Abstract
Typically developing infants rapidly acquire a sophisticated array of social skills within the first year of life. These social skills are largely learned within the context of day-to-day interactions with caregivers. While social neuroscience has made great gains in our knowledge of the underlying neural circuitry of social cognition and behavior, much of this work has focused on experiments that sacrifice ecological validity for experimental control. Functional near-infrared spectroscopy (fNIRS) is a promising methodology for measuring brain activity in the context of naturalistic social interactions. Here, we review what we have learned from fNIRS studies that have used traditional experimental stimuli to study social development during infancy. We then discuss recent infant fNIRS studies that have utilized more naturalistic social stimuli, followed by a discussion of applications of this methodology to the study of atypical social development, with a focus on infants at risk for autism spectrum disorder. We end with recommendations for applying fNIRS to studies of typically developing and at-risk infants in naturalistic social situations.
Keywords: fNIRS, infancy, social development, social interaction
1. Introduction
1.1. General outline
Humans are inherently social creatures. Within the first year of life, typically developing infants develop an amazingly sophisticated repertoire of social behaviors, which provide an avenue for learning across a wide array of developmental domains. Social neuroscience has made great gains in our knowledge of the underlying neural circuitry of social cognition and behavior; however, much of this work, often due to methodological challenges, has focused on older children and adults, with experiments that are far removed from the complex social situations that occur in the natural world. A relatively new neuroimaging technique, functional near-infrared spectroscopy (fNIRS), has been touted as a method that is both well suited to measurement of infant brain responses (Lloyd-Fox et al., 2010), and a promising methodology for measuring brain functioning within more naturalistic contexts (Liu & Pelowski, 2014; Nishiyori, 2016).
In this review, we focus first on what we have learned from fNIRS studies that have used traditional experimental stimuli to study social development during infancy. We then discuss recent infant fNIRS studies that have utilized more naturalistic social stimuli, followed by a discussion of applications of this methodology to the study of atypical social development, with a focus on autism spectrum disorder (ASD). We end with recommendations for applying fNIRS to studies of infants in naturalistic social situations and the potential of this technology in the study of infants at risk for atypical social development.
1.2. fNIRS Overview
Previous reviews have provided comprehensive information on fNIRS methodology and its use with infants (Aslin et al., 2015; Lloyd-Fox et al., 2010; Vanderwert & Nelson, 2014). As such, we provide a relatively brief overview of the technology, particularly as it relates to advantages for the use of fNIRS with behaving infants, and refer interested readers to these previous review articles for more in depth explanations of how fNIRS works and its unique strengths and limitations.
fNIRS is a non-invasive technology that uses near-infrared light to indirectly assess neural activity by measuring local changes in blood oxygenation levels. Emitters and detectors of near-infrared light are secured to a participant’s scalp, forming channels that index activity occurring in the cortex below the channel. Similar to functional magnetic resonance imaging (fMRI), fNIRS measures the hemodynamic response, which is a change in blood oxygenation levels driven by blood flow changes in response to neural metabolic demand (Lloyd-Fox et al., 2010). Uniquely, fNIRS obtains relative measures of concentrations of oxy-hemoglobin (oxy-Hb), deoxy-hemoglobin (deoxy-Hb), and total hemoglobin (total-Hb). Typically, increases in neural activity are accompanied by increases in oxy-Hb and total-Hb concentration, and slight decreases in deoxy-Hb concentration.
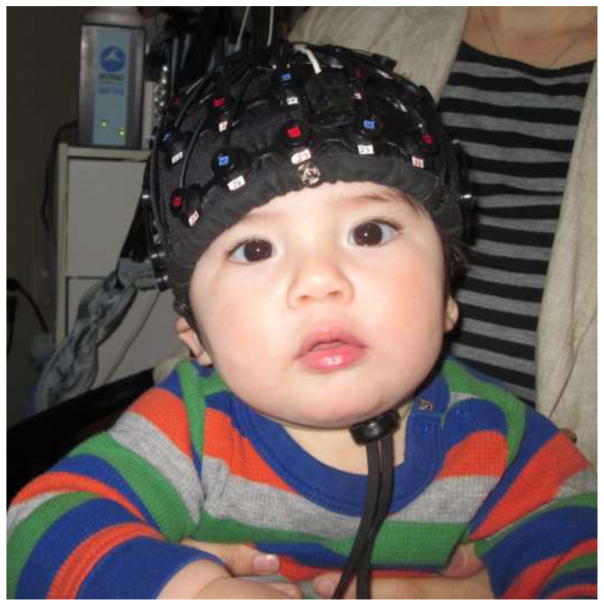
While fNIRS has been used across a wide range of ages, it has several advantages that are most relevant for research with infants. Most significantly for the current review, it allows for the measurement of localized brain responses while infants are awake and behaving. In comparison to other brain imaging technologies that require infants to be still (in particular, fMRI), fNIRS has a relatively robust tolerance for infant motion due to the head-mounted emitters and detectors (Lloyd-Fox et al., 2010). Additionally, depending on the specific headgear and number of channels, the comfort level and ease of setup are relatively infant friendly (Lloyd-Fox et al., 2010). In a typical fNIRS study, infants are seated comfortably on their parent’s lap and the fNIRS headgear is secured quickly to the infant’s head with minimal distress (see Figure 1 for a depiction of infant fNIRS headgear placement). fNIRS is silent, allowing for the use of both audio and visual stimuli. Infants are also an ideal population for fNIRS, as the relative thinness of their skulls (Beauchamp et al., 2011) allows for good propagation of light into the brain, and frequent lack of hair avoids poor signals due to impaired optical coupling between probe and scalp. One additional strength of fNIRS as applied to social neuroscience is the timescale of the measured hemodynamic response, on the order of seconds, is particularly well suited to social interactions and allows for experimental designs with audio, video, and/or moving stimuli.
Figure 1.
Infant with fNIRS headgear
Along with these strengths come some weaknesses as compared to other functional neuroimaging technologies. While fNIRS has better spatial resolution than electroencephalography (EEG), it lacks the exquisite temporal precision of EEG. Likewise, it does not as readily map onto specific brain structures as fMRI. Rather, localization of activity is typically presumed based on external fiducial markers and the position of the channels on the scalp. fNIRS is also only sensitive to cortical activity that is relatively close to the surface of brain, so the investigation of deeper cortical areas (e.g., fusiform gyrus) or subcortical structures is not currently possible with fNIRS. To date, fNIRS studies have typically focused on a subset of cortical regions, most often temporal and/or frontal areas, related to a particular experimental hypothesis, rather than full head coverage, although technical developments are making fuller coverage possible (Homae et al., 2010). Despite these limitations, fNIRS studies have contributed important complementary knowledge to our understanding of social brain development during infancy, adding important spatial information to electrophysiological studies and allowing for a more flexible and diverse array of social stimuli than infant fMRI studies (for a review of infant social brain development across methods see Grossmann, 2015).
2. Foundational fNIRS studies of infant brain responses to pre-recorded social stimuli
One of the fundamental questions that has been asked by the infant fNIRS literature is what regions of the developing brain preferentially respond to social vs. non-social (or less social) stimuli. During real-life social interactions, infants have to simultaneously process information across visual, auditory, and tactile domains, including such varied elements as biological motion, eye gaze, facial expressions, vocalizations, speech prosody, and social touch. Studies that have taken a traditional, tightly controlled experimental approach to the study of the infant social brain provide a foundation of knowledge that can be used to inform studies that sacrifice some level of experimental control for the increased ecological validity of more naturalistic and interactive designs.
In this section, we discuss the methodology and major findings of fNIRS experiments that examined typically developing infant neural responses to pre-recorded social stimuli. We have included both seminal and more recent studies that use a traditional block design to examine neural responses to social stimuli that are particularly relevant for social interaction (e.g., joint attention, infant-directed speech). In this way, we highlight methods, findings, and regions of interest that can inform the design and interpretation of studies using more naturalistic stimuli. We focus on studies in the visual and auditory domains given the dearth of research in other modalities (although see Kida & Shinohara, 2013). Given the limitations of fNIRS with regard to scalp coverage, particularly with less recent studies, researchers have commonly examined regions of interest based upon the adult fMRI literature; most commonly bilateral frontal-temporal and prefrontal cortices. Detailed information about the included studies is presented in Table 1.
Table 1.
Summary of fNIRS studies on social development
| Author (year) | Age | N | Number of channels | Channel location | Chromophore | Analysis level | Multiple comparison correction? | Main findings |
|---|---|---|---|---|---|---|---|---|
| Pre-recorded visual social stimuli | ||||||||
| Farroni et al. (2013) | 1–5 days | 15 | 20 | Bilateral temporal | HbO, HbR | Channel | Bonferroni- corrected (uncorrected also interpreted) | ↑ HbO to dynamic social stimuli (vs. non-social images) in R & L posterior temporal chs. R posterior temporal activation ↑ with age. No HbR changes. No activation to non- social. |
| Grossmann et al. (2008) | 4 mos | 12 | 26 | Frontal & Bilateral temporal | HbO, HbR | Channel | FDR correction | ↑ HbO to dynamic mutual gaze (vs. non-social & averted gaze) in one R posterior temporal and one R PFC ch. No HbR changes. |
| Grossmann & Johnson (2010) | 5 mos | 15 | 24 | Frontal | HbO | Region | Uncorrected (p < .05; one-tailed, planned comparisons) | ↑ HbO to joint attention (vs. no referent & no eye contact) in L dorsal PFC region. |
| Grossmann et al. (2010b) | 5 mos | 20 | 24 | Frontal | HbO | Channel | Uncorrected (p < .05) | ↑ HbO to static mutual gaze (vs. averted gaze) in one L frontal ch. |
| Grossmann et al. (2013) | 4 mos | 15 | 24 | Bilateral temporal | HbO, HbR | Region | Uncorrected (p < .05) | ↑ HbO to robot-like motion (vs. human-like motion) in bilateral anterior ROI (premotor cortex). ↑ HbO to conditions when form and motion matched (human-human movement, robot-robot movement) in L inferior ROI (superior temporal area). No HbR changes. |
| Honda et al. (2010) | 7–8 mos | 13 | 24 | Bilateral temporal- occipital | HbO, HbT | Hemisphere (ch data in figure) | Uncorrected (p < .05) | ↑ HbT to canonical faces in RH. ↑ HbR to scrambled faces in LH. No changes in HbO. |
| Kobayashi et al. (2016) | 9 mos | 12 | 24 | Bilateral temporal- occipital | HbO, HbR, HbT | Hemisphere | Uncorrected (p < .05) | ↑ HbO to adult faces vs. vegetable baseline in R temporal, ↓ HbR to adult faces vs. vegetable baseline in L temporal. No change in response to infant faces. |
| Lloyd-Fox et al. (2009) | 5 mos | Exp 1: 24 Exp 2: 12 |
20 | Bilateral temporal | HbO, HbR | Channel | Uncorrected (p < .05) | ↑ HbO to dynamic social stimuli (vs. non-social images) in 2 R & L posterior temporal chs. No HbR changes. No activation to non- social. |
| Lloyd-Fox et al. (2011) | 5 mos | 13 | 45 | Frontal & Bilateral temporal | HbO, HbR | Channel | Activation reported if 2+ near chs significant at p < .05 (uncorrected) | ↑ HbO to biological motion (vs. mechanical motion) overall in R (7 chs) & L (4 chs) hemisphere. Preferential response to eye movement in R & L frontal-temporal, hand in R & L posterior temporal and prefrontal, and mouth in R middle-temporal. No HbR changes. |
| Lloyd-Fox et al. (2015) | 4–6 mos | 24 | 26 | Bilateral frontal-temporal | HbO, HbR | Region | FDR correction | ↑ HbO to hand movement in R posterior STS-TPJ ROI correlated with fine motor abilities. No HbR changes survived correction. |
| Lloyd-Fox et al. (2016) | Cohort 1: 0–2 mos Cohort 2: 4–8, 9–13, 12–16 mos Cohort 3: 18–24 mos |
C1: 18 C2: 19–24 C3: 16 |
12 | Right temporal | HbO, HbR | Channel | FDR correction (uncorrected also reported) | ↑ HbO to dynamic social stimuli in R posterior temporal chs across ages. No HbR changes. |
| Minagawa- Kawai et al. (2009) | 9–13 mos | 15 | 4 | Frontal | HbO, HbR, HbT | Channel | Bonferroni correction | ↑ HbO to own mother and unfamiliar mother smiling (marginal) in one ch corresponding to OFC. No HbR changes. |
| Nakato et al. (2011) | 6–7 mos | 12 | 24 | Bilateral temporal- occipital | HbO, HbR, HbT | Hemisphere & Channel | Uncorrected (p < .05) | ↑ HbO & HbT to happy faces in LH and to angry faces in RH. More rapid responses to angry faces and more gradual to happy faces. |
| Otsuka et al. (2007) | 5–8 mos | 10 | 24 | Bilateral temporal- occipital | HbO, HbR, HbT | Hemisphere & Channel | Uncorrected (p < .05) | Widespread ↑ HbO & HbT to upright, but not inverted, faces in R temporal area. LH response similar for inverted & upright faces. |
| Ravicz et al. (2015) | 7 mos | 24 | 22 | Frontal | HbO, HbR | Channel | Uncorrected (p < .05) | ↓ HbO to happy faces in two frontal chs. ↑ HbR to happy faces in one frontal ch. Several chs correlated with temperament ratings. Low negative temperament group ↑ HbO to happy faces than high negative group in LH. |
| Xu et al. (2017) | 5–6 mos | 19 | 22 | Frontal | HbO | Channel | FDR correction (uncorrected reported) | ↓ HbO to peek-a-boo by animated character (vs. baseline) in 1 mPFC ch (after correction). No correlation between fNIRS data & looking behavior. |
| Pre-recorded auditory social stimuli | ||||||||
| Cristia et al. (2014) | 0–6 days | 40 | 28 | Bilateral frontal- temporal | HbO | Channel | Uncorrected (p < .05) & Bootstrap resampling | ↑ HbO to emotional vocalizations (vs. silence) in 3 L temporal chs (uncorrected). Similar responses to different types of sounds. |
| Grossmann et al. (2010a) | Exp 1: 4 & 7 mos Exp 2: 7 mos |
E1: 16 (4m) & 16 (7m) E2: 18 |
24 | Bilateral temporal | HbO, HbR | Channel | Uncorrected (p < .05) | E1: At 7 mos, ↑ HbO to vocal (vs. non-vocal) sounds in 2 R & 1 L posterior temporal ch. At 4 mos, ↑ HbO to non-vocal (vs. vocal) sounds in 1 R posterior temporal ch. No HbR changes. E2: ↑ HbO to happy (vs. neutral & angry) vocalizations in 1 R inferior frontal ch. ↑ HbO to angry (vs. neutral & happy) vocalizations in 1 R posterior temporal ch. No HbR changes. |
| Grossmann et al. (2010b) | 5 mos | 20 | 24 | Frontal | HbO | Channel | Uncorrected (p < .05) | ↑ HbO to own name being called (vs. other name) in one L frontal ch (adjacent to channel that activated to mutual gaze). |
| Homae et al. (2006) | 3 mos | 21 | 48 | Bilateral frontal- temporal | HbO | Channel | Uncorrected (p < .001) | ↑ HbO to normal prosody speech (vs. flattened speech) in 1 R posterior temporal ch in sleeping infants. |
| Homae et al. (2007) | 10 mos | 21 | 48 | Bilateral frontal- temporal | HbO, HbR | Channel | FDR correction & Uncorrected (p < .01) | ↑ HbO to flattened speech (vs. normal prosody speech) in 4 R posterior temporal-parietal chs, 1 R frontal ch, & 1 L frontal ch. HbR changes not fully reported. |
| Imafuku et al. (2014) | 6 mos | 17 | 22 | Frontal | HbO | Channel | FDR correction | ↑ HbO to own name by mother (vs. baseline), own name (vs. other name), & mother (vs. stranger) in two chs in dorsal medial PFC (dmPFC). Behavioral preference for mother’s voice associated with ↑ HbO in dmPFC. |
| Lloyd-Fox et al. (2012) | 4–7 mos | 33 | 38 | Bilateral temporal | HbO, HbR | Channel | Uncorrected (p < .05) | ↑ HbO & HbR to voice (vs. silence) in middle bilateral temporal chs. Widespread activation to non-voice (vs. silence). ↓ HbR to voice (vs. non- voice in L anterior STS area. ↑ response to voice, but not non- voice, with age. |
| Lloyd-Fox et al. (2016) | Cohort 1: 0–2 mos Cohort 2: 4–8, 9–13, 12–16 mos Cohort 3: 18–24 mos |
C1: 18 C2: 19–24 C3: 16 |
12 | Right temporal | HbO, HbR | Channel | FDR correction (uncorrected also reported) | ↑ HbO to non-social (vs. social) at 0–2 mos & 4–8 mos in R posterior temporal chs. ↑ HbO to social (vs. non-social) at 9–13 mos, 12–16 mos, & 18–24 mos in anterior temporal chs. Minimal changes in HbR. |
| Naoi et al. (2012) | 4–13 mos | 57 temporal, 48 frontal | 30 | Frontal & Bilateral temporal | HbO, HbR | Channel | Uncorrected | Temporal: ↑ HbO to IDS (vs. ADS) in most R & L temporal chs. Some changes in HbR. Minimal differences between own and unfamiliar mother’s voice. Minimal age-related differences. Frontal: ↑ HbO to IDS (vs. ADS) in most frontal chs to own mother condition. Some HbR changes. ↑ HbO to own mother IDS (vs. unfamiliar IDS) in 1 L superior frontal ch. Infants at 7–9 mos ↑ HbO to own (vs. unfamiliar) in 1 inferior frontal ch. |
| Zhang et al. (2014) | 2–6 days | 18 | 20 | Bilaternal frontal- temporal | HbO, HbR | Channel | FDR correction | ↑ HbO to emotional (vs. neutral) vocalizations in R middle- superior temporal gyrus (1 ch). ↑ HbO to fearful (vs. happy & neutral) vocalizations in R supramarginal gyrus (1 ch). No HbR changes. |
| Live social stimuli | ||||||||
| Lloyd-Fox et al. (2015) | 6 mos | Exp 1: 24 Exp 2: 24 |
E1: 17 E2: 33 |
E1: Frontal & Right temporal E2: Frontal & Bilateral temporal |
HbO, HbR | Channel | Uncorrected (p < .05) | E1: ↑ HbO or ↓ HbR to infant- directed gaze & speech of live social partner in 4 adjacent R inferior frontal & temporal chs. E2: ↑ HbO to IDS (vs. ADS) of live social partner in 3 R inferior frontal-temporal chs (overlapping with E1) & ↑ HbO or ↓ HbR in 3 L temporal chs. |
| Naoi et al. (2009) | 7–12 mos | 17 | 22 | Frontal | HbO | Channel | Uncorrected (p < .05) | ↓ HbO to IJA and/or RJA with live examiner in 3 frontal chs. ↑ HbO to IJA with live social partner in 1 L frontal ch. HbO changes in L frontal channel correlated with % of trials with infant IJA behavior. |
| Urakawa et al. (2015) | 7 mos | 11 | 17 | Frontal | HbO, HbR | Region | Uncorrected (p < .05) | ↑ HbO to direct gaze (vs. averted gaze) of live social partner during peek-a-boo in mPFC. HbR changes unrelated to gaze condition. Infants looked more toward the eyes during direct than averted gaze condition. |
| High-risk infants | ||||||||
| Fox et al. (2013) | 7 mos | 10 HRA, 10 LRC | 24 | Frontal & Right temporal- occipital | HbO, HbR | Channel | Bonferroni- corrected | ↑ HbO or ↓ HbR to smiling (vs. neutral) faces in 6 frontal chs across all infants. ↑ HbO to mother (vs. stranger) in 1 middle frontal ch and ↓ HbR to stranger (vs. mother) in 1 R frontal ch. 7 frontal and R temporal chs differed by group. Several significant interactions between group and condition suggesting differential response to faces in HRA vs. LRC. |
| Keehn et al. (2013) | 3, 6, 9, 12 mos | 27 HRA, 37 LRC (differs by age) | 24 | Bilateral temporal | HbO | Region | Bootstrap analysis to confirm t- test results | At 3 mos, ↑ L anterior-R posterior ROI intrinsic connectivity and ↑ co-activation connectivity intra- hemispheric in HRA vs. LRC. No differences at 6 & 9 mos. At 12 mos, ↓ intra-hemispheric intrinsic and co-activation connectivity for HRA vs. LRC. |
| Lloyd-Fox et al. (2013) | 4–6 mos | 18 HRA, 16 LRC | 26 | Bilateral frontal- temporal | HbO, HbR | Channel | Uncorrected (p < .05) | ↑ HbO to visual social (vs. non- social) stimuli in 2 L posterior temporal chs and 1 R posterior temporal ch in LRC. ↑ HbO to visual social (vs. non-social) stimuli in 1 R posterior temporal ch in HRA. ↑ HbO to vocal (vs. non-vocal) stimuli in 1 R middle temporal ch for LRC. Responses to non-vocal (vs. vocal) stimuli similar across groups. |
Note. HbO = Oxygenated hemoglobin. HbR = Deoxygenated hemoglobin. HbT = Total hemoglobin. FDR = False discovery rate. R = Right. L = Left. Ch = Channel. PFC = Prefrontal cortex. ROI = Region of interest. RH = Right hemisphere. LH = Left hemisphere. STS = Superior temporal sulcus. IDS = Infant-directed speech. ADS = Adult-directed speech. HRA = High-risk for autism. LRC = Low-risk controls.
2.1. Pre-recorded visual social stimuli
The majority of fNIRS studies of infant social development have focused on the visual domain. The visual system comes online early in infancy and is an important aspect of infants’ daily social experiences and interactions. A great deal of social information is obtained through the visual system, in particular through faces, to which infants tend to preferentially attend from early in life (Nelson, 2001) and the processing of which rapidly increases in sophistication over the first year (Frank et al., 2009). The study of neural correlates of social information processing in the visual domain has been limited, at least in part, due to the difficulty of collecting fMRI data with awake infants (although see Deen et al., 2017). fNIRS thus provides an ideal method for localizing cortical activity in response to visual social stimuli in awake infants. Here, we review fNIRS studies that examine brain responses to human movement, faces, facial emotion, and eye gaze (see Table 1 for detailed summaries of included studies).
Several fNIRS studies have asked which areas of the infant brain respond preferentially to dynamic visual social stimuli. From the first days of life, areas of the right and left posterior temporal cortex selectively respond to dynamic face stimuli (i.e., videos of an adult playing peek-a-boo; Farroni et al., 2013). Across this first week of life, there appears to be a rapidly developing sensitivity to social experience in the right posterior temporal cortex, with slightly older newborns more likely to show increased activation to dynamic social stimuli than younger newborns (Farroni et al., 2013). At 5 months, infants continue to show increased bilateral activation to videos of social movement in the posterior temporal cortex (Lloyd-Fox et al., 2009), with the right hemisphere appearing to respond more strongly to visual social stimuli at this age (Lloyd-Fox et al., 2011). Different patterns of brain activation have been found in response to specific socially-relevant body movements in 5-month-old infants. Namely, eye movement is associated with increased activation in bilateral inferior frontal regions, hand movement with increased activity in the bilateral posterior temporal and prefrontal cortices, and mouth movements with right-lateralized activation in the middle temporal region. This line of work was extended in an impressive semi-longitudinal study examining responses to dynamic social stimuli over the first two years of life in a Gambian cohort of infants (Lloyd-Fox et al., 2016). Over 4 to 24 months of age, infants consistently showed increased activation to dynamic visual social stimuli in the right posterior temporal region (only right hemisphere measured due to practical limitations). This study highlights the strength of fNIRS portability for use in cross-cultural studies of infant brain development, in addition to providing a model for future longitudinal investigations of infant social brain development.
Infant brain responses to dynamic visual social stimuli have also been investigated in the context of action perception studies using fNIRS. By 4 months of age, infants differentiate between typical human movements and more robotic movements in areas of the frontal and temporal cortices (Grossmann et al., 2013). Interestingly, infants at this age may respond more strongly to less familiar, robotic movements than more familiar human movements in an area corresponding to the premotor cortex, while a region in the superior temporal cortex responded most strongly to conditions where the type of motion was congruent with the type of figure (e.g., human making human movements; Grossmann et al., 2013). There is further evidence that infant brain responses to perceived action predict individual differences in corresponding motor abilities. Specifically, Lloyd-Fox et al. (2015) found that stronger responses to observed hand movements in the posterior superior temporal sulcus (STS)-temporoparietal junction (TPJ) area (particularly in the right hemisphere) correlate with infants’ observed fine motor abilities at 4–6 months of age.
In addition to human movements, images of faces have been used to examine infant brain responses to faces and facial expressions. Examining neural responses to faces and facial expressions using fNIRS is somewhat limiting, both given the utility of event-related potential (ERP) paradigms in this domain and the depth limitations of fNIRS (e.g., cannot measure fusiform gyrus, amygdala); however, results from these investigations have yielded some valuable information regarding localization of response in infants and provide useful data from which to develop hypotheses for the social interaction paradigms to be discussed later in this review. Studies using fNIRS to contrast infant brain responses to canonical vs. scrambled or inverted faces further suggest preferential responding of the right temporal area in social perception by mid-way through the first year of life (Honda et al., 2010; Otsuka et al., 2007). Infants may also begin to specialize in the type of face over the first year. Kobayashi et al. (2016) examined bilateral temporal area responses to adult and infant faces in 9-month-old infants, with results indicating that the right temporal region activated in response to adult but not to infant faces. This study also contrasted infants’ ability to behaviorally discriminate novel vs. familiar infant and adult faces at 3 and 9 months of age, finding that 3-month-old infants discriminated both types of faces, while 9-month-old infants only distinguished adult faces. Together, this study provides evidence for a putatively experience-dependent increased sensitivity to adult faces that is supported by right-lateralized brain activity by 9 months of age.
fNIRS has also been used to contrast infant brain responses to faces with differing emotional expressions. In a study of 6- to 7-month-old infants, different patterns of brain responses to facial emotion were observed with regard to both the localization and timing of activity (Nakato et al., 2011). Specifically, happy faces were associated with increased activity in fNIRS channels covering the left STS area and a more extended hemodynamic response, while angry faces were associated with increased activity in the right STS area and a more rapid hemodynamic response (Nakato et al., 2011). The prefrontal cortex (PFC) has also been shown to respond to happy faces at around 7 months of age (Ravicz et al., 2015). Higher levels of oxy-Hb in the left PFC while viewing happy faces also showed associations with parent-reported temperament; the left PFC of infants with low parent-reported negative emotionality preferentially responded to happy faces, while infants with high negative emotionality had less activation overall and did not show this lateralization effect. In a unique study of both mother and infant (9–13 months) responses to happy faces that varied in familiarity, Minagawa-Kawai et al. (2009) found that mothers and infants evidenced increased activation in the anterior orbital frontal cortex associated with the own-infant and own-mother condition, respectively. For the infants, this effect was specific to the viewing of images of their own mother smiling. Along with increasing our knowledge of the neural correlates of face processing in the frontal lobe, this study provides a useful basis upon which to design more naturalistic fNIRS experiments within the mother-infant dyad.
Grossmann and colleagues have conducted a series of fNIRS experiments examining infant brain responses to aspects of joint attention using animated videos of adult faces shifting gaze. They found that already by 4–5 months of age, infants distinguish between mutual vs. averted gaze conditions in areas of the frontal and temporal cortices. At 4 months of age, an area corresponding to the right posterior STS and the right frontal polar cortex showed increased activation in response to mutual gaze (Grossmann et al., 2008). At 5 months of age, there is evidence that the left dorsal PFC responds to both mutual gaze and joint attention (i.e., animated face making eye contact, then referencing an object), suggesting that this area is sensitive to critical dyadic and triadic interactions from early in life (Grossmann et al., 2010b; Grossmann and Johnson, 2010).
2.2. Pre-recorded auditory social stimuli
There have been a growing number of fNIRS studies focused on examining infant brain responses to pre-recorded auditory social stimuli. Infants are responsive to auditory information in the social environment, in particular human voices, even earlier in development than visual stimuli. Fetuses have been shown to change their behavior in response to their mother’s voice by the third trimester of pregnancy (Marx & Nagy, 2015), and newborns show a preference for their mother’s voice at birth (DeCasper & Fifer, 1980). Infants also show a behavioral preference for a particular type of speech, termed infant-directed speech, which is slower and of higher and more varying pitch than adult-directed speech, within the first several months of life (Pegg et al., 1992). While fMRI studies have contributed important information regarding the neural underpinnings of auditory social perception using infant sleep fMRI procedures (e.g., Shultz et al., 2014), fNIRS has several advantages that complement the use of fMRI in localizing neural responses to auditory social stimuli. These advantages include the relative silence and increased accessibility of fNIRS systems, and, as noted above, the ability to assess brain responses while infants are awake. Here, we review fNIRS studies that investigate brain responses to human vocalizations, differences in speech prosody (including infant-directed speech), and socially-relevant speech (i.e., response to name; see Table 1 for detailed summaries of included studies). We do not include studies that were focused specifically on language development, which, while of interest, are outside of the scope of the current review (see Vanderwert & Nelson, 2014).
Numerous studies have contrasted infant brain responses to human vocalizations (e.g., laughing, coughing) vs. non-social sounds (e.g., rattle, running water), consistently finding that infants begin responding preferentially to social vs. non-social sounds in the second half of the first year of life. Both Grossmann et al. (2010a) and Lloyd-Fox et al. (2012) found that younger infants (~4 months) responded more strongly to non-vocal vs. vocal sounds, while older infants (~7 months) showed increased activation to social stimuli in the left and right temporal cortex. In the Gambian cohort cited above, findings were largely consistent with these studies; younger infants preferentially responded with more posterior temporal activation in response to the non-social stimuli, while infants at older ages (9–24 months) typically responded more strongly to human vocalizations in more anterior portions of the temporal cortex (Lloyd-Fox et al., 2016).
Differential responses to variations in prosody have also been examined in infants using fNIRS. In the first days following birth, infants show non-selective responses to emotional vocalizations in the left temporal cortex (Cristia et al., 2014), with preliminary evidence that the right temporal cortex is sensitive to vocalizations with emotional (vs. neutral) prosody in the first week of life (Zhang et al., 2014). At 7 months of age, infants further differentiate emotional tone, with responses to angry vocalizations occurring in the right posterior temporal cortex and happy vocalizations in the right inferior frontal region (Grossmann et al., 2010a).
Infant-directed speech (IDS) is another aspect of prosody that has been examined in infants with fNIRS. Naoi et al. (2012) contrasted IDS with adult-directed speech (ADS) spoken by the infants’ own vs. an unfamiliar mother at 4–13 months of age. Infants responded more strongly to IDS vs. ADS in left and right temporal areas, while the left superior frontal cortex responded specifically to own-mother IDS condition. There was also an age effect, with 7- to 9-month-old infants showing particular sensitivity to their own mother’s voice in the IDS condition, which corresponds with a time period that is critical for the development of the mother-infant attachment relationship. Further work suggests that the right posterior temporal cortex shows increased activation to speech with normally varying prosody vs. flattened speech at 3 months (Homae et al., 2006), although stronger responses to the more novel flattened speech is observed by 10 months of age (Homae et al., 2007).
In addition to studying basic vocalizations and prosody, fNIRS has also been used to study the neural underpinnings of infants hearing their name being called. Response to name is considered an important developmental milestone in social development, particularly as it relates to the developing conception of the self. At 5 months, an age at which clear behavioral indicators of response to name are often not evident, infants have shown increased activation in response to their own (vs. another) name in an fNIRS channel in the left dorsal PFC (Grossmann et al., 2010b). Notably, this channel was different, but adjacent to the area that was sensitive to shared gaze in the visual experiment of this study, and PFC responses to the more socially relevant conditions (mutual gaze and own name) were positively correlated. Imafuku et al. (2014) found that two adjacent channels in the dorsal-medial PFC (dmPFC), an area adjacent to the region found in Grossmann et al. (2010b), selectively activated when 6-month-old infants heard their name spoken by their mother. Infants who showed greater responses in the dmPFC to the own mother condition were also more likely to show a behavioral preference for their own mother’s voice as measured by a head-turn preference paradigm (Imafuku et al., 2014).
2.3. Conclusions: pre-recorded visual and auditory social stimuli
In summary, there have been a number of well-conducted fNIRS studies that have used tightly controlled experimental approaches to study infant social brain development. Areas that are involved in social information processing during adulthood appear to rapidly become sensitive to social stimuli within the first year of life, including the STS-TPJ region and areas of the PFC. Some shortcomings of the infant fNIRS studies to date are that these studies frequently have small sample sizes, disallowing for statistical correction of multiple comparisons, lack longitudinal follow-up, and often do not relate findings to concurrent or longitudinal behavioral indicators of social development. While there are notable exceptions (e.g., Imafuku et al., 2014; Lloyd-Fox et al., 2015; Lloyd-Fox et al., 2016; Ravicz et al., 2015; also see Xu et al., 2017), these factors currently limit the impact of the findings. At the same time, the sophistication of the methodology of fNIRS experiments has grown at an astonishing pace, and the knowledge gained from the studies describe above inform research efforts that seek to overcome these challenges and the initial limitations of infant fNIRS work.
3. fNIRS studies of infant brain responses to live social stimuli
While we have learned a great deal about early social brain development with the more traditional experiments described above, increasing numbers of studies have been taking advantage of the relative flexibility of fNIRS to examine neural responses to more ecologically valid, naturalistic social stimuli. These studies range from the use of live actors within the context of a block design to more dynamic interactive social experiences within the adult fNIRS literature. Although studies using more naturalistic stimuli present additional technical challenges (e.g., less standardization, more motion artifact, challenges with data analysis), results from these studies suggest that fNIRS recording during live social interaction is doable and provides unique and rich data on neural responses to actual social experiences. Furthermore, studies contrasting infant brain responses to live vs. pre-recorded stimuli using both fNIRS (Shimada & Hiraki, 2006) and EEG (Jones et al., 2015) suggest that the use of live social stimuli may prove to be a more powerful elicitor of social brain responses, despite the potential loss of experimental control. Here, we focus on studies from the fNIRS literature that examined infant brain responses to live social stimuli (see Table 1 for detailed summaries of included studies).
In an initial study of 7- to 12-month-old infants, Naoi et al. (2009) examined behavioral and brain responses to trials during which a live actor shifted their gaze toward an object (response to joint attention [RJA] condition) or gazed at the infant while an object was presented. In the latter condition, if the infant looked from the actor toward the object (initiation of joint attention [IJA]), the actor would follow their gaze. They found that adjacent and overlapping areas of the frontal cortex showed significant changes following both the RJA and IJA trials. Additionally, increased activity in a channel in the left frontal cortex, which responded only to the IJA condition, was correlated with a greater proportion of trials during which the infant initiated joint attention bids.
Urakawa et al. (2015) examined PFC responses to “peek-a-boo” interactions with a live social partner in a small group of 7-month-old infants using fNIRS. They contrasted a baseline condition with no interaction and two conditions in which the interactive partner had her eyes directed toward or averted away from the infant. The right lateral PFC showed increased activation relative to the baseline condition similarly across gaze conditions, suggesting that this area was generally sensitive to social interaction, while the mPFC responded more strongly to the direct gaze condition. Interestingly, this study simultaneously collected eye tracking data, which revealed that infants looked more toward the eyes during the direct gaze condition and more toward lower portions of the face during the averted eyes condition. This finding further suggests that the mPFC is preferentially activated when infants are looking at a social partner’s eyes, and exemplifies the utility of combining behavioral methods in studies of infant brain functioning.
Lloyd-Fox et al. (2015) conducted a study of 6-month-old infants that included live actors. In the first experiment, infants were exposed to an adult singing nursery rhymes in a foreign language that was either directed at the infant or another infant in the room vs. baseline periods with no interaction. In a second experiment, infants were exposed to adults engaging in adult-directed and infant-directed speech. The results from both experiments suggest that brain activation in inferior frontal, anterior temporal, and temporo-parietal regions is associated with multimodal (speech and gaze) infant-directed social stimuli, in comparison to baseline and unimodal conditions. While this study identified challenges that may be more prevalent in naturalistic social scenarios (e.g., increased data loss possibly due to reduced standardization of stimuli), it provides a unique and well-conceived example of using fNIRS to study infant brain responses in social contexts that more closely reflect those experienced in everyday life.
3.1. Conclusions: live social stimuli
Initial fNIRS studies investigatinginfant brain responses to live social stimuli suggest the feasibility and potential of this methodology. In our final section, we present ideas and recommendations for furthering this line of research into a more truly interactive social context. First, however, we review the literature on the use of fNIRS in infants at risk for social deficits—an area in which interactive neuroscience paradigms may be particularly fruitful.
4. Clinical applications: infants at risk for autism
Given the practical and scientific advantages of fNIRS, this methodology has increasingly been used in studies of clinical populations, including autism spectrum disorder (ASD). ASD is a complex neurodevelopmental disorder that is behaviorally defined based upon deficits in social-communication and the presence of restricted and repetitive behaviors and interests (American Psychiatric Association, 2013). While the ASD phenotype is well characterized, the underlying biological deficits are less well understood. In particular, the differences in brain function that underlie ASD as it first emerges in the infant brain have been a challenge to study, largely due to methodological constraints related to identifying infants with emerging ASD prior to full behavioral expression, as well as the previously noted challenges with measuring brain function during infancy. Researchers have addressed the first challenge by studying infants with known risk factors, such as an older sibling with the disorder (high-risk siblings) and those with ASD-associated genetic syndromes (e.g., tuberous sclerosis complex). The latter challenge has been addressed by using infant-friendly measures of brain activity, including EEG and fNIRS, both of which offer unique contributions to the study of high-risk infants. Here, we focus on the contributions of fNIRS to this burgeoning literature (see Table 1 for detailed summaries of included studies).
A few studies have used fNIRS to examine differences in brain development associated with ASD risk status in high-risk siblings using pre-recorded visual and auditory social stimuli. In the first of these investigations, Fox et al. (2013) measured bilateral frontal and temporal responses to smiling and neutral faces that varied in familiarity (mother vs. stranger) in high-risk and low-risk infants at 7 months of age. Overall, infants showed increased responses to smiling faces in areas of the frontal cortex, and low-risk infants demonstrated stronger responses to faces in comparison to high-risk siblings in both the frontal and right temporal cortices. Closer inspection of the data identified differences in the qualities of the hemodynamic response in frontal regions between the groups, which they posited may reflect early frontal cortex enlargement.
Lloyd-Fox et al. (2013) extended this research by contrasting brain responses to visual and auditory social stimuli in bilateral frontal-temporal regions in high-risk and low-risk infants at 4 to 6 months of age. As in previous studies (Lloyd-Fox et al., 2016), the visual stimuli contrasted videos of adults engaging in social play with non-social images and the auditory stimuli contrasted recordings of human vocalizations with non-vocal sounds. Both groups showed activation in the posterior STS region in response to the visual social stimuli, although the low-risk infants showed more extensive and bilateral activation in comparison to the high-risk sibling group, which showed increased activation in only one channel in the right STS area. There were also risk group-related differences in the auditory condition. The low-risk group evidenced increased activation to the vocal vs. non-vocal condition in an anterior portion of the right STS region, along with areas of the left STS to the non-vocal vs. vocal condition. The high-risk group, in contrast, only showed activation in response to the non-vocal vs. vocal condition in bilateral mid-posterior STS regions. In sum, the high-risk infants, at a group level, showed reduced responses to both visual and auditory social stimuli at 4 to 6 months of age.
Keehn et al. (2013) took a slightly different approach, using fNIRS to examine differences in functional connectivity in high-risk and low-risk infants at 3, 6, 9, and 12 months of age during a language processing task. Intrinsic connectivity measured correlations between time courses of regions of interest after regressing out task-related fluctuations in the signal, while co-activation connectivity similarly examined correlations without regressing out task-related signal. At 3 months, high-risk siblings had higher levels of primarily intra-hemispheric connectivity than low-risk infants during a language processing task. Intrinsic connectivity was largely similar between groups at this age. At 6 and 9 months, there were no differences in connectivity. However, at 12 months, high-risk siblings had decreased intra-hemispheric functional connectivity compared to low-risk infants. Generally, a shift from increased to reduced connectivity was observed between 3 to 12 months of age in the high-risk vs. the low-risk infants. In addition to suggesting the promise of fNIRS as a measure of functional connectivity, these results indicate differential brain development in these high-risk infants that may reflect an endophenotype of ASD.
4.1. Conclusions: infants at risk for social deficits
While further research is necessary, including with diagnostic follow-up, these initial studies indicate that fNIRS can be used successfully in research on infants with elevated risk for ASD, providing important and unique contributions to the literature. Future studies of high-risk infants would benefit from the use of more naturalistic social stimuli (Rolison et al., 2015), which may capture more subtle deficits associated with emerging ASD that may only be evident in complex, interactive social situations.
5. Conclusions and recommendations for future research
In the current review, we discussed findings from fNIRS studies of infant social brain development, including experiments that utilized pre-recorded and live social stimuli, as well as studies of social brain activity in the context of typical development and increased risk for social deficits. Results of this focused review suggest several initial conclusions from this growing body of work: 1) fNIRS is well tolerated by infants and well suited to measuring localized brain activity in response to pre-recorded, multi-modal social stimuli in standardized laboratory contexts; 2) fNIRS is a promising methodology for measuring infant brain responses in more naturalistic social contexts; 3) typically developing infants begin to show preferential responses to multi-modal social stimuli in areas of the temporal and frontal cortices within the first 6 months of life; 4) infants at elevated risk for social deficits demonstrate differential responses to pre-recorded social stimuli during the first year of life, prior to observable behavioral differences (Jones et al., 2014). Along with these significant contributions of the infant fNIRS literature to date, there are many remaining opportunities and challenges for future research. Here, we discuss several approaches for increasing the ecological validity of the literature on infant social brain development using fNIRS.
5.1. Integration of behavioral and fNIRS data
Despite the growing number of studies, fNIRS findings are rarely grounded in measures of infant social behavior. It is unclear to what degree this is due to methodological limitations of collecting multiple measures with infants, a divergence in expertise between traditional neuroscience researchers and behavioral researchers, unpublished null findings, or some other factor. While establishing foundational information on normative brain responses to social stimuli is essential, given the work that has now been conducted it is important for future studies to relate brain imaging findings to relevant measures of infant social behavior.
Research that integrates behavioral and neuroimaging methods can take many forms. One method is to relate patterns of brain activation to concurrent behavior within the same task (e.g., Urakawa et al., 2015). This approach can increase understanding of experiential factors that may have led to differences in brain activation (e.g., eye tracking data revealing increased attention to the eyes during conditions associated with mPFC activation).
A second method is to relate the magnitude (or timing) of brain responses to same-age measures of related behavior within a different task (e.g., Imafuku et al., 2014; Ravicz et al., 2015), which can help to determine whether the identified brain responses explain meaningful differences in social development (e.g., infants with stronger oxy-Hb responses when hearing their name called are also more likely to behaviorally respond to their name being called). This approach provides support for the practical relevance of a study’s brain imaging findings.
A third method is to longitudinally predict individual differences in infant social behavior from earlier brain responses to social stimuli (e.g., infants with increased oxy-Hb responses to mutual eye gaze at 6 months showing more joint attention behaviors at 12 months). This approach has two advantages. First, it is possible that differences in brain development may precede observable differences in a behavior of interest, leading to null findings when examining concurrent social behaviors. Second, longitudinal prediction increases the potential clinical relevance of a study. If an earlier difference in the magnitude or timing of a brain response to social stimuli predicts a later individual difference in an important aspect of social behavior, this brain response may hold promise as a biomarker of risk and optimal development in studies of clinically relevant populations. It is important to note, however, that special attention must be paid in studies of this kind to ensure sufficient power to detect longitudinal correlations between brain and behavior.
5.2. Interactive social paradigms
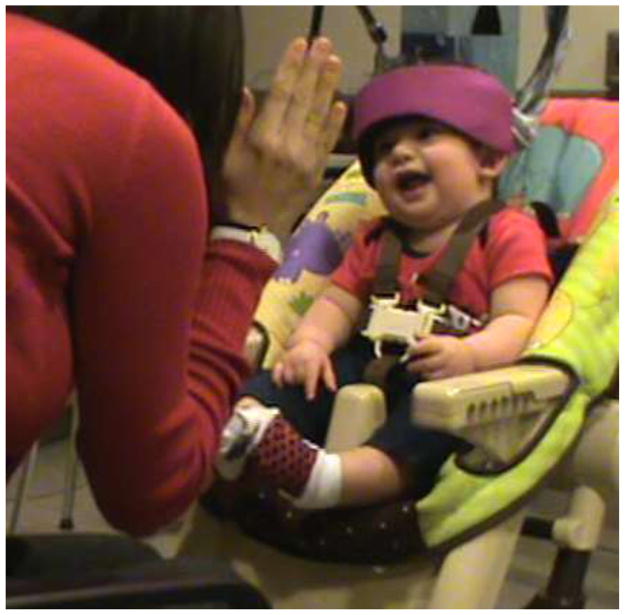
In addition to integrating measures of social behavior when examining infant social brain development, future studies in this area would benefit from the use of a more naturalistic social context while collecting infant brain imaging data. Brain responses to more naturalistic social stimuli may be more reflective of functioning during daily life, and may, therefore, be more closely related to behavior. While fNIRS is well suited to use in such situations, very few studies are currently published in the infant fNIRS literature that have used live social stimuli and none have yet been published with more interactive paradigms (although see Jones et al., 2015; Reid et al., 2011). We propose several study designs that could be used independently or in conjunction to measure infant brain responses in more socially interactive contexts (see Figure 2 for a depiction of a sample infant social interaction paradigm using fNIRS).
Figure 2.
Parent-child interaction with fNIRS
5.2.1. Traditional block design with a live social partner
Studies to date of infants in a live social context have largely replicated traditional block design stimulus presentation, but replaced a pre-recorded stimulus with a live actor (e.g., Lloyd-Fox et al., 2015). This approach is an important step towards interactive social neuroscience in that it allows for brain measurements while the infant is engaged with another person, adding ecological validity to the experimental conditions that is not present with pre-recorded stimuli. The live stimulus presentation in a block design retains a degree of experimental control, allowing for data analysis that is similar to studies with pre-recorded stimulus presentation. However, this approach does not allow for truly naturalistic interactions, and the data are implicitly being analyzed according to the intent of the interaction rather than according to the infant’s actual behavior during the experimental conditions. In addition, using a scripted live presentation of stimuli may require that trained experimenters perform the task conditions in order to standardize across participants, limiting the types of social interactions that can be studied to those that are between the infant and a stranger. However, with special attention to training other presenters, such as parents, along with post-hoc review for standardization of presentation, this limitation could be overcome.
5.2.2. Spontaneous behaviors as event markers
A contrasting approach to assessing the brain response to a particular social interaction would be to allow for a more naturalistic, less scripted interaction, but analyze brain responses to particular events. This approach would require neuroimaging and simultaneous measures of behavior such as eye-tracking (as in Urakawa et al., 2015) and/or video recordings of interactions. Behaviors of interest such as eye contact or smiles could be marked as events, and a standard event-related approach to analyzing the fNIRS brain responses could be employed. This experimental design would allow for a direct analysis of behaviors of interest in a naturalistic context and would be a particularly intriguing approach to examining whether neural underpinnings of external behaviors differ during atypical neurodevelopment.
While the scientific questions about brain-behavior relationships that could be addressed using this approach are compelling, there remain many challenges to implementing this method. Technical challenges would include synchronizing neural and behavioral measures, as well as determining and marking events, although recent advances in automated video content analysis could perhaps enable this type of analysis. Other experimental challenges would be less standardization in the number of events or trials between participants, which could impact interpretation of results. Pilot work regarding the ideal length of such an interaction, considering both the tolerance of the infant and the necessary time for ensuring sufficient events for a variety of children, is needed.
5.2.3. Examine global features of brain network activation
Other experimental designs that do not rely on specific event timing for assessing the impact of social interaction could also be considered, drawing both from studies of infants and children using fNIRS in a non-social context and the adult social neuroscience literature. Instead of linking the brain responses to specific events, measures of overall brain connectivity in social and non-social conditions could be explored. These approaches would require longer periods of social interaction for connectivity measures, such as correlations between channels in low-frequency fluctuations or small world network properties, to be calculated. The connectivity or network properties of the brain could then be compared in social and non-social conditions. While this approach has not been attempted in infants yet, to the best of our knowledge, some fNIRS studies have measured infant functional connectivity during a language task (Keehn et al., 2013), and have contrasted infant connectivity during a language task vs. silence (Homae et al., 2011), or a working memory task vs. rest in children (Perlman et al., 2016). While this approach would not allow for strong statements to be made about any particular behavior in relation to social brain network activity, it would allow for more general contrasts between social and non-social conditions while sidestepping many of the technical difficulties involved in defining events in naturalistic interactions. This approach would also avoid the need for extensive training of an experimenter to provide a scripted live interaction.
5.2.4. Measure neural synchrony during “hyperscanning”
An alternate approach, called “hyperscanning,” simultaneously measures the brain responses of two interacting individuals (for a recent review of the use of hyperscanning to study the social brain see Babiloni & Astolfi, 2014). In this study design, the degree of synchronization between participants’ brain activity is the measure of interest, which eliminates the necessity of other behavioral measures (although if those measures do exist they can provide important context for interpreting results). Several studies within the adult fNIRS literature have used this approach, but, to our knowledge, there have not yet been any published studies with infants.
One common method used in fNIRS hyperscanning studies is to contrast the degree of neural synchrony in a given region of interest of two adults during different types of interactions, such as cooperation and competition, or no interaction (Cui et al., 2012; Liu et al., 2016). Another method has been to contrast neural synchrony between social partners during conversation (Jiang et al., 2015; Jiang et al., 2012) or when making eye contact with a live social partner vs. an image of a face (Hirsch et al., 2017). In a particularly interesting approach, Jiang et al. (2015) measured neural synchrony within the context of conversations between groups of three adults. Based on videotapes of the interactions a conversational leader and followers were defined, and the neural synchrony of leader-follower and follower-follower pairs were analyzed. They found that leader-follower pairs were more synchronous in the left TPJ region, and, when dynamically analyzing the fNIRS data, that the leaders and followers appeared to influence each other.
A hyperscanning approach would in many ways be particularly well suited to research on infant social development. Infants learn to socialize primarily through one-on-one interactions with caregivers. An aspect of these interactions, often termed synchrony, indexes the degree to which an infant and parent’s behavior is synchronized in time (Feldman, 2007b). Parent-infant synchrony during the first year of life longitudinally predicts important aspects of social development, such as empathy (Feldman, 2007a), self-control (Feldman et al., 1999), and attachment security (Jaffe et al., 2001). Using fNIRS to measure neural synchrony between parents and infants would provide unique information regarding the degree to which brain and behavior are synchronized, and whether more synchronous brain activity during infancy predicts more optimal social outcomes. fNIRS is also particularly well suited to hyperscanning studies due to the ease of recording data for multiple participants by simply splitting the sources and detectors into two sets and applying one set to each participant.
While some technical difficulties of interactive social neuroscience paradigms such as the need to mark events of interest or have highly scripted interactions can be avoided with the hyperscanning approach, there are still numerous technical difficulties that would have to be addressed in order to successfully apply the technique with infants. For instance, particular care would need to be taken to measure from the same brain regions in adults and infants, requiring thoughtful placement of optodes and consideration of source-detector spacing. However, there appear to be some similarities in brain responses to social stimuli between adults and infants (e.g., mPFC and STS-TPJ) that would allow for carefully constructed hypotheses that could inform optode placement, reducing the impact of this issue.
5.2.5. “Brain first”: use hemodynamic responses themselves to define events of interest
Last, an intriguing new approach to naturalistic fNIRS data analysis developed for adults suggests that instead of the standard approach of starting from behavior and using that to infer neural underpinnings, it may be fruitful to automatically detect brain responses and then determine the behaviors with which they are correlated (see Pinti et al., 2017 for details of this method). This approach has the potential to provide new insights into the infant social brain as it does not require experimenters to a priori define behaviors of interest in the limited behavioral repertoire of infants.
5.3. Interactive social neuroscience with clinical populations
In addition to providing information about social brain development, increased integration of behavioral data and an interactive social neuroscience approach could provide unique information to studies of infant risk populations and early intervention. Initial evidence from fNIRS studies of high-risk siblings using pre-recorded social stimuli indicates that infants with elevated risk for ASD and subclinical social-communication deficits differ from low-risk infants in aspects of early social brain functioning, although longitudinal ASD outcome data are still needed to assess the degree to which early social brain abnormalities are specific to an ASD diagnosis vs. familial risk. Studies of infants with elevated risk for ASD would benefit from examining whether differences in brain responses to social stimuli predict individual differences in related aspects of social functioning. Such analyses would provide initial evidence that early abnormalities in these brain responses may be a meaningful way to differentiate which infants are at highest risk for ASD. It is also possible that differential brain responses may be more likely to predict differences in more proximal aspects of social functioning (e.g., brain response to gaze shifting and behavioral response to joint attention bids) rather than the complex set of behaviors that comprise ASD.
While ASD is associated with broad deficits in social-communicative functioning, these deficits are most apparent during unstructured social interactions that require the integration of multi-modal stimuli and the complex coordination of reciprocal social initiations and responses. In fact, observation of an individual’s social and communication behaviors in the context of a relatively unstructured social interaction (e.g., with the Autism Diagnostic Observation Schedule [ADOS]; Lord et al., 2000) is an essential part of a comprehensive autism assessment. It follows then that studies that focus on brain responses to disembodied, unimodal social stimuli (e.g., faces) may sometimes fail to find expected differences in brain function (e.g., Hadjikhani et al., 2004; Webb et al., 2010). Rather, as Rolison et al. (2015) argue, an interactive social neuroscience approach in which brain responses of individuals with ASD are assessed in the context of actual social interaction has the potential to further our understanding of underlying neural mechanisms and potential treatment targets for individuals with ASD.
5.4. Final conclusions
The current review focused on the contribution of fNIRS to the study of infant social brain development, highlighting the potential of this methodology for studies using more ecologically valid social stimuli. We conclude that while initial studies using traditional pre-recorded social stimuli have provided a strong basis for understanding infant brain responses to isolated or highly controlled forms of social information, future studies should strive to take advantage of the flexibility of fNIRS by examining infant brain responses in the context of live social interaction. These studies can focus on individual brain responses or inter-brain coherence through the measurement of both social partners. We further argue that an interactive social neuroscience approach may be particularly well suited to the study of autism, given the difficulties with which individuals with ASD have in dynamic social situations requiring social and emotional reciprocity. While the use of live social stimuli necessarily limits experimental control, studies examining infant social brain function within naturalistic social contexts using fNIRS offer an important addition to this growing literature.
Highlights.
fNIRS is well tolerated by infants and well suited to measuring localized brain activity in response to pre-recorded, multi-modal social stimuli.
fNIRS is a promising methodology for measuring infant brain activity during naturalistic social interactions.
Recommendations for the use of fNIRS to study infants in naturalistic social situations and the potential of this approach in the study of autism are discussed.
Acknowledgments
We thank Shafali Jeste for her valuable feedback on this review, as well as Kevin Pelphrey and Charles Nelson, III for their guidance and mentorship. The first author’s work on this review was supported by the National Institute of Mental Health (F32 MH108283-01). The second author’s work was supported by the National Institute of Mental Health (4R01 MH078829) and the Bill and Melinda Gates Foundation (OPP1111625). The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- Aslin RN, Shukla M, Emberson LL. Hemodynamic correlates of cognition in human infants. Annu Rev Psychol. 2015;66:349–379. doi: 10.1146/annurev-psych-010213-115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni F, Astolfi L. Social neuroscience and hyperscanning techniques: past, present and future. Neurosci Biobehav Rev. 2014;44:76–93. doi: 10.1016/j.neubiorev.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Beurlot MR, Fava E, Nath AR, Parikh NA, Saad ZS, … Oghalai JS. The developmental trajectory of brain-scalp distance from birth through childhood: implications for functional neuroimaging. PLoS One. 2011;6(9):e24981. doi: 10.1371/journal.pone.0024981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristia A, Minagawa Y, Dupoux E. Responses to vocalizations and auditory controls in the human newborn brain. PLoS ONE. 2014;9(12):e115162. doi: 10.1371/journal.pone.0115162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Bryant DM, Reiss AL. NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage. 2012;59(3):2430–2437. doi: 10.1016/j.neuroimage.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers’ voices. Science. 1980;208(4448):1174–1176. doi: 10.1126/science.7375928. doi: http://dx.doi.org/10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Deen B, Richardson H, Dilks DD, Takahashi A, Keil B, Wald LL, … Saxe R. Organization of high-level visual cortex in human infants. Nature Communications. 2017;8:13995. doi: 10.1038/ncomms13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farroni T, Chiarelli AM, Lloyd-Fox S, Massaccesi S, Merla A, Di Gangi V, … Johnson MH. Infant cortex responds to other humans from shortly after birth. Sci Rep. 2013;3:2851. doi: 10.1038/srep02851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Mother-infant synchrony and the development of moral orientation in childhood and adolescence: direct and indirect mechanisms of developmental continuity. Am J Orthopsychiatry. 2007a;77(4):582–597. doi: 10.1037/0002-9432.77.4.582. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry. 2007b;48(3–4):329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Greenbaum CW, Yirmiya N. Mother-infant affect synchrony as an antecedent of the emergence of self-control. Dev Psychol. 1999;35(1):223–231. doi: 10.1037//0012-1649.35.1.223. [DOI] [PubMed] [Google Scholar]
- Fox SE, Wagner JB, Shrock CL, Tager-Flusberg H, Nelson CA. Neural processing of facial identity and emotion in infants at high-risk for autism spectrum disorders. Front Hum Neurosci. 2013;7:89. doi: 10.3389/fnhum.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MC, Vul E, Johnson SP. Development of infants’ attention to faces during the first year. Cognition. 2009;110(2):160–170. doi: 10.1016/j.cognition.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T. The development of social brain functions in infancy. Psychol Bull. 2015;141(6):1266–1287. doi: 10.1037/bul0000002. [DOI] [PubMed] [Google Scholar]
- Grossmann T, Cross ES, Ticini LF, Daum MM. Action observation in the infant brain: the role of body form and motion. Soc Neurosci. 2013;8(1):22–30. doi: 10.1080/17470919.2012.696077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH. Selective prefrontal cortex responses to joint attention in early infancy. Biol Lett. 2010;6(4):540–543. doi: 10.1098/rsbl.2009.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH, Lloyd-Fox S, Blasi A, Deligianni F, Elwell C, Csibra G. Early cortical specialization for face-to-face communication in human infants. Proc Biol Sci. 2008;275(1653):2803–2811. doi: 10.1098/rspb.2008.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Oberecker R, Koch SP, Friederici AD. The developmental origins of voice processing in the human brain. Neuron. 2010a;65(6):852–858. doi: 10.1016/j.neuron.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Parise E, Friederici AD. The detection of communicative signals directed at the self in infant prefrontal cortex. Front Hum Neurosci. 2010b;4:201. doi: 10.3389/fnhum.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, … Tager- Flusberg H. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22(3):1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hirsch J, Zhang X, Noah JA, Ono Y. Frontal temporal and parietal systems synchronize within and across brains during live eye-to-eye contact. Neuroimage. 2017;157:314–330. doi: 10.1016/j.neuroimage.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Asakawa K, Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neurosci Res. 2006;54:276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Taga G. Prosodic processing in the developing brain. Neurosci Res. 2007;59:29–39. doi: 10.1016/j.neures.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Otobe T, Nakano T, Go T, Konishi Y, Taga G. Development of global cortical networks in early infancy. J Neurosci. 2010;30(14):4877–4882. doi: 10.1523/jneurosci.5618-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Taga G. Large-scale brain networks underlying language acquisition in early infancy. Front Psychol. 2011;2:93. doi: 10.3389/fpsyg.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Nakato E, Otsuka Y, Kanazawa S, Kojima S, Yamaguchi MK, Kakigi R. How do infants perceive scrambled face?: A near-infrared spectroscopic study. Brain Res. 2010;1308:137–146. doi: 10.1016/j.brainres.2009.10.046. [DOI] [PubMed] [Google Scholar]
- Imafuku M, Hakuno Y, Uchida-Ota M, Yamamoto J, Minagawa Y. “Mom called me!” Behavioral and prefrontal responses of infants to self-names spoken by their mothers. Neuroimage. 2014;103:476–484. doi: 10.1016/j.neuroimage.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Jaffe J, Beebe B, Feldstein S, Crown CL, Jasnow MD. Rhythms of dialogue in infancy: coordinated timing in development. Monogr Soc Res Child Dev. 2001;66(2):i–viii. 1–132. [PubMed] [Google Scholar]
- Jiang J, Chen C, Dai B, Shi G, Ding G, Liu L, Lu C. Leader emergence through interpersonal neural synchronization. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(14):4274–4279. doi: 10.1073/pnas.1422930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Dai B, Peng D, Zhu C, Liu L, Lu C. Neural synchronization during face-to-face communication. J Neurosci. 2012;32(45):16064–16069. doi: 10.1523/jneurosci.2926-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJ, Venema K, Lowy R, Earl RK, Webb SJ. Developmental changes in infant brain activity during naturalistic social experiences. Dev Psychobiol. 2015;57(7):842–853. doi: 10.1002/dev.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJH, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience and. 2014 doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Wagner JB, Tager-Flusberg H, Nelson CA. Functional connectivity in the first year of life in infants at-risk for autism: a preliminary near-infrared spectroscopy study. Front Hum Neurosci. 2013;7:444. doi: 10.3389/fnhum.2013.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida T, Shinohara K. Gentle touch activates the prefrontal cortex in infancy: an NIRS study. Neurosci Lett. 2013;541:63–66. doi: 10.1016/j.neulet.2013.01.048. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Macchi Cassia V, Kanazawa S, Yamaguchi MK, Kakigi R. Perceptual narrowing towards adult faces is a cross-cultural phenomenon in infancy: a behavioral and near-infrared spectroscopy study with Japanese infants. Dev Sci. 2016 doi: 10.1111/desc.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Mok C, Witt EE, Pradhan AH, Chen JE, Reiss AL. NIRS-based hyperscanning reveals inter-brain neural synchronization during cooperative Jenga game with face-to-face communication. Front Hum Neurosci. 2016;10:82. doi: 10.3389/fnhum.2016.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Pelowski M. A new research trend in social neuroscience: Towards an interactive-brain neuroscience. Psych J. 2014;3(3):177–188. doi: 10.1002/pchj.56. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Begus K, Halliday D, Pirazzoli L, Blasi A, Papademetriou M, … Elwell CE. Cortical specialisation to social stimuli from the first days to the second year of life: A rural Gambian cohort. Dev Cogn Neurosci. 2016 doi: 10.1016/j.dcn.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci Biobehav Rev. 2010;34(3):269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Elwell CE, Charman T, Murphy D, Johnson MH. Reduced neural sensitivity to social stimuli in infants at risk for autism. Proc Biol Sci. 2013;280(1758):20123026. doi: 10.1098/rspb.2012.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Everdell N, Elwell CE, Johnson MH. Selective cortical mapping of biological motion processing in young infants. J Cogn Neurosci. 2011;23(9):2521–2532. doi: 10.1162/jocn.2010.21598. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Mercure E, Elwell CE, Johnson MH. The emergence of cerebral specialization for the human voice over the first months of life. Soc Neurosci. 2012;7(3):317–330. doi: 10.1080/17470919.2011.614696. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Volein A, Everdell N, Elwell CE, Johnson MH. Social perception in infancy: a near infrared spectroscopy study. Child Dev. 2009;80(4):986–999. doi: 10.1111/j.1467-8624.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Szeplaki-Kollod B, Yin J, Csibra G. Are you talking to me? Neural activations in 6-month-old infants in response to being addressed during natural interactions. Cortex. 2015;70:35–48. doi: 10.1016/j.cortex.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Wu R, Richards JE, Elwell CE, Johnson MH. Cortical activation to action perception is associated with action production abilities in young infants. Cereb Cortex. 2015;25(2):289–297. doi: 10.1093/cercor/bht207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, … Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Marx V, Nagy E. Fetal behavioural responses to maternal voice and touch. PLoS One. 2015;10(6):e0129118. doi: 10.1371/journal.pone.0129118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Matsuoka S, Dan I, Naoi N, Nakamura K, Kojima S. Prefrontal activation associated with social attachment: facial-emotion recognition in mothers and infants. Cereb Cortex. 2009;19(2):284–292. doi: 10.1093/cercor/bhn081. [DOI] [PubMed] [Google Scholar]
- Nakato E, Otsuka Y, Kanazawa S, Yamaguchi MK, Kakigi R. Distinct differences in the pattern of hemodynamic response to happy and angry facial expressions in infants--a near-infrared spectroscopic study. Neuroimage. 2011;54(2):1600–1606. doi: 10.1016/j.neuroimage.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Naoi N. Cerebral responses to joint attention episodes in infants. In: Minagawa-Kawai Y, Naoi N, Kojima S, editors. A new approach to functional neuroimaging: Near-infrared spectroscopy. Keio University Press; 2009. pp. 82–86. [Google Scholar]
- Naoi N, Minagawa-Kawai Y, Kobayashi A, Takeuchi K, Nakamura K, Yamamoto J, Kojima S. Cerebral responses to infant-directed speech and the effect of talker familiarity. Neuroimage. 2012;59(2):1735–1744. doi: 10.1016/j.neuroimage.2011.07.093. [DOI] [PubMed] [Google Scholar]
- Nelson CA. The development and neural bases of face recognition. Infant and Child Development. 2001;10(1–2):3–18. doi: http://dx.doi.org/10.1002/icd.239. [Google Scholar]
- Nishiyori R. fNIRS: An emergent method to document functional cortical activity during infant movements. Front Psychol. 2016;7:533. doi: 10.3389/fpsyg.2016.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka Y, Nakato E, Kanazawa S, Yamaguchi MK, Watanabe S, Kakigi R. Neural activation to upright and inverted faces in infants measured by near infrared spectroscopy. Neuroimage. 2007;34(1):399–406. doi: 10.1016/j.neuroimage.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Pegg JE, Werker JF, McLeod PJ. Preference for infant-directed over adult-directed speech: Evidence from 7-week-old infants. Infant Behav Dev. 1992;15(3):325–345. doi: 10.1016/0163-6383(92)80003-D. [DOI] [Google Scholar]
- Perlman SB, Huppert TJ, Luna B. Functional near-infrared spectroscopy evidence for development of prefrontal engagement in working memory in early through middle childhood. Cereb Cortex. 2016;26(6):2790–2799. doi: 10.1093/cercor/bhv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti P, Merla A, Aichelburg C, Lind F, Power S, Swingler E, … Tachtsidis I. A novel GLM-based method for the Automatic IDentification of functional Events (AIDE) in fNIRS data recorded in naturalistic environments. Neuroimage. 2017;155:291–304. doi: 10.1016/j.neuroimage.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravicz MM, Perdue KL, Westerlund A, Vanderwert RE, Nelson CA. Infants’ neural responses to facial emotion in the prefrontal cortex are correlated with temperament: a functional near-infrared spectroscopy study. Front Psychol. 2015;6:922. doi: 10.3389/fpsyg.2015.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid VM, Striano T, Iacoboni M. Neural correlates of dyadic interaction during infancy. Dev Cogn Neurosci. 2011;1(2):124–130. doi: 10.1016/j.dcn.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolison MJ, Naples AJ, McPartland JC. Interactive Social Neuroscience to Study Autism Spectrum Disorder. The Yale Journal of Biology and Medicine. 2015;88(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Hiraki K. Infant’s brain responses to live and televised action. Neuroimage. 2006;32(2):930–939. doi: 10.1016/j.neuroimage.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Shultz S, Vouloumanos A, Bennett RH, Pelphrey K. Neural specialization for speech in the first months of life. Developmental Science. 2014;17(5):766–774. doi: 10.1111/desc.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa S, Takamoto K, Ishikawa A, Ono T, Nishijo H. Selective Medial Prefrontal Cortex Responses During Live Mutual Gaze Interactions in Human Infants: An fNIRS Study. Brain Topogr. 2015;28(5):691–701. doi: 10.1007/s10548-014-0414-2. [DOI] [PubMed] [Google Scholar]
- Vanderwert RE, Nelson CA. The use of near-infrared spectroscopy in the study of typical and atypical development. Neuroimage. 2014;85(Pt 1):264–271. doi: 10.1016/j.neuroimage.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJ, Merkle K, Murias M, Greenson J, Richards T, … Dawson G. Response to familiar faces, newly familiar faces, and novel faces as assessed by ERPs is intact in adults with autism spectrum disorders. Int J Psychophysiol. 2010;77(2):106–117. doi: 10.1016/j.ijpsycho.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Hoshino E, Yatabe K, Matsuda S, Sato H, Maki A, … Minagawa Y. Prefrontal function engaging in external-focused attention in 5- to 6-month-old infants: a suggestion for default mode network. Front Hum Neurosci. 2017;10 doi: 10.3389/fnhum.2016.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Sun J, Sun B, Luo Q, Gong H. Studying hemispheric lateralization during a Stroop task through near-infrared spectroscopy-based connectivity. J Biomed Opt. 2014;19(5):57012. doi: 10.1117/1.jbo.19.5.057012. [DOI] [PubMed] [Google Scholar]