Abstract
DNA replication fidelity relies on base selectivity of the replicative DNA polymerases, exonucleolytic proofreading, and postreplicative DNA mismatch repair (MMR). Ultramutated human cancers without MMR defects carry alterations in the exonuclease domain of DNA polymerase ε (Polε). They have been hypothesized to result from defective proofreading. However, modeling of the most common variant, Polε-P286R, in yeast produced an unexpectedly strong mutator effect that exceeded the effect of proofreading deficiency by two orders of magnitude and indicated the involvement of other infidelity factors. The in vivo consequences of many additional Polε mutations reported in cancers remain poorly understood. Here, we genetically characterized 13 cancer-associated Polε variants in the yeast system. Only variants directly altering the DNA binding cleft in the exonuclease domain elevated the mutation rate. Among these, frequently recurring variants were stronger mutators than rare variants, in agreement with the idea that mutator phenotype has a causative role in tumorigenesis. In nearly all cases, the mutator effects exceeded those of an exonuclease-null allele, suggesting that mechanisms distinct from loss of proofreading may drive the genome instability in most ultramutated tumors. All mutator alleles were semidominant, supporting the view that heterozygosity for the polymerase mutations is sufficient for tumor development. In contrast to the DNA binding cleft alterations, peripherally located variants, including a highly recurrent V411L, did not significantly elevate mutagenesis. Finally, the analysis of Polε variants found in MMR-deficient tumors suggested that the majority cause no mutator phenotype alone but some can synergize with MMR deficiency to increase the mutation rate.
Keywords: DNA polymerase ε, POLE, cancer, mutator, proofreading
In normal cells, DNA is replicated with great accuracy to avoid mutations that can lead to disease. High-fidelity DNA replication is achieved through the correct nucleotide selection by replicative DNA polymerases α (Polα), δ (Polδ), and ε (Polε), exonucleolytic proofreading by Polδ and Polε, and postreplicative DNA mismatch repair (MMR) (Ganai and Johansson 2016). It has long been known that germline mutations in MMR genes cause predisposition to colorectal cancer (CRC) and an increased risk of several other cancer types in Lynch syndrome (Peltomäki 2003). Somatic MMR defects are also common in sporadic CRC, endometrial cancer (EC), and gastric cancer. By contrast, although impaired nucleotide selectivity and proofreading promote tumorigenesis in mouse models (Goldsby et al. 2001; Venkatesan et al. 2007; Albertson et al. 2009), the association between replicative DNA polymerase defects and cancer in humans has not been established until recently. Several years ago, large-scale molecular characterization of sporadic CRC and EC by the Cancer Genome Atlas Network identified a subset of tumors that were significantly hypermutated (>10 mutations per megabase). Many of them, as expected, displayed microsatellite instability (MSI) indicative of MMR defects. However, the most hypermutated tumors were microsatellite stable (MSS) and contained somatic mutations in the POLE gene encoding the catalytic subunit of Polε (Cancer Genome Atlas Network 2012; Cancer Genome Atlas Research Network 2013). Many subsequent whole-exome and targeted sequencing studies of CRC and EC samples similarly reported somatic POLE mutations, together revealing that at least 6% of colorectal and 7% of endometrial tumors contain these changes (Barbari and Shcherbakova 2017). POLE mutations have also been observed, although less frequently, in other types of gastrointestinal cancer, as well as tumors of the brain, breast, ovary, prostate, lung, kidney, cervix, and bone (Cerami et al. 2012; Forbes et al. 2015; Grossman et al. 2016; Campbell et al. 2017). In addition to somatic defects, germline POLE mutations were found in patients with hereditary CRC, with strong evidence for the causative role of a highly penetrant POLE-L424V variant predisposing to multiple colorectal adenomas and carcinomas (Palles et al. 2013).
POLE is an essential gene, and nearly all variants reported in cancers are missense mutations, for which the pathogenicity cannot be definitively predicted in the absence of functional analyses. The amino acid substitutions, however, tend to cluster in the exonuclease domain, with recurring hotspots at highly conserved residues. Many of these substitutions were predicted by in silico analysis to affect DNA binding and/or exonuclease activity (Church et al. 2013; Palles et al. 2013; Rohlin et al. 2014; Hansen et al. 2015; Rayner et al. 2016). These findings have led to the notion that faulty proofreading is the main consequence of the polymerase alterations and is therefore responsible for the hypermutation observed in the tumors. Indeed, in vitro assays have shown that several cancer-associated Polε variants have reduced exonuclease activity and fidelity (Shinbrot et al. 2014). Additionally, the presence of these variants in tumors is significantly correlated with a hypermutated phenotype and a unique mutation signature, consistent with a primary role of the polymerase defects in driving the hypermutation (Alexandrov et al. 2013; Church et al. 2013; Shinbrot et al. 2014; Shlien et al. 2015; Campbell et al. 2017). Yet, several observations are difficult to reconcile with the idea that defective proofreading is the sole consequence of Polε mutations. First, some variants are seen at a vastly greater frequency than others, despite similar effects on exonuclease activity. Second, changes of catalytic residues in the exonuclease domain are rarely reported. Functional studies in vivo have only been performed for the most frequently recurring Polε-P286R substitution (Kane and Shcherbakova 2014). When modeled in yeast, this variant has an extraordinary mutator effect that exceeds the effect of proofreading deficiency by two orders of magnitude, supporting a pathogenic role but also suggesting a consequence beyond defective proofreading alone. This striking phenotype indicates that further mechanistic and functional in vivo studies are required to fully understand the impact of cancer-associated Polε mutations.
Although Polε-P286R is the most common variant in cancers, additional, less frequent variants likely account for a large proportion of disease cases. In this work, we assess the functional consequences of 13 additional Polε variants in the genetically tractable yeast system. We show that only mutations affecting residues near the DNA binding cleft in the exonuclease domain have mutator effects. The magnitude of the mutator effects is highly variable, but in the vast majority of cases it exceeds the effect of proofreading deficiency. Furthermore, frequently recurring DNA binding cleft variants had stronger mutator effects than rarer variants, in line with the idea that the likelihood of developing cancer is proportional to the severity of the mutator phenotype. This finding suggests that the vastly different frequencies of Polε variants in sporadic tumors can be explained, at least in part, by differences in their mutator effects. We further validate the pathogenicity of DNA binding cleft mutations by demonstrating their ability to confer a mutator phenotype in the heterozygous state, which mimics the state of Polε alterations in tumors. Finally, analysis of several Polε variants found in MMR− tumors suggests that although many such variants are likely to be neutral passenger mutations, some could be weak mutators that synergize with MMR deficiency to promote genome instability.
Materials and Methods
Yeast strains and plasmids
All Saccharomyces cerevisiae strains (Supplemental Material, Table S1 in File S1) were derived from TM30 (MATa ade5-1 lys2-Tn5-13 trp1-289 his7-2 leu2-3,112 ura3-4 CAN1::LEU2) and/or TM44 (MATα ade5-1 lys2::InsEA14trp1-289 his7-2 leu2-3,112 ura3-52 can1Δ::loxP) (Mertz et al. 2015). Mutations in the POL2 gene encoding the catalytic subunit of Polε were created by site-directed mutagenesis in one of the following URA3-based integrative vectors. YIpDK1 contains the 2.1-kb N-terminal HpaI-EcoRI fragment of POL2, which includes the promoter and exonuclease domain regions (Kane and Shcherbakova 2014). p173 contains the 5.5-kb C-terminal BamHI-BspEI fragment of POL2 including the DNA polymerase domain and downstream flanking DNA (Pavlov et al. 2001). p174 contains the 9.8-kb C-terminal BamH1-SstI fragment of POL2 including the DNA polymerase domain and a more extended downstream flanking region (Pavlov et al. 2001). YIpDK1 was used to make pol2-F139L, pol2-R252H, pol2-D290V, pol2-P301H, pol2-N351S, pol2-F382S, pol2-V426L, pol2-L439V, pol2-P451R, and pol2-S474F; p173 was used to make pol2-R778W and pol2-A979V; and p174 was used to make pol2-D1757N.
Haploid strains were constructed by one of two methods, both of which consider the possibility that the variant alleles might result in lethality if expressed as the sole source of the polymerase. In both methods, the mutant alleles are first introduced into cells that also express the wild-type POL2, and the viability of the mutants is assessed after a loss of the wild-type allele is allowed to occur in nonselective conditions. Both methods ultimately result in variant alleles expressed at their natural chromosomal locations from the endogenous promoter. In the first method, TM63 diploid (created by crossing TM30 with TM44) was transformed with an integrative plasmid (YIpDK1-pol2-x, p173-pol2-x, or p174-pol2-x) linearized with the appropriate restriction enzyme (Table S2 in File S1) such that integration into one of the two POL2 loci placed the URA3 selection marker between a full-length copy of POL2 with the mutation and a truncated copy of POL2 without the mutation. These diploids were then sporulated, and haploids were generated by tetrad dissection. The presence of four viable haploid spores in each tetrad indicated that the pol2 mutation was not lethal. Derivatives of the Ura+ (mutant) haploids that underwent recombination to lose the URA3 marker and restore a single, full-length POL2 or pol2-x allele were then selected for on media containing 5-fluoroorotic acid. Clones with the pol2-x mutant allele were identified by sequencing. Diploid strains heterozygous or homozygous for the pol2-x mutation were created by crossing CAN1::LEU2 pol2-x haploids with can1Δ POL2 or can1Δ pol2-x haploids of the opposite mating type, respectively. The diploids contain the Kluyveromyces lactis LEU2 gene downstream of the CAN1 gene in one chromosome and a deletion of CAN1 in the homologous chromosome, allowing for the selection of recessive can1 mutants on medium containing canavanine and lacking leucine (Mertz et al. 2015).
In the second method, haploids (TM30 and TM44) were transformed with an integrative plasmid linearized with the appropriate restriction enzyme (Table S2 in File S1) such that integration would produce strains containing the URA3 selection marker between a full-length copy of POL2 without the mutation and a truncated copy of POL2 with the mutation. These strains were further transformed with a YEpPOL2-trp plasmid constructed by cloning the 12.1-kb XhoI-SstI fragment from YEpPOL2 (Araki et al. 1992) containing the entire open reading frame of POL2 plus flanking DNA into the SalI and SstI sites of YEplac112 (Gietz and Sugino 1988). The YEpPOL2-trp plasmid provided ectopic expression of wild-type POL2. Clones that underwent recombination at the chromosomal POL2 locus to lose the URA3 marker and restore a single full-length POL2 or pol2-x allele were then selected for on media containing 5-fluoroorotic acid. Recombinants with the pol2-x mutant allele were identified by sequencing. Their ability to lose the YEpPOL2-trp plasmid on nonselective media was determined as previously described (Daee et al. 2010). A high frequency of plasmid loss indicated that the pol2-x mutation was not lethal. The Trp− derivatives containing only the chromosomal mutant allele were used for the mutation rate measurements. Diploids heterozygous or homozygous for the pol2-x mutations were created by crossing TM30 pol2-x with TM44 POL2 or TM44 pol2-x, respectively.
To generate strains lacking MLH1, wild-type and pol2-x mutant haploid strains were transformed with a PCR-generated mlh1Δ::hphMX4 deletion cassette as previously described (Goldstein and McCusker 1999). Diploid strains homozygous for the MLH1 deletion were generated by crossing CAN1::LEU2pol2-x mlh1Δ::hphMX4 strains with can1Δ POL2mlh1Δ::hphMX4 strains of the opposite mating type.
Spontaneous mutation rate measurements
The rate of spontaneous mutation to canavanine resistance (CanR) was measured by fluctuation analysis as described (Northam et al. 2010). The rates of his7-2 and lys2::InsEA14 reversion were determined similarly, except that the revertants were selected on synthetic complete medium lacking histidine or lysine, respectively. To measure the rate of CanR mutation in diploids, mutants were selected on synthetic complete medium lacking arginine and leucine, and containing 60 mg/liter L-canavanine. Mutation rates are reported as the median for at least 18 independent cultures of each strain. The 95% confidence interval for the median was determined as previously described (Dixon and Massey 1969; Van der Parren 1970). The significance of differences between mutation rates was determined by the Wilcoxon–Mann–Whitney nonparametric test.
Frequency of POLE variants in tumors
The POLE variant frequency was calculated from published studies of sporadic CRC and EC (a total of >13,000 tumors; Table S4 in File S1). The number of documented occurrences of each variant was divided by the total number of tumors in which the corresponding exon of POLE was sequenced. Data on CRC and EC were combined. P values for pairwise comparison of frequencies of individual variants were calculated by Fisher’s exact test.
Data availability
Strains and plasmids are available upon request. All mutation rate and variant frequency data used to reach the conclusions are presented fully within the article and the supplemental material.
Results
Cancer-associated Polε mutations have varying mutator effects
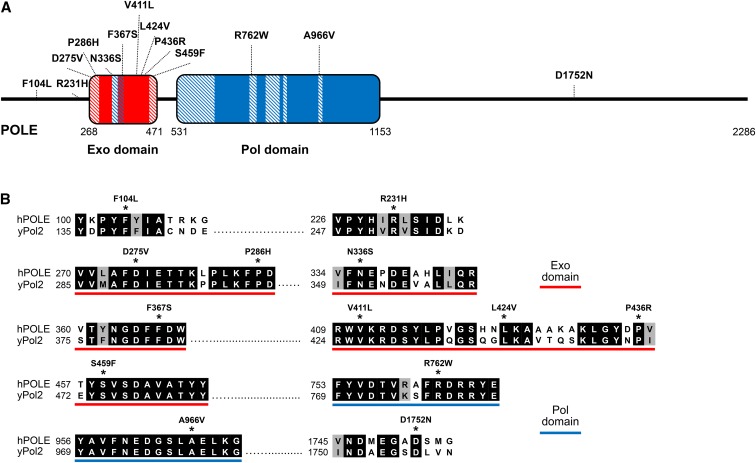
The DNA replication machinery is structurally and functionally conserved among eukaryotes, and the yeast and human Polε show a high degree of amino acid sequence similarity. Thus, in vivo consequences of human Polε variants can be evaluated by studying analogous mutations in yeast, which is easily amenable to genetic manipulation and offers a variety of well-controlled mutagenesis assays. Several hundred distinct Polε mutations have been reported in human tumors (Barbari and Shcherbakova 2017; Campbell et al. 2017). The set of 13 Polε variants studied in this work (Figure 1 and Table 1) included seven variants within the exonuclease domain (D275V, P286H, F367S, V411L, L424V, P436R, and S459F); five variants outside the exonuclease domain that were found in hypermutated MMR-deficient (MSI) tumors (R231H, R762W, and A966V) or hypermutated MMR-proficient (MSS) tumors (F104L and D1752N); and a single nucleotide polymorphism (SNP) in the exonuclease domain, N336S, which is present in the general population at a frequency of 0.9%. Most exonuclease domain variants have been suggested to be pathogenic based on their recurrence in hypermutated tumors, predicted effects on DNA binding or catalysis, and/or demonstrated exonuclease defects in vitro (Church et al. 2013; Shinbrot et al. 2014; Rayner et al. 2016; Campbell et al. 2017).
Figure 1.
Cancer-associated Polε mutations studied in this work. (A) Schematic of human POLE showing the exonuclease (Exo) and polymerase (Pol) domains, conserved Exo and Pol motifs (hatched boxes), and the locations of mutations. (B) Alignment of amino acid sequences of human POLE and yeast Pol2 around the mutation sites.
Table 1. Characteristics of Polε mutations studied in this work.
Indicates the number of times the variant has been reported in whole-exome or targeted sequencing studies of all cancer types. Because many studies analyzed only selected exons of POLE, these numbers do not necessarily reflect the relative frequency of each variant.
AA, anaplastic astrocytoma; BrC, breast carcinoma; CRC, colorectal cancer; DuC, duodenal carcinoma; EC, endometrial cancer; GBM, glioblastoma; HGG, high grade glioma; KC, kidney cancer; LC, liver cancer; LuC, lung cancer; OC, ovarian cancer; PC, prostate cancer; PXA, pleomorphic xanthoastrocytoma; STAD, stomach adenocarcinoma; USC, uterine serous carcinoma.
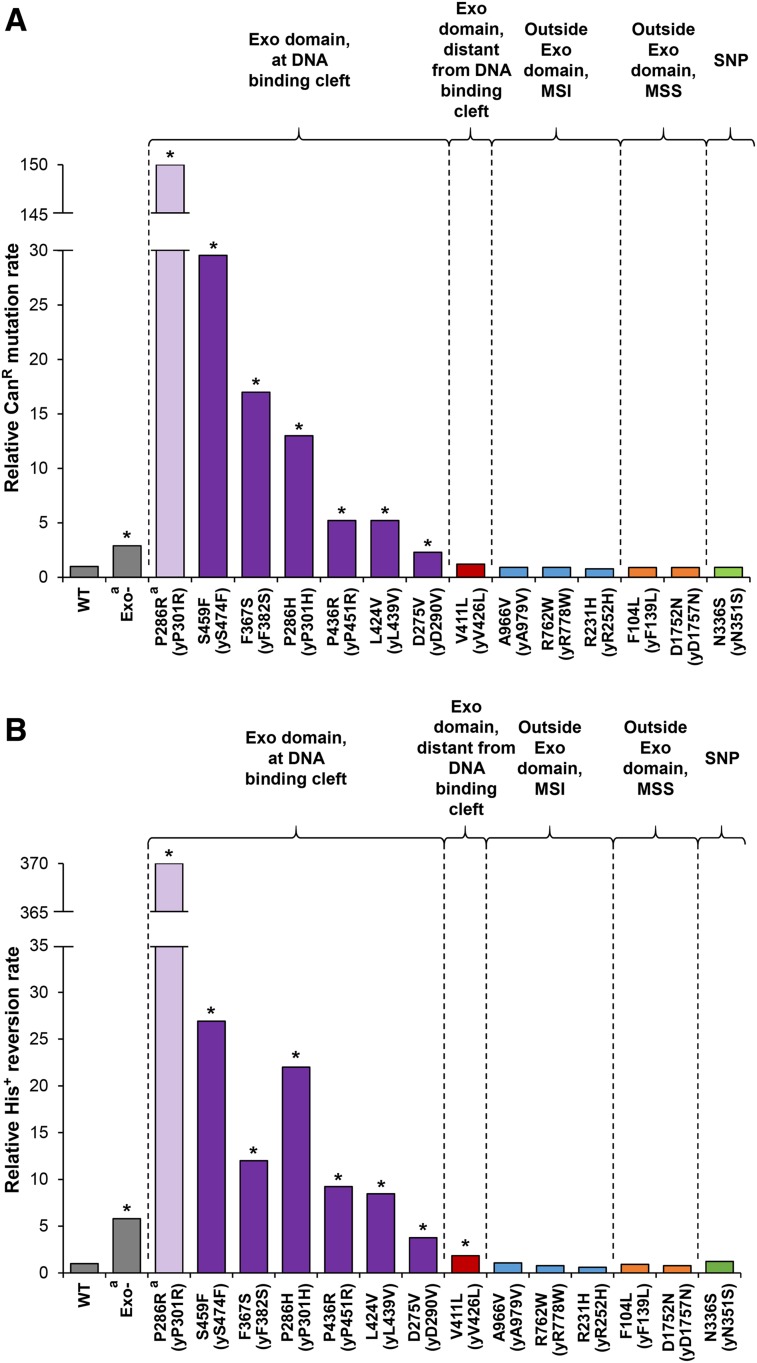
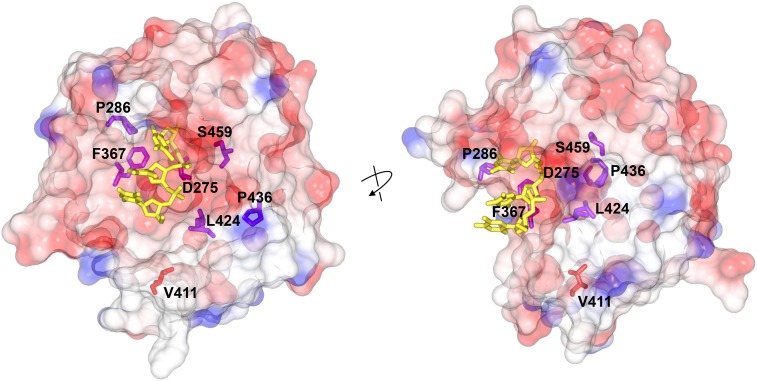
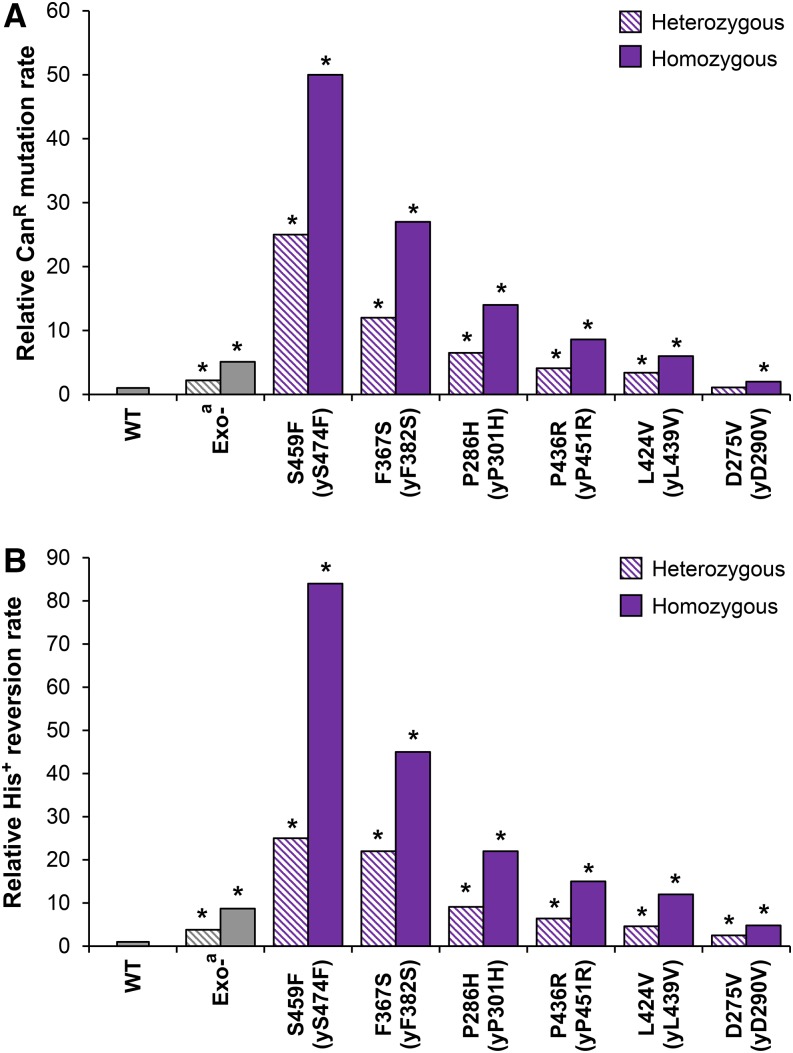
We constructed haploid yeast strains with the corresponding chromosomal pol2 alleles and measured the mutation rate using two primary assays. The CanR forward mutation assay detects a variety of base substitutions, frameshifts, and complex mutations that deactivate the CAN1 gene. The His+ reversion assay scores +1 events that revert the frameshift mutation his7-2, which is a single-base deletion in a run of eight As in the HIS7 gene (Shcherbakova and Kunkel 1999). Most exonuclease domain variants had significant, although highly variable, mutator effects (Figure 2; purple bars). Although none were near the magnitude of the previously studied pol2-P301R allele (human POLE-P286R mimic; Kane and Shcherbakova 2014), the mutator effects still substantially exceeded the effect of the pol2-4 (pol2-D290A,E292A) mutation, which completely inactivates Polε proofreading (Morrison et al. 1991) (Figure 2; Exo−). Thus, the vast majority of exonuclease domain variants must increase the mutation rate through mechanisms other than simple loss of exonuclease activity. One exception was pol2-D290V (mimicking human POLE-D275V), which had a rather weak mutator effect nearly identical to that of pol2-4 (Figure 2). This was not completely unexpected, since pol2-D290V eliminates one of the two catalytic carboxylates affected by the pol2-4 mutation. The main consequence of the pol2-D290V variant may indeed be defective proofreading. The only exonuclease domain variant that showed no significant increase in CanR mutation and only marginally increased His+ reversion was pol2-V426L, mimicking human POLE-V411L (Figure 2; red bars). This was surprising, because V411L is the second most frequently observed variant after P286R, and it is clearly associated with high levels of hypermutation in tumors. While trying to explain the lack of a mutator effect in yeast, we noted that all variants showing mutator effects (Figure 2) are suggested by the crystal structure of Polε to directly alter the DNA binding cleft within the exonuclease domain (Hogg et al. 2014; Rayner et al. 2016; Figure 3). By contrast, V411L is located further away from the exonuclease active site (Figure 3), and neither the side chain of leucine nor that of valine at this position is expected to contact the DNA substrate. It has been reported previously that the mutational spectrum in V411L tumors is somewhat different from the spectrum in other POLE-mutant tumors (Church et al. 2013). Taken together, these observations suggest that the molecular mechanisms underlying the pathogenicity of V411L and DNA binding cleft mutations may not be the same (see Discussion).
Figure 2.
Mutator effects of cancer-associated Polε variants in haploid yeast strains. Mutation rates were measured in haploid strains in which the chromosomal POL2 gene was replaced with mutant pol2 alleles mimicking human POLE variants. The human variants are listed on the x-axis with the analogous yeast substitutions in parentheses. Exo−, exonuclease-deficient variant encoded by the pol2-4 allele. (A) CanR mutation relative to wild type (WT). (B) Reversion of the his7-2 allele relative to WT. Data are from Table S3 in File S1. Asterisks indicate P < 0.05 by Wilcoxon–Mann–Whitney test compared with WT. aData from Kane and Shcherbakova (2014).
Figure 3.
Location of cancer-associated Polε mutations within the exonuclease domain. Structure of the exonuclease domain of Saccharomyces cerevisiae Polε (PBD ID: 4M8O) is from Hogg et al. (2014). Single-stranded DNA (yellow) was modeled in the exonuclease active site by alignment with the structure of T4 polymerase–DNA complex (PBD ID: 1NOY) as done previously (Rayner et al. 2016). The protein surface is colored according to electrostatic potential, revealing the predominantly negatively charged exonuclease active site cleft. Two views of the same structure are shown, with the arrow indicating the approximate direction of rotation. Note the clustering of six cancer-associated mutations at the active site cleft (side chains shown in purple sticks) and the distant position of V411 (red sticks). The figure was generated with CCP4MG molecular graphics software.
The pol2 mutations affecting amino acid residues outside the exonuclease domain and the pol2-N351S allele mimicking the SNP showed no mutator effect (Figure 2; blue, orange, and green bars). In addition to the CAN1 and his7-2 mutagenesis reporters, all strains also allowed for the measurement of lys2::InsEA14 allele reversion, which scores −1 frameshifts in a run of 14 As, thus providing a readout for instability of mononucleotide repeats (Tran et al. 1997). Consistent with the lack of association between POLE mutations and an MSI phenotype in tumors, Lys+ reversion was barely, if at all, affected in the pol2 mutants (Figure S1 in File S1).
Mutator effects of DNA binding cleft variants correlate with their incidence in tumors
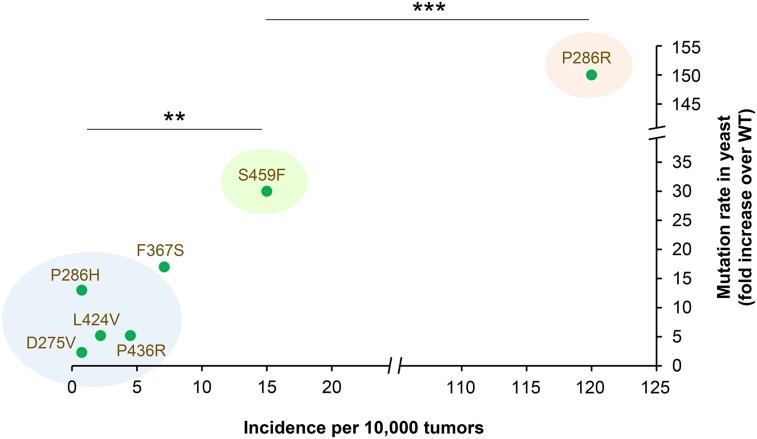
We hypothesized previously that the higher incidence of the P286R variant in cancers in comparison with other exonuclease domain changes is due to its exceptionally strong mutator effect and the resulting increased chances of accumulating cancer-driving mutations (Kane and Shcherbakova 2014). Indeed, the less common variants studied here were substantially weaker mutators (Figure 2). For the variants that caused a significant elevation of the mutation rate over wild-type levels (the DNA binding cleft variants), we further examined the relationship between their incidence in tumors and the mutator phenotype. We calculated the frequency of each variant using published sequencing data on >13,000 sporadic colorectal and endometrial tumors (Table S4 in File S1). Although the incidence estimates for the rarest variants are likely to be imprecise, in general, we observed that stronger mutator effects tended to be associated with higher variant frequency (Figure 4). The incidence of P286R was significantly higher than the incidence of the moderate mutator S459F, and the incidence of S459F significantly exceeded that of the weakest mutators P286H, P436R, L424V, and D275V. It is interesting that the correlation between the mutator effect and variant frequency was only observed for the DNA binding cleft variants. As described earlier, the very common but peripherally located V411L failed to produce any mutator effect in yeast, indicating that other factors must determine its high incidence.
Figure 4.
Relationship between the mutator effects of DNA binding cleft variants and their frequency in tumors. Relative CanR mutation rates for the yeast analogs of cancer-associated variants (Figure 2 and Table S3 in File S1) were plotted against the frequency at which the corresponding variant has been reported in CRC and EC (Table S4 in File S1). The mutation rate for the yeast P286R mimic is from Kane and Shcherbakova (2014). The breaks in the x and y axes remove equal fractions of the two axes. Blue, green, and pink ovals highlight variants, for which statistical significance of differences in frequency are indicated by asterisks. **P < 0.01 for the frequency of S459F vs. frequency of each of the variants in the blue oval. ***P < 0.0001. The P values for pairwise comparisons of all variants are shown in Table S5 in File S1.
The DNA binding cleft mutations are also known to variably affect the exonuclease activity of purified human Polε, ranging from a twofold reduction to a complete loss of exonuclease (Shinbrot et al. 2014). However, no correlation was seen between the extent of exonuclease deficiency or in vitro fidelity of Polε variants and their frequency in tumors (Figure S2 in File S1). These findings indicate that the preferential occurrence of some but not other DNA binding cleft variants in tumors is likely explained by the differences in the mutator effects in vivo and not by the extent of exonuclease deficiency. They also further confirm that the effects of exonuclease domain variants on the mutation rate are separable from their effects on proofreading.
Mutator Polε variants are semidominant
Polε mutations are almost exclusively present in the heterozygous state in sporadic human tumors. Loss of heterozygosity (LOH) is also not required for carriers of germline POLE mutations to develop tumors (Palles et al. 2013). We found previously that heterozygosity for the pol2-P301R allele used to mimic the most common POLE-P286R variant confers a strong increase in mutagenesis, comparable to the effect of MMR deficiency (Kane and Shcherbakova 2014). However, it remained unclear whether the other, weaker mutator alleles could cause a substantial enough increase in mutagenesis when present in the heterozygous state. We constructed diploid strains heterozygous for the mutator pol2 alleles and measured the rate of CanR mutation and His+ reversion. All DNA binding cleft variants significantly increased the mutation rate at both reporter loci when present in the heterozygous state, with the exception of CanR mutation in the weakest mutator strain pol2-D290V (Figure 5; hatched purple bars). These results further validate the pathogenicity of the majority of DNA binding cleft variants and explain why LOH is not necessary for hypermutability and tumorigenesis. Because LOH is still occasionally seen in both sporadic and hereditary cancers, and could potentially be associated with higher levels of hypermutation and earlier onset of the disease (Barbari and Shcherbakova 2017), we also investigated the impact of homozygosity of the mutator pol2 alleles on mutagenesis. The mutation rate in homozygous diploid strains was approximately twice as high as that in the corresponding heterozygous strains (Figure 5; solid purple bars). Thus, the mutant and wild-type polymerases must equally contribute to DNA replication in the heterozygotes, and the LOH would likely further accelerate the accumulation of mutations and tumorigenesis in humans. Mutator effects of pol2 alleles at the lys2-InsEA14 homonucleotide repeat locus were weak, if present at all, similar to the results with haploids, but semidominance could still be noted in the cases where mutagenesis was significantly increased over the wild-type level (Figure S3 in File S1).
Figure 5.
Mutator effects of cancer-associated Polε variants in diploid yeast strains. Mutation rates were measured in diploid yeast strains heterozygous or homozygous for pol2 mutations mimicking the human POLE variants. Relative mutation rates are shown compared with wild type (WT). The human variants are listed on the x-axis with the analogous yeast substitutions in parentheses. Exo−, exonuclease-deficient variant encoded by the pol2-4 allele. (A) CanR mutation was measured in strains containing a single copy of the CAN1 gene as described in Materials and Methods. (B) His+ reversion was measured in strains homozygous for the his7-2 allele. Asterisks indicate P < 0.05 by Wilcoxon–Mann–Whitney test compared with WT. Data are from Table S6 in File S1. aData from Kane and Shcherbakova (2014).
Weak Polε mutators present in MMR− tumors can act synergistically with MMR defects to increase mutation rate
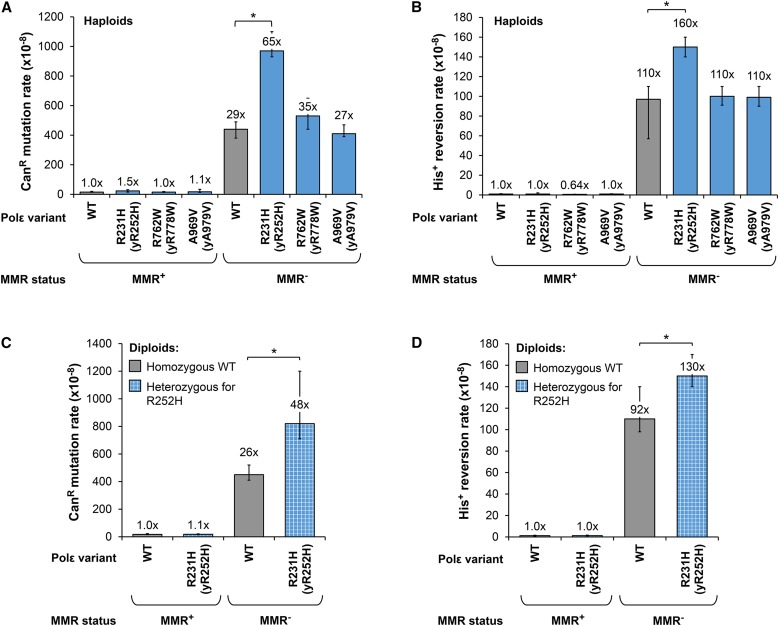
Three pol2 variants mimicking human POLE mutations reported in MMR-deficient MSI tumors had no mutator effect in MMR-proficient strains (Figure 2, blue bars). To determine whether these variants could make a meaningful contribution to genome instability in a MMR-deficient background, we measured their effects on the rate of CanR mutation and His+ reversion in strains with a deletion of MLH1. This deletion mimics the MMR deficiency caused by the hypermethylation of the MLH1 promoter, which is typically responsible for the MSI phenotype of tumors. The pol2-R252H variant caused a synergistic increase in the mutation rate in combination with the MMR defect. The synergy was observed both when pol2-R252H was present as the only POL2 allele in haploids (Figure 6, A and B) and in the heterozygous state in diploids (Figure 6, C and D), suggesting a potentially significant role of the corresponding human variant when MMR is compromised. By contrast, the pol2-R252H allele caused no significant increase in the rate of lys2-InsEA14 reversion beyond the expected dramatic increase conferred by the MLH1 deletion (Figure S4 in File S1). These assays predict that the corresponding human variant, R231H, would not further increase MSI in the MMR-deficient tumor but could accelerate the accumulation of mutations in nonrepetitive sequences. The other two alleles mimicking POLE variants R762W and A966V found in MSI tumors had no effect on mutation rate at any of the three reporter loci in the MMR-deficient background (Figure 6, A and B and Figure S4A in File S1). Therefore, it is probable that these two variants are neutral passenger mutations that arose as a consequence of the hypermutation caused by defective MMR. Taken together, these results suggest that although many of the POLE mutations in MMR-deficient tumors may have no functional significance, some could result in mildly error-prone polymerase variants that can act synergistically with faulty MMR to further elevate mutagenesis.
Figure 6.
Effects of Polε variants found in MMR-deficient tumors on the mutation rate in MMR-deficient background. Mutation rates were measured in haploid (A and B) or diploid (C and D) strains containing chromosomal pol2 mutations indicated on the x-axis. The MMR defect was mimicked by deleting the yeast MLH1 gene. Mutation rates are given as the median for at least 18 independent cultures, with error bars indicating 95% confidence intervals. Fold increase in mutation rate relative to the strain with wild-type POL2 and MLH1 genes is shown above each bar. Asterisks indicate P < 0.05 by Wilcoxon–Mann–Whitney test. Data are from Table S7 in File S1.
Discussion
Of the hundreds of somatic Polε mutations reported in tumors to date, the functional impact in vivo has only been assessed for the most frequently recurring Polε-P286R variant (Kane and Shcherbakova 2014). To gain a comprehensive understanding of the consequences of these mutations, we set out to characterize 13 additional cancer-associated Polε variants in the yeast model system. We show that amino acid substitutions near the DNA binding cleft in the exonuclease domain (the yeast mimics of D275V, P286H, F367S, L424V, P436R, and S459F) increase mutagenesis (Figure 2 and Figure 5). The increased mutation rate strongly argues that these variants drive the genomic instability in tumors, as previous in silico and in vitro analyses suggested (Church et al. 2013; Palles et al. 2013; Shinbrot et al. 2014; Rayner et al. 2016; Campbell et al. 2017). The magnitude of the mutator effect is highly variable and correlates with the incidence of these variants in tumors (Figure 4). This correlation is consistent with the idea that the chance of any particular POLE mutant cell lineage developing into a tumor (and, thus, the chance of the POLE mutation being detected by sequencing of tumor samples) is proportional to the severity of the mutator phenotype. Although different DNA binding cleft variants could also conceivably arise at different rates, our data suggest that the cancer risk conferred by each variant is a major determinant of its ultimate frequency in tumors.
The magnitude of the mutator effects we observed argues against the common view that the cancer-associated Polε mutations act by disabling proofreading. With the exception of the mutation affecting the catalytic aspartic acid residue (pol2-D290V mimicking human POLE-D275V), all other DNA binding cleft variants increased the mutation rate more than the pol2-4 mutation that completely eliminates proofreading (Figure 2). Most of these variants only partially reduce the exonuclease activity of human Polε in vitro (Shinbrot et al. 2014); therefore, if this was the only consequence at the protein level, the yeast mimics would be expected to be weaker mutators than pol2-4, not stronger. The disconnect between the exonuclease defect and the mutator phenotype is further apparent from the observation that the frequency of DNA binding cleft variants in tumors does not correlate with the degree of proofreading impairment (Figure S2 in File S1), whereas it does correlate with the mutator effect in vivo (Figure 4). Thus, the mutations must impact the polymerase in some additional way(s). It is likely that the pathogenicity of DNA binding cleft mutations is determined by these additional defects, and not by the loss of proofreading per se. The exact nature of these defects remains to be investigated.
The rather mild mutator effect of the yeast analog of POLE-L424V (Figure 2) deserves a special comment. Although it is consistent with the relatively low frequency of this variant in sporadic tumors, L424V is the most frequent germline DNA polymerase variant in patients with hereditary CRC. It has been reported in >20 families (Palles et al. 2013; Valle et al. 2014; Chubb et al. 2015; Elsayed et al. 2015; Spier et al. 2015) and has also been observed as a de novo germline variant in some CRC cases (Valle et al. 2014; Elsayed et al. 2015). The high prevalence of this specific variant in hereditary CRC was puzzling. It could be argued that stronger mutators escaped detection because they do not result in the specific clinical syndrome—a high-penetrance predisposition to colorectal adenomas and carcinomas—in which L424V is implicated. However, other modest mutators of the same range as L424V have not been detected in hereditary cancers either. A possible clue is offered by the genomic DNA sequence context of the mutation site, which likely represents a mutational hotspot (Barbari and Shcherbakova 2017). The mutation is a C to G transversion occurring in close proximity to a 10-nt GC-rich palindromic sequence that can form a hairpin-type structure [Figure 3 in Barbari and Shcherbakova (2017)]. We have shown previously that such short hairpins impede DNA synthesis by replicative DNA polymerases and promote mutations in the nearby region, particularly C→G transversions (Northam et al. 2014). The mechanism of these mutations involves the recruitment of translesion synthesis DNA polymerases that bypass the replication-blocking structures in an error-prone manner. Thus, it is likely that the POLE-L424V variant is seen frequently in cancers not because it is more pathogenic than other POLE mutations, but because it is generated more frequently at the DNA level, either during normal DNA replication or during various stress responses. The exceptionally high recurrence of L424V as a germline variant, but not as much as a somatic one, suggests an intriguing possibility that the bypass of small hairpin structures is more problematic in germline cells.
Unexpected based on the known properties of POLE mutant tumors was the absence of a readily detectable mutator effect of the POLE-V411L analog. V411L is one of the two most frequent variants in hypermutated sporadic tumors. A recent study of mutation burden in >81,000 tumors, the largest collection yet, confirmed its invariable association with hypermutation (Campbell et al. 2017). From a mechanistic point of view, the lack of striking phenotypic consequences of this conservative amino acid substitution is not overly surprising. Human Polε-V411L showed only a threefold reduction in exonuclease activity in vitro compared with the wild-type Polε, in contrast to an up to 20-fold decrease in some other cancer-associated variants (Shinbrot et al. 2014). Also, because V411 is located further away from the DNA binding interface (Figure 3), this mutation may not cause that additional unidentified change in properties that makes the other exonuclease domain variants strong mutators. If the mild exonuclease defect is the only consequence of the V411L substitution, it may not produce a statistically significant increase in mutagenesis in our yeast assays. Although this provides a plausible explanation for the lack of a mutator effect, the reasons for the high recurrence of this variant in tumors remain unclear. One possibility is that even a very weak mutator phenotype may be sufficient to accumulate a large number of mutations over many years. The high prevalence could then be explained if this genomic site behaved as a mutational hotspot, similar to the L424V case. We could not immediately pinpoint any specific DNA sequence features around the mutation site that would support the hotspot hypothesis. A recent study also suggested that cells with the V411L mutation may, in fact, take a shorter path to cancer: a germline V411L variant was identified in a pediatric CRC case with an onset at least 10 yr earlier than is typical for carriers of other POLE mutations (Wimmer et al. 2017). An alternative explanation is that the V411L substitution causes a defect in a protein–protein interaction important for replication fidelity that cannot be modeled in yeast. It is also possible that expression of the mutator phenotype by V411L cells requires some additional factors that are not present during growth under standard laboratory conditions, such as exposure to DNA-damaging agents or replication stress. Although highly speculative, a fourth possibility is that the V411L variant is not the primary driver of hypermutability in tumors but arises concomitantly with another genome maintenance defect. As mentioned previously, the spectrum of mutations accumulating in tumors with the V411L variant differs somewhat from the mutational specificity of other POLE mutant tumors (Church et al. 2013), suggesting different mutagenesis mechanisms. Further studies are needed to determine the reasons for the pathogenicity of this atypical variant and its high recurrence, including analysis in human cells to assess potential consequences that may not be detected in yeast.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.118.200042/-/DC1.
Acknowledgments
We thank Krista Brown for technical assistance and Youri Pavlov for the YEpPOL2 plasmid. This work was supported by the National Institutes of Health grant ES015869 to P.V.S. S.R.B. was supported by the Cancer Biology Training Grant T32CA009476 from the National Cancer Institute.
Footnotes
Communicating editor: B. Calvi
Literature Cited
- Albertson T. M., Ogawa M., Bugni J. M., Hays L. E., Chen Y., et al. , 2009. DNA polymerase ε and δ proofreading suppress discrete mutator and cancer phenotypes in mice. Proc. Natl. Acad. Sci. USA 106: 17101–17104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov L. B., Nik-Zainal S., Wedge D. C., Aparicio S. A., Behjati S., et al. , 2013. Signatures of mutational processes in human cancer. Nature 500: 415–421 (erratum: Nature 502: 258). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianova M. A., Chetan G. K., Sibin M. K., McKee T., Merkler D., et al. , 2017. Germline PMS2 and somatic POLE exonuclease mutations cause hypermutability of the leading DNA strand in biallelic mismatch repair deficiency syndrome brain tumours. J. Pathol. 243: 331–341. [DOI] [PubMed] [Google Scholar]
- Araki H., Ropp P. A., Johnson A. L., Johnston L. H., Morrison A., et al. , 1992. DNA polymerase II, the probable homolog of mammalian DNA polymerase ε, replicates chromosomal DNA in the yeast Saccharomyces cerevisiae. EMBO J. 11: 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbari S. R., Shcherbakova P. V., 2017. Replicative DNA polymerase defects in human cancers: consequences, mechanisms, and implications for therapy. DNA Repair (Amst.) 56: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingsley C. C., Cohn D. E., Mutch D. G., Stephens J. A., Suarez A. A., et al. , 2015. Polymerase ε (POLE) mutations in endometrial cancer: clinical outcomes and implications for Lynch syndrome testing. Cancer 121: 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. B., Light N., Fabrizio D., Zatzman M., Fuligni F., et al. , 2017. Comprehensive analysis of hypermutation in human cancer. Cell 171: 1042–1056.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network , 2012. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network , 2013. Integrated genomic characterization of endometrial carcinoma. Nature 497: 67–73 (erratum: Nature 500: 242). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E., Gao J., Dogrusoz U., Gross B. E., Sumer S. O., et al. , 2012. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2: 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb D., Broderick P., Frampton M., Kinnersley B., Sherborne A., et al. , 2015. Genetic diagnosis of high-penetrance susceptibility for colorectal cancer (CRC) is achievable for a high proportion of familial CRC by exome sequencing. J. Clin. Oncol. 33: 426–432. [DOI] [PubMed] [Google Scholar]
- Church D. N., Briggs S. E., Palles C., Domingo E., Kearsey S. J., et al. , 2013. DNA polymerase ε and δ exonuclease domain mutations in endometrial cancer. Hum. Mol. Genet. 22: 2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church D. N., Stelloo E., Nout R. A., Valtcheva N., Depreeuw J., et al. , 2015. Prognostic significance of POLE proofreading mutations in endometrial cancer. J. Natl. Cancer Inst. 107: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daee D. L., Mertz T. M., Shcherbakova P. V., 2010. A cancer-associated DNA polymerase δ variant modeled in yeast causes a catastrophic increase in genomic instability. Proc. Natl. Acad. Sci. USA 107: 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W. J., Massey F. J., Jr, 1969. Introduction to Statistical Analysis. McGraw-Hill, New York. [Google Scholar]
- Domingo E., Freeman-Mills L., Rayner E., Glaire M., Briggs S., et al. , 2016. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: a retrospective, pooled biomarker study. Lancet Gastroenterol. Hepatol. 1: 207–216. [DOI] [PubMed] [Google Scholar]
- Elsayed F. A., Kets C. M., Ruano D., van den Akker B., Mensenkamp A. R., et al. , 2015. Germline variants in POLE are associated with early onset mismatch repair deficient colorectal cancer. Eur. J. Hum. Genet. 23: 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erson-Omay E. Z., Çağlayan A. O., Schultz N., Weinhold N., Omay S. B., et al. , 2015. Somatic POLE mutations cause an ultramutated giant cell high-grade glioma subtype with better prognosis. Neuro-oncol. 17: 1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa I., Lee C. H., D’Angelo E., Palacios J., Prat J., 2017. Undifferentiated and dedifferentiated endometrial carcinomas with POLE exonuclease domain mutations have a favorable prognosis. Am. J. Surg. Pathol. 41: 1121–1128. [DOI] [PubMed] [Google Scholar]
- Forbes S. A., Beare D., Gunasekaran P., Leung K., Bindal N., et al. , 2015. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 43: D805–D811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganai R. A., Johansson E., 2016. DNA replication—a matter of fidelity. Mol. Cell 62: 745–755. [DOI] [PubMed] [Google Scholar]
- Giannakis M., Mu X. J., Shukla S. A., Qian Z. R., Cohen O., et al. , 2016. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 15: 857–865 (erratum: Cell Rep. 17: 1206). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A., 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74: 527–534. [DOI] [PubMed] [Google Scholar]
- Goldsby R. E., Lawrence N. A., Hays L. E., Olmsted E. A., Chen X., et al. , 2001. Defective DNA polymerase-δ proofreading causes cancer susceptibility in mice. Nat. Med. 7: 638–639. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., McCusker J. H., 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15: 1541–1553. [DOI] [PubMed] [Google Scholar]
- Gong J., Wang C., Lee P. P., Chu P., Fakih M., 2017. Response to PD-1 blockade in microsatellite stable metastatic colorectal cancer harboring a POLE mutation. J. Natl. Compr. Canc. Netw. 15: 142–147. [DOI] [PubMed] [Google Scholar]
- Grossman R. L., Heath A. P., Ferretti V., Varmus H. E., Lowy D. R., et al. , 2016. Toward a shared vision for cancer genomic data. N. Engl. J. Med. 375: 1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. F., Johansen J., Bøjrnevoll I., Sylvander A. E., Steinsbekk K. S., et al. , 2015. A novel POLE mutation associated with cancers of colon, pancreas, ovaries and small intestine. Fam. Cancer 14: 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang L. N., McConechy M. K., Köbel M., Anglesio M., Senz J., et al. , 2015. Polymerase epsilon exonuclease domain mutations in ovarian endometrioid carcinoma. Int. J. Gynecol. Cancer 25: 1187–1193. [DOI] [PubMed] [Google Scholar]
- Hogg M., Osterman P., Bylund G. O., Ganai R. A., Lundström E. B., et al. , 2014. Structural basis for processive DNA synthesis by yeast DNA polymerase ε. Nat. Struct. Mol. Biol. 21: 49–55. [DOI] [PubMed] [Google Scholar]
- Jansen A. M., van Wezel T., van den Akker B. E., Ventayol Garcia M., Ruano D., et al. , 2016. Combined mismatch repair and POLE/POLD1 defects explain unresolved suspected Lynch syndrome cancers. Eur. J. Hum. Genet. 24: 1089–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanns T. M., Miller C. A., Dorward I. G., Tsien C., Chang E., et al. , 2016. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. 6: 1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane D. P., Shcherbakova P. V., 2014. A common cancer-associated DNA polymerase ε mutation causes an exceptionally strong mutator phenotype, indicating fidelity defects distinct from loss of proofreading. Cancer Res. 74: 1895–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köbel M., Meng B., Hoang L. N., Almadani N., Li X., et al. , 2016. Molecular analysis of mixed endometrial carcinomas shows clonality in most cases. Am. J. Surg. Pathol. 40: 166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConechy M. K., Talhouk A., Leung S., Chiu D., Yang W., et al. , 2016. Endometrial carcinomas with POLE exonuclease domain mutations have a favorable prognosis. Clin. Cancer Res. 22: 2865–2873. [DOI] [PubMed] [Google Scholar]
- Mehnert J. M., Panda A., Zhong H., Hirshfield K., Damare S., et al. , 2016. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J. Clin. Invest. 126: 2334–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng B., Hoang L. N., McIntyre J. B., Duggan M. A., Nelson G. S., et al. , 2014. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol. Oncol. 134: 15–19. [DOI] [PubMed] [Google Scholar]
- Mertz T. M., Sharma S., Chabes A., Shcherbakova P. V., 2015. Colon cancer-associated mutator DNA polymerase δ variant causes expansion of dNTP pools increasing its own infidelity. Proc. Natl. Acad. Sci. USA 112: E2467–E2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A., Bell J. B., Kunkel T. A., Sugino A., 1991. Eukaryotic DNA polymerase amino acid sequence required for 3′→5′ exonuclease activity. Proc. Natl. Acad. Sci. USA 88: 9473–9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northam M. R., Robinson H. A., Kochenova O. V., Shcherbakova P. V., 2010. Participation of DNA polymerase ζ in replication of undamaged DNA in Saccharomyces cerevisiae. Genetics 184: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northam M. R., Moore E. A., Mertz T. M., Binz S. K., Stith C. M., et al. , 2014. DNA polymerases ζ and Rev1 mediate error-prone bypass of non-B DNA structures. Nucleic Acids Res. 42: 290–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palles C., Cazier J. B., Howarth K. M., Domingo E., Jones A. M., et al. , 2013. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat. Genet. 45: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov Y. I., Shcherbakova P. V., Kunkel T. A., 2001. In vivo consequences of putative active site mutations in yeast DNA polymerases α, ε, δ, and ζ. Genetics 159: 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltomäki P., 2003. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J. Clin. Oncol. 21: 1174–1179. [DOI] [PubMed] [Google Scholar]
- Rayner E., van Gool I. C., Palles C., Kearsey S. E., Bosse T., et al. , 2016. A panoply of errors: polymerase proofreading domain mutations in cancer. Nat. Rev. Cancer 16: 71–81. [DOI] [PubMed] [Google Scholar]
- Rohlin A., Zagoras T., Nilsson S., Lundstam U., Wahlström J., et al. , 2014. A mutation in POLE predisposing to a multi-tumour phenotype. Int. J. Oncol. 45: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshagiri S., Stawiski E. W., Durinck S., Modrusan Z., Storm E. E., et al. , 2012. Recurrent R-spondin fusions in colon cancer. Nature 488: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbakova P. V., Kunkel T. A., 1999. Mutator phenotypes conferred by MLH1 overexpression and by heterozygosity for mlh1 mutations. Mol. Cell. Biol. 19: 3177–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinbrot E., Henninger E. E., Weinhold N., Covington K. R., Göksenin A. Y., et al. , 2014. Exonuclease mutations in DNA polymerase ɛ reveal replication strand specific mutation patterns and human origins of replication. Genome Res. 24: 1740–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlien A., Campbell B. B., de Borja R., Alexandrov L. B., Merico D., et al. , 2015. Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra-hypermutated cancers. Nat. Genet. 47: 257–262. [DOI] [PubMed] [Google Scholar]
- Spier I., Holzapfel S., Altmüller J., Zhao B., Horpaopan S., et al. , 2015. Frequency and phenotypic spectrum of germline mutations in POLE and seven other polymerase genes in 266 patients with colorectal adenomas and carcinomas. Int. J. Cancer 137: 320–331. [DOI] [PubMed] [Google Scholar]
- Stenzinger A., Pfarr N., Endris V., Penzel R., Jansen L., et al. , 2014. Mutations in POLE and survival of colorectal cancer patients–link to disease stage and treatment. Cancer Med. 3: 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk A., McConechy M. K., Leung S., Li-Chang H. H., Kwon J. S., et al. , 2015. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 113: 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H. T., Keen J. D., Kricker M., Resnick M. A., Gordenin D. A., 1997. Hypermutability of homonucleotide runs in mismatch repair and DNA polymerase proofreading yeast mutants. Mol. Cell. Biol. 17: 2859–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle L., Hernández-Illán E., Bellido F., Aiza G., Castillejo A., et al. , 2014. New insights into POLE and POLD1 germline mutations in familial colorectal cancer and polyposis. Hum. Mol. Genet. 23: 3506–3512. [DOI] [PubMed] [Google Scholar]
- Van der Parren J. L., 1970. Tables for distribution-free confidence limits for the median. Biometrika 57: 613–617. [Google Scholar]
- Venkatesan R. N., Treuting P. M., Fuller E. D., Goldsby R. E., Norwood T. H., et al. , 2007. Mutation at the polymerase active site of mouse DNA polymerase δ increases genomic instability and accelerates tumorigenesis. Mol. Cell. Biol. 27: 7669–7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer K., Beilken A., Nustede R., Ripperger T., Lamottke B., et al. , 2017. A novel germline POLE mutation causes an early onset cancer prone syndrome mimicking constitutional mismatch repair deficiency. Fam. Cancer 16: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A., Kuick C. H., Wong W. L., Tham J. M., Mansor S., et al. , 2016. Mutation spectrum of POLE and POLD1 mutations in South East Asian women presenting with grade 3 endometrioid endometrial carcinomas. Gynecol. Oncol. 141: 113–120. [DOI] [PubMed] [Google Scholar]
- Yoshida R., Miyashita K., Inoue M., Shimamoto A., Yan Z., et al. , 2011. Concurrent genetic alterations in DNA polymerase proofreading and mismatch repair in human colorectal cancer. Eur. J. Hum. Genet. 19: 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehir A., Benayed R., Shah R. H., Syed A., Middha S., et al. , 2017. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23: 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Choi M., Overton J. D., Bellone S., Roque D. M., et al. , 2013. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl. Acad. Sci. USA 110: 2916–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains and plasmids are available upon request. All mutation rate and variant frequency data used to reach the conclusions are presented fully within the article and the supplemental material.