Abstract
Understanding the organizing and activating effects of gonadal steroids on adult physiology can guide insight into sex differences in and hormonal influences on health and disease, ranging from diabetes and other metabolic disorders, emotion and stress regulation, substance abuse, pain perception, immune function and inflammation, cognitive function and dysfunction accompanying neurological disorders. Because the brain is highly sensitive to many forms of estrogens, it is not surprising that many adult behaviors, including cognitive function, are modulated by estrogens. Estrogens are known for their facilitating effects on learning and memory, but it becoming increasingly clear that they also can impair learning and memory of some classes of tasks and may do so through direct actions on specific neural systems. This review takes a multiple memory systems approach to understanding how estrogens can at the same time enhance hippocampus-sensitive place learning and impair striatum-sensitive response learning by exploring the role estrogen receptor signaling may play in the opposing cognitive effects of estrogens. Accumulating evidence suggests that neither receptor subtype nor the timing of treatment, i.e. rapid vs slow, explain the bidirectional effects of estrogens on different types of learning. New findings pointing to neural metabolism and the provision of energy substrates by astrocytes as a candidate mechanism for cognitive enhancement and impairment are discussed.
Keywords: Hippocampus, Striatum, Memory systems, Estrogen signaling, Metabolism
1. Introduction
The clinical importance of elucidating the contributions of estrogens and other reproductive hormones to neurological and behavioral functions is far reaching and encompasses issues ranging from studying sex as a biological variable in health and disease to deciphering the impact of environmental endocrine disruptors on neural health. Understanding the developmental and adult consequences of estrogen exposures can advance understanding of independent and interacting contributions of genes, hormones, and environment to individual differences in brain function and dysfunction that may relate to sex differences or similarities. Moreover, understanding the extent of estrogen effects on basic neurobiological functions, such as neural transmission, has the potential to inform mechanisms of neurological disorders, particularly those that have endocrine underpinnings and promote health practices for all sexes [1]. For example, knowledge about estrogen regulation of neural transmission, tissue excitability, and seizure development has translational value for understanding and treating catamenial epilepsy and other seizure disorders in males and females. Understanding hormonal regulation of affective behaviors, including effects on mood states and neurochemistry will undoubtedly promote diagnosis and treatment of post-partum depression. Endocrine consequences of non-neural health problems, such as reproductive senescence and polycystic ovarian syndrome, and their treatments that typically mimic or disrupt estrogen signaling, may inadvertently modulate brain function. Finally, we currently are experiencing excessively high exposures to environmental estrogens and other endocrine disrupting chemicals [2] that may mimic or block endogenous hormone function in both males and females across different stages of the lifespan, creating equally unintended neural and behavioral health outcomes.
Understanding the etiology and mechanisms of these outcomes will allow for development of effective treatments and preventions.
Estrogens are no longer solely considered to be female sex hormones given their broad reaching effects on a variety of non-reproductive behaviors in both males and females [3]. Similarly, the pervasive nature of estrogens is evident in their regulation of form and function across a wide array of tissues and cellular processes. The brain is one estrogenic target that has received considerable attention over the last several decades given how robustly estrogens can organize and activate behaviors in males and females [4] comprising not only mating and parenting [5–7] but also those related to feeding and energy balance [8], navigation [3, 9], emotion, aggression, and stress regulation ([5, 10–13 THIS ISSUE], and cognition ([9, 14–17]. Thus, estrogens are thought to act quite robustly across the brain but may do so in very different ways depending upon the sex of the organism [17– 20], dose and timing of hormone exposure [6, 7, 16, 21], and brain region of interest [16].
Perhaps one of the more compelling arguments for pursuing the effects of hormones on brain and cognition comes from the gender bias in Alzheimer’s disease (AD). AD is the most prevalent form of dementia and the only top-ten lethal condition in the United States without effective treatments or known etiologies. The incidence and prevalence of AD are higher in women who are two to three times as likely as men to be living with AD even after accounting for longevity, suggesting a sex difference in the risk and etiology of the disease [22]. When combined with the evidence that age is the biggest risk factor for sporadic AD [22], the sex difference in demographics highlights the possibility that menopause and the accompanying decline in circulating ovarian hormones contribute to higher risk in women. Despite the hotly debated results from the Women’s Health Initiative, [23, 24], converging lines of clinical and preclinical evidence strongly confirm that naturally occurring estrogens given around the time of natural or surgical menopause confer neural protection against dementia and that women who take hormone replacement therapies are at lower risk for AD [25–27].
Estrogens facilitate learning and memory in tasks believed to rely on brain regions such as the hippocampus and entorhinal cortex that are ravaged by degeneration associated with AD [28 THIS ISSUE]. However, as will be discussed in detail below, estrogens not only enhance hippocampus-sensitive forms of learning and memory but also impair learning and memory in non-hippocampal tasks that depend on the integrity of other neural systems such as the caudate/putamen (striatum) and frontal cortex [16]. Thus, loss of ovarian hormones during menopause may indeed enhance cognitive function and may relate to changes in structure and function of the striatum. Interestingly, not all brain areas are damaged by AD and, in some regions such as the caudate nucleus, may actually be enlarged. A recent report using magnetic resonance imaging demonstrated that compared to non-demented people with mild cognitive impairment those with AD had increases in caudate volume accompanying the decline in hippocampal volume [29]. Detailed analysis of contributing variables suggested that the demographic factors of gender and age, but not an AD diagnosis, may have driven the effects, as caudate enlargement was positively associated with older age and being female, and most certainly being post-menopausal. Thus, elucidating the dynamic interplay between hormone status and function of different neural systems will undoubtedly advance our understanding of healthy aging along with AD and other neural disorders.
The work described herein is based on the philosophy that there are brain states or contexts that can enhance or impair the ability to process information needed to solve specific tasks. Hormones can create these brain states responsible for improvements or impairments in cognition depending upon the specific attributes of the task at hand [30]. This review will highlight findings showing that estrogens bidirectionally modulate learning and memory in young adult female rats depending on the type of problem to be solved and the memory system engaged during learning; the focus here will be on the hippocampus and striatum. Recognizing early in the evolution of this work that female subjects were explicitly omitted from behavioral neuroscience studies, and perhaps even more so from those focused on neural mechanisms of learning and memory, our research program was borne out of a need to study hormonal modulation of learning in the female in its own right, as its own paradigm. This task of focusing on estrogen modulation of cognition is not so much a protest against the unwarranted male bias in neuroscience [31] as it is an attempt to equalize the foundation of knowledge based on females before moving towards the important goal of assessing the impact of sex on brain and cognitive health, an imperative now recognized by researchers, funders, and editors [32–34]. Nearly all of the work discussed here was conducted with females alone; however, in some cases we have data from male rats that can be used as points of comparisons even though the experiments themselves were not explicitly designed as direct tests of sex influences and did not examine estrogen effects in males. Notwithstanding these limitations, these exploratory approaches might provide a foundation upon which we can build studies to assess sex as a biological variable for the selectivity of estrogen modulation of learning [34, 35].
2. A focus on estrogens and learning strategy using a memory systems lens
Just as males and females express qualitatively different reproductive behaviors as a result of both organizational and activational effects of hormones, there are sex and gender differences in cognitive functions such as declarative memory detected by recall of information from narratives [36], in sensory-motor dexterity [37, 38], and in spatial information processing [9, 37–40]. To solve a navigation problem based on using visual cues, males tend to rely on room geometry while females have a propensity to use landmark cues, suggesting that sex differences reflect the quality of information used and not necessarily the amount of information that is stored [9]. In humans, abilities that are sensitive to biological sex tend to fluctuate across the menstrual cycle [37, 39], such that male-preferred abilities are high during low-hormone stages and low during high hormone stages while female-preferred strategies are high during high-hormone stages and low during low hormone stages [41]. It follows that ovarian hormone status, which fluctuates across the reproductive cycle, may maximize or minimize sex differences, in some cases creating and in other cases eliminating differences depending on the type of task being assessed. For example, with high hormone status, young adult female rats do well on spatial navigation tasks but poorly on place preference learning or delayed alternation conditioning tasks [42–44], suggesting that testing during high hormone phases might produce apparent sex differences in conditioning but not in spatial navigation. Thus, better resolution of sex differences in cognition might be obtained if regular fluctuations in hormones across the estrous cycle are considered.
Because of the numerous findings that estrogens increase hippocampal plasticity [45], considered by many to underlie memory, a positive relationship between estrogens and cognition has been readily presumed and supported by many results that estrogens enhance memory. However, a finer analysis of decades of work is that estrogens produce robust yet mixed effects on learning and memory – at times enhancing, at times impairing and at other times having no measurable effects on cognition. The direction of effect seems to vary with task, treatment, and subject factors including subject sex, age, and reproductive status [17, 28 THIS ISSUE, 46, 47], the type of estrogen, its dose, and regimen [16, 48], and task attributes such as type of memory probed, stressful elements, or phase of learning [14, 16, 30, 42, 49, 50]. Given these varied effects, it is possible that estrogens up- or down-regulate cognition by modulating function of select neural systems that mediate learning and memory during the training experience.
The diverse effects of estrogens on learning and memory fit well with a multiple memory system framework developed by many [51–59] positing that individuals can and do use different brain regions to solve tasks with different cognitive attributes. Theoretically, any brain region can be considered a memory system if it plays a key role in cognition; however, not all brain regions show selective engagement under different task conditions and thus cannot be dissociated based on task attributes. The hippocampus and striatum are two brain regions in particular that have features making them particularly good prototypes for tests of hormonal modulation of multiple memory systems. First, both are large structures that are histologically separate and therefore relatively easy to manipulate and to assay. Secondly, the involvement of hippocampus and striatum in different types of learning and memory is readily dissociable through cognitive tasks that tap one system over the other. Shown largely in rats, with parallel findings using virtual tasks and imaging in humans [60], hippocampus-sensitive tasks include spatial working memory or “cognitive” tasks, win-shift strategies, context conditioning, object placement memory tests (also called novel object location tasks), and place or allocentric learning in mazes. In contrast, striatum-sensitive tasks include reference memory or “habit” tasks, win-stay strategies, cue conditioning, and response, cued, or egocentric learning in mazes.
The dissociable features of different memory systems have been elegantly tested and characterized by several investigators, with many examples showing independence of these memory systems such that disruption of functioning of hippocampus tends to impair selectively place but not response learning while lesions to striatum selectively impair response but not place learning [51, 54, 57, 62]. Further support for the hippocampus and striatum being distinct and dissociable memory systems comes from partitioning task-dependent changes in metabolic substrates [63], cell signaling, and gene transcription [64, 65] across the two structures. In practice, however, the effects are more complex than a simple double dissociation [57, 66–69] in that manipulations that disable one structure can actually improve cognitive functions shown to depend on intact functioning of the other system [55, 56, 68], suggesting ongoing competition between or across these two memory systems. Whether anatomical or operational, competitive interactions between memory systems may not necessarily be under direct, monosynaptic control but instead may reflect interactions across outputs of structures, information transfer between systems, or modulated input to each memory system from shared cortical afferents, all of which are explored in depth and articulated eloquently in a recent review [68].
One scenario suggests the prefrontal cortex, a region that receives afferents from hippocampus and striatum, may integrate relevant task information from the two memory systems and ultimately decide which system gains control during task acquisition, retention, or retrieval. Similarly, the hippocampus and striatum both receive inputs from the same cortical regions, with reciprocal afferents to those regions. Thus, representations from each memory system may modulate cortical input to the other system, creating a context in which information processing in one structure is able to modulate functional output of the other. The neural mechanisms underlying these regulatory processes are poorly understood but may include plasticity in neurochemical release, neuronal firing patterns, cell signaling pathways, and gene transcription [67–70]. In addition to competitive interactions, examples of collaboration across hippocampus and striatum, where both structures appear to contribute to optimal task performance have also been described, for example using electrophysiological correlates [69] and manipulations to up- and down-regulate functional CREB [70].
When viewed from this memory system vantage point, it is likely that estrogens enhance and impair learning and memory through opposing actions on different brain regions, possibly through competitive interactions; that is, both the enhancement and impairment in learning is produced through direct actions on only one structure, the hippocampus or the striatum. To test the question of whether and how estrogens modulate learning strategy we conducted a series of experiments assessing hippocampus-sensitive place learning, which requires rats to find food in a plus- or T-shaped maze by always navigating to the arm in the same room position and striatum-sensitive egocentric response learning [71–73], which requires the use of the same body turn (e.g. right or left) to find food in the maze (Figure 1). Training procedures for each learning task are identical except for the strategy required to solve the task. We have developed two general paradigms that use the same plus-shaped land maze, one paradigm that is a dual-solution task (Figure 1A) allowing for the selection of either place or response strategies, and the other a pair of single-solution tasks (Figure 1B), one that requires a place strategy and the other that requires a response strategy for optimal performance. Training in both paradigms is confined to a single session ~1–4 hrs, within one day, thereby allowing comparisons across individual estrous cycle stages, which typically last from ~ 6 – 24 hours in the rat, or across narrow windows of exogenous estrogen treatments.
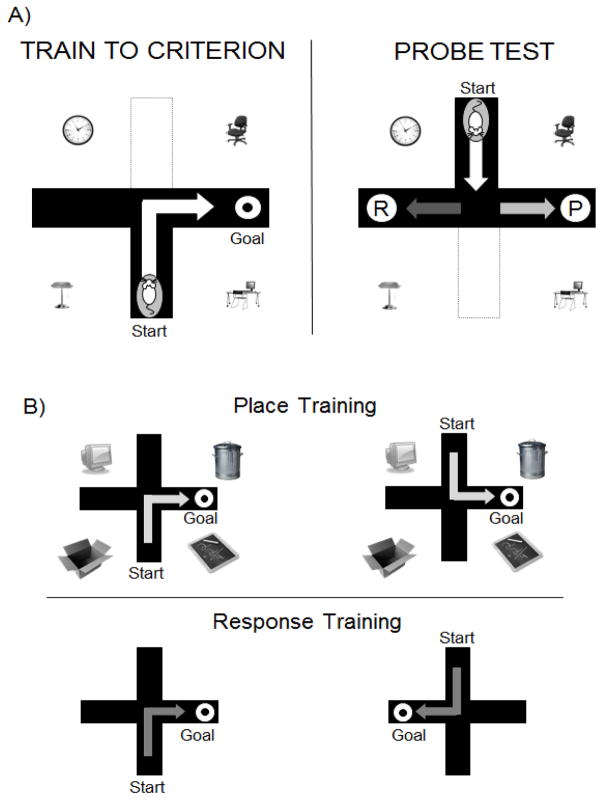
FIGURE 1.
Schematic of behavioral paradigms used to test allocentric place and egocentric response strategies. Rats are trained to find food on land-based mazes. A) Dual solution T-maze task in which both place and response strategies are equally effective. During training, the goal and start arms are held constant relative to each other and to room cues. The rat can learn by relying on a specific body turn or by relying on the arrangement of room cues. The strategy used during training is determined with a probe test where the start arm is rotated 180° from the start arm used during training. A response strategy (R) is assigned if the rat turns in the direction rewarded during training, while a place strategy (P) is assigned if the rat chooses the arm in the spatial position rewarded during training. B) Single solution maze tasks that tap either place (upper panel) or response (lower panel) learning.
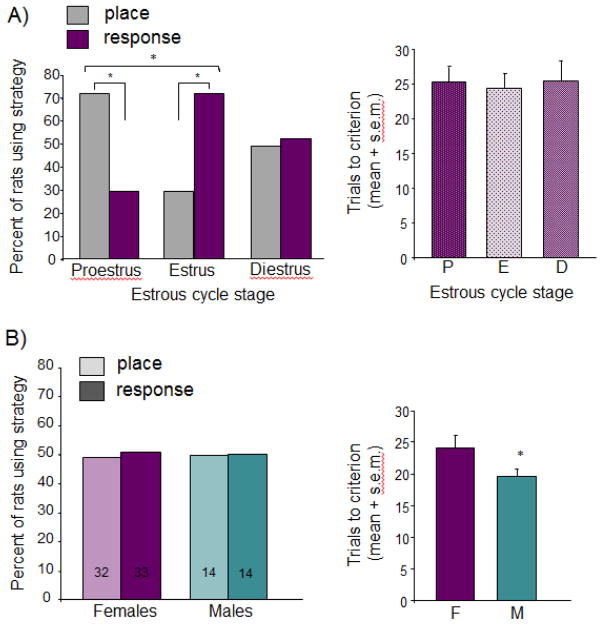
We tested whether the fluctuation of hormones across the estrous cycle in young adult rats produced corresponding fluctuations in the use of learning strategies during task acquisition. In the dual-solution task, where strategy is chosen by the individual, rats showed dramatic shifts in strategy use across the cycle and biases in strategies within cycle stage (Figure 2A; [74]). As predicted from prior work showing that elevated levels of estrogens support hippocampal functions [45], at proestrus, significantly more rats used place versus response strategies. No bias was seen in rats at met/diestrous stages, when hormone levels are intermediate between proestrous and estrous states. However, at estrus when hormone levels were relatively low, rats expressed a significant bias towards response strategies, suggesting a shift in the choice of strategies from place to response with the changing hormonal milieu. Importantly, the same number of trials was needed to meet criterion performance (~25) across all groups, highlighting the novel finding that cognitive strategy and not necessarily learning ability per se fluctuates across the estrous cycle.
FIGURE 2.
Learning strategy selection in the dual solution task. A) Effects of estrous cycle on strategy selection. Left graph depicts percent of rats using place and response strategies; right graph shows learning speed using the number of trials to reach criterion. Note a substantial bias within stage and shift between stages in the proportion of rats using place and response strategies without any evidence of cycle effects on learning speed. B) Comparisons across age-matched males and females without regard to estrous status. When data from females are collapsed across cycle stage there are no strategy biases or sex differences in strategy use (left panel) or learning speed (right panel). Adapted from [74, 75]. * = p < 0.05.
When the data in females are collapsed across all three stages of the estrous cycle and compared to males trained in the same maze with the same training conditions [75], there are no significant sex differences in strategy, with half of the rats of each sex displaying a propensity to use place learning and the other half response learning. Males showed a subtle but significant increase in learning speed, seen by the reduction in trials to reach criterion, a surprising finding given that learning speeds are typically the same across treatment groups even when strategies shift [74, 75] (Figure 2A). As such, little is known regarding the cell biology controlling acquisition speed in the dual solution task, and thus it is difficult to speculate what may contribute to this sex difference. Perhaps sex differences in provisions of astrocytic lactate, a metabolite known for its critical role in learning and memory (see section 3.3 below), allow for faster learning in males, a possibility in need of further investigation. Indeed, the established sex differences in the many neurobiological processes elucidated throughout this Special Issue point to a myriad of viable cell and molecular candidates, such as estrogen receptor distribution, aromatase activity, and immune function, for future studies on sex differences in learning and memory.
When tested in humans, a similar small but consistent male advantage for acquisition rate was detected in an analogous virtual dual solution maze task [76] that primed the participants with aerial views of the training environment and probed for spatial vs response strategies twice during training. Moreover, in contrast to prior findings by the same investigator who reported response biases in both genders after extensive training [c.f. 76], there was a higher bias towards using spatial strategies in men than in women. Particularly because men also demonstrated superior mental rotation skills, it is possible that the aerial priming promoted the selection of spatial strategies in men more readily than in women [76]. Because results in rats suggest that hormone-induced modulation of strategy choice is notably stronger than any apparent sex difference in strategy choice, it is unfortunate that menstrual cycle status was not used as a variable to discern hormone contribution to virtual maze learning strategies in women, which in turn may clarify discrepancies across reports.
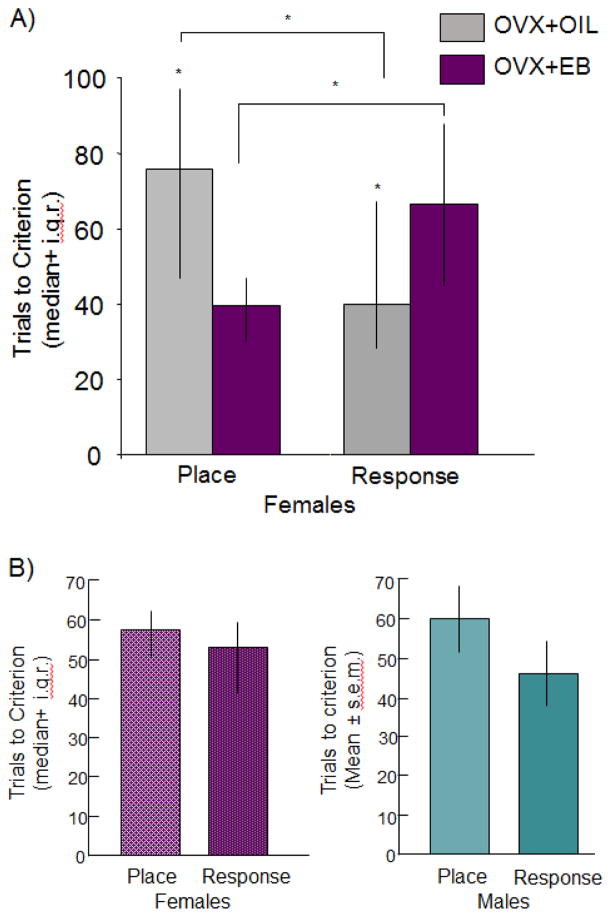
The role estrogens play in mediating the shift in learning styles was demonstrated by assaying strategy selection in ovariectomized rats treated acutely with systemic estradiol benzoate for two days to mimic the rise in circulating estrogens experienced across the estrous cycle. Compared to oil-treated controls, young adult female rats with relatively high circulating estradiol show preferences for place strategies over response strategies [16, 77], with a pattern of biases similar to those detected across the estrous cycle [74, 78, 79]. Given these preferences, estrogens may regulate the ability to use one style over another, e.g. improve performance on hippocampus-sensitive place learning but impair striatum-sensitive response learning.
Direct tests of this possibility were made using single solution place and response tasks that require rats to use one strategy over the other. Female rats that were ovariectomized and received hormone replacement with relatively short exposures of estradiol benzoate, 48 and 24 hours prior to training, solve place tasks more quickly than response tasks and more quickly than did rats with control oil treatments (Figure 3A; [80]). Consistent with the response strategy selection at estrus (Figure 2A; [74, 78]) or in rats given injections of oil-vehicle after ovariectomy [77], oil-treated rats significantly outperformed estradiol-treated rats on response learning, again pointing to an estradiol-induced shift in learning abilities towards the use of place learning and away from the use of response learning, a finding corroborated by others using chronic treatments, training across days, or different striatum-sensitive navigational tasks [16, 81, 82]. When evaluated without regard to estradiol status, place and response learning scores in young adult females are equivalent to each other and to respective place and response scores in age-matched males (Figure 3B; [63]). Thus, like strategy selection, the ability to solve place and response tasks also seems to rely more heavily on hormone status at the time of training than on sex status.
FIGURE 3.
Effects of A) estradiol and B) sex on place and response learning in single solution tasks. A) In young adult ovariectomized female rats, estradiol benzoate (10 ug/rat) give 48 and 24 hours prior to training enhanced place learning but impaired response learning as measured by number of trials to reach criterion. B) When data from females are collapsed across treatment groups, there are no differences in learning across tasks or between age-matched males and females. Adapted from [80, 63]. * = p < 0.05.
There is a dearth of studies exploring the role of androgens in learning strategies, one of the few areas of behavioral neuroscience lagging far behind similar investigations in females. Even in the handful of published reports the findings are mixed, which, as in females, may result from differences in tasks. When tested in a dual-solution task, gonadally intact males showed place learning biases undetected in our experiments (see Figure 2B) that were unaffected by castration [83], supporting the possibility that androgens are unimportant for spatial strategy selection [84]. However, like estrogens, androgen modulation of navigation is sensitive to dose, with lower doses (0.125 mg/rat) biasing towards response and cued strategies and higher doses (0.5 mg/rat) prompting place strategies [85]. It is important to note that the cue density or configuration can be manipulated to foster the selection of one or the other strategy (unpublished findings; [72]) and thus comparing specific results across studies from different laboratories with different training environments might be difficult.
3. Deciphering the biology underlying bidirectional effects of estrogens on learning strategy
It is now established that physiological doses of estrogens enhance hippocampus-sensitive learning and memory but interrupt other forms of cognition, particularly those that rely on the striatum [16]. While unlikely, the bidirectional cognitive effects of estrogens may result from nonmnemonic or extrabrain influences on motivational state such as hunger or thirst [86] or sensory function affecting cue acuity or use [87]; if so, central manipulations of estrogen signaling would essentially be ineffective. Evidence that estrogens do indeed enhance place learning and impair response learning through direct action at the hippocampus comes from direct pretraining application of estrogens or antiestrogens into each brain site followed by training on a single solution place or response maze. Hippocampus infusions of estradiol sulfate enhanced learning of the place task with no effect on response learning while striatal infusions impaired learning of the response task without modulating place learning, pointing to a clean double dissociation between site of estradiol infusion and task performance [88]. Furthermore, implants of the estrogen receptor (ER) blocker ICI 182,780 into the canonical brain region attenuated the effect of systemic estradiol on place and response learning: ER blockade in the hippocampus reversed the estradiol-induced place learning enhancement while implants into the striatum reversed response learning impairments [89, 90]. Thus, in females, estradiol works to enhance and impair place and response learning, respectively, through site-specific, independent, ER-mediated mechanisms during task acquisition and not via competitive or collaborative interactions between the two structures. Interactions between memory systems seem to be the norm for males, thereby revealing a sex difference in memory systems characteristics. For example, as alluded to above, interventions that disrupt hippocampal structure or function in male young adult male rats facilitate response learning while those that enhance hippocampal function interrupt response learning [55, 56, 58, 68]; the converse is seen with manipulations to the striatum [66, 67, 91]. Collaboration across hippocampal and non-hippocampal memory systems has also been described in males [59, 69, 70, 92, 93], but not in females [88] where data are relatively lacking, highlighting the possibility that mechanisms of hormonal modulation of multiple memory systems may be qualitatively different between males and females.
The cellular and molecular mechanisms mediating these opposing actions remain obscure but are likely to include site-specific differences in ER distribution along with inter- and intra-cellular signaling events. Sorting these mechanisms undoubtedly has important implications for human health because of the wide use of estrogenic agents for cancer treatments, for prevention of osteoporosis, and in everyday use such as consuming diets high in phytoestrogens and taking oral contraceptives. That is, if the bidirectional effects dissociate by some identifiable aspect of estrogen signaling, it will be possible to develop estrogenic treatments – either anti-estrogens or agonists – targeting one or other mechanism that avoid the deleterious effects on cognition while maintaining the beneficial intended effects. For example, if we find that estrogens improve cognition via a select set of receptors or molecular signaling pathways yet impair via a different set of cell signaling mechanisms, new compounds that target one receptor or molecule in the pathway could be developed to avoid the deleterious effects on cognition or even promote the beneficial actions.
Receptor signaling
The brain uses many different types of estrogen responsive pathways involving classical ERs and novel signaling molecules identified on neurons, glia, and cells of the cerebrovasculature [94, 95]. The distribution of classical and novel ERs is vastly different for the hippocampus and striatum. The hippocampus has been described as having essentially all types of estrogen responsive proteins, including ERα, ERβ, the g-protein coupled receptor estrogen receptor, GPER/GPR30, and ERX, which unlike other ERs is activated by 17-α estradiol [96, 97], that localize to the nucleus (ERα, ERβ), plasma and mitochondrial membranes (ERα, ERβ, ERX), and endoplasmic reticulum (GPER) [98–101]. In contrast, the striatum only contains extranuclear receptors (ERs and GPER) largely confined to axon terminals and astrocytes [102–104], and thus striatal cells can only initiate estrogen signaling through non-genomic events by activation of membrane ERs or transactivation of other membrane receptors such as metabotropic glutamate or trophic factor receptors. Estrogens can still exert durable effects in the striatum that outlast the rapid initiation of signaling and may do so by co-opting other downstream cellular processes e.g. MAPK, PLC, PI3-K, PKA, that lead to transcriptional regulation [105–107].
The distinct pattern of ER distribution across the hippocampus and striatum makes estrogen signaling a good candidate for mediating estrogen induced-cognitive enhancements and impairments. To decipher the contribution of estrogen signaling mechanisms we determined if the impairments and enhancements in learning dissociated either by ER subtypes or timing of exposure, i.e. just prior to training vs hours or days prior to training. Results from these two approaches would provide insight into signaling pathways that have specific downstream molecular targets and temporal properties.
3.1 ERalpha vs ERbeta
Early reports using in situ hybridization and immunohistochemistry [98, 100, 108] showed considerable ERβ in cortex and hippocampus and less so in other areas. Conversely, ERα labeling was weak in hippocampus but heavy throughout the hypothalamus and other “reproductive” areas such as medial amygdala, leading to the speculation that ERα mediates reproductive behavior and ERβ mediates cognitive activity [108–110]; a separation in function by receptor was also suggested by findings from studies of reproductive and nonreproductive behaviors using ERα and ERβ knock-out (ERαKO, ERβKO) models [111]. However, the ERα vs ERβ distinction for sex vs cognition was weakened by refined detection methods, revealing that many brain areas have both classical ERs in addition to other estrogen receptors including GPER, but that they distribute across different cell types, different compartments, and with different densities [4]. Moreover, ERα activation during development may be critical for establishing appropriate neural circuitry important for cognition [112] and, in the adult state, for mediating estrogenic modulation of learning and memory [113].
A growing body of evidence mostly from studies of hippocampus-sensitive cognition comparing efficacy of both ERα and ERβ agonists points to the involvement of both receptors, the specificity of which may depend on the animal model used, type of task, and the timing of treatments with respect to different phases of learning and memory (see also 3.2). For example, in wild-type ovariectomized mice activation of either receptor using the ERα-selective agonist propyl pyrazole triol (PPT) or ERβ-selective agonist diarylpropionitrile (DPN) immediately after training improved memory formation for novel objects or object placement when tested one to two days later [114]. However, when recognition was tested only 4 hours later in ovariectomized rats the results are mixed, with evidence of only one or the other receptor agonist effectively enhancing object recognition or object placement memory [115, 116].
When given over two to three days prior to training on hippocampus-sensitive object recognition (rats) or social transmission of food preference tasks (mice), PPT failed to enhance subsequent memory despite the robust enhancement by estradiol and ERβ-selective agonists, DPN [116, 117, 118] and WAY-200070 [117, 118]. In fact, social learning of a food preference in mice might actually be impaired by chronic ERα activation per se [117, 118]. Other findings, however, indicate that selective signaling through ERα supports better learning, but only when agonist treatments are given just before recognition memory training [119]. In contrast, when ovariectomized rats were tested in a T-maze task, continuous replacement with either PPT or DPN for two to three weeks facilitated acquisition of a delayed match-to-sample working memory task [120]. Thus, at face value, it is difficult to derive a unitary cognitive function for either ER subtype, but it is important to keep in mind the wide variations in the behavioral paradigms, treatment regimens ranging from acute (minutes to hours), sub-acute (one-two days), chronic (days to weeks), and timing of treatments (before and during training or post-training thereby modulating acquisition, memory, or both) that can produce equally varying effects, which may indeed reflect the memory system activated. This position is supported by evidence of ER-specific effects on different types of social learning [117–119].
A special role for ERα in learning has been suggested in ER knockout (ERKO) models. Speed of acquisition in the spatial version of the swim task was attenuated by estradiol in wild-type mice but not in ERαKOs. ERα stimulation may thus mediate learning impairments [113, 121] while signaling through other estrogen binding proteins including ERβ may promote or facilitate learning, similar to mixed effects described for social learning [118]. Thus, while any or all receptors might be involved in cognition, it is possible that selectivity in ER signaling leads to the bidirectional effects of estrogens on learning per se.
If this receptor-mediated dissociation between enhancement and impairment holds true for our cognitive tasks, we would expect to find particularly robust place learning enhancements with ERβ-selective compounds and response learning impairments with ERα-selective compounds. Tests of this possibility were made in an extensive set of experiments using young adult female rats given different ER agonists. Separate groups of rats received one of five doses of PPT (ERα-selective agonist), DPN (ERβ-selective agonist), the more highly selective ERβ agonist, Br-ERB-041, that more closely matches the kinetics of PPT) and were tested on either place or response learning mazes [122]. Instead of observing distinct receptor-selective enhancements and impairments, all three agonists as well as oral genistein [123], a soy isoflavone known to bind preferentially to ERβ, enhanced place learning and impaired response learning, but did so with dose-response curves that were largely non-monotonic and specific for that compound. Interestingly, for each agonist, the optimal impairing and enhancing doses were similar across response and place tasks, respectively, suggesting that ER-specific events alone fail to account for the bidirectional effects of estrogens on learning.
3.2 The timing of treatments
Estrogens induce changes in neural structure and function through estrogen receptor-mediated processes that take seconds, minutes, hours, or days to accomplish depending on the endpoint measure. For example, modulation of ion currents in principle neurons, enhancements in neuronal excitability and synaptic plasticity, actin cytoskeleton restructuring, and activation of MAPKinase and CREB occur within seconds to minutes of estradiol application [124–126]. Within 30 minutes following estradiol exposure, increased dendritic spines and synaptic elements can be detected on CA1 hippocampal pyramidal cells [127], originally shown to peak 48 hours after two days of systemic estradiol treatment [128]. Paralleling these cellular and molecular changes, estrogens modulate learning and memory within minutes to hours of exposures. Various forms of social learning are rapidly modulated by estrogens but the direction of effects depends on the receptor subtype and timing [118, 119]. Both object and place recognition are improved after only minutes of estrogen exposure [129] through mechanisms that enhance acquisition, retention, or both. Estrogens given directly into the brain immediately after training, but not hours later, enhance recognition memory for objects and locations [130] suggesting that estrogens can modulate the neural mechanisms underlying memory formation that are proximal but not distal to the training experience. Estrogen-mediated enhancement of memory formation has also been demonstrated in males using a similar post-training design in the spatial version of the swim task [14], pointing to similar memory retention actions in males and females. In all, the findings highlight the role of cell signaling events in the absence of substantial gene transcription for cognitive enhancements with little known about estrogen-induced cognitive impairments.
We asked whether the hippocampus-dependent enhancement in place learning and striatum-dependent impairment in response learning would sort by the timing of exposures. Ovariectomized young adult rats prepared with bilateral guide cannulae aimed at either the hippocampus or striatum were given direct infusions of estradiol-sulfate (used for aqueous solubility) across two days (48, 24, 2 hrs), 2 hours, or 15 min before training on either place or response learning. The doses and timing were chosen to mimic brain levels presumed to occur in previous studies using systemic injections. For both place and response learning, a single 2-hr exposure to estradiol in hippocampus or striatum was sufficient to enhance place learning [131] and impair response learning [132], respectively, suggesting that long-term exposures of two days are not necessary to observe the opposing estradiol effects on learning.
Findings from maze training 15 minutes following infusions were quite surprising. No enhancement in place learning was seen when estradiol was administered into the hippocampus. In fact, place learning was actually impaired by this short pre-training exposure [131], contrasting the considerable evidence showing increased hippocampal plasticity within minutes of estrogen treatment [125, 130]. Moreover, infusions of estradiol-sulfate into the striatum just 15 minutes prior to response training produced no measureable impairments on response learning. These results were unexpected because estradiol produces very rapid, i.e. within minutes of exposure, decreases in cellular signaling including calcium influx and CREB activation [124, 125]. Moreover, and perhaps more surprising, we recently found that systemic injections of estradiol-benzoate (4.5 ug/kg) 15 minutes prior to training did impair response learning, demonstrating that the striatum can indeed respond to the impairing effects of estrogens within seconds or minutes. Perhaps the null effects from direct striatum injections of estradiol-sulfate resulted from insufficient amounts of activated, unsulfated estradiol; that is, it may take steroid sulfatase longer than 15 minutes to produce impairing concentrations of active estradiol. This explanation, however, fails to account for our non-canonical impairing effects of hippocampal estradiol, the underlying mechanisms of which are currently under study.
In sum, we found that relatively short exposures to estradiol produce both place learning enhancement and response learning impairments. Thus, it appears that the bidirectional effects of estrogens on place and response learning are not explained by receptor type or timing of exposure alone, but instead may result from an interaction between the two where rapid enhancements and impairments might dissociated by ER subtype or ER subtype efficacy might dissociate by short vs long exposures, e.g. similar to impairments of social learning following PPT, an ERα-selective agonist, when given just prior to training but not earlier [117–119].
3.3 Estrogenic regulation of cerebral metabolism
Estrogens are thought to control peripheral metabolism and protect against metabolic dysfunction through behavioral means, i.e. regulation of food intact and animal activity levels, as well as through cellular processes such as regulation of glucose metabolism, pancreatic β-cell function, and insulin sensitivity in the periphery [8, 133]. Low estrogen levels can lead to host of health problems associated with altered energy balance and cellular metabolism, including obesity, metabolic syndrome, immune dysfunction, inflammatory diseases, and liver disease. The health consequences of metabolic dysregulation are exacerbated by increasing age [133–135], creating a double indemnity for women as they grow older and transition to a postmenopausal state, by combining the cost of hormone loss with advanced age. The importance of estrogens and androgens in maintaining healthy metabolic state is seen in sex and gender differences in incidence and risk of obesity, diabetes, and other metabolism-related disorders [8, 133, 134].
Ovarian steroids are also believed to protect neural health through balance of energy metabolism and provision of energy substrates [134, 135]. Estrogen replacement in women attenuates menopause-related declines in regional cerebral blood flow and glucose metabolism and protects against neural dysregulation and hypometabolism associated with Alzheimer’s disease [136–140]. Moreover, women taking tamoxifen, a breast cancer treatment that functions as an estrogen receptor antagonist, have relatively lower regional glucose metabolism, supporting the notion that estrogens up-regulate cerebral metabolism [141]. However, there exists the chicken or the egg problem: it is unclear whether estrogens regulate metabolism, which then modulates cognition, or whether estrogens modulate cognition that, in turn, regulates the magnitude of cerebral blood flow and glucose metabolism. Sorting out the level of action of estrogens on brain metabolism may lead to lifestyle interventions for cognitive aging, particularly in women, that focus on maintaining healthy brain metabolism independent of hormone therapies.
The brain is a greedy organ, consuming considerably more energy on average (~20%) than its proportionate mass (2% of body) would indicate. During times of high neural activity, for example solving complex cognitive tasks, glucose provision might fall short of high energy demands because of the relatively low concentration of glucose in the extracellular fluid of brain relative to blood and facilitated transport required to move glucose into the brain. This lag was detected with in vivo microdialysis while male rats were tested on a hippocampus-sensitive working memory task. The magnitude of depletion in extracellular glucose concentrations in hippocampus corresponded to task difficulty [142, 143]. Systemic injections of glucose reversed the depletion and restored memory scores on the harder tasks, indicating that glucose provision across the blood brain barrier can facilitate cognition.
A role for energy substrates other than glucose in regulating learning and memory is also becoming well established. Recent studies using males demonstrate that sufficient levels of brain lactate may also critical for optimal learning and memory [63, 144–146], illuminating a new role for astrocytes as providers of metabolic substrates during cognition [147–149] (Figure 4; however, see [150] for alternative viewpoint stressing glucose utilization). For fuel, neurons can use glucose provided by the cerebral vasculature or lactate provided by astrocytes [63, 144, 145], particularly during times of high neural activity [151, 152]. Ketone bodies such as 3-β-hydroxybutyrate, provided by blood or astrocytes, are also effective substrates for brain metabolism, creating multiple reservoirs for brain energy needs that may, under some conditions such as insulin insensitivity, be more efficient for ATP production than is glucose [153].
FIGURE 4.
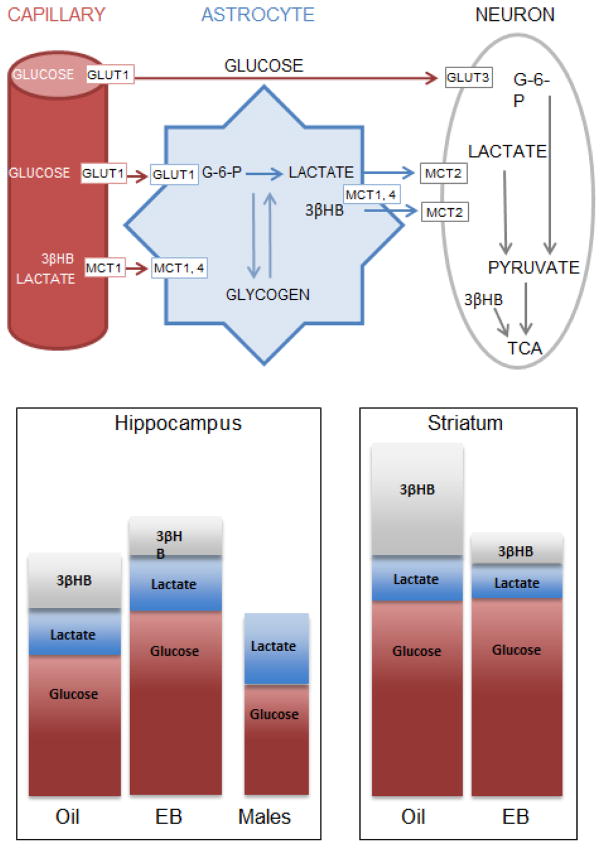
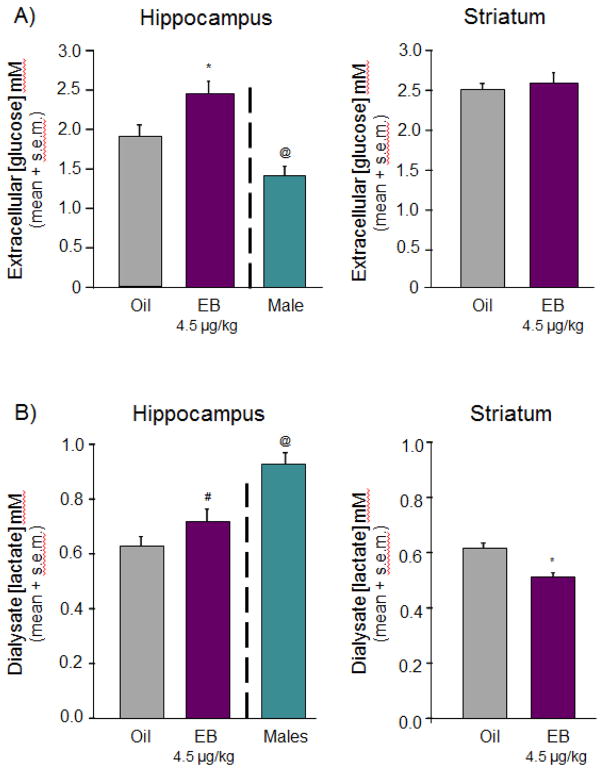
A) Graphic showing reservoirs of metabolic substrates in brain and provision routes based on the lactate-shuttle hypothesis. GlUT = glucose transporter; MCT = monocarboxylate transporter; 3βHB = 3-β hydroxybutyrate; TCA = tricarboxylic acid cycle; G-6-P = glucose-6-phosphate. Adapted from [144]. B) Schematic of data collected in ovariectomized females with and without 4.5 ug/kg estradiol benzoate and age-matched males. Bars depict relative brain concentrations of glucose and lactate measured from extracellular fluid dialysate and the ketone 3βHB from whole tissue homogenates.
Considering the astrocyte lactate shuttle model, it is not surprising that peripheral and central injections of glucose and lactate enhance memory in young adult male rats and mice [144, 146]. Moreover, brain lactate responses to training, measured with indwelling bioprobe technology that allows for second-by-second readouts of extracellular lactate during behavioral testing, reveal a task by brain structure double dissociation. Compared to control male rats given reward only, hippocampal lactate rises substantially during place but not response training, whereas striatal lactate rises selectively during response training [63]. The selective rise in extracellular lactate is likely a result of transport from the blood or from the astrocytes via training-induced hydrolysis of glycogen that can subsequently be converted to lactate or conversion of astrocytic glucose to lactate that is then shuttled into the extracellular space and used either for energy metabolism by neurons or for cell signaling [146–149]. Recently discovered g-protein coupled lactate receptors, GPR81, on vascular cells or neurons [154] are thought to regulate blood flow, angiogenesis, or other profiles of metabolic status of large ensembles of neurons [155], providing an indirect means for lactate to regulate brain metabolism and energy state. Whether providing energy substrates or signaling molecules, the astrocytes play key roles in regulation of cognition by controlling extracellular levels of metabolic substrates and may reflect the degree to which a brain region responds to or participates in cognitive training.
Keeping in mind the pervasive opportunity for metabolism to regulate cognition, it is possible that bidirectional regulation of metabolic substrates in the hippocampus and striatum by estrogens contribute to the opposing effects on cognitive strategy. Estrogens increase glucose transport across the blood brain barrier in cortex and hippocampus within four hours of exposure [156–158] perhaps through enhanced expression or membrane insertion of glucose transporters (GluT) [157–160]. Astrocyte processes in close contact with endothelial cells of the blood brain barrier control endothelial permeability [161]. In addition, astrocytes express membrane and cytosolic ERs that respond rapidly to estrogens, making them well positioned to mediate the effects of estrogens on brain metabolism [95, 162]. In female mouse models of Alzheimer’s disease, molecular profiles in hippocampus of ovariectomized and gonadally intact mice suggest that available brain energy substrates shift from lactate and ketone bodies in the absence of estradiol towards glucose in its presence [159]. Similar trends were observed across aging in wild-type mice, which were preceded by reductions in GluTs [160], and suggesting that proper glucose transport into the brain may be a precursor for neural health.
To determine whether regulation of energy availability is a viable candidate for our opposing effects or estrogens on learning, we measured glucose and lactate concentrations in hippocampal and striatal extracellular fluid of ovariectomized (21 days) young adult female rats treated with two days of systemic estradiol (4.5 μg/kg) or oil vehicle using microdialysis techniques and zero-net flux analysis (for descriptions of zero-net flux, see [163]). We also measured ketone and glycogen concentrations in tissue samples from hippocampus and striatum of uncannulated but similarly treated rats. Estradiol treatments regulated substrate availability in hippocampus and striatum but with a pattern specific to the substrate of interest, summarized graphically in Figure 4A. For instance, estradiol significantly increased ECF glucose in the hippocampus but not in striatum, increasing basal hippocampal concentrations above those of males (Figures 4B, 5). Basal levels of extracellular lactate, on the other hand, responded to estradiol treatment with a decrease in striatum and an increase in the hippocampus. One of the most robust effects was on striatal ketone availability where estradiol substantially decreased tissue content in striatum but not hippocampus; concentrations of 3βHB were significantly higher in the striatum of oil-treated controls than in any other tissue, suggesting differential regulation of metabolites across these two brain regions by hormone depletion and repletion that might explain the task- and site-specific improvements and impairments in learning.
FIGURE 5.
A) Glucose and B) lactate concentrations in extracellular fluid of hippocampus and striatum collected from ovariectomized female rats treated systemically with two days of either oil vehicle (grey bars) or estradiol benzoate (EB; purple bars). Data correspond to schematized results shown in Figure 4. Estradiol increased glucose in hippocampus but not striatum and increased lactate in hippocampus but decreased lactate in striatum. Hippocampal concentrations of glucose A) are substantially lower in males compared to females while hippocampal concentrations of lactate B) are significantly lower in females compared to males. # = p = 0.07; * = p < 0.05; @ = p < 0.05 males vs oil or EB.
Sex differences in extracellular hippocampal glucose and lactate concentrations have also been noted between gonadally intact males and ovariectomized females treated with or without estradiol (Figures. 4B, 5). Compared to both groups of female rats, i.e. those ovariectomized and replaced with estradiol or replaced with oil vehicle for two days, males have significantly lower ECF glucose concentrations. However, these lower levels of glucose in males are balanced by significantly higher levels of lactate (Figures 4B, 5). The sum of relative concentrations of lactate and glucose in males seems to match that in hormone-deprived females (Figure 4B), but is substantially lower than the sum from estradiol-treated females, suggesting that estradiol may elevate available energy resources in the hippocampus. Parallel comparisons from the striatum of males and females or from the hippocampus of estrogen-treated males have not yet been made.
Our observed estradiol-induced shift in the brain’s metabolic profile is somewhat different from that proposed by Diaz-Brinton and colleagues [135, 159, 160]. For example, treatments of five weeks of daily high doses of estradiol given to ovariectomized 3xTgAD female mice were hypothesized to upregulate glucose but downregulate lactate availability in hippocampus, partially restoring levels to those of sham-operated gonadally intact mice. Differences in species, treatment and methods of measures may have contributed to discrepancies in findings. First, we gave two days of moderate doses of estradiol compared to the five weeks of high doses. Moreover, we collected ECF and tissue samples of substrates, whereas substrate availability in mice was largely inferred from expression patterns of glucose and monocarboxylate transporters and metabolic enzymes using semiquantitative western blot techniques [159, 160].
Because extracellular measures reflect the dynamic between provision and consumption, it is possible that altered tissue/ECF concentrations of substrates result from contributions from changes in cellular activity, energy consumption, and energy provision, the calculus of which is unknown and hard to untangle. In any event, it is important to note that estradiol had no effect on circulating levels of glucose, lactate, or ketones and thus most likely regulates substrate availability or energy consumption through direct actions in the hippocampus and striatum. In the hippocampus, one possibility is that estradiol alters glucose transport across the blood brain barrier by increasing number of the 55 kD glucose transporters, GluT155, on the vascular endothelial cells [159, 160]. It is also possible, and not mutually exclusive, that glucose transport into astrocytes through the lower molecular weight GluT145, for storage as glycogen or use by astrocytes for their own energy needs, is decreased by estradiol signaling through astrocytic ERs, leading to higher extracellular glucose concentrations overall. Estradiol might also facilitate production of hippocampal lactate by astrocytes through augmentation of glycogen breakdown or lactate dehydrogenase 5 expression or activity despite reports suggesting the converse [159, 160]. In a similar vein, estrogens may impair striatum-sensitive learning by decreasing availability or increased consumption of so-called alternative substrates such as ketones and lactate via astrocytic and neuronal metabolism. Whether and how estrogen-induced changes in hippocampal and striatal energy substrates translate to cognition is unknown, but initial tests of training-related alterations in glycogen, glucose, and lactate during training are underway.
As discussed above, astrocytes are poised to mediate the opposing effects of estrogens on learning through regulation of metabolic resources, adding a new dimension to investigations of hormonal, sex, and age-related control over neural mechanisms of learning and memory. Indeed, cognition is undoubtedly an emergent property of the concerted efforts of many general and specific processes that include not only neurons and astrocytes but also microglia and endothelial cells and a portfolio of mechanisms requiring metabolism, intracellular signaling and intercellular signaling. Exciting new findings suggest that astrocytes increase estradiol synthesis through cytokine-dependent upregulation of aromatase and release cytokines, as do microglia, all of which can alter metabolism and neural plasticity essential for learning and memory [164, 165 THIS ISSUE]. As such, glia and other non-neuronal tissues now have starring roles in the growing story of endocrine control of brain function and dysfunction and will likely take center stage for future studies on sex as a biological variable in brain and cognitive health and disease.
4. Closing remarks
Studies of sex as a biological variable, together with studies of estrogen effects on non-reproductive functions on brain and behavior, have undergone an exciting transition in the past decade or so. The field has moved well beyond occasional examinations of male vs. female behaviors and demonstrations of hormone effects on behavior to development of rich coherent frameworks that are becoming more and more mechanistic [166]. Key variables that interact with sex, gender, and estrogen effects on cognition include cognitive attribute and associated brain region, treatment dose and timing, age, and receptor signaling pathway to produce varying effects on learning and memory [167] that can be unified through a multiple memory systems framework. The findings reported here highlighted contributions of the hippocampus and striatum as tests for a memory systems framework that can be used for future explorations to expand to other task attributes that engage different brain areas. This will allow us to gain a broader view of hormone regulation on nervous system function. The field has made great strides to probe mechanisms for the enhancing effects of estrogens, but our understanding of the cell and molecular biology related to learning impairments with respect to timing, receptor systems and downstream signaling is lacking. Regulation of brain site-specific metabolism as a mechanism of estrogenic modulation of learning and memory is a likely prospect and reflects an emerging field of brain bioenergetics and cognition.
Findings of differences by task and brain area open opportunities to inform our understanding of sex as a biological variable for the control over cognition and of neural mechanisms of learning and memory regardless of sex. Moreover, this multiple memory systems approach to understanding estrogenic regulation of cognition has applications to human health and gender-specific medicine beyond elucidating the consequences of menopause by guiding development of medications that not only target a specific organ, e.g. breast or bone vs brain, but also target a specific brain region.
Highlights.
Different memory systems are used to solve different types of cognitive tasks
In females, estrogens enhance and impair learning depending on the memory system
Hormone effects on learning strategy are greater than sex differences in strategy
Opposing effects of estrogens fail to dissociate by estrogen receptor type or timing
A role for cellular metabolism and astrocytes in these opposing effects is discussed
Acknowledgments
We would like to thank Colin Saldanha, Terry Davidson, and Bernadette Storey-Laubach for organizing the American University Symposium on Sex Differences: From Neuroscience to the Clinic and Beyond (April 20–21, 2017) and for the invitation to participate in the conference. This work was supported by National Institutes of Health [P50 AT006268-05; P30 AG034464 through the Center for Aging and Policy Studies] and National Science Foundation [IOS 1318490 and IOB 0520876].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, McCarthy MM, Sohrabji F, Schiebinger L, Wetherington CL, Makris S. Considering sex as a biological variable in preclinical research. FASEB J. 2017;31:29–34. doi: 10.1096/fj.201600781R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Report on State of the Science of Endocrine Disrupting Chemicals. 2012 http://www.who.int/ceh/publications/endocrine/en/
- 3.Beatty WW. Sexual differentiation. Springer US; 1992. Gonadal hormones and sex differences in nonreproductive behaviors; pp. 85–128. [Google Scholar]
- 4.McEwen BS, Milner TA. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. 2017;95:24–39. doi: 10.1002/jnr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci U S A. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vesudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Cornil CA, Ball GF, Balthazart J. The dual action of estrogen hypothesis. Trends Neurosci. 2015;38:408–416. doi: 10.1016/j.tins.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- 10.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 11.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laredo SA, Villalon Landeros R, Trainor BC. Rapid effects of estrogens on behavior: Environmental modulation and molecular mechanisms. Front Neuroendocrinol. 2014;35:447–458. doi: 10.1016/j.yfrne.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bangasser DA, Eck SR, Telenson AM, Salvatore M. Sex differences in stress regulation of arousal and cognition. Physiol Behav. doi: 10.1016/j.physbeh.2017.09.025. In press. THIS ISSUE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- 15.Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korol DL, Pisani SL. Estrogens and cognition: Friends or Foes? An evaluation of the opposing effects of estrogens on learning and memory. Horm Behav. 2015;74:105–115. doi: 10.1016/j.yhbeh.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koss WA, Frick KM. Sex differences in hippocampal function. J Neurosci Res. 2017;95:539–562. doi: 10.1002/jnr.23864. [DOI] [PubMed] [Google Scholar]
- 18.Scharfman HE, MacLusky NJ. Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol Dis. 2014;72:180–192. doi: 10.1016/j.nbd.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharmacol Rev. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmoud R, Wainwright SR, Galea LA. Sex hormones and adult hippocampal neurogenesis: regulation, implications, and potential mechanisms. Front Neuroendocrinol. 2016;41:129–152. doi: 10.1016/j.yfrne.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Sheppard PA, Koss WA, Frick KM, Choleris E. Rapid actions of estrogens and their receptors on memory acquisition and consolidation in females. J Neuroendocrinol. 2017 doi: 10.1111/jne.12485. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Launer LJ, Andersen K, Dewey M, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JRM, Dartigues JF, Kragh-Sorensen P, Lobo A. Rates and risk factors for dementia and Alzheimer’s disease results from EURODEM pooled analyses. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- 24.Sherwin BB. Estrogen and memory in women: how can we reconcile the findings? Horm Behav. 2005;47:371–375. doi: 10.1016/j.yhbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, McCleary CA, Klein RA, Hake AM, Farlow MR. Estrogen for Alzheimer’s disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000;54:295–295. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- 26.Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA, J Am Med Assoc. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- 27.Merlo S, Spampinato SF, Sortino MA. Estrogen and Alzheimer’s disease: still an attractive topic despite disappointment from early clinical results. Eur J Pharmacol. 2017 doi: 10.1016/j.ejphar.2017.05.059. in press. [DOI] [PubMed] [Google Scholar]
- 28.Frick KM, Tuscher JJ, Koss WA, Kim J, Taxier LR. Estrogenic regulation of memory consolidation: A look beyond the hippocampus, ovaries, and females. Physiol Behav. 2017 doi: 10.1016/j.physbeh.2017.07.028. in press. THIS ISSUE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persson K, Bohbot VD, Bogdanovic N, Selbæk G, Brækhus A, Engedal K. Finding of increased caudate nucleus in patients with Alzheimer’s disease. Acta Neurol Scand. 2017 doi: 10.1111/ane.12800. in press. [DOI] [PubMed] [Google Scholar]
- 30.Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cahill L. An issue whose time has come. J Neurosci Res. 2017;95:12–13. doi: 10.1002/jnr.23972. [DOI] [PubMed] [Google Scholar]
- 34.Prager EM. Addressing sex as a biological variable. J Neurosci Res. 2017;95:11–11. doi: 10.1002/jnr.23979. [DOI] [PubMed] [Google Scholar]
- 35.Klein SL, Schiebinger L, Stefanick ML, Cahill L, Danska J, De Vries GJ, Kibbe MR, McCarthy MM, Mogil JS, Woodruff TK, Zucker I. Opinion: sex inclusion in basic research drives discovery. Proc Nat Acad Sci. 2015;112:5257–5258. doi: 10.1073/pnas.1502843112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen SE, Ahmed I, Cahill L. Sex and menstrual cycle phase at encoding influence emotional memory for gist and detail. Neurobiol Learn Mem. 2013;106:56–65. doi: 10.1016/j.nlm.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hampson E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain Cogn. 1990a;14:26–43. doi: 10.1016/0278-2626(90)90058-v. [DOI] [PubMed] [Google Scholar]
- 38.Kimura D. Sex differences in the brain. Sci Amer. 1992;267:118–125. doi: 10.1038/scientificamerican0992-118. [DOI] [PubMed] [Google Scholar]
- 39.Hampson E, Kimura D. Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behav Neurosci. 1988;102:456–459. doi: 10.1037//0735-7044.102.3.456. [DOI] [PubMed] [Google Scholar]
- 40.Galea LA, Kimura D. Sex differences in route-learning. Pers Individ Dif. 1993;14:53–65. [Google Scholar]
- 41.Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 1990b;15:97–111. doi: 10.1016/0306-4530(90)90018-5. [DOI] [PubMed] [Google Scholar]
- 42.Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- 43.Wang VC, Sable HJK, Ju YH, Allred CD, Helferich HG, Korol DL, Schantz SL. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behav Neurosci. 2008;122:794–804. doi: 10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang VC, Neese SL, Korol DL, Schantz SL. Chronic estradiol replacement impairs performance on an operant delayed spatial alternation task in young, middle-aged, and old rats. Horm Behav. 2009;56:382–390. doi: 10.1016/j.yhbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- 46.Barha CK, Lieblich SE, Chow C, Galea LA. Multiparity-induced enhancement of hippocampal neurogenesis and spatial memory depends on ovarian hormone status in middle age. Neurobiol Aging. 2015;36:2391–2405. doi: 10.1016/j.neurobiolaging.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Wang VCc, Neese SL, Korol DL, Schantz SL. Estradiol impairs response inhibition in young and middle-aged, but not old rats. Neurotoxicol Teratol. 2011;33:405–414. doi: 10.1016/j.ntt.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holmes MM, Wide JK, Galea LA. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- 49.Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman J, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: Influence of estrous cycle. Pharmacol Biochem Behav. 2004;78:569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 50.Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- 53.McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- 54.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 55.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 56.Chang Q, Gold PE. Intra-hippocampal lidocaine injections impair acquisition of a place task and facilitate acquisition of a response task in rats. Behav Brain Res. 2003;144:19–24. doi: 10.1016/s0166-4328(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 57.Gold PE. Coordination of multiple memory systems. Neurobiol Learn Mem. 2004;82:230–242. doi: 10.1016/j.nlm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Gold PE, Newman LA, Scavuzzo CJ, Korol DL. Modulation of multiple memory systems: from neurotransmitters to metabolic substrates. Hippocampus. 2013;23:1053–1065. doi: 10.1002/hipo.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mizumori SJ, Jo YS. Homeostatic regulation of memory systems and adaptive decisions. Hippocampus. 2013;23:1103–1124. doi: 10.1002/hipo.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci. 2003;23:5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- 62.Brightwell JJ, Smith CA, Neve RL, Colombo PJ. Transfection of mutant CREB in the striatum, but not the hippocampus, impairs long-term memory for response learning. Neurobiol Learn Mem. 2008;89:27–35. doi: 10.1016/j.nlm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Newman LA, Scavuzzo CS, Gold PE, Korol DL. Training-induced elevations in extracellular lactate in hippocampus and striatum: dissociations by cognitive strategy and type of reward. Neurobiol Learn Mem. 2017;137:142–153. doi: 10.1016/j.nlm.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colombo PJ, Brightwell JJ, Countryman RA. Cognitive strategy-specific increases in phosphorylated cAMP response element-binding protein and c-Fos in the hippocampus and dorsal striatum. J Neurosci. 2003;23:3547–3554. doi: 10.1523/JNEUROSCI.23-08-03547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gill KM, Bernstein IL, Mizumori SJ. Immediate early gene activation in hippocampus and dorsal striatum: effects of explicit place and response training. Neurobiol Learn Mem. 2007;87:583–596. doi: 10.1016/j.nlm.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 66.McDonald RJ, Devan BD, Hong NS. Multiple memory systems: the power of interactions. Neurobiol Learn Mem. 2004;82:333–346. doi: 10.1016/j.nlm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Packard MG, Goodman J. Factors that influence the relative use of multiple memory systems. Hippocampus. 2013;23:1044–1052. doi: 10.1002/hipo.22178. [DOI] [PubMed] [Google Scholar]
- 68.White NM, Packard MG, McDonald RJ. Dissociation of memory systems: The story unfolds. Behav Neurosci. 2013;127:813–834. doi: 10.1037/a0034859. [DOI] [PubMed] [Google Scholar]
- 69.Mizumori SJ. Self regulation of memory processing centers of the brain. In: Ragozzino ME, Jackson P, Chiba A, Berman R, editors. The Neurobiological Basis of Memory: A System, Attribute, and Process Analysis. Springer; New York: 2016. pp. 199–225. [Google Scholar]
- 70.Kathirvelu B, Colombo PJ. Effects of lentivirus- mediated CREB expression in the dorsolateral striatum: Memory enhancement and evidence for competitive and cooperative interactions with the hippocampus. Hippocampus. 2013;23:1066–1074. doi: 10.1002/hipo.22188. [DOI] [PubMed] [Google Scholar]
- 71.Tolman EC, Ritchie BF, Kalish D. Studies in spatial learning; place learning versus response learning. J Exp Psychol. 1946;36:221–229. doi: 10.1037/h0060262. [DOI] [PubMed] [Google Scholar]
- 72.Restle F. Discrimination of cues in mazes: A resolution of the “place-vs.-response” question. Psychol Rev. 1957;64:217–228. doi: 10.1037/h0040678. [DOI] [PubMed] [Google Scholar]
- 73.Potegal M. The caudate nucleus egocentric localization system. Acta Neurobiol Exp (Wars) 1972;32:479–494. [PubMed] [Google Scholar]
- 74.Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo JM. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 75.McIntyre CK, Marriott LK, Gold PE. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol Learn Mem. 2003;79:177–183. doi: 10.1016/s1074-7427(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 76.Astur RS, Purton AJ, Zaniewski MJ, Cimadevilla J, Markus EJ. Human sex differences in solving a virtual navigation problem. Behav Brain Res. 2016;308:236–243. doi: 10.1016/j.bbr.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 77.Quinlan MG, Hussain D, Brake WG. Use of cognitive strategies in rats: the role of estradiol and its interaction with dopamine. Horm Behav. 2008;53:185–191. doi: 10.1016/j.yhbeh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 78.McElroy MW, Korol DL. Intrahippocampal muscimol shifts learning strategy in gonadally intact young adult female rats. Learn Mem. 2005;12:150–158. doi: 10.1101/lm.86205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pleil KE, Williams CL. The development and stability of estrogen-modulated spatial navigation strategies in female rats. Horm Behav. 2010;57:360–367. doi: 10.1016/j.yhbeh.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- 81.Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Daniel JM, Lee CD. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol Learn Mem. 2004;82:142–149. doi: 10.1016/j.nlm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 83.Hawley WR, Grissom EM, Barratt HE, Conrad TS, Dohanich GP. The effects of biological sex and gonadal hormones on learning strategy in adult rats. Physiol Behav. 2012;105:1014–1020. doi: 10.1016/j.physbeh.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 84.Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spritzer MD, Fox EC, Larsen GD, Batson CG, Wagner BA, Maher J. Testosterone influences spatial strategy preferences among adult male rats. Horm Behav. 2013;63:800–812. doi: 10.1016/j.yhbeh.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dember WN, Richman CL. Spontaneous alternation behavior. Springer-Verlag; New York: 1989. [Google Scholar]
- 87.Sava S, Markus EJ. Intramaze cue utilization in the water maze: effects of sex and estrous cycle in rats. Horm Behav. 2005;48:23–33. doi: 10.1016/j.yhbeh.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 88.Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiol Learn Mem. 2006;86:336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 90.Korol DL. Estrogens have their ups and downs: A multiple memory systems approach to the bidirectional effects of estrogens on learning strategy. In: Frick K, Ball G, Balthazart J, Nelson R, editors. Estrogens and Memory: Basic Research and Clinical Implications. Oxford University Press; 2017. Oxfords’ Series in Behavioral Neuroendocrinology. under review. [Google Scholar]
- 91.Pych JC, Kim M, Gold PE. Effects of injections of glucose into the dorsal striatum on learning of place and response mazes. Behav Brain Res. 2006;167:373–378. doi: 10.1016/j.bbr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 92.O’Reilly RC, Norman KA. Hippocampal and neocortical contributions to memory: Advances in the complementary learning systems framework. Trends Cog Sci. 2002;6:505–510. doi: 10.1016/s1364-6613(02)02005-3. [DOI] [PubMed] [Google Scholar]
- 93.Yeshenko O, Guazzelli A, Mizumori SJY. Context-dependent reorganization of spatial and movement representations by simultaneously recorded hippocampal and striatal neurons during performance of allocentric and egocentric tasks. Behav Neurosci. 2004;118:751–769. doi: 10.1037/0735-7044.118.4.751. [DOI] [PubMed] [Google Scholar]
- 94.Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Toran-Allerand CD. Estrogen and the Brain: Beyond ER- α, ER- β, and 17β-Estradiol. Ann N Y Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- 98.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 99.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 100.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 101.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz E. RA transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 102.Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology. 2012;153:5373–5383. doi: 10.1210/en.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav. 2015;74:125–138. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 105.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 106.Gold PE, Korol DL. Hormones and Memory. In: Koob GF, Le Moal M, Thompson RF, Dantzer R, editors. Encyclopedia of Behavioral Neuroscience. Vol. 2. Academic Press; Oxford: 2010. pp. 57–64. [Google Scholar]
- 107.Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011;42:236–241. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor- α and- β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 109.Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence of the colocalization of estrogen receptor-β mRNA and estrogen receptor-α immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- 110.Shughrue PJ, Merchenthaler I. Estrogen is more than just a “sex hormone”: novel sites for estrogen action in the hippocampus and cerebral cortex. Front Neuroendocrinol. 2000;21:95–101. doi: 10.1006/frne.1999.0190. [DOI] [PubMed] [Google Scholar]
- 111.Bodo C, Rissman EF. New roles for estrogen receptor β in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Fugger HN, Foster TC, Gustafsson J-A, Rissman EF. Novel effects of estradiol and estrogen receptor α and β on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- 113.Fugger HN, Cunningham SG, Rissman EF, Foster TC. Sex differences in the activational effect of ERα on spatial learning. Horm Behav. 1998;34:163–170. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- 114.Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clipperton AE, Spinato JM, Chernets C, Pfaff DW, Choleris E. Differential effects of estrogen receptor alpha and beta specific agonists on social learning of food preferences in female mice. Neuropsychopharmacology. 2008;33:2362. doi: 10.1038/sj.npp.1301625. [DOI] [PubMed] [Google Scholar]
- 118.Ervin KSJ, Lymer JM, Matta R, Clipperton-Allen AE, Kavaliers M, Choleris E. Estrogen involvement in social behavior in rodents: rapid and long-term actions. Horm Behav. 2015;74:53–76. doi: 10.1016/j.yhbeh.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 119.Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- 120.Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor α. Brain Res. 1999;835:80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- 122.Pisani SL, Neese SL, Katzenellenbogen JA, Schantz SL, Korol DL. Estrogen receptor-selective agonists modulate learning in female rats in a dose-and task-specific manner. Endocrinology. 2016;157:292–303. doi: 10.1210/en.2015-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pisani SL, Neese SL, Doerge DR, Helferich WG, Schantz SL, Korol DL. Acute genistein treatment mimics the effects of estradiol by enhancing place learning and impairing response learning in young adult female rats. Hormo Behav. 2012;62:491–499. doi: 10.1016/j.yhbeh.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boulware MI, Mermelstein PG. Membrane estrogen receptors activate metabotropic glutamate receptors to influence nervous system physiology. Steroids. 2009;74:608–613. doi: 10.1016/j.steroids.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 128.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 130.Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Korol DL, Scavuzzo CJ, Collier RL. Intrahippocampal infusions of estradiol can enhance or impair place learning depending on timing of treatment. Soc Neurosci Abst. 2010;36:296.12. [Google Scholar]
- 132.Zurkovsky L, Serio SJ, Korol DL. Intra-striatal estradiol in female rats impairs response learning within two hours of treatment. Horm Behav. 2011;60:470–477. doi: 10.1016/j.yhbeh.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 133.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Diff. 2015;6:14. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35:8–30. doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34:171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- 137.Eberling JL, Reed BR, Coleman JE, Jagust WJ. Effect of estrogen on cerebral glucose metabolism in postmenopausal women. Neurology. 2000;55:875–877. doi: 10.1212/wnl.55.6.875. [DOI] [PubMed] [Google Scholar]
- 138.Maki PM, Resnick SM. Effects of estrogen on patterns of brain activity at rest and during cognitive activity: A review of neuroimaging studies. NeuroImage. 2001;14:789–801. doi: 10.1006/nimg.2001.0887. [DOI] [PubMed] [Google Scholar]
- 139.Rasgon NL, Silverman D, Siddarth P, Miller K, Ercoli LM, Elman S, Lavretsky H, Huang S-C, Phelps ME, Small GW. Estrogen use and brain metabolic change in postmenopausal women. Neurobiol Aging. 2005;26:229–235. doi: 10.1016/j.neurobiolaging.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 140.Silverman DHS, Geist CL, Kenna HA, Williams K, Wroolie T, Powers B, Brooks J, Rasgon NL. Differences in regional brain metabolism associated with specific formulations of hormone therapy in postmenopausal women at risk for AD. Psychoneuroendocrinology. 2011;36:502–513. doi: 10.1016/j.psyneuen.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxifen-associated effects on brain structure and function. NeuroImage. 2004;21:364–371. doi: 10.1016/j.neuroimage.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 142.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Nat Acad Sci. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McNay EC, Gold PE. Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behav Cog Neurosci Rev. 2002;1:264–280. doi: 10.1177/1534582302238337. [DOI] [PubMed] [Google Scholar]
- 144.Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PloS ONE. 2011;6:e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gao V, Suzuki A, Magistretti PJ, Lengacher S, Pollonini G, Steinman MQ, Alberini CM. Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc Nat Acad Sci. 2016;113:8526–8531. doi: 10.1073/pnas.1605063113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- 148.Magistretti PJ. Neuron–glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 149.Steinman MQ, Gao V, Alberini CM. The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front Integr Neurosci. 2016;10:10. doi: 10.3389/fnint.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dienel GA. Lack of appropriate stoichiometry: Strong evidence against an energetically important astrocyte–neuron lactate shuttle in brain. J Neurosci Res. 2017 doi: 10.1002/jnr.24015. [DOI] [PubMed] [Google Scholar]
- 151.Brown AM, Tekkök SB, Ransom BR. Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem Int. 2004;45:529–536. doi: 10.1016/j.neuint.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 152.Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 153.Achanta LB, Rae CD. β-Hydroxybutyrate in the brain: one molecule, multiple mechanisms. Neurochem Res. 2017;42:35–49. doi: 10.1007/s11064-016-2099-2. [DOI] [PubMed] [Google Scholar]
- 154.Bozzo L, Puyal J, Chatton JY. Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PloS one. 2013;8:e71721. doi: 10.1371/journal.pone.0071721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bergersen LH, Gjedde A. Is lactate a volume transmitter of metabolic states of the brain? Front Neuroenerg. 2012;4:5. doi: 10.3389/fnene.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bishop J, Simpkins JW. Estradiol enhances brain glucose uptake in ovariectomized rats. Brain Res Bull. 1995;36:315–320. doi: 10.1016/0361-9230(94)00208-i. [DOI] [PubMed] [Google Scholar]
- 157.Cheng CM, Cohen M, Wang JIE, Bondy CA. Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex. FASEB J. 2001;15:907–915. doi: 10.1096/fj.00-0398com. [DOI] [PubMed] [Google Scholar]
- 158.Shi J, Simpkins JW. 17 beta-Estradiol modulation of glucose transporter 1 expression in blood-brain barrier. Am J Physiol: Endocrinol Metab. 1997;272:E1016–E1022. doi: 10.1152/ajpendo.1997.272.6.E1016. [DOI] [PubMed] [Google Scholar]
- 159.Ding F, Yao J, Zhao L, Mao Z, Chen S, Brinton RD. Ovariectomy induces a shift in fuel availability and metabolism in the hippocampus of the female transgenic model of familial Alzheimer’s. PloS one. 2013a;8:e59825. doi: 10.1371/journal.pone.0059825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ding F, Yao J, Rettberg JR, Chen S, Brinton RD. Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and alzheimer’s mouse brain: implication for bioenergetics intervention. PLoS ONE. 2013b;8:e79977. doi: 10.1371/journal.pone.0079977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 162.Fuente-Martin E, Garcia-Caceres C, Morselli E, Clegg DJ, Chowen JA, Finan B, Brinton RD, Tschöp MH. Estrogen, astrocytes and the neuroendocrine control of metabolism. Rev Endocr Metab Disord. 2013;14:331–338. doi: 10.1007/s11154-013-9263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McNay EC, Gold PE. Extracellular glucose concentrations in the rat hippocampus measured by zero-net-flux. J Neurochem. 1999;72:785–790. doi: 10.1046/j.1471-4159.1999.720785.x. [DOI] [PubMed] [Google Scholar]
- 164.Pederson AL, Nelson LH, Saldanha CJ. Centrally synthesized estradiol is a potent anti-inflammatory in the injured zebra finch brain. Endocrinol. 2016;157:2041–2051. doi: 10.1210/en.2015-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pederson AL, Brownrout JL, Saldanha CJ. Neuroinflammation and neurosteroidogenesis: Reciprocal modulation during injury to the adult zebra finch brain. Physiol Behav. doi: 10.1016/j.physbeh.2017.10.013. in press. THIS ISSUE. [DOI] [PubMed] [Google Scholar]
- 166.Shansky RM, Woolley CS. Considering sex as a biological variable will be valuable for neuroscience research. J Neurosci. 2016;36:11817–11822. doi: 10.1523/JNEUROSCI.1390-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Galea LA, Frick KM, Hampson E, Sohrabji F, Choleris E. Why estrogens matter for behavior and brain health. Neurosci Biobehav Rev. 2017;76:363–379. doi: 10.1016/j.neubiorev.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]