Abstract
Background
There is conflicting evidence on the effect of obesity on lung function in adults and children with and without asthma. We aimed to evaluate the relation between overweight or obesity and lung function, and whether such relationship varies by age, sex, or asthma status.
Methods
We searched PubMed, Scopus, CINAHL, Cochrane, and EMBASE for all studies (in English) reporting on obesity status (by BMI) and lung function, from 2005 to 2017. Main outcomes were FEV1, FVC, FEV1/FVC, FEF25-75, TLC, RV, and FRC. Random-effects models were used to calculate the pooled risk estimates; each study was weighed by the inverse effect size variance. For each outcome, we compared overweight or obese (“obese”) subjects with those of normal weight.
Results
All measures of lung function were decreased among obese subjects. Obese adults showed a pattern (lower FEV1, FVC, TLC, and RV) different from obese children (more pronounced FEV1/FVC deficit with unchanged FEV1 or FVC). There were also seemingly different patterns by asthma status, in that subjects without asthma had more marked decreases in FEV1, TLC, RV and FRC than subjects with asthma. Subjects who were obese (as compared to overweight) had even further decreased FEV1, FVC, TLC, RV, and FRC.
Conclusion
Obesity is detrimental to lung function, but specific patterns differ between children and adults. Physicians should be aware of adverse effects of obesity on lung function, and weight-control should be considered in the management of airway disease among the obese.
Keywords: Obesity, childhood obesity, lung function, asthma, meta-analysis
INTRODUCTION
The worldwide epidemic of obesity is a major public health problem, particularly in industrialized countries. Obesity is associated with numerous complications, including multiple detrimental effects on the respiratory system1. Several plausible mechanisms have been proposed to explain the observed association between obesity and respiratory symptoms, such as decreased total respiratory system compliance, increased airway resistance, reduced lung volumes, and altered ventilation and gas exchange2, 3. Overweight and obesity have also been associated with higher incidence of asthma4, asthma morbidity, and resistance to therapy5. Evidence shows that weight gain precedes the development of asthma symptoms6 and that obese individuals have later decreases in lung function7. Conversely, weight loss results in improvement of asthma-related health outcomes in adults 8, 9.
In adults, an inverse association between BMI and lung volumes indicates that obesity leads to a restrictive lung deficit10, 11. However, some studies have also reported slightly lower FEV1/FVC in adults with asthma12. On the other hand, several studies in children have reported that increased BMI is associated with normal –or even increasing– FEV1 and FVC but low FEV1/FVC, consistent with either an obstructive deficit or airway dysanapsis13, 14, with some reports showing differences by sex15. Yet other studies have shown findings in children that may be suggestive of a restrictive deficit, which may be more similar to findings reported in adults16–18. Such inconsistencies may partly be due to differences among study populations and outcome measures. More importantly, they suggest that the association between obesity and lung function may differ between children and adults. In this meta-analysis, we aim to further elucidate the relationship between overweight or obesity and lung function, and whether such relationship differs by age group or by asthma status.
METHODS
We prospectively registered the protocol for this meta-analysis on 10/07/2015 at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015023193.
Search and selection criteria
We searched PubMed, Scopus, CINAHL, and the Cochrane Database for all studies reporting obesity status and lung function in human subjects, published in English from 2005 (standardization of spirometry19) to January of 2017, using the following keywords: (“Body Mass Index” OR “BMI” OR “percent body fat” OR “body fat distribution” OR “Waist Circumference” OR “Obesity” OR “Overweight” OR “Waist-hip ratio” OR “Adiposity” OR “abdominal obesity”) AND [(“FEV1” OR “FVC” OR “FEV1/FVC” OR “FEF2575” OR “PEFR” OR “TLC” OR “RV/TLC” OR “FRC” OR “lung function” OR “spirometry”) NOT (“cystic fibrosis” OR “COPD” OR “cancer”)].
Inclusion criteria were: 1) observational studies of children or adults with assessments of obesity and lung function; or 2) baseline data from experimental studies focused on obesity and lung function. Exclusion criteria were: 1) studies focused on cystic fibrosis or chronic obstructive pulmonary disease (COPD); 2) studies that included diseases or therapies that may affect the lung function of subjects (e.g. cancer, radiotherapy); and 3) obesity secondary to specific diseases (e.g. hypothyroidism, hypertension) or medical treatments. Our primary outcomes were spirometry measures (FEV1, FVC, FEV1/FVC, and FEF25-75). Secondary outcomes were measures of lung volume (TLC, RV, RV/TLC, and FRC). Study subjects were classified as “normal weight (reference group)”, “overweight”, or “obese” by BMI (z-score in children and kg/m2 in adults). Central or abdominal obesity was defined by waist circumference (WC) or waist-to-hip ratio (WHR).
Data abstraction and data analysis
The study was performed following the recommendations for reporting meta-analyses of observational studies by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group20 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines21. Titles, abstracts, contexts, and citations were independently assessed and analyzed by two investigators (YYH and JM). Disagreements were resolved through a mediator (EF).
For all outcomes analyzed, we calculated the pooled β coefficients (weighted mean difference [WMD]) and 95% confidence intervals (CI) using random-effects models to address the heterogeneity across the included studies. Each study was weighted by its inverse effect size variance. Some studies were included more than once when they had several comparisons (e.g. overweight vs. normal weight, and obese vs. normal weight) or strata (e.g. males and females, children [≤ 18 years] and adults [>18 years]). Egger’s test and funnel plots with pseudo 95% CI were used to examine small-study effects and publication bias. When possible, meta-regression was performed to explore potential sources of heterogeneity and test whether certain characteristics (e.g. age-group, sex distribution) modify the effect of obesity on lung function; meta-regression was performed only when ≥10 studies reported a specific characteristic. All analyses were performed using STATA v13 (StataCorp, College Station, TX).
RESULTS
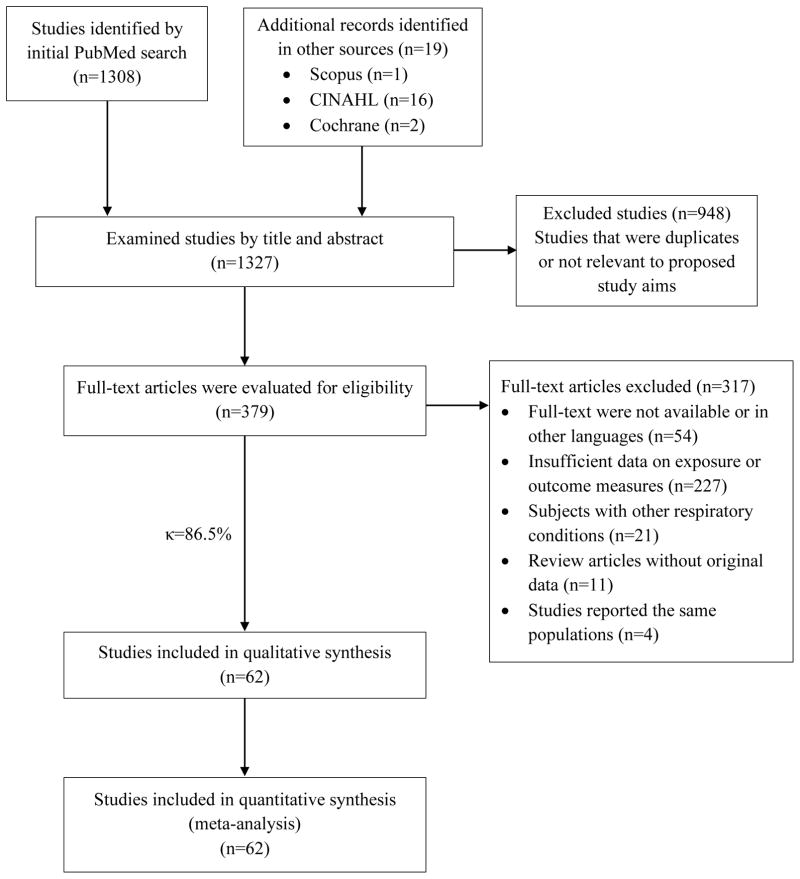
A total of 1327 articles were initially identified (Figure 1). After removal of duplicates and non-relevant studies, 379 studies were evaluated. Based on full-text screening, the authors agreed on 328 of 379 articles (inter-reader κ = 86.5%). After arbitration, and based on inclusion and exclusion criteria, a total of 62 studies were included for meta-analysis. Because very few studies reported WC or WHR, only studies using BMI to define overweight/obesity were included.
Figure 1. Study selection flowchart.
κ = Kappa agreement coefficient.
Forced expiratory volume in 1 second (FEV1)
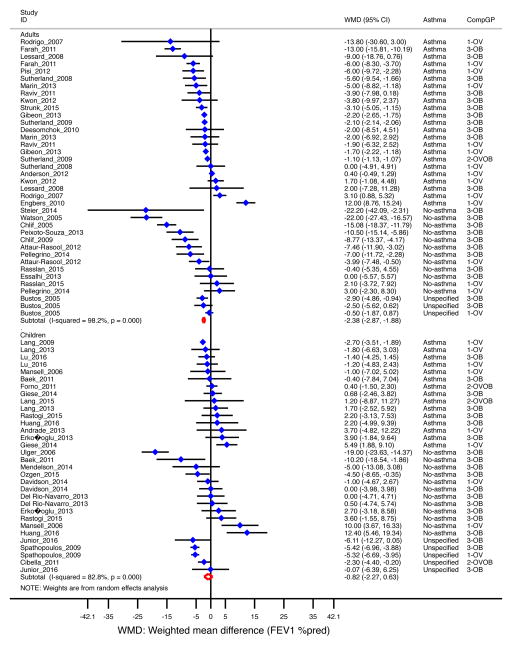
When analyzing all selected studies (44 studies, n=23,460 subjects)12, 15–18, 22–60, overweight/obese subjects had 2.2% lower percent-predicted FEV1 [WMD −2.2, 95% CI=−2.6 to −1.8] than subjects of normal weight (Table 1). The decrement was significant in adults (WMD −2.4%, 95% CI= −2.9 to −1.9]) but not in children (−0.8%, 95% CI= −2.3 to 0.6], Figure 2); and more pronounced among overweight/obese subjects without asthma (−3.9%, 95% CI=−7.1 to −0.7) than in those with asthma (−1.4% [−1.9, −0.9]) (Supplemental Figure E1). Similar results were found when analyzing adults only by asthma status: overweight/obese adults with asthma had 1.7% (95% CI= −2.3 to −1.2) lower FEV1; while those without asthma had 6.9% (95% CI= −11.1 to −2.8) lower FEV1 (Table 1). These results were driven mainly by obesity (−2.5%, 95% CI= −3.4 to −1.5%) in subjects with asthma and −5.3% (95% CI= −8.8 to −1.8) in those without) rather than by overweight (non-significant; data not shown).
Table 1.
Pooled estimates of overweight or obesity and lung function by age group and asthma.
| FEV1 (%pred) | FVC (%pred) | FEV1/FVC (%) | FEF25-75 (%pred) | TLC (%pred) | RV (%pred) | FRC (%pred) | |
|---|---|---|---|---|---|---|---|
| All | −2.2 (−2.6, −1.8) | −2.2 (−3.7, −0.6) | −1.5 (−1.9, −1.2) | −5.4 (−7.3, −3.5) | −4.2 (−5.4, −3.0) | −6.6 (−9.3, −3.8) | −17.1 (−25.2, −9.0) |
| By age | |||||||
| Adult | −2.4 (−2.9, −1.9) | −4.6 (−6.9, −2.2) | −1.0 (−1.4, −0.6)* | −3.3 (−10.1, 3.6) | −4.4 (−5.7, −3.0) | −5.4 (−8.2, −2.7) | −16.9 (−25.4, −8.2) |
| Child | −0.8 (−2.3, +0.6) | 0.3 (−1.7, +2.3) | −2.4 (−3.0, −1.8)* | −4.7 (−6.9, −2.6) | −3.7 (−5.8, −1.5) | −33.0 (−44.2, −21.7)1 | −18.5 (−24.4, −12.6)1 |
| By asthma | |||||||
| Asthma | −1.4 (−1.9, −0.9) | −1.7 (−3.7, +0.3) | −1.5 (−1.9, −1.0) | −3.2 (−6.3, −0.1) | −3.3 (−5.1, −1.5) | −5.3 (−9.4, −1.3) | −3.7 (−15.0, +7.6) |
| No asthma | −3.9 (−7.1, −0.7) | −2.8 (−7.8, +2.3) | −1.6 (−2.3, −0.8) | −4.4 (−10.0, +1.2) | −4.8 (−7.1, −2.5) | −7.6 (−11.7, −3.5) | −23.2 (−30.3, −16.1) |
| By age and asthma | |||||||
| Adult, asthma | −1.7 (−2.3, −1.2)* | −3.2 (−6.0, −0.4) | −1.2 (−1.8, −0.6) | −3.3 (−10.1, +3.6) | −3.3 (−5.2, −1.4) | −4.1 (−8.2, −0.1) | −0.7 (−12.5, +11.0)* |
| Adult, no asthma | −6.9 (−11.1, −2.8)* | −7.5 (−11.4, −3.7) | −0.9 (−1.9, +0.1) | -- | −5.3 (−8.2, −2.4) | −6.5 (−10.2, −2.7) | −23.6 (−31.1, −16.1)* |
| Child, asthma | 0.4 (−1.2, +2.0) | 0.4 (−0.6, +1.5) | −1.8 (−2.4, −1.3) | −2.5 (−6.6, +1.5) | −3.6 (−8.0, +0.8) | -- | -- |
| Child, no asthma | −0.9 (−5.4, +3.6) | 2.8 (−6.8, +12.5) | −2.8 (−3.9, −1.8) | −4.4 (−10.0, +1.2) | −3.7 (−6.2, −1.3) | -- | -- |
| By weight group | |||||||
| Overweight | 0.37 (−1.7, +0.9) | −0.3 (−1.8, +1.2) | −1.2 (−1.9, −0.6) | −4.8 (−7.2, −2.4) | −2.0 (−4.1, +0.1) | −5.8 (−12.9, +1.4) | −8.2 (−19.7, +3.4) |
| Obese | −3.9 (−4.8, −2.9) | −3.2 (−5.2, −1.2) | −1.7 (−2.5, −0.9) | −4.6 (−8.1, −1.2) | −5.4 (−7.0, −3.9) | −7.4 (−11.6, −3.2) | −21.2 (−30.9, −11.5) |
Shown are weighted mean differences (WMDs) for overweight/obese vs normal weight. Significant WMDs in bold.
WMDs are significantly different between the groups (e.g. WMD for adult vs. child, or asthma vs. no asthma).
Only one study and thus only that study’s effect size reported (not a pooled estimate).
Figure 2. Obesity and FEV1 (as percent of predicted) by age group.
Forest plot for FEV1 %pred, showing weighted mean difference (WMD) by study, for each comparison group (CompGP) vs normal-weight subjects. OV: Overweight. OB: Obese. OVOB: Overweight or obese.
When analyzing studies that reported FEV1 as an absolute value (liters)61–69, overweight/obese children had 410 mL [95% CI= 280mL to 540mL] higher FEV1, while overweight/obese adults had 150mL [95% CI= −260mL to −40mL] lower FEV1 (Supplemental Figure E2).
Forced vital capacity (FVC)
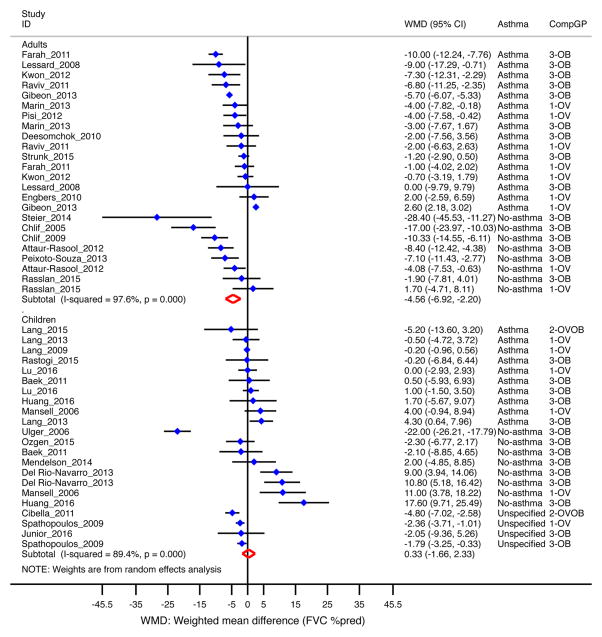
In 30 studies included (n=16,913), percent-predicted FVC decreased by 2.2% [95% CI= −3.7 to −0.6%] among overweight/obese subjects compared to those of normal weight 15–18, 23–29, 32–34, 36, 37, 39, 40, 45, 46, 48, 50, 52–59. The decrement was significant in adults (−4.6% [95% CI= −6.9 to −2.2%]) but not in children (Figure 3); and more pronounced among obese subjects (−3.2% [95% CI= −5.2 to −1.2%] than in overweight subjects (Supplemental Figure E3). When stratifying by asthma status, FVC decreased further in adults without asthma (−7.5% [95% CI= −11.4 to −3.7%]) than in those with asthma (−3.2% [95% CI= −6.0 to −0.4%]). Among studies that reported FVC as absolute value (liters)51, 61–70, overweight/obese children had an increased FVC (250mL [95% CI= 190mL to 300mL]), while overweight/obese adults had a decreased FVC (−140mL [95% CI= −250mL to −40mL], data not shown).
Figure 3. Obesity and FVC (as percent of predicted) by age group.
Forest plot for FVC %pred, showing weighted mean difference (WMD) by study, for each comparison group (CompGP) vs normal-weight subjects. OV: Overweight. OB: Obese. OVOB: Overweight or obese.
FEV1/FVC
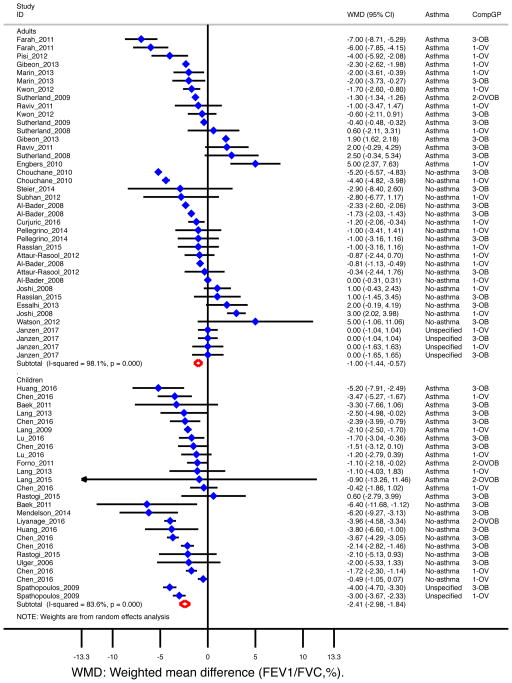
For all 34 studies combined (n=28,494), FEV1/FVC was 1.5% [95% CI= −1.9 to −1.2%] lower among overweight/obese subjects12, 15–18, 23–29, 31, 34, 35, 38, 40, 45, 47, 48, 50, 53, 55, 57, 59, 61–63, 66–71 (Table 1). The decrement was more pronounced in children (−2.4%, [95% CI= −3.0 to −1.8%]) than in adults (−1.0% [95% CI= −1.4 to −0.6%]) (Figure 4); and it was similar in subjects with (−1.5% [95% CI= −1.9 to −1.0%]) and without asthma (−1.6% [95% CI= −2.3 to −0.8%]) (Table 1). When reviewing twelve studies that reported FEV1/FVC as percent of predicted, 10, 32, 33, 36, 37, 39, 44, 54, 56,58, 60, 72, 73 FEV1/FVC was lower among overweight/obese children (−1.7% [95% CI= −3.2 to −0.2%]) but slightly increased among overweight/obese adults (1.3% [95% CI= 0.01 to 2.6%], data not shown).
Figure 4. Obesity and FEV1/FVC (%) by age group.
Forest plot for FEV1/FVC, showing weighted mean difference (WMD) by study, for each comparison group (CompGP) vs normal-weight subjects. OV: Overweight. OB: Obese. OVOB: Overweight or obese.
Maximum mid-expiratory flow (MMEF or FEF25-75)
Percent-predicted FEF25-75 was significantly decreased (−5.4% [95% CI= −7.3 to −3.5%]) among overweight/obese subjects in 16 studies (n=13,627) 16–18, 28, 32, 33, 43, 45, 46, 50–52, 54, 55, 58, 74. The decrement was significant in children (−4.7% [95% CI= −6.9 to −2.6%]) but not in adults (Supplemental Figure E4). It was significant only subjects with asthma (−3.2%, [−6.3 to −0.1%]); and after stratifying by weight, it was similarly decreased in overweight (−4.8%, [95% CI= −7.2 to −2.4%]) and obese (−4.6%, [95% CI= −8.1 to −1.2%]) subjects (Table 1).
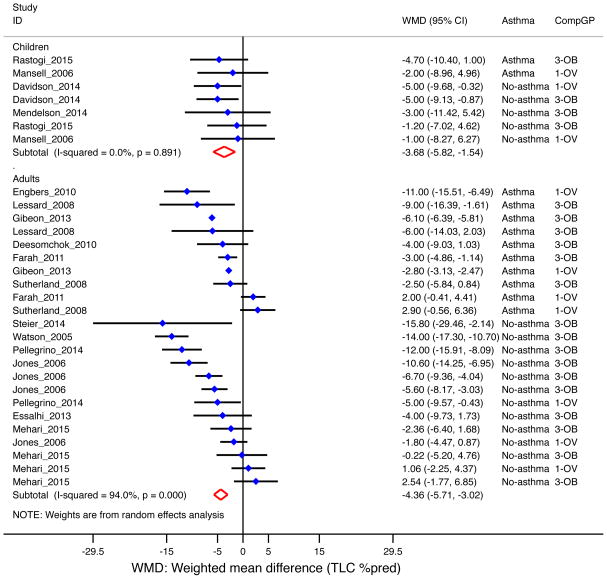
Total lung capacity (TLC)
Overall, overweight/obese subjects had 4.2% [95% CI= −5.4 to −3.0%] lower percent-predicted TLC based on 16 studies (n=2,678)10, 23–25, 31–33, 35, 38, 40, 41, 46, 50, 51, 53, 73, with similar findings in children (−3.7% [95% CI= −5.8 to −1.5%]) and adults (−4.4% [95% CI= −5.7 to −3.0%], Figure 5). The decrease was more pronounced in subjects without asthma (−4.8% [95% CI= −7.1 to −2.5%]) than among those with asthma (−3.3% [95% CI= −5.1 to −1.5%], Supplemental Figure E5); and in the obese (−5.4% [95% CI= −7.0 to −3.9%]) than in the overweight (−2.0% [95% CI= −4.1 to 0.1%], Supplemental Figure E6).
Figure 5. Obesity and TLC (as percent of predicted) by age group.
Forest plot for TLC %pred, showing weighted mean difference (WMD) by study, for each comparison group (CompGP) vs normal-weight subjects. OV: Overweight. OB: Obese. OVOB: Overweight or obese.
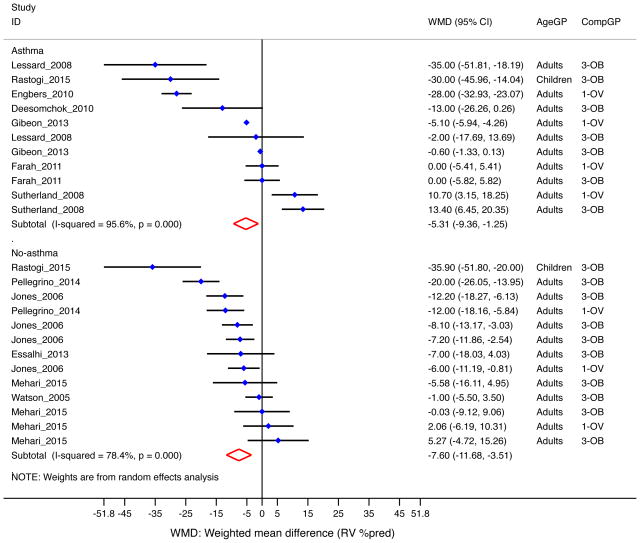
Residual volume (RV)
In 12 studies combined (n=2,085), overweight/obese subjects had 6.6% [95% CI= −9.3 to −3.8%] lower percent-predicted RV10, 23–25, 31–33, 35, 38, 41, 50, 73. The decrement in RV was significant in adults (−5.4% [95% CI= −8.2 to −2.7%]) (Table 1); only one study reported RV in children50, and thus no pooled analysis was performed. The effect was more pronounced in subjects without asthma (−7.6% [95% CI= −11.7 to −3.5%]) than in those with asthma (Figure 6), and in the obese (−7.4% [−11.6 to −3.2%]) than in the overweight (Table 1). Four studies reported RV/TLC ratio measures10, 40, 50, 73; there was no significant change in RV/TLC by overweight/obesity (data not shown).
Figure 6. Obesity and RV (as percent of predicted) by asthma status.
Forest plot for RV %pred, showing weighted mean difference (WMD) by study, for each comparison group (CompGP) vs normal-weight subjects. OV: Overweight. OB: Obese. OVOB: Overweight or obese.
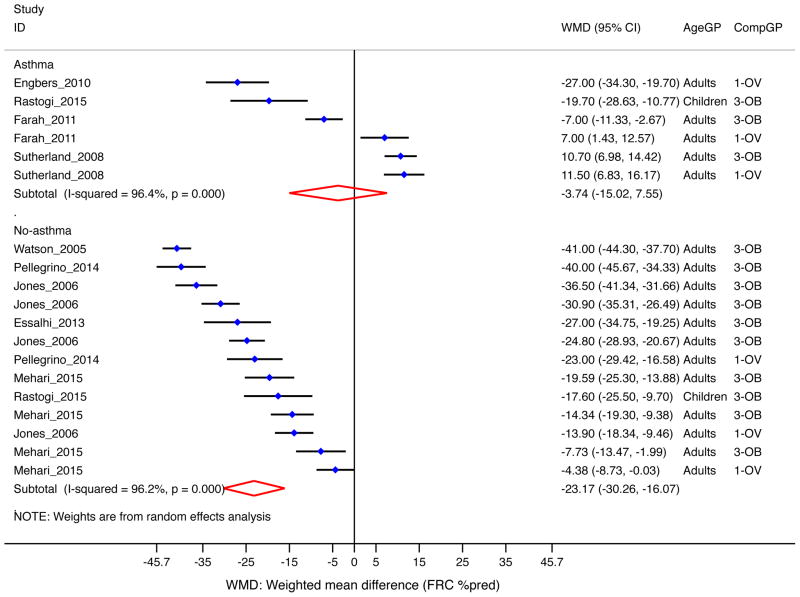
Functional residual capacity (FRC)
Overall, percent-predicted FRC was significantly lower among overweight/obese subjects than in those normal weight (−17.1% [−25.2, −9.0], 9 studies, n=1,235)10, 23, 24, 31, 35, 38, 41, 50, 73. The decrement was more pronounced in subjects without asthma (−23.2% [−30.3, −16.1], Figure 7); and in the obese (−21.2% [−30.9%, −11.5], Supplemental Figure E7).
Figure 7. Obesity and FRC (as percent of predicted) by asthma status.
Forest plot for FRC %pred, showing weighted mean difference (WMD) by study, for each comparison group (CompGP) vs normal-weight subjects. OV: Overweight. OB: Obese. OVOB: Overweight or obese.
Assessment of publication bias and meta-regression
Egger tests showed no evidence of publication bias in any of the measures, except for RV (p=0.003, Supplemental Figure E8). There was marked heterogeneity (I2>70%) in all outcomes and analyses, except for TLC in children (I2=0%). Meta-regression analyses showed that age and sex distributions explain part of this heterogeneity. Studies with higher proportion of males showed a more pronounced effect of obesity on FEV1 (β=−0.09, p=0.019) in adults, and on FEV1/FVC in subjects with asthma (β=−0.04, p=0.002). Studies with higher mean age showed a more significant effect of obesity on FVC (β=−0.17, p=0.013) in all subjects, and FEV1/FVC in all subjects (β=0.06, p=0.005) (Supplemental Figure E9).
DISCUSSION
In this meta-analysis, overweight/obesity is shown to be detrimental to lung function across age groups, regardless of asthma status. However, these detrimental effects differ between adults and children: obese adults have a more pronounced decline in FEV1, FVC, TLC, and RV; whereas obese children show a more pronounced decline in FEV1/FVC and FEF25-75. Likewise, more marked decrements in FEV1, TLC, RV and FRC were found in subjects without asthma than in those with asthma. Compared to overweight subjects, the obese also had more marked decrements in FEV1, FVC, TLC, RV, and FRC.
FEV1, the most frequently used spirometric index, is a function of airway resistance and total lung compliance. Overweight or obesity12, 24, 25, 27, 28, 34–37, 39–42, 56, 58 was significantly associated with lower FEV1 in the overall meta-analysis. However, the effect differed by age, with results driven by adults and no significant association in children. In fact, only a few individual studies reported a decreased FEV1 in overweight/obese children16–18, 45, 56, 58, and the pooled analysis of studies reporting FEV1 in liters showed that overweight/obese children had slightly higher absolute values. On the contrary, very few studies have shown an increased FEV1 in overweight adults23, 30 compared to adults of normal weight.
FVC, the total volume of air exhaled with maximally forced effort from a maximal inspiration, reflects the total compliance from both the lung and chest wall. Similar to FEV1, overweight or obesity 24–28, 33, 34, 36, 37, 39, 40, 67 was negatively associated with FVC in adults, but there was no significant association in children. Likewise, our pooled analysis of studies reporting absolute FVC showed a slight increase in FVC in overweight46, 66 or obese15, 52, 66 children. Thus, overweight/obesity is associated with lower FEV1 and FVC in adults, but with either normal or higher FEV1 and FVC in children. We have recently reported that overweight/obese children have increased risk of airway dysanapsis14, a phenomenon in which an asymmetrical growth of the lungs and airways lead to higher FEV1 and FVC but with a more pronounced effect on FVC. Subjects with dysanapsis thus have a low FEV1/FVC ratio but normal or supranormal FEV1 and FVC. We found an increase in FEV1 that appeared to be larger than the increase in FVC (WMD 410 mL vs 250 mL), but the current analysis is based on published study means rather than individual-level data, and thus we were not able to directly assess the association between obesity and dysanapsis.
Interestingly, the effect sizes for FEV1 and FVC were much larger among subjects without asthma than among those with asthma, suggesting that obesity may have stronger effects on FEV1 and FVC in healthy subjects. Although the reasons for this are beyond the scope of this analysis, mean FEV1 and FVC in all included studies was markedly higher among non-asthmatics than among asthmatics (see Supplementary Table E1), and thus we hypothesize that the effect of obesity is more readily apparent in subjects without asthma because of their normal lung function, whereas subjects with asthma already have lower lung function due to their disease, and thus the effect of obesity may not be as prominent.
In contrast to FEV1 and FVC, the overall decrease of FEV1/FVC among overweight or obese subjects was more pronounced in children than in adults. Similar results were observed for FEF25-75, which is the mean forced expiratory flow between 25% and 75% of the FVC: FEF25-75 decreased significantly among overweight or obese children16, 17, 45, 52, 58, 74 with an overall 5.4% decrement, but there was no significant associations in adults. Only one individual study reported a 9% decrease in FEF25-75 in asthmatic overweight adults28. FEF25-75 can be highly variable and thus should be interpreted with caution, but it has been correlated with bronchodilator responsiveness in asthmatic children with normal FEV1, suggesting clinically relevant reversible airflow obstruction75. Interestingly, the declines in FEV1/FVC and FEF25-75 were similar between overweight and obesity.
The effects of obesity on lung volumes have been better studied in adults. TLC is often used to identify a restrictive ventilatory deficit76. While TLC may be preserved in most obese subjects, other than those with morbid obesity or with excessive central adiposity77, our results indicate a reduction in TLC overall, with several individual studies showing that overweight or obesity diminished TLC both in adults10, 23–25, 33, 35, 40, 41 and –to a lesser degree– in children51. A more pronounced decrement in TLC was reported in obesity than in overweight, and in non-asthmatics than among asthmatics. Similarly, we observed that obesity was associated with lower RV in adults, and this association was seen both in asthmatics23–25, 32, 33, 50 and non-asthmatics10, 35, 38, 40, 41, 50, 51. Obesity was also associated with lower FRC among adults, which may be secondary to changes in the elastic properties of the chest wall that can lead to reduced airway caliber and increased airways resistance78. FRC was decreased only in overweight or obese non-asthmatics (~23.2% lower compared to normal weight asthmatics), but not in asthmatics, suggesting again that obesity may have a more noticeable detrimental effect on lung volumes in otherwise healthy individuals. Only one study reported RV and FRC in children, and we were thus unable to perform a pooled analysis.
Strengths and limitations
To the best of our knowledge, this is the first meta-analysis to encompass all published studies on obesity and lung function. Because of large sample size, we were able to stratify the analysis by age group, asthma, and weight category. We were also able to perform meta-regression to evaluate potential effect modifiers such as age or gender.
We also recognize several limitations. First, few studies of obesity and lung function have used indices other than BMI (e.g. percent body fat, waist-to-hip ratio, etc.), and thus we had to focus on BMI. Future efforts should aim to determine whether adiposity distribution has different effects on lung function. Second, most studies included in the analysis were cross-sectional, and thus a causal relationship between overweight or obesity and lung function cannot be determined. Third, most studies reported joint findings for males and females, and we could thus only evaluate the potential effect of sex by using the proportions from each individual study. Given the small number of studies reporting details on smoking, atopy and race/ethnicity, we were unable to assess whether these factors modify the effect of obesity on lung function; moreover, we had no data on pubertal status and thus could not assess whether it constitutes an effect modifier among children. Because we did not have individual-level data for these studies, we were unable to perform more detailed analyses, including the association between obesity and airway dysanapsis. Finally, we had insufficient data on RV and FRC among children to perform a pooled analysis of these measures.
Conclusion
Overweight and obesity have significant effects on lung function, including spirometric parameters and lung volumes. Overall, this association is observed regardless of age group and asthma status. However, specific patterns differed between children and adults. While obesity affected lung function in subjects with asthma, subjects without asthma had even more pronounced reductions –particularly for lung volumes. Physicians should be aware of the adverse effects of obesity on lung function, and weight control should be considered in the management of airway diseases among obese individuals.
Supplementary Material
What is known about the topic?
Overweight and obesity are associated with changes in lung function in children, adolescents, and adults. However, there is some conflicting evidence on whether these changes differ by age group, sex, or asthma status.
What does this article add to our knowledge?
We provide comprehensive pooled estimates of the effect of obesity on lung function, stratified by age group (pediatrics vs. adults) and asthma status. Moreover, we use meta-regression to evaluate the role of age and gender in these associations.
How does this study impact current management guidelines?
Detrimental changes in lung function constitute yet another complication of obesity. These changes differ by age, sex, and asthma status; clinicians should take these characteristics into account when evaluating obesity and lung function.
Acknowledgments
Funding: Dr. Forno’s contribution was supported by NIH grant HL125666 and by a grant from Children’s Hospital of Pittsburgh of UPMC. Dr. Celedón’s contribution was supported by NIH grants HL079966, HL117191 and HL119952, and by an endowment from the Heinz Foundation.
ABBREVIATIONS
- 95% CI
95% confedence interval
- BMI
Body mass index
- FEF25-75
Forced expiratory flow between 25th and 75th of the forced vital capacity
- FEV1
Forced expiratory volume in the 1st second
- FRC
Functional residual capacity
- FVC
Forced vital capacity
- RV
Residual volume
- TLC
Total lung capacity
- WC
Waist circumference
- WHR
Waist-to-hip ratio
- WMD
Weighted mean difference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McClean KM, Kee F, Young IS, Elborn JS. Obesity and the lung: 1. Epidemiology Thorax. 2008;63:649–54. doi: 10.1136/thx.2007.086801. [DOI] [PubMed] [Google Scholar]
- 2.Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13:203–10. doi: 10.1155/2006/834786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol (1985) 2010;108:206–11. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 4.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–35. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 6.Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006;91:334–9. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thyagarajan B, Jacobs DR, Jr, Apostol GG, Smith LJ, Jensen RL, Crapo RO, et al. Longitudinal association of body mass index with lung function: the CARDIA study. Respir Res. 2008;9:31. doi: 10.1186/1465-9921-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juel CT, Ali Z, Nilas L, Ulrik CS. Asthma and obesity: does weight loss improve asthma control? a systematic review. J Asthma Allergy. 2012;5:21–6. doi: 10.2147/JAA.S32232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulrik CS. Asthma and obesity: is weight reduction the key to achieve asthma control? Curr Opin Pulm Med. 2016;22:69–73. doi: 10.1097/MCP.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 10.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–33. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 11.King GG, Brown NJ, Diba C, Thorpe CW, Munoz P, Marks GB, et al. The effects of body weight on airway calibre. Eur Respir J. 2005;25:896–901. doi: 10.1183/09031936.05.00104504. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland ER, Lehman EB, Teodorescu M, Wechsler ME. Body mass index and phenotype in subjects with mild-to-moderate persistent asthma. J Allergy Clin Immunol. 2009;123:1328–34. e1. doi: 10.1016/j.jaci.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL Childhood Asthma Management Program Research G. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–41. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forno E, Weiner DJ, Mullen J, Sawicki G, Kurland G, Han YY, et al. Obesity and Airway Dysanapsis in Children with and without Asthma. Am J Respir Crit Care Med. 2017;195:314–23. doi: 10.1164/rccm.201605-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang JE, Holbrook JT, Wise RA, Dixon AE, Teague WG, Wei CY, et al. Obesity in children with poorly controlled asthma: Sex differences. Pediatr Pulmonol. 2013;48:847–56. doi: 10.1002/ppul.22707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulger Z, Demir E, Tanac R, Goksen D, Gulen F, Darcan S, et al. The effect of childhood obesity on respiratory function tests and airway hyperresponsiveness. Turk J Pediatr. 2006;48:43–50. [PubMed] [Google Scholar]
- 17.Spathopoulos D, Paraskakis E, Trypsianis G, Tsalkidis A, Arvanitidou V, Emporiadou M, et al. The effect of obesity on pulmonary lung function of school aged children in Greece. Pediatr Pulmonol. 2009;44:273–80. doi: 10.1002/ppul.20995. [DOI] [PubMed] [Google Scholar]
- 18.Baek HS, Kim YD, Shin JH, Kim JH, Oh JW, Lee HB. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann Allergy Asthma Immunol. 2011;107:14–21. doi: 10.1016/j.anai.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 22.Anderson WJ, Lipworth BJ. Does body mass index influence responsiveness to inhaled corticosteroids in persistent asthma? Ann Allergy Asthma Immunol. 2012;108:237–42. doi: 10.1016/j.anai.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Engbers M, Vachier I, Sterk P, Bourdin A, Gras D, Godard P, et al. Mild asthma in overweight women: A new phenotype? Respir Med. 2010;104:1138–44. doi: 10.1016/j.rmed.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Farah CS, Kermode JA, Downie SR, Brown NJ, Hardaker KM, Berend N, et al. Obesity is a determinant of asthma control independent of inflammation and lung mechanics. Chest. 2011;140:659–66. doi: 10.1378/chest.11-0027. [DOI] [PubMed] [Google Scholar]
- 25.Gibeon D, Batuwita K, Osmond M, Heaney LG, Brightling CE, Niven R, et al. Obesity-associated severe asthma represents a distinct clinical phenotype: analysis of the British Thoracic Society Difficult Asthma Registry Patient cohort according to BMI. Chest. 2013;143:406–14. doi: 10.1378/chest.12-0872. [DOI] [PubMed] [Google Scholar]
- 26.Kwon JW, Kim SH, Kim TB, Park HW, Chang YS, Jang AS, et al. Airway hyperresponsiveness is negatively associated with obesity or overweight status in patients with asthma. Int Arch Allergy Immunol. 2012;159:187–93. doi: 10.1159/000335926. [DOI] [PubMed] [Google Scholar]
- 27.Marin G, Gamez AS, Molinari N, Kacimi D, Vachier I, Paganin F, et al. Distal airway impairment in obese normoreactive women. Biomed Res Int. 2013;2013:707856. doi: 10.1155/2013/707856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pisi R, Aiello M, Tzani P, Marangio E, Olivieri D, Pisi G, et al. Overweight is associated with airflow obstruction and poor disease control but not with exhaled nitric oxide change in an asthmatic population. Respiration. 2012;84:416–22. doi: 10.1159/000340038. [DOI] [PubMed] [Google Scholar]
- 29.Raviv S, Dixon AE, Kalhan R, Shade D, Smith LJ. Effect of obesity on asthma phenotype is dependent upon asthma severity. J Asthma. 2011;48:98–104. doi: 10.3109/02770903.2010.534220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigo GJ, Plaza V. Body mass index and response to emergency department treatment in adults with severe asthma exacerbations: a prospective cohort study. Chest. 2007;132:1513–9. doi: 10.1378/chest.07-0936. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland TJ, Cowan JO, Taylor DR. Dynamic hyperinflation with bronchoconstriction: differences between obese and nonobese women with asthma. Am J Respir Crit Care Med. 2008;177:970–5. doi: 10.1164/rccm.200711-1738OC. [DOI] [PubMed] [Google Scholar]
- 32.Deesomchok A, Fisher T, Webb KA, Ora J, Lam YM, Lougheed MD, et al. Effects of obesity on perceptual and mechanical responses to bronchoconstriction in asthma. Am J Respir Crit Care Med. 2010;181:125–33. doi: 10.1164/rccm.200906-0934OC. [DOI] [PubMed] [Google Scholar]
- 33.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134:317–23. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 34.Attaur-Rasool S, Shirwany TA. Body mass index and dynamic lung volumes in office workers. J Coll Physicians Surg Pak. 2012;22:163–7. [PubMed] [Google Scholar]
- 35.Pellegrino R, Gobbi A, Antonelli A, Torchio R, Gulotta C, Pellegrino GM, et al. Ventilation heterogeneity in obesity. J Appl Physiol (1985) 2014;116:1175–81. doi: 10.1152/japplphysiol.01339.2013. [DOI] [PubMed] [Google Scholar]
- 36.Chlif M, Keochkerian D, Mourlhon C, Choquet D, Ahmaidi S. Noninvasive assessment of the tension-time index of inspiratory muscles at rest in obese male subjects. Int J Obes (Lond) 2005;29:1478–83. doi: 10.1038/sj.ijo.0803030. [DOI] [PubMed] [Google Scholar]
- 37.Chlif M, Keochkerian D, Choquet D, Vaidie A, Ahmaidi S. Effects of obesity on breathing pattern, ventilatory neural drive and mechanics. Respir Physiol Neurobiol. 2009;168:198–202. doi: 10.1016/j.resp.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Essalhi M, Gillaizeau F, Chevallier JM, Ducloux R, Chevalier-Bidaud B, Callens E, et al. Risk factors for airway hyperresponsiveness in severely obese women. Respir Physiol Neurobiol. 2013;186:137–45. doi: 10.1016/j.resp.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Peixoto-Souza FS, Piconi-Mendes C, Baltieri L, Rasera-Junior I, Barbalho-Moulim MC, Lima Montebelo MI, et al. Lung age in women with morbid obesity. Rev Assoc Med Bras. 2013;59:265–9. doi: 10.1016/j.ramb.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Steier J, Lunt A, Hart N, Polkey MI, Moxham J. Observational study of the effect of obesity on lung volumes. Thorax. 2014;69:752–9. doi: 10.1136/thoraxjnl-2014-205148. [DOI] [PubMed] [Google Scholar]
- 41.Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol (1985) 2005;98:512–7. doi: 10.1152/japplphysiol.00430.2004. [DOI] [PubMed] [Google Scholar]
- 42.Bustos P, Amigo H, Oyarzun M, Rona RJ. Is there a causal relation between obesity and asthma? Evidence from Chile. Int J Obes (Lond) 2005;29:804–9. doi: 10.1038/sj.ijo.0802958. [DOI] [PubMed] [Google Scholar]
- 43.Andrade LS, Araujo AC, Cauduro TM, Watanabe LA, Castro AP, Jacob CM, et al. Obesity and asthma: association or epiphenomenon? Rev Paul Pediatr. 2013;31:138–44. doi: 10.1590/s0103-05822013000200002. [DOI] [PubMed] [Google Scholar]
- 44.Giese JK. Pediatric obesity and its effects on asthma control. J Am Assoc Nurse Pract. 2014;26:102–9. doi: 10.1111/1745-7599.12029. [DOI] [PubMed] [Google Scholar]
- 45.Lang JE, Feng H, Lima JJ. Body mass index-percentile and diagnostic accuracy of childhood asthma. J Asthma. 2009;46:291–9. doi: 10.1080/02770900802712963. [DOI] [PubMed] [Google Scholar]
- 46.Mansell AL, Walders N, Wamboldt MZ, Carter R, Steele DW, Devin JA, et al. Effect of body mass index on response to methacholine bronchial provocation in healthy and asthmatic adolescents. Pediatr Pulmonol. 2006;41:434–40. doi: 10.1002/ppul.20368. [DOI] [PubMed] [Google Scholar]
- 47.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedon JC, et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127:741–9. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang JE, Hossain MJ, Lima JJ. Overweight children report qualitatively distinct asthma symptoms: analysis of validated symptom measures. J Allergy Clin Immunol. 2015;135:886–93. e3. doi: 10.1016/j.jaci.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erkocoglu M, Kaya A, Ozcan C, Akan A, Vezir E, Azkur D, et al. The effect of obesity on the level of fractional exhaled nitric oxide in children with asthma. Int Arch Allergy Immunol. 2013;162:156–62. doi: 10.1159/000351454. [DOI] [PubMed] [Google Scholar]
- 50.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, et al. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am J Respir Crit Care Med. 2015;191:149–60. doi: 10.1164/rccm.201409-1587OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davidson WJ, Mackenzie-Rife KA, Witmans MB, Montgomery MD, Ball GD, Egbogah S, et al. Obesity negatively impacts lung function in children and adolescents. Pediatr Pulmonol. 2014;49:1003–10. doi: 10.1002/ppul.22915. [DOI] [PubMed] [Google Scholar]
- 52.Del Rio-Navarro BE, Blandon-Vijil V, Escalante-Dominguez AJ, Berber A, Castro-Rodriguez JA. Effect of obesity on bronchial hyperreactivity among Latino children. Pediatr Pulmonol. 2013;48:1201–5. doi: 10.1002/ppul.22823. [DOI] [PubMed] [Google Scholar]
- 53.Mendelson M, Michallet AS, Perrin C, Levy P, Wuyam B, Flore P. Exercise training improves breathing strategy and performance during the six-minute walk test in obese adolescents. Respir Physiol Neurobiol. 2014;200:18–24. doi: 10.1016/j.resp.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Cibella F, Cuttitta G, La Grutta S, Melis MR, Bucchieri S, Viegi G. A cross-sectional study assessing the relationship between BMI, asthma, atopy, and eNO among schoolchildren. Ann Allergy Asthma Immunol. 2011;107:330–6. doi: 10.1016/j.anai.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Huang F, Del-Rio-Navarro BE, Torres-Alcantara S, Perez-Ontiveros JA, Ruiz-Bedolla E, Saucedo-Ramirez OJ, et al. Adipokines, asymmetrical dimethylarginine, and pulmonary function in adolescents with asthma and obesity. J Asthma. 2016 doi: 10.1080/02770903.2016.1200611. [DOI] [PubMed] [Google Scholar]
- 56.Costa D, Junior, Peixoto-Souza FS, Araujo PN, Barbalho-Moulin MC, Alves VC, Gomes EL, et al. Influence of Body Composition on Lung Function and Respiratory Muscle Strength in Children With Obesity. J Clin Med Res. 2016;8:105–10. doi: 10.14740/jocmr2382w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu KD, Phipatanakul W, Perzanowski MS, Balcer-Whaley S, Matsui EC. Atopy, but not obesity is associated with asthma severity among children with persistent asthma. J Asthma. 2016;53:1033–44. doi: 10.3109/02770903.2016.1174259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozgen IT, Cakir E, Torun E, Gules A, Hepokur MN, Cesur Y. Relationship Between Functional Exercise Capacity and Lung Functions in Obese Chidren. J Clin Res Pediatr Endocrinol. 2015;7:217–21. doi: 10.4274/jcrpe.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rasslan Z, Stirbulov R, Junior RS, Curia ST, da Conceicao Lima CA, Perez EA, et al. The impact of abdominal adiposity measured by sonography on the pulmonary function of pre-menopausal females. Multidiscip Respir Med. 2015;10:23. doi: 10.1186/s40248-015-0018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strunk RC, Colvin R, Bacharier LB, Fuhlbrigge A, Forno E, Arbelaez AM, et al. Airway Obstruction Worsens in Young Adults with Asthma Who Become Obese. J Allergy Clin Immunol Pract. 2015;3:765–71. e2. doi: 10.1016/j.jaip.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Bader WR, Ramadan J, Nasr-Eldin A, Barac-Nieto M. Pulmonary ventilatory functions and obesity in Kuwait. Med Princ Pract. 2008;17:20–6. doi: 10.1159/000109585. [DOI] [PubMed] [Google Scholar]
- 62.Chouchane A, Miadi-Messaoud H, Ghannouchi I, Rouatbi S, Zbidi A, Tabka Z, et al. Obesity induced bronchopulmonary hyperresponsiveness in Tunisian women. Int J Obes (Lond) 2010;34:1078–85. doi: 10.1038/ijo.2010.22. [DOI] [PubMed] [Google Scholar]
- 63.Subhan MM, Ali SA, Bokhari SS, Khan MN, Ahmad HR. Underweight and overweight men have greater exercise-induced dyspnoea than normal weight men. Ups J Med Sci. 2012;117:383–9. doi: 10.3109/03009734.2012.714416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drumond SC, Fontes MJ, Assis I, Duarte MA, Lamounier JA, Lopes Orlandi LC, et al. Comparison of three sets of reference equations for spirometry in children and adolescents with distinct body mass indices. J Bras Pneumol. 2009;35:415–22. doi: 10.1590/s1806-37132009000500005. [DOI] [PubMed] [Google Scholar]
- 65.Bennett WD, Ivins S, Alexis NE, Wu J, Bromberg PA, Brar SS, et al. Effect of Obesity on Acute Ozone-Induced Changes in Airway Function, Reactivity, and Inflammation in Adult Females. PLoS One. 2016;11:e0160030. doi: 10.1371/journal.pone.0160030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen YC, Huang YL, Ho WC, Wang YC, Yu YH. Gender differences in effects of obesity and asthma on adolescent lung function: Results from a population-based study. J Asthma. 2016:1–7. doi: 10.1080/02770903.2016.1212367. [DOI] [PubMed] [Google Scholar]
- 67.Curjuric I, Imboden M, Bridevaux PO, Gerbase MW, Haun M, Keidel D, et al. Common SIRT1 variants modify the effect of abdominal adipose tissue on aging-related lung function decline. Age (Dordr) 2016;38:52. doi: 10.1007/s11357-016-9917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janzen B, Karunanayake C, Rennie D, Pickett W, Lawson J, Kirychuk S, et al. Gender Differences in the Association of Individual and Contextual Exposures with Lung Function in a Rural Canadian Population. Lung. 2017;195:43–52. doi: 10.1007/s00408-016-9950-8. [DOI] [PubMed] [Google Scholar]
- 69.Liyanage G, Jayamanne BD, Aaqiff M, Sriwardhana D. Effect of body mass index on pulmonary function in children. Ceylon Med J. 2016;61:163–6. doi: 10.4038/cmj.v61i4.8382. [DOI] [PubMed] [Google Scholar]
- 70.Joshi AR, Singh R. Correlation of pulmonary function tests with body fat percentage in young individuals. Indian J Physiol Pharmacol. 2008;52:383–8. [PubMed] [Google Scholar]
- 71.Watson RA, Pride NB, Thomas EL, Ind PW, Bell JD. Relation between trunk fat volume and reduction of total lung capacity in obese men. J Appl Physiol (1985) 2012;112:118–26. doi: 10.1152/japplphysiol.00217.2011. [DOI] [PubMed] [Google Scholar]
- 72.Yoo S, Kim HB, Lee SY, Kim BS, Kim JH, Yu JH, et al. Association between obesity and the prevalence of allergic diseases, atopy, and bronchial hyperresponsiveness in Korean adolescents. Int Arch Allergy Immunol. 2011;154:42–8. doi: 10.1159/000319207. [DOI] [PubMed] [Google Scholar]
- 73.Mehari A, Afreen S, Ngwa J, Setse R, Thomas AN, Poddar V, et al. Obesity and Pulmonary Function in African Americans. PLoS One. 2015;10:e0140610. doi: 10.1371/journal.pone.0140610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeon YH, Yang HJ, Pyun BY. Lung function in Korean adolescent girls: in association with obesity and the menstrual cycle. J Korean Med Sci. 2009;24:20–5. doi: 10.3346/jkms.2009.24.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simon MR, Chinchilli VM, Phillips BR, Sorkness CA, Lemanske RF, Jr, Szefler SJ, et al. Forced expiratory flow between 25% and 75% of vital capacity and FEV1/forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010;126:527–34. e1–8. doi: 10.1016/j.jaci.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 77.Sood A. Altered resting and exercise respiratory physiology in obesity. Clin Chest Med. 2009;30:445–54. vii. doi: 10.1016/j.ccm.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–93. doi: 10.1016/j.jaci.2008.03.004. quiz 94–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.