Abstract
A series of isopropanol-bridged carbazole azoles as potential antimicrobial agents were designed and synthesized from commercial carbazoles. Bioassay revealed that 3,6-dichlorocarbazolyl triazole 3f could effectively inhibit the growth of E. faecalis with minimal inhibitory concentration of 2 μg/mL. The active molecule 3f showed lower propensity to trigger the development of resistance in bacteria than norfloxacin and exerted rapidly bactericidal ability. Compound 3f also exhibited low cytotoxicity to normal mammalian RAW264.7 cells. Further mechanism exploration indicated that conjugate 3f was membrane active against E. faecalis and could form 3f–DNA complex by intercalating into DNA of resistant E. faecalis, which might be responsible for its antimicrobial action. Molecular docking showed an efficient binding of triazole derivative 3f with DNA gyrase enzyme through noncovalent interactions.
Keywords: Carbazole, antibacterial, resistance, cytotoxicity, E. faecalis, DNA
The excessive reliance on antibiotics has promoted the steady emergence of multidrug-resistant bacteria and concurrently rendered most important weapons against pathogens ineffective on a worldwide scale.1 The increasingly prevalent Enterococcus faecalis (E. faecalis) as a member of “ESKAPE” in hospitals has become a leading cause of serious complications in patient treatments, such as urinary tract infection, abdominal infection, and pelvic infection and could even result in septicemia and internal carditis.2E. faecalis could also change the manner of cell growth together with division and is facile to develop intrinsical resistance to many clinical antibiotics like vancomycin, linezolid, and daptomycin, ascribed to the thick cell wall.3 The toxicity, resistance, and dissemination of E. faecalis have continuously necessitated the pursuit of new curatively effective antibacterial agents with novel targets to address the growing medical need.
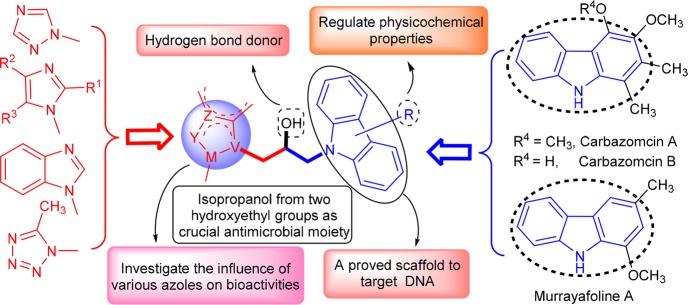
Natural and synthetic congeners of carbazole comprise a large group of therapeutically useful agents in medicinal areas,4 and especially antimicrobial carbazomycin5 and murrayafoline A6 have been marketed successfully.7 Mechanism investigation found that carbazoles with large π-conjugated backbone could noncovalently bind with DNA through intercalation with base pairs, minor groove binding, or electrostatic interactions.8 Carbazoles have also been evidenced to be membrane active by disrupting bacterial membrane integrity and mislocalizing essential membrane-associated protein.9 These cases greatly raise the spirits of exploiting potential antimicrobial carbazoles with multiple targets, which will provide an important step forward in the medicinal field. It has been indicated that azole ring functionalized carbazoles at N-position showed comparable or even superior inhibition against the tested microbes to the reference drugs.10,11 Very recently, the modification of the 3- and 6-positions in carbazole has opened new avenues to exploit novel effective antimicrobial candidates.12 However, the poor solubility of carbazole, which is probably resulted from the rigidly planar structure, has imposed serious restriction on its further application. It has been reported that the introduction of a hydroxy group as hydrogen bond donor to carbazole derivatives could ameliorate physicochemical property and improve binding affinity with bioactive molecules, thus enhancing biological activities.13
Alcohols from natural and artificial sources have long been employed as disinfectants with potent antimicrobial potentiality in daily life because of the ubiquity, convenience, and high efficiency.14 Particularly, aminoalcohols have been announced to exert prominent antibacterial abilities.15 Currently, azoles with advantages like high therapeutic index, favorable pharmacokinetic parameters, and good safety profile have achieved considerable success in the treatment of infective diseases by interacting with biomacromolecules in microorganisms.16,17 Structurally simple metronidazole as a combination of alcohol and nitroimidazole exerts excellent inhibitory efficacies against obligate anaerobes, and this remarkable success quickly diverts continuous efforts toward the development of other hydroxyethyl azoles in clinic to treat pathogenic infections.18,19 Some nonclinical hydroxyethyl azoles have also been developed with superior antibacterial or antifungal potencies.20,21 These researches clearly demonstrated the enormous potentiality of hydroxyethyl azole in the antimicrobial aspect.
In view of these, various azoles were incorporated into carbazole backbone through molecular hybridization22 with an isopropanol linker, which is usually regarded as a combination of two hydroxyethyl fragments, to generate a series of potential antimicrobial carbazole compounds, where the nitrogen atom, hydroxy group, and aromatic heterocycle are able to exert various interactions with active sites in a biological system and might simultaneously bind with several targets, thus displaying multiple action modes (Figure 1). Therefore, this kind of new compound may be conducive to overcome gradually severe drug resistance and broaden antimicrobial spectrum. Meanwhile, the modification of carbazole by functional groups with varying degrees of electronegativities could regulate pharmacological properties to possibly decrease or eliminate the side effects.
Figure 1.
Design of isopropanol-conjugated carbazole azoles as novel potential antimicrobial agents.
Target molecules were assessed for antimicrobial activities, and the structure–activity relationship (SAR) was presented. Bacterial resistance, bactericidal kinetics, and cytotoxicity of the active molecule were also evaluated. The further exploration for possible antibacterial mechanism of active molecule was done by studying membrane disruption potentiality, interaction with DNA, and molecular docking with DNA gyrase.
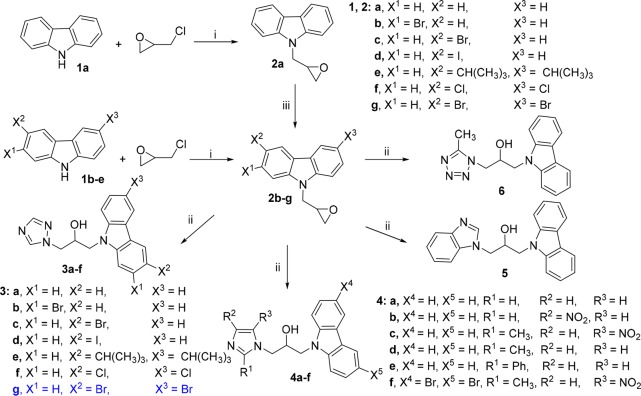
The target isopropanol-conjugated carbazole azoles were synthesized according to Scheme 1 (see also Supporting Information).
Scheme 1. Synthesis of Compounds 3–6.
Reagents and conditions: (i) KOH, DMF, 4 °C; (ii) potassium carbonate, CH3CH2OH, 80 °C; (iii) NCS or NBS, DMF, 0–25 °C.
It has been verified that in vitro bioactivities and in vivo pharmacokinetic properties of antimicrobial agents are sensitive to the pH value of culture medium and human body fluid since pH values could greatly affect water solubility of compounds and thus further have an impact on transportation, absorption, distribution, and metabolism.23 Nitroimidazolyl carbazole 4c with poor solubility was investigated as a guiding compound to evaluate its antimicrobial activities under different pH conditions. Although the water solubility of compound 4c was slightly improved in strong basic solution, which might result from the release of a trace amount of H+ by the hydroxyl group, the inhibitory action of compound 4c (Figures S1 and S2) was not responsive to pH conditions, and no prominent change in minimal inhibitory concentration (MIC) was observed. Previous work has also evidenced that antimicrobial agents with easily ionized groups are more sensitive to the variation of pH.24 Hence, the absence of fragment that is facile to ionize might account for the insensitivity of compound 4c to pH, which did not enhance suppressive action against the tested bacteria and fungi. In view of this, neutral aqueous media that simulated to the internal environment was chosen as a measuring condition to evaluate the actual antimicrobial effect.
Table S2 showed that the type of azoles played a significant role in the biological activity. Triazolyl carabzoles 3a–g had higher levels of growth inhibition against the tested bacteria among the final compounds. Among triazole series 3a–g, some compounds showed moderate to high activities toward Gram-positive bacteria of E. faecalis and S. aureus ATCC 29213, and Gram-negative E. coli ATCC 25922 in comparison with norfloxacin, where the halogenated carbazoles 3b–d and 3f–g generally revealed higher suppressive capacities than the unsubstituted one (3a), manifesting that the introduction of halogen was constructive to the antibacterial activities. Among monosubstituted series, the monobromocarbazoles 3b–c revealed stronger inhibitory action against the bacterial strains than 3-iodocarbazolyl triazole 3d, and the transformation of bromine at 2-position of triazolyl carbazole 3b to 3-position produced 3-bromocarbazolyl triazole 3c with slightly improved potency, which indicated that halogen and its position on carbazole backbone were closely related with the efficacies. However, carbazole derivative 3e with two electron-donating tertiary butyl groups was observed with a significant loss of activity, even being weaker than the unsubstituted one (3a), while the bacteriostatic abilities of dibromocarbazole 3g and dichlorocarbazole 3f were sharply increased in comparison with 3a, manifesting that the functionalization by electron-withdrawing halogens at 3- and 6-positions of carbazole backbone was a structural requirement for antibacterial potentialities. The comparison of activities between compounds 3f and 3g showed that chlorine with stronger electronegativity was more positive for activities.
Nitroimidazolyl carbazole 4b in the imidazole series was the relatively most active one, and the insertion of methyl to imidazole gave compound 4c with marginally lower antibacterial potencies, suggesting that the nitro group was pivotal and that the insertion of methyl group was disadvantageous. Methylimidazolyl carbazole 4d with obviously decreased potencies further proved the deduction. The substitution of the methyl group by a phenyl substituent generated highly hydrophobic compound 4e with worse repressive activities, revealing the adverse effect of hydrophobicity for activities. The anti-P. aeruginosa ATCC 27853 potency of compound 4f was 8-fold more potent than compound 4c (MIC = 32 μg/mL), further indicating that the introduction of electron-withdrawing bromine was beneficial. Both benzimidazolyl carbazole 5 and tetrazolyl carbazole 6 displayed weak or no obvious activity in inhibiting the growth of the tested bacteria, suggesting that benzimidazole and tetrazole were negative to the antibacterial potency. Noticeably, triazole derivative 3f was efficient against E. faecalis at 2 μg/mL, and compound 3g bearing triazole ring also showed comparable inhibition against Gram-positive S. aureus 29213 to norfloxacin, while compound 4f possessing 2-methyl-5-nitroimidazole moiety was more active against Gram-negative P. aeruginosa 27853 with tough permeable outer membrane. Nitroimidazoles were known to exert an antibacterial effect through the reduction of nitro group to give reactive radical species and penetrate cell membrane by passive diffusion.25 This explained the superiority of compound 4f toward Gram-negative strain and also implied a possible membrane associated mechanism.
Some target compounds (Table S4) also revealed antifungal potentialities. It was noticeable that compound 3f had comparable anti-C. parapsilosis ATCC 22019 (MIC = 4 μg/mL) and superior anti-A. fumigatus (MIC = 32 μg/mL) abilities to clinical fluconazole.
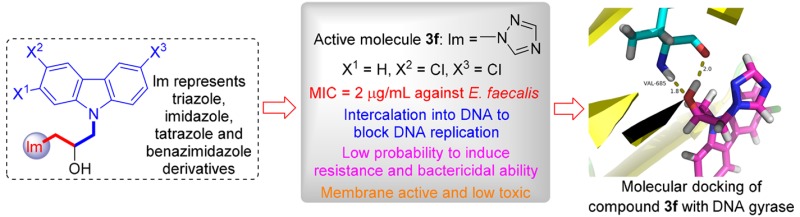
The development of drug resistance in bacteria for conventional antibiotics has become as a major public health concern and also an important attribute of antimicrobial agents for further development as clinical candidates.26 Therefore, the drug resistance developing ability of E. faecalis against 3,6-dichlorocarbazolyl triazole 3f was investigated with norfloxacin as a positive control. Figure 2 showed MIC values ranging from 2 to 8 μg/mL for compound 3f after 11 passages, whereas the anti-E. faecalis potency for norfloxacin was decreased 16-fold after just seven passages. This result indicated that compound 3f did not permit the development of drug resistance as easily as norfloxacin.
Figure 2.
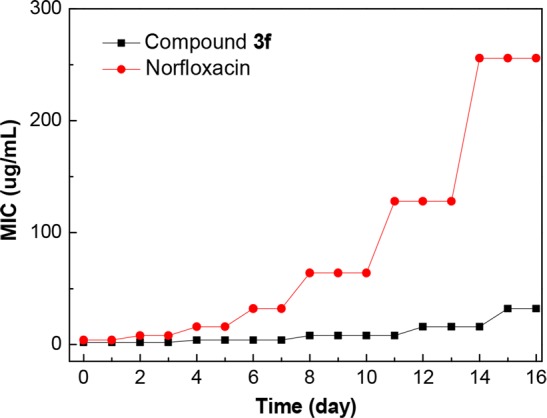
Drug resistance test of compound 3f toward E. faecalis.
The emerging resistance of antibacterial agents could impair the competence of rapidly killing bacteria.27 Given the excellent bacteriostatic performance of compound 3f toward E. faecalis, the bactericidal activity of active molecule 3f was also investigated to provide more information about the acting rate of compound against the tested bacteria. Figure S3 revealed that compound 3f produced more than 3 log (CFU/mL) reduction of bacteria within an hour at a concentration of 4 × MIC as compared to positive control with an increasing tendency, manifesting that compound 3f had a rapid killing effect toward E. faecalis.
Bacterial membrane serves as the first barrier that precludes drug accumulation.28 The inhibition of specific enzymatic processes in membrane, the decrease of transmembrane potentiality, and the increase of phospholipid bilayer permeability are found to be helpful for perturbing bacterial physiology, facilitating the penetration of free radicals, and thus enhancing drug uptake.29 These facts adequately illustrate the significance of membrane active molecules, which are intimately coupled with the development of drug resistance. Herein, bacterial membrane disruption ability of compound 3f was accessed by utilizing propidium iodide (PI) dye, which can pass through the membrane of compromised cells and emit fluorescence upon binding to DNA.30 Fluorescence intensity of the mixtures 3f and PI (Figure S4) exhibited rapid augment and became steady after 60 min, while the two control groups showed no variation, indicating that compound 3f was capable of efficiently increasing membrane permeability of both Gram-positive (E. faecalis) and Gram-negative (E. coli ATCC 25922) bacteria.
The absence of cytotoxicity is one of the essential criteria to estimate the potential of the membrane active agent.31,32 The in vitro cytotoxicity of bioactive molecule 3f was determined toward normal mammalian cells (RAW264.7) via MTT assay. Cytotoxicity results (Figure 3) showed that the cell viability of compound 3f remained greater than 80% even if incubated at a concentration of 128 μg/mL for 24 h. It indicated that RAW264.7 cells possessed good tolerance to dichlorocarbazole 3f at high concentration, thus revealing the therapeutic potentiality of 3f.
Figure 3.
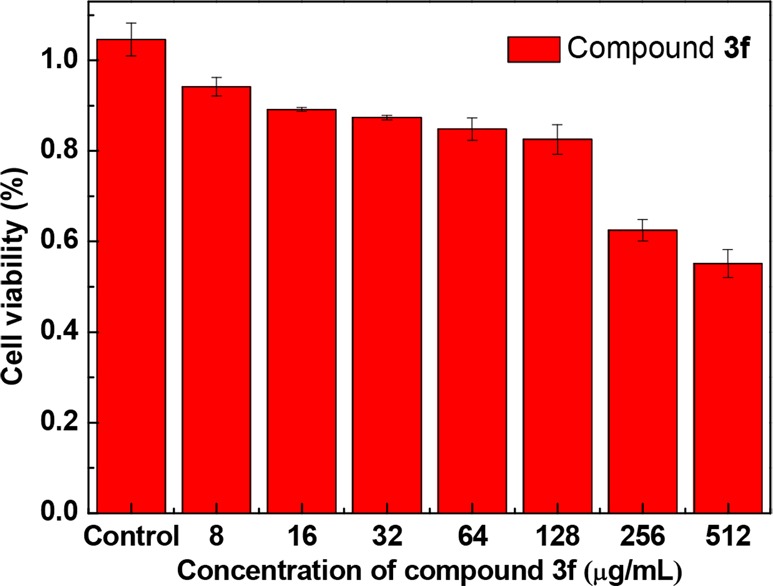
Cytotoxic assay of compound 3f on mouse macrophages cells tested by MTT methodology. Each data bar represents an average of three parallels, and error bars indicate one standard deviation from the mean.
DNA is a potential drug target due to the presence of multiple sites for drug interaction and usually employed in the design of newly promising antimicrobial drugs.33 Carbazole derivatives were reported to be able to efficiently interact with DNA.34 Herein, DNA isolated from resistant E. faecalis strains was investigated for its interaction with compound 3fin vitro by UV–vis spectroscopic and fluorescence methods (Supporting Information). Final results demonstrated that compound 3f could bind with DNA in an intercalative type and form compound 3f–DNA complex, which might block DNA replication and thus reveal antibacterial activities. Herein, triazolyl carbazole 3f might be a promising DNA-targeting candidate for the administration of bacterial infection.
DNA gyrase (PDB ID: 1Zi0) as an attractive target to investigate the antibacterial mechanism was subjected into ligand–receptor docking to rationalize the observed antibacterial activity and understand the possible mechanism.35 Docking results (Figure S11) demonstrated that active molecule 3f could bind with amino acid residues in the active pocket of the enzyme with binding energy of −6.82 kcal/mol (S-enantiomer) and −10.13 kcal/mol (R-enantiomer), indicating that the R-enantiomer of 3f was likely to be a more favorable stereoisomer in inhibiting the function of DNA gyrase. Noticeably, the hydroxy group of compound 3f was adjacent to VAL685 (S-enantiomer) and TYR-64 (R-enantiomer) by forming hydrogen bonds, suggesting the significance of the hydroxy moiety on the antimicrobial ability. Additional hydrophobic interactions existed between both stereoisomers of 3f and DNA gyrase. van der Waals force also made contribution to the binding of S-enantiomer with enzyme. These noncovalent bonds might be helpful for the structural stability of compound 3f–DNA gyrase complex, which further accounted for the strong inhibitory efficacy of compound 3f.
This work developed isopropanol-conjugated carbazole azoles by a convenient and efficient procedure to combat the clinically infectious pathogens. Predominantly, compound 3f could effectively inhibit the growth of E. faecalis, S. aureus ATCC 29213, and C. parapsilosis ATCC 22019 with MIC values of 2, 4, and 4 μg/mL, respectively. SAR suggested that both triazole ring and substituents on carbazole scaffold were important influence factors on antimicrobial potency, and the introduction of halogen with strong electronegativity to the 3,6-positions of carbazole ring was helpful for inhibitory activities. Conjugate 3f displayed lower tendency to trigger resistance in bacteria than norfloxacin, killed bacteria in short time, and exerted low toxicity against RAW264.7 cells. Triazolyl carbazole 3f was also membrane active and could interact with E. faecalis DNA through intercalation, which might account for its antibacterial potentiality. Further molecular docking indicated that compound 3f could form supramolecular complex with DNA gyrase through noncovalent interactions. Therefore, this work discovered a potential candidate with potential dual targets, which might be an encouraging starting point in the discovery of novel antibacterial agents to overcome drug resistance.
Acknowledgments
We are grateful to Clinical Laboratory Department, Sichuan Academy of Medical Sciences, Sichuan Provincial People’s Hospital, for providing us with bacteria and fungi isolates used for this study.
Glossary
ABBREVIATIONS
- SAR
structure–activity relationship
- NBS
N-bromosuccinimide
- NCS
N-chlorosuccinimide
- MIC
minimal inhibitory concentration
- PI
propidium iodide
- NR
neutral red
- TLC
thin-layer chromatography
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00514.
Full experimental procedures, characterization, structural spectra, antibacterial and antifungal activity data, resistance study, bactericidal curve, bacterial membrane disruption, cytotoxicity evaluation, interaction of compound 3f with DNA, and molecular docking (PDF)
Author Contributions
All authors have given approval to the final version of the manuscript.
This work was partially supported by the National Natural Science Foundation of China (No. 21672173), Chongqing Special Foundation for Postdoctoral Research Proposal (No. Xm2016039), and Program for Overseas Young Talents from State Administration of Foreign Experts Affairs, China (No. WQ2017XNDX047).
The authors declare no competing financial interest.
Supplementary Material
References
- Brown E. D.; Wright G. D. Antibacterial Drug Discovery in the Resistance Era. Nature 2016, 529, 336–343. 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- Mohammad H.; Younis W.; Chen L.; Peters C. E.; Pogliano J.; Pogliano K.; Cooper B.; Zhang J. N.; Mayhoub A.; Oldfield E.; Cushman M.; Seleem M. N. Phenylthiazole Antibacterial Agents Targeting Cell Wall Synthesis Exhibit Potent Activity in vitro and in vivo against Vancomycin-resistant Enterococci. J. Med. Chem. 2017, 60, 2425–2438. 10.1021/acs.jmedchem.6b01780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp B.; Lázár V. New Recipe for Targeting Resistance. Nat. Chem. Biol. 2016, 12, 891–892. 10.1038/nchembio.2215. [DOI] [PubMed] [Google Scholar]
- Schmidt A. W.; Reddy K. R.; Knölker H. J. Occurrence, Biogenesis, and Synthesis of Biologically Active Carbazole Alkaloids. Chem. Rev. 2012, 112, 3193–3328. 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]
- Chakraborty S.; Saha C. Total Synthesis of Carbazomycin G. Eur. J. Org. Chem. 2013, 2013, 5731–5736. 10.1002/ejoc.201300467. [DOI] [Google Scholar]
- Cuong N. M.; Wilhelm H.; Porzel A.; Arnold N.; Wessjohann L. 1-O-Substituted Derivatives of Murrayafoline A and their Antifungal Properties. Nat. Prod. Res. 2008, 22, 1428–1432. 10.1080/14786410802006033. [DOI] [PubMed] [Google Scholar]
- Thomas S. M.; Purmal A.; Pollastri M.; Mensa-Wilmot K. Discovery of A Carbazole-derived Lead Drug for Human African Trypanosomiasis. Sci. Rep. 2016, 6, e32083. 10.1038/srep32083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Głuszyńska A. Biological Potential of Carbazole Derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. 10.1016/j.ejmech.2015.02.059. [DOI] [PubMed] [Google Scholar]
- Eun Y. J.; Foss M. H.; Kiekebusch D.; Pauw D. A.; Westler W. M.; Thanbichler M.; Weibel D. B. DCAP: A Broad-spectrum Antibiotic That Targets the Cytoplasmic Membrane of Bacteria. J. Am. Chem. Soc. 2012, 134, 11322–11325. 10.1021/ja302542j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addla D.; Wen S. Q.; Gao W. W.; Maddili S. K.; Zhang L.; Zhou C. H. Design, Synthesis, and Biological Evaluation of Novel Carbazole Aminothiazoles as Potential DNA Targeting Antimicrobial Agents. MedChemComm 2016, 7, 1988–1994. 10.1039/C6MD00357E. [DOI] [Google Scholar]
- Zhang F. F.; Gan L. L.; Zhou C. H. Synthesis, Antibacterial and Antifungal Activities of Some Carbazole Derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 1881–1884. 10.1016/j.bmcl.2010.01.159. [DOI] [PubMed] [Google Scholar]
- Maji B.; Kumar K.; Kaulage M.; Muniyappa K.; Bhattacharya S. Design and Synthesis of New Benzimidazole-carbazole Conjugates for the Stabilization of Human Telomeric DNA, Telomerase Inhibition, and Their Selective Action on Cancer Cells. J. Med. Chem. 2014, 57, 6973–6988. 10.1021/jm500427n. [DOI] [PubMed] [Google Scholar]
- Xu W.; Huang J. J.; Shao B. H.; Xu X. J.; Jiang R. W.; Yuan M. Design, Synthesis, Crystal Structure, Biological Evaluation and Molecular Docking Studies of Carbazole-arylpiperazine Derivatives. Bioorg. Med. Chem. 2016, 24, 5565–5572. 10.1016/j.bmc.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Kubo I.; Cespedes C. L. Antifungal Activity of Alkanols: Inhibition of Growth of Spoilage Yeasts. Phytochem. Rev. 2013, 12, 961–977. 10.1007/s11101-013-9325-1. [DOI] [Google Scholar]
- Cheng C. Y.; Chang C. P.; Lauderdale T. L. Y.; Yu G. Y.; Lee J. C.; Jhang Y. W.; Wu C. H.; Ke Y. Y.; Sadani A. A.; Yeh C. F.; Huang I. W.; Kuo Y. P.; Tsai D. J.; Yeh T. K.; Tseng C. T.; Song J. S.; Liu Y. W.; Tsou L. K.; Shia K. S. Bromomethylthioindole Inspired Carbazole Hybrids as Promising Class of Anti-MRSA Agents. ACS Med. Chem. Lett. 2016, 7, 1191–1196. 10.1021/acsmedchemlett.6b00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. W.; Zhou C. H. Antimicrobial 2-Aminothiazolyl Quinolones: What Is Their Potential in the Clinic?. Future Med. Chem. 2017, 9, 1461–1464. 10.4155/fmc-2017-0108. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Damu G. L. V.; Cui S. F.; Mi J. L.; Tangadanchu V. K. R.; Zhou C. H. Discovery of Potential Antifungal Triazoles: Design, Synthesis, Biological Evaluation, and Preliminary Antifungal Mechanism Exploration. MedChemComm 2017, 8, 1631–1639. 10.1039/C7MD00112F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Peng X. M.; Damu G. L. V.; Geng R. X.; Zhou C. H. Comprehensive Review in Current Developments of Imidazole-based Medicinal Chemistry. Med. Res. Rev. 2014, 34, 340–437. 10.1002/med.21290. [DOI] [PubMed] [Google Scholar]
- Gao W. W.; Gopala L.; Bheemanaboina R. R. Y.; Zhang G. B.; Li S.; Zhou C. H. Discovery of 2-Aminothiazolyl Berberine Derivatives as Effectively Antibacterial Agents toward Clinically Drug-resistant Gram-negative Acinetobacter baumanii. Eur. J. Med. Chem. 2018, 146, 15–37. 10.1016/j.ejmech.2018.01.038. [DOI] [PubMed] [Google Scholar]
- Peng X. M.; Peng L. P.; Li S.; Avula S. R.; Kannekanti V. K.; Zhang S. L.; Tam K. Y.; Zhou C. H. Quinazolinone Azolyl Ethanols: Potential Lead Antimicrobial Agents with Dual Action Modes Targeting MRSA DNA. Future Med. Chem. 2016, 8, 1927–1940. 10.4155/fmc-2016-0002. [DOI] [PubMed] [Google Scholar]
- Fang X. F.; Li D.; Tangadanchu V. K. R.; Gopala L.; Gao W. W.; Zhou C. H. Novel Potentially Antifungal Hybrids of 5-Flucytosine and Fluconazole: Design, Synthesis and Bioactive Evaluation. Bioorg. Med. Chem. Lett. 2017, 27, 4964–4969. 10.1016/j.bmcl.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Chen Y. Y.; Gopala L.; Bheemanaboina R.; R Y.; Liu H. B.; Cheng Y.; Geng R. X.; Zhou C. H. Novel Naphthalimide Aminothiazoles as Potential Multi-targeting Antimicrobial Agents. ACS Med. Chem. Lett. 2017, 8, 1331–1335. 10.1021/acsmedchemlett.7b00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnusch C. J.; Albada H. B.; van Vaardegem M.; Liskamp R. M. J.; Sahl H. G.; Shadkchan Y.; Osherov N.; Shai Y. Trivalent Ultrashort Lipopeptides Are Potent pH Dependent Antifungal Agents. J. Med. Chem. 2012, 55, 1296–1302. 10.1021/jm2014474. [DOI] [PubMed] [Google Scholar]
- Blagodatskikh I. V.; Kulikov S. N.; Vyshivannaya O. V.; Bezrodnykh E. A.; Tikhonov V. E. N-Reacetylated Oligochitosan: pH Dependence of Self-assembly Properties and Antibacterial Activity. Biomacromolecules 2017, 18, 1491–1498. 10.1021/acs.biomac.7b00039. [DOI] [PubMed] [Google Scholar]
- Sutherland H. S.; Blaser A.; Kmentova I.; Franzblau S. G.; Wan B. J.; Wang Y. H.; Ma Z. K.; Palmer B. D.; Denny W. A.; Thompson A. M. Synthesis and Structure-activity Relationships of Antitubercular 2-Nitroimidazooxazines Bearing Heterocyclic Side Chains. J. Med. Chem. 2010, 53, 855–866. 10.1021/jm901378u. [DOI] [PubMed] [Google Scholar]
- Jeyakkumar P.; Liu H. B.; Gopala L.; Cheng Y.; Peng X. M.; Geng R. X.; Zhou C. H. Novel Benzimidazolyl Tetrahydroprotoberberines: Design, Synthesis, Antimicrobial Evaluation and Multi-targeting Exploration. Bioorg. Med. Chem. Lett. 2017, 27, 1737–1743. 10.1016/j.bmcl.2017.02.071. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Avula S. R.; Gao W. W.; Addla D.; Tangadanchu V. K. R.; Zhang L.; Lin J. M.; Zhou C. H. Multi-targeting Exploration of New 2-Aminothiazolyl Quinolones: Synthesis, Antimicrobial Evaluation, Interaction with DNA, Combination with Topoisomerase IV and Penetrability into Cells. Eur. J. Med. Chem. 2016, 124, 935–945. 10.1016/j.ejmech.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Hoque J.; Konai M. M.; Gonuguntla S.; Manjunath G. B.; Samaddar S.; Yarlagadda V.; Haldar J. Membrane Active Small Molecules Show Selective Broad Spectrum Antibacterial Activity with No Detectable Resistance and Eradicate Biofilms. J. Med. Chem. 2015, 58, 5486–5500. 10.1021/acs.jmedchem.5b00443. [DOI] [PubMed] [Google Scholar]
- Hurley K. A.; Heinrich V. A.; Hershfield J. R.; Demons S. T.; Weibel D. B. Membrane-targeting DCAP Analogues with Broad-spectrum Antibiotic Activity against Pathogenic Bacteria. ACS Med. Chem. Lett. 2015, 6, 466–471. 10.1021/acsmedchemlett.5b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y. F.; Yang X. A.; Goswami S.; Gorityala B. K.; Idowu T.; Domalaon R.; Zhanel G. G.; Shan A.; Schweizer F. Amphiphilic Tobramycin-lysine Conjugates Sensitize Multidrug Resistant Gram-negative Bacteria to Rifampicin and Minocycline. J. Med. Chem. 2017, 60, 3684–3702. 10.1021/acs.jmedchem.6b01742. [DOI] [PubMed] [Google Scholar]
- Konai M. M.; Ghosh C.; Yarlagadda V.; Samaddar S.; Haldar J. Membrane Active Phenylalanine Conjugated Lipophilic Norspermidine Derivatives with Selective Antibacterial Activity. J. Med. Chem. 2014, 57, 9409–9423. 10.1021/jm5013566. [DOI] [PubMed] [Google Scholar]
- Cui S. F.; Addla D.; Zhou C. H. Novel 3-Aminothiazolquinolones: Design, Synthesis, Bioactive Evaluation, SARs, and Preliminary Antibacterial Mechanism. J. Med. Chem. 2016, 59, 4488–4510. 10.1021/acs.jmedchem.5b01678. [DOI] [PubMed] [Google Scholar]
- Li X. L.; Hu Y. J.; Wang H.; Yu B. Q.; Yue H. L. Molecular Spectroscopy Evidence of Berberine Binding to DNA: Comparative Binding and Thermodynamic Profile of Intercalation. Biomacromolecules 2012, 13, 873–880. 10.1021/bm2017959. [DOI] [PubMed] [Google Scholar]
- Peng X. M.; Cai G. X.; Zhou C. H. Recent Developments in Azole Compounds as Antibacterial and Antifungal Agents. Curr. Top. Med. Chem. 2013, 13, 1963–2010. 10.2174/15680266113139990125. [DOI] [PubMed] [Google Scholar]
- Ahmed M.; Kelley S. O. Enhancing the Potency of Nalidixic Acid toward A Bacterial DNA Gyrase with Conjugated Peptides. ACS Chem. Biol. 2017, 12, 2563–2569. 10.1021/acschembio.7b00540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.