Abstract
Background
Vaccination that prevents tuberculosis (TB) disease, particularly in adolescents, would have the greatest impact on the global TB epidemic. Safety, reactogenicity and immunogenicity of the vaccine candidate M72/AS01E was evaluated in healthy, HIV-negative adolescents in a TB endemic region, regardless of Mycobacterium tuberculosis (M.tb) infection status.
Methods
In a phase II, double-blind randomized, controlled study (NCT00950612), two doses of M72/AS01E or placebo were administered intramuscularly, one month apart. Participants were followed-up post-vaccination, for 6 months. M72-specific immunogenicity was evaluated by intracellular cytokine staining analysis of T cells and NK cells by flow cytometry.
Results
No serious adverse events were recorded. M72/AS01E induced robust T cell and antibody responses, including antigen-dependent NK cell IFN-γ production. CD4 and CD8 T cell responses were sustained at 6 months post vaccination. Irrespective of M.tb infection status, vaccination induced a high frequency of M72-specific CD4 T cells expressing multiple combinations of Th1 cytokines, and low level IL-17. We observed rapid boosting of immune responses in M.tb-infected participants, suggesting natural infection acts as a prime to vaccination.
Conclusions
The clinically acceptable safety and immunogenicity profile of M72/AS01E in adolescents living in an area with high TB burden support the move to efficacy trials.
Keywords: Tuberculosis, Vaccine, T cell, NK cell, Cytokine, M72/AS01E
1. Introduction
Tuberculosis (TB) is the second leading cause of mortality world-wide due to a single infectious agent, leading to 9.0 million incident cases and 1.5 million deaths a year [1]. TB results in substantial personal, social, public health, and economic cost. South Africa faces a particularly high burden of TB disease, with the second highest annual incidence of TB cases in the world. Globally, the TB epidemic is compounded by the emergence of drug resistance; novel vaccination strategies may impact both drug sensitive and resistant disease.
Bacille Calmette-Guérin (BCG) is the only currently licensed vaccine against TB disease. Although BCG has been in use since 1921, it provides highly variable and mostly poor protection against pulmonary TB disease in adolescents and adults [1–5]. Adolescents and adults with TB disease spread Mycobacterium tuberculosis (M.tb), and should therefore be the main target populations of novel TB vaccination strategies [6].
Fourteen TB vaccine candidates are currently in clinical testing [7]. Most novel TB vaccine candidates aim to boost or modulate pre-existing T cell responses against M.tb. M72/AS01E, one such vaccine candidate, is a recombinant fusion protein (M72) derived from Mtb32 and Mtb39, adjuvanted with AS01E[8]. M72/AS01E has shown promise in multiple Phase I and IIa clinical trials in adults [9–14], including M.tb-infected persons living in a high TB burden setting. In these studies, the vaccine had a clinically acceptable safety profile and induces high magnitude M72-specific CD4 T cell responses, including a complex pattern of Th1 cytokines. However, the vaccine has not been evaluated in adolescents, a major target population for novel vaccination strategies. The aim of this study was to assess safety, reactogenicity and immunogenicity of two doses of M72/AS01E vaccination in healthy, Human Immunodeficiency Virus (HIV) uninfected adolescents living in a TB endemic setting.
2. Methods
2.1. Study design
This Phase II, double-blind randomized, controlled trial was approved by the University of Cape Town Health Sciences Human Research Ethics Committee (ClinicalTrials.gov, NCT00950612), and conducted in accordance with the Helsinki Declaration and Good Clinical Practices. Informed consent was obtained from the legal guardians and assent from the participants prior to screening.
2.2. Participants and vaccination
Greater detail of all procedures, including the vaccine, assays and statistical analysis, can be found in the supplementary material. Briefly, we aimed to enroll 60 adolescents aged 13–17 years from the Cape Town region of South Africa if they were healthy, HIV-negative, with no previous or current TB disease, and regardless of M.tb infection status (determined by QuantiFERON TB Gold In-Tube test (QFT)). Screening procedures included physical examination, chest X-ray, blood tests for hematology and biochemistry and a pregnancy test in females. Following blinded randomization, 40 participants were allocated to receive 2 doses of M72/AS01E (10 μg M72 adjuvanted with AS01E, an adjuvant system containing 25 μg 3-O-desacyl-4′-monophosphoryl lipid A (MPL), 25 μg QS-21 Stimulon® [Quillaja saponaria Molina, fraction 21; licensed by GSK from Antigenics Inc., a wholly owned subsidiary of Agenus Inc., a Delaware, USA corporation] and liposome) and 20 to receive 2 doses of placebo (saline), on study days 0 and 30, administered intramuscularly.
2.3. Safety and reactogenicity evaluation
Injection site reactions, solicited and unsolicited systemic adverse events (AEs), and safety blood abnormalities were evaluated by diary card completion, physical examination and laboratory testing. Follow up clinic visits were performed 1 and 7 days after each vaccination, and on days 60 and 210 after the first vaccination.
2.4. Antibody ELISA
On study days 0, 30, 60 and 210, total anti-M72 IgG was measured in serially-diluted serum by ELISA, as previously described [10,14].
2.5. T cell intracellular cytokine staining assay
Two intracellular cytokine staining (ICS) assays were completed on samples collected on study days 0, 7, 30, 37, 60, and 210. First, whole blood was incubated with an M72 peptide pool, or with recombinant M72 fusion protein, as previously described [15,16]. Expression of IFN-γ, IL-2, TNF-α, IL-17, Ki67 and PD-1 was determined in CD4 and CD8 T cells. Second, isolated and stored PBMC were later thawed and incubated with the M72 peptide pool, as previously described [10,17]. Expression of CD40L, IFN-γ, IL-2 and TNF-α were determined in CD4 and CD8 T cells. Cells were acquired on a LSR II flow cytometer (BD Biosciences).
2.6. NK cell intracellular cytokine staining assay
CD56+CD16+/− NK cell expression of IFN-γ and CD69 was measured following PBMC incubation with an M72 peptide pool, using an adapted ICS as previously described [18,19].
2.7. Data analysis
Frequency of AEs was described per number of administered doses, by type (injection site, systemic, laboratory), and by severity, seriousness and causality. Frequency and pattern of expression of different markers were outcomes of the ICS; data were analyzed using FlowJo software (TreeStar). Specific responses were calculated by subtraction of response frequencies in unstimulated samples from stimulated samples. Antibody results were described as geometric mean concentrations (GMC); a response was defined as >2.8 ELISA units/mL. Statistical comparisons between groups and time points were assessed with nonparametric tests, using GraphPad Prism 6.0d (GraphPad Software). Analysis were per protocol unless otherwise indicated.
3. Results
3.1. Participants
Sixty healthy, HIV-negative adolescents (median age 15.0 years, interquartile range – IQR – 14.1–16.3) were enrolled (Table 1). All participants had documented evidence of BCG vaccination or BCG scar. On Day 0 and Day 30, forty participants received M72/AS01E vaccine, and twenty received placebo. Demographic characteristics and reasons for exclusion did not differ between groups at baseline (Table 1 and Fig. S1).
Table 1.
Demographic characteristics of enrolled participants.
| M72/AS01E (n = 40) | Placebo (n = 20) | Overall (n = 60) | |
|---|---|---|---|
| Male, n (%) | 22 (55.0) | 9 (45.0) | 31 (51.7) |
| Median age in years (range, IQRa) | 15.0 (13–17, 14–16) | 14.5 (14–17, 14–15) | 15.0 (13–17, 14–16) |
| Race, n (%) | |||
| Black | 11 (27.5%) | 6 (30.0%) | 17 (28.3%) |
| White | 3 (7.5%) | 1 (5.0%) | 4 (6.7%) |
| Mixed race | 26 (65.0%) | 13 (65.0%) | 39 (65.0%) |
| QuantiFERON status at baseline, n (%) | |||
| Negative | 22 (55.0%) | 10 (50.0%) | 32 (53.3%) |
| Positive | 18 (45.0%) | 10 (50.0%) | 28 (46.7%) |
IQR, Interquartile range.
n (%) = number (percentage) of participants enrolled.
3.2. M72/AS01E had a clinically acceptable safety profile
No participant experienced a serious adverse event (SAE) or withdrew due to an AE. AEs were reported in the 7 day post-vaccination period after 93.8% of all doses in the M72/AS01E group and after 57.9% of all doses in the placebo group (Table S1). In the M72/AS01E group, local AEs were reported after 90% of doses and general AEs after 75% of doses. In the placebo group, local AEs were reported after 26.3% of doses and general AEs after 44.7% of doses. 92.5% of M72/AS01E recipients had AEs after dose 1 and 95% after dose 2; these frequencies were 61.1% and 55% in placebo recipients, respectively.
The most common M72/AS01E associated local AE was pain, after 90% of doses, followed by swelling and redness, after 34% and 21% of doses, respectively (Table 2). Pain occurred after 21% of placebo doses, and swelling and redness each after 5% of doses.
Table 2.
Frequency of solicited local adverse events (AEs) and general AEs reported during the 7-day follow-up periods following first or second vaccination n (%).a General AE include AEs considered related and not-related to vaccination.
| M72/AS01E | Placebo | |||
|---|---|---|---|---|
|
|
|
|||
| (N = 80)b | 95% CId | (N = 38)b | 95% CId | |
| Local AE | ||||
| Pain | ||||
| All | 72 (90) | 81.2–95.6 | 8 (21.1) | 9.6–37.3 |
| Grade 3c | 10 (12.5) | 6.2–21.8 | 0 (0.0) | 0–9.3 |
| Redness | ||||
| All | 17 (21.3) | 12.9–31.8 | 2 (5.3) | 0.6–17.7 |
| Grade 3c | 1 (1.3) | 0.0–6.8 | 0 (0.0) | 0.0–9.3 |
| Swelling | ||||
| All | 27 (33.8) | 23.6–45.2 | 2 (5.3) | 0.6–17.7 |
| Grade 3c | 0 (0.0) | 0.0–4.5 | 0 (0.0) | 0.0–9.3 |
| General AE | ||||
| Fatigue | ||||
| All | 31 (38.8) | 28.1–50.3 | 5 (13.2) | 4.4–28.1 |
| Grade 3 | 3 (3.8) | 0.8–10.6 | 0 (0.0) | 0.0–9.3 |
| Gastrointestinal symptoms | ||||
| All | 22 (27.5) | 18.1–38.6 | 5 (13.2) | 4.4–28.1 |
| Grade 3 | 1 (1.3) | 0.0–6.8 | 2 (5.3) | 0.6–17.7 |
| Headache | ||||
| All | 43 (53.8) | 42.2–65.0 | 6 (15.8) | 6.0–31.3 |
| Grade 3 | 9 (11.3) | 5.3–20.3 | 0 (0.0) | 0.0–9.3 |
| Malaise | ||||
| All | 19 (23.8) | 14.9–34.6 | 3 (7.9) | 1.7–21.4 |
| Grade 3 | 3 (3.8) | 0.8–10.6 | 0 (0.0) | 0.0–9.3 |
| Myalgia | ||||
| All | 9 (11.3) | 5.3–20.3 | 3 (7.9) | 1.7–21.4 |
| Grade 3 | 2 (2.5) | 0.3–8.7 | 0 (0.0) | 0.0–9.3 |
| Fever | ||||
| All | 36 (45) | 33.8–56.5 | 2 (5.3) | 0.6–17.7 |
| Grade 3c | 4 (5.0) | 1.4–12.3 | 0 (0.0) | 0.0–9.3 |
n (%) = number (percentage) of doses followed by at least one type of AE.
N = number of documented doses (2 dose schedule per protocol. Two subjects did not return diary cards after both injections and are not included in this table.
Grade 3 defined as >50 mm (redness, swelling) or symptom severe enough to limit normal daily activity (pain), or a temperature >39.5 °C (fever).
Exact 95% confidence interval, lower limit and upper limit range of % proportion.
Headache, fever, and fatigue were the most frequently reported systemic AEs among M72/AS01E recipients, after 54%, 45%, and 39% of doses, respectively, compared to 16%, 5%, 13% in the placebo group (Table 2).
Unsolicited AEs 30 days post-vaccination, including abnormal safety laboratory findings, were infrequent, occurring after 15% of all doses in the M72/AS01E group, and 15% in placebo group (Table S2).
3.3. M.tb infection might be associated with a higher frequency of adverse events
As expected for an endemic region, 45% of M72/AS01E vaccinees and 50% of placebo recipients were QFT-positive at baseline (Table 1) [20]. Post hoc analyses showed that the frequency of injection site reactions and unsolicited symptoms were similar between participants who were QFT-positive and -negative at enrollment (data not shown). Some solicited general symptoms (fever, headache and gastro-intestinal symptoms) occurred more frequently in QFT-positive participants receiving M72/AS01E (Table S3).
3.4. M72/AS01E induces a specific CD4 T cell response, which is boosted and sustained by a second vaccine dose
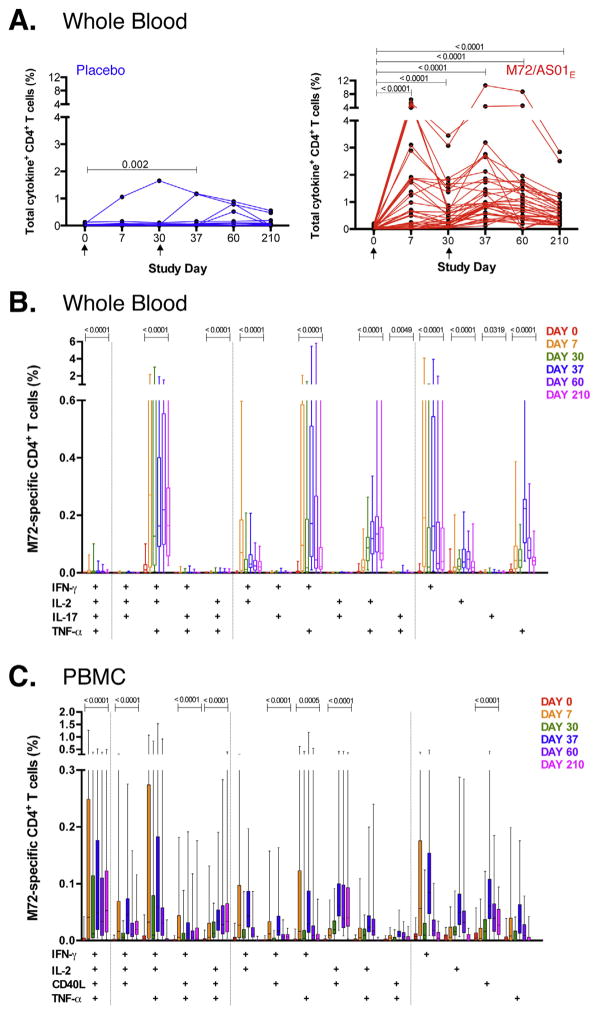
T cell responses to vaccination were measured with a whole blood ICS assay and flow cytometry (Fig. S2). The highest observed frequencies of M72-specific CD4 T cells producing IFN-γ, TNF-α, IL-2 and/or IL-17 occurred 7 days post-vaccination (Fig. 1A), with significant boosting after the second dose. M72-specific CD4 T cell frequencies remained higher than pre-vaccination levels at all the time points following vaccination (p < 0.0001, Day 210 vs. pre-vaccination).
Fig. 1.
M72/AS01E induces a robust CD4 T cell response that is boosted with a second vaccination. Longitudinal CD4 T cell responses to pooled M72 peptides in whole blood (A and B) and cryopreserved PBMC (C) from individual participants. (A) Total cytokine responses were calculated as the frequencies of CD4 T cells expressing any of IFN-γ, IL-2, TNF-α and/or IL-17. Each line represents a different individual in either the M72/AS01E vaccinated (n = 40, in red) or placebo vaccinated group (n = 19, in blue). Arrows represent the time of vaccination. Results are shown after background subtraction of frequencies of cytokine-expressing CD4 T cells in the unstimulated negative control sample. Wilcoxon matched pairs signed rank test was used to compare frequencies of M72-specific total cytokine response between two time points. Wilcoxon matched pairs signed rank test p values are shown, compared to pre-vaccination time point. (B) Vaccination induced a complex profile of cytokine expression and multiple distinct subsets of CD4 T cell responses. Frequencies of M72-specific CD4 T cells expressing IFN-γ, IL-2, TNF-α and IL-17 were measured by ICS assay after 12 h stimulation of whole blood with M72 peptide pools. For each plot, only the M72/AS01E vaccinated participants are shown. The median is represented by the horizontal line, the interquartile range by the box and the range by the whiskers. Durability of response of each subset of CD4 T cell population was measured by Wilcoxon matched-pairs signed rank test comparing Day 210 to pre-vaccination responses; only values <0.05 are shown. Representative flow cytometry plots and gating strategy are shown in Supplementary Fig. S2. (C) Frequencies of CD4 T cells expressing IL-2, TNF-α, IFN-γ and CD40L in response to M72 peptide pool stimulation measured in cryopreserved PBMC by ICS. For B and C, only the M72/AS01E vaccinated participants are shown. Results are shown after background subtraction of cytokine production in the unstimulated control. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In vaccinees, M72-specific CD4 T cells expressed multiple combinations of IFN-γ, IL-2, TNF-α and/or IL-17 (Fig. 1B): 11 distinct subsets maintained higher cytokine expression at Day 210, compared with pre-vaccination. Cytokine combinations expressed at greatest frequency were IFN-γ/IL-2/TNF-α together, TNF-α/IFN-γ together, or TNF-α/IL-2 together. IL-17-producing CD4 T cells were detected with a peak measured response 7 days after a second vaccine dose (Fig. S3).
The pattern and kinetics of M72-specific Th1 cytokine production was independently confirmed by PBMC ICS (Fig. 1C). With the exception of CD40L expression, not assessed in whole blood, lower responses were observed in PBMC (Fig. 1C).
The peak response of the cell cycling marker Ki67 was at 7 days after each vaccination (Fig. S4A); a large proportion of Ki67+ cells did not express any of the classical Th1 or Th17 cytokines, and vice versa (Fig. S4B). Expression of PD-1, a negative regulator of activated T cells, was elevated on M72-specific CD4 T cells at all time points post-vaccination, with kinetics similar to those of cytokine expression (Fig. S5A).
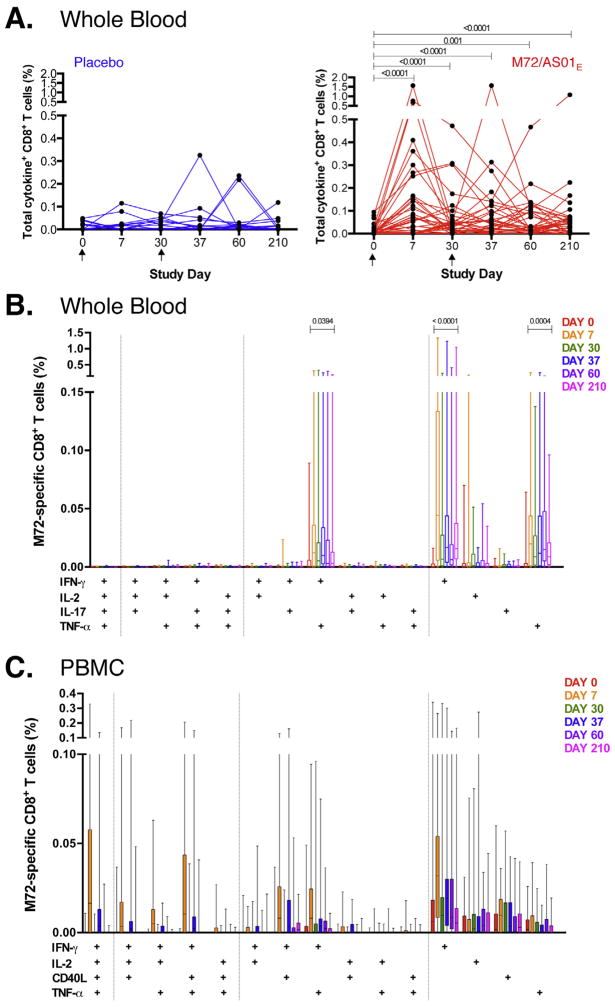
3.5. M72/AS01E induces a sustained CD8 T cell response
Kinetics of the M72-induced CD8 T cell response was similar to that of CD4 T cells, but of lower magnitude and greater individual variability after both doses of vaccine (Fig. 2A). In the majority of vaccinated participants, vaccination boosted detectable CD8 T cells frequencies above pre-vaccination levels, at all time points (p < 0.0001, Day 210 vs. pre-vaccination). M72-specific CD8 T cell responses in placebo recipients did not increase significantly over time.
Fig. 2.
M72/AS01E induces a sustained CD8 T cell response. Longitudinal CD8 T cell responses to pooled M72 peptides in whole blood (A and B) and cryopreserved PBMC (C) from individual participants. (A) Total cytokine responses were calculated as the frequencies of CD8 T cells expressing any of IFN-γ, IL-2, TNF-α and/or IL-17. Each line represents a different individual in either the M72/AS01E vaccinated (n = 40, red) or placebo-vaccinated group (n = 19, blue). Arrows represent the time of vaccination. Results are shown after background subtraction of frequencies of cytokine-expressing CD8 T cells in the unstimulated negative control sample. Wilcoxon matched pairs signed rank test was used to compare frequency of M72-specific total cytokine response between two time points. Wilcoxon matched pairs signed rank test p values are shown after adjustment for multiple comparisons, compared to pre-vaccination time point. Friedman test was used to calculate significant differences across time. (B) Frequencies of M72-specific CD8 T cells expressing IFN-γ, IL-2, TNF-α and IL-17 measured by ICS assay after stimulation of whole blood with M72 peptide pools. For each plot, only M72/AS01E vaccinated participants are shown. The median, interquartile range and range are shown. Durability of response of each CD8 T cell subset was measured by Wilcoxon matched-pairs signed rank test comparing Day 210 to pre-vaccination responses; only values <0.05 are shown. (C) Frequencies of CD8 T cells expressing IL-2, TNF-α, IFN-γ and CD40L in response to M72 peptide pool stimulation measured in cryopreserved PBMC by ICS. For B and C, only the M72/AS01E vaccinated participants are shown. Results are shown after background subtraction of cytokine production in the unstimulated control. No subsets showed any significance in expression between Day 0 and Day 210 in the PBMC assay when performing Wilcoxon matched pairs signed rank test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Overall, M72-specific CD8 T cell responses detected in cryopreserved PBMC were low compared to whole blood (Fig. 2B and C), as seen previously [9,10,12]. M72/AS01E induced predominantly IFN-γ single-positive CD8 T cells, and, in whole blood, TNF-α single-positive CD8 T cells. In whole blood, Day 210 frequencies were elevated above baseline for IFN-γ single-positive (p < 0.0001), TNF-α single-positive (p = 0.0004) and IFN-γ+TNF-α+ subsets (p = 0.0394).
PD-1 expression on M72-specific IFN-γ+ CD8 T cells was low at all time points post-vaccination (Fig. S5B). Expression patterns of Ki67 were similar to those observed for CD4 T cells (Fig. S4A and D).
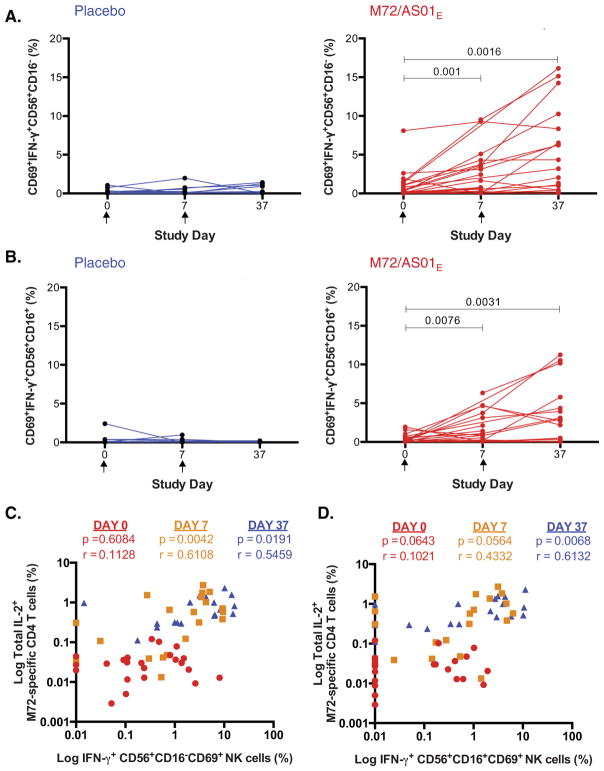
3.6. Vaccination with M72/AS01E activates NK cells
The effect of M72/AS01E on NK cell responses immediately post-vaccination was measured in a subset of participants PBMC (M72/AS01E: n = 27; placebo: n = 15) post hoc. Recent observations suggest that CD4 T cells, through IL-2, can activate NK cells to produce IFN-γ [18,19,21]. By stimulating cryopreserved PBMC with M72 peptide pool, we observed IFN-γ production by both CD56+CD16− and CD56+CD16+ subsets of NK cells (Fig. 3A and B). Further, activated NK cells co-expressing CD69 and IFN-γ were increased 7 days after each vaccination, compared with placebo. At day 37, frequencies of IL-2-expressing M72-specific CD4 T cells correlated with frequencies of IFN-γ-expressing NK cell subsets (Fig. 3C and D).
Fig. 3.
Vaccination with M72/AS01E activates NK cells. IFN-γ production by activated CD69+CD56+ NK cells assessed in cryopreserved PBMC in response to 18 h M72 peptide pool stimulation. Frequencies of IFN-γ producing cells measured in CD16− (A) and CD16+ (B) NK cells at the pre-vaccination time point (Day 0) and 7 days post first vaccination (Day 7) and second vaccination (Day 37), from placebo vaccinated (blue) and M72/AS01E vaccinated participants (red). Wilcoxon matched pairs signed rank test p values are shown. The Kruskal–Wallis test was used to compare all time points, and Wilcoxon matched pairs signed rank test used to compare each time point to Day 0. Correlation is shown between IL-2 production by M72-specific CD4 T cells in PBMC and IFN-γ expressing CD56+CD16−CD69+ NK cells (C) and CD56+CD16+CD69+ NK cells (D). Spearman correlation p and r values are shown, comparing correlation at Day 0 (red circles), Day 7 (yellow squares) and Day 37 (blue triangles). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
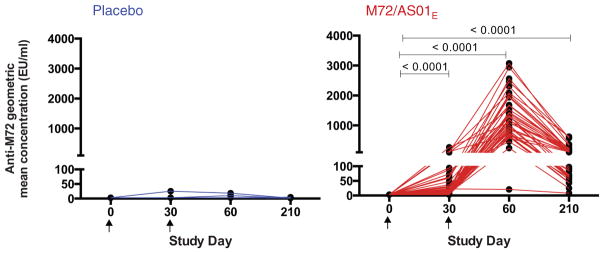
3.7. M72 induces an antibody response
Anti-M72 IgG antibody response against the recombinant M72 protein was measured in serum by ELISA. Prior to vaccination, all participants except one in the M72/AS01E group were seronegative. After 2 vaccine doses, all participants had converted to seropositivity; 2 placebo recipients had detectable but low titer anti-M72 IgG. By Day 210, M72-specific IgG was still detectable in all vaccinees (p < 0.0001 Day 210 vs. pre-vaccination) and in 1 placebo recipient (Fig. 4).
Fig. 4.
M72/AS01E induces an antibody response. Geometric mean concentrations (GMC) of anti-M72 IgG antibodies measured by ELISA in serially diluted serum from placebo (blue) and M72-vaccinated participants (red). The Friedman test was used to compare all time points, and Wilcoxon matched pairs signed rank test used to compare each time point to Day 0.
3.8. M72 induces a higher response in individuals already exposed to M.tb
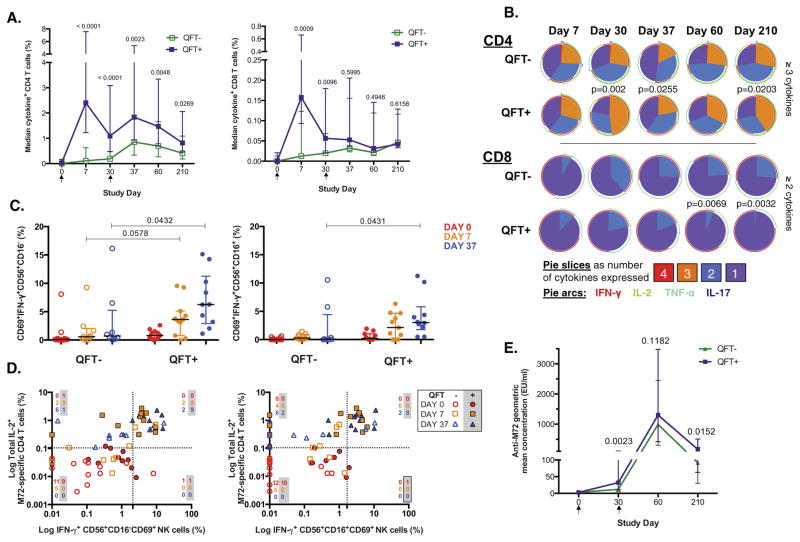
To determine the effects of M.tb infection on vaccination-induced immune responses, we stratified participants according to QFT status (Table 1). Baseline total cytokine responses of M72-specific CD4 and CD8 T cells were uniformly low in both groups. M72-specific T cell responses following vaccine priming were much higher in M.tb-infected individuals compared with M.tb-uninfected individuals (Fig. 5A); in the latter group, the specific responses were more prominent after the second dose. There was a strong correlation between M72-specific T cell cytokine production and IFN-γ detected by QFT (Fig. S6).
Fig. 5.
M72/AS01E induces a higher response in individuals already exposed to M.tb. (A) Median total CD4 (left) and CD8 (right) M72-specifc T cell responses in whole blood are shown in M72/AS01E vaccinated adolescents stratified according to their M.tb infection status using the QuantiFERON-TB Gold In-Tube test. Bars represent interquartile range. Background values (unstimulated) were subtracted. For all analyses, a Mann–Whitney U test was performed to compare the response of each group at specific time points post-vaccination. Closed squares (purple) represent M.tb-infected (QFT-positive) individuals and open squares (green) represent M.tb-uninfected (QFT-negative) individuals. (B) Pie charts representing the median proportion of cells co-expressing cytokines, among all M72-specific CD4 and CD8 T cells expressing cytokines, after whole blood stimulation with M72 peptide pool. A Mann–Whitney test was performed to compare the proportion (%) of CD4 T cells expressing 3 or more cytokines, and CD8 T cells expressing 2 or more cytokines. Only p values <0.05 are shown. (C) IFN-γ response in CD56+CD16− (left) and CD56+CD16+ (right) CD69+ activated NK cells are shown for QFT-positive and QFT-negative individuals. Median and interquartile ranges are shown. Mann–Whitney p values comparing QFT-negative and QFT-positive participants at each time point are shown. (D) IFN-γ production by CD56+CD16− (left) and CD56+CD16+ (right) activated NK cells at day 37 associated with M72-specific CD4 T-cells expressing IL-2 among the QFT-positive participants. Data are reported as the association between frequencies of CD4 T cells producing IL-2 and frequencies of activated NK cells producing IFNγ after M72 peptide pool stimulation, in QFT-positive and QFT-negative participants vaccinated with M72/AS01E. A cut-off for the proportion of cytokine producing cells, shown by dotted lines, was established based on the 95th percentile observed in pre-vaccination samples from all vaccinated participants. The number of QFT-negative and QFT-positive (shaded) participants in each quadrant at each study day is indicated. At study days 0, 7 and 37, the number of QFT-positive M72/AS01E vaccinated participants with samples available was n = 11, 11 and 10, respectively, and the number of QFT-negative M72/AS01E vaccinated participants with samples available was n = 12, 9 and 8, respectively. (E) Antibody response kinetics to M72 in QFT-negative and QFT-positive individuals after one or two immunizations of M72/AS01E. Median and interquartile ranges of anti-M72 GMC are shown. Mann–Whitney p value comparing QFT-negative and QFT-positive participants at each time point are shown. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In whole blood assays, M72-specific CD4 T cell responses in M.tb-uninfected persons never reached the magnitude of those achieved in M.tb-infected persons; however, in PBMC, the response was equivalent at Day 60 and Day 210 (data not shown).
As for CD4 T cells, similar kinetics were observed for CD8 T cell responses in the M.tb-infected group, except that lesser boosting was observed following the second vaccination (Fig. 5A). After boosting, CD8 T cell responses were similar in both groups, and maintained up to Day 210 at levels significantly higher than those observed pre-vaccination.
Proportions of M72-specific CD4 T cells expressing ≥3 cytokines together were higher in M.tb-infected individuals on Day 30, 37 and 210, compared with M.tb-uninfected individuals (Fig. 5B). In contrast, M.tb-infected persons showed lower proportions of induced CD8 T cells expressing ≥2 cytokines at Day 60 and 210, compared with uninfected individuals.
Ki67 expression on both CD4 and CD8 T cells showed similar kinetics 7 days post-vaccination (Fig. S4C and E). PD-1 expression on CD4 T cells was higher in M.tb-uninfected participants at all time points post-vaccination (Fig. S5C). No difference was seen in PD-1 expression on CD8 T cells among the groups (Fig S5D).
M.tb infection also resulted in a much greater boost of IFN-γ production by both CD16− and CD16+ activated NK cells in vaccinees (Fig. 5C), and strong association with IL-2 production by M72-specific CD4 T cells (Fig. 5D). The proportion of participants with high frequencies of both IL-2-producing CD4 T cells and IFN-γ-producing NK cells increased with vaccinations. By Day 37, all M.tb-infected participants with high frequencies of IL-2-producing CD4 T cells also had increased proportions of IFN-γ-producing CD56+CD16− NK cells, and 8 of 10 M.tb-infected participants had high proportions of IFN-γ-producing CD56+CD16+NK cells. A similar, although less striking association was also seen in M.tb-uninfected participants.
M.tb-infected adolescents responded to the first vaccination with higher anti-M72 IgG antibody titers at Day 30, compared to M.tb-uninfected adolescents, but not at the other time points (Fig. 5E).
4. Discussion
In this Phase II trial, conducted in a TB endemic setting, we showed that two doses of M72/AS01E candidate vaccine, delivered intramuscularly 30 days apart to healthy, HIV-uninfected adolescents, had a clinically acceptable safety and reactogenicity profile. The vaccine was immunogenic and induced sustained antigen-specific CD4 and CD8 T cell and IgG responses up to 6 months post last vaccination. These findings were independent of prior M.tb infection, although natural priming with M.tb resulted in faster and quantitatively higher whole blood immune responses to M72/AS01E. These findings complement safety and immunological observations in trials conducted in M.tb-infected adults in South Africa [14] and the Philippines [13], and M.tb-uninfected adults in Europe and the USA [9–12].
M72/AS01E appeared well tolerated, although a higher frequency of mild to moderate local AEs and severe pain at injection site was reported in the vaccinated, compared to the placebo group. The systemic AE profile was analogous to a flu-like syndrome in the first 24 h post-vaccination. AEs were typically transient and resolved within 7 days. Certain solicited general symptoms occurred more frequently in M72/AS01E than in placebo recipients who were M.tb-infected at enrollment, compared with uninfected vaccinees. The relatively small sample sizes in these early phase studies preclude definitive conclusions to be drawn about the safety and immunogenicity results.
In our experience, magnitudes of the total M72-specific CD4 T cell response induced by vaccination are among the highest seen in recent literature when compared with responses in adolescents and adults induced by two viral vectored vaccines, MVA85A and Aeras-402, in the same whole blood assay using different M.tb antigens for stimulation (Ag85A, Ag85B, and TB10.4) [22–24]. In the absence of immune correlates of protection, we hypothesize that both magnitude and quality of cellular immune responses may be important components of protective immunity. M72/AS01E vaccination induced a potent and durable M72-specific CD4 T cell response, with frequencies of cells expressing ≥3 cytokines higher at Day 210 than pre-vaccination. In animal models, multifunctional CD4 T cells have correlated with protection against M.tb challenge and other pathogens [25–31], although the role of cytokine co-expression in protection of humans against TB remains unclear [22,32,33]. For example, although low frequencies of multifunctional CD4 T cells expressing IFN-γ, IL-2, TNF-α and IL-17 were induced by MVA85A, the vaccine failed to improve on BCG-induced protective immunity against TB disease in infants [23,32]. Furthermore, frequencies of multifunctional BCG-specific CD4 T cell responses did not correlate with risk of TB disease in infants [33]. Nevertheless, CD4 T cells, and TNF-α and IFN-γ cytokine production are considered critical for control of M.tb infection in humans [34–39].
Vaccination induced a durable M72-specific CD8 T cell response in whole blood. CD8 T cells may contribute to clearance of M.tb-infected cells [40,41]. Although CD8 responses were moderate in magnitude compared to CD4 T cells, vaccination induced significantly higher frequencies of IFN-γ+, TNF-α+, and IFN-γ+TNF-α+ CD8 T cell subsets at Day 210, compared to pre-vaccination. Notably, the second vaccine dose appeared to have little, if any, boosting effect on M72-specific CD8 T cell responses in adolescents already M.tb-infected, but was necessary to boost responses in uninfected participants to equivalent levels.
Here, we show an association between IL-2-producing M72-specific CD4 T cells and IFN-γ-producing NK cells, suggesting that NK cells might act as effectors of acquired immune responses [18,21]. This NK cell recall response to peptide stimulation, identified in malaria, rabies and HIV-1 vaccination [18,19,21,42], may play an important role in vaccination strategies and allow us to further interrogate the host immune response to M.tb vaccination beyond classical Th1 sources of IFN-γ [18,19,43,44]. The CD4 T cell-associated NK cell responses detected after M72/AS01E vaccination suggest that NK cells might act as “effectors of acquired immune responses” [18,21]. The link between CD4 T cell responses, NK cell activation and QFT status shown here is consistent with the model proposed by Riley and others [18,21,43,44] and could bring further understanding into the mode of action of M72/AS01E. Clearly, further work is needed to elucidate the precise mechanisms that link CD4 T cell responses and NK cell activation and the role of pre-existing memory in this pathway.
Overall, our data indicate that M72/AS01E vaccination has an acceptable clinical safety and reactogenicity profile and induces potent and sustained CD4 and CD8 T cell responses, and CD4 T cell dependent IFN-γ recall responses in NK cells, in both M.tb-infected and uninfected healthy adolescents from a TB endemic area. However, different kinetics of immune responses observed in M.tb-infected and uninfected participants suggests that, in the context of M.tb vaccination, vaccine “priming” could modulate preexisting immune and epitope recognition already present in an M.tb-infected and antigen exposed population. It raises important implications for effective approaches of vaccination to enhance protective immunity or prevent recurrent TB in high burden settings. The clinically acceptable safety and reactogenicity profile, combined with the potent and sustained T cell responses, and NK recall responses induced by vaccination, suggests that M72/AS01E is a good candidate to advance into efficacy trials in this key target population.
Supplementary Material
Acknowledgments
The authors thank the participating volunteers and their families, and acknowledge the clinicians, nurses, and laboratory technologists at South African Tuberculosis Vaccine Initiative and laboratory technicians at GSK Vaccines. In addition, the authors thank Sofia Dos Santos Mendes and Sophie Vanwetswinkel (XPE Pharma & Science on behalf of GSK Vaccines) for providing publication coordination; Janani Murali (previously at GSK Vaccines) and An Ranquin (XPE Pharma & Science on behalf of GSK Vaccines) for protocol and report writing support, respectively; Elodie Tournay (GSK Vaccines) for statistical input; Itumeleng Siweya (GSK Vaccines) for study monitoring coordination; Patricia Bourguignon (GSK Vaccines) for coordinating laboratory activities, E. Jane Hughes for lab management, Michele van Rooyen (SATVI) for study coordination and Nazma Mansoor (SATVI) for flow cytometry acquisition.
Funding: This work was supported by co-funding from Glaxo-SmithKline Biologicals S.A. and AERAS. GlaxoSmithKline Biologicals S.A., as sponsor of the study, was involved in all stages of the study conduct and analysis. A.P.-N. is supported in part by The Carnegie Corporation of New York and The Claude Leon Foundation. At time of submission, part of the data from this manuscript had been presented at the Keystone Symposium on Host Response to Tuberculosis, 2015 (J3).
Authors contribution: All authors participated in the design, or implementation, or analysis and interpretation of the study results, as well as in the development of this manuscript. All authors had full access to the data and gave final approval before submission. H.G. was the coordinating investigator and together with M.T. and A.L. was responsible for the conduct of the trial. A.B. was responsible for the statistical analyses. O.O.-A. and D.L. led the clinical team; E.D. was responsible for study management activities; M.-A.D. was the project leader; and A.P-N, W.H., T.S., C.D., W.B., E.J., P.M. and R.vd.M. led the laboratory analysis. The corresponding author was responsible for submission of the publication.
Conflicts of interest: W.B., E.J., P.M., A.B., M.-A.D., E.D., D.L., R. vd.M. and O.O.-A. are employees of the GSK group of companies and E.J., P.M., M.-A.D., D.L., R. vd.M. and O.O.-A. own company stocks or restricted shares. All other authors report no potential conflicts.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2015.05.088
Footnotes
Vaccine Study Team: Anne Bollaerts b, Marie-Ange Demoitie b, Angelique Kany Kany Luabeya a, Evi De Ruymaeker b, Michele Tameris a, Didier Lapierre b, Thomas J. Scriba a
References
- 1.World Health Organization. Global tuberculosis report 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.Soysal A, Millington KA, Bakir M, Dosanjh D, Aslan Y, Deeks JJ, et al. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet. 2005;366:1443–51. doi: 10.1016/S0140-6736(05)67534-4. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. J Am Med Assoc. 1994;271:698–702. [PubMed] [Google Scholar]
- 4.Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–80. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 5.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 6.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271–86. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]
- 7.Frick M. [accessed March 2014];TB vaccines pipeline. 2013 Jun; Available from: http://www.treatmentactiongroup.org/sites/g/files/g450272/f/201306/2013%20Pipeline%20Report.pdf.
- 8.Garç on N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011;10:471–86. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 9.Leroux-Roels I, Leroux-Roels G, Ofori-Anyinam O, Moris P, De Kock E, Clement F, et al. Evaluation of the safety and immunogenicity of two antigen concentrations of the Mtb72F/AS02(A) candidate tuberculosis vaccine in purified protein derivative-negative adults. Clin Vaccine Immunol. 2010;17:1763–71. doi: 10.1128/CVI.00133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leroux-Roels I, Forgus S, De Boever F, Clement F, Demoitié M-A, Mettens P, et al. Improved CD4+ T cell responses to Mycobacterium tuberculosis in PPD-negative adults by M72/AS01 as compared to the M72/AS02 and Mtb72F/AS02 tuberculosis candidate vaccine formulations: a randomized trial. Vaccine. 2013;31:2196–206. doi: 10.1016/j.vaccine.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Spertini F, Audran R, Lurati F, Ofori-Anyinam O, Zysset F, Vandepapelière P, et al. The candidate tuberculosis vaccine Mtb72F/AS02 in PPD positive adults: a randomized controlled phase I/II study. Tuberculosis [Edinb] 2013;93:179–88. doi: 10.1016/j.tube.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Eschen Von K, Morrison R, Braun M, Ofori-Anyinam O, De Kock E, Pavithran P, et al. The candidate tuberculosis vaccine Mtb72F/AS02A: tolerability and immunogenicity in humans. Hum Vaccin. 2009;5:475–82. doi: 10.4161/hv.8570. [DOI] [PubMed] [Google Scholar]
- 13.Montoya J, Solon JA, Cunanan SRC, Acosta L, Bollaerts A, Moris P, et al. A randomized, controlled dose-finding phase II study of the M72/AS01 candidate tuberculosis vaccine in healthy PPD-positive adults. J Clin Immunol. 2013;33:1360–75. doi: 10.1007/s10875-013-9949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day CL, Tameris M, Mansoor N, van Rooyen M, de Kock M, Geldenhuys H, et al. Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults. Am J Respir Crit Care Med. 2013;188:492–502. doi: 10.1164/rccm.201208-1385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, et al. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods. 2004;291:185–95. doi: 10.1016/j.jim.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Kagina BM, Mansoor N, Kpamegan EP, Penn-Nicholson A, Nemes E, Smit E, et al. Qualification of a whole blood intracellular cytokine staining assay to measure mycobacteria-specific CD4 and CD8 T cell immunity by flow cytometry. J Immunol Methods. 2014 doi: 10.1016/j.jim.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moris P, van der Most R, Leroux-Roels I, Clement F, Dramé M, Hanon E, et al. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol. 2011;31:443–54. doi: 10.1007/s10875-010-9490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol. 2010;185:2808–18. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 19.Horowitz A, Hafalla JCR, King E, Lusingu J, Dekker D, Leach A, et al. Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS, S/AS01 malaria vaccine. J Immunol. 2012;188:5054–62. doi: 10.4049/jimmunol.1102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahomed H, Hawkridge T, Verver S, Geiter L, Hatherill M, Abrahams DA, et al. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis. 2011;15:331–6. [PubMed] [Google Scholar]
- 21.Jost S, Tomezsko PJ, Rands K, Toth I, Lichterfeld M, Gandhi RT, et al. CD4+ T-cell help enhances NK cell function following therapeutic HIV-1 vaccination. J Virol. 2014;88:8349–54. doi: 10.1128/JVI.00924-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scriba TJ, Tameris M, Smit E, van der Merwe L, Hughes EJ, Kadira B, et al. A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV- and/or Mycobacterium tuberculosis-infected adults. Am J Respir Crit Care Med. 2012;185:769–78. doi: 10.1164/rccm.201108-1548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Isaacs F, et al. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol. 2010;40:279–90. doi: 10.1002/eji.200939754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, et al. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am J Respir Crit Care Med. 2010;181:1407–17. doi: 10.1164/rccm.200910-1484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 26.Lindenstrøm T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009;182:8047–55. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- 27.Aagaard C, Hoang T, Dietrich J, Cardona P-J, Izzo A, Dolganov G, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. 2011;369:20130437–47. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 28.Aagaard CS, Hoang TTKT, Vingsbo-Lundberg C, Dietrich J, Andersen P. Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis. J Immunol. 2009;183:2659–68. doi: 10.4049/jimmunol.0900947. [DOI] [PubMed] [Google Scholar]
- 29.Darrah PA, Patel DT, De Luca PM, Lindsay RWB, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 30.Forbes EK, Sander C, Ronan EO, McShane H, Hill AVS, Beverley PCL, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–64. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derrick SC, Yabe IM, Yang A, Morris SL. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine. 2011;29:2902–9. doi: 10.1016/j.vaccine.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–8. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kagina BMN, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette–Guérin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–9. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 35.Alcaïs A, Fieschi C, Abel L, Casanova J-L. Tuberculosis in children and adults: two distinct genetic diseases. J Exp Med. 2005;202:1617–21. doi: 10.1084/jem.20052302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alcaïs A, Quintana-Murci L, Thaler DS, Schurr E, Abel L, Casanova J-L. Life-threatening infectious diseases of childhood: single-gene inborn errors of immunity? Ann N Y Acad Sci. 2010;1214:18–33. doi: 10.1111/j.1749-6632.2010.05834.x. [DOI] [PubMed] [Google Scholar]
- 37.Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave EJ, et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med. 2010;207:2869–81. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geldmacher C, Schuetz A, Ngwenyama N, Casazza JP, Sanga E, Saathoff E, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis. 2008;198:1590–8. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 40.Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5:e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 1992;89:12013–7. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol. 2010;184:6043–52. doi: 10.4049/jimmunol.1000106. [DOI] [PubMed] [Google Scholar]
- 43.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 44.He X-S, Draghi M, Mahmood K, Holmes TH, Kemble GW, Dekker CL, et al. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest. 2004;114:1812–9. doi: 10.1172/JCI22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.