Abstract
Essentially all animals with nervous systems utilize electrical synapses as a core element of communication. Electrical synapses, formed by gap junctions between neurons, provide rapid, bidirectional communication that accomplishes tasks distinct from and complementary to chemical synapses. These include coordination of neuron activity, suppression of voltage noise, establishment of electrical pathways that define circuits, and modulation of high order network behavior. In keeping with the omnipresent demand to alter neural network function in order to respond to environmental cues and perform tasks, electrical synapses exhibit extensive plasticity. In some networks, this plasticity can have dramatic effects that completely remodel circuits or remove the influence of certain cell types from networks. Electrical synaptic plasticity occurs on three distinct time scales, ranging from milliseconds to days, with different mechanisms accounting for each. This essay highlights principles that dictate the properties of electrical coupling within networks and the plasticity of the electrical synapses, drawing examples extensively from retinal networks.
Keywords: Electrical synapse, connexin, phosphorylation, phosphatase, circadian rhythm
Introduction
The fundamental organizing principle of a nervous system is synaptic communication between its elements. This is accomplished primarily by two types of synapses, chemical and electrical, which have distinct properties and serve complementary functions. Chemical synapses require release of a transmitter from one cell, providing selective, unidirectional transmission that is highly versatile. They can produce sign-conserving or sign-inverting responses; they can amplify; they can modulate other synapses. We tend to think of electrical synapses, gap junctions between neurons, as simple structures that merely provide quicker, bidirectional transmission without a transmitter. In reality, electrical synapses are sophisticated elements of neural circuits that support emergent properties of network behavior [1, 2]. Some recent reviews have discussed the characteristics of electrically-coupled networks in detail [2–4].
Fundamental to the functioning of a nervous system is plasticity of its synapses. Plasticity is essential to adapt to environmental stimuli, to produce complex behavior, and to learn. While plasticity is often considered the domain of chemical synapses, it has become clear that plasticity is also a fundamental property of electrical synapses. A great deal of recent work has contributed to understanding the mechanisms that underlie plasticity. In this review, I will discuss the mechanisms of electrical synaptic plasticity, making extensive use of examples from the vertebrate retina, in which electrical synaptic plasticity has been studied in detail. However, the principles proposed apply generally throughout the central nervous system, and indeed also to non-vertebrate nervous systems that use innexins rather than connexins to form electrical synapses.
Electrical synapses comprise a surprisingly large fraction of synapses. In the rabbit retinal connectome RC1 [5, 6] about 20 electrical synapses have been found for every 100 chemical synapses of all types combined, with about ¼ of the volume annotated (Robert Marc, personal communication). Consequently, their contributions to neural network functions are profound. Over the years, many electrically-coupled networks have been studied. From this body of work, it is possible to derive several principles that describe the characteristics of electrical coupling and the mechanisms that control its plasticity.
1. The magnitude of electrical coupling can vary dramatically between different neurons or with different states of plasticity
In order to understand electrical synapses, it is important to understand that electrical synapses do not represent a single entity with a single purpose. Rather, electrical synapses in different neurons can have dramatically different properties in order to serve disparate functions. Some neurons can have massive coupling. Junctional conductance of up to 500 nS has been measured between isolated pairs of retinal horizontal cells [7]. These neurons operate in a network that is, at times, effectively syncytial. Light responses arising in a single cell are distributed widely through the network, contributing to a broad average measure of local lighting conditions that is subtracted from photoreceptor output through inhibitory feedback to photoreceptor synapses. In contrast, most neurons are coupled to a much smaller extent. More typical measurements of junctional conductance are an average of 6 nS between pairs of fairly well-coupled MesV neurons [8] and 0.8 nS between pairs of hippocampal interneurons [9]. This low coupling serves different purposes, such as synchronizing the spiking activity of nearest neighbor cells.
Three factors intrinsic to the gap junctions contribute to differences in junctional conductance between cells: 1) the number of gap junction channels between the cells, 2) the fraction of those channels that are “active” or open, and 3) the single channel conductance of the individual channels. The first two factors change with time, contributing to forms of plasticity. The third factor is a characteristic property of each connexin type, and for connexins with distinct subconductance states, can also change with time. Use of different connexins in a neural circuit contributes to a design strategy to achieve specific circuit outcomes. In mammals, 5 types of connexin have been identified expressed in neurons: Cx36, Cx45, Cx57, Cx50, and Cx30.2 [10–15]. A number of others have been found in fish and amphibian neurons, but for the most part are evolutionarily closely related to the 5 in mammals [16]. These connexins vary substantially in their properties. Single channel conductance ranges from very large 220 pS channels made by Cx50 [17] to 10–15 pS channels made by Cx36 [18, 19] and 9 pS channels made by Cx30.2 [20].
The effect of expression of different connexin types has been specifically evaluated in retinal horizontal cell networks, which vary substantially in their receptive field size and tracer permeability properties between different types of horizontal cell. In theoretical calculations based on measured gap junction area, cell type coverage factor (overlap within the network), connexin type single channel conductance, and the estimated fraction of open connexin channels, Pan et al. [21] estimated that the junctional conductance between adjacent pairs of rabbit A-type horizontal cells expressing Cx50 was 20-fold larger than that of B-type horizontal cell axon terminals expressing Cx57. Of this, ~4-fold was attributable to connexin single channel conductance, ~3-fold to gap junction area, and ~2-fold to coverage factor of the cell network.
The extent of electrical coupling in any network is not constant, but changes dynamically in response to network activity and signaling. For example, in isolated retinal horizontal cell pairs, application of dopamine, nitric oxide and various drugs that altered intracellular signaling pathways reversibly changed junctional conductance by 40–90% [7, 22]. This is in keeping with the scale of plasticity observed by tracer coupling in horizontal cell networks in the intact retina in response to dopamine administration or light adaptation [23–25]. Such changes in coupling significantly alter the network performance of the neurons. For horizontal cells, transition from the poorly-coupled state to the well-coupled state effectively removes the inhibitory influence of the horizontal cell network on photoreceptor synapses in response to localized stimuli [26] due to dissipation of the small signal through the extensive network, resulting in decreased feedback strength.
2. Different forms of plasticity are developed on three time scales
Electrical synapse plasticity, defined broadly, can encompass any changes that alter the degree of coupling between neurons. Electrical coupling is most simply described by the effect a voltage change in one cell has on its coupled neighbor, termed the coupling coefficient (C):
| (1) |
where V1 is the voltage of the “driver” cell and V2 is the voltage of the “follower” cell. Figure 1A diagrams the electrical circuit represented by two coupled cells in the steady state. Current (I) injected into cell 1 passes toward ground (arrows) either across the resistance of its own membrane (R1), or across the gap junctional resistance (Rj) and the membrane resistance of cell 2 (R2) in series. Because Rj and R2 are organized in series, they act as a voltage divider, so that cell 2 voltage depends on cell 1 voltage according to:
| (2) |
This can be rearranged:
| (3) |
From this it is evident that the coupling coefficient depends not only on the junctional resistance but also on the membrane resistance of the postsynaptic cell, and more specifically on the relative magnitudes of the two. Any changes in these parameters alter C.
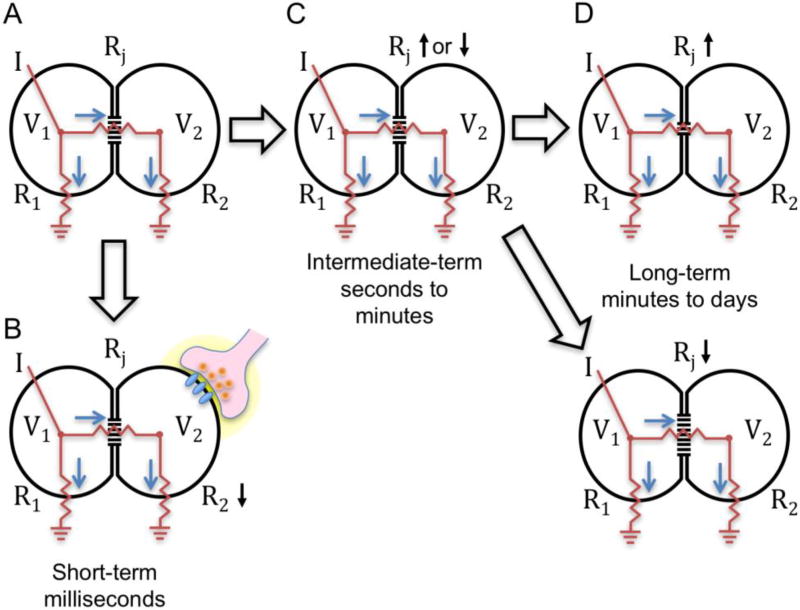
Figure 1. Mechanisms that alter coupling on three time scales.
A. Equivalent circuit for a pair of gap junction-coupled cells during current injection into cell 1 (I), where R1 and R2 represent the membrane resistance of cell 1 and cell 2 respectively, and Rj represents the junctional resistance. Current flows toward ground following the blue arrows, influencing the voltage of cell 1 (V1) and cell 2 (V2). B. Short-term change in coupling resulting from activation of post-synaptic receptors on cell 2 and resulting drop in R2. These changes take milliseconds to develop and may last up to a few seconds. C. Intermediate-term changes in coupling resulting from modification of existing gap junction channels, such as phosphorylation or de-phosphorylation, and concomitant decrease or increase in Rj. These changes occur in seconds to minutes and may remain at a stable steady state for hours. D. Long-term changes in coupling resulting from a decrease (top) or increase (bottom) in the number of gap junction channels between the cells. These changes result from expression or turnover changes.
Plasticity of electrical coupling is elicited by mechanisms that change either R2 or Rj. These mechanisms are most easily grouped by time scales in which they act:
Short-term plasticity occurs on a milliseconds time scale. This is most often achieved by transient changes in R2 as a result of plasma membrane channels opening or closing (figure 1B), but may also result from voltage gating of the connexin channels themselves. Some authors consider dynamic changes of membrane resistance not to constitute electrical synapse “plasticity” because they do not change junctional conductance. They are included here nonetheless because of their important role in modulation of electrical coupling.
Intermediate-term plasticity takes seconds to minutes to alter the state. These changes are dominated by changes in Rj in response to a variety of synaptic and extracellular cues (figure 1C). Intermediate-term plasticity often results in pseudo-stable state changes that are referred to in literature as ‘long-term potentiation’ or ‘long-term depression.’ I refer to them as intermediate-term here to acknowledge their dynamic nature and to distinguish from mechanistically-different long-term changes.
Long-term plasticity occurs on a minutes to hours time scale as result of protein turnover and gene expression changes. These changes may last hours, days, weeks, or a lifetime, and primarily influence Rj (figure 1D).
Short-term plasticity can occur in any circuit due to shunting effects of ion channel activation (figure 1B). The classic example of this occurs in the dendritic electrical synapses of inferior olive (IO) neurons. These electrical synapses occur in glomeruli densely surrounded by inhibitory and excitatory chemical synapses [27], an arrangement that prompted Llinas et al. [28] to propose that activation of inhibitory synapses arising from the deep cerebellar nuclei (DCN) would transiently decouple IO neurons due to current shunting (a decrease in R2). This was indeed demonstrated recently in a study in which optogenetic stimulation of DCN neurons transiently reduced IO coupling [29].
Intermediate term plasticity, occurring on a time scale of seconds to minutes, is a prominent form of plasticity in most neurons in which electrical synaptic plasticity has been observed. This results from changes in Rj as a result of modulation of the activity of the connexin channels present (figure 1C). This is the dominant form of plasticity in retinal circuits in the context of circadian and light-driven changes in adaptation state that occur every day (cf. [24, 25, 30, 31]). Indeed, such plasticity occurs in retinal circuits even at a developmental stage when only intrinsically-photosensitive retinal ganglion cells (ipRGCs) contribute to light-dependent behaviors [32]. At this time, glutamatergic activity waves strongly modulate coupling in the ipRGC circuit, directly influencing the number of ganglion cells that can respond to light. Although well-studied in the retina, intermediate-term plasticity is by no means restricted to retinal circuits. Prominent plasticity of the electrical synaptic component of the Mauthner cell mixed synapse [33–36], and of electrical synapses in the supraoptic nucleus [37], thalamic reticular nucleus [38–40], inferior olive [41, 42], and many other areas [43] depend on this type of mechanism. Many of the principles discussed in this essay relate to intermediate-term plasticity, and mechanisms will be discussed in detail in the sections that follow.
Long-term plasticity results from changes in gene expression that alter the expression level or possibly the type of connexin present in a circuit (figure 1D). Gap junctional coupling among neurons changes throughout the course of development, with a pattern of increased coupling established gradually during development and then a tapering off toward adulthood [44]. This developmental expression pattern plays important roles in organizing activity waves in the developing retina that contribute to patterning of projections and synaptic contacts in the lateral geniculate and visual cortex [45, 46]. Gene expression changes may also alter coupling on shorter time scales. Katti et al. [47] have found that the expression level of Cx36, as reflected by transcript and protein abundance, approximately doubles at night in mouse photoreceptors over the daytime level. This change may contribute to the circadian change in coupling among photoreceptors. Changes in connexin expression level and coupling have also been identified in response to injury [48–51], and may play important roles in the developing pathology of the injured nervous system.
Finally, changes in protein turnover or stability may induce long-term plasticity. Flores et al. [52] demonstrated experimentally that interfering with vesicle trafficking machinery involved in exocytosis or endocytosis of cargo vesicles in Mauthner neurons progressively decreased or increased, respectively, electrical synaptic coupling. The calculated half-life of 1–3 hr [52] is in reasonable agreement to the 3.1 hr half-life of Cx35 in gap junctions measured in a cell culture system [53]. In the same study, Flores et al. found that interfering with the interactions of Cx35 with its C-terminal anchored scaffold proteins destabilized gap junctions and resulted in their loss. These observations demonstrate that a steady state level of electrical coupling is maintained by continuous insertion and removal of connexins and stabilization of the gap junctions by accessory protein complexes. All of these processes may be regulated.
3. Intermediate term plasticity of most electrical synapses is controlled by a balance of protein kinase and phosphatase activities that control phosphorylation state of the connexins
Intermediate-term plasticity is a dominant factor in activity- and neurotransmitter-driven changes in electrical coupling that reshape neural circuits. As such, it has received a lot of attention and will command a large fraction of the discussion of design principles in this review. Changes in coupling in many neural circuits driven by environmental cues and activity have been linked to changes in protein kinase activities. It has been assumed that this results in changes in phosphorylation of the connexin(s) that form the electrical synapses cf. [22–24, 36, 54]. Surprisingly, among connexins that form electrical synapses, this has only been shown to be specifically true for Cx36 [55–57] and Cx50 [58]. Because of the extensive work that has been done with Cx36 in the last decade, most of the principles discussed will use this connexin as the example.
To study plasticity of retinal electrical synapses, Kothmann et al. [59] developed phospho-specific antibodies directed to two evolutionarily conserved regulatory phosphorylation sites of Cx35 and Cx36. This study showed that phosphorylation of both sites was strongly regulated by light in certain gap junctions. In subsequent studies, Kothmann et al. [60] and Li et al. [61, 62] showed that phosphorylation of Cx36 was directly related to coupling in AII amacrine cells and photoreceptors, respectively. The phosphorylation level of Cx36 showed a direct, positive correlation to functional tracer coupling over slightly more than an order of magnitude in both neural networks, without changes in number or size of gap junctions [60, 62]. These studies revealed that activities of protein kinases [61–63] or phosphatases [60] were critical to set the level of coupling in the network, and controlled the pattern of plasticity.
A key feature of intermediate-term plasticity is that it is intrinsically dynamic. While plasticity of this type may be referred to as ‘long-term potentiation’ (cf. [36, 39]) or ‘long-term depression’ (cf. [39, 64]), the resulting states are pseudo-stable. The underlying mechanism that creates them depends on continuous phosphorylation and de-phosphorylation of the population of connexin channels in order to maintain a steady state. As a result, experimental manipulations that result in a change in the rate of either process alter coupling in seconds to minutes. The signaling pathways that control these processes are central to the behavior of each type of electrical synapse, and determine the pattern of plasticity observed. Examples of these signaling pathways will be described in more detail in the following sections.
A different mechanism has been described recently that may also contribute to plasticity of electrical synapses. Connexin 36 has been found to be inhibited by physiological concentrations of intracellular magnesium [65]. It is proposed that physiological changes in metabolism that alter intracellular ATP, the primary buffer for Mg2+, can modulate coupling [65, 66]. While this has yet to be demonstrated physiologically, dynamic changes in coupling have been induced by changing intracellular Mg2+ and ATP in neurons of the trigeminal mesencephalic nucleus [65], and in interneurons of the thalamic reticular nucleus [66].
4. Organization of signaling pathways determines the pattern of plasticity
Each electrical synapse undergoes plasticity in a pattern that is stereotyped for a specific neural circuit. While the pattern may be a simple, monophasic “opened-closed” shift, this is not necessarily true for all circuits. Some retinal circuits show biphasic responses to the driving environmental factor, light intensity. For example, AII amacrine cell coupling has been observed to be very low in complete darkness, elevated at low to moderate light intensities, and reduced again in bright light [30]. The same pattern has also been observed in horizontal cells [67, 68]. Other neurons show different patterns. Detailed studies in photoreceptors and AII amacrine cells have shown that the organization of signaling pathways that control connexin phosphorylation are responsible for these different patterns [60–63]. From these studies, certain principles governing the pattern of plasticity can be deduced.
A. Convergence of signaling pathways on a single point of control results in monophasic regulation of plasticity
The simplest, and perhaps intuitively most expected pattern of plasticity is a monophasic shift from an open to a closed state or from a closed to an open state in response to a physiological cue. Retinal photoreceptors provide a well-studied example of this pattern of plasticity. Coupling among mammalian and fish photoreceptors is high at night in darkness and shifts to low in the daytime and in light [31, 61]. This process, as assessed by the coupling of rods to cones, is controlled by a circadian rhythm as well as light [31, 69–71], and has been linked to the circadian rhythm of dopamine secretion. Some of these studies showed that dopamine D4 receptor activation was responsible for the uncoupling driven by subjective day or by light adaptation, when extracellular dopamine levels are high [31, 70, 71]. D4 receptor activation inhibits adenylate cyclase through Gi signaling, reducing the production of cAMP and reducing the activity of cAMP-dependent protein kinase (PKA). The activity of PKA, in turn, directly controls the phosphorylation of Cx36 and photoreceptor coupling [61] (figure 2).
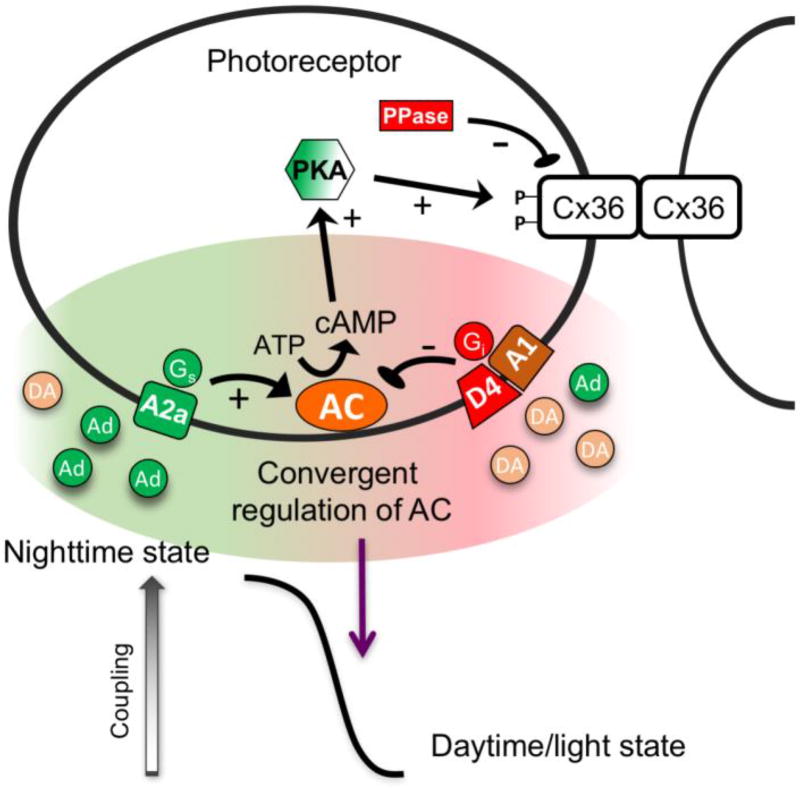
Figure 2. Mechanisms controlling plasticity of photoreceptor electrical synapses.
Light adaptation and circadian rhythms control the state of photoreceptor coupling through transduction of extracellular dopamine and adenosine signals via G-protein coupled receptors. Adenosine A2a receptors are stimulatory through Gs signaling and dopamine D4 and adenosine A1 receptors are inhibitory through Gi signaling. All three receptor types converge on adenylate cyclase (AC) to control protein kinase A (PKA) activity and Cx36 phosphorylation. The action of an unidentified phosphatase (PPase) is required to achieve the daytime, dephosphorylated condition. Convergence of the key regulating signals on a single point of control (AC) results in a monophasic transition from the nighttime to daytime states. The dynamic range achieved by these mechanisms is at least an order of magnitude.
Recent studies have revealed that this signaling pathway is substantially more complicated than had been believed. Li et al. [62, 72] have found that photoreceptor coupling is controlled not only by dopamine but also by adenosine, acting through both adenosine A2a and A1 receptors (figure 2). Like dopamine, extracellular adenosine in the retina is also controlled by light and a circadian rhythm, with extracellular levels highest in darkness in the subjective night [73]. Adenosine A2a receptors are activated by the nighttime level of adenosine to activate adenylate cyclase through Gs, enhancing PKA activity and increasing Cx36 phosphorylation [62]. Conversely, the Gi-coupled A1 receptor is also present. This receptor has slightly higher affinity for adenosine than does A2a and is active in the light-adapted daytime state, when the A2a receptor is not [72]. The Gi-coupled A1 receptor inhibits adenylate cyclase, reinforcing the inhibitory effect of the dopamine D4 receptor. Although it has not been specifically tested, it is presumed that the A1 receptor is also active at night, but is either present at lower abundance or has lower efficacy than the A2a receptor that dominates the control of adenylate cyclase activity.
With at least three G-protein coupled receptors controlling photoreceptor coupling, is conceivable that the pattern of plasticity could be very complex. However, all three receptors converge on a single point of control, adenylate cyclase (figure 2). This leads to the monophasic plasticity curve that is observed experimentally. In principle, the convergence of several signaling pathways on a single point of control should affect the steepness, or Hill coefficient, of the plasticity curve; this has not been tested experimentally to date. Finally, it should be noted that the plasticity of photoreceptor electrical synapses requires the activity of a phosphatase that has not been identified (figure 2). Tonic activity of a phosphatase would support the simple, monophasic plasticity pattern observed. However, if the phosphatase activity was regulated, more complex, though perhaps subtle, patterns of plasticity may emerge. Such complexity is explained in more detail in the following section.
B. Independent phosphorylating and dephosphorylating pathways result in biphasic or higher order patterns of plasticity
There is no a priori reason to expect any set of signaling cues to converge on a common point of regulation. Indeed, if separate signaling pathways regulate activities of the protein kinase that phosphorylates a connexin and the protein phosphatase activity that de-phosphorylates it, a biphasic pattern of plasticity is to be expected. This pattern of plasticity is well demonstrated by the electrical synapses of retinal AII amacrine cells. AII amacrine cells are known to display biphasic plasticity of receptive field size and intercellular coupling with respect to background light intensity [30, 74]. Recent studies have demonstrated how independent signaling pathways controlling Cx36 phosphorylation and de-phosphorylation result in this pattern of plasticity (figure 3). On the activating leg of the plasticity curve, Cx36 phosphorylation is controlled by the activity of glutamatergic bipolar neurons that are pre-synaptic to, or very nearby, AII amacrine cells [63]. Non-synaptic NMDA receptors that are directly associated with the electrical synapses respond to spillover glutamate, triggering a Ca2+-dependent signaling cascade that activates CaM-Kinase II and phosphorylates Cx36 (figure 3). Direct synaptic activation of the AII amacrine cells (via AMPA receptors) also contributes to this signaling by depolarizing the AII amacrine cells and relieving Mg2+ block of the NMDA receptors (figure 3) [63]. This activating leg depends on the activity of either rod-driven or cone-driven On bipolar neurons, and can be activated at relatively low light intensity.
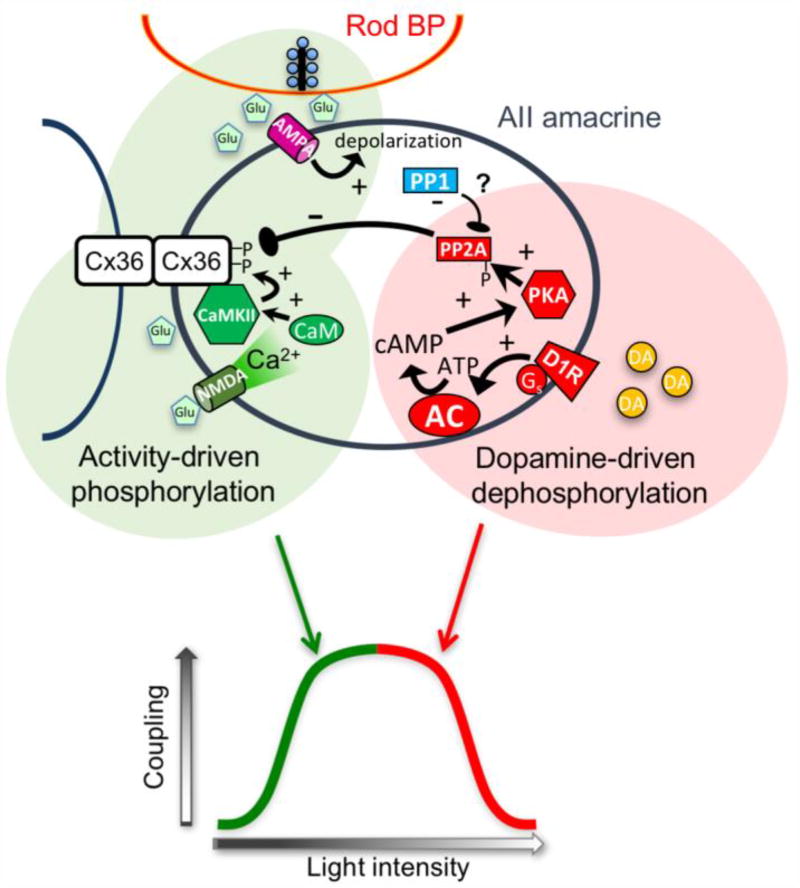
Figure 3. Mechanisms controlling plasticity of AII amacrine cell electrical synapses.
Light intensity controls AII amacrine cell coupling via two independent pathways. Glutamatergic activity close to electrical synapses, including that of presynaptic rod bipolar cells and likely nearby cone bipolar cells, activate dedicated non-synaptic NMDA receptors associated with the gap junctions via glutamate spillover. This initiates a calcium signaling cascade that activates CaM Kinase II and phosphorylates Cx36 to increase coupling. Direct synaptic activation of AII amacrine cells via the synaptic AMPA receptors facilitates this process by depolarizing AIIs and relieving Mg2+ block of the NMDA receptors. This pathway can be supported by dim light that activates the rod pathway. At high light intensities, dopamine secretion from dopaminergic amacrine cells activates D1 receptors on AIIs, initiating a cAMP signaling cascade that activates PKA and, in turn, Protein Phosphatase 2A (PP2A). This results in net dephosphorylation of Cx36 and reduction in coupling. The widely separated light intensity thresholds of the two independent pathways leads to a distinct biphasic plasticity curve.
In the AII amacrine cell, the dephosphorylating leg of the plasticity curve is driven by a separate pathway that depends on extracellular dopamine and activation of dopamine D1 receptors [54, 60]. Activation of D1 receptors increases adenylate cyclase activity through Gs signaling, similar to the action of adenosine A2a receptors in photoreceptors, and activates PKA (figure 3). However, unlike the situation in mammalian and fish photoreceptors, PKA in AII amacrine cells activates the protein phosphatase PP2A [60]. This potent phosphatase dephosphorylates Cx36, presumably while PKA phosphorylates it, resulting in a net de-phosphorylation of Cx36 and reduction in coupling (figure 3). A second phosphatase that is active in this pathway, PP1, appears to target PP2A to put the brakes on the pathway [60]. The dopamine secretion that activates the dephosphorylating leg of the plasticity curve requires bright light [30, 74]. Consequently, the light thresholds required to activate the two legs of the plasticity curve are well separated, resulting in the clear biphasic plasticity curve (figure 3).
5. The deck is stacked to achieve certain outcomes
When electrical synaptic plasticity has been studied in detail by any techniques it has become evident that each circuit possesses its own distinct mechanisms and displays reproducible plasticity that can be profound. In our studies of electrical synaptic plasticity, a final design principle has emerged as a governing factor that works in concert with all of the signaling mechanisms that combine to give each circuit its unique character. The example for this principle is the heterogeneous population of electrical synapses among photoreceptors. Section 4A and figure 2 have documented the signaling pathways that control coupling among photoreceptors in some detail. In recent studies, Jin et al. [75] have found that the rod light response in CBA/CaJ mice shows circadian modulation of amplitude with a sharp, almost step-like increase in the hour before subjective dawn that is attributable to a sharp reduction of electrical coupling (figure 4A). The CBA/CaJ strain of mouse has an intact melatonin biosynthetic pathway and consequently shows a circadian rhythm of retinal dopamine secretion [76, 77], which contributes to the regulation of coupling in constant darkness. Thus, it is expected that this circadian rhythm-controlled process remains intact in constant darkness.
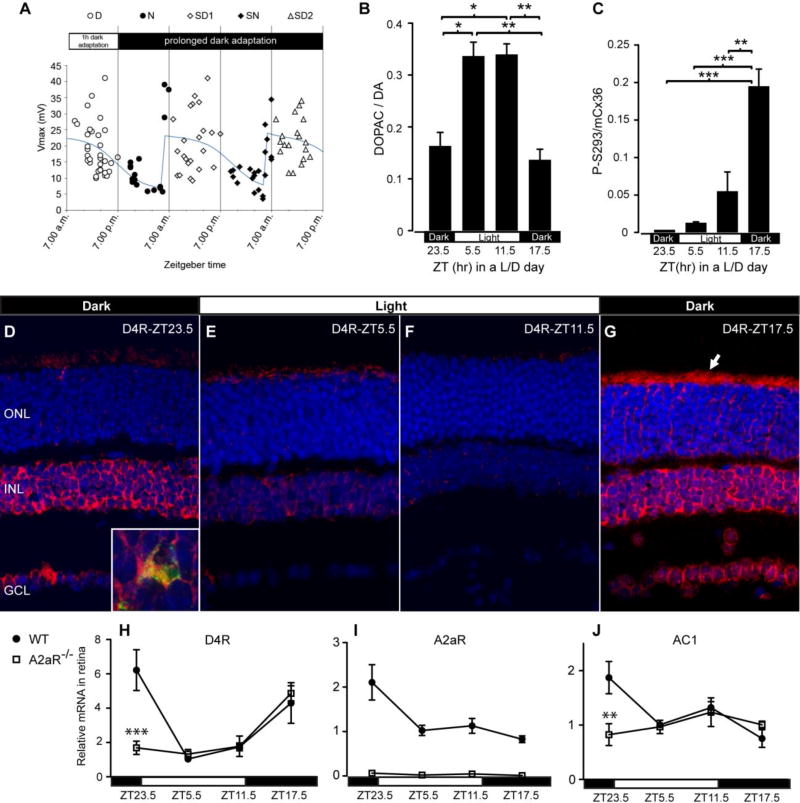
Figure 4. Gene expression cycles stack the deck to achieve certain outcomes at specific times of day.
A. Light responses of CBA/CaJ mouse rods show a circadian pattern in amplitude resulting in a step-like transition to higher amplitude responses in anticipation of subjective dawn. The variation of voltage response is due to electrical coupling, with smaller amplitudes resulting from dissipation of response current into the network through coupling. B. Dopamine release in C57Bl/6 mouse retina through a light-dark day. Dopamine release remains low in darkness and high in light in this strain. C. Cx36 phosphorylation in photoreceptors of C57Bl/6 mouse retina through a light-dark day. Note that phosphorylation is lowest in darkness in anticipation of dawn despite continued low dopamine levels. D–G. In situ hybridization for dopamine D4 receptor mRNA at the same time points during a light-dark day as in C. Photoreceptor D4 transcripts are among the outer row of nuclei (ONL) and in the adjacent photoreceptor inner segments just above that row (arrow in G). Note strongly cyclic expression pattern. H–J. Q-rtPCR analysis of transcript levels of dopamine D4 receptor (H), adenosine A2a receptor (I) and adenylate cyclase 1 (J) through a light-dark day. All three transcripts show cyclic expression. The cycles are largely abolished in an A2a receptor knockout. Panel A reproduced with permission from Jin et al., 2015 [75]. Panels B–J adapted with permission from Li et al., 2013 [60].
In contrast to the CBA/CaJ strain of mice, the widely-used C57Bl/6 strain harbors a natural mutation in serotonin N-acetyltransferase that suppresses melatonin biosynthesis [78] and results in the failure to produce a circadian rhythm of retinal dopamine release [76]. In these animals, retinal dopamine release remains at the nighttime level even shortly before light onset (figure 4B) [62], while light-driven dopamine release is intact. Nonetheless, in these animals, photoreceptor Cx36 phosphorylation achieves its lowest level in darkness, just prior to light onset (figure 4C) [62]. This suppression of phosphorylation is not due to release of dopamine, and so must result from other factors. Li et al. [62] have found that expression of many elements of the signaling pathway is regulated in a cyclic fashion. This includes the dopamine D4 receptor (mRNA in situ hybridization: figure 4D–G; qPCR: figure 4H), adenosine A2a receptor (figure 4I) and adenylate cyclase 1 (figure 4J). Knockout of the adenosine A2a receptor disrupts these rhythms (figure 4H–J) [62], as does knockout of dopamine D4 receptor [79], and greatly disrupts regulation of photoreceptor Cx36 phosphorylation [62].
The cumulative evidence from Li et al. [62] suggest that the signaling pathway that regulates photoreceptor coupling is not as simple as that described in figure 2. Expression of elements of the signaling pathway varies rhythmically so that abundance of some elements, and likely relative abundance of activating components and inactivating components, changes with time of day. Thus, it is a forgone conclusion that photoreceptors will be uncoupled in anticipation of dawn. Note, however, that this state does not last throughout the day. It is relaxed through the course of the day so that Cx36 phosphorylation, and by inference coupling, is quite elevated prior to dusk in the continued presence of light and dopamine (figure 4B, C). At present, it is not known which components of the signaling pathway have dominant effects on coupling and uncoupling at different times of the day, but it appears that their balance shifts during the course of each day.
Photoreceptors provide an enlightening example of the effect of rhythmic variation in signaling pathway components on control of electrical coupling. However, it cannot be assumed that it will be the only example. A glance at the dopamine D4 receptor mRNA expression pattern (figure 4D–G) reveals that this signal transducer varies rhythmically in abundance in all of the layers of the retina, representing dozens of neuron types. It can be expected that a similar dependence of coupled state on time of day occurs in many other neural circuits. Beyond the retina, the lesson learned from this example is to expect that the balance of signaling pathway components will be set to achieve a specific state that optimizes a certain type of network performance. This state may not remain the same at all times of day or under all conditions; environmental factors likely manipulate the poise of the system to achieve different optimal states.
Conclusions
For every nervous system, from simple nerve nets to sophisticated brains, plasticity of connections that refine the strength of communication and the network within which it is distributed is essential to interpret complex environmental stimuli and create complex behavioral output. It has become clear that electrical synapses carry their weight in these processes. All electrical synapses are capable of plasticity, and indeed this plasticity may be quite sophisticated. Plasticity can occur on time scales ranging from milliseconds to days and can produce changes in coupling of more than an order of magnitude. The design principles I have described govern different aspects of plasticity including the magnitude of coupling achieved within a network, the time scale on which plasticity occurs, the pattern of plasticity, and even the time of day at which certain forms of plasticity occur. Although I have primarily described retinal circuits, these principles are general and can apply to all electrically-connected neural circuits within the confines of their own physiological demands.
Highlights.
Electrical synapses exhibit a high degree of plasticity.
Plasticity develops in scales of milliseconds, seconds-minutes, or hours-days.
Different mechanisms underlying plasticity determine its time scale and pattern.
Mechanisms underlying plasticity can change with time of day, favoring certain states.
Acknowledgments
I wish to thank Dr. Christophe Ribelayga for constructive comments on this manuscript.
Funding
This work was supported by the National Institutes of Health (grant EY012857 to JO), the Louisa Stude Sarofim endowment, and a challenge grant to the Department of Ophthalmology and Visual Science from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Marder E. Electrical synapses: beyond speed and synchrony to computation. Curr Biol. 1998;8:R795–797. doi: 10.1016/s0960-9822(07)00502-7. [DOI] [PubMed] [Google Scholar]
- 2.Curti S, O'Brien J. Characteristics and plasticity of electrical synaptic transmission. BMC Cell Biol. 2016;17(Suppl 1):13. doi: 10.1186/s12860-016-0091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereda AE, Curti S, Hoge G, Cachope R, Flores CE, Rash JE. Gap junction-mediated electrical transmission: Regulatory mechanisms and plasticity. Biochim Biophys Acta. 2013;1828:134–146. doi: 10.1016/j.bbamem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulon P, Landisman CE. The Potential Role of Gap Junctional Plasticity in the Regulation of State. Neuron. 2017;93:1275–1295. doi: 10.1016/j.neuron.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JR, Jones BW, Watt CB, Shaw MV, Yang JH, Demill D, Lauritzen JS, Lin Y, Rapp KD, Mastronarde D, Koshevoy P, Grimm B, Tasdizen T, Whitaker R, Marc RE. Exploring the retinal connectome. Mol Vis. 2011;17:355–379. [PMC free article] [PubMed] [Google Scholar]
- 6.Lauritzen JS, Sigulinsky CL, Anderson JR, Kalloniatis M, Nelson NT, Emrich DP, Rapp C, McCarthy N, Kerzner E, Meyer M, Jones BW, Marc RE. Rod-cone crossover connectome of mammalian bipolar cells. J Comp Neurol. 2016 doi: 10.1002/cne.24084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985;82:3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curti S, Hoge G, Nagy JI, Pereda AE. Synergy between electrical coupling and membrane properties promotes strong synchronization of neurons of the mesencephalic trigeminal nucleus. J Neurosci. 2012;32:4341–4359. doi: 10.1523/JNEUROSCI.6216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zsiros V, Maccaferri G. Noradrenergic modulation of electrical coupling in GABAergic networks of the hippocampus. J Neurosci. 2008;28:1804–1815. doi: 10.1523/JNEUROSCI.4616-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien J, Al-Ubaidi MR, Ripps H. Connexin 35: a gap-junctional protein expressed preferentially in the skate retina. Molecular Biology of the Cell. 1996;7:233–243. doi: 10.1091/mbc.7.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condorelli DF, Parenti R, Spinella F, Salinaro AT, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–1208. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- 12.Guldenagel M, Sohl G, Plum A, Traub O, Teubner B, Weiler R, Willecke K. Expression patterns of connexin genes in mouse retina. J Comp Neurol. 2000;425:193–201. [PubMed] [Google Scholar]
- 13.Hombach S, Janssen-Bienhold U, Sohl G, Schubert T, Bussow H, Ott T, Weiler R, Willecke K. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur J Neurosci. 2004;19:2633–2640. doi: 10.1111/j.0953-816X.2004.03360.x. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien JJ, Li W, Pan F, Keung J, O'Brien J, Massey SC. Coupling between A-type horizontal cells is mediated by connexin 50 gap junctions in the rabbit retina. J Neurosci. 2006;26:11624–11636. doi: 10.1523/JNEUROSCI.2296-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller LP, Dedek K, Janssen-Bienhold U, Meyer A, Kreuzberg MM, Lorenz S, Willecke K, Weiler R. Expression and modulation of connexin 30.2, a novel gap junction protein in the mouse retina. Vis Neurosci. 2010;27:91–101. doi: 10.1017/S0952523810000131. [DOI] [PubMed] [Google Scholar]
- 16.Cruciani V, Mikalsen SO. The vertebrate connexin family. Cell Mol Life Sci. 2006;63:1125–1140. doi: 10.1007/s00018-005-5571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivas M, Costa M, Gao Y, Fort A, Fishman GI, Spray DC. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. J Physiol (Lond) 1999;517:673–689. doi: 10.1111/j.1469-7793.1999.0673s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. Functional properties of channels formed by the neuronal gap junction protein connexin36. J Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teubner B, Degen J, Sohl G, Guldenagel M, Bukauskas FF, Trexler EB, Verselis VK, De Zeeuw CI, Lee CG, Kozak CA, Petrasch-Parwez E, Dermietzel R, Willecke K. Functional expression of the murine connexin 36 gene coding for a neuron-specific gap junctional protein. J Membr Biol. 2000;176:249–262. doi: 10.1007/s00232001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreuzberg MM, Sohl G, Kim JS, Verselis VK, Willecke K, Bukauskas FF. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ Res. 2005;96:1169–1177. doi: 10.1161/01.RES.0000169271.33675.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan F, Keung J, Kim IB, Snuggs MB, Mills SL, O'Brien J, Massey SC. Connexin 57 is expressed by the axon terminal network of B-type horizontal cells in the rabbit retina. J Comp Neurol. 2012;520:2256–2274. doi: 10.1002/cne.23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeVries SH, Schwartz EA. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccolino M, Neyton J, Gerschenfeld HM. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984;4:2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampson EC, Weiler R, Vaney DI. pH-gated dopaminergic modulation of horizontal cell gap junctions in mammalian retina, Proceedings of the Royal Society of London - Series B. Biological Sciences. 1994;255:67–72. doi: 10.1098/rspb.1994.0010. [DOI] [PubMed] [Google Scholar]
- 25.Bloomfield SA, Xin D, Persky SE. A comparison of receptive field and tracer coupling size of horizontal cells in the rabbit retina. Vis Neurosci. 1995;12:985–999. doi: 10.1017/s0952523800009524. [DOI] [PubMed] [Google Scholar]
- 26.Pandarinath C, Bomash I, Victor JD, Prusky GT, Tschetter WW, Nirenberg S. A novel mechanism for switching a neural system from one state to another. Front Comput Neurosci. 2010;4:2. doi: 10.3389/fncom.2010.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sotelo C, Llinas R, Baker R. Structural study of inferior olivary nucleus of the cat: morphological correlates of electrotonic coupling. J Neurophysiol. 1974;37:541–559. doi: 10.1152/jn.1974.37.3.541. [DOI] [PubMed] [Google Scholar]
- 28.Llinas R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- 29.Lefler Y, Yarom Y, Uusisaari MY. Cerebellar inhibitory input to the inferior olive decreases electrical coupling and blocks subthreshold oscillations. Neuron. 2014;81:1389–1400. doi: 10.1016/j.neuron.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 30.Bloomfield SA, Xin D, Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Vis Neurosci. 1997;14:565–576. doi: 10.1017/s0952523800012220. [DOI] [PubMed] [Google Scholar]
- 31.Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arroyo DA, Kirkby LA, Feller MB. Retinal Waves Modulate an Intraretinal Circuit of Intrinsically Photosensitive Retinal Ganglion Cells. J Neurosci. 2016;36:6892–6905. doi: 10.1523/JNEUROSCI.0572-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolszon LR, Faber DS. The effects of postsynaptic levels of cyclic AMP on excitatory and inhibitory responses of an identified central neuron. J Neurosci. 1989;9:784–797. doi: 10.1523/JNEUROSCI.09-03-00784.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XD, Faber DS. Initial synaptic efficacy influences induction and expression of long-term changes in transmission. Proc Natl Acad Sci U S A. 1991;88:4299–4303. doi: 10.1073/pnas.88.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereda AE, Nairn AC, Wolszon LR, Faber DS. Postsynaptic modulation of synaptic efficacy at mixed synapses on the Mauthner cell. J Neurosci. 1994;14:3704–3712. doi: 10.1523/JNEUROSCI.14-06-03704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereda AE, Bell TD, Chang BH, Czernik AJ, Nairn AC, Soderling TR, Faber DS. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gap-junctional conductance and glutamatergic transmission. Proc Natl Acad Sci U S A. 1998;95:13272–13277. doi: 10.1073/pnas.95.22.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatton GI, Yang QZ. Ionotropic histamine receptors and H2 receptors modulate supraoptic oxytocin neuronal excitability and dye coupling. J Neurosci. 2001;21:2974–2982. doi: 10.1523/JNEUROSCI.21-09-02974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas JS, Landisman CE. Bursts modify electrical synaptic strength. Brain Res. 2012;1487:140–149. doi: 10.1016/j.brainres.2012.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Neely R, Landisman CE. Activation of Group I and Group II Metabotropic Glutamate Receptors Causes LTD and LTP of Electrical Synapses in the Rat Thalamic Reticular Nucleus. J Neurosci. 2015;35:7616–7625. doi: 10.1523/JNEUROSCI.3688-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sevetson J, Fittro S, Heckman E, Haas JS. A calcium-dependent pathway underlies activity-dependent plasticity of electrical synapses in the thalamic reticular nucleus. J Physiol. 2017;595:4417–4430. doi: 10.1113/JP274049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathy A, Clark BA, Hausser M. Synaptically induced long-term modulation of electrical coupling in the inferior olive. Neuron. 2014;81:1290–1296. doi: 10.1016/j.neuron.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turecek J, Yuen GS, Han VZ, Zeng XH, Bayer KU, Welsh JP. NMDA Receptor Activation Strengthens Weak Electrical Coupling in Mammalian Brain. Neuron. 2014;81:1375–1388. doi: 10.1016/j.neuron.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas JS, Greenwald CM, Pereda AE. Activity-dependent plasticity of electrical synapses: increasing evidence for its presence and functional roles in the mammalian brain. BMC Cell Biol. 2016;17(Suppl 1):14. doi: 10.1186/s12860-016-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belousov AB, Fontes JD. Neuronal gap junctions: making and breaking connections during development and injury. Trends Neurosci. 2013;36:227–236. doi: 10.1016/j.tins.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roerig B, Feller MB. Neurotransmitters and gap junctions in developing neural circuits. Brain Res Brain Res Rev. 2000;32:86–114. doi: 10.1016/s0165-0173(99)00069-7. [DOI] [PubMed] [Google Scholar]
- 46.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katti C, Butler R, Sekaran S. Diurnal and circadian regulation of connexin 36 transcript and protein in the mammalian retina. Invest Ophthalmol Vis Sci. 2013;54:821–829. doi: 10.1167/iovs.12-10375. [DOI] [PubMed] [Google Scholar]
- 48.Nakase T, Sohl G, Theis M, Willecke K, Naus CC. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am J Pathol. 2004;164:2067–2075. doi: 10.1016/S0002-9440(10)63765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohsumi A, Nawashiro H, Otani N, Ooigawa H, Toyooka T, Yano A, Nomura N, Shima K. Alteration of gap junction proteins (connexins) following lateral fluid percussion injury in rats. Acta neurochirurgica. Supplement. 2006;96:148–150. doi: 10.1007/3-211-30714-1_33. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Song JH, Denisova JV, Park WM, Fontes JD, Belousov AB. Neuronal gap junction coupling is regulated by glutamate and plays critical role in cell death during neuronal injury. J Neurosci. 2012;32:713–725. doi: 10.1523/JNEUROSCI.3872-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paschon V, Higa GS, Resende RR, Britto LR, Kihara AH. Blocking of connexin-mediated communication promotes neuroprotection during acute degeneration induced by mechanical trauma. PLoS One. 2012;7:e45449. doi: 10.1371/journal.pone.0045449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores CE, Nannapaneni S, Davidson KG, Yasumura T, Bennett MV, Rash JE, Pereda AE. Trafficking of gap junction channels at a vertebrate electrical synapse in vivo. Proc Natl Acad Sci U S A. 2012;109:E573–582. doi: 10.1073/pnas.1121557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang HY, Lin YP, Mitchell CK, Ram S, O'Brien J. Two-color fluorescent analysis of connexin 36 turnover: relationship to functional plasticity. J Cell Sci. 2015;128:3888–3897. doi: 10.1242/jcs.162586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hampson EC, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. Journal of Neuroscience. 1992;12:4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitropoulou G, Bruzzone R. Modulation of perch connexin35 hemi-channels by cyclic AMP requires a protein kinase A phosphorylation site. J Neurosci Res. 2003;72:147–157. doi: 10.1002/jnr.10572. [DOI] [PubMed] [Google Scholar]
- 56.Ouyang X, Winbow VM, Patel LS, Burr GS, Mitchell CK, O'Brien J. Protein kinase A mediates regulation of gap junctions containing connexin35 through a complex pathway. Brain Res Mol Brain Res. 2005;135:1–11. doi: 10.1016/j.molbrainres.2004.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Del Corsso C, Iglesias R, Zoidl G, Dermietzel R, Spray DC. Calmodulin dependent protein kinase increases conductance at gap junctions formed by the neuronal gap junction protein connexin36. Brain Res. 2012 doi: 10.1016/j.brainres.2012.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J, Ek Vitorin JF, Weintraub ST, Gu S, Shi Q, Burt JM, Jiang JX. Phosphorylation of connexin 50 by protein kinase A enhances gap junction and hemichannel function. J Biol Chem. 2011;286:16914–16928. doi: 10.1074/jbc.M111.218735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kothmann WW, Li X, Burr GS, O'Brien J. Connexin 35/36 is phosphorylated at regulatory sites in the retina. Vis Neurosci. 2007;24:363–375. doi: 10.1017/S095252380707037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kothmann WW, Massey SC, O'Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–14911. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Chuang AZ, O'Brien J. Photoreceptor coupling is controlled by connexin 35 phosphorylation in zebrafish retina. J Neurosci. 2009;29:15178–15186. doi: 10.1523/JNEUROSCI.3517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP, O'Brien J. Adenosine and Dopamine Receptors Coregulate Photoreceptor Coupling via Gap Junction Phosphorylation in Mouse Retina. J Neurosci. 2013;33:3135–3150. doi: 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kothmann WW, Trexler EB, Whitaker CM, Li W, Massey SC, O'Brien J. Nonsynaptic NMDA Receptors Mediate Activity-Dependent Plasticity of Gap Junctional Coupling in the AII Amacrine Cell Network. J Neurosci. 2012;32:6747–6759. doi: 10.1523/JNEUROSCI.5087-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haas JS, Zavala B, Landisman CE. Activity-dependent long-term depression of electrical synapses. Science. 2011;334:389–393. doi: 10.1126/science.1207502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palacios-Prado N, Hoge G, Marandykina A, Rimkute L, Chapuis S, Paulauskas N, Skeberdis VA, O'Brien J, Pereda AE, Bennett MV, Bukauskas FF. Intracellular magnesium-dependent modulation of gap junction channels formed by neuronal connexin36. J Neurosci. 2013;33:4741–4753. doi: 10.1523/JNEUROSCI.2825-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palacios-Prado N, Chapuis S, Panjkovich A, Fregeac J, Nagy JI, Bukauskas FF. Molecular determinants of magnesium-dependent synaptic plasticity at electrical synapses formed by connexin36. Nature communications. 2014;5:4667. doi: 10.1038/ncomms5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mangel SC, Dowling JE. Responsiveness and receptive field size of carp horizontal cells are reduced by prolonged darkness and dopamine. Science. 1985;229:1107–1109. doi: 10.1126/science.4035351. [DOI] [PubMed] [Google Scholar]
- 68.Baldridge WH. Triphasic adaptation of teleost horizontal cells. Prog Brain Res. 2001;131:437–449. doi: 10.1016/s0079-6123(01)31035-x. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Mangel SC. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proc Natl Acad Sci U S A. 1996;93:4655–4660. doi: 10.1073/pnas.93.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ribelayga C, Wang Y, Mangel SC. Dopamine mediates circadian clock regulation of rod and cone input to fish retinal horizontal cells. J Physiol. 2002;544:801–816. doi: 10.1113/jphysiol.2002.023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ribelayga C, Mangel SC. Identification of a circadian clock-controlled neural pathway in the rabbit retina. PLoS One. 2010;5:e11020. doi: 10.1371/journal.pone.0011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li H, Chuang AZ, O'Brien J. Regulation of photoreceptor gap junction phosphorylation by adenosine in zebrafish retina. Vis Neurosci. 2014;31:237–243. doi: 10.1017/S095252381300062X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ribelayga C, Mangel SC. A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J Neurosci. 2005;25:215–222. doi: 10.1523/JNEUROSCI.3138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bloomfield SA, Volgyi B. Function and plasticity of homologous coupling between AII amacrine cells. Vision Res. 2004;44:3297–3306. doi: 10.1016/j.visres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 75.Jin NG, Chuang AZ, Masson PJ, Ribelayga CP. Rod electrical coupling is controlled by a circadian clock and dopamine in mouse retina. J Physiol. 2015;593:1597–1631. doi: 10.1113/jphysiol.2014.284919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- 77.Kasahara T, Abe K, Mekada K, Yoshiki A, Kato T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci U S A. 2010;107:6412–6417. doi: 10.1073/pnas.0914399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC. Natural melatonin 'knockdown' in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63:189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 79.Jackson CR, Chaurasia SS, Zhou H, Haque R, Storm DR, Iuvone PM. Essential roles of dopamine D4 receptors and the type 1 adenylyl cyclase in photic control of cyclic AMP in photoreceptor cells. J Neurochem. 2009;109:148–157. doi: 10.1111/j.1471-4159.2009.05920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]