Abstract
The thyroid hormone receptors, TRα1, TRβ1, and other subtypes, are members of the nuclear receptor superfamily that mediate the action of thyroid hormone signaling in numerous tissues to regulate important physiological and developmental processes. Their most well-characterized role is as ligand-dependent transcription factors; TRs bind thyroid hormone response elements in the presence or absence of thyroid hormone to facilitate the expression of target genes. Although primarily residing in the nucleus, TRα1 and TRβ1 shuttle rapidly between the nucleus and cytoplasm. We have identified multiple nuclear localization signals and nuclear export signals within TRα1 and TRβ1 that interact with importins and exportins, respectively, to mediate translocation across the nuclear envelope. More recently, enigmatic cytoplasmic functions have been ascribed to other TR subtypes, expanding the diversity of the cellular response to thyroid hormone. By integrating data on localization signal motifs, this review provides an overview of the complex interplay between TR’s dynamic transport pathways and thyroid hormone signaling activities. We examine the variation in TR subtype response to thyroid hormone signaling, and what is currently known about regulation of the variety of tissue-specific localization patterns, including targeting to the nucleus, the mitochondrion, and the inner surface of the plasma membrane.
Keywords: thyroid hormone, thyroid hormone receptor, nuclear import, nuclear export, mitochondrial import
Introduction
Thyroid hormone is essential for many diverse processes in nearly all vertebrate tissues, and abnormal thyroid hormone signaling underpins several human diseases (Chen, et al. 2013; Kim and Cheng 2013; Laudet and Gronemeyer 2002; Mendoza and Hollenberg 2017; Mondal, et al. 2016; Mullur, et al. 2014; van der Spek, et al. 2017). Much of thyroid hormone action is mediated by the thyroid hormone receptors (TRs), members of the nuclear receptor superfamily that act as ligand-dependent transcription factors. By modulating the transcription of target genes in response to ligand, TRs play key physiological roles in the regulation of many aspects of development, growth, and metabolism, including regulation of mitochondrial activity (Bernal 2017; Flamant and Gauthier 2013; Pascual and Aranda 2013; Skah, et al. 2017; Vella and Hollenberg 2017; Wrutniak-Cabello, et al. 2017). Thyroid hormone signaling is typically classified into two distinct pathways, nongenomic and genomic; however, these designations do not fully capture the subtleties of thyroid hormone action. To address the complexity of thyroid hormone signaling, a more precise nomenclature has recently been formulated (Flamant, et al. 2017). In this new classification scheme, four types of thyroid hormone signaling are defined: type 1 is the canonical pathway in which liganded TR binds directly to DNA; type 2 describes liganded TR tethered to chromatin-associated proteins, but not bound to DNA directly; type 3 suggests that liganded TR can exert its function without recruitment to chromatin in either the nucleus or cytoplasm; and type 4 proposes that thyroid hormone acts at the plasma membrane or in the cytoplasm without binding TR, a mechanism of action that is emerging as a key component of thyroid hormone signaling (Davis, et al. 2016; Kalyanaraman, et al. 2014).
The biological effect of thyroid hormone in a given tissue depends on a number of factors: the amount of available hormone, the levels of different TR subtypes and their post-translational modifications, the type of heterodimerization partner, and their interaction with corepressors and coactivators (Morte and Bernal 2014). In addition, accurate translocation of TRs from their synthesis in the cytosol to their ultimate destination is essential for maintaining proper cellular functions and activities (Bonamy and Allison 2006; Bonamy, et al. 2005; Bondzi, et al. 2011; Fernandez-Majada, et al. 2007; Wang and Li 2014). The thyroid hormone receptors are remarkably dynamic proteins. Although primarily residing in the nucleus TRα1 and TRβ1 shuttle rapidly between the nucleus and cytoplasm, and recent characterization of TRα1 isoforms with cytoplasmic functions adds a surprising twist to the intricacies of the receptor’s subcellular trafficking. The fine balance between nuclear import and export of TRs has emerged as a critical control point for modulating thyroid hormone-responsive gene expression (Roggero, et al. 2016; Subramanian, et al. 2015), while an additional layer of complexity is added by multiple modular, often overlapping, functional domains. General understanding of nuclear localization signal (NLS) and nuclear export signal (NES) structure, mitochondrial and membrane targeting signals, and how these motifs are regulated will assist in refining understanding of the mechanism of action of TRs. In this review we will focus on mechanisms regulating the journey of TR from its site of synthesis in the cytoplasm to its final localization in target tissues, and how the receptor integrates gene expression across multiple levels in the cellular response to hormone. Before considering the cellular response to thyroid hormone, it is important to first examine the pathway by which thyroid hormone reaches target tissues and gains access to its intracellular receptors.
Thyroid hormone signaling
Thyroid hormone is produced through a feedback loop that includes the hypothalamus, pituitary, and thyroid gland, commonly referred to as the hypothalamic-pituitary-thyroid (HPT) axis (Medici, et al. 2015; Mendoza and Hollenberg 2017). The HPT axis involves a series of signal transduction cascades, where a signal sent from the hypothalamus eventually arrives at the thyroid gland, triggering release of thyroid hormone. In the circulatory system, the majority of total 3,5,3′,5′-L-tetraiodothyronine (thyroxine, T4) and 3,5,3′-L-triiodothyronine (T3) are bound with three different thyroid hormone carrying proteins: thyroxine-binding globulin, transthyretin, and human serum albumin (Mondal et al. 2016; Pappa, et al. 2015). Upon reaching the target tissue, thyroid hormones enter cells via uptake through specific membrane transporters, including the monocarboxylate transporters MCT8 and MCT10 (Abe, et al. 2012; Bernal, et al. 2015). The most extensively characterized transporter, MCT8, transports thyroid hormone exclusively and preferentially binds T3; however, secondary thyroid hormone transporters have been described that can compensate for loss of MCT8 expression, including the heterodimeric L-type amino acid transporters (LATs), LAT1 and LAT2, and the organic anion-transporting polypeptide (OATP) family (Mendoza and Hollenberg 2017).
Once in the cell, the intracellular concentration of thyroid hormone can be modified by the action of a suite of deiodinases. The prohormone T4 can be converted to the physiologically active hormone T3, or inactivated via conversion to 3,3′,5′-L-triiodothyronine (reverse T3, or rT3) within the cell. T3 and rT3 can be modified to form the physiologically active 3,5′-L-diiodothyronine (T2), or the inactive 3,3′-L-diiodothyronine (3,3′-T2), respectively, to protect tissues from excess hormone (Dentice, et al. 2013; Mondal et al. 2016; Orozco, et al. 2014). Whether T4 is directly involved in mediating gene expression remains a subject of debate. T4 is thought to primarily influence gene expression indirectly by cross-talk with other cell signaling pathways at the plasma membrane (Davis et al. 2016); however, there also is accumulating evidence that T4 can directly modulate gene expression, dependent on the TR subtype and other cellular cofactors (Galton 2017). T3 is directly involved in mediating gene expression by binding to TR in either the cytoplasm or nucleus of the cell (Bunn, et al. 2001). The intricate balance between thyroid hormone production and deiodination is critical for regulation of TR-mediated gene expression, and the dysregulation of this process may contribute to type II diabetes mellitus, obesity, cardiovascular disease, and some types of cancer (Brent 2012; Kim and Cheng 2013; Ruiz-Llorente, et al. 2011).
In addition to the type 1 canonical response mediated by nuclear TRs, thyroid hormone also has effects not exerted through the nuclear TRs; such effects were puzzled over early on to explain observations that thyroid hormone can, in some cases, initiate cellular responses that are too rapid to be attributed to transcription and translation (Davis et al. 2016; Flamant 2016). Although detailed coverage of type 4 actions of thyroid hormone is beyond the scope of this review, it is worth noting the existence of a hormone receptor that is associated with the plasma membrane structural protein ανβ3 integrin, a regulator of cell-cell and cell-extracellular matrix interactions (Cvoro, et al. 2016; Davis et al. 2016; Lin, et al. 2016; Martin, et al. 2014; Mullur et al. 2014). This receptor binds T3 and T4 and stimulates certain cellular responses, such as the remodeling of the actin cytoskeleton that is a vital component of brain development in neurons and glial cells (Leonard and Farwell 1997), and changes in the morphology of breast cancer cells (Flamini, et al. 2017). The ανβ3 integrin-associated receptor has two thyroid hormone binding sites, S1 and S2, that lead to activation of the phosphatidylinositol 3-OH kinase (PI3K) and ERK1/2 signaling pathways, respectively. The receptor is structurally unrelated and has no sequence homology to nuclear TR and, although it could be referred to as a “thyroid hormone receptor,” this nomenclature should be avoided to prevent misconceptions about the nature of this noncanonical receptor. Type 3 signaling, mediated by transcriptionally inactive cytoplasmic TR isoforms, will be addressed later in this review.
Nuclear localization and function of thyroid hormone receptors
The type 1 genomic effects of TRs are two-fold; TRs can act as repressors of specific genes in the absence of ligand and activators of these same genes in the presence of ligand. For some genes, the reverse is the case: unliganded TR acts as an activator, while liganded TR is a repressor. This dual role of TRs implies constitutive nuclear localization. Many studies early on in the field supported this restricted subcellular distribution for TR (Andersson and Vennstrom 1997; Kumara-Siri, et al. 1986; Lee and Mahdavi 1993; Macchia, et al. 1992; Zhang and Lazar 2000; Zhu, et al. 1998). However, we and others have shown that even though TRα1 and TRβ1 appear to be predominantly nuclear at steady state, in fact, the receptors are undergoing rapid nucleocytoplasmic shuttling in both the presence and absence of T3 (Baumann, et al. 2001; Bunn et al. 2001), movement which can be visualized by heterokaryon assays or fluorescence recovery after photobleaching (Grespin, et al. 2008; Subramanian et al. 2015). Detailed investigation of TRs has revealed distinct, dynamic localization patterns for some variants. Analysis of the intracellular localization of TRs by biochemical fractionation, immunocytochemistry, or indirect immunofluorescence assays has proved challenging overall, because of a lack of validated isoform-specific antibodies, and the difficulty in detecting endogenous TR subtypes that are less abundant in cells. Many studies have thus relied on transient transfection assays and expression of fluorescent protein-tagged TRs. With regards to nuclear localization, in our hands, there is no indication that overexpressing TRs leads to a more cytoplasmic localization by saturating the capacity of cells to transport proteins into the nucleus, or that fluorescent protein tags alter localization. For example, in transfected NIH-3T3 (mouse) cells or HeLa (human) cells, neither of which express detectable levels of endogenous TR, both exogenous GFP-tagged TRα1 and untagged TRα1 detected by antibody staining show a primarily nuclear distribution at steady state (Bonamy et al. 2005; Bunn et al. 2001).
Thyroid hormone receptor subtypes
The thyroid hormone receptors are well conserved throughout vertebrate evolution, originating from a single TR gene early in animal evolution (Manzon, et al. 2014); and there is evidence for nuclear TR-mediated responses to thyroid hormone in non-vertebrate lineages, including molluscs, echinoderms, cephalochordates, and ascidians (Darras, et al. 2011; Huang, et al. 2015; Laudet and Gronemeyer 2002; Taylor and Heyland 2017). The vertebrate thyroid hormone receptors are encoded by two genes located on different chromosomes, NR1A1 and NR1A2, although due to ancestral gene duplication, some nonmammalian vertebrate species, including teleost fish, have two TRα-encoding genes (Darras et al. 2011; Galay-Burgos, et al. 2008). From these loci, a surprisingly diverse set of TR proteins are produced, through alternative splicing, alternative promoter usage, and internal initiation codons. Intense investigation of rodent and human TRs continues to reveal new subtypes, while the number of subtypes identified in other species, as of yet, is more restricted (Buchholz, et al. 2006; Kanaho, et al. 2006; Nelson and Habibi 2008; Politis, et al. 2017). For example, chickens and ducks have at least three subtypes (TRα, TRβ2, TRβ0) (Bishop, et al. 2000); zebrafish produces two TRβ variants and at least three TRα isoforms that all act as functional nuclear receptors (Darras et al. 2011); two distinct TRα transcripts and one TRβ transcript have been isolated from the American alligator (Helbing, et al. 2006); and the Atlantic halibut has two TRα and two TRβ isoforms (Galay-Burgos et al. 2008). The main focus of this review is on the well-characterized mammalian receptors, in particular TRα1 and TRβ1.
Not all of the mammalian TR proteins produced act as nuclear receptors, however, and the physiological significance of many of the nonreceptor isoforms remains a subject of investigation (Flamant and Gauthier 2013; Mullur et al. 2014; Vella and Hollenberg 2017). What is currently known about the intracellular localization and function of the mammalian TRs is summarized in Table 1, and further described herein. The predominant isoforms generated by alternative splicing mechanisms include the bona fide nuclear receptors TRα1, TRβ1, TRβ2, TRβ3, and TRβ4 (Moriyama, et al. 2016; Tagami, et al. 2010); and the nonreceptor TR variants that lack T3 binding ability, TRα2, TRα3, and TRα-ΔE6 (Casas, et al. 2006).
Table 1.
Cellular localization and function of mammalian TRα and TRβ isoforms
| Receptors | Cellular localization | Known or hypothetical function(s) |
|---|---|---|
| TRα (NR1A1) | ||
| TRα1 | ||
| p46 (full-length) | Nuclear | Transcriptional activation/repression |
| p43 | Mitochondrial matrix | Transcriptional activation/repression |
| p33 | Unknown | Unknown |
| p30 | Plasma membrane | Signaling cascade |
| p28 | Mitochondrial inner membrane | Signaling cascade |
| TRα2 | Nuclear | Possible antagonist of TR action |
| TRα3 | Nuclear | Possible antagonist of TR action |
| TRΔα1 | Unknown | Possible antagonist of TR action |
| TRΔα2 | Unknown | Possible antagonist of TR action |
| TRα-ΔE6 | Cytoplasm | Inhibitor of TR activity |
| TRβ (NR1A2) | ||
| TRβ1 | Predominantly nuclear | Transcriptional activation/repression |
| TRβ2 | Predominantly nuclear | Transcriptional activation/repression |
| TRβ2Δ | Predominantly nuclear | Possible transcriptional regulation |
| TRβ3 | Predominantly nuclear | Transcriptional activation/repression |
| TRΔβ3 | Predominantly nuclear | Dominant negative antagonist |
| TRβ4 | Predominantly nuclear | Weak antagonist of TR action |
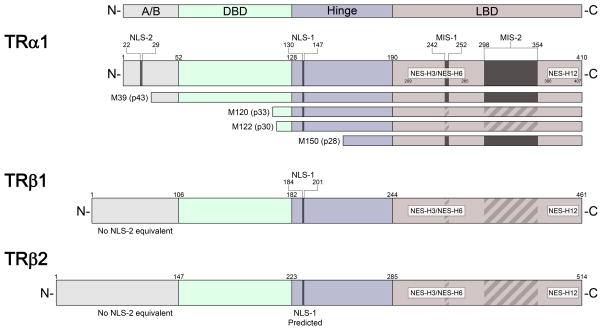
TRα1 has the highest expression in bone, the gastrointestinal tract, cardiac and skeletal muscle, and the central nervous system; TRα2 and TRα3 are predominant in the brain, kidney, testis, brown adipose tissue, and skeletal muscle (Guissouma, et al. 2014; Skah et al. 2017). TRα-ΔE6 is expressed in all tissues tested, and can sequester TRα1 in the cytoplasm (Casas et al. 2006). TRα2 is found consistently in mammals but not in other species. Although a dominant negative function has been attributed to mammalian TRα2, which is widely co-expressed with TRα1, the physiological relevance has remained a puzzle, particularly since it is unexplained why it would be necessary for TRα2 to counter-balance normal TR activity in mammals but not in non-mammalian species (Vennstrom et al., 2010). There is recent compelling evidence, however, that TRα2 modulates thyrotropin releasing hormone gene expression in the hypothalamus (Guissouma et al. 2014). In addition, four truncated forms of TRα1 (full-length, 46 kD) originate from alternative internal AUG translation initiation codons in TRα1 mRNA and are named based on their molecular masses: p43 starts at the equivalent of methionine-39 (Met39) in the full-length receptor, p33 starts with Met120, p30 starts with Met122, and p28 starts with Met150 (Kalyanaraman et al. 2014; Wrutniak-Cabello et al. 2017) (Fig. 1). Finally, other truncated forms of TRα, TRΔα1 and TRΔα2, are produced from an internal promoter in intron 7; they contain only the C-terminus of the LBD and are expressed in the brain, lung, and gut (Davis et al. 2016; Chassande et al. 1997). TRΔα1 has been proposed as a candidate mediator of T4-binding in the cytoplasm, potentially playing a role in regulating actin polymerization (Davis et al. 2016).
Figure 1.
Thyroid hormone receptor (TR) major subtypes and localization signals. The structural diagram (not to scale) of TRα1, TRβ1, and TRβ2 shows nuclear localization signal (NLS), nuclear export signal (NES), and mitochondrial import sequence (MIS) motifs, where known (solid bar) or predicted (striped bar) based on sequence homology. The positions of localization signals are indicated in relation to the respective individual domains of TR: N-terminal A/B domain (A/B), DNA-binding domain (DBD), hinge domain, and ligand-binding domain (LBD). The TRα1 mRNA encodes several forms of truncated TR by translation initiation from internal AUG sites encoding methionine (M); amino acid residue numbers correspond to the position in full-length TRα1.
TRβ1 is most abundant in the liver, kidney, and the inner ear; TRβ2 is predominant in the hypothalamus, pituitary, cochlea, and retina; and TRβ4 is ubiquitously expressed, with relatively high expression in the brain and kidney (Flamant and Gauthier 2013; Hahm and Privalsky 2013; Mullur et al. 2014; Vella and Hollenberg 2017). Other minor isoforms of TRβ1 (52-kD) also exist; for example, two isoforms are alternatively translated from TRβ1 mRNA, with TRβ3 (44.6-kD) appearing to act as a functional receptor in rat (Flamant and Gauthier 2013), and TRΔβ3 (32.8-kD) functioning as a ligand-responsive dominant negative antagonist (Williams 2000). In addition, an elongated form of TRβ2, termed TRβ2Δ, has been proposed to function as a nuclear receptor in the rat pituitary gland (Zhao, et al. 2014). In this review, we will focus on the TRα and TRβ isoforms where intracellular localization and targeting signals have been investigated in more detail.
Functional domains of the thyroid hormone receptor
The thyroid hormone receptor consists of four modular domains that are evolutionarily conserved among the nuclear receptor superfamily (Fig. 1): a variable N-terminal A/B domain, which contains a region involved in transactivation, activation function-1 (AF-1); a central DNA binding domain (DBD) comprised of two zinc fingers; a C-terminal ligand-binding domain (LBD), which also includes dimerization interfaces and activation function-2 (AF-2); and a linker or hinge region between the LBD and DBD that contributes to DNA binding, activation function and repression, ligand binding, and corepressor interactions (Mondal et al. 2016; Nascimento, et al. 2006; Pawlak, et al. 2012; Zhang et al. 2017). TRα1 and TRβ1 both contain AF-1 domains involved in the transcriptional response to hormone; while the TRβ2 isoform, which differs from TRβ1 in the A/B domain, has a unique hormone-independent AF-1 domain that recruits coactivators (Tomura, et al. 1995; Oberste-Berghas et al. 2000).
Ligand-binding domain conformation
The LBD of TR is composed of 12 α-helices that form a hollow pocket lined with hydrophobic residues. The ligand binding site is highly flexible, and the structural details underpinning receptor activation after T3 binding are complex (Schweizer, et al. 2017). The twelfth helix contains the ligand-dependent activation domain, AF2 (Figueira, et al. 2011). Helix 12 forms a short pivoting structure that can adopt different conformations. In the absence of T3, helix 12 is in an extended position and the corepressor binding groove is occupied by the corepressor nuclear receptor (CoRNR)-box helical motifs found in silencing mediator for retinoid or thyroid-hormone receptors (SMRT) and nuclear receptor co-repressor 1 (N-CoR1). Binding of T3 may induce a hormone-dependent “mouse-trap” mechanism (Flamant 2016; Moras and Gronemeyer 1998; Sonoda, et al. 2008), where helix 12 rotates to swing shut and close off the pocket around T3. As a result of this conformational change, a novel docking surface forms for interaction with LXXLL motifs (L denotes leucine; X denotes an undetermined amino acid) of a transcriptional coactivator (Rosen and Privalsky 2011). A refinement of this model suggests that TR helix 12 functions as a “selective gatekeeper” that actively discriminates between different forms of corepressor even in the unliganded receptor (Rosen and Privalsky 2009); and other models propose that rearrangements in a mobile part of the LBD comprising helix 3, the loop between helix 1 and helix 2, and nearby β-sheets, play a greater role in ligand dissociation than repositioning of helix 12 (Martinez, et al. 2006). Mutations that disrupt helix 12 alter corepressor specificity as well as T3-mediated release of corepressors (Rosen and Privalsky 2009). Recent X-ray crystallographic structural studies have revealed a second ligand binding site in TR located between helices 9–11 that may interact with T4 (Souza, et al. 2014).
Nuclear import and export signals
The nuclear transport process provides a central regulatory point for coordinating cell signaling and gene expression. Macromolecules known as nuclear pore complexes (NPCs) are the regulatory gatekeepers of the entry and exit of nuclear proteins, and allow for the passive diffusion of small molecules less than 40-kD (Li, et al. 2016). NPCs are distributed throughout the nuclear envelope, embedded at sites within the luminal space between the outer and inner membrane of the nuclear envelope (Cautain, et al. 2015; Tran, et al. 2014). They are octagonally symmetric cylindrical structures made up of proteins termed nucleoporins or Nups, that act to anchor the NPC in the nuclear envelope and provide interaction domains for nuclear proteins to translocate through a central channel (Hayama, et al. 2017). The translocation of nuclear proteins through the NPCs is typically facilitated by karyopherin β-like family members (importins and exportins), with each member performing a distinct nuclear import, export, or bidirectional transport function (Chook and Suel 2011; Kimura and Imamoto 2014).
Our systematic characterization of nuclear export signal (NES) and nuclear localization signal (NLS) motifs by site-directed mutagenesis has elucidated the mechanics of TR nuclear localization (Mavinakere, et al. 2012). In depth analysis of TRα1 and TRβ1 structure reveals that the two subtypes both contain a classical bipartite NLS, named NLS-1, residing in the hinge region, and a second monopartite NLS, termed NLS-2, located in the A/B domain of TRα1 that is absent in TRβ1 (Fig. 1) (Mavinakere et al. 2012). RNAi and coimmunoprecipitation assays show that members of the importin family of karyopherins, specifically importin 7, importin β1, and adapter importin α1 recognize these NLSs and directly mediate the nuclear import of TRs through the NPC (Roggero et al. 2016) (Fig. 2). In support of the importance of NLS-1 for efficient nuclear localization, an isoform that lacks the hinge domain, TRα-ΔE6, has a strikingly altered localization compared with TRα1; TRα-ΔE6-GFP was shown to be predominantly expressed in the cytoplasm with minor nuclear fluorescence (Casas et al. 2006). In addition, TRβ4 is primarily localized to the nucleus, and mutation of two putative NLSs near the hinge region results in a whole cell distribution of the receptor (Moriyama et al. 2016).
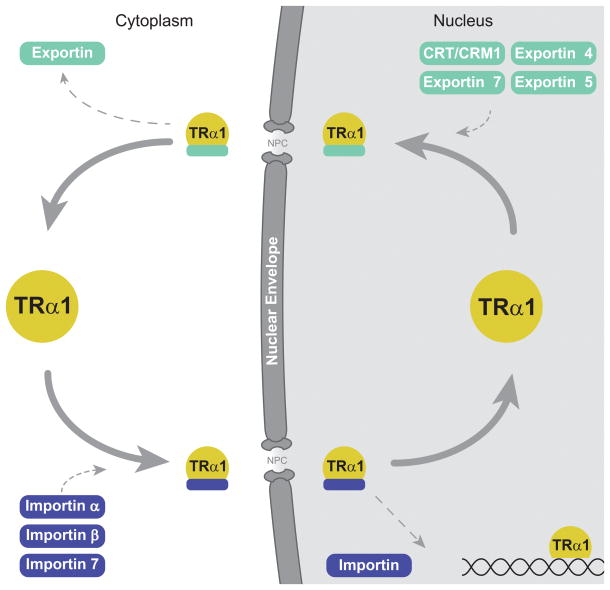
Figure 2.
Thyroid hormone receptor nucleocytoplasmic shuttling pathway. The well-characterized pathway for TRα1 is depicted. TRα1 binds to specific importins in the cytoplasm, as indicated. The TRα1-importin complex passes through a nuclear pore complex (NPC) embedded in the nuclear envelope into the nucleus, where the complex is disassembled and TRα1 binds to target genes. TRα1 exits the nucleus through the NPC in association with specific exportins or calreticulin (CRT)/CRM1. TRβ1 follows a similar nucleocytoplasmic shuttling pathway, but nuclear import is solely mediated by the importin α1/importin β1 complex.
In an earlier study, we showed that TRα1 exits the nucleus through two pathways, one dependent on the export factors CRM1 and calreticulin, and the other CRM1-independent (Grespin et al. 2008). In a subsequent study, we also identified a novel NES in helix 12 of the ligand-binding domain of TR (NES-H12). Another novel NES motif spans helix 3 and helix 6 (NES-H3/H6) (Mavinakere et al. 2012) (Fig. 1). Notably, these NES motifs are not sensitive to leptomycin B, a specific inhibitor of CRM1, suggesting that they mediate the CRM1-independent export pathway followed by TR. Follow-up work by RNAi has shown that multiple exportins influence TR export, including exportins 4, 5, and 7 (Subramanian et al. 2015). Not surprisingly, the two NLSs found in TRα1 act to confer strong nuclear localization to the receptor; we hypothesize that TRβ1’s small cytosolic population (Baumann et al. 2001; Zhu et al. 1998) may reflect an altered balance of NLS and NES activity (Mavinakere et al. 2012; Zhang et al. 2017) (Fig. 3). Although multiple NLS and NES motifs exist in a variety of nuclear proteins, how these multiple signals interact in collective remains unclear (Bonaldi, et al. 2003; Dai, et al. 2015; Lu, et al. 2014; Mavinakere et al. 2012; Panayiotou, et al. 2016; Umemoto and Fujiki 2012). Once TRs are directed into the nucleus and released from importin, they can then interact with target genes to modulate gene expression in response to hormone.
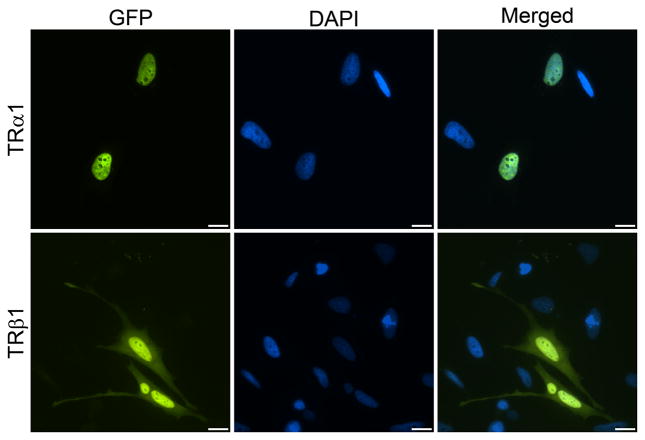
Figure 3.
Distinct intracellular localization patterns for TRα1 and TRβ1. HeLa cells transfected with expression plasmids for green fluorescent protein (GFP)-tagged TRα1 and TRβ1 were analyzed by fluorescence microscopy after staining for DNA with DAPI to visualize the nucleus. GFP-TRα1 predominantly localizes to the nucleus; GFP-TRβ1 also localizes to the nucleus but has a slight cytoplasmic population. Scale bar, 10 μm.
Thyroid hormone receptor gene activation and silencing
A multifaceted cascade of events results in binding of TRs to thyroid hormone response elements (TREs) and culminates in the modulation of target gene expression in response to thyroid hormone (Ayers, et al. 2014; Bernal and Morte 2013; Brent 2012; Vella and Hollenberg 2017). Thyroid hormone receptors often heterodimerize with the retinoid X receptor (RXR), expanding the range of T3 responsiveness for genes within the same cell (Diallo, et al. 2007; Flamant 2016). On positive TREs, corepressors, such as N-CoR1 or N-CoR2 (also known as SMRT) and histone deacetylase (HDAC), are bound in the absence of ligand to TR, leading to repression of target gene expression (Mendoza, et al. 2017; Oberoi, et al. 2011; Xu, et al. 1999). Upon ligand binding, TR undergoes a conformational change, resulting in a new set of activator proteins bound to the receptor, such as SRC-1 (p160/steroid receptor coactivator 1) and histone acetyltransferase (HAT). This leads to changes in chromatin structure and the subsequent transcription of the target gene (Dasgupta and O’Malley 2014; McKenna, et al. 1999; Soriano, et al. 2011). In addition to unliganded TR bound to positive TREs, chromatin immunoprecipitation sequencing (ChIP-seq) analysis of endogenous TR in mouse liver tissue suggests that the receptor’s interaction with chromatin is highly dynamic and that it can be recruited to chromatin in a ligand-dependent manner (Grontved, et al. 2015). These findings align with an earlier report that used fluorescence recovery after photobleaching (FRAP) to show that TRβ1 moves rapidly within the nucleus, and that ligand binding does not affect its mobility (Maruvada, et al. 2003). A recent study in mice suggests that TR target genes respond to T3 based on the availability of specific corepressors and coactivators, providing an explanation for tissue-specific responses to similar amounts of T3 (Vella, et al. 2014). In addition to activating transcription on positive TREs, TRs can also repress gene expression, possibly by binding to putative negative TREs in a T3-dependent manner (Bernal and Morte 2013). In this instance, N-CoR1 and SMRT appear to play a role in determining T3-sensitivity, suggesting that corepressors can be recruited to TR in the presence of T3 (Astapova and Hollenberg 2013; Astapova, et al. 2011; Shimizu, et al. 2015). The mechanism remains unclear, however, and a recent genome-wide analysis of chromatin occupancy of TRs in neural cells does not appear to support the hypothesis that liganded TR acts directly as a transcription repressor (Chatonnet, et al. 2013). Further, ChIP-seq studies in hypothyroid and hyperthyroid mouse liver cells suggest that negative regulation instead may be mediated by diminished TR recruitment in the presence of T3 (Ramadoss, et al. 2014).
Cytoplasmic Functions of the Thyroid Hormone Receptor
For many years the focus in the field was on characterizing the nuclear function of TRs, but now their emerging roles in the cytoplasm also must be considered. Study of the functional domains of full-length TRα1 (p46) and the truncated isoforms p43, p33, p30, and p28 has revealed conflicting intracellular targeting signals within TRα1 that can direct the proteins to the nucleus, mitochondria, or the inner surface of the plasma membrane (Kalyanaraman et al. 2014; Mavinakere et al. 2012; Wrutniak-Cabello et al. 2017) (Figs. 1 and 4). TRα1 p43 and p28 are targeted to the mitochondrial matrix and mitochondrial inner membrane, respectively. The biological function of TRα1 p33 remains unknown, but p30 is post-translationally modified via palmitoylation and colocalizes with caveolin-1 at the inner surface of the plasma membrane. Upon binding T3 the nitric oxide (NO)-cyclic guanosine monophosphate (cGMP)-protein kinase G (PKG) signaling cascade is activated, and stimulates proliferation and survival in multiple cell types (Hiroi, et al. 2006; Kalyanaraman et al. 2014; Wrutniak-Cabello et al. 2017).
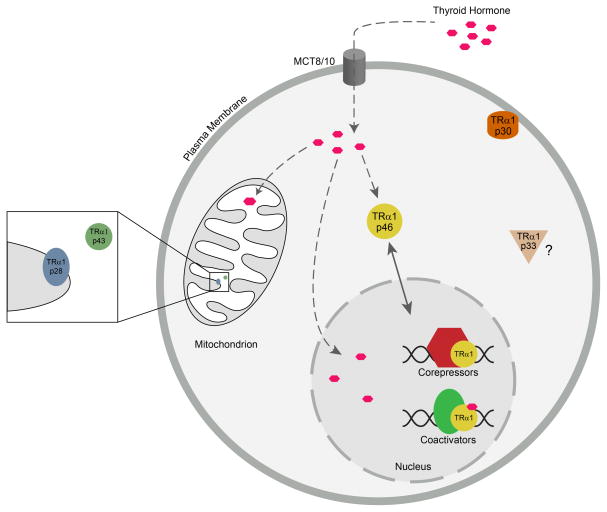
Figure 4.
Model for localization of TRα1 isoforms to the mitochondria, plasma membrane, and nucleus. TRα1 mRNA yields different forms of truncated TR by internal translation initiation. Once synthesized in the cytosol, the different forms localize to different intracellular compartments. TRα1 p28 and TRα1 p43 localize to the mitochondrial inner membrane and matrix, respectively. TRα1 p30 localizes to the inner surface of the plasma membrane, where it can bind to thyroid hormone to mediate thyroid hormone signaling. The specific localization and function of TRα1 p33 remains unknown. Full-length TRα1 (TRα1 p46) localizes to the nucleus where it modulates target gene expression in response to thyroid hormone, in association with corepressors and coactivators. Thyroid hormone enters the cell through the monocarboxylate 8 and 10 transporters (MCT8/10).
Studies in diverse cells types, including human adipose-derived stem cells (hADSC), human primary osteoblasts, mouse osteoblast-like MC3T3 cells, monkey kidney cells (CV-1), neonatal rat ventricular myocytes (NRVM), and mouse cardiomyocytes (HL-1), have revealed TR subtypes localized to the mitochondria, plasma membrane, and cytoplasmic compartments in a tissue-specific manner (Carazo, et al. 2012; Cvoro et al. 2016; Kalyanaraman et al. 2014; Wadosky, et al. 2016). Of particular interest, human ADSCs are multipotent adult stem cells with the capacity to differentiate into adipocytes, chondrocytes, and osteocytes, and they express TRα1, TRα2, and TRβ1 at variable levels. TR intracellular localization was investigated by indirect immunofluorescence assay and, interestingly, all subtypes showed cytoplasmic localization. Further examination via double immunostaining of TRα1 and TRα2 with a mitochondrial marker showed a predominantly mitochondrial localization for TRα1 proteins (Carazo et al. 2012; Psarra and Sekeris 2008; Wadosky et al. 2016). Although western blot analysis was not performed to visualize protein size, these findings suggest that truncated forms of TR were reliably being detected by the antibodies used in this study.
Mitochondrial targeting
A major compartment of thyroid hormone accumulation within the cell is the mitochondria (Bassett, et al. 2003; Davis et al. 2016; Psarra and Sekeris 2008; Wrutniak-Cabello et al. 2017). The major effect of thyroid hormone on mitochondrial activity has been partially explained by reports of truncated TRα1 variants localizing to the mitochondria of different mammalian tissues, such as liver, brown and white adipose tissue, red and white muscle, heart, tongue, and testis (Carazo et al. 2012; Fumel, et al. 2013; Wrutniak-Cabello et al. 2017). In addition, a truncated TRβ (TRβA1) localizes to the mitochondria in Xenopus laevis (South African clawed frog) oocytes (Saelim, et al. 2007). TRα1 p43 is targeted to the mitochondrial matrix, while TRα1 p28 is targeted to the mitochondrial inner membrane (Carazo et al. 2012; Kalyanaraman et al. 2014; Wrutniak-Cabello et al. 2017) (Fig. 4). TRα1 p43 displays an N-terminal deletion that lacks NLS-2, but still possesses NLS-1 in the hinge region (Fig. 1). In contrast, TRα1 p28 displays an N-terminal deletion of the A/B domain, the DBD, and NLS-1. Neither p43 or p28 possess a canonical mitochondrial import signal (MIS). Nonetheless, sequences within helices 5, 10, and 11 in the C-terminal LBD of p43 and p28 have been identified that are necessary for mitochondrial import (Carazo et al. 2012) (Fig. 1). Helix 5, spanning amino acids 242–252 of TRα1, was found to drive an atypical mitochondrial import process independent of ATP and the mitochondrial membrane potential; whereas helices 10–11, spanning amino acids 298–354, induced a typical mitochondrial import process sensitive to ATP and the mitochondrial membrane potential. Whether these two mitochondrial import sequences, MIS1 and MIS2, are functional or not, is proposed to depend on the “permissive” role of the N-terminus of TRα1 (Carazo et al. 2012). In this model, conformational changes of the protein, dependent on the flexibility of the hinge region, would disrupt the functionality of NLS-1 in the hinge region and induce the activity of the mitochondrial import sequences (Wrutniak-Cabello et al. 2017). Interestingly, TRβ1 harbors these conserved MIS1 and MIS2 motifs and lacks NLS-2 in the N-terminal A/B domain (Fig. 1), but there is no evidence of functionality of the MIS motifs. It is of interest to determine the exact nature of the N-terminal A/B domain sequence in regulating localization of TRs to the mitochondrial or nuclear compartments.
Plasma membrane targeting
Beyond type 1 genomic actions within the mitochondria, type 3 actions of TR are primarily associated with its localization to the plasma membrane. The alternative translation product TRα1 p30 is targeted to the plasma membrane where it is proposed to play a key role in mediating signaling pathways involved in cell survival and proliferation (Carazo et al. 2012; Kalyanaraman et al. 2014; Wrutniak-Cabello et al. 2017). Further, there is tissue-specific variation in p30’s localization to the plasma membrane (Kalyanaraman et al. 2014). In murine primary osteoblasts, TRα1 p30 associates with lipid rafts (cholesterol-rich plasma membrane microdomains that contain caveolin-1) to function as a unique signal transduction platform. In contrast, in MC3T3 cells TRα1 p30 associated with caveolin-1, nitric oxide synthase 3 (NOS3), protein kinase G type II (PKGII), and the tyrosine kinase Src. These data point to the possibility that TRα1 p28 localizes to the mitochondrial inner membrane following a similar mechanism (Kalyanaraman et al. 2014), and provide an understanding of how certain membrane-targeted proteins interact with caveolin to reach the plasma membrane (Hayer, et al. 2010).
A role for posttranslational modification in TR localization
Post-translational modifications (PTMs) play a significant role in the regulation of protein structure, enzymatic activity, stability or degradation, subcellular localization, protein-protein interactions, and diverse cell signaling (Azevedo and Saiardi 2016; Drazic, et al. 2016; Lin, et al. 2015; Rodriguez 2014). Many amino acid side chains such as cysteine (C), serine (S), threonine (T), and tyrosine (Y) are post-translationally modified; however, the amino acid lysine (K) is targeted by an extremely high number of PTMs including methylation, ubiquitination, sumoylation, and acetylation. Thyroid hormone receptors, and other nuclear receptors, undergo PTMs that influence transcriptional activity and subcellular localization (Abdel-Hafiz and Horwitz 2014; Cui, et al. 2004; Faresse 2014; Lin, et al. 2005; Sanchez-Pacheco, et al. 2009). For example, the association of TRα1 p30 with the plasma membrane is mediated by palmitoylation, a post-translational lipid modification. Consequently, it has been predicted that cysteine (Cys)254 and Cys255 palmitoylation is necessary to localize p30 to the plasma membrane (Kalyanaraman et al. 2014).
For nuclear TRs, phosphorylation regulates DNA binding and transcriptional activation, and it has been shown that phosphorylation of one or more sites in TRα1 enhances nuclear retention or inhibits nuclear export but is not directly involved in nuclear import (Nicoll, et al. 2003). Intriguingly, a recent study suggests the phosphorylation of TRβ1 may play a role in promoting nuclear localization in serum-starved Chinese hamster ovary (CHO) cells. FLAG-tagged TRβ1 was shown to form a cytoplasmic complex with the p85 regulatory subunit of PI3K and the Src family kinase Lyn (Martin et al. 2014). Complex formation was dependent on two phosphotyrosine motifs in the second zinc finger of TRβ1 that are not conserved in TRα1. When hormone was added, the complex dissociated, allowing PI3K activity to increase and TRβ1 to move into the nucleus to regulate transcription. It will be of interest to extend these studies to tracking receptor movement in live cells. The authors suggest that dramatic shifts in localization may not be observable with GFP-tagged receptors, because the GFP tag might interfere with PI3K association; however, their qualitative observations of receptor distribution are consistent with the variability we see in populations of cells expressing GFP-TRβ1. As shown in Fig. 3, GFP-TRβ1 typically has a greater cytosolic population than GFP-TRα1, and we find TRβ1 distributions ranging from whole cell to primarily nuclear. For critical analysis of the fine nuances of receptor localization, rigorous quantification of the nucleus versus cytoplasmic distribution by fluorescence intensity measurements will be essential.
Acetylation sites that are important for transcriptional activity have been identified in the hinge domain of TR, corresponding to K130, K134, and K136 in human TRα1 (Sanchez-Pacheco et al. 2009), and to K184, K188, and K190 in TRβ1 (Lin et al. 2005). These lysines are integral components of NLS-1 (Mavinakere et al. 2012), suggesting that acetylation state could have an impact on NLS activity. Whether this PTM is important for modulating the nuclear localization of TR subtypes is under investigation.
It is known that ubiquitination of liganded TRα1 targets the receptor for rapid proteasome-mediated degradation (Bondzi et al. 2011). Recently, it was reported that monoubiquitination of TRα1 within its LBD results in a shift in the diffuse intranuclear localization of TRα1 toward the nuclear periphery in cardiomyocytes (Wadosky et al. 2016). TRα1 activity stimulates hypertrophy in cardiomyocytes, and although TRα2 and TRβ1 are present in this cell type, they lack this function. Muscle-specific ubiquitin ligase muscle ring finger-1 (MuRF1) (Rodriguez, et al. 2015) was shown to monoubiquitinate TRα1 in vitro; however, specific lysine sites have not yet been identified and monoubiquitinated forms have not been detected in vivo (Wadosky et al. 2016). Whether polyubiquitination or monoubiquitination directly modulates TR nucleocytoplasmic shuttling remains to be determined.
Several studies have provided evidence that sumoylation of TR plays an essential role in fine-tuning TR regulation of gene expression. SUMO modification sites have been identified at K283 and K389 of TRα1 (positioned in NES-H3/H6); and at K50 (A/B domain within AF1), K146 (DBD) and at K443 (near the NES-H12 motif) of TRβ1 (Liu, et al. 2015; Liu, et al. 2012; Weitzel 2016). Given the proximity of the SUMO-modified lysines to NES motifs, sumoylation is also under study for its impact on NES activity and TR nuclear localization.
Taken together, these reports provide insights into the possible interplay of TR post-translational modification with TR localization: palmitoylation directs p30 to the membrane; phosphorylation promotes nuclear retention; acetylation occurs within the hinge NLS-1; and ubiquitination and sumoylation occur within the NES-containing LBD of TR. Although not yet reported to be post-translationally modified, TRα1 p43 contains mitochondrial import sequences and thus has a high probability of also containing PTM sites that modulate trafficking.
Mislocalization of thyroid hormone receptors and disease
In addition to diseases correlated with dysregulated hormone production, mutations in TR can give rise to disease, most notably the autosomal dominant Resistance to Thyroid Hormone (RTH) syndrome; and mutations can contribute to certain types of cancer, including human hepatocellular carcinoma, renal clear cell carcinoma, breast cancer, pituitary tumor, and thyroid cancer (Astapova et al. 2011). Early evidence to suggest that mutated TR could be involved in carcinogenesis came from the discovery that TRα1 is the cellular counterpart of the retroviral v-ErbA carried by the avian erythroblastosis virus involved in acute erythroleukemia and sarcomas (Sap, et al. 1986). Many of these TR mutants have lost T3 binding and transactivation capacity and some exhibit dominant negative activity (Chan and Privalsky 2010; Conde, et al. 2006; Kim and Cheng 2013; Lin, et al. 2013; Martinez-Iglesias, et al. 2009; Rosen, et al. 2011; Rosen and Privalsky 2009, 2011; Wojcicka, et al. 2014). The question is thus raised, does receptor localization impact disease pathology? So far, the answer appears to be, yes. Dominant negative TR mutants, such as v-ErbA, have been shown to localize to both the nuclear and cytoplasmic compartments in cells (Boucher, et al. 1988), are recruited to aggresomes, display altered transport activity and mislocalize TRα1 to these cytosolic inclusions (Bonamy and Allison 2006; Bonamy et al. 2005; Bondzi et al. 2011; Bunn et al. 2001; DeLong, et al. 2004; Takalo, et al. 2013; Zhang et al. 2017). The altered localization of v-ErbA appears to be enhanced by acquisition of the N-terminal viral Gag sequence, which harbors a strong CRM1-dependent NES (DeLong et al. 2004).
The factors that determine whether a given amino acid substitution causes endocrine disruption or cancer remain enigmatic, particularly for changes within the LBD. Typically, human cancers have multiple TR mutations, while single mutations are characteristic of RTH, and it has been proposed that synergistic interactions of these mutations strengthen the dominant negative activity (Rosen and Privalsky 2009, 2011). RTH syndromes exist due to mutations in the respective TR isoforms, TRα1 and TRβ1, and the variability in symptomatic phenotype is characterized by the tissues in which these isoforms are highly expressed (Mendoza and Hollenberg 2017; Mullur et al. 2014; Vella and Hollenberg 2017; Vella et al. 2014). Clinical phenotypes of RTH include elevated thyroid hormone levels, goiter, short stature, decreased weight, tachycardia, hearing loss, attention-deficit hyperactivity disorder, decreased IQ, and dyslexia (Bochukova, et al. 2012; Dumitrescu and Refetoff 2013; Moran, et al. 2013; Parrilla, et al. 1991; Schoenmakers, et al. 2013). Interestingly, the highest frequency of mutations occurs in the region corresponding to NES-H12, with another cluster of mutations occurring within NES-H3/H6 (Fig. 1).
Except for our prior studies, there is little information on the contribution of altered nucleocytoplasmic shuttling dynamics to the phenotype of RTH and cancer-promoting mutants. Two of our recent findings stand out: a R26H substitution in NLS-2 of the oncoprotein v-ErbA abrogates the activity of NLS-2, while mutagenesis studies on NES-H12 point to the intriguing possibility that altered shuttling of TRβ1 may be a contributing factor in RTH (Mavinakere et al. 2012). Based on these data, we hypothesize that intracellular mislocalization of TR is a crucial factor to consider in pathogenesis (Bonamy and Allison 2006; Bonamy et al. 2005).
Concluding remarks
Thyroid hormone receptor subtypes mediate the actions of thyroid hormone in a variety of cellular compartments, including the nucleus, the mitochondria, and at the inner surface of the plasma membrane (Fig. 4). Within the nucleus, TRα1 and TRβ1 bind to the TREs of target genes, in the presence or absence of thyroid hormone, to influence an astonishing number of cellular processes, including cell proliferation, oxygen consumption, protein synthesis, and carbohydrate, lipid, and vitamin metabolism. The physiological significance of TRα1 and TRβ1 nucleocytoplasmic shuttling may, at least in part, be to serve as a “ferry boat” (Kolodkin, et al. 2010) to increase the rate of T3 (and possibly T4) nuclear entry, relative to simple diffusion through the cytosol, or to circumvent localization of T3 to the mitochondria. Furthermore, important PTMs have been reported that suggest an increasingly complex interplay with TR’s NLS and NES motifs, and possibly MIS motifs, that may affect TR’s ultimate localization in target tissues. There is a dynamic balance between nuclear import, retention, and export of shuttling transcription factors and, in the case of TR, localization to cytosolic compartments as well. These observations, coupled with the multiplicity of thyroid hormone signaling within the cell, may provide important insights into the development of treatments for RTH and some types of cancer.
Acknowledgments
Funding
This work was supported in part by National Institutes of Health grant 2R15DK058028 and National Science Foundation grant MCB 1120513 to L.A.A.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
References
- Abdel-Hafiz HA, Horwitz KB. Post-translational modifications of the progesterone receptors. J Steroid Biochem Mol Biol. 2014;140:80–89. doi: 10.1016/j.jsbmb.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe S, Namba N, Abe M, Fujiwara M, Aikawa T, Kogo M, Ozono K. Monocarboxylate transporter 10 functions as a thyroid hormone transporter in chondrocytes. Endocrinology. 2012;153:4049–4058. doi: 10.1210/en.2011-1713. [DOI] [PubMed] [Google Scholar]
- Andersson ML, Vennstrom B. Chicken thyroid hormone receptor α requires the N-terminal amino acids for exclusive nuclear localization. FEBS Lett. 1997;416:291–296. doi: 10.1016/s0014-5793(97)01223-4. [DOI] [PubMed] [Google Scholar]
- Astapova I, Hollenberg AN. The in vivo role of nuclear receptor corepressors in thyroid hormone action. Biochim Biophys Acta. 2013;1830:3876–3881. doi: 10.1016/j.bbagen.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astapova I, Vella KR, Ramadoss P, Holtz KA, Rodwin BA, Liao XH, Weiss RE, Rosenberg MA, Rosenzweig A, Hollenberg AN. The nuclear receptor corepressor (NCoR) controls thyroid hormone sensitivity and the set point of the hypothalamic-pituitary-thyroid axis. Mol Endocrinol. 2011;25:212–224. doi: 10.1210/me.2010-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers S, Switnicki MP, Angajala A, Lammel J, Arumanayagam AS, Webb P. Genome-wide binding patterns of thyroid hormone receptor beta. PLoS One. 2014;9:e81186. doi: 10.1371/journal.pone.0081186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo C, Saiardi A. Why always lysine? The ongoing tale of one of the most modified amino acids. Adv Biol Regul. 2016;60:144–150. doi: 10.1016/j.jbior.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Bassett JH, Harvey CB, Williams GR. Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocrinol. 2003;213:1–11. doi: 10.1016/j.mce.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Baumann CT, Maruvada P, Hager GL, Yen PM. Nuclear cytoplasmic shuttling by thyroid hormone receptors. J Biol Chem. 2001;276:11237–11245. doi: 10.1074/jbc.M011112200. [DOI] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormone regulated genes in cerebral cortex development. J Endocrinol. 2017;232:R83–R97. doi: 10.1530/JOE-16-0424. [DOI] [PubMed] [Google Scholar]
- Bernal J, Guadano-Ferraz A, Morte B. Thyroid hormone transporters--functions and clinical implications. Nat Rev Endocrinol. 2015;11:406–417. doi: 10.1038/nrendo.2015.66. [DOI] [PubMed] [Google Scholar]
- Bernal J, Morte B. Thyroid hormone receptor activity in the absence of ligand: physiological and developmental implications. Biochim Biophys Acta. 2013;1830:3893–3899. doi: 10.1016/j.bbagen.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Bishop CM, McCabe CJ, Gittoes NJ, Butler PJ, Franklyn JA. Tissue-specific regulation of thyroid hormone receptor mRNA isoforms and target gene proteins in domestic ducks. J Endocrinol. 2000;165:607–615. doi: 10.1677/joe.0.1650607. [DOI] [PubMed] [Google Scholar]
- Bochukova E, Schoenmakers N, Agostini M, Schoenmakers E, Rajanayagam O, Keogh JM, Henning E, Reinemund J, Gevers E, Sarri M, et al. A mutation in the thyroid hormone receptor α gene. N Engl J Med. 2012;366:243–249. doi: 10.1056/NEJMoa1110296. [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. Embo J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonamy GM, Allison LA. Oncogenic conversion of the thyroid hormone receptor by altered nuclear transport. Nucl Recept Signal. 2006;4:e008. doi: 10.1621/nrs.04008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonamy GM, Guiochon-Mantel A, Allison LA. Cancer promoted by the oncoprotein v-ErbA may be due to subcellular mislocalization of nuclear receptors. Mol Endocrinol. 2005;19:1213–1230. doi: 10.1210/me.2004-0204. [DOI] [PubMed] [Google Scholar]
- Bondzi C, Brunner AM, Munyikwa MR, Connor CD, Simmons AN, Stephens SL, Belt PA, Roggero VR, Mavinakere MS, Hinton SD, et al. Recruitment of the oncoprotein v-ErbA to aggresomes. Mol Cell Endocrinol. 2011;332:196–212. doi: 10.1016/j.mce.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher P, Koning A, Privalsky ML. The avian erythroblastosis virus erbA oncogene encodes a DNA-binding protein exhibiting distinct nuclear and cytoplasmic subcellular localizations. J Virol. 1988;62:534–544. doi: 10.1128/jvi.62.2.534-544.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122:3035–3043. doi: 10.1172/JCI60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Fu L, Shi YB. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol. 2006;145:1–19. doi: 10.1016/j.ygcen.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Bunn CF, Neidig JA, Freidinger KE, Stankiewicz TA, Weaver BS, McGrew J, Allison LA. Nucleocytoplasmic shuttling of the thyroid hormone receptor α. Mol Endocrinol. 2001;15:512–533. doi: 10.1210/mend.15.4.0619. [DOI] [PubMed] [Google Scholar]
- Carazo A, Levin J, Casas F, Seyer P, Grandemange S, Busson M, Pessemesse L, Wrutniak-Cabello C, Cabello G. Protein sequences involved in the mitochondrial import of the 3,5,3′-L-triiodothyronine receptor p43. J Cell Physiol. 2012;227:3768–3777. doi: 10.1002/jcp.24085. [DOI] [PubMed] [Google Scholar]
- Casas F, Busson M, Grandemange S, Seyer P, Carazo A, Pessemesse L, Wrutniak-Cabello C, Cabello G. Characterization of a novel thyroid hormone receptor α variant involved in the regulation of myoblast differentiation. Mol Endocrinol. 2006;20:749–763. doi: 10.1210/me.2005-0074. [DOI] [PubMed] [Google Scholar]
- Cautain B, Hill R, de Pedro N, Link W. Components and regulation of nuclear transport processes. FEBS J. 2015;282:445–462. doi: 10.1111/febs.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan IH, Privalsky ML. A conserved lysine in the thyroid hormone receptor-α1 DNA-binding domain, mutated in hepatocellular carcinoma, serves as a sensor for transcriptional regulation. Mol Cancer Res. 2010;8:15–23. doi: 10.1158/1541-7786.MCR-09-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatonnet F, Guyot R, Benoit G, Flamant F. Genome-wide analysis of thyroid hormone receptors shared and specific functions in neural cells. Proc Natl Acad Sci U S A. 2013;110:E766–775. doi: 10.1073/pnas.1210626110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Tsai MM, Chi HC, Lin KH. Biological significance of a thyroid hormone-regulated secretome. Biochim Biophys Acta. 2013;1834:2271–2284. doi: 10.1016/j.bbapap.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Chook YM, Suel KE. Nuclear import by karyopherin-βs: recognition and inhibition. Biochim Biophys Acta. 2011;1813:1593–1606. doi: 10.1016/j.bbamcr.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde I, Paniagua R, Zamora J, Blanquez MJ, Fraile B, Ruiz A, Arenas MI. Influence of thyroid hormone receptors on breast cancer cell proliferation. Ann Oncol. 2006;17:60–64. doi: 10.1093/annonc/mdj040. [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhang M, Pestell R, Curran EM, Welshons WV, Fuqua SA. Phosphorylation of estrogen receptor α blocks its acetylation and regulates estrogen sensitivity. Cancer Res. 2004;64:9199–9208. doi: 10.1158/0008-5472.CAN-04-2126. [DOI] [PubMed] [Google Scholar]
- Cvoro A, Bajic A, Zhang A, Simon M, Golic I, Sieglaff DH, Maletic-Savatic M, Korac A, Webb P. Ligand independent and subtype-selective actions of thyroid hormone receptors in human adipose derived stem cells. PLoS One. 2016;11:e0164407. doi: 10.1371/journal.pone.0164407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Bercury KK, Jin W, Macklin WB. Olig1 Acetylation and nuclear export mediate oligodendrocyte development. J Neurosci. 2015;35:15875–15893. doi: 10.1523/JNEUROSCI.0882-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras VM, Van Herck SL, Heijlen M, De Groef B. Thyroid hormone receptors in two model species for vertebrate embryonic development: chicken and zebrafish. J Thyroid Res. 2011;2011:402320. doi: 10.4061/2011/402320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, O’Malley BW. Transcriptional coregulators: emerging roles of SRC family of coactivators in disease pathology. J Mol Endocrinol. 2014;53:R47–59. doi: 10.1530/JME-14-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12:111–121. doi: 10.1038/nrendo.2015.205. [DOI] [PubMed] [Google Scholar]
- DeLong LJ, Bonamy GM, Fink EN, Allison LA. Nuclear export of the oncoprotein v-ErbA is mediated by acquisition of a viral nuclear export sequence. J Biol Chem. 2004;279:15356–15367. doi: 10.1074/jbc.M308214200. [DOI] [PubMed] [Google Scholar]
- Dentice M, Marsili A, Zavacki A, Larsen PR, Salvatore D. The deiodinases and the control of intracellular thyroid hormone signaling during cellular differentiation. Biochim Biophys Acta. 2013;1830:3937–3945. doi: 10.1016/j.bbagen.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo EM, Wilhelm KG, Jr, Thompson DL, Koenig RJ. Variable RXR requirements for thyroid hormone responsiveness of endogenous genes. Mol Cell Endocrinol. 2007;264:149–156. doi: 10.1016/j.mce.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazic A, Myklebust LM, Ree R, Arnesen T. The world of protein acetylation. Biochim Biophys Acta. 2016;1864:1372–1401. doi: 10.1016/j.bbapap.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Dumitrescu AM, Refetoff S. The syndromes of reduced sensitivity to thyroid hormone. Biochim Biophys Acta. 2013;1830:3987–4003. doi: 10.1016/j.bbagen.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faresse N. Post-translational modifications of the mineralocorticoid receptor: How to dress the receptor according to the circumstances? J Steroid Biochem Mol Biol. 2014;143:334–342. doi: 10.1016/j.jsbmb.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Fernandez-Majada V, Pujadas J, Vilardell F, Capella G, Mayo MW, Bigas A, Espinosa L. Aberrant cytoplasmic localization of N-CoR in colorectal tumors. Cell Cycle. 2007;6:1748–1752. doi: 10.4161/cc.6.14.4429. [DOI] [PubMed] [Google Scholar]
- Figueira AC, Saidemberg DM, Souza PC, Martinez L, Scanlan TS, Baxter JD, Skaf MS, Palma MS, Webb P, Polikarpov I. Analysis of agonist and antagonist effects on thyroid hormone receptor conformation by hydrogen/deuterium exchange. Mol Endocrinol. 2011;25:15–31. doi: 10.1210/me.2010-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamant F. Futures challenges in thyroid hormone signaling research. Front Endocrinol (Lausanne) 2016;7:58. doi: 10.3389/fendo.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamant F, Cheng SY, Hollenberg AN, Moeller LC, Samarut J, Wondisford FE, Yen PM, Refetoff S. Thyroid hormone signaling pathways: time for a more precise nomenclature. Endocrinology. 2017;158:2052–2057. doi: 10.1210/en.2017-00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamant F, Gauthier K. Thyroid hormone receptors: the challenge of elucidating isotype-specific functions and cell-specific response. Biochim Biophys Acta. 2013;1830:3900–3907. doi: 10.1016/j.bbagen.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Flamini MI, Uzair ID, Pennacchio GE, Neira FJ, Mondaca JM, Cuello-Carrion FD, Jahn GA, Simoncini T, Sanchez AM. Thyroid hormone controls breast cancer cell movement via integrin αV/β3/SRC/FAK/PI3-Kinases. Horm Cancer. 2017;8:16–27. doi: 10.1007/s12672-016-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumel B, Roy S, Fouchecourt S, Livera G, Parent AS, Casas F, Guillou F. Depletion of the p43 mitochondrial T3 receptor increases Sertoli cell proliferation in mice. PLoS One. 2013;8:e74015. doi: 10.1371/journal.pone.0074015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galay-Burgos M, Power DM, Llewellyn L, Sweeney GE. Thyroid hormone receptor expression during metamorphosis of Atlantic halibut (Hippoglossus hippoglossus) Mol Cell Endocrinol. 2008;281:56–63. doi: 10.1016/j.mce.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Galton VA. The ups and downs of the thyroxine pro-hormone hypothesis. Mol Cell Endocrinol. 2017;458:105–111. doi: 10.1016/j.mce.2017.01.029. [DOI] [PubMed] [Google Scholar]
- Grespin ME, Bonamy GM, Roggero VR, Cameron NG, Adam LE, Atchison AP, Fratto VM, Allison LA. Thyroid hormone receptor α1 follows a cooperative CRM1/calreticulin-mediated nuclear export pathway. J Biol Chem. 2008;283:25576–25588. doi: 10.1074/jbc.M710482200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grontved L, Waterfall JJ, Kim DW, Baek S, Sung MH, Zhao L, Park JW, Nielsen R, Walker RL, Zhu YJ, et al. Transcriptional activation by the thyroid hormone receptor through ligand-dependent receptor recruitment and chromatin remodelling. Nat Commun. 2015;6:7048. doi: 10.1038/ncomms8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guissouma H, Ghaddab-Zroud R, Seugnet I, Decherf S, Demeneix B, Clerget-Froidevaux MS. TR α2 exerts dominant negative effects on hypothalamic Trh transcription in vivo. PLoS One. 2014;9:e95064. doi: 10.1371/journal.pone.0095064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm JB, Privalsky ML. Research resource: identification of novel coregulators specific for thyroid hormone receptor-β2. Mol Endocrinol. 2013;27:840–859. doi: 10.1210/me.2012-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama R, Rout MP, Fernandez-Martinez J. The nuclear pore complex core scaffold and permeability barrier: variations of a common theme. Curr Opin Cell Biol. 2017;46:110–118. doi: 10.1016/j.ceb.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayer A, Stoeber M, Bissig C, Helenius A. Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic. 2010;11:361–382. doi: 10.1111/j.1600-0854.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- Helbing CC, Crump K, Bailey CM, Kohno S, Veldhoen N, Bryan T, Bermudez D, Guillette LJ., Jr Isolation of the alligator (Alligator mississippiensis) thyroid hormone receptor α and β transcripts and their responsiveness to thyroid stimulating hormone. Gen Comp Endocrinol. 2006;149:141–150. doi: 10.1016/j.ygcen.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, et al. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci U S A. 2006;103:14104–14109. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Xu F, Qu T, Zhang R, Li L, Que H, Zhang G. Identification of thyroid hormones and functional characterization of thyroid hormone receptor in the Pacific Oyster Crassostrea gigas provide insight into evolution of the thyroid hormone system. PLoS One. 2015;10:e0144991. doi: 10.1371/journal.pone.0144991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman H, Schwappacher R, Joshua J, Zhuang S, Scott BT, Klos M, Casteel DE, Frangos JA, Dillmann W, Boss GR, et al. Nongenomic thyroid hormone signaling occurs through a plasma membrane-localized receptor. Sci Signal. 2014;7:ra48. doi: 10.1126/scisignal.2004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaho YI, Endo D, Park MK. Molecular characterization of thyroid hormone receptors from the leopard gecko, and their differential expression in the skin. Zoolog Sci. 2006;23:549–556. doi: 10.2108/zsj.23.549. [DOI] [PubMed] [Google Scholar]
- Kim WG, Cheng SY. Thyroid hormone receptors and cancer. Biochim Biophys Acta. 2013;1830:3928–3936. doi: 10.1016/j.bbagen.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Imamoto N. Biological significance of the importin-β family-dependent nucleocytoplasmic transport pathways. Traffic. 2014;15:727–748. doi: 10.1111/tra.12174. [DOI] [PubMed] [Google Scholar]
- Kolodkin AN, Bruggeman FJ, Plant N, Mone MJ, Bakker BM, Campbell MJ, van Leeuwen JP, Carlberg C, Snoep JL, Westerhoff HV. Design principles of nuclear receptor signaling: how complex networking improves signal transduction. Mol Syst Biol. 2010;6:446. doi: 10.1038/msb.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumara-Siri MH, Shapiro LE, Surks MI. Association of the 3,5,3′-triiodo-L-thyronine nuclear receptor with the nuclear matrix of cultured growth hormone-producing rat pituitary tumor cells (GC cells) J Biol Chem. 1986;261:2844–2852. [PubMed] [Google Scholar]
- Laudet V, Gronemeyer H. The Nuclear Receptor : Factsbook. San Diego: Academic Press; 2002. [Google Scholar]
- Lee Y, Mahdavi V. The D domain of the thyroid hormone receptor α1 specifies positive and negative transcriptional regulation functions. J Biol Chem. 1993;268:2021–2028. [PubMed] [Google Scholar]
- Leonard JL, Farwell AP. Thyroid hormone-regulated actin polymerization in brain. Thyroid. 1997;7:147–151. doi: 10.1089/thy.1997.7.147. [DOI] [PubMed] [Google Scholar]
- Li C, Goryaynov A, Yang W. The selective permeability barrier in the nuclear pore complex. Nucleus. 2016;7:430–446. doi: 10.1080/19491034.2016.1238997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Chin YT, Yang YC, Lai HY, Wang-Peng J, Liu LF, Tang HY, Davis PJ. Thyroid hormone, cancer, and apoptosis. Compr Physiol. 2016;6:1221–1237. doi: 10.1002/cphy.c150035. [DOI] [PubMed] [Google Scholar]
- Lin HY, Hopkins R, Cao HJ, Tang HY, Alexander C, Davis FB, Davis PJ. Acetylation of nuclear hormone receptor superfamily members: thyroid hormone causes acetylation of its own receptor by a mitogen-activated protein kinase-dependent mechanism. Steroids. 2005;70:444–449. doi: 10.1016/j.steroids.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Lin W, Gao L, Chen X. Protein-specific imaging of posttranslational modifications. Curr Opin Chem Biol. 2015;28:156–163. doi: 10.1016/j.cbpa.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Lin YH, Liao CJ, Huang YH, Wu MH, Chi HC, Wu SM, Chen CY, Tseng YH, Tsai CY, Chung IH, et al. Thyroid hormone receptor represses miR-17 expression to enhance tumor metastasis in human hepatoma cells. Oncogene. 2013;32:4509–4518. doi: 10.1038/onc.2013.309. [DOI] [PubMed] [Google Scholar]
- Liu YY, Ayers S, Milanesi A, Teng X, Rabi S, Akiba Y, Brent GA. Thyroid hormone receptor sumoylation is required for preadipocyte differentiation and proliferation. J Biol Chem. 2015;290:7402–7415. doi: 10.1074/jbc.M114.600312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Kogai T, Schultz JJ, Mody K, Brent GA. Thyroid hormone receptor isoform-specific modification by small ubiquitin-like modifier (SUMO) modulates thyroid hormone-dependent gene regulation. J Biol Chem. 2012;287:36499–36508. doi: 10.1074/jbc.M112.344317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Antoine DJ, Kwan K, Lundback P, Wahamaa H, Schierbeck H, Robinson M, Van Zoelen MA, Yang H, Li J, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci U S A. 2014;111:3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchia E, Falcone M, Giorgilli G, Bogazzi F, Antonangeli L, Baccarini S, Fontanini G, Torresani J, DeGroot LJ, Pinchera A. Site-specific anti-c-erb A antibodies recognizing native thyroid hormone receptors: their use to detect the expression and localization of α and β c-erb A proteins in rat liver. J Recept Res. 1992;12:201–215. doi: 10.3109/10799899209074792. [DOI] [PubMed] [Google Scholar]
- Manzon LA, Youson JH, Holzer G, Staiano L, Laudet V, Manzon RG. Thyroid hormone and retinoid X receptor function and expression during sea lamprey (Petromyzon marinus) metamorphosis. Gen Comp Endocrinol. 2014;204:211–222. doi: 10.1016/j.ygcen.2014.05.030. [DOI] [PubMed] [Google Scholar]
- Martin NP, Marron Fernandez de Velasco E, Mizuno F, Scappini EL, Gloss B, Erxleben C, Williams JG, Stapleton HM, Gentile S, Armstrong DL. A rapid cytoplasmic mechanism for PI3 kinase regulation by the nuclear thyroid hormone receptor, TRβ, and genetic evidence for its role in the maturation of mouse hippocampal synapses in vivo. Endocrinology. 2014;155:3713–3724. doi: 10.1210/en.2013-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Iglesias O, Garcia-Silva S, Tenbaum SP, Regadera J, Larcher F, Paramio JM, Vennstrom B, Aranda A. Thyroid hormone receptor β1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res. 2009;69:501–509. doi: 10.1158/0008-5472.CAN-08-2198. [DOI] [PubMed] [Google Scholar]
- Martinez L, Webb P, Polikarpov I, Skaf MS. Molecular dynamics simulations of ligand dissociation from thyroid hormone receptors: evidence of the likeliest escape pathway and its implications for the design of novel ligands. J Med Chem. 2006;49:23–26. doi: 10.1021/jm050805n. [DOI] [PubMed] [Google Scholar]
- Maruvada P, Baumann CT, Hager GL, Yen PM. Dynamic shuttling and intranuclear mobility of nuclear hormone receptors. J Biol Chem. 2003;278:12425–12432. doi: 10.1074/jbc.M202752200. [DOI] [PubMed] [Google Scholar]
- Mavinakere MS, Powers JM, Subramanian KS, Roggero VR, Allison LA. Multiple novel signals mediate thyroid hormone receptor nuclear import and export. J Biol Chem. 2012;287:31280–31297. doi: 10.1074/jbc.M112.397745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- Medici M, Visser WE, Visser TJ, Peeters RP. Genetic determination of the hypothalamic-pituitary-thyroid axis: where do we stand? Endocr Rev. 2015;36:214–244. doi: 10.1210/er.2014-1081. [DOI] [PubMed] [Google Scholar]
- Mendoza A, Astapova I, Shimizu H, Gallop MR, Al-Sowaimel L, MacGowan SMD, Bergmann T, Berg AH, Tenen DE, Jacobs C, et al. NCoR1-independent mechanism plays a role in the action of the unliganded thyroid hormone receptor. Proc Natl Acad Sci U S A. 2017;114:E8458–E8467. doi: 10.1073/pnas.1706917114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza A, Hollenberg AN. New insights into thyroid hormone action. Pharmacol Ther. 2017;173:135–145. doi: 10.1016/j.pharmthera.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S, Raja K, Schweizer U, Mugesh G. Chemistry and biology in the biosynthesis and action of thyroid hormones. Angew Chem Int Ed Engl. 2016;55:7606–7630. doi: 10.1002/anie.201601116. [DOI] [PubMed] [Google Scholar]
- Moran C, Schoenmakers N, Agostini M, Schoenmakers E, Offiah A, Kydd A, Kahaly G, Mohr-Kahaly S, Rajanayagam O, Lyons G, et al. An adult female with resistance to thyroid hormone mediated by defective thyroid hormone receptor alpha. J Clin Endocrinol Metab. 2013;98:4254–4261. doi: 10.1210/jc.2013-2215. [DOI] [PubMed] [Google Scholar]
- Moras D, Gronemeyer H. The nuclear receptor ligand-binding domain: structure and function. Curr Opin Cell Biol. 1998;10:384–391. doi: 10.1016/s0955-0674(98)80015-x. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Yamamoto H, Futawaka K, Atake A, Kasahara M, Tagami T. Molecular characterization of human thyroid hormone receptor β isoform 4. Endocr Res. 2016;41:34–42. doi: 10.3109/07435800.2015.1066801. [DOI] [PubMed] [Google Scholar]
- Morte B, Bernal J. Thyroid hormone action: astrocyte-neuron communication. Front Endocrinol (Lausanne) 2014;5:82. doi: 10.3389/fendo.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento AS, Dias SM, Nunes FM, Aparicio R, Ambrosio AL, Bleicher L, Figueira AC, Santos MA, de Oliveira Neto M, Fischer H, et al. Structural rearrangements in the thyroid hormone receptor hinge domain and their putative role in the receptor function. J Mol Biol. 2006;360:586–598. doi: 10.1016/j.jmb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Nelson ER, Habibi HR. Functional significance of a truncated thyroid receptor subtype lacking a hormone-binding domain in goldfish. Endocrinology. 2008;149:4702–4709. doi: 10.1210/en.2008-0107. [DOI] [PubMed] [Google Scholar]
- Nicoll JB, Gwinn BL, Iwig JS, Garcia PP, Bunn CF, Allison LA. Compartment-specific phosphorylation of rat thyroid hormone receptor α1 regulates nuclear localization and retention. Mol Cell Endocrinol. 2003;205:65–77. doi: 10.1016/s0303-7207(03)00199-0. [DOI] [PubMed] [Google Scholar]
- Oberoi J, Fairall L, Watson PJ, Yang JC, Czimmerer Z, Kampmann T, Goult BT, Greenwood JA, Gooch JT, Kallenberger BC, et al. Structural basis for the assembly of the SMRT/NCoR core transcriptional repression machinery. Nat Struct Mol Biol. 2011;18:177–184. doi: 10.1038/nsmb.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste-Berghaus C, Zanger K, Hashimoto K, Cohen RN, Hollenberg AN, Wondisford FE. Thyroid hormone-independent interaction between the thyroid hormone receptor β2 amino terminus and coactivators. J Biol Chem. 2000;275:1787–1792. doi: 10.1074/jbc.275.3.1787. [DOI] [PubMed] [Google Scholar]
- Orozco A, Navarrete-Ramirez P, Olvera A, Garcia GC. 3,5-Diiodothyronine (T2) is on a role. A new hormone in search of recognition. Gen Comp Endocrinol. 2014;203:174–180. doi: 10.1016/j.ygcen.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Panayiotou R, Miralles F, Pawlowski R, Diring J, Flynn HR, Skehel M, Treisman R. Phosphorylation acts positively and negatively to regulate MRTF-A subcellular localisation and activity. Elife. 2016:5. doi: 10.7554/eLife.15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa T, Ferrara AM, Refetoff S. Inherited defects of thyroxine-binding proteins. Best Pract Res Clin Endocrinol Metab. 2015;29:735–747. doi: 10.1016/j.beem.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrilla R, Mixson AJ, McPherson JA, McClaskey JH, Weintraub BD. Characterization of seven novel mutations of the c-erbA beta gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two “hot spot” regions of the ligand binding domain. J Clin Invest. 1991;88:2123–2130. doi: 10.1172/JCI115542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A, Aranda A. Thyroid hormone receptors, cell growth and differentiation. Biochim Biophys Acta. 2013;1830:3908–3916. doi: 10.1016/j.bbagen.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Pawlak M, Lefebvre P, Staels B. General molecular biology and architecture of nuclear receptors. Curr Top Med Chem. 2012;12:486–504. doi: 10.2174/156802612799436641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis SN, Servili A, Mazurais D, Zambonino-Infante JL, Miest JJ, Tomkiewicz J, Butts IAE. Temperature induced variation in gene expression of thyroid hormone receptors and deiodinases of European eel (Anguilla anguilla) larvae. Gen Comp Endocrinol. 2017 doi: 10.1016/j.ygcen.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Psarra AM, Sekeris CE. Steroid and thyroid hormone receptors in mitochondria. IUBMB Life. 2008;60:210–223. doi: 10.1002/iub.37. [DOI] [PubMed] [Google Scholar]
- Ramadoss P, Abraham BJ, Tsai L, Zhou Y, Costa-e-Sousa RH, Ye F, Bilban M, Zhao K, Hollenberg AN. Novel mechanism of positive versus negative regulation by thyroid hormone receptor β1 (TRβ1) identified by genome-wide profiling of binding sites in mouse liver. J Biol Chem. 2014;289:1313–1328. doi: 10.1074/jbc.M113.521450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JA. Interplay between nuclear transport and ubiquitin/SUMO modifications in the regulation of cancer-related proteins. Semin Cancer Biol. 2014;27:11–19. doi: 10.1016/j.semcancer.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Rodriguez JE, Liao JY, He J, Schisler JC, Newgard CB, Drujan D, Glass DJ, Frederick CB, Yoder BC, Lalush DS, et al. The ubiquitin ligase MuRF1 regulates PPARα activity in the heart by enhancing nuclear export via monoubiquitination. Mol Cell Endocrinol. 2015;413:36–48. doi: 10.1016/j.mce.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggero VR, Zhang J, Parente LE, Doshi Y, Dziedzic RC, McGregor EL, Varjabedian AD, Schad SE, Bondzi C, Allison LA. Nuclear import of the thyroid hormone receptor α1 is mediated by importin 7, importin β1, and adaptor importin α1. Mol Cell Endocrinol. 2016;419:185–197. doi: 10.1016/j.mce.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MD, Chan IH, Privalsky ML. Mutant thyroid hormone receptors (TRs) isolated from distinct cancer types display distinct target gene specificities: a unique regulatory repertoire associated with two renal clear cell carcinomas. Mol Endocrinol. 2011;25:1311–1325. doi: 10.1210/me.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MD, Privalsky ML. Thyroid hormone receptor mutations found in renal clear cell carcinomas alter corepressor release and reveal helix 12 as key determinant of corepressor specificity. Mol Endocrinol. 2009;23:1183–1192. doi: 10.1210/me.2009-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MD, Privalsky ML. Thyroid hormone receptor mutations in cancer and resistance to thyroid hormone: perspective and prognosis. J Thyroid Res. 2011;2011:361304. doi: 10.4061/2011/361304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Llorente L, Martinez-Iglesias O, Garcia-Silva S, Tenbaum S, Regadera J, Aranda A. The thyroid hormone receptors as tumor suppressors. Horm Mol Biol Clin Investig. 2011;5:79–89. doi: 10.1515/HMBCI.2010.045. [DOI] [PubMed] [Google Scholar]
- Saelim N, Holstein D, Chocron ES, Camacho P, Lechleiter JD. Inhibition of apoptotic potency by ligand stimulated thyroid hormone receptors located in mitochondria. Apoptosis. 2007;12:1781–1794. doi: 10.1007/s10495-007-0109-1. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pacheco A, Martinez-Iglesias O, Mendez-Pertuz M, Aranda A. Residues K128, 132, and 134 in the thyroid hormone receptor-α are essential for receptor acetylation and activity. Endocrinology. 2009;150:5143–5152. doi: 10.1210/en.2009-0117. [DOI] [PubMed] [Google Scholar]
- Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennstrom B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986;324:635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Schoenmakers N, Moran C, Peeters RP, Visser T, Gurnell M, Chatterjee K. Resistance to thyroid hormone mediated by defective thyroid hormone receptor α. Biochim Biophys Acta. 2013;1830:4004–4008. doi: 10.1016/j.bbagen.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Schweizer U, Towell H, Vit A, Rodriguez-Ruiz A, Steegborn C. Structural aspects of thyroid hormone binding to proteins and competitive interactions with natural and synthetic compounds. Mol Cell Endocrinol. 2017;458:57–67. doi: 10.1016/j.mce.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Astapova I, Ye F, Bilban M, Cohen RN, Hollenberg AN. NCoR1 and SMRT play unique roles in thyroid hormone action in vivo. Mol Cell Biol. 2015;35:555–565. doi: 10.1128/MCB.01208-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skah S, Uchuya-Castillo J, Sirakov M, Plateroti M. The thyroid hormone nuclear receptors and the Wnt/β-catenin pathway: An intriguing liaison. Dev Biol. 2017;422:71–82. doi: 10.1016/j.ydbio.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Sonoda MT, Martinez L, Webb P, Skaf MS, Polikarpov I. Ligand dissociation from estrogen receptor is mediated by receptor dimerization: evidence from molecular dynamics simulations. Mol Endocrinol. 2008;22:1565–1578. doi: 10.1210/me.2007-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano FX, Leveille F, Papadia S, Bell KF, Puddifoot C, Hardingham GE. Neuronal activity controls the antagonistic balance between peroxisome proliferator-activated receptor-γ coactivator-1α and silencing mediator of retinoic acid and thyroid hormone receptors in regulating antioxidant defenses. Antioxid Redox Signal. 2011;14:1425–1436. doi: 10.1089/ars.2010.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza PC, Puhl AC, Martinez L, Aparicio R, Nascimento AS, Figueira AC, Nguyen P, Webb P, Skaf MS, Polikarpov I. Identification of a new hormone-binding site on the surface of thyroid hormone receptor. Mol Endocrinol. 2014;28:534–545. doi: 10.1210/me.2013-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian KS, Dziedzic RC, Nelson HN, Stern ME, Roggero VR, Bondzi C, Allison LA. Multiple exportins influence thyroid hormone receptor localization. Mol Cell Endocrinol. 2015;411:86–96. doi: 10.1016/j.mce.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami T, Yamamoto H, Moriyama K, Sawai K, Usui T, Shimatsu A, Naruse M. Identification of a novel human thyroid hormone receptor β isoform as a transcriptional modulator. Biochem Biophys Res Commun. 2010;396:983–988. doi: 10.1016/j.bbrc.2010.05.038. [DOI] [PubMed] [Google Scholar]
- Takalo M, Salminen A, Soininen H, Hiltunen M, Haapasalo A. Protein aggregation and degradation mechanisms in neurodegenerative diseases. Am J Neurodegener Dis. 2013;2:1–14. [PMC free article] [PubMed] [Google Scholar]
- Taylor E, Heyland A. Evolution of thyroid hormone signaling in animals: Non-genomic and genomic modes of action. Mol Cell Endocrinol. 2017;459:14–20. doi: 10.1016/j.mce.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Tomura H, Lazar J, Phyillaier M, Nikodem VM. The N-terminal region (A/B) of rat thyroid hormone receptors α 1, β 1, but not β 2 contains a strong thyroid hormone-dependent transactivation function. Proc Natl Acad Sci U S A. 1995;92:5600–5604. doi: 10.1073/pnas.92.12.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran EJ, King MC, Corbett AH. Macromolecular transport between the nucleus and the cytoplasm: Advances in mechanism and emerging links to disease. Biochim Biophys Acta. 2014;1843:2784–2795. doi: 10.1016/j.bbamcr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T, Fujiki Y. Ligand-dependent nucleo-cytoplasmic shuttling of peroxisome proliferator-activated receptors, PPARα and PPARγ. Genes Cells. 2012;17:576–596. doi: 10.1111/j.1365-2443.2012.01607.x. [DOI] [PubMed] [Google Scholar]
- van der Spek AH, Fliers E, Boelen A. Thyroid hormone metabolism in innate immune cells. J Endocrinol. 2017;232:R67–R81. doi: 10.1530/JOE-16-0462. [DOI] [PubMed] [Google Scholar]
- Vella KR, Hollenberg AN. The actions of thyroid hormone signaling in the nucleus. Mol Cell Endocrinol. 2017;458:127–135. doi: 10.1016/j.mce.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella KR, Ramadoss P, Costa ESRH, Astapova I, Ye FD, Holtz KA, Harris JC, Hollenberg AN. Thyroid hormone signaling in vivo requires a balance between coactivators and corepressors. Mol Cell Biol. 2014;34:1564–1575. doi: 10.1128/MCB.00129-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennstrom B, Liu H, Forrest D. Thyroid hormone receptors. In: Bunce CM, Campbell MJ, editors. Nuclear Receptors. Current Concepts and Future Challenges. London: Springer; 2010. [Google Scholar]
- Wadosky KM, Berthiaume JM, Tang W, Zungu M, Portman MA, Gerdes AM, Willis MS. MuRF1 mono-ubiquitinates TRα to inhibit T3-induced cardiac hypertrophy in vivo. J Mol Endocrinol. 2016;56:273–290. doi: 10.1530/JME-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li S. Protein mislocalization: mechanisms, functions and clinical applications in cancer. Biochim Biophys Acta. 2014;1846:13–25. doi: 10.1016/j.bbcan.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel JM. Impaired repressor function in SUMOylation-defective thyroid hormone receptor isoforms. Eur Thyroid J. 2016;5:152–163. doi: 10.1159/000447232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR. Cloning and characterization of two novel thyroid hormone receptor β isoforms. Mol Cell Biol. 2000;20:8329–8342. doi: 10.1128/mcb.20.22.8329-8342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcicka A, Piekielko-Witkowska A, Kedzierska H, Rybicka B, Poplawski P, Boguslawska J, Master A, Nauman A. Epigenetic regulation of thyroid hormone receptor β in renal cancer. PLoS One. 2014;9:e97624. doi: 10.1371/journal.pone.0097624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrutniak-Cabello C, Casas F, Cabello G. Mitochondrial T3 receptor and targets. Mol Cell Endocrinol. 2017;458:112–120. doi: 10.1016/j.mce.2017.01.054. [DOI] [PubMed] [Google Scholar]
- Xu L, Glass CK, Rosenfeld MG. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- Zhang J, Roggero VR, Allison LA. Nuclear import and export of the thyroid hormone receptor. Vitamins and Hormones. 2017:106. doi: 10.1016/bs.vh.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Zhao RL, Sun B, Liu Y, Li JH, Xiong WL, Liang DC, Guo G, Zuo AJ, Zhang JY. Cloning and identification of a novel thyroid hormone receptor β isoform expressed in the pituitary gland. Mol Cell Biochem. 2014;389:141–150. doi: 10.1007/s11010-013-1935-9. [DOI] [PubMed] [Google Scholar]
- Zhu XG, Hanover JA, Hager GL, Cheng SY. Hormone-induced translocation of thyroid hormone receptors in living cells visualized using a receptor green fluorescent protein chimera. J Biol Chem. 1998;273:27058–27063. doi: 10.1074/jbc.273.42.27058. [DOI] [PubMed] [Google Scholar]