Abstract
Introduction
Arterial stiffness, measured by the augmentation index (AIX) from radial artery tonometry, and endothelial dysfunction, measured by brachial-artery flow-mediated vasodilation (FMD), have each been associated with increased risk of cardiovascular events. However, their interrelationship in PAD patients is poorly understood.
Materials and Methods
In a cross-sectional analysis of 123 vascular surgery outpatients, the association between FMD and AIX was examined in controls with atherosclerotic risk factors (n=32) and patients with PAD (n=91). PAD was defined as claudication symptoms with an ankle-brachial index (ABI) of <.9 or a history of revascularization for symptomatic PAD. Controls had an ABI ≥.9 and no history of atherosclerotic vascular disease.
Results
Compared to controls, patients with PAD had lower FMD (6.3 ± 3.8 vs. 8.4 ± 3.7, p=.008), while central AIX normalized to 75bpm (25.5 ± 9.0 vs. 19.3 ± 8.6, p=.001) and peripheral AIX (91.3 ± 14.5 vs. 81.3 ± 11.4, p=.001) were higher. FMD was not significantly correlated with either central or peripheral AIX (central AIX: p=.58; peripheral AIX: p=.89) across the entire cohort, or in either the patients with PAD (central AIX: p=.48; peripheral AIX: p=.23) or controls (central AIX: p=.43; peripheral AIX: p=.92). In a multivariate model including FMD, higher AIX remained independently associated with PAD.
Conclusions
In an analysis of vascular surgery outpatients, no correlation between FMD and AIX was detected. Larger prospective studies are needed to determine whether the inclusion of both parameters improves predictive models for the early identification and potential risk stratification of PAD patients.
Keywords: flow mediated vasodilation, radial artery tonometry, endothelial function, arterial stiffness, peripheral artery disease (PAD)
INTRODUCTION
Peripheral artery disease (PAD), an atherosclerotic process of the peripheral arterial tree, is a significant public health concern due to its increasing worldwide prevalence and high economic burden for society.1, 2 In the United States, critical limb ischemia, the end-stage of PAD, accounts for over 300,000 inpatient admissions annually.3 Additionally, affected individuals suffer from decreased quality of life and increased mortality.4, 5 Non-invasive measures of specific physiological processes play an important role in understanding the pathophysiology of PAD. They also have the potential to play a clinical role in the identification and risk stratification of individuals with PAD, and the subsequent measurement of their response to treatment. Brachial artery flow-mediated vasodilation (FMD) for endothelial function and radial artery tonometry for arterial stiffness are two such measures.6, 7
FMD involves a measurement of change in the brachial artery diameter in response to a hyperemic stimulus, which triggers endothelial cells to release nitric oxide and cause arterial dilation.8 A higher FMD corresponds to better endothelial function, and two large meta-analyses have shown that cardiovascular disease (CVD) risk increases by approximately 10% for each 1% decrease in FMD.9, 10 Lower FMD has also been associated with increased risk of post-operative cardiovascular events following vascular surgery.11 Despite concerns of using FMD in clinical practice due to labor-intensiveness and reproducibility issues, standardization is improving its reliability.12
Arterial tonometry involves placing a small tonometer over a target artery to detect the underlying pulse waveforms, which are influenced by the stiffness of the arterial wall. Radial artery tonometry can be used to calculate a central and peripheral augmentation index (AIX), which refers to the ratio of the augmented pressure, resulting from wave reflections, to the pulse pressure, expressed as a percentage.13 Tonometry at two arteries of a measured distance apart, most commonly the common carotid and femoral arteries, can be used to calculate the pulse-wave velocity (PWV) through the central arteries, with higher velocity corresponding to greater arterial stiffness. AIX is an indirect measure of stiffness14, 15 and PWV is a direct measure.16 Arterial stiffness is associated with adverse vascular outcomes and mortality,17, 18 while AIX specifically is also predictive of CVD risk.19
Although increased arterial stiffness and decreased endothelial function are associated with worse vascular outcomes, suggesting an inverse correlation, studies have not consistently shown a correlation between the two measures.20–23 Furthermore, studies investigating the relationship between arterial stiffness and endothelial function in the PAD population are limited.24 As these assessments become more widely utilized as measures of future risk, it is important to understand how they relate to each other and how they can be used together to improve accuracy. Additionally, FMD and AIX are measuring different vascular functions that could each play unique roles in increasing vascular risk. Understanding how arterial stiffness and endothelial function relate to predict outcomes in PAD is meaningful to understand how all of these factors can be prevented or treated. Therefore, the present study provides a robust dataset to study the association between arterial stiffness, as measured by the AIX, and endothelial function, as measured by FMD, in the PAD population. Since both arterial stiffness and endothelial function are involved in the pathophysiology and outcomes of PAD, this study tests the hypothesis that there is an inverse correlation between FMD and augmentation index.
MATERIALS AND METHODS
Study Participants
From February 2012 to September 2016, a cross-sectional sample of 123 veterans was enrolled from the San Francisco VA Medical Center (SFVAMC) outpatient vascular surgery clinic. Participants were identified as having PAD (n=91) if they had an abnormal ankle-brachial index (ABI) (<0.9) plus symptoms of claudication or if they had a history of peripheral revascularization for symptomatic PAD. Controls (n=32) had a normal ABI and no history of atherosclerotic vascular disease. To be eligible for inclusion in this study, all participants had to have complete radial artery tonometry and FMD data. All participants were at least 35 years of age, reflecting the population of veterans with PAD. Potential participants were excluded if they had a creatinine ≥ 2 mg/dL, or a history of significant hepatic impairment (Child-Pugh ≥ B), non-vascular inflammatory disorders (e.g. requiring immunosuppressive medications), or other concurrent severe acute disease. These exclusion criteria were used so that data from participants could be used for other studies on the role of inflammation in PAD.
Demographic information including age, sex, and race was recorded for all study participants. History of smoking was assessed, including pack years (defined as the number of years smoking multiplied by average number of packs per day), as well as history of major comorbidities including hypertension, hyperlipidemia, diabetes mellitus, or coronary artery disease. Measurements taken included body mass index (BMI), blood pressure at the brachial artery, and an ABI for each lower extremity using established techniques.25 Participants completed the PTSD checklist – civilian version (PCL-C) and patient health questionnaire (PHQ-9), with PTSD defined as a PCL ≥ 4026 and depression defined as a score ≥ 10.27 These mental health measures were included because FMD has been shown to be independently associated with PTSD.28 Current use of the following medications was recorded: aspirin, ace-inhibitor, beta-blocker, and statin. Finally, blood was obtained for laboratory assessment of high-sensitivity C-reactive protein (hsCRP), hemoglobin A1c, lipids (total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides), and estimated glomerular filtration rate (eGFR). The investigator-initiated protocol was approved by the University of California, San Francisco (UCSF) Committee on Human Research as well as the SFVAMC Research and Development Office with all participants giving informed written consent.
Flow Mediated Dilation
Brachial artery FMD was performed as previously described by our group and consistent with current standards.8, 28 Participants were instructed to fast as well as to avoid nicotine and caffeine for several hours prior to the exam. Prior to recording, participants were placed in a supine position for 10-minutes and allowed to acclimate to the exam environment. Using the upper arm technique supported by current guidelines, 29 a tourniquet blood pressure cuff was placed on the upper arm just distal to deltoid insertion. Using B-mode ultrasound (Philips HD11) with a broadband linear array transducer with a 3–12 MHz range (Philips L12–3), the target artery was examined to locate a segment for further imaging. Criteria include identifying a straight arterial segment with a “double line sign,” signifying visualization of the near and far intima media, and a landmark such as a crossing vein. To record the baseline diameter of the brachial artery, an EKG-gated image capture software system was employed (Brachial Imager, Medical Imaging Applications LLC, Coralville, IA). Additionally, using an isonation angle of 60°, a baseline Doppler spectral waveform was recorded. Baseline data was collected for 60 seconds to calculate mean diameter and velocity.
To induce a state of hyperemia, the blood pressure cuff was first inflated to 50mmHg above the patient’s systolic blood pressure or 250mmHg, whichever first induced complete brachial artery occlusion as confirmed by ultrasound. The occlusion cuff was kept in place for 5 minutes. Then the cuff was deflated and post-hyperemic diameter and spectral waveforms were recorded for 3 minutes.
Continuous edge-detection software (Brachial Analyzer, Medical Imaging Applications LLC, Coralville, IA) was used for image analysis and calculation of hemodynamic parameters relevant to FMD at baseline and post-cuff release. Luminal diameter was measured between the near and far wall. The velocity-time integral (VTI) was calculated by integrating the spectral waveform over four cardiac cycles. Using the VTI and cross-section area of the vessel, the blood flow through the brachial artery could be calculated. Then the Hagen-Poiseuille equation (Equation 1) was used to calculate mean shear stress, where Tw is shear stress in dynes/cm2, μ is viscosity of the blood and set at 0.0035, Q is mean flow, and r is the radius (cm) of the brachial artery lumen. Equation 1. Shear Stress formula
Hyperemic parameters were calculated using data recorded for 55–65 seconds after cuff release, including FMD, which is defined as [(hyperemia diameter – baseline diameter)/baseline diameter] multiplied by 100 to express the result as a percentage.
For quality control, images were evaluated by two people and graded on a 6-point scale for the following: the presence of a landmark structure, a horizontally directed artery, correct longitudinal alignment, clearly visualized “double line sign”, and at least 5 mm of visualized artery. The inter-observer variability was 0.05 ± 0.16% and the intra-observer variability was 0 ± 0.15%, as previously published.28
Radial Artery Tonometry
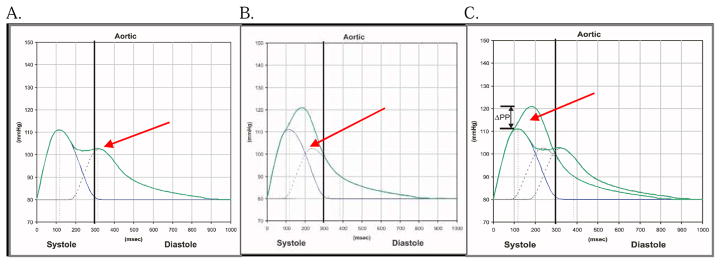
AIX is calculated from pulse wave analysis (PWA) owing to the observation that the arterial waveform is a composite of each forward pulse wave, plus a component of the preceding wave, which was reflected off the arterial wall in a retrograde fashion. Stiffer arteries cause the reflected wave to collide with the succeeding wave earlier in diastole leading to a higher augmented pressure (Figure 1). 30, 31
FIGURE 1. The Augmentation Index.
A) In healthy arteries, the reflected wave returns late in diastole. B) In stiffer arteries, the reflected wave returns earlier in diastole, and C) augments the pressure of the combined waveform. The augmentation index (AIX) represents the augmented pressure as a percentage of the overall pulse pressure such that [augmented pressure/(systolic blood pressure – diastolic blood pressure) × 100].
Note: Figure was modified and reproduced with permission from AtCor Medical.
While in a supine position, participants were instructed to rest for five minutes to equilibrate to the exam environment. The brachial artery blood pressure was measured and entered into the SphygmoCor® system (AtCor Medical, Sydney, Australia). Tonometric assessment was then performed by applying the SphygmoCor® applanation tonometer to the skin above the radial artery at the right distal arm/wrist. The device’s proprietary software calculated the AIX, defined as [augmented pressure/(systolic blood pressure – diastolic blood pressure)]. The peripheral AIX was calculated directly and the software used previously validated transfer equations to approximate the central AIX.32 The use of radial artery measurements to approximate central stiffness are now routinely conducted.6, 33, 34 Since central AIX is sensitive to variations in heart rate, all measurements were normalized to 75 beats per minute (bpm).13, 16 The normalization process is conducted automatically such that for each increase of 10 bpm, central AIX is inversely decreased by 4.8%.6 The device’s software also calculated a quality index, which takes into account multiple parameters of variation in the recorded waveforms (e.g. pulse height variation, diastolic length variation, etc.). Less variation leads to a higher quality index, with a manufacturer set threshold of 80 for acceptable quality. Up to four recordings were conducted for each participant and the measurement with the highest quality index was recorded.
Following a protocol developed in compliance with manufacturer guidelines, two reviewers (GZ and KS) rated the quality of all tonometry data and achieved 100% inter-rater agreement. All data that had a quality index <80 or failed to meet two of the following three criteria were discarded: 1) pulse height ≥ 80, 2) height variation ≤ 5%, and 3) diastolic variation ≤ 5%. Using these standards, all 123 participants included in this study had high quality central AIX data, but peripheral AIX data was obtained on only 119 because peripheral AIX was missing for two participants and dropped for two others due to extreme values (i.e., greater than 3 standard deviations above the study mean).
Statistical Analysis
Data analysis was primarily performed using Stata version 14 (StataCorp, College Station, Texas). MATLAB (MathWorks, Natick, Massachusetts) was used for Fisher transformations and to confirm correlation coefficients. Unadjusted, between-group differences were compared using two-tailed unpaired t-tests and Fisher’s exact test. Three separate multivariable logistic regression models were built to predict PAD using either central AIX at 75bpm, peripheral AIX, or FMD as the primary predictor. Covariates to include in the model were determined using a univariate screening analysis where each potential covariate was added one at a time while adjusting for the primary predictor of interest (i.e., central AIX, peripheral AIX, or FMD). All covariates with a p<.10 were included in the final model in addition to race (Caucasian vs. other) and age, which were forced into the models. All variables in Table 1 were screened for potential inclusion except for CAD, history of prior revascularization, and ABI; these latter measures were used to define whether each participant had PAD or was a control. When related variables met criteria for inclusion (e.g. LDL and hyperlipidemia), the more time invariant variable (e.g. the underlying disorder such as hyperlipidemia) was preferentially selected for inclusion in the final model. 2
TABLE 1.
Characters of Patients with PAD and Controls (n=123)
| PAD (n=91) | Controls (n=32) | ||||
|---|---|---|---|---|---|
| Characteristics | n/mean | %/(SD) | n/mean | %/(SD) | P-valuea |
| Demographics | |||||
| Age | 69.9 | (6.7) | 65.7 | (9.2) | .01 |
| Male Sex | 88 | 97% | 31 | 97% | 1.00 |
| Caucasian | 62 | 69% | 21 | 66% | .83 |
| Comorbidities and Risk Factors | |||||
| Body Mass Index (kg/m2) | 28.7 | (5.4) | 28.3 | (3.7) | .69 |
| Pack Years | 44 | (31) | 20 | (24) | <.001 |
| Hypertension | 83 | 91% | 19 | 59% | <.001 |
| Hyperlipidemia | 77 | 85% | 21 | 66% | .04 |
| Diabetes Mellitus | 34 | 37% | 4 | 13% | .01 |
| Coronary Artery Disease | 38 | 42% | 0 | 0% | <.001b |
| Depression (PHQ-9 score ≥ 10) | 12 | 16% | 2 | 8% | .51 |
| PTSD (PCL-C ≥ 40) | 16 | 21% | 2 | 9% | .23 |
| Systolic Blood Pressure (mm Hg) | 144 | (19) | 135 | (15) | .02 |
| Diastolic Blood Pressure (mm Hg) | 78 | (11) | 82 | (8) | .13 |
| Ankle Brachial Index (ABI) | 0.73 | (.15) | 1.11 | (.12) | <.001b |
| History of Revascularization | 36 | 40% | 0 | 0% | <.001b |
| Medications | |||||
| Aspirin | 69 | 76% | 18 | 56% | .04 |
| Ace-inhibitor | 46 | 51% | 11 | 34% | .15 |
| Beta-blocker | 54 | 59% | 6 | 19% | <.001 |
| Statin | 75 | 82% | 18 | 56% | <.01 |
| Laboratory Studies | |||||
| Total Cholesterol (mg/dL) | 162 | (43) | 177 | (35) | .10 |
| LDL (mg/dL) | 86 | (35) | 105 | (29) | .01 |
| HDL (mg/dL) | 48 | (13) | 48 | (13) | .77 |
| Triglycerides (mg/dL) | 137 | (95) | 128 | (112) | .68 |
| eGFR (mL/min) | 77 | (23) | 88 | (23) | .02 |
| HbA1C (%) | 6.2 | (1.3) | 5.9 | (1.5) | .35 |
| log(hsCRP) | 0.83 | (1.02) | 0.72 | (1.29) | .62 |
Continuous characteristics are summarized by mean (SD) with between-groups p-values calculated using a t-test. Categorical variables were summarized by number (%) with p-values calculated using Fisher’s exact test.
Per selection criteria, control subjects did not have CAD or a history of peripheral revascularization. ABI was also used to differentiate PAD patients and controls.
Scatterplots were generated and Pearson correlation coefficients were then calculated for FMD and AIX for the entire cohort. This analysis was repeated separately for the PAD subgroup as well as the controls. To better understand the range of correlation coefficients the study was powered to detect based on the observed pattern of data, 95% confidence intervals for all Pearson coefficients were calculated. To determine the confidence intervals, the Pearson product moment coefficient had to be converted to a normally distributed quantity using a Fisher transformation. Once the confidence interval was calculated, the values were then transformed back to rho. A final multivariable logistic regression model was built to predict PAD using both FMD and tonometry as primary predictors. All the covariates from the previously built tonometry and FMD models to predict PAD were included in this final model. Finally, to examine whether including both AIX and FMD in a single model improved its ability to predict PAD, an exploratory area under the curve (AUC) analysis was performed along with a likelihood ratio test. All multivariable models were audited for influential values using Pregibon’s dbeta and appropriate sensitivity analyses were performed.
RESULTS
The unadjusted, between-groups analysis revealed that patients with PAD (n=91) were older and more likely to have several comorbidities including hypertension, hyperlipidemia, and diabetes when compared to controls (n=32). Patients with PAD smoked a greater number of pack years, and had a lower LDL, which was likely an indicator of greater statin use. Additionally, participants with PAD had higher blood pressure (systolic), lower ABI, lower eGFR, and were more likely to take beta-blockers and aspirin (Table 1). All the above comparisons reached statistical significance. Furthermore, there were several unadjusted differences between groups on FMD and tonometry parameters. Most notably, brachial FMD was lower in PAD patients (6.3 ± 3.8 vs. 8.4 ± 3.7, p=.008), while both central AIX normalized to 75bpm (25.5 ± 9.0 vs. 19.3 ± 8.6, p=.001) and peripheral AIX (91.3 ± 14.5 vs. 81.3 ± 11.4, p=.001) were higher in PAD (Table 2).
TABLE 2.
Tonometry Parameters in Patients with PAD and Controls
| PAD (n=91) | Controls (n=32) | ||||
|---|---|---|---|---|---|
| Characteristics | mean | (SD) | mean | (SD) | P-valuea |
| Central Artery Stiffness | |||||
| Central Systolic Pressure (mm/Hg) | 135 | (19) | 126 | (16) | .03 |
| Central Diastolic Pressure (mm/Hg) | 79 | (14) | 83 | (8) | .11 |
| Central Augmentation Index | 31.5 | (9.4) | 25.5 | (8.9) | .002 |
| Central Augmentation Index at 75bpm | 25.5 | (9.0) | 19.3 | (8.6) | .001 |
| Peripheral Artery Stiffnessb | |||||
| Peripheral Systolic Pressure (mm/Hg) | 145 | (19) | 136 | (16) | .03 |
| Peripheral Diastolic Pressure (mm/Hg) | 79 | (11) | 82 | (8) | .13 |
| Peripheral Mean Pressure (mm/Hg) | 101 | (13) | 101 | (9) | .88 |
| Peripheral Augmentation Index | 91.3 | (14.5) | 81.3 | 11.4 | .001 |
| Brachial Artery Flow Mediated Dilation | |||||
| Baseline Diameter (cm) | 0.39 | (.06) | 0.43 | (.07) | .01 |
| Reactive Hyperemia Diameter (cm) | 0.42 | (.06) | 0.47 | (.07) | .001 |
| % Change from Baseline (brachial FMD) | 6.3 | (3.8) | 8.4 | (3.7) | .008 |
| Baseline Velocity (m/s) | 0.17 | (.07) | 0.16 | (.05) | .24 |
| Reactive Hyperemia Velocity (m/s) | 0.72 | (.28) | 0.86 | (.28) | .02 |
| Change from Baseline with RH (m/s) | 0.55 | (.28) | 0.71 | (.27) | .007 |
| Baseline Flow (mL/min) | 130 | (68) | 138 | (55) | .57 |
| Reactive Hyperemia Flow (mL/min) | 628 | (313) | 882 | (341) | <.001 |
| Change from Baseline with RH (mL/min) | 498 | (283) | 744 | (313) | <.001 |
| Baseline Shear Stress (Dynes/cm2) | 12.7 | (7.0) | 10.5 | (3.9) | .09 |
| Reactive Hyperemia Shear Stress (Dynes/cm2) | 48.9 | (20.6) | 53.5 | (21.0) | .28 |
| Change from Baseline with RH (Dynes/cm2) | 36.2 | (19.3) | 43.0 | (19.4) | .09 |
Continuous characteristics are summarized by mean (SD) with between-groups p-values calculated using a t-test.
Peripheral tonometry data summarizes all values after two outliers >3SD from the mean were dropped.
To determine whether central AIX, peripheral AIX, and FMD are each independently associated with PAD, three separate multivariable models were built. Each model controlled for age, race, hypertension, diabetes, hyperlipidemia, pack years, and eGFR. Higher central (OR 1.07, 95% CI 1.01–1.15, p=.030) and peripheral AIX (OR 1.07, 95% CI 1.02–1.13, p=.008) were associated with higher odds of PAD (Table 3). While FMD was significantly lower in PAD in the unadjusted analysis, it did not reach statistical significance in the multivariable analysis (OR .91, 95% CI .78–1.05, p=.20). Sensitivity analyses which controlled for ACE inhibitor and beta-blocker use revealed similar results (central AIX: 1.10, 95% CI 1.02–1.18, p=.015; peripheral AIX: OR 1.09, 95% CI 1.02–1.16, p=.007; FMD: OR .90, 95% CI .77–1.05, p=.18).
TABLE 3.
Bivariate and Multivariable Associations with PAD (n=123)
| Bivariate Analysisa | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Predictor | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Central Augmentation Index | ||||||
| Central AIX @ 75bpmb | 1.08 | (1.03–1.13) | <.01 | 1.07 | (1.01–1.15) | .03 |
| Age (years) | 1.06 | (1.00–1.13) | .06 | 1.02 | (.94–1.11) | .63 |
| Caucasian | 1.39 | (.56–3.42) | .48 | 1.41 | (.42–54.77) | .58 |
| Hypertension | 6.66 | (2.30–19.3) | <.01 | 4.07 | (1.04–15.9) | .04 |
| Diabetes Mellitus | 4.76 | (1.46–15.5) | .01 | 2.84 | (.68–11.9) | .15 |
| Hyperlipidemia | 3.22 | (1.19–8.70) | .02 | 1.76 | (.43–7.27) | .43 |
| Pack Years (units of 5) | 1.17 | (1.06–1.29) | <.01 | 1.15 | (1.01–1.31) | .03 |
| eGFR (10 mL/min) | 0.79 | (.65–.96) | .02 | 0.79 | (.62–1.01) | .06 |
| Peripheral Augmentation Index | ||||||
| Peripheral AIXb | 1.06 | (1.02–1.10) | <.01 | 1.07 | (1.02–1.12) | .008 |
| Age (years) | 1.05 | (.99–1.12) | .12 | 1.02 | (.93–1.11) | .35 |
| Caucasian | 1.27 | (.51–3.14) | .61 | 1.27 | (.37–4.38) | .38 |
| Hypertension | 5.57 | (1.94–16.0) | <.01 | 3.61 | (.91–14.3) | .07 |
| Diabetes Mellitus | 6.03 | (1.72–21.2) | .01 | 4.72 | (.98–22.7) | .05 |
| Hyperlipidemia | 2.66 | (1.99–7.15) | .05 | 1.45 | (.35–6.90) | .61 |
| Pack Years (units of 5) | 1.18 | (1.06–1.30) | <.01 | 1.16 | (1.02–1.32) | .02 |
| eGFR (10 mL/min) | 0.82 | (.67–1.02) | .07 | 0.83 | (.65–1.06) | .13 |
| Flow Mediated Dilation | ||||||
| FMDb | 0.86 | (.77–.97) | .01 | 0.91 | (.78–1.05) | .20 |
| Age (years) | 1.07 | (1.00–1.13) | .04 | 1.04 | (.96–1.13) | .34 |
| Caucasian | 1.32 | (.54–3.23) | .54 | 1.16 | (.36–3.76) | .80 |
| Hypertension | 5.81 | (2.06–16.4) | <.01 | 3.61 | (.97–13.4) | .06 |
| Diabetes Mellitus | 3.71 | (1.17–11.7) | .03 | 2.94 | (.68–12.7) | .15 |
| Hyperlipidemia | 2.90 | (1.11–7.54) | .03 | 1.26 | (.34–4.71) | .73 |
| Pack Years (units of 5) | 1.20 | (1.08–1.33) | <.01 | 1.20 | (1.06–1.36) | <.01 |
| eGFR (10 mL/min) | 0.84 | (.69–1.02) | .08 | 0.82 | (.64–1.04) | .11 |
The bivariate logistic regression model estimates the odds (95% confidence interval) of PAD associated with a one unit change in the predictor when controlling for the primary predictor of interest (i.e., central AIX, peripheral AIX, or FMD). All variables from table 1 were run through a bivariate analysis to screen for potential confounders. All variables with a p-value <.10 in the bivariate analysis were included in the multivariable model. Age and Caucasian race were also included, despite not achieving a p-value <.10 in the bivariate analysis, since they are common confounders. Sex was omitted due to the small number of female subjects. ABI, CAD, and history of peripheral revascularization were also omitted.
The primary predictor was run as a univariate logistic regression prior to inclusion in the multivariable model.
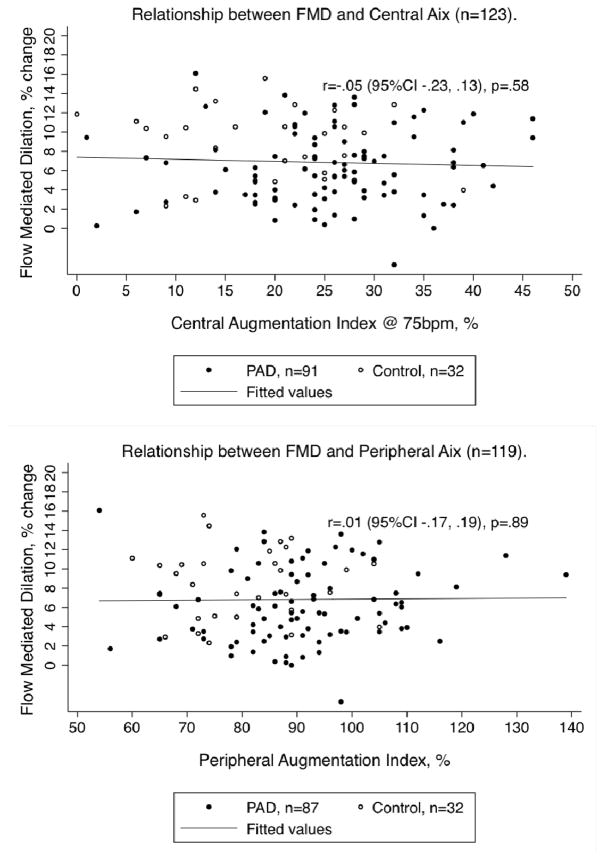
Pearson correlations between FMD and central AIX (r=−.05, 95% CI −.23, .13, p=.58) as well as peripheral AIX (r=.01, 95% CI −.17, .19, p=.89) revealed no significant relationship across the entire sample (Figure 2). Separate subgroup analyses for PAD patients (central AIX: r=.07, 95% CI −.13–.28, p=.48; peripheral AIX: r=.13, 95% CI −.08, .33, p=.23) and controls (central AIX: r=−.14, 95% CI −.47,.22, p=.43; peripheral AIX: r=−.02, 95% CI −.36, .33, p=.92) revealed no significant correlations between FMD and either tonometry parameter.
FIGURE 2.
FMD and Tonometry Correlations
Finally, to determine whether FMD and tonometry were independently associated with PAD in a model including both measures, two additional multivariable models were built that included all the covariates from Table 3. Central AIX remained independently associated with PAD (OR 1.08, 95%CI 1.01–1.15, p=.030) whereas FMD did not reach significance (OR .91, 95% CI .78–1.05, p=.20). Similarly, higher peripheral AIX remained independently associated with PAD (OR 1.08, 95% CI 1.02–1.14, p=.005), while lower FMD trended toward significance (OR .88, 95% CI .76–1.03, p=.11) (Table 4). These results were unchanged with the addition of ACE inhibitor and beta-blocker use to the base model for both central AIX (AIX: OR 1.09, 95% CI 1.01–1.18, p=.019; FMD: OR .91, 95% CI .78–1.07, p=.26) and peripheral AIX (AIX: OR 1.08, 95% CI 1.02–1.15, p=.008; FMD: .91, 95% CI .77–1.06, p=.23). A likelihood ratio test determined that the multivariable model with FMD was improved with the addition of either central AIX (p=.02) or peripheral AIX (p=.002). Conversely, the multivariable models with AIX were marginally improved with the addition of FMD (central aix: p=.20 & peripheral aix: p=.10). Additionally, in an exploratory AUC analysis, the combined model performed marginally better than the model for FMD alone (AUC .89; 95% CI .80–.97; vs. .85; 95% CI .76–.95; p=.11), although this result did not reach statistical significance.
TABLE 4.
Multivariable Model to Predict PAD with Tonometry and FMD as Predictors (n=123)
| Univariate Analysis | |||
|---|---|---|---|
| Predictor | OR | 95% CI | P-value |
| Central AIX @ 75bpm | 1.08 | (1.03–1.13) | .002 |
| Peripheral AIX | 1.06 | (1.02–1.10) | .001 |
| FMD | 0.86 | (.77–.97) | .01 |
| Age (years) | 1.08 | (1.02–1.14) | .01 |
| Caucasian | 1.16 | (.49–2.72) | .73 |
| Hypertension | 7.10 | (2.58–19.5) | <.001 |
| Diabetes Mellitus | 4.18 | (1.35–12.9) | .01 |
| Hyperlipidemia | 2.88 | (1.14–7.27) | .03 |
| Pack Years (units of 5) | 1.19 | (1.08–1.31) | .001 |
| eGFR (10 mL/min) | 0.81 | (.67–.97) | .02 |
| Multivariable Analysisa | |||
| OR | 95% CI | P-value | |
| Central Augmentation Index | |||
| Central AIX @ 75bpm | 1.08 | (1.01–1.15) | .03 |
| FMD | 0.91 | (.78–1.05) | .20 |
| Age (years) | 1.01 | (.92–1.10) | .82 |
| Caucasian | 1.56 | (.45–5.44) | .48 |
| Hypertension | 3.78 | (.95–15.0) | .06 |
| Diabetes Mellitus | 2.74 | (.66–11.4) | .17 |
| Hyperlipidemia | 1.77 | (.43–7.41) | .43 |
| Pack Years (units of 5) | 1.15 | (1.01–1.31) | .03 |
| eGFR (10 mL/min) | 0.80 | (.62–1.03) | .08 |
| Peripheral Augmentation Index | |||
| Peripheral AIX | 1.08 | (1.02–1.13) | .005 |
| FMD | 0.88 | (.76–1.03) | .11 |
| Age (years) | 1.00 | (.91–1.10) | .98 |
| Caucasian | 1.42 | (.40–5.09) | .59 |
| Hypertension | 3.26 | (.79–13.5) | .10 |
| Diabetes Mellitus | 4.83 | (.98–23.7) | .05 |
| Hyperlipidemia | 1.47 | (.35–6.18) | .60 |
| Pack Years (units of 5) | 1.17 | (1.03–1.34) | .02 |
| eGFR (10 mL/min) | 0.85 | (.66–1.09) | .20 |
Multivariable models built using covariates from Table 3.
A Pregibon’s dbeta analysis found that the same control participant was highly influential in all multivariable models. This participant was a 68yo male with much stiffer arteries (central AIX 39% and peripheral AIX 109%) and a lower FMD (3.96%) than would otherwise be expected. He also had a very elevated CRP of 11.8 and a heavy smoking history of 91 pack years. The patient’s high CRP at the time of participation plus subsequent clinical history of sub-acute, chronic fatigue raises the possibility of an occult, chronic inflammatory process at the time of participation. Therefore, a series of sensitivity analyses were performed with this influential participant removed from the multivariable models. The conclusions for tonometry models were unaffected as the sensitivity analysis revealed that the already statistically significant independent association between central AIX and peripheral AIX only became slightly stronger when the participant was excluded (central AIX: OR 1.09 vs. 1.07; peripheral AIX: OR 1.09 vs. 1.07). The FMD model trended closer toward statistical significance with FMD independently and inversely associated with PAD (OR 0.88, p=.11 vs. OR 0.91, p=.20). Additionally, FMD reached statistical significance when combined with peripheral AIX in a combined model (OR 0.84, 95%CI .71–1.00, p=.049). In the AUC analysis, this combined model performed marginally better than the model with FMD alone (AUC .89; 95% CI .81–.99; vs. .85; 95% CI .76–.95; p=.056). Excluding this control participant did not affect the lack of correlation between AIX and FMD (central: r=−.04, p=.65; peripheral: r=.02, p=.82).
DISCUSSION
The present study found that in a cohort of vascular surgery outpatients, FMD and AIX were not correlated across the entire sample or in either the PAD or control subgroups. The study was adequately powered to detect a clinically meaningful correlation, which is suggested by the Pearson coefficient’s 95% confidence intervals being tightly centered around 0. Even the upper and lower limits of these confidence intervals are small enough as to not be clinically relevant. This lack of correlation is despite an independent association between higher AIX and PAD as well as a trend toward an independent association between lower FMD and PAD. The results also demonstrate the robust association between AIX and PAD in combined models with FMD. Therefore, the present study adds to the literature by showing no association between arterial stiffness as measured by AIX and FMD in a sample of vascular surgery outpatients. Additionally, it suggests that predictive models with FMD might be improved with the inclusion of AIX.
Previous studies have demonstrated an inconsistent association between FMD and various tonometric parameters. The reactive hyperemic index (RHI), a measure of endothelial function in the microvasculature obtained using arterial tonometry, was shown to have a small correlation with FMD in the Gutenberg Heart Study35 and Kuvin et al. (2003) found a modest positive correlation between RHI and FMD in 89 patients presenting with chest pain.36 However, two large studies, one from the Framingham Offspring cohort and the other from a sample of patients with CAD, failed to find a statistically significant relationship between RHI and FMD.21, 22
Similarly, the literature on the potential association between FMD and arterial stiffness suggests a small negative correlation, if one exists at all. Studies correlating FMD and PWV have come to inconsistent conclusions ranging from a small statistically significant negative correlation,37 which was eliminated in multivariable analysis for one of the studies,38 to a small positive correlation.39 Examining AIX and FMD, several studies have found either no correlation,40 or small, marginally significant, negative correlations.23, 33 In a sample of 100 patients (83 with CVD and 17 healthy controls), Soga et al. (2008) also found a small negative correlation between central AIX and FMD, which held in multivariable regression.34
Understanding the pathophysiology of arterial stiffness and endothelial function provides further evidence for why there might be a lack of association. Like endothelial dysfunction, arterial stiffness is a feature of atherosclerosis,41 but it is heavily influenced by physical forces like hypertension and renin-angiotensin activation,30 as well as inflammation. Inflammation is thought to increase stiffness through several mechanisms including decreased nitric oxide (NO) production, elastin and collagen degradation, increased smooth muscle cell migration to the intima, and swelling of the extra-cellular matrix.42 While endothelial function is also affected by NO and inflammation, stiffness depends on structural rather than just functional changes.7 Endothelial function, by comparison, plays a more functional than structural role. The endothelium maintains vascular tone through the release of a variety of vasoactive mediators.43 A particularly important mediator released by the endothelium is NO, which is a vasodilator that also prevents platelet aggregation. A decrease in NO production is a hallmark of endothelial dysfunction, which precedes the development of atherosclerosis.44 It is hypothesized that smoking impairs endothelial function, as measured via FMD, by impairing these mechanisms.45, 46 Additionally, patients with PAD are more likely to be smokers, which is supported by the baseline cohort data indicating a greater mean of pack years in the PAD patients (44 vs 20 p=<.001). Given the relationship between smoking and FMD, and the very strong relationship between smoking and PAD, the multivariable model consistently demonstrates a loss of significance after adjusting for pack years. These finding suggest that differences in FMD between PAD and controls are largely mediated by risk factors. It is likely that the small sample size prevented the detection of any smaller independent differences in FMD between the PAD patients and controls. Differences in baseline brachial artery diameter between patients with PAD and controls were likely attributable to differences in comorbidities47 between the groups. Variations in baseline brachial artery diameter did not alter how FMD was measured or calculated.
Several studies using FMD and AIX as outcome variables have found diverging results between the two. For example, specific biomarkers of cardiac stress such as growth differentiation factor-15 (GDF-15) are negatively correlated with FMD, but not AIX.48 FMD is also lower in patients with heart failure who have a preserved ejection fraction, but AIX is unchanged.49 In a randomized controlled trial with CKD patients treated with tetrahydrobiopterin to increase bioavailability of NO, there was an observed improvement in AIX, but no change in FMD.50 Additionally, treatment with valsartan was associated with improved arterial stiffness as measured by PWV and some parameters of radial artery tonometry, but not FMD.51 Finally, in a study of adherence to dietary guidelines among nearly 6,000 community-based subjects from the Framingham Heart cohort, better diet was associated with lower AIX, even when controlling for cardiac risk factors, but not FMD.52 Therefore, the previous literature in predominately non-PAD cohorts suggests that any association between AIX and FMD is likely small.
As far as their potential role in prediction models, both FMD and AIX have been correlated with measures of disease severity in PAD as well as future risk of CAD or death.19, 53, 54 Additionally, each measure has shown the ability to be improved with specific clinical interventions.55, 56 While our results demonstrate that tonometry is strongly associated with PAD, even in combined models, the pattern of results suggest that the study was not adequately powered to determine whether FMD was significantly associated with PAD in multivariable models with and without tonometry included. While the sensitivity analysis demonstrated a significant inverse association between FMD and PAD in the combined model with a single influential participant excluded, this result should be interpreted with caution due to the lack of absolute justification for excluding this participant. Both tonometry and FMD provide valuable investigative information on the pathophysiologic effects of PAD on the vasculature and improve our understanding of chronic vascular injury. As these methods of predicting risk become more standardized and easily performed, they have the potential for playing a more significant clinical role in outpatient and preoperative clinics. Including these measures in the assessment of patients with PAD may help direct the level of prevention and timing or method of intervention. Larger, prospective studies could help determine whether models with either or both parameters can improve the capacity for identifying disease, tracking its progression, anticipating future risk, and monitoring treatment response.
Limitations
Limitations of the present study include its cross-sectional design, from which only an association between either FMD or AIX and PAD can be determined. Utilization of aspirin, beta-blockers, statins, and ACE-inhibitors differed between the two groups and could have altered FMD, preventing the detection of a statistically significant relationship between FMD and PAD in the multivariable model. Each of these medications have been reported to improve FMD57–60, although their combined effect is not as well known. It would be ideal to compare FMD in groups with similar use of these medications, however, these medications are commonly taken by patients with clinical atherosclerosis and selection of controls for this kind of study would be difficult. Additional analyses controlling for ACE-inhibitor and beta-blocker use revealed no differences in associations between parameters of interest (i.e., AIX and FMD) with PAD. Additionally, we did not use nitroglycerin to test NO-independent dilation to determine whether FMD was influenced by altered properties of underlying smooth muscle cells. Lastly, our sample was predominately male, reflecting the demographics of the VA population, and may not be generalizable to women.
CONCLUSION
In summary, the present study found no association between FMD and AIX. Higher AIX was independently associated with PAD and lower FMD trended toward an independent association with PAD. Adding AIX to multivariable models with FMD improved their predictive ability. However, the study was not adequately powered to determine whether FMD was independently associated with PAD, although the pattern of results, plus prior research, suggest that such an inverse relationship is likely. If each measure is independently associated with PAD, but not each other, this would suggest that models combining both AIX and FMD might be superior for identifying and risk-stratifying patients with PAD. Larger studies are needed to make such a determination and future prospective studies are needed to determine whether FMD and radial artery tonometry have a potential role in the clinical setting.
Acknowledgments
Funding: This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1 TR001871 with additional student research support from the Society for Vascular Surgery Student Research Fellowship Award and the American Heart Association Student Scholarship (GZ). Furthermore, this work was supported by start-up funds from the University of California San Francisco and the Northern California Institute for Research and Education, by Award Number KL2RR024130 from the National Center for Research Resources, Award Number 1K23HL122446-01 from the National Institute of Health/NHLBI, and a Society for Vascular Surgery Seed Grant and Career Development Award (MG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The funding organizations were not involved in the design and conduct of the study, collection, management, analysis, and interpretation of the data, or preparation, review or approval of the manuscript.
Footnotes
Author Contributions: GJZ, SMG, and WJG were involved in the conception and the design of the study. GJZ and KAS were involved in the collection of the data. GJZ, KAS, JLR, MSS, SCW, WJG, and SMG were involved in the analysis and interpretation of the data. GJZ and NKH were involved in the statistical analysis. GJZ and KAS wrote the initial manuscript. All of the authors were involved in critically revising and editing the article. All of the authors gave a final approval of the manuscript. SMG maintained overall responsibility of the study and the manuscript.
The Author Disclosure Statement: The authors have no conflicts of interest to report.
Disclosure: The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. The Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. DOI: //dx.doi.org/10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Hartman L, Town RJ, et al. National health care costs of peripheral arterial disease in the Medicare population. Vascular Medicine. 2008;13:209–215. doi: 10.1177/1358863X08089277. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal S, Sud K, Shishehbor MH. Nationwide Trends of Hospital Admission and Outcomes Among Critical Limb Ischemia Patients: From 2003–2011. Journal of the American College of Cardiology. 2016;67:1901–1913. doi: 10.1016/j.jacc.2016.02.040. DOI: //dx.doi.org/10.1016/j.jacc.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, Langer RD, Fronek A, et al. Mortality over a Period of 10 Years in Patients with Peripheral Arterial Disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Ninomiya JK, Wingard DL, et al. Progression of Peripheral Arterial Disease Predicts Cardiovascular Disease Morbidity and Mortality. Journal of the American College of Cardiology. 2008;52:1736–1742. doi: 10.1016/j.jacc.2008.07.060. DOI: https://doi.org/10.1016/j.jacc.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoner L, Young JM, Fryer S. Assessments of Arterial Stiffness and Endothelial Function Using Pulse Wave Analysis. International Journal of Vascular Medicine. 2012;2012:903107. doi: 10.1155/2012/903107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widlansky ME, Gokce NAKJJF, Vita JA. The clinical implications of endothelial dysfunction. Journal of the American College of Cardiology. 2003;42:1149–1160. doi: 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 8.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2010 Oct 19;300:H2–12. doi: 10.1152/ajpheart.00471.2010. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ras RT, Streppel MT, Draijer R, et al. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. International journal of cardiology. 2013;168:344–351. doi: 10.1016/j.ijcard.2012.09.047. DOI: //dx.doi.org/10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Arora RC, Hiebert BM, et al. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. European Heart Journal - Cardiovascular Imaging. 2014 doi: 10.1093/ehjci/jet256. [DOI] [PubMed] [Google Scholar]
- 11.Gokce N, Keaney JF, Hunter LM, et al. Risk Stratification for Postoperative Cardiovascular Events via Noninvasive Assessment of Endothelial Function. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 12.Ghiadoni L, Salvetti M, Muiesan ML, et al. Evaluation of Endothelial Function by Flow Mediated Dilation: Methodological Issues and Clinical Importance. High Blood Pressure & Cardiovascular Prevention. 2015;22:17–22. doi: 10.1007/s40292-014-0047-2. [DOI] [PubMed] [Google Scholar]
- 13.Beckmann M, Jacomella V, Kohler M, et al. Risk Stratification of Patients with Peripheral Arterial Disease and Abdominal Aortic Aneurysm Using Aortic Augmentation Index. PLoS ONE. 2015;10:e0139887. doi: 10.1371/journal.pone.0139887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brant LCC, Hamburg NM, Barreto SM, et al. Relations of Digital Vascular Function, Cardiovascular Risk Factors, and Arterial Stiffness: The Brazilian Longitudinal Study of Adult Health (ELSAΓÇÉBrasil) Cohort Study. J Am Heart Assoc. 2014:3. doi: 10.1161/JAHA.114.001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 16.Husmann M, Jacomella V, Thalhammer C, et al. Markers of arterial stiffness in peripheral arterial disease. Vasa. 2015;44:341–348. doi: 10.1024/0301-1526/a000452;02. [DOI] [PubMed] [Google Scholar]
- 17.Mattace-Raso F, van dC, Hofman A, et al. Arterial Stiffness and Risk of Coronary Heart Disease and Stroke. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 18.Kals J, Lieberg J, Kampus P, et al. Prognostic Impact of Arterial Stiffness in Patients with Symptomatic Peripheral Arterial Disease. European Journal of Vascular and Endovascular Surgery. 2014;48:308–315. doi: 10.1016/j.ejvs.2014.05.018. DOI: //dx.doi.org/10.1016/j.ejvs.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Weber T, Auer J, O’Rourke MF, et al. Arterial Stiffness, Wave Reflections, and the Risk of Coronary Artery Disease. Circulation. 2004;109:184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 20.Heffernan KS, Karas RH, Mooney PJ, et al. Pulse wave amplitude is associated with brachial artery diameter: Implications for gender differences in microvascular function. Vascular Medicine. 2009 doi: 10.1177/1358863X09349523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamburg NM, Palmisano J, Larson MG, et al. Relation of Brachial and Digital Measures of Vascular Function in the Community: The Framingham Heart Study. Hypertension. 2011;57:390–396. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin B-J, Gurtu V, Chan S, et al. The relationship between peripheral arterial tonometry and classic measures of endothelial function. Vascular Medicine. 2013;18:13–18. doi: 10.1177/1358863X12468194. [DOI] [PubMed] [Google Scholar]
- 23.Poredoš P, Bešič H, Jeraj L. Relationship between endothelial function of micro- and macrocirculation in patients with peripheral arterial disease. Vasa. 2017;46:17–22. doi: 10.1024/0301-1526/a000587. [DOI] [PubMed] [Google Scholar]
- 24.Allan RB, Delaney CL, Miller MD, et al. A Comparison of Flow-mediated Dilatation and Peripheral Artery Tonometry for Measurement of Endothelial Function in Healthy Individuals and Patients with Peripheral Arterial Disease. European Journal of Vascular and Endovascular Surgery. 2013;45:263–269. doi: 10.1016/j.ejvs.2012.12.002. DOI: //dx.doi.org/10.1016/j.ejvs.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Grenon SM, Gagnon J, Hsiang Y. Ankle–Brachial Index for Assessment of Peripheral Arterial Disease. N Engl J Med. 2009;361:e40. doi: 10.1056/NEJMvcm0807012. [DOI] [PubMed] [Google Scholar]
- 26.Blanchard EB, Jones-Alexander J, Buckley TC, et al. Psychometric properties of the PTSD checklist (PCL) Behaviour research and therapy. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. DOI: //dx.doi.org/10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a Brief Depression Severity Measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grenon SM, Owens CD, Alley H, et al. Posttraumatic Stress Disorder Is Associated With Worse Endothelial Function Among Veterans. Journal of the American Heart Association. 2016:5. doi: 10.1161/JAHA.115.003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002 Jan 15;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 30.Benetos A, Waeber B, Izzo J, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. American Journal of Hypertension. 2002;15:1101–1108. doi: 10.1016/S0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell GF, Hwang S-J, Vasan RS, et al. Arterial Stiffness and Cardiovascular Events. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C-H, Nevo E, Fetics B, et al. Estimation of Central Aortic Pressure Waveform by Mathematical Transformation of Radial Tonometry Pressure. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 33.Ravikumar R, Deepa R, Shanthirani CS, et al. Comparison of carotid intima-media thickness, arterial stiffness, and brachial artery flow mediated dilatation in diabetic and nondiabetic subjects (The Chennai Urban Population Study [CUPS-9]) The American Journal of Cardiology. 2002;90:702–707. doi: 10.1016/s0002-9149(02)02593-6. DOI: //dx.doi.org/10.1016/S0002-9149(02)02593-6. [DOI] [PubMed] [Google Scholar]
- 34.Soga J, Nakamura S, Nishioka K, et al. Relationship between Augmentation Index and Flow-Mediated Vasodilation in the Brachial Artery. Hypertension research : official journal of the Japanese Society of Hypertension. 2008;31:1293–1298. doi: 10.1291/hypres.31.1293. [DOI] [PubMed] [Google Scholar]
- 35.Schnabel RB, Schulz A, Wild PS, et al. Non-Invasive Vascular Function Measurement in the Community: Cross-Sectional Relations and Comparison of Methods. Circulation: Cardiovascular Imaging. 2011 doi: 10.1161/CIRCIMAGING.110.961557. [DOI] [PubMed] [Google Scholar]
- 36.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. American Heart Journal. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. DOI: //dx.doi.org/10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 37.McEniery CM, Wallace S, Mackenzie IS, et al. Endothelial Function Is Associated With Pulse Pressure, Pulse Wave Velocity, and Augmentation Index in Healthy Humans. Hypertension. 2006;48:602–608. doi: 10.1161/01.HYP.0000239206.64270.5f. [DOI] [PubMed] [Google Scholar]
- 38.Koivistoinen T, Virtanen M, Hutri-Kähönen N, et al. Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: The Cardiovascular Risk in Young Finns Study and the Health 2000 Survey. Atherosclerosis. 2012;220:387–393. doi: 10.1016/j.atherosclerosis.2011.08.007. DOI: //dx.doi.org/10.1016/j.atherosclerosis.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Massmann A, Stemler J, Fries P, et al. Automated oscillometric blood pressure and pulse-wave acquisition for evaluation of vascular stiffness in atherosclerosis. Clinical Research in Cardiology. 2017:1–11. doi: 10.1007/s00392-017-1080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nigam A, Mitchell GF, Lambert J, et al. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. The American Journal of Cardiology. 2003;92:395–399. doi: 10.1016/s0002-9149(03)00656-8. DOI: //dx.doi.org/10.1016/S0002-9149(03)00656-8. [DOI] [PubMed] [Google Scholar]
- 41.Catalano M, Scandale G, Carzaniga G, et al. Aortic Augmentation Index in Patients With Peripheral Arterial Disease. The Journal of Clinical Hypertension. 2014;16:782–787. doi: 10.1111/jch.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain S, Khera R, Corrales–Medina VF, et al. Inflammation and arterial stiffness in humans. Atherosclerosis. 2014;237:381–390. doi: 10.1016/j.atherosclerosis.2014.09.011. DOI: //dx.doi.org/10.1016/j.atherosclerosis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Poredos P, Jezovnik MK. Testing Endothelial Function and its Clinical Relevance. Journal of Atherosclerosis and Thrombosis. 2013;20:1–8. doi: 10.5551/jat.14340. [DOI] [PubMed] [Google Scholar]
- 44.Lekakis J, Abraham P, Balbarini A, et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. European Journal of Cardiovascular Prevention & Rehabilitation. 2011;18:775–789. doi: 10.1177/1741826711398179. [DOI] [PubMed] [Google Scholar]
- 45.Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996 Jan 18;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 46.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993 Nov 01;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 47.Holewijn S, den Heijer M, Swinkels DW, et al. Brachial artery diameter is related to cardiovascular risk factors and intima-media thickness. Eur J Clin Invest. 2009 May 21;39:554–560. doi: 10.1111/j.1365-2362.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- 48.Andersson C, Enserro D, Sullivan L, et al. Relations of circulating GDF-15, soluble ST2, and troponin-I concentrations with vascular function in the community: The Framingham Heart Study. Atherosclerosis. 2016;248:245–251. doi: 10.1016/j.atherosclerosis.2016.02.013. DOI: //dx.doi.org/10.1016/j.atherosclerosis.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maréchaux S, Samson R, van Belle E, et al. Vascular and Microvascular Endothelial Function in Heart Failure With Preserved Ejection Fraction. Journal of cardiac failure. 2016;22:3–11. doi: 10.1016/j.cardfail.2015.09.003. DOI: //dx.doi.org/10.1016/j.cardfail.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Park J, Liao P, Sher S, et al. Tetrahydrobiopterin lowers muscle sympathetic nerve activity and improves augmentation index in patients with chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2015;308:R208. doi: 10.1152/ajpregu.00409.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajagopalan S, Kariisa M, Dellegrottaglie S, et al. Angiotensin Receptor Blockade Improves Vascular Compliance in Healthy Normotensive Elderly Individuals: Results From a Randomized Double-Blind Placebo-Controlled Trial. Journal of clinical hypertension. 2006;8:783–790. doi: 10.1111/j.1524-6175.2006.05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sauder KA, Proctor DN, Chow M, et al. Endothelial function, arterial stiffness, and adherence to the 2010 Dietary Guidelines for Americans: a cross-sectional analysis. The British journal of nutrition. 2015;113:1773–1781. doi: 10.1017/S0007114515000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brevetti G, Silvestro A, Schiano V, et al. Endothelial Dysfunction and Cardiovascular Risk Prediction in Peripheral Arterial Disease. Circulation. 2003;108:2093. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 54.Khaleghi M, Kullo IJ. Aortic augmentation index is associated with the ankle-brachial index: A community-based study. Atherosclerosis. 2007;195:248–253. doi: 10.1016/j.atherosclerosis.2006.12.017. DOI: //dx.doi.org/10.1016/j.atherosclerosis.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Husmann M, Dörffler-Melly J, Kalka C, et al. Successful lower extremity angioplasty improves brachial artery flow-mediated dilation in patients with peripheral arterial disease. Journal of Vascular Surgery. 2008;48:1211–1216. doi: 10.1016/j.jvs.2008.06.039. DOI: //dx.doi.org/10.1016/j.jvs.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 56.Jacomella V, Shenoy A, Mosimann K, et al. The Impact of Endovascular Lower-limb Revascularisation on the Aortic Augmentation Index in Patients with Peripheral Arterial Disease. European Journal of Vascular and Endovascular Surgery. 2013;45:497–501. doi: 10.1016/j.ejvs.2013.01.026. DOI: //dx.doi.org/10.1016/j.ejvs.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 57.Kabaklic A, Fras Z. Moderate-dose atorvastatin improves arterial endothelial function in patients with angina pectoris and normal coronary angiogram: a pilot study. Arch Med Sci. 2017 Jul 20;13:827–836. doi: 10.5114/aoms.2017.68238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peller M, Ozieranski K, Balsam P, et al. Influence of beta-blockers on endothelial function: A meta-analysis of randomized controlled trials. Cardiol J. 2015 Jul 24;22:708–716. doi: 10.5603/CJ.a2015.0042. [DOI] [PubMed] [Google Scholar]
- 59.Magen E, Viskoper JR, Mishal J, et al. Effects of low-dose aspirin on blood pressure and endothelial function of treated hypertensive hypercholesterolaemic subjects. J Hum Hypertens. 2005 Jul 22;19:667–673. doi: 10.1038/sj.jhh.1001910. [DOI] [PubMed] [Google Scholar]
- 60.Miyamoto M, Kotani K, Ishibashi S, et al. The effect of antihypertensive drugs on endothelial function as assessed by flow-mediated vasodilation in hypertensive patients. Int J Vasc Med. 2012 Apr 11;2012:453264. doi: 10.1155/2012/453264. [DOI] [PMC free article] [PubMed] [Google Scholar]