Abstract
Background & objective:
Multidrug-resistant Acinetobacter baumannii (MDR-AB) is an important nosocomial pathogen which is associated with significant morbidity and mortality, particularly in high-risk populations. Aminoglycoside-modifying enzymes (AMEs) and 16S ribosomal RNA (16S rRNA) methylation are two important mechanisms of resistance to aminoglycosides. The aim of this study was to determine the prevalence of 16S rRNA methylase (armA, rmtA, rmtB, rmtC, and rmtD), and the AME genes [aac(6′)-Ib, aac(3)-I, ant(3′′)-I, aph(3′)-I and aac(6')-Id], among clinical isolates of A. baumannii in Tehran, Iran.
Methods:
Between November 2015 to July 2016, a total of 110 clinical strains of A. baumannii were isolated from patients in two teaching hospitals in Tehran, Iran. Antimicrobial susceptibility testing was performed according to Clinical and Laboratory Standards Institute guidelines. The presence of genes encoding the AMEs and 16S rRNA methylases responsible for resistance was investigated by multiplex polymerase chain reaction.
Results:
The results showed that colistin was an effective antibiotic and could be used as a last-resort treatment of infections caused by MDR-AB. The resistance rate to aminoglycosides were 100%, 96.36% and 90.9% for tobramycin, gentamicin and amikacin, respectively. In this study, AME genes of aac(6′)-Ib, aac(3)-I and ant(3′′)-I were most prevalent among the isolated strains.
Conclusion
Markedly high resistance to tobramycin, gentamicin and amikacin was noted in current study. Our results suggested that modifying enzyme genes in conjunction with methylation of 16S rRNA might contribute to aminoglycoside resistance induced in vivo in A. baumannii. Further studies are required to determine the prevalence of the aminoglycoside resistance genes in other hospitals of Iran.
Key Words: Acinetobacter baumannii, Multidrug resistant, Aminoglycoside-Modifying Enzymes, Iran
Introduction
Multidrug-resistant Acinetobacter baumannii (MDR-AB) have recently emerged as a life-threatening pathogen which is responsible for a variety of healthcare-associated infections (HAIs) and large epidemics in hospitals (1). Due to MDR-strains are resistant to almost all routinely used antimicrobial agents such as; aminoglycosides, fluoroquinolones, tetracyclines, and cephalosporines, they are becoming a major problem in hospitalized patients in hospital environments all around the world (2, 3, 4). Aminoglycosides are broad-spectrum bactericidal antibiotics which are commonly prescribed for the treatment of infections with Gram-negative bacteria (5, 6). In general, aminoglycosides such as tobramycin, gentamicin and amikacin have extensively been used for ttreatment of A.baumannii infections (7, 8). Aminoglycoside-modifying enzymes (AMEs) and 16S ribosomal RNA (16S rRNA) methylation are two important mechanisms for antibiotic inactivation and lead to resistance to multiple aminoglycosides in A. baumannii (9, 10). According to their functions, AMEs are generally categorized to three types: aminoglycoside phosphotransferase (APS), aminoglycoside acetyltransferase (AAC), and aminoglycoside nucleotidyltransferase (ANT). All three types of AMEs have been identified in clinical isolates of Acinetobacter spp. (10). Furthermore, a high number of genes encoding these enzymes are associated with plasmids and transposons, which help in the rapid spreading of antimicrobial resistance through species boundaries. Several AMEs have been recognized in Acinetobacter spp., including variants of phosphotransferases APH(3')-I, APH(3')-II, and APH(3')-VI, the acetyltransferases AAC(3)-I, AAC(3)-II, AAC(3)-III, AAC(6')-I, AAC(6')-II, and AAC(6')-III, and the nucleotidyltransferases ANT(3-)-I, ANT(4')-I, and ANT(2")-I (11,12,13). The aminoglycoside antibiotics bind to the A-position of 16S rRNA in the 30S ribosomal small subunit and interact with protein synthesis. At present, ten types of 16S rRNA methylase genes (armA, rmtA, rmtB, rmtC, rmtD, rmtE, rmtF, rmtG, rmtH and npmA) have been described as another main mechanism of aminoglycoside resistance in A. baumannii. Hence, these genes can simply move to other bacteria since their resistance genes are commonly located on small plasmids (14, 15). As well as, the 16S rRNA methyltransferase genes are important factors in the increasing prevalence of aminoglycoside resistance among A. baumannii strains, the analysis of the acquisition of these genes by clinical isolates is essential for the treatment and prevention of their infections (15). Additionally, the emergence of AMEs and 16S rRNA methylases among A. baumannii strains is a serious global threat for the future of antibacterial chemotherapy. With respect to studies such as, Aliakbarzade et al. (16), Karah et al. (17), and Joshi et al. (18), in this field and also, high dissemination and high prevalence of resistance-related genes, it is important to pay greater attention for choosing the best therapy options for patients and optimizing preventive policies to avoid spreading resistance-encoding genes. Therefore, the aim of this study was to investigate the prevalence of 16S rRNA methylase [armA, rmtA, rmtB, rmtC, and rmtD], and the AME genes [aac(6′)-Ib, aac(3)-I, ant(3′′)-I, aph(3′)-I and aac(6')-Id], among clinical isolates of A. baumannii from Tehran, Iran.
Materials and Methods
Inclusion/exclusion criteria
All clinical samples which contain Acinetobacter isolates, including both gender, and from all age groups including infants to elderly without considering risk factor were involved in this study. Hence, all duplicated clinical isolates were excluded from this study. All isolates harboring mixed microorganisms (which contains Acinetobacter) were also excluded.
Specimen collection and strain identification
The current study was a descriptive cross-sectional study. A total of 110 consecutive non-duplicate strains of A. baumannii were collected from two teaching hospitals between November 2015 to July 2016. These clinical strains were isolated aseptically from wound, tracheal tube, pleural fluid, blood, urine and sputum of hospitalized patients in Milad and Shahid Motahari hospitals, Tehran, Iran. The isolates were identified based on standard bacteriological tests including gram staining, motility, oxidase, methyl red, voges-proskauer, simmon's citrate, urease and grown on MacConkey agar (16). The confirmation was done using the Microgen identification kit (Microgen Bioproducts Co., UK). In addition, all isolates were examined by PCR amplification for the presence of the blaOXA-51-like beta-lactamase gene intrinsic to A. baumannii, according to previously described method (19), and then the isolates were stored at –70°C in glycerol skim milk broth.
Antimicrobial susceptibility testing
Antimicrobial susceptibility was performed using Kirby-Bauer disc diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (20). The discs and contents which were used were as follows: tetracycline (TE: 10 μg), meropenem (MEM: 10 μg), amikacin (AK: 30 μg), imipenem (IPM: 10 μg), cefotaxime (CTX: 30 μg), ceftriaxone (CRO: 30 μg), piperacillin/tazobactam (PTZ: 100/10 μg), piperacillin (PIP: 100 μg), ceftazidime (CAZ: 30 μg), ciprofloxacin (CIP: 5 μg), cefepime (FEP: 30 μg), trimethoprim-sulfamethoxazole (SXT: 2.5 μg), gentamicin (GEN: 10 μg) and Tobramycin ( TOB: 10 μg) (All purchased from Mast Group, Merseyside, UK). Briefly, a microbial suspension was obtained from overnight cultures. The turbidity of each bacterial suspension was adjusted equivalent to a turbidity of 0.5 McFarland standard and then inoculated onto Müller-Hinton agar plates (Merck, Darmstadt, Germany). The diameter zone of inhibition (mm) around the discs were measured after incubation at 37ºC for 20 - 24 hours.
Minimum Inhibitory Concentration (MIC)
For colistin, amikacin and gentamicin MIC was determined by the broth microdilution protocol according to the CLSI. Antibiotic powders were dissolved in an appropriate solvent according to the manufacturer’s recommendations. MICs for amikacin/gentamicin ranging from 0.25 to 512 μg/ml and 0.25 to 128 μg/ml for colistin, were tested. Each well of a 96-well microtiter plate (Extra Gene-Company) contained a total volume of 100 μL of the antibiotic dilution and Müller-Hinton broth medium. Then, the 0.5 McFarland suspension was diluted 1:20 to yield 5 × 106 CFU/ml. When 0.01 ml of this suspension was inoculated into the broth, the final test concentration of the bacteria was approximately 5 × 105 CFU/ml. The correct density of the turbidity standard was verified by measuring absorbance using a spectrophotometer. The microtiter plates were incubated at 37°C for 20-24 hours. The MIC was taken as the lowest concentration of the antibiotic which inhibited the growth of the isolates. The MIC50 and MIC90 of the antibiotics were calculated by MIC that inhibited 50% and 90% of the isolates, respectively. Escherichia coli ATCC 25922 carried out as quality control (QC) strain for all suspectibility testing assays.
DNA extraction
Genomic DNA was extracted from fresh overnight cultures grown on Luria-Bertani (LB) agar plates (Difco Laboratories, Detroit, Mich.) by the phenol-chloroform method (21). Extracted DNA was resolved in 100 µL of TE buffer (10 mM Tris, 1 mM EDTA [pH 8.0]) plus 10 µL of RNase (Sigma, St. Louis, Mo.) for removal of contaminating RNA. The concentration and quality of the extracted DNA were evaluated using a Nanodrop spectrophotometer (ND-1000; Thermo Scientific; Wilmington, DE, USA). Purified DNA was aliquoted and preserved at -20°C.
Screening for the presence of aminoglycoside resistance genes
The specific primer sequences sets used in this study are listed in Table 1.
Table 1.
The primer sequences used in this study
| Reference | Amplicon size (bp) |
Primer | Sequence (5′–3′) | Target gene | |
|---|---|---|---|---|---|
| 22 | 169 | aac(3)-I -F | ACCTACTCCCAACATCAGCC | aac(3)-I | aminoglycoside-modifying enzymes |
| aac(3)-I -R | ATATAGATCTCACTACGCGC | ||||
| 22 | 435 | aac(6')-Id -F | ATGATTAGAAAAGCAACTGTCCAAG | aac(6')-Id | |
| aac(6')-Id -R | TTAAAGTTGCTTTGTAAAACAAATC | ||||
| 22 | 519 | aac(6')-Ib -F | ATGACTGAGCATGACCTTGC | aac(6')-Ib | |
| aac(6')-Ib -R | TTAGGCATCACTGCGTGTTC | ||||
| 22 | 284 | ant(3'')-I -F | TGATTTGCTGGTTACGGTGAC | ant(3'')-I | |
| ant(3'')-I -R | CGCTATGTTCTCTTGCTTTTG | ||||
| 22 | 816 | aph(3')-I -F | ATGTGCCATATTCAACGGGAAACG | aph(3')-I | |
| aph(3')-I -R | TCAGAAAAACTCATCGAGCATCAA | ||||
| 22 | 774 | armA -F | ATGGATAAGAATGATGTTGTTAAG | armA | 16S rRNA methylases |
| armA -R | TTATTTCTGAAATCCACTAGTAATTA | ||||
| 22 | 315 | rmtA -F | CCTAGCGTCCATCCTTTCCTC | rmtA | |
| rmtA-I -R | AGCGATATCCAACACACGATGG | ||||
| 23 | 173 | rmtB -F | GCT TTC TGC GGG CGA TGT AA | rmtB | |
| rmtB -R | ATG CAA TGC CGC GCT CGT AT | ||||
| 22 | 846 | rmtC -F | ATGAAAACCAACGATAATTATC | rmtC | |
| rmtC -R | TTACAATCTCGATACGATAAAATAC | ||||
| 23 | 401 | rmtD -F | CGG CAC GCG ATT GGG AAG C | rmtD | |
| rmtD -R | CGG AAA CGA TGC GAC GAT |
The Primer Basic Local Alignment Search Tool (NCBI BLAST) software and MFEprimer-2.0 server, a fast thermodynamics-based program, was used for checking PCR primer specificity. To aid recognition in a multiplex format, the length of PCR products were selected so that there was ˷100 bp difference amongst each subgroup. Multiplex-PCR was performed with PCR system (Eppendorf Co., Germany) for detection of AMEs (aac (6′)-Ib, aac (3)-I, ant (3′′)-I, aph (3′)-I, aac (6')-Id) and 16S rRNA methylases (armA, rmtA, rmtB, rmtC, and rmtD) genes. The M-PCR I was undertaken in a final volume of 25 µl containing 2.5 µl of 10X PCR buffer (Thermo Fisher Scientific, Inc.), 2 µl MgCl2 (0.8 mM), 1.5 µl of mixed dNTP (10 μM), 1.5 µl of each the AMEs primer (10 pmol), 1 µl of DNA Taq polymerase (2 U) (Takara Bio, Inc., Dalian, China), 1 μl of template DNA and sterile distilled water. The nucleic acid amplification was performed with the following conditions: 94˚C for 3 minutes, followed by 30 cycles of 94˚C for 50 seconds, 56˚C for 40 seconds and 72˚C for 1 minute and final extension for 6 minutes. The mix for the detection of 16S rRNA methylase genes contained 2 µl of mixed dNTP (10 mM), 2 μl of 10X PCR buffer, 0.4 µl of DNA Taq polymerase (5 U), 1.5 µl MgCl2 (0.8 mM), 1.25 µl of each primer (10 pmol), 1μL of template, DNA and sterile distilled water to a final volume of 25 μl. The M-PCR II assay was performed, using the following parameters: initial denaturation for 1 minute at 95°C, 33 cycles at 94˚C for 45 seconds, 55˚C for 45 seconds, and 72˚C for 1 minute, followed by a final extension during 4 minutes at 72˚C. The PCR products were electrophoresed (Bio Rad, USA) in 1.5% (W/V) agarose gels (SinaClon, Iran) in TAE buffer (0.04 M Tris-acetate, 0.001 M EDTA [pH 8.2]) for 60 minutes at 120 V. Gels were stained with 2 μg/ml ethidium bromide (EtBr) and visualized by irradiation with UV light. Amplicons were identified by estimation of their lengths (in base pairs) using a DNA ladder (Fermentase, CA). Positive and negative controls were included with each reaction.
Statistical analysis
The Minitab16 software (Minitab Inc., Minnesota, USA) was used for the statistical analyses. A P-value of <0.05 and 95% confidence intervals were used to determine significant.
Ethics statement
Ethical approval was not needed for the survey, since there was no direct patient involvement and only bacterial isolates were retrospectively studied. Additionally, all clinical samples were unidentified and no recognizable patient information was available.
Results
Overall, 51 (46.36%) and 59 (53.63%) strains were collected from Shahid Motahari and Milad hospitals respectively. All 110 A. baumanni isolates had positive results for blaOXA-51-like gene. These strains were mostly isolated from wound samples (n=51, 46.36%), followed by tracheal tube (n=37, 33.63). The other samples were isolated from pleural fluid (n=8, 7.27%), blood (n=8, 7.27%), urine (n=4, 3.63%), and sputum (n=2, 1.81%). Seventy-one (64.5%) strains were isolated from male patients and 39 (35.45 %) from female patients. The patients infected with MDR-AB ranged in age from 17 to 100 years and the average age of patients was 52 +/- 17 years (Mean +/- SD). In the current study, colistin exhibited good activity against the MDR-AB strains (MIC50, 0.5 μg/mL, and MIC90, 1 μg/ml). Also, according to antibiotics susceptibility testing by disc diffusion, all isolates of A. baumannii were defined as MDR and resistant to almost all available antibiotics (Table 2).
Table 2.
Antibiotic susceptibility testing results
| Antimicrobial class | Antibiotic | Resistant No (%) | Intermediate No (%) | Susceptible No (%) |
|---|---|---|---|---|
| Aminoglycosides | Gentamicin | 106 (96.36%) | 0 (0%) | 4 (3.63%) |
| Amikacin | 100 (90.9%) | 5 (4.54%) | 5 (4.54%) | |
| Tobramycin | 110 (100%) | 0 (0%) | 0 (0%) | |
| Folate pathway inhibitors | Trimethoprim-sulfamethoxazole | 110 (100%) | 0 (0%) | 0 (0%) |
| Carbapenems | Imipenem | 108 (98.18%) | 2 (1.81%) | 0(0%) |
| Meropenem | 110(100%) | 0 (0%) | 0 (0%) | |
| Cephems | Cefotaxime | 110 (100%) | 0 (0%) | 0 (0%) |
| Ceftriaxone | 110 (100%) | 0 (0%) | 0(0%) | |
| Cefepime | 110(100%) | 0 (0%) | 0 (0%) | |
| Ceftazidime | 110 (100%) | 0 (0%) | 0 (0%) | |
| Fluoroquinolones | Ciprofloxacin | 110 (100%) | 0 (0%) | 0(0%) |
| Tetracyclines | Tetracycline | 95 (86.36%) | 12 (10.9%) | 3(2.72%) |
| β-lactam/β-lactamase inhibitor combinations | Piperacillin/Tazobactam | 110 (100%) | 0 (0%) | 0 (0%) |
| Penicillins | Piperacillin | 110 (100%) | 0 (0%) | 0(0%) |
The Acinetobacter isolate was specified as MDR if it was resistant to at least 3 classes of antibiotics (24). The rate of resistance to aminoglycosides was as follows; tobramycin 100%, gentamicin 96.36%, and amikacin 90.9%. The MIC50 and MIC90 of gentamicin was determined as 128 μg/ml and 512 μg/ml, respectively and MIC50 and MIC90 of amikacin was determined >128 μg/ml. High MIC values would also predict the limited efficacy of those antibiotics against MDR-AB infections. The frequency of aac(6′)-Ib, aac(3)-I, ant(3′′)-I, aph(3′)-I and aac(6')-Id genes were 75.45% (83/110), 69.09% (76/110), 57.27% (63/110), 38.18% (42/110) and 1.81% (2/110), respectively. Furthermore, armA and rmtA genes were detected in 26.36% (29/110) and 2.72% (3/110) of the strains repectively. However, none of the strains carried rmtB, rmtC, and rmtD genes. Figures 1 and 2 show the agarose gel electrophoresis MPCR-amplified products of AMEs and 16S rRNA methylase genes, respectively. Coexistence of aminoglycoside resistance genes among the isolates are shown in Table 3.
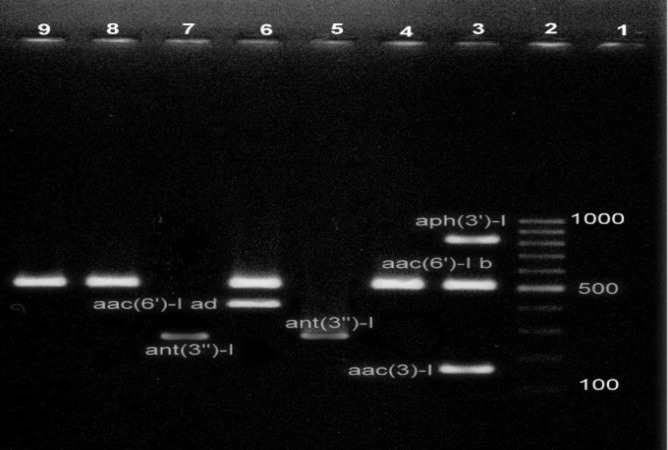
Figure 1.
M-PCR I simultaneously amplified aac(3)-I (169 bp), aac(6')-Id (435 bp), aac(6')-Ib (519 bp), ant(3'')-I (284) and aph(3')-I (816 bp). Lane 1, was the negative control. Lane 2, 100 bp DNA Ladder, Lanes 3-9 amplified products of studied AME genes
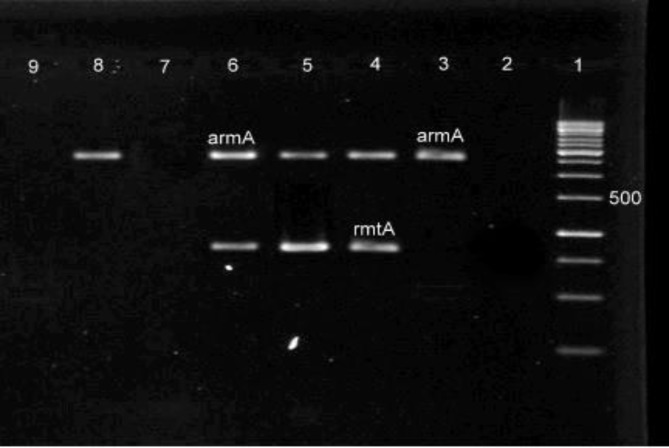
Figure 2.
M-PCR II simultaneously amplified armA (774 bp) and rmtA (315 bp). Lane 1, 100 bp DNA Ladder. Lane 2, negative control. Lanes 3-6 and 8, amplified products of studied 16S rRNA methylase genes, Lanes 7 and 9, had negative results for all 16S rRNA methylase genes
Table 3.
Coexistence of aminoglycoside resistance genes among MDR-AB isolates
| Patterns | No. of isolates | Positive rate (%) |
|---|---|---|
| aac(6′)-Ib + aac(3)-I + ant(3′′ )-I | 22 | 20 |
| aac(3)-I + aac(6′)-Ib + aph(3′)-I | 21 | 19.09 |
| aac(6′)-Ib + ant(3′′ )- I | 20 | 18.18 |
| ant(3′′ )- I + armA+ aph(3′)-I | 14 | 12.72 |
| aac(3)-I + armA | 13 | 11.81 |
| aac(6′)-Ib + aac(3)-I | 6 | 5.45 |
| aac(3)-I + aph(3′)-I + aac(6′)-Ib | 5 | 4.54 |
| aac(6′)-Ib + ant(3′′ )- I + aac(3)-I | 4 | 3.63 |
| armA + aac(6’)-Ib + aac(6')-Id | 2 | 1.81 |
| aac(3)-I + ant(3′′ )- I + aph(3′)-I + rmtA | 2 | 1.81 |
| aac(6′)-Ib + rmtA +ant(3′′ )- I + aac(3)-I | 1 | 0.9 |
Discussion
A. baumannii is a ubiquitous opportunistic pathogen that is especially successful at colonizing and persisting in hospital environments (25). Emerging importance of MDR-AB critically ill patients and their capacity to be more resistant to antimicrobial agents necessitates immediate action (4, 25).
In spite of the emergence of new antibiotics, aminoglycosides are still used in combination with β-lactams for treatment of MDR-AB infections in hospitalized patients (26). The results of the current study showed that all tested isolates were significantly resistant to most accessible treatment options such as gentamicin (96.36%) and amikacin (90.9%). This rate of antibiotic resistance in developing countries has increased significantly compared to developed countries during the recent years (27, 28). This may be due to antibiotic stewardship programs and infection control policies in developed countries. In this study, 100% of the isolates were resistant to tobramycin, trimethoprim-sulfamethoxazole, meropenem, cefotaxime, ceftriaxone, cefepime, ceftazidime, ciprofloxacin, piperacillin/tazobactam and piperacillin. These results were consistent with previous Iranian studies on drug resistance of A. baumannii (29, 30), as well as other countries such as China, Turkey and Pakistan (31, 32, 33). As expected, the most effective antibiotic with appreciable activity against the studied isolates was colistin. In agreement with our data, the results of previous studies in Iran (29, 34, 35) have shown that colistin-based therapy for treatment of diseases caused by MDR-AB had an appropriate clinical manifestation and decreased mortality.
Many factors affect the dissemination of antibiotic resistance genes. Although some of Acinetobacter spp., have shown intrinsically resistance to some aminoglycosides, resistance genes have also been found in transposons, plasmids and integrons. The rapid emergence of resistance to aminoglycosides in clinical isolates of Acinetobacter has been linked to their ability to acquire these resistance determinants. A wide range of the aminoglycoside resistance genes have been reported in A. baumannii (36). Several factors such as geographical regions, misuse of antibiotics, and inappropriate prescribing of aminoglycosides can play a significant role in the prevalence of aminoglycoside resistance genes (37). For instance, in Belgium the aac(3)-Ia gene (contribut to the gentamicin and tobramycin resistance) was commonly identified in Acinetobacter strains (38). Additionally, it was shown that the distribution of amikacin resistance among A. baumannii isolated in Spain was correlated with an epidemic strain harboring the aph(3′)-VIa (conferring resistance to kanamycin and neomycin) gene (39). In the current study, MPCR test was used to determine 16S rRNA methylase and AME encoding genes in clinically relevant A. baumannii isolates. Our study shows that the multiplex PCR method is a fast, reliable, and powerful technique for simultaneous detection of multiple factors associated with aminoglycoside-resistance. For epidemiologic analysis, MPCR method may be very helpful than using a conventional PCR for targeting each gene. The results of this study are consistent with those reported by Dillon et al. (40) and Poirel et al. (41) and show that the MPCR is a suitable and fast diagnostic application for the management and screening of genes that confer resistance to antibiotics. Our findings indicate that the aac(6′)-Ib, aac(3)-I, aph(3′)-I, and armA genes are more prevalent than other genes; aac(6')-Id and rmtA genes were found only at a very low incidence in our tested isolates. In a study conducted by Wen JT et al. in China, the prevalence of genes encoding aac(3')-I, aac(6')-Ib, ant(3'')-I, and aph(3')-I were 90%, 90%, 85%, and 35.0%, respectively, although other types of 16S rRNA methylase including rmtA, rmtB, rmtC, rmtD and armA were not detected among the MDR-AB isolates (22). A study conducted by Xiao et al. on A. baumannii isolates showed that the prevalence of aac(3)-I, aac(6ʹ)-Ib, ant(2ʺ)-I, and aph(3')-I genes were 10.7%, 17.9%, 14.3%, and 17.9%, respectively, and except armA (17.9%), other types of 16S rRNA methylase genes were not detected in any of the isolates (31). Aliakbarzade et al. (16) reported among the 103 A. baumannii isolates in Tabriz (Northwest Iran), 65.11 % and 60.46 % were positive for aacC1 and aph(3')-VIa, respectively. However in another study performed by Moniri et al. in Kashan (Isfahan Province, Iran) the frequency of aph(3')-VIa, aac(3)-Ia, ant(3')-Ia and ant(2")-Ia genes were 65%, 63.3%, 41.7%, and 3.3%, respectively (42). The 16S rRNA methylases could confer high-level resistance to aminoglycosides (15). In our study, armA was the most prevalent 16S rRNA methylase gene (26.36%), which is in concordance with reports of Vajihe Sheikhalizadeh et al. (44). This gene could be moved to other bacteria by conjugation and conferred high-level resistance to kanamycin, tobramycin, amikacin and gentamicin. These results exhibited that clinical isolates of Acinetobacter in different regions, harboring various types of aminoglycoside resistance genes and coexistence of resistance genes could be found in MDR-AB. In conclusion, the current study revealed that high prevalence of aminoglycoside resistance genes among MDR-AB isolates may be associated with AMEs genes, and 16S rRNA methylase genes were not prevalent in the tested isolates. The other mechanisms of resistance to aminoglycosides such as efflux pumps in MDR-AB strains should be considered in further investigations. Finally, paying attention to the recommended guidelines and implementing infection control policies to decrease the rate of MDR strains must be prioritized in therapeutic centers.
Acknowledgments
The authors are thankful to the staff of the Department of Microbiology, Iran University of Medical Sciences. The assistance of the staff of Shahid Motahari Burns Hospital and Milad Hospital of Tehran, Iran is appreciated.
Conflict of interest
The authors declared no conflicts of interest.
References
- 1.Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;6:25. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields RK, Clancy CJ, Gillis LM, Kwak EJ, Silveira FP, Massih RCA, et al. Epidemiology, clinical characteristics and outcomes of extensively drug-resistant Acinetobacter baumannii infections among solid organ transplant recipients. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–33. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K, Yong D, Jeong SH, Chong Y. Multidrug-resistant Acinetobacter spp: increasingly problematic nosocomial pathogens. Yonsei Med J. 2011;52(6):879–91. doi: 10.3349/ymj.2011.52.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bejestani FB, Hakemi-Vala M, Momtaheni R, Bejestani OB, Gholami M. The frequency of imp and vim genes among Pseudomonas aeruginosa isolates from children’s medical center of Tehran. Arch Clin Infect Dis. 2015;10(1):1–4. [Google Scholar]
- 6.Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: An Overview. Cold Spring Harb Perspect Med. 2016;6(6):a027029. doi: 10.1101/cshperspect.a027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis. 2010;51(1):79–84. doi: 10.1086/653120. [DOI] [PubMed] [Google Scholar]
- 8.Fournier P-E, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2(1):e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigro SJ, Post V, Hall RM. Aminoglycoside resistance in multiply antibiotic-resistant Acinetobacter baumannii belonging to global clone 2 from Australian hospitals. J Antimicrob Chemother. 2011;66(7):1504–9. doi: 10.1093/jac/dkr163. [DOI] [PubMed] [Google Scholar]
- 10.Shaw K, Rather P, Hare R, Miller G. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57(1):138–63. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atasoy AR, Ciftci IH, Petek M. Modifying enzymes related aminoglycoside: analyses of resistant Acinetobacter isolates. Int J Clin Exp Med. 2015;8(2):2874. [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist Updat. 2010;13(6):151–71. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemec A, Dolzani L, Brisse S, van den Broek P, Dijkshoorn L. Diversity of aminoglycoside-resistance genes and their association with class 1 integrons among strains of pan-European Acinetobacter baumannii clones. J Med Microbiol. 2004;53(12):1233–40. doi: 10.1099/jmm.0.45716-0. [DOI] [PubMed] [Google Scholar]
- 14.Al Sheikh YA, Marie MAM, John J, Krishnappa LG, Dabwab KHM. Prevalence of 16S rRNA methylase genes among β-lactamase-producing Enterobacteriaceae clinical isolates in Saudi Arabia. Libyan J Med. 2014;9(1) doi: 10.3402/ljm.v9.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Yu H, Guo Q, Xu X, Ye X, Wu S, et al. Distribution of 16S rRNA methylases among different species of Gram-negative bacilli with high-level resistance to aminoglycosides. Eur J Clin Microbiol Infect Dis. 2010;29(11):1349–53. doi: 10.1007/s10096-010-1004-1. [DOI] [PubMed] [Google Scholar]
- 16.Aliakbarzade K, Farajnia S, Nik AK, Zarei F, Tanomand A. Prevalence of aminoglycoside resistance genes in Acinetobacter baumannii isolates. Jundishapur J Microbiol. 2014;7(10) doi: 10.5812/jjm.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karah N, Dwibedi CK, Sjöström K, Edquist P, Johansson A, Wai SN, et al. Novel aminoglycoside resistance transposons and transposon-derived circular forms detected in carbapenem-resistant Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother. 2016;60(3):1801. doi: 10.1128/AAC.02143-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi PR, Acharya M, Kakshapati T, Leungtongkam U, Thummeepak R, Sitthisak S. Co-existence of bla OXA-23 and bla NDM-1 genes of Acinetobacter baumannii isolated from Nepal: antimicrobial resistance and clinical significance. Antimicrob Resist Infect Control. 2017;6(1):21. doi: 10.1186/s13756-017-0180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol. 2006;44(8):2974–6. doi: 10.1128/JCM.01021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CLSI. M100-S25 performance standards for antimicrobial susceptibility testing. Twenty-fifth informational supplement. 2015 [Google Scholar]
- 21.Ghatak S, Muthukumaran RB, Nachimuthu SK. A simple method of genomic DNA extraction from human samples for PCR-RFLP analysis. J Biomol Tech. 2013;24(4):224–31. doi: 10.7171/jbt.13-2404-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen J, Zhou Y, Yang L, Xu Y. Multidrug-resistant genes of aminoglycoside-modifying enzymes and 16S rRNA methylases in Acinetobacter baumannii strains. Genet Mol Res. 2014;13(2):3842–9. doi: 10.4238/2014.May.16.9. [DOI] [PubMed] [Google Scholar]
- 23.Doi Y, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis. 2007;45(1):88–94. doi: 10.1086/518605. [DOI] [PubMed] [Google Scholar]
- 24.Manchanda V, Sanchaita S, Singh N. Multidrug resistant acinetobacter. J Global Infect Dis. 2010;2(3) doi: 10.4103/0974-777X.68538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C-H, Lin L-C, Chang Y-J, Chen Y-M, Chang C-Y, Huang C-C. Infection control programs and antibiotic control programs to limit transmission of multi-drug resistant Acinetobacter baumannii infections: evolution of old problems and new challenges for institutes. Int J Environ Res Public Health. 2015;12(8):8871–82. doi: 10.3390/ijerph120808871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed A, Azim A, Gurjar M, Baronia AK. Current concepts in combination antibiotic therapy for critically ill patients. Indian J Crit Care Med. 2014;18(5):310–314. doi: 10.4103/0972-5229.132495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gholipourmalekabadi M, Bandehpour M, Mozafari M, Hashemi A, Ghanbarian H, Sameni M, et al. Decellularized human amniotic membrane: more is needed for an efficient dressing for protection of burns against antibiotic-resistant bacteria isolated from burn patients. Burns. 2015;41(7):1488–97. doi: 10.1016/j.burns.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadtaheri Z, Pourpaki M, Mohammadi F, Namdar R, Masjedi M-R. Surveillance of antimicrobial susceptibility among bacterial isolates from intensive care unit patients of a tertiary-care university hospital in Iran: 2006–2009. Chemotherapy. 2010;56(6):478–84. doi: 10.1159/000321032. [DOI] [PubMed] [Google Scholar]
- 29.Vakili B, Fazeli H, Shoaei P, Yaran M, Ataei B, Khorvash F, et al. Detection of colistin sensitivity in clinical isolates of Acinetobacter baumannii in Iran. J Res Med Sci. 2014;19(3) [PMC free article] [PubMed] [Google Scholar]
- 30.Gholami M, Hashemi A, Hakemi-Vala M, Goudarzi H, Hallajzadeh M. Efflux pump inhibitor phenylalanine-arginine Β-naphthylamide effect on the minimum inhibitory concentration of imipenem in Acinetobacter baumannii strains isolated from hospitalized patients in Shahid Motahari burn hospital, Tehran, Iran. Jundishapur J Microbiol. 2015;8(10) doi: 10.5812/jjm.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao-min X, You-fen F, Wei-yun F, Zu-huang M, Xing-bei W. Antibiotic resistance determinants of a group of multidrug-resistant Acinetobacter baumannii in China. J Antibiot. 2014;67(6):439–44. doi: 10.1038/ja.2014.18. [DOI] [PubMed] [Google Scholar]
- 32.Direkel Ş, Uzunoğlu E, Keleş S, Yapar K. Antibiotic Resistance Rates of Acinetobacter Baumannii Strains Isolated from Various Clinical Samples in Giresun Prof Dr Atilla ilhan Ozdemir State Hospital. Gazi Med J. 2015;26(3) [Google Scholar]
- 33.Shamim S, Abbas M, Qazi MH. Prevalence of Multidrug Resistant Acinetobacter baumannii in Hospitalized Patients in Lahore, Pakistan. Pakistan J Mol Med. 2015;2(1):23–8. [Google Scholar]
- 34.Khoshnood S, Eslami G, Hashemi A, Bahramian A, Heidary M, Yousefi N, et al. Distribution of aminoglycoside resistance genes among Acinetobacter baumannii strains isolated from burn patients in Tehran, Iran. Arch Pediatr Infect Dis. 2017;5(3) [Google Scholar]
- 35.Hakemi-vala M, Ostadasadi-malayeri H, Gholami M, Goudarzi H. Survey on the role of AdeRS and oxa23 genes among imipenem resistant Acinetobacter baumannii isolates from hospitalized patients of Tehran. Clin Chem Lab Med. 2016;54(7):eA50–eA1. [Google Scholar]
- 36.Lin MF, Lan CY. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J Clin Cases. 2014;16;2(12):787–814. doi: 10.12998/wjcc.v2.i12.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ventola CL. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharmacol Ther. 2015;40(4):277–83. [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw K, Hare R, Sabatelli F, Rizzo M, Cramer C, Naples L, et al. Correlation between aminoglycoside resistance profiles and DNA hybridization of clinical isolates. Antimicrob Agents Chemother. 1991;35(11):2253–61. doi: 10.1128/aac.35.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vila J, Marcos A, Marco F, Abdalla S, Vergara Y, Reig R, et al. In vitro antimicrobial production of beta-lactamases, aminoglycoside-modifying enzymes, and chloramphenicol acetyltransferase by and susceptibility of clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 1993;37(1):138–41. doi: 10.1128/aac.37.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dillon B, Thomas L, Mohmand G, Zelynski A, Iredell J. Multiplex PCR for screening of integrons in bacterial lysates. J Microbiol Methods. 2005;62(2):221–32. doi: 10.1016/j.mimet.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–23. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Moniri R, Farahani RK, Shajari G, Shirazi MN, Ghasemi A. Molecular epidemiology of aminoglycosides resistance in Acinetobacter spp With emergence of multidrug-resistant strains. Iran J Public Health. 2010;39(2) [PMC free article] [PubMed] [Google Scholar]
- 43.Sheikhalizadeh V, Hasani A, Rezaee MA, Rahmati-yamchi M, Hasani A, Ghotaslou R, et al. Comprehensive study to investigate the role of various aminoglycoside resistance mechanisms in clinical isolates of Acinetobacter baumannii. J Infect Chemother. 2017;23(2):74–9. doi: 10.1016/j.jiac.2016.09.012. [DOI] [PubMed] [Google Scholar]