b70bf. [DOI] [PMC free article] [PubMed] [Google Scholar]


