Abstract
Context:
Aberrant biomechanics may affect force attenuation at the knee during dynamic activities, potentially increasing the risk of sustaining a knee injury or hastening the development of osteoarthritis after anterior cruciate ligament reconstruction (ACLR). Impaired quadriceps neuromuscular function has been hypothesized to influence the development of aberrant biomechanics.
Objective:
To determine the association between quadriceps neuromuscular function (strength, voluntary activation, and spinal-reflex and corticomotor excitability) and sagittal-plane knee biomechanics during jump landings in individuals with ACLR.
Design:
Cross-sectional study.
Setting:
Research laboratory.
Patients or Other Participants:
Twenty-eight individuals with unilateral ACLR (7 men, 21 women; age = 22.4 ± 3.7 years, height = 1.69 ± 0.10 m, mass = 69.4 ± 10.1 kg, time postsurgery = 52 ± 42 months).
Main Outcome Measure(s):
We quantified quadriceps spinal-reflex excitability via the Hoffmann reflex normalized to maximal muscle response (H : M ratio), corticomotor excitability via active motor threshold, strength as knee-extension maximal voluntary isometric contraction (MVIC), and voluntary activation using the central activation ratio (CAR). In a separate session, sagittal-plane kinetics (peak vertical ground reaction force [vGRF] and peak internal knee-extension moment) and kinematics (knee-flexion angle at initial contact, peak knee-flexion angle, and knee-flexion excursion) were collected during the loading phase of a jump-landing task. Separate bivariate associations were performed between the neuromuscular and biomechanical variables.
Results:
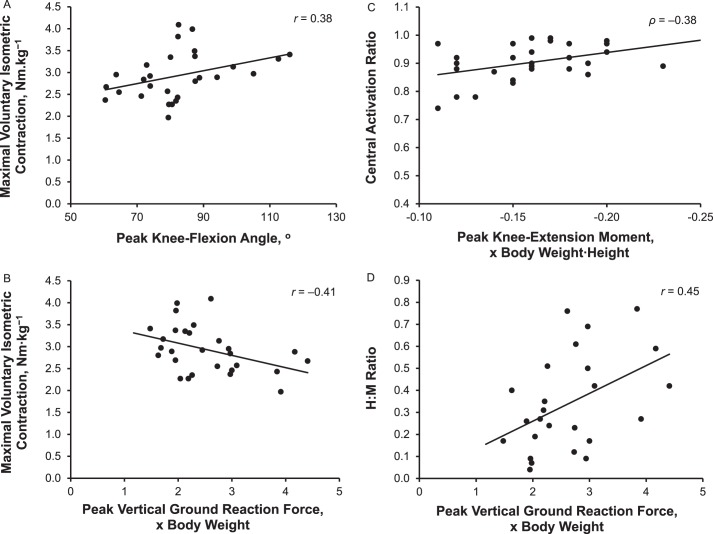
In the ACLR limb, greater MVIC was associated with greater peak knee-flexion angle (r = 0.38, P = .045) and less peak vGRF (r = −0.41, P = .03). Greater CAR was associated with greater peak internal knee-extension moment (ρ = −0.38, P = .045), and greater H : M ratios were associated with greater peak vGRF (r = 0.45, P = .02).
Conclusions:
Greater quadriceps MVIC and CAR may provide better energy attenuation during a jump-landing task. Individuals with greater peak vGRF in the ACLR limb possibly require greater spinal-reflex excitability to attenuate greater loading during dynamic movements.
Key Words: knee flexion, ground reaction force, spinal-reflex excitability, corticomotor excitability
Quadriceps muscle weakness is common after anterior cruciate ligament reconstruction (ACLR) and may persist for years after patients complete formal rehabilitation and return to sport.1−3 It is influenced, in part, by diminished voluntary activation,4 which is commonly demonstrated after ACLR.5 Whereas the exact causes of diminished voluntary activation are not fully understood, altered spinal reflex and corticomotor excitability after ACLR1,6,7 have been suggested8,9 to contribute to this inability to fully activate the quadriceps. Given that the quadriceps are imperative for both generating force and attenuating energy about the knee during dynamic activities, diminished neuromuscular function of the quadriceps may lead to aberrant lower extremity biomechanics that increase the risk of reinjury.10,11
Landing from a jump is a highly dynamic physical task that requires the development of sufficient internal knee-extension moment (KEM), generated by eccentric action of the quadriceps, to control knee-joint motion and attenuate impact forces.11,12 Quadriceps weakness and decreased voluntary activation were common after ACLR,2,13,14 and individuals demonstrated substantially reduced KEMs and peak knee-flexion angles during walking, jogging,15 and landing tasks.16,17 Quadriceps strength deficits are also associated with a stiffer, or more extended, knee on the ACLR side during jump landing.17,18 Experimentally induced quadriceps muscle inhibition has been shown to result in increased ground reaction forces and reduced KEM and knee-flexion angle during jump landing.12 The reductions in KEM and knee-flexion angle were hypothesized to be influenced by the inability to fully activate the quadriceps muscle after ACLR.12 Taken together, these results suggest that poor quadriceps function may result in altered sagittal-plane knee biomechanics during walking and landing.12,13,15,19
Individuals who have sustained an anterior cruciate ligament (ACL) injury and undergone ACLR are at greater risk for a subsequent ACL injury in both the involved and uninvolved limbs than individuals with no history of ACL injury.10,20 Secondary ACL injury risk appears to be strongly related to between-limbs asymmetries in sagittal-plane knee moments during jump landing.10,20 However, few researchers have evaluated how quadriceps function is associated with knee-joint sagittal-plane kinetics, vertical ground reaction force (vGRF), and kinematics during jump landings in individuals with unilateral ACLR. Understanding the associations between quadriceps function and knee biomechanics informs the development of clinical interventions that facilitate a safe return to high-risk activities and potentially reduce the risk of subsequent injury. Therefore, the purpose of our study was to evaluate the associations between indices of quadriceps neuromuscular function (strength, voluntary activation, and spinal-reflex and corticomotor excitability) and sagittal-plane knee kinetics (peak KEM), kinematics (knee-flexion angle at initial contact [IC], peak knee-flexion angle, and knee-flexion excursion), and peak vGRF during jump landings after ACLR. We hypothesized that greater quadriceps strength, voluntary activation, and spinal-reflex and corticomotor excitability would be associated with greater peak KEM, knee-flexion angle at IC, peak knee-flexion angle, and knee-flexion excursion and lower vGRF bilaterally during a jump-landing task.
METHODS
Participants
Individuals with a history of primary unilateral ACLR were recruited for this cross-sectional laboratory study. All 28 participants were a minimum of 6 months (range, 10–161 months) post-ACLR at the time of data collection and had been cleared for unrestricted participation in physical activity by an orthopaedic surgeon (Table 1). All participants reported that they engaged in at least 20 minutes of moderate physical activity 3 times per week. We excluded individuals with a history of lower extremity orthopaedic surgery other than unilateral ACLR, bilateral ACLR, multiligament reconstruction to the ACLR knee, or ACLR revision surgery. We also excluded individuals who self-reported a previous diagnosis of knee osteoarthritis or current osteoarthritis symptoms (eg, pain, swelling, and stiffness), balance or neuromuscular disorder, or an orthopaedic injury in either limb during the 6 months before the study. Given the use of transcranial magnetic stimulation (TMS) testing in the study, we excluded individuals with a history of concussion or head injury in the 6 months before the study, stroke, cardiac condition, epilepsy, cranial surgery, diagnosed migraines, cancer in the brain or thigh musculature, or diagnosed psychiatric disorder. All participants provided written informed consent, and the Institutional Review Board at the University of North Carolina at Chapel Hill approved the study.
Table 1. .
Participant Demographicsa
| Variable |
Mean ± SD |
| Age, y | 22.4 ± 3.7 |
| Height, m | 1.69 ± 0.1 |
| Mass, kg | 69.4 ± 10.1 |
| Body mass index, kg·m2 | 24.3 ± 2.8 |
| International Knee Documentation Committee Scale score, % | 88.8 ± 8.9 |
| Tegner Activity Scale score (range = 0–10) | 7.3 ± 1.8 |
| Time since surgery, mo | 52 ± 42 |
Men = 7, women = 21.
Procedures
Participants self-reported age, sex, graft type, date of ACLR, and whether a concomitant meniscectomy or meniscal repair was performed at the time of ACLR. Self-reported level of physical activity was assessed using the Tegner Activity Scale, and self-reported disability was assessed via the subjective section of the International Knee Documentation Committee Scale. Height and mass were measured before testing. To decrease the risk that participants would be fatigued from strength testing during the jump-landing assessment, we performed neuromuscular (spinal-reflex and corticomotor excitability, strength, and voluntary activation) and biomechanical (peak vGRF, peak KEM, knee-flexion angle at IC, peak knee-flexion angle, and knee-flexion excursion) assessments bilaterally during separate sessions. We tested the neuromuscular measures in the following order: (1) spinal-reflex excitability, (2) corticomotor excitability, and (3) strength and voluntary activation. We did not randomize the order of testing, as collecting multiple maximal-effort outcomes (strength and voluntary activation) before outcomes that were collected during submaximal effort or a resting state (spinal reflex and corticomotor excitability) might have influenced the outcomes collected during a submaximal or resting state.21 The order of limbs tested for neuromuscular measures was randomized between ACLR and uninjured limbs.
Quadriceps Spinal-Reflex Excitability
Quadriceps spinal-reflex excitability was assessed bilaterally via the Hoffmann reflex (H-reflex) normalized to the maximal muscle response (H : M ratio).22 Participants were positioned supine on a padded plinth with their upper extremities relaxed by their sides and their test knee supported in 15° of flexion. Electromyography (EMG) data were collected using 2 pregelled Ag/AgCl electrodes (model EL503; BIOPAC Systems, Inc, Goleta, CA) positioned 1.75 mm apart over the distal belly of the vastus medialis and the reference electrode positioned over the medial malleolus.7 The skin sites were prepared for EMG by shaving where necessary, debriding, and cleaning with alcohol before electrode placement. An MP150 data-acquisition system and Acqknowledge software (both BIOPAC Systems, Inc) were used to collect and visualize the EMG signals at 2000 Hz and administer the electrical stimulus. To elicit the quadriceps H-reflex, a 1-millisecond square-wave stimulus was delivered to the femoral nerve where it passed through the femoral triangle.23 The maximal H-reflex was determined by increasing the stimulus in 0.2-V increments until the maximal peak-to-peak amplitude was obtained. Maximal muscle response was determined by increasing the stimulus intensity in 1.0-V increments until the peak-to-peak amplitude of the muscle response did not rise with 3 consecutive increases. The 5 largest H-reflexes were averaged and normalized to the average of 5 maximal muscle responses to determine the H : M ratio.22
Quadriceps Corticomotor Excitability
The TMS was used to assess the active motor threshold (AMT), which we used to quantify corticomotor excitability. The participants were seated in a dynamometer (model HUMAC Norm; CSMi Solutions, Stoughton, MA) with their hips and knees in 85° and 90° of flexion, respectively, and the shank secured to the dynamometer arm using a hook-and-loop strap.7 The EMG electrodes remained in situ from spinal-reflex excitability testing. A synthetic elastic swim cap with predrawn frontal- and midsagittal-plane lines was placed on the scalp in a standardized position.6 A predrawn grid system on the cap with points separated by 1 cm was used to determine the coil position.
Participants performed a series of submaximal graded warm-up isometric contractions between 25% and 75% of maximal effort to become familiar with the procedures. Next, they performed practice maximal voluntary isometric contractions (MVICs) until the peak torque ceased to increase 10% from the previous trial.24 These data were used to calculate the 5% of MVIC that would be used as the torque threshold for AMT testing.
A MagStim BiStim with a double-cone TMS coil (Magstim Company, Whitland, United Kingdom) was used to systematically deliver stimuli at 50% of maximum stimulator output throughout the grid to determine the point that produced the largest motor-evoked potential (MEP).25 This point was defined as the hot spot where the AMT was established for the vastus medialis muscle, and the coil position was traced onto the cap to ensure a consistent location of the stimulation site.26 At the hot spot, the stimulus intensity was adjusted until the AMT was identified. We defined AMT as the minimum stimulus intensity required to elicit a peak-to-peak MEP amplitude equal to or greater than 100 μV in 5 of 10 consecutive trials while the participant performed a quadriceps contraction at 5% of MVIC during delivery of the stimulus.25,27 The MEP amplitudes were collected with the same MP150 data-acquisition system (BIOPAC Systems, Inc) as described for the spinal-reflex excitability testing.
Quadriceps Strength and Voluntary Activation
Quadriceps strength and voluntary activation data were collected during 2 maximal-effort testing trials while participants were positioned on the dynamometer as described for the corticomotor-excitability assessment with two 7- × 13-cm self-adhesive stimulating electrodes placed proximally over the vastus lateralis and distally over the vastus medialis. The torque signal was sampled at 600 Hz while participants extended the knee maximally and as rapidly as possible. Data from these 2 trials were averaged and used for the final data analysis. Quadriceps voluntary activation was assessed using the superimposed burst (SIB) technique and quantified as the central activation ratio (CAR).5
We used an automated stimulus-triggering system to ensure that participants provided maximal effort (intraclass correlation coefficient = 0.981−0.984).28 A custom LabVIEW program (National Instruments, San Antonio, TX) was used to display the participant's MVIC values on the screen concurrently with the torque signals in real time.28 The participants were instructed to extend the knee into the torque lever arm as hard and fast as possible to attain the MVIC value. When the torque value reached the MVIC and subsequently dropped by 1 Nm, the computer automatically triggered the delivery of the SIB at a 10-pulse train, 0.6-millisecond pulse duration, 100-Hz frequency, and 125-V intensity to the quadriceps by an electrical stimulator (model S48 Grass Stimulator and SIU8T isolation unit; Natus Neurology, West Warwick, RI), and the resulting increase in torque was recorded.28 The MVIC torque was extracted by determining the data point of the greatest torque before CAR stimulation. We calculated CAR as the ratio of the MVIC torque to the maximum torque after the SIB [CAR = MVIC/(MVIC+SIB)].1,7,28,29
Jump-Landing Biomechanics
Participants wore spandex shorts and their own athletic shoes for testing. In addition, female participants wore a tight-fitting tank top supplied by the laboratory, and male participants did not wear a shirt. They were outfitted with 25 retroreflective markers on the following bony landmarks: acromion processes, manubrium of the sternum, anterior-superior iliac spines, greater trochanters, anterior thighs, medial and lateral femoral condyles, anterior shanks, medial and lateral malleoli, calcanei, and the first and fifth metatarsal heads.30 We also attached a rigid cluster of 3 markers to the sacrum and 1 additional marker at the L4-5 vertebral space. Before motion capture, a static trial was captured with the participant standing with the upper extremities positioned at 90° of abduction to estimate the locations of the landmarks needed to calculate joint centers. After the static trial, the medial condyle and malleolus markers were removed for data collection. Participants performed a jump-landing task from a 30-cm box positioned at 50% of the participant's height from the front edge of the force plates.31 We instructed them to jump forward off the box to a double-legged landing with 1 foot on each force plate and then immediately jump vertically as high as possible.31 Each participant performed a minimum of 3 practice trials. Five jump-landing trials were conducted, and data from the first and last trials were excluded from data analysis. A trial was considered successful if the participant left the box with both feet at the same time, landed on the force plates, and jumped straight up in the air. If the trial was unsuccessful, a subsequent trial was collected for analysis.
Key Points
Measures of quadriceps neuromuscular function and sagittal-plane knee biomechanics during jump landing were associated in the involved limb of individuals with anterior cruciate ligament reconstruction.
Individuals with better quadriceps neuromuscular function were better able to attenuate lower extremity loading during jump landing.
Greater spinal-reflex excitability was associated with higher peak vertical ground reaction force.
Spinal-reflex pathways may be more associated than corticomotor pathways in individuals who have had anterior cruciate ligament reconstruction and demonstrate high levels of loading during dynamic activities.
Three-dimensional kinematic data were collected using a 7-camera motion-capture system (Vicon Motion Systems, Centennial, CO), sampled at 120 Hz, and filtered using a fourth-order low-pass Butterworth filter with a 12-Hz cutoff frequency. Kinetic data were collected at 1200 Hz using 2 embedded 40- × 60-cm force plates (model FP406010; Bertec Corp, Columbus, OH). Knee- and ankle-joint centers were defined as the midpoints between the medial and lateral condyles and malleoli, respectively. Hip-joint–center coordinates were estimated from the coordinates of the L4-5, right anterior-superior iliac spine, and left anterior-superior iliac spine markers using the method of Bell et al.32 Joint angles were defined based on the position of the distal segment relative to the proximal segment using the Euler method with the following planes of rotational motion: sagittal (y-axis), frontal (x-axis), and transverse (z-axis).
Lower extremity biomechanics were evaluated over the loading phase, which was defined as the interval from IC (vGRF ≥ 10 N) to peak knee flexion. Knee-flexion excursion was calculated for each trial by subtracting the IC joint angle from the peak knee-flexion joint angle during the loading phase. Knee-joint moments were calculated using an inverse-dynamics approach. Internal KEM was normalized by dividing by the product of body weight and height (×BW·H) and expressed as a negative value, whereby a greater negative value indicated a greater KEM. Peak vGRF was normalized by dividing by body weight (×BW).
Statistical Analysis
Means and standard deviations were calculated for all outcome measures. All data were assessed for normality using the Shapiro-Wilk test before the primary analysis. Between-limbs comparisons were conducted using paired-samples t tests. For the primary analysis, separate Pearson product moment correlations (r) were used to assess the associations between biomechanical and neuromuscular variables if both variables were normally distributed for the ACLR limb and the uninvolved limb. Spearman rank correlations (ρ) were used if one variable was nonnormally distributed. We classified the strength of all bivariate associations as negligible (0.0–0.3), low (0.31−0.5), moderate (0.51−0.70), high (0.71−0.90), or very high (0.91−1.0).33 We excluded missing data from analyses in a pairwise manner, thereby including as many participants in each analysis as the available data allowed. For bivariate associations, we also conducted univariate regression analyses and reported r2 to determine the variance in each biomechanical variable that was associated with the variance in the neuromuscular variable. Furthermore, additional correlation analyses were conducted to examine the associations between the neuromuscular variables in each limb. If an association was found between 2 neuromuscular variables, we performed multiple regression analyses to account for the correlated neuromuscular variables and examine the unique variance explained in the biomechanical variable of interest by each of the separate neuromuscular variables.
To better discuss our results, we performed a post hoc analysis to examine the influence of meniscal procedure on the associations between the biomechanical and neuromuscular variables. Two groups were created based on self-reported meniscal procedures performed at the time of ACLR. For each group, the Pearson product moment correlation (r) was used to assess the associations between biomechanical and neuromuscular variables if both were normally distributed for the ACLR limb. Spearman rank correlations (ρ) were used if one of the variables was nonnormally distributed. All statistical analyses were performed using SPSS (version 21.0; IBM Corp, Armonk, NY), and the α level was set a priori at .05 for all tests.
RESULTS
The means and standard deviations for the neuromuscular and biomechanical outcome measures are presented in Table 2. Quadriceps MVIC and CAR were obtained from all participants, and quadriceps H-reflex and AMT were successfully assessed in 89% (n = 25) and 93% (n = 26) of participants, respectively. All outcome measures were normally distributed except CAR in the ACLR limb (P < .001). We did not observe between-limbs differences for quadriceps neuromuscular outcome measures. Individuals demonstrated greater peak vGRF (P = .005) and knee-flexion excursion (P = .049) and lower peak KEM (P = .001) and knee-flexion angle at IC (P = .03) in the involved limb. We observed negligible nonsignificant associations between the neuromuscular variables in the ACLR limb (r range = −0.19 to 0.13, P > .05). Within the uninvolved limb, we noted a low positive association between CAR and MVIC (ρ = 0.463, P = .051). The remaining associations in the uninvolved limb were negligible (r range = −0.10 to 0.17).
Table 2. .
Quadriceps Neuromuscular and Biomechanical Outcome Measures
| Variable |
Limb, Mean ± SD |
P Value |
|
| Anterior Cruciate Ligament Reconstruction |
Uninvolved |
||
| Maximal voluntary isometric contraction, Nm·kg−1 | 2.9 ± 0.6 | 3.1 ± 0.6 | .09 |
| Central activation ratio, % | 90.0 ± 6.0 | 89.0 ± 9.0 | .99 |
| Spinal-reflex excitability, H : M ratio | 0.3 ± 0.2 | 0.2 ± 0.2 | .09 |
| Active motor threshold, % T | 46.4 ± 9.9 | 43.9 ± 8.6 | .24 |
| Peak vertical ground reaction force, × body weight | 2.6 ± 0.8 | 2.2 ± 0.6 | .005a |
| Knee-flexion angle at initial contact, ° | 18.5 ± 6.4 | 21.6 ± 5.8 | .03a |
| Peak knee-flexion angle, ° | 82.9 ± 14.1 | 84.5 ± 14.5 | .21 |
| Knee-flexion excursion, ° | 64.4 ± 10.6 | 62.9 ± 11.3 | .049a |
| Peak knee-extension moment, × body weight × height | −0.2 ± 0.03 | −0.2 ± 0.03 | .001a |
Abbreviations: H : M, Hoffman reflex normalized to maximal muscle response; T, Tesla (maximum stimulator output).
Different between limbs (P < .05).
Associations Between Neuromuscular Outcomes and Jump-Landing Biomechanics in the ACLR Limb
Associations between the biomechanical and neuromuscular outcomes are presented in Table 3. We observed a low positive association between MVIC and peak knee-flexion angle (n = 28; r = 0.38, r2 = 0.14, P = .045), and a negative association between MVIC and peak vGRF (n = 28; r = −0.41, r2 = 0.17, P = .03). Similarly, a low negative association was found between CAR and peak KEM during landing (n = 28; ρ = −0.38, r2 = 0.14, P = .045). In addition, the H : M ratio had a low positive association with the peak vGRF for the injured limb (n = 25; r = 0.45, r2 = 0.20, P = .02). Negligible to low associations were present between AMT and knee sagittal-plane kinetics and kinematics during the landing phase of the jump landing (r range = −0.32 to 0.29, P > .05). The significant associations are illustrated in the Figure.
Table 3. .
Bivariate Associations Between Quadriceps Neuromuscular Outcome Measures and Sagittal-Plane Knee Kinetics and Kinematics for the Involved Limb During the Landing Phase of a Drop Jump
| Variable |
Maximal Voluntary Isometric Contractiona |
Central Activation Ratiob |
Spinal-Reflex Excitabilitya |
Active Motor Thresholda |
| Knee angle at initial contact | 0.27 | −0.06 | 0.09 | −0.29 |
| Peak knee-flexion angle | 0.38c | 0.11 | 0.003 | −0.32 |
| Knee-flexion excursion | 0.34 | 0.18 | −0.05 | −0.25 |
| Peak vertical ground reaction force | −0.41c | −0.05 | 0.45c | −0.14 |
| Peak knee-extension moment | −0.23 | −0.38c | 0.30 | 0.29 |
Values are Pearson product moment correlation coefficients (r).
Values are Spearman rank correlations (ρ).
Indicates association (P < .05).
Figure.
Scatter plots of the associations between the following: A, Quadriceps strength and peak knee flexion. B, Quadriceps strength and peak vertical ground reaction force. C, Quadriceps voluntary activation and peak knee-extension moment. D, Spinal-reflex excitability and peak vertical ground reaction force in the involved limb. Abbreviation: H : M, Hoffmann reflex normalized to maximal muscle response.
Associations Between Neuromuscular Outcomes and Jump-Landing Biomechanics in the Uninvolved Limb
We observed negligible to low nonsignificant associations between quadriceps neuromuscular function outcome measures and sagittal-plane knee kinetics and kinematics during jump landing in the uninvolved limb (r range = −0.35 to 0.28, P > .05; Table 4).
Table 4. .
Bivariate Associations Between Quadriceps Neuromuscular Outcome Measures and Sagittal-Plane Knee Kinetics and Kinematics for the Uninvolved Limb During the Landing Phase of a Drop Jumpa
| Variable |
Maximal Voluntary Isometric Contraction |
Central Activation Ratio |
Spinal-Reflex Excitability |
Active Motor Threshold |
| Knee angle at initial contact | 0.21 | 0.14 | 0.02 | −0.19 |
| Peak knee-flexion angle | 0.28 | −0.08 | −0.12 | −0.20 |
| Knee-flexion excursion | 0.25 | −0.17 | −0.17 | −0.17 |
| Peak vertical ground reaction force | −0.26 | −0.35 | 0.14 | −0.08 |
| Peak knee-extension moment | 0.01 | −0.09 | −0.19 | 0.30 |
Values are Pearson product moment correlation coefficients (r).
Post Hoc Analysis
A moderate negative association was observed between CAR and peak KEM in individuals who reported undergoing a concomitant meniscal procedure (n = 14; ρ = −0.53, P = .049). The association between CAR and peak KEM in participants who did not undergo a meniscal procedure was not significant (n = 14; ρ = 0.240, P = .45). We noted negligible to moderate nonsignificant associations between the remaining quadriceps neuromuscular function outcome measures and sagittal-plane knee kinetics and kinematics during jump landing for participants with or without a reported meniscal procedure (r range = −0.32 to 0.54; P > .05; Table 5).
Table 5. .
Bivariate Associations Between Quadriceps Neuromuscular Outcome Measures and Sagittal-Plane Knee Kinetics and Kinematics for the Involved Limb During the Landing Phase of a Drop Jump in Individuals Who Have Had a Meniscal Procedure
| Variable |
Maximal Voluntary Isometric Contractiona |
Central Activation Ratiob |
Spinal-Reflex Excitabilitya |
Active Motor Thresholda |
| Knee angle at initial contact | 0.31 | −0.20 | 0.15 | −0.32 |
| Peak knee-flexion angle | 0.49 | 0.16 | 0.03 | −0.30 |
| Knee-flexion excursion | 0.47 | 0.19 | −0.03 | −0.22 |
| Peak vertical ground reaction force | −0.32 | −0.23 | 0.40 | −0.15 |
| Peak knee-extension moment | −0.28 | −0.53c | 0.54 | 0.28 |
Values are Pearson product moment correlation coefficients (r).
Values are Spearman rank correlations (ρ).
Indicates association (P < .05).
DISCUSSION
Contrary to previous researchers,1,6,34 we found no between-limbs differences in quadriceps neuromuscular function in our study cohort. However, individuals demonstrated a greater peak vGRF and lower KEM and peak knee-flexion angle in the ACLR limb than in the uninvolved limb during jump landing. Quadriceps strength, voluntary activation, and spinal-reflex excitability were associated with knee sagittal-plane kinetics and kinematics during jump landings in individuals after ACLR, even once these individuals returned to full physical activity. As we hypothesized, individuals with stronger quadriceps in the ACLR limb landed with greater peak knee-flexion angles and lower peak vGRF, suggesting that they may be able to more effectively attenuate loads on the lower extremity during landing. Given that greater KEM was indicated by a more negative value, participants with greater quadriceps voluntary activation demonstrated greater KEM, indicating that they were able to activate their quadriceps to resist greater external knee-flexion moments created during the loading phase of the jump landing. Overall, these associations suggest that greater quadriceps strength after ACLR may reduce the risk of rerupture. Greater spinal-reflex excitability was associated with greater vGRF in the ACLR limb, whereas corticomotor excitability was not associated with any biomechanical outcome that we analyzed. Reflexive excitability may be more influential during jump landings in those with ACLR than corticomotor excitability is but still an inadequate strategy to attenuate loading on landing.
Quadriceps weakness has been associated with aberrant movement patterns and asymmetries in knee-joint biomechanics after ACLR.10,20,35 Landing from a jump produces external knee-flexion moments that act to flex the knee. Effective landing requires resisting these external knee-flexion moments with a KEM created by muscles that work eccentrically to absorb kinetic energy through the lower extremity.36 Adequate quadriceps function, therefore, is crucial for controlling knee flexion and attenuating energy during a jump landing. After the early postoperative phases (>6 months post-ACLR), individuals appear to walk with smaller knee-flexion angles than their uninjured counterparts.37 Persistent reductions in knee-flexion angle during jump landings have been linked to rerupture of the ACL,10 which may be due to a reduced ability to attenuate energy through the lower limb joints. In our study, greater quadriceps strength was associated with greater peak knee-flexion angle and lower peak vGRF on landing. However, quadriceps strength explained only 14% and 17% of the variance in peak knee-flexion angle and peak vGRF, respectively. The low association may be a result of the participants in our study demonstrating symmetric quadriceps neuromuscular function, particularly in strength and activation. These individuals may use different muscle groups to control jump-landing forces due to a lack of adequate bilateral quadriceps function. Previous studies support this finding, with investigators reporting that knee-flexion angle on IC was associated with greater energy absorption36 and reduced ground reaction forces38 compared with stiff landings and smaller knee-flexion angles. Devita and Skelly36 demonstrated that 19% more energy was attenuated with landing strategies that used greater knee flexion, suggesting that the muscles were able to absorb more load during a landing with greater knee-flexion excursion. These findings indicate that, whereas a stronger quadriceps may minimize the kinetic energy through knee tissues and potentially reduce the risk of injury and joint degeneration,36 other factors may influence knee flexion and energy attenuation during jump landing. Whereas we focused on the knee joint during jump landing, other lower extremity and spine joints may influence how the knee joint attenuates loads during jump landing. Limited ankle movement39 and a more upright trunk position during jump landing have been linked to deleterious landing kinetics40 and lower limb jump-landing mechanics.41
Diminished voluntary activation contributes to quadriceps weakness.4 Persistent alterations in ascending signals from the knee joint to the motor cortex result in inhibitory signals to the quadriceps α-motoneuron pool and, thus, a reduced ability to voluntarily activate the muscle.4,42 Diminished voluntary activation has been linked with reduced KEM and a stiff knee during the stance phase of the gait cycle.4,11 Stiffening the knee joint may be a compensatory adaptation to diminished voluntary activation and quadriceps strength.36,38 It is possible that a reduced KEM on landing would result in increased load through the passive structures in the knee joint, including the ACL graft.36 We found an association between quadriceps voluntary activation and KEM on jump landing: individuals who had greater voluntary activation demonstrated greater KEM. Greater voluntary activation indicated that individuals were able to recruit more motor units at higher firing frequencies,5 suggesting that they may have been better able to use their quadriceps to eccentrically control the knee and attenuate loading forces during jump landings. Similar to quadriceps strength, voluntary quadriceps activation explained only 14% of the variance in KEM during jump landing. The association between KEM and quadriceps activation remained significant, albeit only moderately, in individuals who reported a meniscal procedure.
Contraction of skeletal muscle is generated via 2 mechanisms: (1) voluntary contraction from the motor cortex and the descending motor pathways and (2) the spinal reflex pathways.43 Whereas researchers1,6,7 have reported alterations in quadriceps corticomotor excitability in individuals with ACLR, we found no association between quadriceps corticomotor excitability and sagittal-plane knee kinetics and kinematics during jump landing. The low magnitude of the association between corticomotor excitability and knee-joint biomechanics may have resulted from the assessment techniques used in our study. Corticomotor excitability was assessed in a nonfunctional seated position during a low-grade isometric contraction (5% MVIC), which may not accurately represent the activity or neuromuscular function during a more dynamic movement, such as a jump landing. The AMT has been shown to be different between limbs at 6 months post-ACLR.6 However, the heterogeneity in time since surgery (range = 10−161 months) in our study may have washed out the effects of changes in neuromuscular function at different time points post-ACLR. In addition, the clinical relevance of alterations in AMT after ACLR is not completely understood. Reduced corticomotor excitability after ACLR possibly results in individuals using greater spinal-reflex excitability. Pietrosimone et al7 hypothesized that increased spinal-reflex excitability after ACLR is a mechanism used to maintain voluntary activation. They suggested that individuals with ACLR who demonstrate full quadriceps neuromuscular function (voluntary activation ≥ 95%) have increased spinal-reflex excitability.7 We found an association between spinal-reflex excitability and peak vGRF, such that increased excitability was associated with greater peak vGRF on landing. This association may represent an up-regulation of spinal-reflex excitability due to diminished quadriceps strength, which may be a neuromuscular strategy to increase the ability of weaker muscles to react to an external stimulus. Conversely, the association between higher spinal-reflex excitability and greater peak vGRF may also be a protective mechanism in individuals who land with greater vGRF normalized to body weight. Up-regulated reflexes may be necessary to respond to a perturbation in individuals who land with higher vGRF.
We were surprised to observe no associations between neuromuscular variables and sagittal-plane kinetics or kinematics in the uninvolved limb. Participants did not demonstrate between-limbs differences in quadriceps neuromuscular function. By maintaining neuromuscular symmetry, perhaps the effects of the selected outcome variables on knee-joint biomechanics were minimized in this cohort of individuals with ACLR. It is also feasible that the uninvolved limb was organizing neuromuscular control in a different manner than the ACLR limb, resulting in the lack of associations in the uninvolved limb.
The sagittal-plane kinetics and kinematics that we assessed are potentially modifiable risk factors that could be targeted with rehabilitation interventions, possibly using feedback to correct aberrant biomechanics. Lepley et al6 showed that individuals who received instruction in landing could land with less ground reaction force than those relying on previous experience alone. Therefore, instruction in jump-landing techniques that includes cueing an increase in knee-flexion angle at IC to train “soft-landing” techniques could be incorporated into rehabilitation programs. Furthermore, traditional strengthening programs could be coupled with disinhibitory techniques to address voluntary activation deficits.44 The associations among quadriceps strength, voluntary activation, and knee-joint biomechanics suggest that improvements in quadriceps voluntary activation may lead to increased strength and more favorable knee-joint biomechanics in the ACLR limb during activities that require jump landings.
Given the nature of our study, we cannot determine if altered landing patterns were present before the injury and therefore resulted in the initial injury or if they were due to the injury. We did not obtain all neuromuscular variables from all participants. We examined only quadriceps isometric strength, but this has been shown to predict self-reported function45 and disability24 after ACLR. The jump-landing task was an anticipated double-legged landing, which may account for the low associations observed in our study. A potential avenue for future research would be to examine associations between biomechanics and neuromuscular function during a more dynamic movement, such as a single-legged landing or landing from an unknown height. Researchers should also evaluate associations between neuromuscular function and the spatial-temporal outcomes of movement, including time to peak kinetics and kinematics. Our participants were a sample of convenience, and we cannot account for their postoperative rehabilitation. Residual impairments may have been present in some participants due to different rehabilitation protocols and individual recovery patterns. The reported associations also may have been influenced by heterogeneity in time since ACLR and postoperative recovery.
CONCLUSIONS
We found associations between measures of quadriceps neuromuscular function and sagittal-plane knee biomechanics during jump landing in the involved limbs of individuals with ACLR. Greater quadriceps strength was associated with lower peak vGRF and increased peak knee-flexion angle, whereas greater quadriceps voluntary activation was associated with greater peak KEM on jump landing. Taken together, these results suggest that individuals with better quadriceps neuromuscular function are better able to use the muscle to attenuate lower extremity loading during jump landing. Greater quadriceps spinal-reflex excitability was associated with higher peak vGRF, suggesting that spinal-reflex pathways were more associated than corticomotor pathways in individuals with ACLR who demonstrated high levels of loading during dynamic activities.
REFERENCES
- 1. Kuenze CM, Hertel J, Weltman A, Diduch D, Saliba SA, Hart JM. . Persistent neuromuscular and corticomotor quadriceps asymmetry after anterior cruciate ligament reconstruction. J Athl Train. 2015; 50 3: 303– 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lepley LK. . Deficits in quadriceps strength and patient-oriented outcomes at return to activity after ACL reconstruction: a review of the current literature. Sports Health. 2015; 7 3: 231– 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palmieri-Smith RM, Lepley LK. . Quadriceps strength asymmetry after anterior cruciate ligament reconstruction alters knee joint biomechanics and functional performance at time of return to activity. Am J Sports Med. 2015; 43 7: 1662– 1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pietrosimone B, Lepley AS, Murray AM, Thomas AC, Bahhur NO, Schwartz TA. . Changes in voluntary quadriceps activation predict changes in muscle strength and gait biomechanics following knee joint effusion. Clin Biomech (Bristol, Avon). 2014; 29 8: 923– 929. [DOI] [PubMed] [Google Scholar]
- 5. Kent-Braun JA, Le Blanc R. . Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996; 19 7: 861– 869. [DOI] [PubMed] [Google Scholar]
- 6. Lepley AS, Gribble PA, Thomas AC, Tevald MA, Sohn DH, Pietrosimone BG. . Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: a 6-month longitudinal investigation. Scand J Med Sci Sports. 2015; 25 6: 828– 839. [DOI] [PubMed] [Google Scholar]
- 7. Pietrosimone BG, Lepley AS, Ericksen HM, Clements A, Sohn DH, Gribble PA. . Neural excitability alterations after anterior cruciate ligament reconstruction. J Athl Train. 2015; 50 6: 665– 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pietrosimone BG, McLeod MM, Lepley AS. . A theoretical framework for understanding neuromuscular response to lower extremity joint injury. Sports Health. 2012; 4 1: 31– 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ward S, Pearce AJ, Pietrosimone B, Bennell K, Clark R, Bryant AL. . Neuromuscular deficits after peripheral joint injury: a neurophysiological hypothesis. Muscle Nerve. 2015; 51 3: 327– 332. [DOI] [PubMed] [Google Scholar]
- 10. Hewett TE, Di Stasi SL, Myer GD. . Current concepts for injury prevention in athletes after anterior cruciate ligament reconstruction. Am J Sports Med. 2013; 41 1: 216– 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palmieri-Smith R, Thomas A. . A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009; 37 3: 147– 153. [DOI] [PubMed] [Google Scholar]
- 12. Palmieri-Smith RM, Kreinbrink J, Ashton-Miller JA, Wojtys EM. . Quadriceps inhibition induced by an experimental knee joint effusion affects knee joint mechanics during a single-legged drop landing. Am J Sports Med. 2007; 35 8: 1269– 1275. [DOI] [PubMed] [Google Scholar]
- 13. Hart J, Pietrosimone B, Hertel J, Ingersoll C. . Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010; 45 1: 87– 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomas A, Villwock M, Wojtys E, Palmieri-Smith R. . Lower extremity muscle strength after anterior cruciate ligament injury and reconstruction. J Athl Train. 2013; 48 5: 610– 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewek M, Rudolph K, Axe MJ, Snyder-Mackler L. . The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2002; 17 1: 56– 63. [DOI] [PubMed] [Google Scholar]
- 16. Gokeler A, Hof AL, Arnold MP, Dijkstra PU, Postema K, Otten E. . Abnormal landing strategies after ACL reconstruction. Scand J Med Sci Sports. 2010; 20 1: 12– 19. [DOI] [PubMed] [Google Scholar]
- 17. Oberländer KD, Brüggemann GP, Höher J, Karamanidis K. . Altered landing mechanics in ACL-reconstructed patients. Med Sci Sports Exerc. 2013; 45 3: 506– 513. [DOI] [PubMed] [Google Scholar]
- 18. Oberländer KD, Brüggemann GP, Höher J, Karamanidis K. . Reduced knee joint moment in ACL deficient patients at a cost of dynamic stability during landing. J Biomech. 2012; 45 8: 1387– 1392. [DOI] [PubMed] [Google Scholar]
- 19. Hart JM, Ko JW, Konold T, Pietrosimione B. . Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: a systematic review. Clin Biomech (Bristol, Avon). 2010; 25 4: 277– 283. [DOI] [PubMed] [Google Scholar]
- 20. Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010; 38 10: 1968– 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gandevia SC, Petersen N, Butler JE, Taylor JL. . Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol. 1999; 521 pt 3: 749– 759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palmieri RM, Ingersoll CD, Hoffman MA. . The Hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004; 39 3: 268– 277. [PMC free article] [PubMed] [Google Scholar]
- 23. Hopkins JT, Wagie NC. . Intrasession and intersession reliability of the quadriceps Hoffmann reflex. Electromyogr Clin Neurophysiol. 2003; 43 2: 85– 89. [PubMed] [Google Scholar]
- 24. Pietrosimone BG, Lepley AS, Ericksen HM, Gribble PA, Levine J. . Quadriceps strength and corticospinal excitability as predictors of disability after anterior cruciate ligament reconstruction. J Sport Rehabil. 2013; 22 1: 1– 6. [DOI] [PubMed] [Google Scholar]
- 25. Luc BA, Lepley AS, Tevald MA, Gribble PA, White D, Pietrosimone B. . Reliability of corticomotor excitability in leg and thigh musculature at 14 and 28 days. J Sport Rehabil. 2014; 23 4: 330– 338. [DOI] [PubMed] [Google Scholar]
- 26. Groppa S, Oliviero A, Eisen A, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012; 123 5: 858– 882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994; 91 2: 79– 92. [DOI] [PubMed] [Google Scholar]
- 28. Luc BA, Harkey MH, Arguelles G, Blackburn JT, Ryan E, Pietrosimone B. . Measuring voluntary quadriceps activation: effect of visual feedback and stimulus delivery. J Electromyogr Kinesiol. 2016; 26: 73– 81. [DOI] [PubMed] [Google Scholar]
- 29. Hart JM, Fritz JM, Kerrigan DC, Saliba EN, Gansneder BM, Ingersoll CD. . Reduced quadriceps activation after lumbar paraspinal fatiguing exercise. J Athl Train. 2006; 41 1: 79– 86. [PMC free article] [PubMed] [Google Scholar]
- 30. Frank B, Bell DR, Norcross MF, Blackburn JT, Goerger BM, Padua DA. . Trunk and hip biomechanics influence anterior cruciate loading mechanisms in physically active participants. Am J Sports Med. 2013; 41 11: 2676– 2683. [DOI] [PubMed] [Google Scholar]
- 31. Walsh M, Boling MC, McGrath M, Blackburn JT, Padua DA. . Lower extremity muscle activation and knee flexion during a jump-landing task. J Athl Train. 2012; 47 4: 406– 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bell AL, Pedersen DR, Brand RA. . A comparison of the accuracy of several hip center location prediction methods. J Biomech. 1990; 23 6: 617– 621. [DOI] [PubMed] [Google Scholar]
- 33. Mukaka MM. . Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012; 24 3: 69– 71. [PMC free article] [PubMed] [Google Scholar]
- 34. Kuenze CM, Foot N, Saliba SA, Hart JM. . Drop-landing performance and knee-extension strength after anterior cruciate ligament reconstruction. J Athl Train. 2015; 50 6: 596– 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. . Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014; 42 7: 1567– 1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Devita P, Skelly WA. . Effect of landing stiffness on joint kinetics and energetics in the lower extremity. Med Sci Sports Exerc. 1992; 24 1: 108– 115. [PubMed] [Google Scholar]
- 37. Hart HF, Culvenor AG, Collins NJ, et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2016; 50 10: 597– 612. [DOI] [PubMed] [Google Scholar]
- 38. Podraza JT, White SC. . Effect of knee flexion angle on ground reaction forces, knee moments and muscle co-contraction during an impact-like deceleration landing: implications for the non-contact mechanism of ACL injury. Knee. 2010; 17 4: 291– 295. [DOI] [PubMed] [Google Scholar]
- 39. Fong CM, Blackburn JT, Norcross MF, McGrath M, Padua DA. . Ankle-dorsiflexion range of motion and landing biomechanics. J Athl Train. 2011; 46 1: 5– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blackburn JT, Padua DA. . Sagittal-plane trunk position, landing forces, and quadriceps electromyographic activity. J Athl Train. 2009; 44 2: 174– 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blackburn JT, Padua DA. . Influence of trunk flexion on hip and knee joint kinematics during a controlled drop landing. Clin Biomech (Bristol, Avon). 2008; 23 3: 313– 319. [DOI] [PubMed] [Google Scholar]
- 42. Urbach D, Nebelung W, Becker R, Awiszus F. . Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris a prospective twitch interpolation study. J Bone Joint Surg Br. 2001; 83 8: 1104– 1110. [DOI] [PubMed] [Google Scholar]
- 43. Kandel ER, Schwartz JH, Jessell TM. . Principles of Neural Science. 4th ed. New York, NY: McGraw-Hill; 2000. [Google Scholar]
- 44. Pietrosimone B, Blackburn JT, Harkey MS, Luc BA, Pamukoff DN, Hart JM. . Clinical strategies for addressing muscle weakness following knee injury. Clin Sports Med. 2015; 34 2: 285– 300. [DOI] [PubMed] [Google Scholar]
- 45. Pietrosimone B, Lepley AS, Harkey MS, et al. Quadriceps strength predicts self-reported function post-ACL reconstruction. Med Sci Sports Exerc. 2016; 48 9: 1671– 1677. [DOI] [PubMed] [Google Scholar]