Abstract
Objective
Only few studies have investigated cortical thickness in anorexia nervosa (AN), and it is unclear whether patterns of altered cortical thickness can be identified as biomarkers for AN.
Method
Cortical thickness was measured in 19 adult women with restricting-type AN, 24 individuals recovered from restricting-type AN (REC-AN) and 24 healthy controls. Those individuals with current or recovered from AN had previously shown altered regional cortical volumes across orbitofrontal cortex and insula. A linear relevance vector machine-learning algorithm estimated patterns of regional thickness across 24 subdivisions of those regions.
Results
Region-based analysis showed higher cortical thickness in AN and REC-AN, compared to controls, in the right medial orbital (olfactory) sulcus, and greater cortical thickness for short insular gyri in REC-AN versus controls bilaterally. The machine-learning algorithm identified a pattern of relatively higher right orbital, right insular and left middle frontal cortical thickness, but lower left orbital, right middle and inferior frontal, and bilateral superior frontal cortical thickness specific to AN versus controls (74% specificity and 74% sensitivity, χ2p<0.004); predicted probabilities differed significantly between AN and controls (p<0.023). No pattern significantly distinguished the REC-AN group from controls.
Conclusions
Higher cortical thickness in medial orbitofrontal cortex and insula probably contributes to higher gray matter volume in AN in those regions. The machine-learning algorithm identified a mixed pattern of mostly higher orbital and insular, but relatively lower superior frontal cortical thickness in individuals with current AN. These novel results suggest that regional cortical thickness patterns could be state markers for AN.
Keywords: Anorexia nervosa, cortical thickness, machine learning, individualized prediction models, structural MRI
INTRODUCTION
Anorexia nervosa (AN) is a severe psychiatric disorder characterized by fear of weight gain and a pursuit of thinness through dietary restriction, leading to severe underweight (American Psychiatric Association., 2013). Core symptoms of AN also include the so-called body image distortion, that is feeling fat while underweight and disturbances in interoceptive awareness (Garner, Olmstead, & Polivy, 1983). Research indicates that cognitive-emotional processes may drive those behaviors (Treasure et al., 2015).
A variety of brain imaging techniques has been used to investigate brain structure and function to understand AN’s underlying neurobiology. The literature on structural alterations in AN so far yielded partially conflicting results with some studies showing higher or lower or no difference in regional brain volume compared to controls (Frank, 2015a; King, Frank, Thompson, & Ehrlich, 2018; Van den Eynde et al., 2012). Investigating brain structure in patients with AN in brain areas that are related to eating-related functions such as processing of taste and awareness of the body might lead to a better understanding of the neurobiology of eating disorder symptoms. Such insights can inform the recognition of sub-groups and might aid in the development of novel treatments (Frank, 2015b; Kaye, Wierenga, Bailer, Simmons, & Bischoff-Grethe, 2013). Among brain regions that have been implicated in eating disorder pathophysiology are the insula and the orbitofrontal cortex (Frank, 2015a). The insula is involved in the processing of taste and interoceptive awareness among other functions such as pain (Craig, 2002; Uddin, 2015), and it is thought to be a key node in the pathophysiology of eating disorders (Frank, Shott, Hagman, & Mittal, 2013; Kerr et al., 2016; Oberndorfer et al., 2013). The orbitofrontal cortex is important in processing sensory-specific satiety regulating food intake (Frank et al., 2012; Rolls, 2008), and its connectivity with the insula has been shown to be central in interoceptive and taste processing (Kuehn, Mueller, Lohmann, & Schuetz-Bosbach, 2016).
One of the most frequently studied measures of brain structure is brain volume, which is a composite measure that is influenced by two separate factors: cortical thickness, and cortical surface area (Winkler et al., 2010). These two components have been shown to be genetically independent, and cortical thickness might be a more specific and biologically significant measure than volume (Panizzon et al., 2009). It is not known whether cortical thickness alterations contribute to regional volume measures in AN. Relatively few studies have been conducted so far on cortical thickness in AN. A large recent study (King et al., 2015) found widespread lower cortical thickness in women with AN compared to healthy controls. The same group published a subsequent longitudinal study showing normalization of cortical thickness after an average of three months of weight restoration (Bernardoni et al., 2016). Two other studies found reduced cortical thickness in patients with AN in fronto-cingulate and parietal regions (Bar, de la Cruz, Berger, Schultz, & Wagner, 2015; Fuglset et al., 2016). In another study, reductions in cortical thickness were present in AN, but differences did not survive correction for multiple comparisons (Lavagnino et al., 2016). King and colleagues found a negative correlation between cortical thickness and drive for thinness in the extra-striate area, a region involved in body perception. It is unclear, however, whether that region truly processes drive for thinness, or whether lower cortical thickness was the result from high food avoidance due to high drive for thinness. Thus the relationship between eating disorder symptoms and abnormalities in cortical thickness in AN is still largely unknown (Frank, 2015b).
To assess cortical thickness, high-resolution anatomic parcellation of the human cerebral cortex is performed, and different methods can be applied to compare study groups (Destrieux, Fischl, Dale, & Halgren, 2010; Fischl, 2012). One is to select regions of interest and compare thickness measures between groups. Another is to establish how patterns of brain cortical thickness may relate to AN. For the latter, machine-learning analytic approaches can utilize multiple neuroanatomical measurements to classify individual participants and groups and are thus well-positioned to provide this kind of information (Lavagnino et al., 2015; Mwangi et al., 2016). Advantages of machine-learning algorithms include the ability to analyze multiple (regional thickness) measurements simultaneously, and the use of robust cross-validation methods to establish generalizability of the results by testing them on participants that were previously excluded from the analysis (Mwangi et al., 2016).
The aim of the present study was two-fold. First, we wanted to test whether we would find higher cortical thickness in orbitofrontal and insular cortex based on our previous observations in the same sample of individuals in whom we previously found larger volume in individuals ill or recovered from AN compared to controls (Frank, Shott, Hagman, & Mittal, 2013). The second aim was to identify cortical thickness patterns that could be a biomarker for AN and that could point toward altered brain organization. Brain organization refers to how brain regions form networks, and larger or smaller brain regions therefore contribute differently to larger networks that are thought to drive behavior (Pessoa, 2014). Therefore, if we can find in AN brain regions (for instance frontal cortex) that show larger volume or cortical thickness relative to other areas (for instance temporal or parietal cortex), then this could be a characteristic marker for illness state or trait, have implications on specific behaviors, and could indicate brain development that distinguishes individuals with AN from controls. Machine-learning has only rarely been applied in eating disorder brain research, and we did not have strong previous data that would guide hypothesis building. This method does not compare groups for thickness but rather identifies patterns of higher and lower thickness within a group and then tests whether patterns can identify group membership. Such patterns of altered cortical thickness would help identify potential imbalances between brain regions, as well as make inference on behavior that is related to the identified regions (Boen et al., 2014; Metzler-Baddeley, Caeyenberghs, Foley, & Jones, 2016). Based on our previous observation of increased volume in orbitofrontal and insular cortex in this data set, we predicted that orbitofrontal and insular regions would be relatively higher compared to other regional thickness measures in the AN groups and predict membership in the AN or REC-AN group.
METHODS
Participants
Nineteen adult female patients with current anorexia nervosa (AN), restricting-type, 24 women recovered from restricting anorexia nervosa (REC-AN) and 24 healthy control women (CW) participated in the study, individuals who had been assessed for cortical thickness in the past (Frank, Shott, Hagman, & Mittal, 2013). Participants with AN were recruited from Children’s Hospital Colorado and EDCare Denver. The Colorado Multiple Institutional Review Board approved all procedures. All participants provided written informed consent. Participants with current AN were scanned after 1-2 weeks of inpatient or partial hospital treatment, and were closely supervised with regards to calorie intake and hydration, in an attempt to minimize potential effects of starvation and dehydration. All patients were under close supervision by a medical doctor. Only individuals with normal complete blood count, comprehensive metabolic panel and urine analysis including specific gravity were admitted to the study. Abnormal hydration status at entry to treatment was monitored and corrected including fluid adjustment. Healthy control and REC-AN participants were recruited through local advertisements. All participants were administered the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 2002) by a doctoral-level interviewer. Recovered women had a history of restricting-type AN, but maintained normal weight (body mass index, BMI, >18.5), regular menstrual cycle, normal exercise and food intake for at least one year. All participants were right-handed, had no history of past or present neurological diseases, major medical illnesses, psychosis or substance use disorders. Handedness was assessed using the Edinburgh Inventory (Oldfield, 1971).
Behavioral Measures
All study participants completed the Eating Disorder Inventory-3 (EDI-3, Interoceptive awareness, drive for thinness and body dissatisfaction were used in the analyses) (Garner, 2004) as well as the state and trait anxiety inventory (STAI) (Spielberger, 1983).
MRI Acquisition and Image Analysis
Structural brain scans were acquired using a 3T GE Signa MR scanner with an axial three-dimensional, T1-weighted magnetization-prepared rapid acquisition gradient echo spoiled gradient recall (SPGR) sequence with the following parameters: field of view=22 cm, flip angle=10°, slice thickness=1.2 mm, matrix size=256×256, repetition time=10 ms, echo time=3 ms, voxel size=1.2 mm3.
T1-weighted scans were individually inspected to ensure no presence of gross artifacts and subsequently pre-processed using the Freesurfer software library (http://surfer.nmr.mgh.harvard.edu/) version 5.3.0 (Fischl, 2012). The segmentation of the cerebral cortex and the extraction of cortical thickness values was performed using an automated procedure implemented in Freesurfer (Fischl & Dale, 2000). The Freesurfer package is a set of computational tools that allow surface reconstruction and labeling of neuroanatomical structures. In more detail, the Freesurfer brain scan pre-processing stage involved three major steps. 1) Brain scan motion correction and non-uniform intensity normalization (Sled, Zijdenbos, & Evans, 1998). 2) Removal of non-brain tissue (e.g., skull) and transformation of resulting scans into the Talairach standard space. 3) Cortical reconstruction and extraction of volumetric and surface-based measurements (e.g., volume and cortical thickness). In the current study, the Destrieux atlas (Destrieux, Fischl, Dale, & Halgren, 2010) was used for parcellation of the brain into 74 different structures.
Group-level Comparisons and Correlations Between Cortical Thickness and Behavioral Data
We tested for group differences in cortical thickness between individuals with AN, REC-AN and CW in two key cortical regions for which we have recently found increased volume across age groups and states of illness in AN: the orbitofrontal cortex and the insula. The orbitofrontal cortex is parcellated by Freesurfer in orbital gyrus, gyrus rectus, lateral orbital sulcus, medial orbital sulcus (olfactory sulcus), orbital sulci (H-shaped sulci), suborbital sulcus (or supraorbital sulcus). The insula is parcellated in long insular gyrus and central sulcus of the insula, short insular gyri. We tested for group differences in cortical thickness measurements in these regions.
We then selected the sub-regions where significant differences occurred and tested for correlations between cortical thickness and eating disorder symptom scores drive for thinness, body dissatisfaction, and interoceptive awareness separately in the three groups.
Multivariate Machine-Learning Analyses
We selected as predictor variables cortical thickness measurements in areas selected by prior literature. These regions included: orbitofrontal cortex (Frank, Shott, Hagman, & Mittal, 2013; Frank, Shott, Hagman, & Yang, 2013), insula (Frank, Shott, Hagman, & Mittal, 2013; Frank, Shott, Hagman, & Yang, 2013; Friederich et al., 2012; Van den Eynde et al., 2012), frontal cortex (Amianto et al., 2013; Brooks et al., 2011; Friederich et al., 2012; Joos et al., 2010; Suchan et al., 2010), and cingulate cortex (Muhlau et al., 2007; Van den Eynde et al., 2012). The Destrieux atlas (Destrieux, Fischl, Dale, & Halgren, 2010) extracts 24 subdivisions from these regions, translated into 24 predictor variables in each individual which were subsequently used in the machine-learning analysis (the regions are reported in the supplementary materials).
Then a multivariate machine-learning algorithm was applied to estimate an individual’s probability score . This score ranging from zero to unity quantifies the probability of a person belonging to either CW (0), AN (1) or REC-AN (2) groups given regional cortical thickness measurements. Consequently, a linear relevance vector machine (RVM) algorithm (Tipping, 2001) was used to estimate the probability . First, cortical thickness measurements were normalized by subtracting the mean and dividing by the standard deviation (Z-score) and represented as . Corresponding individuals’ categorical labels (CW=0, AN=1) were represented as . The input-target pair of predictor and target variables was represented as , where N represents a number of observations (participants). The current predictive classification problem was represented using a general linear model (GLM) as . . Linear kernel mapping estimated inter-participant similarities during algorithm training; and stand for model bias and measurement noise, respectively. is a vector representing weighting factors estimated during the RVM training process and used in making predictions when the algorithm is exposed to previously ‘unseen’ individual’s data. RVM uses a sparse Bayesian learning framework to estimate optimal weighting factors and other parameters (Mwangi, Ebmeier, Matthews, & Steele, 2012; Mwangi et al., 2016; Tipping, 2001).
In this study, the RVM algorithm was implemented using a MATLAB (The MathWorks, Inc., Natick, Massachusetts) toolbox (Tipping, 2001) and in-house custom routines as described elsewhere (Mwangi, Ebmeier, Matthews, & Steele, 2012; Mwangi, Hasan, & Soares, 2013; Mwangi et al., 2016). To examine the generalization ability (high sensitivity/specificity) of the algorithm in identifying affiliation with a diagnostic group, a leave-one-out cross-validation (LOOCV) approach was used. This process entailed separating training the algorithm with all individuals but one while the left out individual was used as a test sample, a process, which was repeated until all individuals were used as test persons. The performance of the algorithm in distinguishing AN or REC-AN from CW was evaluated with the following standard parameters: accuracy, sensitivity, specificity. Therefore, we set out to establish an RVM algorithm able to discriminate study groups and compute an individual’s probability score of belonging to one of these groups. All individuals were assigned probability scores based on the cortical thickness measures, which were compared across three groups (AN, REC-AN, CW). We hypothesized that individuals’ probability scores would differ among AN and CW groups while the REC-AN individuals would be assigned intermediate probabilities.
Machine-learning algorithms commonly present a problem known as ‘class imbalance’: it consists in having observations (participants) in one class (e.g., CW participants) that exceed observations in the other class (e.g., AN). Machine-learning algorithms trained using imbalanced data sets tend to assign new observations to the majority class (e.g., CW) (Dubey et al., 2014). In this study, we addressed the class imbalance problem by matching the sample size of CW to that of the AN or REC-AN group through the following procedure. Each AN or REC-AN participant was matched with a CW participant based on age. This procedure resulted in having in most AN-CW pairs the CW participant slightly older than the AN participant they were paired with. To eliminate systematic bias, in a second step we modified some of the choices performed in the first matching to address this bias. We obtained equal AN, REC-AN and CW groups of 19 participants.
Statistical Analyses
Demographic variables, extracted cortical thickness, and machine-learning probability scores were analyzed using IBM SPSS Statistics (v. 23.0) (Chicago, IL, USA). Data were tested for normality of distribution using the Kolmogorov-Smirnov test. Correlation analyses were conducted using Pearson correlation procedures. Demographic and behavioral variables were analyzed using one-way ANOVA. Region of interest derived cortical thickness values were analyzed using MANCOVA including age as a covariate and group, anxiety disorder, mood disorder and antidepressant use as co-factors in the model. Wilk’s lambda was calculated to test multivariate effects and estimated marginal means for group effects and corrected for multiple comparisons (Bonferroni). Only three individuals took an atypical antipsychotic, and we did not include this category in the analysis. The machine-learning algorithm used statistics as described above.
RESULTS
Table 1 summarizes demographic and behavioral data. The AN and REC-AN groups didn’t differ from controls with regards to age; however, the REC-AN group was on average older than patients with AN. Individuals with AN had lower BMI compared the other two groups. Drive for thinness, body dissatisfaction and interoceptive deficits scores from EDI-3 were higher in AN compared to both CW and REC-AN (p<0.001), as well as higher in the REC-AN compared to CW.
Table 1.
Demographic variables for study groups.
| Variable | A. Healthy Control Group (N = 24) | B. Anorexia Nervosa Group (N = 19) | C. Recovered Anorexia Nervosa Group (N = 24) | ANOVA | Post-Hoc Comparisonsb | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | SD | Mean | SD | Mean | SD | F | p | ||
| Age (years) | 27.4 | 6.3 | 23.1 | 5.8 | 30.3 | 8.1 | 5.7 | 0.005 | B<C*** |
| BMI (kg/m2)a | 21.6 | 1.3 | 16.0 | 1.1 | 20.8 | 2.4 | 65.0 | <0.001 | A, C>B*** |
| Education (years) | 16.6 | 2.1 | 14.5 | 2.4 | 16.9 | 2.7 | 5.8 | 0.004 | B<A, C* |
| Drive for Thinnessd | 2.6 | 3.4 | 19.2 | 6.7 | 8.5 | 6.4 | 46.6 | <0.001 | B>C>A*** |
| Body Dissatisfactiond | 4.4 | 4.3 | 24.4 | 9.3 | 10.5 | 8.1 | 40.2 | <0.001 | B>C,A***; C>A** |
| Interoceptive Awarenessd | 1.3 | 1.9 | 9.7 | 4.1 | 3.5 | 3.4 | 39.4 | <0.001 | B>C,A***; C>A* |
|
| |||||||||
| N | % | N | % | ||||||
|
| |||||||||
| SSRI | 5 | 26.3 | 6 | 25.0 | |||||
| Antipsychotic use | 1 | 5.3 | 0 | 0.0 | |||||
| SSRI and Antipsychotic use | 2 | 10.5 | 0 | 0.0 | |||||
| Major depression | 2 | 10.5 | 3 | 12.5 | |||||
| Anxiety disorder | 4 | 21.1 | 4 | 16.7 | |||||
| Major depression and Anxiety Disorder | 6 | 31.6 | 2 | 8.3 | |||||
BMI=body mass index
Significance computed with the Dunnett T3 post hoc test
SSRI = Serotonin reuptake inhibitor
From the Eating Disorder Inventory - 3
p < 0.05
p < 0.01
p < 0.005
Group Contrasts for Regionally Extracted Cortical Thickness and Correlations With Behavioral Data
Data were normally distributed, and parametric tests were therefore applied. The multivariate test indicated a significant effect for group (Wilk’s lambda 0.368, p<0.028, partial η2 =0.393) and antidepressant (Wilk’s lambda 0.567, p<0.037, partial η2 =0.433) as well as for the covariate age (Wilk’s lambda 0.566, p<0.037, partial η2 =0.434). Post hoc tests indicated greater cortical thickness for short insular gyri in REC-AN relative to CW on the left (p<0.014, REC-AN=3.933±0.070, CW=3.675±0.052, AN=3.848±0.072) and in the right hemisphere(p<0.002, REC-AN=3.667±0.063, CW=3.383±0.047, AN=3.528±0.064), as well as higher cortical thickness in AN and REC-AN, compared to CW in the right medial orbital (olfactory) sulcus (REC-AN vs CW p<0.002, AN vs CW p<0.004, REC-AN=2.166±0.043, CW=1.974±0.032, AN=2.158±0.044).
In the AN group left short insula gyri cortical thickness correlated negatively with state (r=−0.590, p<0.008) and trait (r=−0.544, p<0.016) anxiety, but no eating disorder measures.
Machine-Learning Model
The RVM model was run with balanced AN and CW samples (19 participants in each group). The model predicted whether a participant belonged to the AN or control group with 74% specificity and 74% sensitivity (χ2=8.5; p<0.004). Regions that were most relevant for model predictions are represented in Figure 1. The ANOVA to test for differences in the probability values of the three groups was significant in the omnibus test (F=4.33, p<0.017); the posthoc test (Bonferroni corrected) indicated that the probabilities separate AN from CW (p<0.023), and there was a trend of a difference between participants with AN and participants recovered (p<0.068), but no difference between controls and participants recovered (see the bar graph in Figure 2). Using age and BMI as confounding variables the ANCOVA omnibus test is still significant (F=3.49, p<0.037), and Bonferroni post-hoc still showed a difference between AN and CW (p<0.034).
Figure 1.
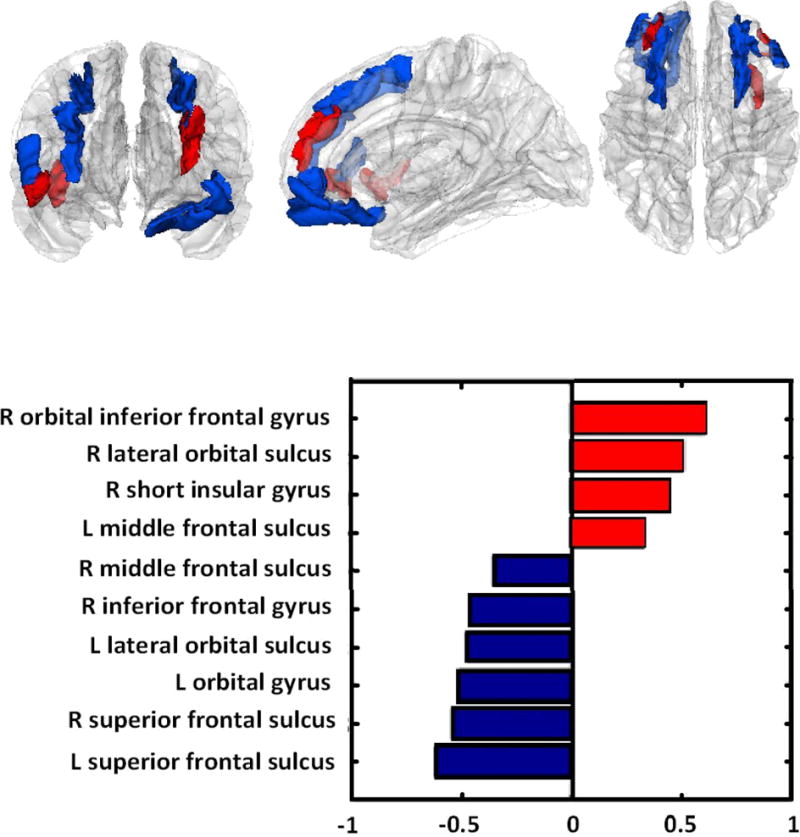
Blue regions in the brain: cortical thickness relatively lower in AN; red regions: cortical thickness relatively higher.
Figure 2.
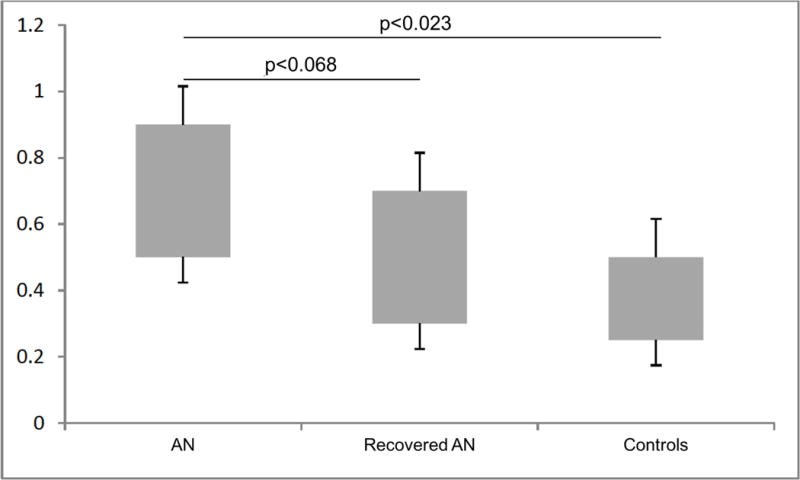
Boxplots representing probability values of having anorexia obtained by the model from cortical thickness of subjects with anorexia nervosa (AN), subjects recovered from anorexia nervosa (recovered AN) and healthy controls. Error bars represent the standard errors of the mean; the relevance vector machine model was run with balanced samples of 19 participants in each group.
DISCUSSION
This study suggests that women with AN and recovered from AN have higher cortical thickness in a sample that previously showed higher brain volume using different methodology. Second, a computational machine-learning model approach that included a broad range of brain regions in this sample suggests that a pattern of both greater and comparatively lower cortical thickness across frontal brain regions characterize AN during its acute state. However, larger studies are needed to support those results.
Studies reporting global (King et al., 2015) and regional (Fuglset et al., 2016) cortical thickness alterations in different stages of weight restoration have provided some insight on cortical thickness in acute AN and after recovery. In this study, we first analyzed cortical thickness in brain regions known to be involved in the processing of taste, reward and body awareness (Craig, 2009; Frank, 2015a; Frank et al., 2012; Rolls, 2008; Wang et al., 2008), regions that we found having larger volume compared to controls in this sample. We found greater cortical thickness in portions of the medial orbital sulcus and the insula in women with AN compared to controls, which suggest that previous findings in the same sample of increased gray matter volume of the medial orbitofrontal cortex and the insula (Frank, Shott, Hagman, & Mittal, 2013) were likely to be at least in part driven by cortical thickness, since cortical thickness is a part of the volume determination. The use of a different methodology (cortical thickness) also provides validation of the previously found greater volume in those regions. However, there was not exact overlap, for instance, we did not find significant differences in gyrus rectus cortical thickness between groups. The reasons for this discrepancy is unclear but indicates that there are different factors that contribute to altered thickness or volume. Nevertheless, elevated cortical thickness across AN and REC-AN is important as this measure is highly heritable and the results could point toward developmental factors of altered brain structure in AN (Panizzon et al., 2009).
We utilized cortical thickness measurements to develop a successful classification of individual participants with AN and healthy controls. The results indicate that patterns of higher and lower cortical thickness in key brain areas (e.g. frontal cortex, orbital cortex, insula) characterize patients with AN. It is important to emphasize that this pattern classification does not identify higher or lower thickness between groups in one single region, but rather patterns within groups and how well specific patterns can then identify group membership, that is identify a pattern that is specific to for instance AN when ill. The machine-learning model achieved an accurate prediction of diagnosis of AN, distinguishing ill participants from controls: the good ability of the model to generalize to novel data is demonstrated by its successful performance in predicting the diagnosis (AN or CW) in participants that were not included in the ‘training’ phase of the analysis (LOOCV approach). Classification in patients and controls relies on a pattern of differences in the measured variables, i.e. increases in the insula and the orbitofrontal cortex, and relatively lower cortical thickness in frontal regions: reductions in the superior frontal gyrus bilaterally are the most relevant contributors to the prediction (Figure 1). This pattern allows generating hypotheses on how cortical thickness may be related to symptoms or behaviors in AN. It is possible that lower cortical thickness in prefrontal cognitive brain areas could be related to altered response inhibition (T. A. Oberndorfer, Kaye, Simmons, Strigo, & Matthews, 2011; Wierenga et al., 2014) or set shifting in adults with AN (Sato et al., 2013). Higher cortical thickness measures in insula and orbitofrontal cortex, on the other hand, could be related to altered reward processing and satiety perception in AN (Frank et al., 2012). However, whether such structure-function relationships exist requires further study.
The lack of differences in the probability index computed from cortical thickness between REC-AN and healthy controls indicates that although group differences in specific gray matter areas could be demonstrated between REC-AN and CW in group comparisons in a previous study on this sample (Frank, Shott, Hagman, & Mittal, 2013), there were no sufficiently distinctive patterns of cortical thickness measurements that allowed our algorithm to differentiate healthy controls from individuals recovered from AN. This could be because gray matter volume (the metric utilized in the analysis of the previous study) is a composite measure of cortical thickness and surface area: the finding of larger gray matter volume in REC-AN in Frank et al. 2013 might be driven by larger surface area. AN-related alterations in surface area are still unknown, and future studies need to clarify this point.
In a recent study a pattern of widespread decreased, instead of increased, cortical thickness was found in acute patients with AN, and no increases were found (King et al., 2015). Cortical thickness was reduced in acute patients with AN in another recent study (although group differences did not survive correction for multiple comparisons) (Lavagnino et al., 2016). We believe that this difference might be due to the characteristics of the sample of the current study, namely the differences in duration of nutritional rehabilitation in these groups of patients. Individuals with eating disorders often have longer periods of severe malnutrition with dehydration or hyper-hydration, food restriction or excessive food intake (American Psychiatric Association., 2013; Hart, Abraham, Franklin, & Russell, 2011; Lowinger, Griffiths, Beumont, Scicluna, & Touyz, 1999). The mechanisms of decrease or increase in localized brain volume in AN is unknown, however fluid intake and hypo- and hyper-alimentation are known to affect the cerebral cortex and could account for those changes (Blasel et al., 2012; Duning et al., 2005; Streitburger et al., 2012). This will require more in-depth research though, before we can understand the underlying mechanism of brain volume changes in AN. In King et al., the patients underwent scanning within 96 hours after beginning a nutritional rehabilitation program; in Lavagnino et al. the patients underwent scanning before beginning nutritional rehabilitation. In the sample studied in this paper, patients were scanned after 1 to 2 weeks of nutritional rehabilitation. The patients recruited in Lavagnino et al. were in an acute state of malnutrition, and as for King et al., it is possible that 96 hours of nutritional rehabilitation might not be enough to restore the nutritional status in patients so that patterns of increased cortical thickness in AN patients couldn’t be observed. If this is the case, in acute AN there might be cortical thickness alterations that are more typical of the acute state of malnourishment (e.g. diffuse cortical thinning); a different pattern of alterations might be seen after short-term nutritional rehabilitation: reductions in cortical thickness are now more localized to the superior frontal gyrus, and increased cortical thickness in the orbital cortex and the insula becomes noticeable. Finally, alterations in cortical thickness might disappear in recovered participants, while other parameters (perhaps surface area) might still show alterations (which would account for the findings of the earlier study (Frank, Shott, Hagman, & Mittal, 2013). Longitudinal studies with larger samples from our group are currently under way to confirm and expand or disprove this hypothesis. Stage-specific alterations might provide neurobiological milestones to existing staging and neuro-progression models of AN (Treasure, Stein, & Maguire, 2015).
This study presents some limitations. The result of elevated cortical thickness in orbitofrontal and insular cortex is specific to this study and cannot be extrapolated to other studies as different studies have recruited participants under different conditions of illness, comorbidities, medication use, etc. Future studies across research labs and comparing various levels of illness will be needed to further investigate cortical thickness in AN. This is the first study on a machine-learning classification model using cortical thickness measurements obtained with an automated procedure (Destrieux, Fischl, Dale, & Halgren, 2010) to separate participants with AN or recovered from AN from healthy controls. Therefore, our results require replication in an independent sample. Nevertheless, it is important to emphasize that the RVM algorithm utilized in this study features robust cross-validation methods (LOOCV) that support the generalizability of results by testing the algorithm on participants that were not included in the training stage. Our findings included both higher and lower cortical thickness measures as part of a signature of AN. To better elucidate this point, future studies should ideally use designs with maybe weekly or every other week brain scanning to be able to describe in detail the changes that occur during nutritional rehabilitation. However, the cost may be prohibitive. The effects of comorbidity also need to be further studied in more complex models to identify truly AN related alterations. We did not find brain cortical thickness – eating disorder behavior correlations that survived multiple comparison corrections and this may be a factor of the modest sample size. Also, we utilized selected regions of interest based on the existing literature; using different brain regions for the analyses could yield different results.
In conclusion, our study indicates in AN a pattern of relatively lower cortical thickness in superior frontal regions when compared to relatively higher cortical thickness in the insula and orbitofrontal cortex in AN; this pattern of alterations could not be demonstrated in individuals recovered from AN and may, therefore, be a state biomarker of the disorder. The discrepancy of higher insular-orbitofrontal cortex but lower superior frontal cortex thickness raises the question whether there is an imbalance of reward and higher-order cognitive processes, which needs further exploration. On the other hand, elevated orbitofrontal cortical thickness in ill and recovered AN could suggest heritable alterations but could also be changes related to the illness. Studying structural alterations in localized and well-characterized brain areas as well as using computerized algorithms will help better understand whether there are patterns of structural brain development that are specific to AN. Such studies may also help elucidate brain structure-behavior relationships when paired with functional studies.
Supplementary Material
Acknowledgments
Supported by NIMH grantsK23 MH080135, R01 MH096777, and MH103436.
Supported in part by the Pat Rutherford, Jr. Endowed Chair in Psychiatry (Jair C. Soares).
Footnotes
Declaration of interest
Prof. Soares has participated in research funded by Forest, Merck, BMS, GSK and has been a speaker for Pfizer and Abbott. All other authors declare that they have no conflict of interests.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5(TM)) 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Amianto F, Caroppo P, D’Agata F, Spalatro A, Lavagnino L, Caglio M, Fassino S. Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: a voxel-based morphometry study. Psychiatry Research. 2013;213(3):210–216. doi: 10.1016/j.pscychresns.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Bar KJ, de la Cruz F, Berger S, Schultz CC, Wagner G. Structural and functional differences in the cingulate cortex relate to disease severity in anorexia nervosa. Journal Psychiatry & Neuroscience. 2015;40(4):269–279. doi: 10.1503/jpn.140193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardoni F, King JA, Geisler D, Stein E, Jaite C, Natsch D, Ehrlich S. Weight restoration therapy rapidly reverses cortical thinning in anorexia nervosa: A longitudinal study. Neuroimage. 2016;130:214–222. doi: 10.1016/j.neuroimage.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Blasel S, Pilatus U, Magerkurth J, von Stauffenberg M, Vronski D, Mueller M, Hattingen E. Metabolic gray matter changes of adolescents with anorexia nervosa in combined MR proton and phosphorus spectroscopy. Neuroradiology. 2012 doi: 10.1007/s00234-011-1001-9. [DOI] [PubMed] [Google Scholar]
- Boen E, Westlye LT, Elvsashagen T, Hummelen B, Hol PK, Boye B, Malt UF. Regional cortical thinning may be a biological marker for borderline personality disorder. Acta Psychiatrica Scandinavica. 2014;130(3):193–204. doi: 10.1111/acps.12234. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Barker GJ, O’Daly OG, Brammer M, Williams SC, Benedict C, Campbell IC. Restraint of appetite and reduced regional brain volumes in anorexia nervosa: a voxel-based morphometric study. BMC Psychiatry. 2011;11:179. doi: 10.1186/1471-244X-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey R, Zhou J, Wang Y, Thompson PM, Ye J, Alzheimer’s Disease Neuroimaging, I Analysis of sampling techniques for imbalanced data: An n = 648 ADNI study. Neuroimage. 2014;87:220–241. doi: 10.1016/j.neuroimage.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duning T, Kloska S, Steinstrater O, Kugel H, Heindel W, Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64(3):548–550. doi: 10.1212/01.WNL.0000150542.16969.CC. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) In: New York State Psychiatric Institute, editor. Biometrics Research. New York, NY: 2002. [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences USA. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK. Advances from neuroimaging studies in eating disorders. CNS Spectrums. 2015a;20(4):391–400. doi: 10.1017/S1092852915000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK. Recent advances in neuroimaging to model eating disorder neurobiology. Current Psychiatry Reports. 2015b;17(4):559. doi: 10.1007/s11920-015-0559-z. [DOI] [PubMed] [Google Scholar]
- Frank GK, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, O’Reilly RC. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology. 2012;37(9):2031–2046. doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Shott ME, Hagman JO, Mittal VA. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. American Journal of Psychiatry. 2013;170(10):1152–1160. doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Shott ME, Hagman JO, Yang TT. Localized brain volume and white matter integrity alterations in adolescent anorexia nervosa. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(10):1066–1075 e1065. doi: 10.1016/j.jaac.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich HC, Walther S, Bendszus M, Biller A, Thomann P, Zeigermann S, Herzog W. Grey matter abnormalities within cortico-limbic-striatal circuits in acute and weight-restored anorexia nervosa patients. Neuroimage. 2012;59(2):1106–1113. doi: 10.1016/j.neuroimage.2011.09.042. [DOI] [PubMed] [Google Scholar]
- Fuglset TS, Endestad T, Hilland E, Bang L, Tamnes CK, Landro NI, Ro O. Brain volumes and regional cortical thickness in young females with anorexia nervosa. BMC Psychiatry. 2016;16(1):404. doi: 10.1186/s12888-016-1126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner DM. Eating Disorder Inventory™-3 (EDI™-3) Lutz, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- Garner DM, Olmstead MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia and bulimia nervosa. International Journal of Eating Disorders. 1983;2:15–34. [Google Scholar]
- Hart S, Abraham S, Franklin RC, Russell J. The reasons why eating disorder patients drink. European Eating Disorders Review. 2011;19(2):121–128. doi: 10.1002/erv.1051. [DOI] [PubMed] [Google Scholar]
- Joos A, Kloppel S, Hartmann A, Glauche V, Tuscher O, Perlov E, Tebartz van Elst L. Voxel-based morphometry in eating disorders: correlation of psychopathology with grey matter volume. Psychiatry Research. 2010;182(2):146–151. doi: 10.1016/j.pscychresns.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends in Neurosciences. 2013;36(2):110–120. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK. Altered Insula Activity during Visceral Interoception in Weight-Restored Patients with Anorexia Nervosa. Neuropsychopharmacology. 2016;41(2):521–528. doi: 10.1038/npp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Frank GKW, Thompson PM, Ehrlich S. Structural Neuroimaging of Anorexia Nervosa: Future Directions in the Quest for Mechanisms Underlying Dynamic Alterations. Biological Psychiatry. 2018;83(3):224–234. doi: 10.1016/j.biopsych.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Geisler D, Ritschel F, Boehm I, Seidel M, Roschinski B, Ehrlich S. Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biological Psychiatry. 2015;77(7):624–632. doi: 10.1016/j.biopsych.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Kuehn E, Mueller K, Lohmann G, Schuetz-Bosbach S. Interoceptive awareness changes the posterior insula functional connectivity profile. Brain Structure & Function. 2016;221(3):1555–1571. doi: 10.1007/s00429-015-0989-8. [DOI] [PubMed] [Google Scholar]
- Lavagnino L, Amianto F, Mwangi B, D’Agata F, Spalatro A, Zunta Soares GB, Soares JC. The relationship between cortical thickness and body mass index differs between women with anorexia nervosa and healthy controls. Psychiatry Research. 2016;248:105–109. doi: 10.1016/j.pscychresns.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Lavagnino L, Amianto F, Mwangi B, D’Agata F, Spalatro A, Zunta-Soares GB, Soares JC. Identifying neuroanatomical signatures of anorexia nervosa: a multivariate machine learning approach. Psychological Medicine. 2015;45(13):2805–2812. doi: 10.1017/S0033291715000768. [DOI] [PubMed] [Google Scholar]
- Lowinger K, Griffiths RA, Beumont PJ, Scicluna H, Touyz SW. Fluid restriction in anorexia nervosa: a neglected symptom or new phenomenon? International Journal of Eating Disorders. 1999;26(4):392–396. doi: 10.1002/(sici)1098-108x(199912)26:4<392::aid-eat4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Caeyenberghs K, Foley S, Jones DK. Task complexity and location specific changes of cortical thickness in executive and salience networks after working memory training. Neuroimage. 2016;130:48–62. doi: 10.1016/j.neuroimage.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlau M, Gaser C, Ilg R, Conrad B, Leibl C, Cebulla MH, Nunnemann S. Gray matter decrease of the anterior cingulate cortex in anorexia nervosa. American Journal of Psychiatry. 2007;164(12):1850–1857. doi: 10.1176/appi.ajp.2007.06111861. [DOI] [PubMed] [Google Scholar]
- Mwangi B, Ebmeier KP, Matthews K, Steele JD. Multi-centre diagnostic classification of individual structural neuroimaging scans from patients with major depressive disorder. Brain. 2012;135(Pt 5):1508–1521. doi: 10.1093/brain/aws084. [DOI] [PubMed] [Google Scholar]
- Mwangi B, Hasan KM, Soares JC. Prediction of individual subject’s age across the human lifespan using diffusion tensor imaging: a machine learning approach. Neuroimage. 2013;75:58–67. doi: 10.1016/j.neuroimage.2013.02.055. [DOI] [PubMed] [Google Scholar]
- Mwangi B, Wu MJ, Cao B, Passos IC, Lavagnino L, Keser Z, Soares JC. Individualized Prediction and Clinical Staging of Bipolar Disorders using Neuroanatomical Biomarkers. Biological Psychiatry: Cognitive Neuroscience & Neuroimaging. 2016;1(2):186–194. doi: 10.1016/j.bpsc.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberndorfer T, Simmons A, McCurdy D, Strigo I, Matthews S, Yang T, Kaye W. Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls. Psychiatry Research. 2013;214(2):132–141. doi: 10.1016/j.pscychresns.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberndorfer TA, Kaye WH, Simmons AN, Strigo IA, Matthews SC. Demand-specific alteration of medial prefrontal cortex response during an inhibition task in recovered anorexic women. International Journal of Eating Disorders. 2011;44(1):1–8. doi: 10.1002/eat.20750. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. Understanding brain networks and brain organization. Physics of Life Reviews. 2014;11(3):400–435. doi: 10.1016/j.plrev.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiologica Hungarica. 2008;95(2):131–164. doi: 10.1556/APhysiol.95.2008.2.1. [DOI] [PubMed] [Google Scholar]
- Sato Y, Saito N, Utsumi A, Aizawa E, Shoji T, Izumiyama M, Fukudo S. Neural basis of impaired cognitive flexibility in patients with anorexia nervosa. PLoS One. 2013;8(5):e61108. doi: 10.1371/journal.pone.0061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trate Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc.; 1983. [Google Scholar]
- Streitburger DP, Moller HE, Tittgemeyer M, Hund-Georgiadis M, Schroeter ML, Mueller K. Investigating structural brain changes of dehydration using voxel-based morphometry. PLoS One. 2012;7(8):e44195. doi: 10.1371/journal.pone.0044195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchan B, Busch M, Schulte D, Gronemeyer D, Herpertz S, Vocks S. Reduction of gray matter density in the extrastriate body area in women with anorexia nervosa. Behavioural Brain Research. 2010;206(1):63–67. doi: 10.1016/j.bbr.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Tipping ME. Sparse bayesian learning and the relevance vector machine. Journal of Machine Learning Research. 2001;1:211–245. [Google Scholar]
- Treasure J, Stein D, Maguire S. Has the time come for a staging model to map the course of eating disorders from high risk to severe enduring illness? An examination of the evidence. Early Intervention Psychiatry. 2015;9(3):173–184. doi: 10.1111/eip.12170. [DOI] [PubMed] [Google Scholar]
- Treasure J, Zipfel S, Micali N, Wade T, Stice E, Claudino A, Wentz E. Anorexia Nervosa. Nature Reviews Disease Primers. 2015;1:15074. doi: 10.1038/nrdp.2015.74. [DOI] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neuroscience. 2015;16(1):55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Van den Eynde F, Suda M, Broadbent H, Guillaume S, Van den Eynde M, Steiger H, Schmidt U. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. European Eating Disorders Review. 2012;20(2):94–105. doi: 10.1002/erv.1163. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, Volkow ND. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39(4):1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Wierenga C, Bischoff-Grethe A, Melrose AJ, Grenesko-Stevens E, Irvine Z, Wagner A, Kaye WH. Altered BOLD response during inhibitory and error processing in adolescents with anorexia nervosa. PLoS One. 2014;9(3):e92017. doi: 10.1371/journal.pone.0092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53(3):1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


