Abstract
Chronic itch is associated with increased stress, anxiety, and other mood disorders. In turn, stress and anxiety exacerbate itch, leading to a vicious cycle that affects patient behavior (scratching) and worsens disease prognosis and quality of life. This cycle persists across chronic itch conditions of different etiologies and even to some extent in healthy individuals, suggesting that the final common pathway for itch processing (the central nervous system) plays a major role in the relationship between itch and anxiety. Pharmacological and nonpharmacological treatments that reduce anxiety have shown promising anti-itch effects. Further research is needed to establish specific central mechanisms of the itch-anxiety cycle and provide new targets for treatment.
1. Introduction
Itch is a complex sensory phenomenon that incorporates discriminative, cognitive, motivational, and affective components. Recent studies have highlighted the importance of the affective dimension of itch. Chronic itch conditions are associated with higher rates of stress, anxiety, depression, and even suicidal ideation, leading to major deficits in quality of life (Ferm et al., 2010; Halvorsen et al., 2012; Matterne et al., 2013; Schneider et al., 2006; Silverberg et al., 2016). In addition, psychological and emotional factors can modulate the perception of itch and affect treatment outcomes (Verhoeven et al., 2008). While negative emotions can accompany any chronic disease, they take on a particular significance in the context of chronic itch because patient behavior (scratching) directly leads to worsening of the skin condition and perpetuation of itch (Bender et al., 2008; Hong et al., 2004; Iking et al., 2013; Kimura and Miyazawa, 1989).
In particular, the interaction of pruritus, stress (the activation of the HPA axis), and anxiety (the subjective experience of fear or threat avoidance) (Shin and Liberzon, 2010) has important implications for patients. Chronic itch is associated with increased anxiety, and, in turn, anxiety and stress tend to exacerbate itch, leading to a vicious cycle not unlike the itch-scratch cycle. This review will examine the links between itch and anxiety, the impact of stress on itch perception, the role of the central nervous system in the itch-anxiety cycle, and potential interventions to break the cycle.
2. Itch in Dermatological Conditions Is Linked to Anxiety
Pruritus is associated with many dermatological conditions, and dermatology outpatients with pruritus experience significantly more anxiety and lower quality of life than those with no pruritus (Marron et al., 2016). In studies of patients with atopic dermatitis, the most common chronic inflammatory skin disease (Griffiths et al., 2017), pruritus (and not Eczema Area and Severity Index or SCORing of Atopic Dermatitis) was correlated with state anxiety and stress susceptibility as measured by validated psychometric questionnaires (Oh et al., 2010; Rasul et al., 2016). Furthermore, when treatment with dupilumab, an interleukin-4 receptor-α inhibitor, reduced pruritus, atopic patients also reported improvements in anxiety and other psychological scores (Simpson et al., 2016). A study of subjects with occupational hand eczema also found a high prevalence (20%) of anxiety symptoms, with itch being an explanatory variable (Boehm et al., 2012).
Similarly, psoriasis patients with a high level of pruritus had significantly higher state and trait anxiety scores than those with a low level of pruritus (Remrod et al., 2015), and psoriatic itch intensity was correlated with current level of psychological stress (Reich et al., 2010) and anxiety (Lesner et al., 2017). Among plaque psoriasis patients with pruritus, severe pruritus was associated with higher anxiety and depression scores (Mrowietz et al., 2015). After treatment with etanercept (a tumor necrosis factor inhibitor), a clinically meaningful reduction of pruritus was also associated with clinically meaningful improvements in anxiety, even after adjusting for overall disease severity. Associations between pruritus and anxiety have also been seen in other dermatological conditions like chronic urticaria (Zachariae et al., 2012) and lichen planus (Welz-Kubiak et al., 2017).
Persistent pruritus after burn injury may also be associated with anxiety. In one study, itch severity and mental health scores were negatively correlated in patients with post-burn pruritus. Furthermore, the decrease in itch severity over time (1, 3, and 6 months after the injury) was correlated with improvements in mental health. This result was independent of the percent total burn surface area (McGarry et al., 2016). The presence of post-burn pruritus has also been correlated with state anxiety, trait anxiety, and stress susceptibility (Willebrand et al., 2004), though another study only found a non-significant (p=0.07) correlation between post-burn pruritus and trait anxiety (Gauffin et al., 2015).
Of course, dermatological conditions include many symptoms besides itch that can influence mental health. Several studies have suggested that chronic itch patients may feel stigmatization and social anxiety due to visible skin damage (Hrehorow et al., 2012; Schmid-Ott et al., 1999). Interestingly, psoriasis and atopic dermatitis patients display higher levels of anxiety than patients with vitiligo, a disorder that produces highly visible areas of hypopigmentation on the skin but no somatosensory symptoms (Kossakowska et al., 2010; Noh et al., 2013). Additionally, subjects with atopic dermatitis, prurigo nodularis, and chronic pruritus of other origin all reported a more negative body concept than healthy controls, despite the fact that subjects with chronic pruritus of other origin had very few scratch lesions (Stumpf et al., 2013b). These results support the idea that itch per se is a major source of anxiety and negative emotions in pruritic dermatological disease.
3. Itch in Systemic Conditions Is Linked to Anxiety
Less research has been done to investigate the link between pruritus and anxiety in systemic diseases. In end-stage kidney disease patients undergoing hemodialysis, the presence of chronic itch was associated with increased anxiety and decreased quality of life (Weiss et al., 2015). For those hemodialysis patients with chronic itch, the level of anxiety, but not depression, was correlated with higher itch severity (Weiss et al., 2016). Nalfurafine hydrochloride, a kappa opioid receptor agonist that has been approved for the treatment of uremic pruritus in Japan, also reduces state anxiety in these patients (Inui et al., 2012).
Chronic itch has been associated with lower mental health scores in cholestatic liver disease (Cheung et al., 2016; Raszeja-Wyszomirska et al., 2015) and systemic sclerosis (Racine et al., 2016). Pruritus also reduces emotional quality of life in HIV (Kaushik et al., 2014) and polycythemia vera (Siegel et al., 2013). Additionally, among patients with polycythemia vera, those with aquagenic pruritus display significantly higher anxiety scores (Lelonek et al., 2017). However, to our knowledge, no studies have yet specifically examined the link between itch and anxiety in those conditions.
4. Psychological Stress Exacerbates Itch
Not only is itch associated with greater anxiety, but stress has also be shown to exacerbate itch, leading to a true itch-anxiety cycle. Many chronic itch patients report that psychological stress is a factor that aggravates their itch. This has been demonstrated in a wide variety of pruritic conditions: atopic dermatitis (71–81% of patients) (Wahlgren, 1991; Yosipovitch et al., 2002b), psoriasis (55–71%) (Yosipovitch et al., 2000; Zachariae et al., 2004), chronic idiopathic urticaria (25%) (Yosipovitch et al., 2002a), lichen planus (43.2%) (Welz-Kubiak et al., 2017), acne-related itch (31%) (Lim et al., 2008), epidermolysis bullosa (63.7%) (Danial et al., 2015), post-burn pruritus (57%) (Parnell et al., 2012), small fiber neuropathy with itch (46%) (Brenaut et al., 2015), uremic pruritus (19–38.8%) (Mistik et al., 2006; Zucker et al., 2003), cholestatic itch (53.3%) (Huesmann et al., 2013), and psychogenic itch (46%) (Misery et al., 2008).
This effect has also been replicated experimentally. Viewing a standardized series of stressful images, such as a snake preparing to strike or a person being pulled from a burning building, increased itch severity in prurigo nodularis and lichen simplex chronicus patients (Kim et al., 2016a). Healthy subjects also reported higher itch from histamine iontophoresis when negative (“tense, uptight, nervous”) emotions were induced with violent film fragments compared to when positive emotions were induced with comedic film fragments(van Laarhoven et al., 2012).
Stress can predispose susceptible individuals to an outbreak of chronic pruritus. In separate studies, 64% of AD patients and 72.5% of psoriasis patients reported that at least one stressful life event occurred in the month prior to itch exacerbation, (Chrostowska-Plak et al., 2013; Reich et al., 2010). In both cases, the retrospective degree of stress was significantly correlated with itch intensity. Similarly, most chronic urticaria patients reported a stressful life event within the six months preceding cutaneous symptoms (Berrino et al., 2006), and many patients identify stress as a trigger in the progression of acute urticaria to chronic urticaria (Comert et al., 2013). Stressful life events are also likely to occur in the months preceding the onset or worsening of pemphigus symptoms (Morell-Dubois et al., 2008). In burn patients, early post-traumatic stress (measured 2 weeks after injury) was a significant predictor of itch intensity at 3, 12, and 24 months post-burn (Van Loey et al., 2008).
A meta-analysis of cohort studies found a bidirectional association between psychological factors (including anxiety) and future atopic disorders as well as between atopic disorders and future poor mental health (Chida et al., 2008). Additionally, a 10-year longitudinal study found that stress levels were linked with development of adult-onset allergic rhinitis and asthma, and the authors suggested that development of atopic dermatitis may be similarly impacted by stress (Rod et al., 2012). After the Great Hanshin Earthquake in Japan, individuals living in areas with greater damage experienced greater levels of stress and a concomitant increase in AD symptoms, including itching (Kodama et al., 1999). Of the factors examined, which included discontinuation of medication, inconvenience in bathing, and inability to clean up living environment, subjective distress had the most impact on AD symptoms.
In some cases, termed functional itch disorder or psychogenic itch, chronic pruritus can be brought on by psychological factors without any identifiable somatic cause. This type of itch is typically associated with stress, major life events, or psychological disorders (Misery et al., 2008), perhaps explaining why high rates of idiopathic itch (32–42%) have been reported in psychiatric inpatients (Kretzmer et al., 2008; Mazeh et al., 2008). As in other conditions, itch severity in psychogenic pruritus negatively affects quality of life (Altunay et al., 2014).
The links between stress, anxiety, and itch also exist in broader populations outside of specific pruritic diagnoses. A cohort study of generally healthy Japanese participants (Yamamoto et al., 2009), a longitudinal study of randomly selected German participants (Matterne et al., 2013), cross-sectional surveys of American and Australian undergraduate students (Schut et al., 2016b; Stewart et al., 2017), and a cross-sectional study of Norwegian adolescents (Halvorsen et al., 2009) all found significant associations between perceived anxiety or stress and itch.
In rodent models, a variety of chronic stressors appear to exacerbate the acute itch response. Mice subjected to 10 days of water avoidance stress, a model of psychological stress wherein mice must stay on a small platform to avoid falling into a pool of water, displayed increased scratching after injection of compound 48/80, a histaminergic pruritogen (Zhao et al., 2013). A 9-day heterotypic chronic intermittent stress protocol, which included cold-restraint stress, water avoidance stress, and forced swim stress, led to increased scratching (but not pain-related behavior) after serotonin injection in rats (Peng et al., 2015).
Stress from morphine withdrawal in mice also led to a time-dependent enhanced scratching response to histamine, which corresponded with an increase in plasma corticosterone levels (Abe et al., 2015). In contrast, when rats were subjected to acute forced swim stress (2–5 minutes, immediately following pruritogen injection), they displayed a reduced scratching response to serotonin (Spradley et al., 2012). This type of acute stress-induced antinociception has been well studied in pain and appears to play a role in itch as well, perhaps by functioning as a distraction. Stress, in combination with genetic or environmental predisposition, can also lead to chronic itch in mice that normally do not develop itch. NC/Nga mice normally develop scratch-induced, atopic-like skin lesions in conventional housing but not specific pathogen-free housing. However, when subjected to four weeks of water avoidance stress, NC/Nga mice in specific pathogen-free housing also developed intense scratching and dermatitis (Amano et al., 2008). BALB/c mice, which are not genetically predisposed to chronic itch, did not scratch under these stress conditions. In allergic contact dermatitis model mice, chronic social isolation stress led to an increase in scratching behavior and idiopathic dermatitis, appearing in areas distinct from the contact dermatitis site (Kitagaki et al., 2014). Furthermore, for socially isolated mice, the itch-scratch response remained active long after the initial skin challenge. Only those mice that were primed with prolonged (60-day) allergic contact dermatitis developed idiopathic dermatitis during social isolation stress.
5. Cognitive Modulation of Itch and Scratching Behavior
It is well-established that cognition can have profound, bidirectional impacts on itch perception. For example, attentional focus to bodily sensations can heighten itch perception, while distraction (audiovisual stimuli, noise, or Stroop test) reduces itch (Leibovici et al., 2009; Stumpf et al., 2013a; van Laarhoven et al., 2010; Yamaguchi et al., 2001) in humans and mice. Faulty cognition may also increase subjective feelings of itch through the nocebo (or negative placebo) effect; when subjects have the expectation that itch will be severe, they may perceive higher levels of itch. Anxiety and stress have been implicated in the nocebo effect for pain hyperalgesia (Benedetti et al., 2006; Bjorkedal and Flaten, 2012; Staats et al., 2001; Woo, 2015) and may also play a role in nocebo itch. In one study of healthy subjects, both nocebo and placebo effects for itch were strongly correlated with worrying and negative affect (anxiety/depression) (Bartels et al., 2014).
Related to nocebo itch is the phenomenon of contagious itch, which has been established in humans, monkeys (Feneran et al., 2013), and very recently in mice (Yu et al., 2017). Provoking cognition about itch via visual cues (for example, images of insects crawling on skin or allergic skin reactions) can induce itch and scratching, even in healthy observers. In particular, images of others scratching evoke scratching in response (Lloyd et al., 2013). After watching itch- and scratch-related videos, healthy individuals reported correlated increases in both itch and anxiety (Ogden and Zoukas, 2009). Subjects with AD were particularly susceptible to the effects of contagious itch, and scratching was directed to many different, non-eczematous locations on the body beyond the itch induction site (Papoiu et al., 2011). While the exact causes of contagious itch are unknown, stress may play a role in contagious itch susceptibility, as in two reports of mass psychogenic itching and scratching that spread among elementary school students who were anxious about achievement tests (Halvorson et al., 2008; Robinson et al., 1984).
In chronic itch, the cognitions or coping mechanisms that patients use to deal with anxiety and stress have major impacts on itch perception and scratching behavior. Resignation, low self-efficacy, or a lack of personal resilience may increase itch (Janowski et al., 2014), while a sense of control and a “fight spirit” was associated with reduced pruritus intensity and less frequent itch (Dalgard et al., 2012; Ograczyk et al., 2014; Ponarovsky et al., 2011). When experiencing high levels of daily stressors, psoriasis patients reported more worrying and scratching. This cognitive and behavioral reactivity was correlated with increases in itch severity four weeks post-stress (Verhoeven et al., 2009). Furthermore, a regression and multiple mediation analysis revealed that, for AD subjects, the itch-scratch cycle was the major coping strategy that linked perceived stress with itch ratings (Schut et al., 2015). This type of maladaptive coping may explain why the presence of anxiety disorder is associated with increased risk of lichen simplex chronicus, which develops from excessive scratching (Liao et al., 2014).
6. Itch, Autonomic Nervous System, and Hypothalamic-Pituitary-Adrenal Axis Function
The mechanisms behind the itch-anxiety cycle are still being explored. Physiological responses to stress are largely controlled by the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis, so we may expect that itch induces changes in these systems. Dysfunctions of the ANS and HPA axis have been reported in patients with some chronic itch conditions, but this evidence is mixed. One study found that AD subjects displayed consistently higher heart rates (indicative of increased sympathetic activity), but no differences in sympathetic or parasympathetic response to mental stress compared to healthy controls (Seiffert et al., 2005). Other studies have found no differences in ANS function at baseline or following stress for patients with AD, psoriasis, or chronic urticaria compared to controls (Buske-Kirschbaum et al., 2002; de Brouwer et al., 2014; Hashiro and Okumura, 1994). While uremic patients displayed significantly decreased heart rate variability compared to controls, it was not correlated with pruritus severity (Zakrzewska-Pniewska and Jedras, 2001).
Vagal nerve stimulation, which activates the parasympathetic nervous system, in subjects without skin disease suppressed itch perception, but not flare, after histamine iontophoresis (Kirchner et al., 2002). However, one study found increased vagal modulation of heart rate variability in atopic dermatitis patients compared to controls (Boettger et al., 2009). Interestingly, subjects with atopic dermatitis showed rigid vagal tone in response to experimentally induced histamine itch and scratching by an investigator (Tran et al., 2010).
Subjects with atopic dermatitis had normal baseline cortisol levels but displayed blunted cortisol response to psychosocial stress (Buske-Kirschbaum et al., 2002). High levels of daily stressors were associated with lower cortisol levels in psoriasis patients, again suggesting a blunted HPA axis response to stress (Evers et al., 2010). Additionally, psoriasis patients who reported that their psoriasis was triggered by stress had higher levels of worry, greater disease severity, and reduced cortisol response to an experimental stressor (Richards et al., 2005). However, another study found similar baseline cortisol but increased cortisol response to stress in patients with psoriasis compared to patients with rheumatoid arthritis or healthy controls (de Brouwer et al., 2014).
7. Itch and Anxiety in the Brain
The itch-anxiety cycle appears to exist for itch of all types: dermatological or systemic origin, histaminergic or nonhistaminergic transmission, and in chronic itch patients or healthy individuals with no pruritic disease. Therefore, it is likely that a key component of the itch-anxiety cycle is the final common pathway for itch of all types: the brain. The brain’s role in the affective components of pain has been explored previously (Han et al., 2015; Neugebauer, 2015). Now, brain imaging and animal studies are providing evidence for the involvement of several anxiety-related structures in the processing of itch. Of course, a major caveat is that these brain areas are activated by many different types of stimulation. Further studies will need to assess whether activations are specifically correlated to anxiety or stress during itch.
The amygdala is the key brain region responsible for generating fear responses and anxiety (Shekhar et al., 2005). Interestingly, amygdala and hippocampus activation appears to go hand-in-hand in most studies of itch, suggesting that the memory of previous itch experiences may be a significant factor in itch-related anxiety. Amygdala and hippocampus were activated in healthy subjects during histamine and/or cowhage itch and deactivated during scratching, particularly active scratching by the participant (Papoiu et al., 2012; Papoiu et al., 2013; Vierow et al., 2015). One study found that the hippocampus was deactivated by scratching cowhage itch in healthy controls and chronic itch (atopic dermatitis, psoriasis, and uremic) patients (Mochizuki et al., 2015). End-stage renal patients with itch had greater activation and increased gray matter density in the amygdala and hippocampus compared to healthy controls (Papoiu et al., 2014). In patients with prurigo nodularis or lichen simplex chronicus, hippocampus activation was significantly increased during stress-induced pruritus (Kim et al., 2016a).
The role of these structures in itch has also been demonstrated in a handful of animal studies. A manganese-enhanced MRI study found that the amygdala and hippocampus were significantly activated in a rat model of chronic itch compared to control rats (Jeong and Kang, 2015). Amygdala and hippocampus were also activated during contagious itch in mice (Yu et al., 2017). Finally, GABAA receptors in the central nucleus of the amygdala appear to play a major role in attenuating or enhancing acute and chronic itch in mice (Chen et al., 2016). Subsets of amygdala neurons have been identified to respond to different types of appetitive or aversive sensory stimuli, such as foot shock, bitter or sweet tasting chemicals (quinine or sucrose), or olfactory cues (peanut oil) (Kim et al., 2016b; Kim et al., 2017; Xiu et al., 2014). Because mice display aversion behavior in response to acute itch stimuli (Mu and Sun, 2017), these types of studies could provide important information about the amygdala neurons and pathways that give negative emotional valence to itch.
Together with the amygdala, the anterior cingulate cortex (ACC) and insular cortex form the “fear network,” which is active during both the acquisition and extinction of fear conditioning (Holzschneider and Mulert, 2011). These areas are also commonly activated during itch and scratching. Histamine, cowhage, and electrically evoked itch all induce robust activation of the ACC and insula in healthy subjects (Mochizuki et al., 2009; Papoiu et al., 2012; Vierow et al., 2015). The insula was also activated following visually evoked itch in healthy subjects (Holle et al., 2012; Mochizuki et al., 2013). In AD subjects, activations in both ACC and insula were seen in during allergen-induced itch (Napadow et al., 2014) and also correlated with histamine itch intensity (Ishiuji et al., 2009). These areas also were significantly activated in uremic patients with chronic pruritus compared to healthy controls (Papoiu et al., 2014). Interestingly, some studies report that scratching an itch resulted in activation of insula and ACC in healthy subjects (Mochizuki et al., 2015; Vierow et al., 2009) and a mixed chronic itch group (Mochizuki et al., 2015), while another study found deactivations of ACC during active and passive scratching of itch in healthy subjects (Papoiu et al., 2013).
The midcingulate cortex (MCC) synthesizes signals about pain, threat, and negative affect in order to modulate anxiety via projections to the amygdala and to motivate behavioral responses (Okon-Singer et al., 2015; Shackman et al., 2011). In a study of temperature-modulated allergen itch in AD subjects, increasing itch sensation was associated with MCC activation (Napadow et al., 2014). Another study found significantly increased MCC activation during histamine-induced itch in healthy controls but not AD subjects (Schneider et al., 2008). MCC was also activated during scratching an itch in both AD subjects and healthy controls (Mochizuki et al., 2015; Vierow et al., 2015). This activation was correlated with pleasure ratings of scratching, and greater activation was seen in chronic itch patients than in healthy controls (Mochizuki et al., 2015). Additionally, end-stage renal disease patients with chronic itch displayed increased MCC gray matter (Papoiu et al., 2014). Taken together, these studies suggest that the MCC may play a role in motivating scratching behavior as a response to the stress of itch. If the MCC is involved in both the itch-anxiety cycle and itch-scratch cycle, it could represent a promising target for treatment.
Finally, the prefrontal cortex (PFC) plays an important role in inhibition of fear, regulation of stress response, and perhaps modulation of chronic itch. While the medial PFC (mPFC) has direct, inhibitory connections with the amygdala (Maroun, 2013), the dorsolateral PFC (dlPFC) provides indirect, top-down regulation of emotion via attention and cognition (Okon-Singer et al., 2015). The dlPFC was activated during allergen-induced itch (Napadow et al., 2014) and histamine itch in atopic subjects, with dlPFC activation correlating to disease severity (Ishiuji et al., 2009). A mixed chronic itch group also displayed increased activation of dorsomedial and lateral PFC during scratching an itch (Mochizuki et al., 2015). The dlPFC was also a key area implicated in both nocebo itch (induced via saline pinprick that the subject believes contains an allergen) and placebo itch relief in atopic dermatitis (Bartels et al., 2016). Few studies report significant PFC response to itch in healthy subjects. In contrast, studies have reported that mPFC was deactivated during combined histamine and cowhage itch (Papoiu et al., 2012) and during scratching cowhage itch in healthy controls (Papoiu et al., 2013). However, healthy subjects did display PFC activation following itch-inducing imagery (Mochizuki et al., 2013). Of note, PFC may effect top-down regulation of itch-related anxiety by coordinating motor responses to scratch the itch. In most fMRI studies of itch, subjects are not allowed to actively scratch themselves.
The central mechanisms of the itch-anxiety cycle are still unclear. It has long been theorized that psychological stress can reduces the itch perception threshold (Cormia, 1952). Additionally, sensitization of itch-signaling pathways is well established in the spinal cord (Davidson et al., 2012; Ikoma et al., 2004) and recently discovered in the ACC of rats with chronic itch (Zhang et al., 2016), so it is plausible that itch-signaling neurons in the amygdala could also be sensitized. Stress is known to induce increased corticotropin-releasing factor (CRF) release into the hypothalamus, amygdala, and other brain areas. Over time, this CRF release leads to long-term synaptic plasticity that contributes to a heightened stress response and chronic anxiety (Kalin et al., 2016; Regev et al., 2012; Shekhar et al., 2005). If chronic itch functions as a chronic stressor, it may cause sensitization of anxiety-related brain areas, leading to increased itch and anxiety over time. The enhanced contagious itch response seen in atopic dermatitis patients provides further support for this idea (Papoiu et al., 2011).
Currently, most itch brain imaging studies are in healthy controls, and itch-related brain activity has not yet been studied in several major pruritic diseases, like psoriasis. Furthermore, there are few animal studies of brain activation during itch. Further studies could elucidate the pathways and neurotransmitters involved in this cycle.
8. Pharmacological and Non-Pharmacological Treatments to Target the Itch-Anxiety Cycle
Several classes of drugs exert both anti-anxiety and anti-itch effects (Table 1). Of these, antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), are the most commonly used for chronic itch. Additionally, the tricyclic antidepressants doxepin and amitriptyline have been used orally and topically to treat itch because they exhibit significant antihistamine effects (Lee et al., 2017; Richelson, 1979). Benzodiazepenes, typically used for acute anxiety attacks, are less studied in the context of itch but may also have beneficial effects. Finally, GABA analogs like gabapentin and pregabalin, which exhibit anti-anxiety effects (Houghton et al., 2017), are now being used to treat chronic itch (Matsuda et al., 2016). Thus far, GABA analogs have mostly been used for pruritus associated with systemic disease or neuropathy.
Table 1.
Examples of anti-anxiety drugs that exhibit anti-pruritic effects.
| Drug | Condition | Sample size | Design | Outcomes | Reference |
|---|---|---|---|---|---|
| SSRIs | |||||
| Paroxetine | Various non-dermatological pruritic conditions | 24 | Prospective, randomized, double-blind, placebo-controlled crossover study | 37.5% of patients had at least 50% reduction in pruritus during paroxetine treatment | (Zylicz et al. 2003) |
| Psychogenic pruritus | 1 | Case report | Complete resolution of itch, scratching, and excoriations | (Biondi et al. 2000) | |
| Paroxetine and fluvoxamine | Chronic pruritus (multiple origins) | 36/group | Prospective, open-label, two-therapy arm study | Maximal antipruritic effect of 67.6% for paroxetine and 64.9% for fluvoxamine | (Ständer et al. 2009) |
| Polycythemia vera | 10 | Case series | Complete or near-complete resolution of aquagenic pruritus for 80% of patients | (Tefferi and Fonseca 2002) | |
| Escitalopram | Psoriasis and psychiatric comorbidity | 19/group | Retrospective, controlled study | Reduced itch, depression, and anxiety scores compared to follow-up only group | (D’Erme et al. 2014) |
| Sertraline | Cholestatic pruritus | 12 | Prospective, randomized, double-blind, placebo-controlled study | Reduction in mean pruritus intensity (−1.86 VAS points), improvement of skin excoriation (83% of subjects), improved mood stability, reduced insomnia | (Mayo et al. 2007) |
| Uremic pruritus | 25 sertraline, 21 placebo | Randomized, double-blind, placebo-controlled study | Reduced itch intensity | (Pakfetrat et al. 2017) | |
| Uremic pruritus | 19 | Open-label, non-controlled study | Reduction in grade of pruritus severity | (Shakiba et al. 2012) | |
| Cholestatic pruritus (pediatric) | 14 | Prospective, multicenter, non-controlled study | Improved itch intensity, skin excoriation, and sleep quality | (Thébaut et al. 2017) | |
| Fluoxetine, citalopram, paroxetine, sertraline, fluvoxamine, or escitalopram | Plaque psoriasis | 1282 | Population-based cohort study | Patients who began SSRI treatment had reduced need for systemic psoriasis treatment | (Thorslund et al. 2013) |
| Tricyclic and Tetracyclic Antidepressants | |||||
| Doxepin | Uremic pruritus | 24 | Prospective, randomized, double-blind, placebo-controlled crossover study | Complete reduction of pruritus in 58.3% of doxepin group; relative improvement in 29.2% | (Pour-Reza-Gholi et al. 2007) |
| Chronic idiopathic urticaria | 16 | Prospective, randomized, double-blind, placebo-controlled crossover study | 64% decrease in itch, 75% decrease in swelling | (Goldsobel et al. 1986) | |
| Cold urticaria | 11 | Randomized, double-blind, placebo-controlled crossover study | Reduced itch in response to ice cube challenge | (Neittaanmäki et al. 1984) | |
| Chronic idiopathic urticaria | 25/group | Randomized, double-blind crossover study | 43% of subjects experienced total clearing of hives and pruritus (5% with diphenhydramine treatment) | (Greene et al. 1985) | |
| Amitriptyline | Lichen amyloidosis | 2 | Case series | Reduced itch intensity and improved quality of life | (Yew and Tey 2014) |
| Notalgia paresthetica | 1 | Case report | Reduced severity and frequency of itch | (Yeo and Tey 2013) | |
| Uremic pruritus | 1 | Case report | Reduced itch intensity | (Yong et al. 2014) | |
| Mirtazapine | Morphine-induced pruritus | 52/group | Prospective, randomized, double-blind, placebo-controlled study | Delayed onset and reduced long-term intensity of itch | (Sheen et al. 2008) |
| Morphine-induced pruritus | 20/group | Prospective, randomized, placebo-controlled study | Reduced itch intensity | (Akhan et al. 2016) | |
| Cancer-related, cholestatic, and uremic pruritus | 4 | Case series | Reduced pruritus and improved sleep | (Davis et al. 2003) | |
| Atopic dermatitis and lichen simplex chronicus | 3 | Case series | Reduced nocturnal pruritus, improved sleep, improved lesions | (Hundley and Yosipovitch 2004) | |
| Cutaneous T-cell lymphoma | Not specified | Case series | Reduced itch intensity and improved sleep | (Demierre and Taverna 2006) | |
| Chronic urticaria | 1 | Case report | Complete resolution of urticaria | (Bigatà et al. 2005) | |
| Benzodiazepenes | |||||
| Midazolam | Morphine-induced pruritus | 40/group | Randomized, double-blind, placebo-controlled study | Reduced incidence of pruritus | (Elhakim et al. 2009) |
| Cholestatic pruritus | 1 | Case report | Reduced itch without causing sedation | (Prieto 2004) | |
| Nitrazepam | Atopic dermatitis | 10 | Randomized, double-blind, placebo-controlled crossover study | No difference in total nocturnal scratching time or mean itch | (Ebata et al. 1998) |
| GABA Analogs | |||||
| Gabapentin | Uremic pruritus | 27/group | Randomized, double-blind, placebo-controlled study | 88.9% of patients on gabapentin had reduced pruritus severity (22.2% placebo) | (Nofal et al. 2016) |
| Morphine-induced pruritus | 20/group | Randomized, double-blind, placebo-controlled study | Delayed onset of itch, reduced itch intensity | (Akhan et al. 2016) | |
| Cholestatic pruritus | 7 gabapentin, 6 placebo | Randomized, double-blind, placebo-controlled study | No difference in itch intensity compared to placebo | (Bergasa et al. 2006) | |
| Uremic pruritus | 15 hemodialysis, 34 conservative treatment | Retrospective single-center cohort study | Reduction in pruritus score across study visits | (Cheikh Hassan et al. 2015) | |
| Post-burn or wound healing itch (pediatric) | 35 | Open-label, non-controlled study | Reduced itch, reduced need for antihistamines | (Mendham 2004) | |
| Notalgia paresthetica | 20 | Open-label, non-controlled study | Reduction in itch (−4.5 VAS points) | (Maciel et al. 2014) | |
| Prurigo nodularis and lichen simplex chronicus | 9 | Case series | Reduced itch intensity and lesions | (Gencoglan et al. 2010) | |
| Cutaneous T-cell lymphoma | Not specified | Case series | Reduced itch intensity | (Demierre and Taverna 2006) | |
| Brachioradial pruritus | 1 | Case report | Reduced itch intensity | (Winhoven et al. 2004) | |
| Brachioradial pruritus | 1 | Case report | Complete reduction of itch | (Yilmaz et al. 2010) | |
| Post-herpetic itch | 1 | Case report | Recovery of lesions and cessation of itch | (Jagdeo and Kroshinsky 2011) | |
| Scalp dysesthesia | 1 | Case report | Resolution of burning, stinging, and itch | (Sarifakioglu and Onur 2013) | |
| Gabapentin or pregabalin | Uremic pruritus | 71 | Open-label, non-controlled study | Reduced itch in 85% of subjects | (Rayner et al. 2012) |
| Pregabalin | Uremic pruritus | 62 pregabalin, 57 placebo | Prospective, randomized, double-blind, placebo-controlled study | Reduced mean itch (−4.6 VAS points) from baseline versus placebo | (Yue et al. 2015) |
| Post-burn itch | 46 pregabalin, 44 placebo | Prospective, randomized, double-blind, placebo-controlled study | Reduced itch and pain | (Gray et al. 2011) | |
| Post-burn itch | 20/group | Randomized, double-blind, placebo-controlled study | 95% reduction of mild/moderate itch; 79% reduction of severe itch | (Ahuja and Gupta 2013) | |
| Prurigo nodularis | 30 | Open-label, non-controlled study | Complete (76% of subjects) or slight (20% of subjects) improvement of itch and nodules | (Mazza et al. 2013) | |
| Generalized chronic pruritus of unknown origin | 22 | Open-label, non-controlled, study | Significant reduction of itch (−2.27 VAS points), improvement of skin manifestations in many subjects | (Park et al. 2012) | |
| Brachioradial pruritus | 3 | Case series | Complete resolution of pruritus | Atış and Bilir Kaya 2017) | |
However, because many of these anti-anxiety drugs interfere with the balance of known itch mediators and neurotransmitters (e.g., serotonin and histamine), it is unclear to what extent these drugs reduce itch through peripheral mechanisms vs. alleviation of itch-related anxiety and/or depression. One study found that chronic itch patients with psychological factors were slightly (but not significantly) more likely to experience itch reduction from SSRI treatment than patients without psychological factors (Stander et al., 2009), but further research is needed. Ideally, treatments will be able to simultaneously target both parts of the itch-anxiety cycle.
In contrast, non-pharmacological treatments can have clear top-down effects on itch. A variety of psychological interventions can target maladaptive itch-related cognitions, emotions, and behaviors. Habit reversal training, relaxation training, and cognitive behavioral therapy have shown success in treating some types of chronic itch. For an excellent recent review of these techniques, see (Schut et al., 2016a).
Noninvasive brain stimulation via repetitive transcranial magnetic stimulation (rTMS) or transcranial direct current stimulation (tDCS) may be another option to block the itch-anxiety cycle. A randomized, controlled clinical trial for low-frequency rTMS of the dlPFC found significant improvements for generalized anxiety disorder patients, including reduced worrying and better emotion regulation (Diefenbach et al., 2016a; Diefenbach et al., 2016b). Noninvasive brain stimulation of motor cortex or dlPFC has also shown success for many types of chronic pain (O'Connell et al., 2014). In particular, stimulation of dlPFC may alter pain perception by modulating pain-related cognitions and emotions, including anxiety (Borckardt et al., 2011; Hosseini Amiri et al., 2016; Maeoka et al., 2012). Early results suggest that tDCS stimulation of the sensorimotor cortex can reduce itch perception (Knotkova et al., 2013; Nakagawa et al., 2016). The effects of noninvasive brain stimulation on itch-related emotions have yet to be explored; however, the dlPFC may be a promising target due to its apparent role in processing chronic itch.
9. Conclusion
The vicious cycle of itch and anxiety plays a significant role in chronic itch intensity and progression. Many types of itch are associated with higher levels of anxiety; in turn, stress appears to exacerbate itch and scratching, perhaps by potentiating itch-related anxiety (Figure 1). Fortunately, there are many treatments that may break the itch-anxiety cycle. These treatments could be especially beneficial for those patients with psychogenic or idiopathic itch, excessive scratching, or itch that is unresponsive to first-line treatments. However, because stress and anxiety can play a role in any type of itch, understanding the itch-anxiety cycle and keeping it in check should be an important part of treatment for all chronic itch patients. Therefore, there is a pressing need for further work with animal models and human subjects to elucidate the neural mechanisms of this cycle and to provide specific targets for treatment.
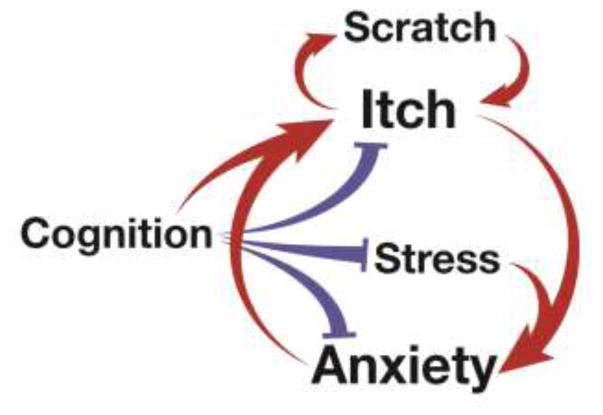
Figure 1.
Itch and anxiety form a vicious cycle, which leads to increased itch perception and scratching behavior. Stress (including the stress of chronic itch) may lead to sensitization of neuronal pathways that exacerbates anxiety. Additionally, cognition can exert either positive or negative feedback within the cycle.
Highlights.
The vicious cycle of itch and anxiety is proposed.
Chronic itch is associated with higher levels of anxiety.
Psychological or emotional stress aggravates itch perception.
The central nervous system plays a major role in the itch-anxiety cycle.
Acknowledgments
The authors are grateful to Hjalte H. Andersen for assistance with figure creation. This work was supported by grants from the National Institutes of Health (AR063228 to T. A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Kobayashi K, Yoshino S, Taguchi K, Nojima H. Withdrawal of repeated morphine enhances histamine-induced scratching responses in mice. Drug Chem Toxicol. 2015;38:167–173. doi: 10.3109/01480545.2014.922095. [DOI] [PubMed] [Google Scholar]
- Ahuja RB, Gupta GK. A four arm, double blind, randomized and placebo controlled study of pregabalin in the management of post-burn pruritus. Burns. 2013;39:24–29. doi: 10.1016/j.burns.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Akhan A, Subasi FD, Bosna G, Ekinci O, Pamuk H, Batan S, Ateser RY, Turan G. Comparison of mirtazapine, gabapentin and ondansetron to prevent intrathecal morphine-induced pruritus. North Clin Istanb. 2016;3:53–59. doi: 10.14744/nci.2016.38233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altunay IK, Atis G, Esen K, Kucukunal A. Impact of functional pruritus compared with mild psoriasis on quality of life: a cross-sectional questionnaire study in Turkey. Am J Clin Dermatol. 2014;15:365–370. doi: 10.1007/s40257-014-0075-7. [DOI] [PubMed] [Google Scholar]
- Amano H, Negishi I, Akiyama H, Ishikawa O. Psychological stress can trigger atopic dermatitis in NC/Nga mice: an inhibitory effect of corticotropin-releasing factor. Neuropsychopharmacology. 2008;33:566–573. doi: 10.1038/sj.npp.1301435. [DOI] [PubMed] [Google Scholar]
- Atis G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459. doi: 10.1111/dth.12459. [DOI] [PubMed] [Google Scholar]
- Bartels DJ, van Laarhoven AI, Haverkamp EA, Wilder-Smith OH, Donders AR, van Middendorp H, van de Kerkhof PC, Evers AW. Role of conditioning and verbal suggestion in placebo and nocebo effects on itch. PLoS One. 2014;9:e91727. doi: 10.1371/journal.pone.0091727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels DJ, van Laarhoven AI, van de Kerkhof PC, Evers AW. Placebo and nocebo effects on itch: effects, mechanisms, and predictors. Eur J Pain. 2016;20:8–13. doi: 10.1002/ejp.750. [DOI] [PubMed] [Google Scholar]
- Bender BG, Ballard R, Canono B, Murphy JR, Leung DY. Disease severity, scratching, and sleep quality in patients with atopic dermatitis. J Am Acad Dermatol. 2008;58:415–420. doi: 10.1016/j.jaad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–12022. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergasa NV, McGee M, Ginsburg IH, Engler D. Gabapentin in patients with the pruritus of cholestasis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2006;44:1317–1323. doi: 10.1002/hep.21370. [DOI] [PubMed] [Google Scholar]
- Berrino AM, Voltolini S, Fiaschi D, Pellegrini S, Bignardi D, Minale P, Troise C, Maura E. Chronic urticaria: importance of a medical-psychological approach. Eur Ann Allergy Clin Immunol. 2006;38:149–152. [PubMed] [Google Scholar]
- Bigata X, Sais G, Soler F. Severe chronic urticaria: response to mirtazapine. J Am Acad Dermatol. 2005;53:916–917. doi: 10.1016/j.jaad.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Biondi M, Arcangeli T, Petrucci RM. Paroxetine in a case of psychogenic pruritus and neurotic excoriations. Psychother Psychosom. 2000;69:165–166. doi: 10.1159/000012386. [DOI] [PubMed] [Google Scholar]
- Bjorkedal E, Flaten MA. Expectations of increased and decreased pain explain the effect of conditioned pain modulation in females. J Pain Res. 2012;5:289–300. doi: 10.2147/JPR.S33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D, Schmid-Ott G, Finkeldey F, John SM, Dwinger C, Werfel T, Diepgen TL, Breuer K. Anxiety, depression and impaired health-related quality of life in patients with occupational hand eczema. Contact Dermatitis. 2012;67:184–192. doi: 10.1111/j.1600-0536.2012.02062.x. [DOI] [PubMed] [Google Scholar]
- Boettger MK, Bar KJ, Dohrmann A, Muller H, Mertins L, Brockmeyer NH, Agelink MW. Increased vagal modulation in atopic dermatitis. J Dermatol Sci. 2009;53:55–59. doi: 10.1016/j.jdermsci.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Reeves ST, Frohman H, Madan A, Jensen MP, Patterson D, Barth K, Smith AR, Gracely R, George MS. Fast left prefrontal rTMS acutely suppresses analgesic effects of perceived controllability on the emotional component of pain experience. Pain. 2011;152:182–187. doi: 10.1016/j.pain.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenaut E, Marcorelles P, Genestet S, Menard D, Misery L. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. 2015;72:328–332. doi: 10.1016/j.jaad.2014.10.034. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Geiben A, Hollig H, Morschhauser E, Hellhammer D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. J Clin Endocrinol Metab. 2002;87:4245–4251. doi: 10.1210/jc.2001-010872. [DOI] [PubMed] [Google Scholar]
- Cheikh Hassan HI, Brennan F, Collett G, Josland EA, Brown MA. Efficacy and safety of gabapentin for uremic pruritus and restless legs syndrome in conservatively managed patients with chronic kidney disease. J Pain Symptom Manage. 2015;49:782–789. doi: 10.1016/j.jpainsymman.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang W, Tan T, Han H, Dong Z. GABA(A) Receptors in the Central Nucleus of the Amygdala Are Involved in Pain- and Itch-Related Responses. J Pain. 2016;17:181–189. doi: 10.1016/j.jpain.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that Influence Health-Related Quality of Life in Patients with Primary Sclerosing Cholangitis. Dig Dis Sci. 2016;61:1692–1699. doi: 10.1007/s10620-015-4013-1. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom Med. 2008;70:102–116. doi: 10.1097/PSY.0b013e31815c1b71. [DOI] [PubMed] [Google Scholar]
- Chrostowska-Plak D, Reich A, Szepietowski JC. Relationship between itch and psychological status of patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2013;27:e239–242. doi: 10.1111/j.1468-3083.2012.04578.x. [DOI] [PubMed] [Google Scholar]
- Comert S, Celebioglu E, Karakaya G, Kalyoncu AF. The general characteristics of acute urticaria attacks and the factors predictive of progression to chronic urticaria. Allergol Immunopathol (Madr) 2013;41:239–245. doi: 10.1016/j.aller.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Cormia FE. Experimental histamine pruritus. I. Influence of physical and psychological factors on threshold reactivity. J Invest Dermatol. 1952;19:21–34. doi: 10.1038/jid.1952.62. [DOI] [PubMed] [Google Scholar]
- D'Erme AM, Zanieri F, Campolmi E, Santosuosso U, Betti S, Agnoletti AF, Cossidente A, Lotti T. Therapeutic implications of adding the psychotropic drug escitalopram in the treatment of patients suffering from moderate-severe psoriasis and psychiatric comorbidity: a retrospective study. J Eur Acad Dermatol Venereol. 2014;28:246–249. doi: 10.1111/j.1468-3083.2012.04690.x. [DOI] [PubMed] [Google Scholar]
- Dalgard F, Stern R, Lien L, Hauser S. Itch, stress and self-efficacy among 18-year-old boys and girls: a Norwegian population-based cross-sectional study. Acta Derm Venereol. 2012;92:547–552. doi: 10.2340/00015555-1309. [DOI] [PubMed] [Google Scholar]
- Danial C, Adeduntan R, Gorell ES, Lucky AW, Paller AS, Bruckner A, Pope E, Morel KD, Levy ML, Li S, et al. Prevalence and characterization of pruritus in epidermolysis bullosa. Pediatr Dermatol. 2015;32:53–59. doi: 10.1111/pde.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ., Jr Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108:1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MP, Frandsen JL, Walsh D, Andresen S, Taylor S. Mirtazapine for pruritus. J Pain Symptom Manage. 2003;25:288–291. doi: 10.1016/s0885-3924(02)00645-0. [DOI] [PubMed] [Google Scholar]
- de Brouwer SJ, van Middendorp H, Stormink C, Kraaimaat FW, Sweep FC, de Jong EM, Schalkwijk J, Eijsbouts A, Donders AR, van de Kerkhof PC, et al. The psychophysiological stress response in psoriasis and rheumatoid arthritis. Br J Dermatol. 2014;170:824–831. doi: 10.1111/bjd.12697. [DOI] [PubMed] [Google Scholar]
- Demierre MF, Taverna J. Mirtazapine and gabapentin for reducing pruritus in cutaneous T-cell lymphoma. J Am Acad Dermatol. 2006;55:543–544. doi: 10.1016/j.jaad.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Diefenbach GJ, Assaf M, Goethe JW, Gueorguieva R, Tolin DF. Improvements in emotion regulation following repetitive transcranial magnetic stimulation for generalized anxiety disorder. J Anxiety Disord. 2016a;43:1–7. doi: 10.1016/j.janxdis.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Diefenbach GJ, Bragdon LB, Zertuche L, Hyatt CJ, Hallion LS, Tolin DF, Goethe JW, Assaf M. Repetitive transcranial magnetic stimulation for generalised anxiety disorder: a pilot randomised, double-blind, sham-controlled trial. Br J Psychiatry. 2016b;209:222–228. doi: 10.1192/bjp.bp.115.168203. [DOI] [PubMed] [Google Scholar]
- Ebata T, Izumi H, Aizawa H, Kamide R, Niimura M. Effects of nitrazepam on nocturnal scratching in adults with atopic dermatitis: a double-blind placebo-controlled crossover study. Br J Dermatol. 1998;138:631–634. doi: 10.1046/j.1365-2133.1998.02174.x. [DOI] [PubMed] [Google Scholar]
- Elhakim M, Abd-Elfattah H, El-Din DN, El-Kabarity R, Atef A, El-Fakey A. Midazolam as an antiemetic in patients receiving epidural morphine for postoperative pain relief. J Opioid Manag. 2009;5:189–195. doi: 10.5055/jom.2009.0020. [DOI] [PubMed] [Google Scholar]
- Evers AW, Verhoeven EW, Kraaimaat FW, de Jong EM, de Brouwer SJ, Schalkwijk J, Sweep FC, van de Kerkhof PC. How stress gets under the skin: cortisol and stress reactivity in psoriasis. Br J Dermatol. 2010;163:986–991. doi: 10.1111/j.1365-2133.2010.09984.x. [DOI] [PubMed] [Google Scholar]
- Feneran AN, O'Donnell R, Press A, Yosipovitch G, Cline M, Dugan G, Papoiu AD, Nattkemper LA, Chan YH, Shively CA. Monkey see, monkey do: contagious itch in nonhuman primates. Acta Derm Venereol. 2013;93:27–29. doi: 10.2340/00015555-1406. [DOI] [PubMed] [Google Scholar]
- Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395–400. doi: 10.2340/00015555-0864. [DOI] [PubMed] [Google Scholar]
- Gauffin E, Oster C, Gerdin B, Ekselius L. Prevalence and prediction of prolonged pruritus after severe burns. J Burn Care Res. 2015;36:405–413. doi: 10.1097/BCR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Gencoglan G, Inanir I, Gunduz K. Therapeutic hotline: Treatment of prurigo nodularis and lichen simplex chronicus with gabapentin. Dermatol Ther. 2010;23:194–198. doi: 10.1111/j.1529-8019.2010.01314.x. [DOI] [PubMed] [Google Scholar]
- Goldsobel AB, Rohr AS, Siegel SC, Spector SL, Katz RM, Rachelefsky GS, Drayton G, Indianer L, Peter JB, Barr RJ, et al. Efficacy of doxepin in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1986;78:867–873. doi: 10.1016/0091-6749(86)90232-0. [DOI] [PubMed] [Google Scholar]
- Gray P, Kirby J, Smith MT, Cabot PJ, Williams B, Doecke J, Cramond T. Pregabalin in severe burn injury pain: a double-blind, randomised placebo-controlled trial. Pain. 2011;152:1279–1288. doi: 10.1016/j.pain.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Greene SL, Reed CE, Schroeter AL. Double-blind crossover study comparing doxepin with diphenhydramine for the treatment of chronic urticaria. J Am Acad Dermatol. 1985;12:669–675. doi: 10.1016/s0190-9622(85)70092-8. [DOI] [PubMed] [Google Scholar]
- Griffiths CE, van de Kerkhof P, Czarnecka-Operacz M. Psoriasis and Atopic Dermatitis. Dermatol Ther (Heidelb) 2017;7:31–41. doi: 10.1007/s13555-016-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen JA, Dalgard F, Thoresen M, Bjertness E, Lien L. Itch and pain in adolescents are associated with suicidal ideation: a population-based cross-sectional study. Acta Derm Venereol. 2012;92:543–546. doi: 10.2340/00015555-1251. [DOI] [PubMed] [Google Scholar]
- Halvorsen JA, Dalgard F, Thoresen M, Thoresen M, Bjertness E, Lien L. Itch and mental distress: a cross-sectional study among late adolescents. Acta Derm Venereol. 2009;89:39–44. doi: 10.2340/00015555-0554. [DOI] [PubMed] [Google Scholar]
- Halvorson H, Crooks J, Lahart DA, Farrell KP. An outbreak of itching in an elementary school-a case of mass psychogenic response. J Sch Health. 2008;78:294–297. doi: 10.1111/j.1746-1561.2008.00303.x. [DOI] [PubMed] [Google Scholar]
- Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD. Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell. 2015;162:363–374. doi: 10.1016/j.cell.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiro M, Okumura M. Anxiety, depression, psychosomatic symptoms and autonomic nervous function in patients with chronic urticaria. J Dermatol Sci. 1994;8:129–135. doi: 10.1016/0923-1811(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Holle H, Warne K, Seth AK, Critchley HD, Ward J. Neural basis of contagious itch and why some people are more prone to it. Proc Natl Acad Sci U S A. 2012;109:19816–19821. doi: 10.1073/pnas.1216160109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschneider K, Mulert C. Neuroimaging in anxiety disorders. Dialogues Clin Neurosci. 2011;13:453–461. doi: 10.31887/DCNS.2011.13.4/kholzschneider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SB, Park JH, Ihm CG, Kim NI. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283–288. doi: 10.3346/jkms.2004.19.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini Amiri M, Tavousi SH, Mazlom SR, Manzari ZS. Effect of transcranial direct current stimulation on pain anxiety during burn wound care. Burns. 2016;42:872–876. doi: 10.1016/j.burns.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Houghton KT, Forrest A, Awad A, Atkinson LZ, Stockton S, Harrison PJ, Geddes JR, Cipriani A. Biological rationale and potential clinical use of gabapentin and pregabalin in bipolar disorder, insomnia and anxiety: protocol for a systematic review and meta-analysis. BMJ Open. 2017;7:e013433. doi: 10.1136/bmjopen-2016-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrehorow E, Salomon J, Matusiak L, Reich A, Szepietowski JC. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67–72. doi: 10.2340/00015555-1193. [DOI] [PubMed] [Google Scholar]
- Huesmann M, Huesmann T, Osada N, Phan NQ, Kremer AE, Stander S. Cholestatic pruritus: a retrospective analysis on clinical characteristics and treatment response. J Dtsch Dermatol Ges. 2013;11:158–168. doi: 10.1111/j.1610-0387.2012.08028.x. [DOI] [PubMed] [Google Scholar]
- Hundley JL, Yosipovitch G. Mirtazapine for reducing nocturnal itch in patients with chronic pruritus: a pilot study. J Am Acad Dermatol. 2004;50:889–891. doi: 10.1016/j.jaad.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Iking A, Grundmann S, Chatzigeorgakidis E, Phan NQ, Klein D, Stander S. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol. 2013;27:550–557. doi: 10.1111/j.1468-3083.2012.04481.x. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62:212–217. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- Inui S, Shirakawa Y, Itami S. Effect of nalfurafine hydrochloride on pruritus and anxiety level in hemodialysis patients. J Dermatol. 2012;39:886–887. doi: 10.1111/j.1346-8138.2011.01442.x. [DOI] [PubMed] [Google Scholar]
- Ishiuji Y, Coghill RC, Patel TS, Oshiro Y, Kraft RA, Yosipovitch G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br J Dermatol. 2009;161:1072–1080. doi: 10.1111/j.1365-2133.2009.09308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagdeo J, Kroshinsky D. A case of post-herpetic itch resolved with gabapentin. J Drugs Dermatol. 2011;10:85–88. [PubMed] [Google Scholar]
- Janowski K, Steuden S, Bogaczewicz J. Clinical and psychological characteristics of patients with psoriasis reporting various frequencies of pruritus. Int J Dermatol. 2014;53:820–829. doi: 10.1111/ijd.12074. [DOI] [PubMed] [Google Scholar]
- Jeong KY, Kang JH. Investigation of the pruritus-induced functional activity in the rat brain using manganese-enhanced MRI. J Magn Reson Imaging. 2015;42:709–716. doi: 10.1002/jmri.24832. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, Tromp do PM, Grabow BP, Olsen ME, Brodsky EK, et al. Overexpressing Corticotropin-Releasing Factor in the Primate Amygdala Increases Anxious Temperament and Alters Its Neural Circuit. Biol Psychiatry. 2016;80:345–355. doi: 10.1016/j.biopsych.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik SB, Cerci FB, Miracle J, Pokharel A, Chen SC, Chan YH, Wilkin A, Yosipovitch G. Chronic pruritus in HIV-positive patients in the southeastern United States: its prevalence and effect on quality of life. J Am Acad Dermatol. 2014;70:659–664. doi: 10.1016/j.jaad.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park JB, Lee JH, Kim IH. How stress triggers itch: a preliminary study of the mechanism of stress-induced pruritus using fMRI. Int J Dermatol. 2016a;55:434–442. doi: 10.1111/ijd.12864. [DOI] [PubMed] [Google Scholar]
- Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat Neurosci. 2016b;19:1636–1646. doi: 10.1038/nn.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron. 2017;93:1464–1479. e1465. doi: 10.1016/j.neuron.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Miyazawa H. The Butterfly Sign in Patients with Atopic-Dermatitis - Evidence for the Role of Scratching in the Development of Skin Manifestations. Journal of the American Academy of Dermatology. 1989;21:579–580. doi: 10.1016/s0190-9622(89)80235-x. [DOI] [PubMed] [Google Scholar]
- Kirchner A, Stefan H, Schmelz M, Haslbeck KM, Birklein F. Influence of vagus nerve stimulation on histamine-induced itching. Neurology. 2002;59:108–112. doi: 10.1212/wnl.59.1.108. [DOI] [PubMed] [Google Scholar]
- Kitagaki H, Hiyama H, Kitazawa T, Shiohara T. Psychological stress with longstanding allergic dermatitis causes psychodermatological conditions in mice. J Invest Dermatol. 2014;134:1561–1569. doi: 10.1038/jid.2014.31. [DOI] [PubMed] [Google Scholar]
- Knotkova H, Portenoy RK, Cruciani RA. Transcranial direct current stimulation (tDCS) relieved itching in a patient with chronic neuropathic pain. Clin J Pain. 2013;29:621–622. doi: 10.1097/AJP.0b013e31826b1329. [DOI] [PubMed] [Google Scholar]
- Kodama A, Horikawa T, Suzuki T, Ajiki W, Takashima T, Harada S, Ichihashi M. Effect of stress on atopic dermatitis: investigation in patients after the great hanshin earthquake. J Allergy Clin Immunol. 1999;104:173–176. doi: 10.1016/s0091-6749(99)70130-2. [DOI] [PubMed] [Google Scholar]
- Kossakowska MM, Ciescinska C, Jaszewska J, Placek WJ. Control of negative emotions and its implication for illness perception among psoriasis and vitiligo patients. J Eur Acad Dermatol Venereol. 2010;24:429–433. doi: 10.1111/j.1468-3083.2009.03432.x. [DOI] [PubMed] [Google Scholar]
- Kretzmer GE, Gelkopf M, Kretzmer G, Melamed Y. Idiopathic pruritus in psychiatric inpatients: an explorative study. Gen Hosp Psychiatry. 2008;30:344–348. doi: 10.1016/j.genhosppsych.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Lee HG, Grossman SK, Valdes-Rodriguez R, Berenato F, Korbutov J, Chan YH, Lavery MJ, Yosipovitch G. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: A retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760–761. doi: 10.1016/j.jaad.2016.10.030. [DOI] [PubMed] [Google Scholar]
- Leibovici V, Magora F, Cohen S, Ingber A. Effects of virtual reality immersion and audiovisual distraction techniques for patients with pruritus. Pain Res Manag. 2009;14:283–286. doi: 10.1155/2009/178751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelonek E, Matusiak L, Wrobel T, Kwiatkowski J, Szepietowski JC. Burden of Aquagenic Pruritus in Polycythaemia Vera. Acta Derm Venereol. 2017 doi: 10.2340/00015555-2812. [DOI] [PubMed] [Google Scholar]
- Lesner K, Reich A, Szepietowski JC, Dalgard FJ, Gieler U, Tomas-Aragones L, Lien L, Poot F, Jemec GB, Misery L, et al. Determinants of Psychosocial Health in Psoriatic Patients: A Multi-national Study. Acta Derm Venereol. 2017;97:1182–1188. doi: 10.2340/00015555-2760. [DOI] [PubMed] [Google Scholar]
- Liao YH, Lin CC, Tsai PP, Shen WC, Sung FC, Kao CH. Increased risk of lichen simplex chronicus in people with anxiety disorder: a nationwide population-based retrospective cohort study. Br J Dermatol. 2014;170:890–894. doi: 10.1111/bjd.12811. [DOI] [PubMed] [Google Scholar]
- Lim YL, Chan YH, Yosipovitch G, Greaves MW. Pruritus is a common and significant symptom of acne. J Eur Acad Dermatol Venereol. 2008;22:1332–1336. doi: 10.1111/j.1468-3083.2008.02828.x. [DOI] [PubMed] [Google Scholar]
- Lloyd DM, Hall E, Hall S, McGlone FP. Can itch-related visual stimuli alone provoke a scratch response in healthy individuals? Br J Dermatol. 2013;168:106–111. doi: 10.1111/bjd.12132. [DOI] [PubMed] [Google Scholar]
- Maciel AA, Cunha PR, Laraia IO, Trevisan F. Efficacy of gabapentin in the improvement of pruritus and quality of life of patients with notalgia paresthetica. An Bras Dermatol. 2014;89:570–575. doi: 10.1590/abd1806-4841.20142777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeoka H, Matsuo A, Hiyamizu M, Morioka S, Ando H. Influence of transcranial direct current stimulation of the dorsolateral prefrontal cortex on pain related emotions: a study using electroencephalographic power spectrum analysis. Neurosci Lett. 2012;512:12–16. doi: 10.1016/j.neulet.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Maroun M. Medial prefrontal cortex: multiple roles in fear and extinction. Neuroscientist. 2013;19:370–383. doi: 10.1177/1073858412464527. [DOI] [PubMed] [Google Scholar]
- Marron SE, Tomas-Aragones L, Boira S, Campos-Rodenas R. Quality of Life, Emotional Wellbeing and Family Repercussions in Dermatological Patients Experiencing Chronic Itching: A Pilot Study. Acta Derm Venereol. 2016;96:331–335. doi: 10.2340/00015555-2263. [DOI] [PubMed] [Google Scholar]
- Matsuda KM, Sharma D, Schonfeld AR, Kwatra SG. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619–625. e616. doi: 10.1016/j.jaad.2016.02.1237. [DOI] [PubMed] [Google Scholar]
- Matterne U, Apfelbacher CJ, Vogelgsang L, Loerbroks A, Weisshaar E. Incidence and determinants of chronic pruritus: a population-based cohort study. Acta Derm Venereol. 2013;93:532–537. doi: 10.2340/00015555-1572. [DOI] [PubMed] [Google Scholar]
- Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45:666–674. doi: 10.1002/hep.21553. [DOI] [PubMed] [Google Scholar]
- Mazeh D, Melamed Y, Cholostoy A, Aharonovitzch V, Weizman A, Yosipovitch G. Itching in the psychiatric ward. Acta Derm Venereol. 2008;88:128–131. doi: 10.2340/00015555-0406. [DOI] [PubMed] [Google Scholar]
- Mazza M, Guerriero G, Marano G, Janiri L, Bria P, Mazza S. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16–18. doi: 10.1111/jcpt.12005. [DOI] [PubMed] [Google Scholar]
- McGarry S, Burrows S, Ashoorian T, Pallathil T, Ong K, Edgar DW, Wood F. Mental health and itch in burns patients: Potential associations. Burns. 2016;42:763–768. doi: 10.1016/j.burns.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Mendham JE. Gabapentin for the treatment of itching produced by burns and wound healing in children: a pilot study. Burns. 2004;30:851–853. doi: 10.1016/j.burns.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Misery L, Wallengren J, Weisshaar E, Zalewska A French Psychodermatology G. Validation of diagnosis criteria of functional itch disorder or psychogenic pruritus. Acta Derm Venereol. 2008;88:503–504. doi: 10.2340/00015555-0486. [DOI] [PubMed] [Google Scholar]
- Mistik S, Utas S, Ferahbas A, Tokgoz B, Unsal G, Sahan H, Ozturk A, Utas C. An epidemiology study of patients with uremic pruritus. J Eur Acad Dermatol Venereol. 2006;20:672–678. doi: 10.1111/j.1468-3083.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Baumgartner U, Kamping S, Ruttorf M, Schad LR, Flor H, Kakigi R, Treede RD. Cortico-subcortical activation patterns for itch and pain imagery. Pain. 2013;154:1989–1998. doi: 10.1016/j.pain.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Inui K, Tanabe HC, Akiyama LF, Otsuru N, Yamashiro K, Sasaki A, Nakata H, Sadato N, Kakigi R. Time course of activity in itch-related brain regions: a combined MEG-fMRI study. J Neurophysiol. 2009;102:2657–2666. doi: 10.1152/jn.00460.2009. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Papoiu ADP, Nattkemper LA, Lin AC, Kraft RA, Coghill RC, Yosipovitch G. Scratching Induces Overactivity in Motor-Related Regions and Reward System in Chronic Itch Patients. J Invest Dermatol. 2015;135:2814–2823. doi: 10.1038/jid.2015.223. [DOI] [PubMed] [Google Scholar]
- Morell-Dubois S, Carpentier O, Cottencin O, Queyrel V, Hachulla E, Hatron PY, Delaporte E. Stressful life events and pemphigus. Dermatology. 2008;216:104–108. doi: 10.1159/000111506. [DOI] [PubMed] [Google Scholar]
- Mrowietz U, Chouela EN, Mallbris L, Stefanidis D, Marino V, Pedersen R, Boggs RL. Pruritus and quality of life in moderate-to-severe plaque psoriasis: post hoc explorative analysis from the PRISTINE study. J Eur Acad Dermatol Venereol. 2015;29:1114–1120. doi: 10.1111/jdv.12761. [DOI] [PubMed] [Google Scholar]
- Mu D, Sun YG. Itch induces conditioned place aversion in mice. Neurosci Lett. 2017;658:91–96. doi: 10.1016/j.neulet.2017.08.046. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Mochizuki H, Koyama S, Tanaka S, Sadato N, Kakigi R. A transcranial direct current stimulation over the sensorimotor cortex modulates the itch sensation induced by histamine. Clin Neurophysiol. 2016;127:827–832. doi: 10.1016/j.clinph.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Napadow V, Li A, Loggia ML, Kim J, Schalock PC, Lerner E, Tran TN, Ring J, Rosen BR, Kaptchuk TJ, et al. The brain circuitry mediating antipruritic effects of acupuncture. Cereb Cortex. 2014;24:873–882. doi: 10.1093/cercor/bhs363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neittaanmaki H, Myohanen T, Fraki JE. Comparison of cinnarizine, cyproheptadine, doxepin, and hydroxyzine in treatment of idiopathic cold urticaria: usefulness of doxepin. J Am Acad Dermatol. 1984;11:483–489. doi: 10.1016/s0190-9622(84)70196-4. [DOI] [PubMed] [Google Scholar]
- Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol. 2015;227:261–284. doi: 10.1007/978-3-662-46450-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofal E, Farag F, Nofal A, Eldesouky F, Alkot R, Abdelkhalik Z. Gabapentin: A promising therapy for uremic pruritus in hemodialysis patients: A randomized-controlled trial and review of literature. J Dermatolog Treat. 2016;27:515–519. doi: 10.3109/09546634.2016.1161161. [DOI] [PubMed] [Google Scholar]
- Noh S, Kim M, Park CO, Hann SK, Oh SH. Comparison of the psychological impacts of asymptomatic and symptomatic cutaneous diseases: vitiligo and atopic dermatitis. Ann Dermatol. 2013;25:454–461. doi: 10.5021/ad.2013.25.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell NE, Wand BM, Marston L, Spencer S, DeSouza LH. The Cochrane, editor. Cochrane Database of Systematic Reviews, C. Chichester, UK: John Wiley & Sons, Ltd; 2014. Non-invasive brain stimulation techniques for chronic pain. [DOI] [PubMed] [Google Scholar]
- Ogden J, Zoukas S. Generating physical symptoms from visual cues: An experimental study. Psychol Health Med. 2009;14:695–704. doi: 10.1080/13548500903311547. [DOI] [PubMed] [Google Scholar]
- Ograczyk A, Miniszewska J, Kepska A, Zalewska-Janowska A. Itch, disease coping strategies and quality of life in psoriasis patients. Postepy Dermatol Alergol. 2014;31:299–304. doi: 10.5114/pdia.2014.40927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SH, Bae BG, Park CO, Noh JY, Park IH, Wu WH, Lee KH. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90:582–588. doi: 10.2340/00015555-0933. [DOI] [PubMed] [Google Scholar]
- Okon-Singer H, Hendler T, Pessoa L, Shackman AJ. The neurobiology of emotion-cognition interactions: fundamental questions and strategies for future research. Front Hum Neurosci. 2015;9:58. doi: 10.3389/fnhum.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakfetrat M, Malekmakan L, Hashemi N, Tadayon T. Sertraline can reduce uremic pruritus in hemodialysis patient: A double blind randomized clinical trial from Southern Iran. Hemodial Int. 2017 doi: 10.1111/hdi.12540. [DOI] [PubMed] [Google Scholar]
- Papoiu AD, Coghill RC, Kraft RA, Wang H, Yosipovitch G. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage. 2012;59:3611–3623. doi: 10.1016/j.neuroimage.2011.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoiu AD, Emerson NM, Patel TS, Kraft RA, Valdes-Rodriguez R, Nattkemper LA, Coghill RC, Yosipovitch G. Voxel-based morphometry and arterial spin labeling fMRI reveal neuropathic and neuroplastic features of brain processing of itch in end-stage renal disease. J Neurophysiol. 2014;112:1729–1738. doi: 10.1152/jn.00827.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoiu AD, Nattkemper LA, Sanders KM, Kraft RA, Chan YH, Coghill RC, Yosipovitch G. Brain's reward circuits mediate itch relief. a functional MRI study of active scratching. PLoS One. 2013;8:e82389. doi: 10.1371/journal.pone.0082389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoiu AD, Wang H, Coghill RC, Chan YH, Yosipovitch G. Contagious itch in humans: a study of visual 'transmission' of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299–1303. doi: 10.1111/j.1365-2133.2011.10318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Jwa SW, Song M, Kim HS, Ko HC, Kim MB, Kwon KS, Kim BS. Efficacy and safety of pregabalin for the treatment of chronic pruritus in Korea. J Dermatol. 2012;39:790–791. doi: 10.1111/j.1346-8138.2012.01572.x. [DOI] [PubMed] [Google Scholar]
- Parnell LK, Nedelec B, Rachelska G, LaSalle L. Assessment of pruritus characteristics and impact on burn survivors. J Burn Care Res. 2012;33:407–418. doi: 10.1097/BCR.0b013e318239d206. [DOI] [PubMed] [Google Scholar]
- Peng XY, Huang Y, Wang XL, Cao LF, Chen LH, Luo WF, Liu T. Adrenergic beta2-receptor mediates itch hypersensitivity following heterotypic chronic stress in rats. Neuroreport. 2015;26:1003–1010. doi: 10.1097/WNR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- Ponarovsky B, Amital D, Lazarov A, Kotler M, Amital H. Anxiety and depression in patients with allergic and non-allergic cutaneous disorders. Int J Dermatol. 2011;50:1217–1222. doi: 10.1111/j.1365-4632.2011.04910.x. [DOI] [PubMed] [Google Scholar]
- Pour-Reza-Gholi F, Nasrollahi A, Firouzan A, Nasli Esfahani E, Farrokhi F. Low-dose doxepin for treatment of pruritus in patients on hemodialysis. Iran J Kidney Dis. 2007;1:34–37. [PubMed] [Google Scholar]
- Prieto LN. The use of midazolam to treat itching in a terminally ill patient with biliary obstruction. J Pain Symptom Manage. 2004;28:531–532. doi: 10.1016/j.jpainsymman.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Racine M, Hudson M, Baron M, Nielson WR Canadian Scleroderma Research G. The Impact of Pain and Itch on Functioning and Health-Related Quality of Life in Systemic Sclerosis: An Exploratory Study. J Pain Symptom Manage. 2016;52:43–53. doi: 10.1016/j.jpainsymman.2015.12.314. [DOI] [PubMed] [Google Scholar]
- Rasul A, El-Nour H, Lonne-Rahm SB, Fransson O, Johansson C, Johansson B, Zubeidi M, Seeberg E, Djurfeldt DR, Azmitia EC, et al. Serotonergic Markers in Atopic Dermatitis. Acta Derm Venereol. 2016;96:732–736. doi: 10.2340/00015555-2354. [DOI] [PubMed] [Google Scholar]
- Raszeja-Wyszomirska J, Wunsch E, Krawczyk M, Rigopoulou EI, Bogdanos D, Milkiewicz P. Prospective evaluation of PBC-specific health-related quality of life questionnaires in patients with primary sclerosing cholangitis. Liver Int. 2015;35:1764–1771. doi: 10.1111/liv.12730. [DOI] [PubMed] [Google Scholar]
- Rayner H, Baharani J, Smith S, Suresh V, Dasgupta I. Uraemic pruritus: relief of itching by gabapentin and pregabalin. Nephron Clin Pract. 2012;122:75–79. doi: 10.1159/000349943. [DOI] [PubMed] [Google Scholar]
- Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Reich A, Hrehorow E, Szepietowski JC. Pruritus is an important factor negatively influencing the well-being of psoriatic patients. Acta Derm Venereol. 2010;90:257–263. doi: 10.2340/00015555-0851. [DOI] [PubMed] [Google Scholar]
- Remrod C, Sjostrom K, Svensson A. Pruritus in psoriasis: a study of personality traits, depression and anxiety. Acta Derm Venereol. 2015;95:439–443. doi: 10.2340/00015555-1975. [DOI] [PubMed] [Google Scholar]
- Richards HL, Ray DW, Kirby B, Mason D, Plant D, Main CJ, Fortune DG, Griffiths CE. Response of the hypothalamic-pituitary-adrenal axis to psychological stress in patients with psoriasis. Br J Dermatol. 2005;153:1114–1120. doi: 10.1111/j.1365-2133.2005.06817.x. [DOI] [PubMed] [Google Scholar]
- Richelson E. Tricyclic antidepressants and histamine H1 receptors. Mayo Clin Proc. 1979;54:669–674. [PubMed] [Google Scholar]
- Robinson P, Szewczyk M, Haddy L, Jones P, Harvey W. Outbreak of itching and rash. Epidemic hysteria in an elementary school. Arch Intern Med. 1984;144:1959–1962. [PubMed] [Google Scholar]
- Rod NH, Kristensen TS, Lange P, Prescott E, Diderichsen F. Perceived stress and risk of adult-onset asthma and other atopic disorders: a longitudinal cohort study. Allergy. 2012;67:1408–1414. doi: 10.1111/j.1398-9995.2012.02882.x.. [DOI] [PubMed] [Google Scholar]
- Sarifakioglu E, Onur O. Women with scalp dysesthesia treated with pregabalin. Int J Dermatol. 2013;52:1417–1418. doi: 10.1111/j.1365-4632.2011.05158.x. [DOI] [PubMed] [Google Scholar]
- Schmid-Ott G, Kuensebeck HW, Jaeger B, Werfel T, Frahm K, Ruitman J, Kapp A, Lamprecht F. Validity study for the stigmatization experience in atopic dermatitis and psoriatic patients. Acta Derm Venereol. 1999;79:443–447. doi: 10.1080/000155599750009870. [DOI] [PubMed] [Google Scholar]
- Schneider G, Driesch G, Heuft G, Evers S, Luger TA, Stander S. Psychosomatic cofactors and psychiatric comorbidity in patients with chronic itch. Clin Exp Dermatol. 2006;31:762–767. doi: 10.1111/j.1365-2230.2006.02211.x. [DOI] [PubMed] [Google Scholar]
- Schneider G, Stander S, Burgmer M, Driesch G, Heuft G, Weckesser M. Significant differences in central imaging of histamine-induced itch between atopic dermatitis and healthy subjects. European Journal of Pain. 2008;12:834–841. doi: 10.1016/j.ejpain.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Schut C, Mollanazar NK, Kupfer J, Gieler U, Yosipovitch G. Psychological Interventions in the Treatment of Chronic Itch. Acta Derm Venereol. 2016a;96:157–161. doi: 10.2340/00015555-2177. [DOI] [PubMed] [Google Scholar]
- Schut C, Mollanazar NK, Sethi M, Nattkemper LA, Valdes-Rodriguez R, Lovell MM, Calzaferri GL, Yosipovitch G. Psychological Stress and Skin Symptoms in College Students: Results of a Cross-sectional Web-based Questionnaire Study. Acta Derm Venereol. 2016b;96:550–551. doi: 10.2340/00015555-2291. [DOI] [PubMed] [Google Scholar]
- Schut C, Weik U, Tews N, Gieler U, Deinzer R, Kupfer J. Coping as mediator of the relationship between stress and itch in patients with atopic dermatitis: a regression and mediation analysis. Exp Dermatol. 2015;24:148–150. doi: 10.1111/exd.12578. [DOI] [PubMed] [Google Scholar]
- Seiffert K, Hilbert E, Schaechinger H, Zouboulis CC, Deter HC. Psychophysiological reactivity under mental stress in atopic dermatitis. Dermatology. 2005;210:286–293. doi: 10.1159/000084752. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiba M, Sanadgol H, Azmoude HR, Mashhadi MA, Sharifi H. Effect of sertraline on uremic pruritus improvement in ESRD patients. Int J Nephrol. 2012;2012:363901. doi: 10.1155/2012/363901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen MJ, Ho ST, Lee CH, Tsung YC, Chang FL, Huang ST. Prophylactic mirtazapine reduces intrathecal morphine-induced pruritus. Br J Anaesth. 2008;101:711–715. doi: 10.1093/bja/aen241. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel FP, Tauscher J, Petrides PE. Aquagenic pruritus in polycythemia vera: characteristics and influence on quality of life in 441 patients. Am J Hematol. 2013;88:665–669. doi: 10.1002/ajh.23474. [DOI] [PubMed] [Google Scholar]
- Silverberg JI, Hinami K, Trick WE, Cella D. Itch in the General Internal Medicine Setting: A Cross-Sectional Study of Prevalence and Quality-of-Life Effects. Am J Clin Dermatol. 2016;17:681–690. doi: 10.1007/s40257-016-0215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EL, Gadkari A, Worm M, Soong W, Blauvelt A, Eckert L, Wu R, Ardeleanu M, Graham NMH, Pirozzi G, et al. Dupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): A phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD) J Am Acad Dermatol. 2016;75:506–515. doi: 10.1016/j.jaad.2016.04.054. [DOI] [PubMed] [Google Scholar]
- Spradley JM, Davoodi A, Carstens MI, Carstens E. Effects of acute stressors on itch- and pain-related behaviors in rats. Pain. 2012;153:1890–1897. doi: 10.1016/j.pain.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staats PS, Staats A, Hekmat H. The additive impact of anxiety and a placebo on pain. Pain Med. 2001;2:267–279. doi: 10.1046/j.1526-4637.2001.01046.x. [DOI] [PubMed] [Google Scholar]
- Stander S, Bockenholt B, Schurmeyer-Horst F, Weishaupt C, Heuft G, Luger TA, Schneider G. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: results of an open-labelled, two-arm proof-of-concept study. Acta Derm Venereol. 2009;89:45–51. doi: 10.2340/00015555-0553. [DOI] [PubMed] [Google Scholar]
- Stewart TJ, Schut C, Whitfeld M, Yosipovitch G. Cross-sectional study of psychological stress and skin symptoms in Australian university students. Australas J Dermatol. 2017 doi: 10.1111/ajd.12640. [DOI] [PubMed] [Google Scholar]
- Stumpf A, Burgmer M, Schneider G, Heuft G, Schmelz M, Phan NQ, Stander S, Pfleiderer B. Sex differences in itch perception and modulation by distraction--an FMRI pilot study in healthy volunteers. PLoS One. 2013a;8:e79123. doi: 10.1371/journal.pone.0079123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf A, Stander S, Phan NQ, Tanneberger A, Heuft G, Schneider G. Body concept of patients with chronic pruritus in relation to scratch lesions and psychic symptoms. Dermatology. 2013b;227:263–269. doi: 10.1159/000354911. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Fonseca R. Selective serotonin reuptake inhibitors are effective in the treatment of polycythemia vera-associated pruritus. Blood. 2002;99:2627. doi: 10.1182/blood.v99.7.2627. [DOI] [PubMed] [Google Scholar]
- Thebaut A, Habes D, Gottrand F, Rivet C, Cohen J, Debray D, Jacquemin E, Gonzales E. Sertraline as an Additional Treatment for Cholestatic Pruritus in Children. J Pediatr Gastroenterol Nutr. 2017;64:431–435. doi: 10.1097/MPG.0000000000001385. [DOI] [PubMed] [Google Scholar]
- Thorslund K, Svensson T, Nordlind K, Ekbom A, Fored CM. Use of serotonin reuptake inhibitors in patients with psoriasis is associated with a decreased need for systemic psoriasis treatment: a population-based cohort study. J Intern Med. 2013;274:281–287. doi: 10.1111/joim.12093. [DOI] [PubMed] [Google Scholar]
- Tran BW, Papoiu AD, Russoniello CV, Wang H, Patel TS, Chan YH, Yosipovitch G. Effect of itch, scratching and mental stress on autonomic nervous system function in atopic dermatitis. Acta Derm Venereol. 2010;90:354–361. doi: 10.2340/00015555-0890. [DOI] [PubMed] [Google Scholar]
- van Laarhoven AI, Kraaimaat FW, Wilder-Smith OH, Evers AW. Role of attentional focus on bodily sensations in sensitivity to itch and pain. Acta Derm Venereol. 2010;90:46–51. doi: 10.2340/00015555-0743. [DOI] [PubMed] [Google Scholar]
- van Laarhoven AI, Walker AL, Wilder-Smith OH, Kroeze S, van Riel PL, van de Kerkhof PC, Kraaimaat FW, Evers AW. Role of induced negative and positive emotions in sensitivity to itch and pain in women. Br J Dermatol. 2012;167:262–269. doi: 10.1111/j.1365-2133.2012.10933.x. [DOI] [PubMed] [Google Scholar]
- Van Loey NE, Bremer M, Faber AW, Middelkoop E, Nieuwenhuis MK. Itching following burns: epidemiology and predictors. Br J Dermatol. 2008;158:95–100. doi: 10.1111/j.1365-2133.2007.08278.x. [DOI] [PubMed] [Google Scholar]
- Verhoeven EW, de Klerk S, Kraaimaat FW, van de Kerkhof PC, de Jong EM, Evers AW. Biopsychosocial mechanisms of chronic itch in patients with skin diseases: a review. Acta Derm Venereol. 2008;88:211–218. doi: 10.2340/00015555-0452. [DOI] [PubMed] [Google Scholar]
- Verhoeven EW, Kraaimaat FW, de Jong EM, Schalkwijk J, van de Kerkhof PC, Evers AW. Individual differences in the effect of daily stressors on psoriasis: a prospective study. Br J Dermatol. 2009;161:295–299. doi: 10.1111/j.1365-2133.2009.09194.x. [DOI] [PubMed] [Google Scholar]
- Vierow V, Forster C, Vogelgsang R, Dorfler A, Handwerker HO. Cerebral Networks Linked to Itch-related Sensations Induced by Histamine and Capsaicin. Acta Derm Venereol. 2015;95:645–652. doi: 10.2340/00015555-2006. [DOI] [PubMed] [Google Scholar]
- Vierow V, Fukuoka M, Ikoma A, Dorfler A, Handwerker HO, Forster C. Cerebral representation of the relief of itch by scratching. J Neurophysiol. 2009;102:3216–3224. doi: 10.1152/jn.00207.2009. [DOI] [PubMed] [Google Scholar]
- Wahlgren CF. Itch and atopic dermatitis: clinical and experimental studies. Acta Derm Venereol Suppl (Stockh) 1991;165:1–53. [PubMed] [Google Scholar]
- Weiss M, Mettang T, Tschulena U, Passlick-Deetjen J, Weisshaar E. Prevalence of chronic itch and associated factors in haemodialysis patients: a representative cross-sectional study. Acta Derm Venereol. 2015;95:816–821. doi: 10.2340/00015555-2087. [DOI] [PubMed] [Google Scholar]
- Weiss M, Mettang T, Tschulena U, Weisshaar E. Health-related quality of life in haemodialysis patients suffering from chronic itch: results from GEHIS (German Epidemiology Haemodialysis Itch Study) Qual Life Res. 2016;25:3097–3106. doi: 10.1007/s11136-016-1340-4. [DOI] [PubMed] [Google Scholar]
- Welz-Kubiak K, Reich A, Szepietowski JC. Clinical Aspects of Itch in Lichen Planus. Acta Derm Venereol. 2017;97:505–508. doi: 10.2340/00015555-2563. [DOI] [PubMed] [Google Scholar]
- Willebrand M, Low A, Dyster-Aas J, Kildal M, Andersson G, Ekselius L, Gerdin B. Pruritus, personality traits and coping in long-term follow-up of burn-injured patients. Acta Derm Venereol. 2004;84:375–380. doi: 10.1080/00015550410032941. [DOI] [PubMed] [Google Scholar]
- Winhoven SM, Coulson IH, Bottomley WW. Brachioradial pruritus: response to treatment with gabapentin. Br J Dermatol. 2004;150:786–787. doi: 10.1111/j.0007-0963.2004.05889.x. [DOI] [PubMed] [Google Scholar]
- Woo KY. Unravelling nocebo effect: the mediating effect of anxiety between anticipation and pain at wound dressing change. J Clin Nurs. 2015;24:1975–1984. doi: 10.1111/jocn.12858. [DOI] [PubMed] [Google Scholar]
- Xiu J, Zhang Q, Zhou T, Zhou TT, Chen Y, Hu H. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nat Neurosci. 2014;17:1552–1559. doi: 10.1038/nn.3813. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Maekawa T, Nishikawa Y, Nojima H, Kaneko M, Kawakita T, Miyamoto T, Kuraishi Y. Characterization of itch-associated responses of NC mice with mite-induced chronic dermatitis. J Dermatol Sci. 2001;25:20–28. doi: 10.1016/s0923-1811(00)00099-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Yamazaki S, Hayashino Y, Takahashi O, Tokuda Y, Shimbo T, Fukui T, Hinohara S, Miyachi Y, Fukuhara S. Association between frequency of pruritic symptoms and perceived psychological stress: a Japanese population-based study. Arch Dermatol. 2009;145:1384–1388. doi: 10.1001/archdermatol.2009.290. [DOI] [PubMed] [Google Scholar]
- Yeo B, Tey HL. Effective treatment of notalgia paresthetica with amitriptyline. J Dermatol. 2013;40:505–506. doi: 10.1111/1346-8138.12154. [DOI] [PubMed] [Google Scholar]
- Yew YW, Tey HL. Itch in familial lichen amyloidosis: effective treatment with amitriptyline in two cases. Dermatol Ther. 2014;27:12–15. doi: 10.1111/dth.12023. [DOI] [PubMed] [Google Scholar]
- Yilmaz S, Ceyhan AM, Baysal Akkaya V. Brachioradial pruritus successfully treated with gabapentin. J Dermatol. 2010;37:662–665. doi: 10.1111/j.1346-8138.2010.00830.x. [DOI] [PubMed] [Google Scholar]
- Yong A, Chong WS, Tey HL. Effective treatment of uremic pruritus and acquired perforating dermatosis with amitriptyline. Australas J Dermatol. 2014;55:e54–57. doi: 10.1111/ajd.12026. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Ansari N, Goon A, Chan YH, Goh CL. Clinical characteristics of pruritus in chronic idiopathic urticaria. Br J Dermatol. 2002a;147:32–36. doi: 10.1046/j.1365-2133.2002.04758.x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Goon A, Wee J, Chan YH, Goh CL. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol. 2000;143:969–973. doi: 10.1046/j.1365-2133.2000.03829.x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Goon ATJ, Wee J, Chan YH, Zucker I, Goh CL. Itch characteristics in Chinese patients with atopic dermatitis using a new questionnaire for the assessment of pruritus. International Journal of Dermatology. 2002b;41:212–216. doi: 10.1046/j.1365-4362.2002.01460.x. [DOI] [PubMed] [Google Scholar]
- Yu YQ, Barry DM, Hao Y, Liu XT, Chen ZF. Molecular and neural basis of contagious itch behavior in mice. Science. 2017;355:1072–1076. doi: 10.1126/science.aak9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Jiao S, Xiao Y, Ren W, Zhao T, Meng J. Comparison of pregabalin with ondansetron in treatment of uraemic pruritus in dialysis patients: a prospective, randomized, double-blind study. Int Urol Nephrol. 2015;47:161–167. doi: 10.1007/s11255-014-0795-x. [DOI] [PubMed] [Google Scholar]
- Zachariae R, Lei U, Haedersdal M, Zachariae C. Itch severity and quality of life in patients with pruritus: preliminary validity of a Danish adaptation of the itch severity scale. Acta Derm Venereol. 2012;92:508–514. doi: 10.2340/00015555-1221. [DOI] [PubMed] [Google Scholar]
- Zachariae R, Zachariae H, Blomqvist K, Davidsson S, Molin L, Mork C, Sigurgeirsson B. Self-reported stress reactivity and psoriasis-related stress of Nordic psoriasis sufferers. J Eur Acad Dermatol Venereol. 2004;18:27–36. doi: 10.1111/j.1468-3083.2004.00721.x. [DOI] [PubMed] [Google Scholar]
- Zakrzewska-Pniewska B, Jedras M. Is pruritus in chronic uremic patients related to peripheral somatic and autonomic neuropathy? Study by R-R interval variation test (RRIV) and by sympathetic skin response (SSR) Neurophysiol Clin. 2001;31:181–193. doi: 10.1016/s0987-7053(01)00257-x. [DOI] [PubMed] [Google Scholar]
- Zhang TT, Shen FY, Ma LQ, Wen W, Wang B, Peng YZ, Wang ZR, Zhao X. Potentiation of synaptic transmission in Rat anterior cingulate cortex by chronic itch. Mol Brain. 2016;9:73. doi: 10.1186/s13041-016-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Hiramoto T, Asano Y, Kubo C, Sudo N. Chronic psychological stress exaggerates the compound 48/80-induced scratching behavior of mice. Pharmacol Biochem Behav. 2013;105:173–176. doi: 10.1016/j.pbb.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Zucker I, Yosipovitch G, David M, Gafter U, Boner G. Prevalence and characterization of uremic pruritus in patients undergoing hemodialysis: uremic pruritus is still a major problem for patients with end-stage renal disease. Journal of the American Academy of Dermatology. 2003;49:842–846. doi: 10.1016/s0190-9622(03)02478-2. [DOI] [PubMed] [Google Scholar]
- Zylicz Z, Krajnik M, Sorge AA, Costantini M. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manage. 2003;26:1105–1112. doi: 10.1016/j.jpainsymman.2003.05.004. [DOI] [PubMed] [Google Scholar]