Abstract
The addition of organic solvents to α-amino acids in aqueous solution could be an effective method in crystallization. We reviewed the available data on the solubility of α-amino acids in water, water–ethanol mixtures, and ethanol at 298.15 K and 0.1 MPa. The solubility of l-alanine, l-proline, l-arginine, l-cysteine, and l-lysine in water and ethanol mixtures and the solubility of l-alanine, l-proline, l-arginine, l-cysteine, l-lysine, l-asparagine, l-glutamine, l-histidine, and l-leucine in pure ethanol systems were measured and are published here for the first time. The impact on the solubility of amino acids that can convert in solution, l-glutamic acid and l-cysteine, was studied. At lower concentrations, only the ninhydrin method and the ultraperfomance liquid chromatography (UPLC) method yield reliable results. In the case of α-amino acids that convert in solution, only the UPLC method was able to discern between the different α-amino acids and yields reliable results. Our results demonstrate that α-amino acids with similar physical structures have similar changes in solubility in mixed water/ethanol mixtures. The solubility of l-tryptophan increased at moderate ethanol concentrations.
1. Introduction
Biobased products made from proteins and α-amino acids (e.g., bioplastics, pharmaceuticals, fine chemicals) could become increasingly important.1−4 One of the challenges is to find a way to separate α-amino acids from industrial residues. The literature data on the use of antisolvents, such as ethanol, to lower the solubility of the α-amino acids to promote crystallization is incomplete. An understanding on the impact of antisolvents on α-amino acids in solution is integral in designing technologies for separating α-amino acids from solution.
Many articles report the solubility measurements of α-amino acids in water5−31 and in mixtures of alcohol and water.32−40 Subsequent research focused on calculating the activity coefficients of these α-amino acids in water, water–ethanol, and ethanol.41−43 Recently, the effect of the addition of ethanol on the solubility of amino acids has also been applied to the crystallization of amino acids after protein hydrolysis.44 However, the solubility of α-amino acids in a two-solvent system cannot be described by a first-degree exponential function with a discrete partition coefficient as espoused in the earliest solubility studies. This is due to the ternary interactions of the solvents to each other and with the solute.
In response to this, models have been developed to explain the solubility of a few α-amino acids in water, water–ethanol, ethanol, and other two solvent systems.7,45−52 However, for several α-amino acids, no data have been published on their solubility in water–ethanol or ethanol systems. For many other α-amino acids, the data are incomplete or unreliable. For this reason, applying the models to all α-amino acids is not possible.
The goal of this article is to understand the solubility of the 20 proteinogenic α-amino acids in water, water–ethanol mixtures, and ethanol. To achieve this goal, three research objectives are pursued. First, the methodologies of bringing the α-amino acids to maximum solubility and the analytical technique of measuring these concentrations in solutions of water, water–ethanol mixtures, and ethanol are evaluated. Second, since recent evidence shows that some α-amino acids can convert to other α-amino acids, the solubility data in solutions of water, water–ethanol mixtures, and ethanol of these α-amino acids at 298.15 K and 0.1 MPa are re-evaluated. Third, the solubility of the α-amino acids l-alanine, l-arginine, l-lysine, l-proline, and l-cysteine in solutions of water, water–ethanol mixtures at 298.15 K and 0.1 MPa is measured. Furthermore, the solubility of l-alanine, l-arginine, l-lysine, l-proline, l-cysteine, l-asparagine, l-aspartic acid, l-glutamine, l-histidine and l-leucine at 298.15 K and 0.1 MPa are measured in ethanol.
1.1. Review of Amino Acid Solubility Data and Methodologies
Data on the solubility of glycine, l-valine, l-serine, l-isoleucine, l-tryptophan, l-tyrosine, l-phenylalanine, and l-threonine in water, water–ethanol mixtures, and ethanol were found in peer reviewed journals. These data were obtained using disparate methodologies in both the dissolution of the α-amino acids as well as in their measurement.
Gravimetric measurement of the dry weight of a solute is a technique that is often used in measuring solubility.7 However, the solubility of some α-amino acids (e.g., l-tyrosine) are extremely low. Furthermore, the solubility of all α-amino acids in ethanol are low. Measuring amino acids with low solubility gravimetrically would consume excessive amounts of ethanol to produce a few milligrams of the solute. Therefore, a spectrophotometric analytical technique using ninhydrin was developed to measure the concentrations of α-amino acids.45 This article will evaluate these two measurement techniques and use a third technique, the ultraperfomance liquid chromatography (UPLC) method.53 The UPLC method is able to detect concentrations of 2.3 μM.
1.2. Impact of Amino Acid Conversions on Their Solubilities
l-Cysteine can form a sulfur bond with itself upon oxidation to form the dimer cystine.54 There is only one piece of solubility data in the literature for the monomer l-cysteine.8 However, the authors do not mention in their work that they took the oxidation reaction with l-cysteine into account when measuring the solubility. This article reports data measured on the solubility of l-cysteine in water, water–ethanol mixtures, and ethanol under sealed oxygen-poor conditions. Furthermore, after measuring the solubility, the samples of l-cysteine in this work were analyzed through mass-spectrometry to show that the formation of the dimer cystine was negligible.
Data on the solubility of l-glutamic acid in an ethanol–water system were found by McMeekin et al. and expanded by other authors.12,23 However, l-glutamic acid has been shown to convert to l-pyroglutamic acid. The conversion to l-pyroglutamic acid increases as the temperature of the solution increases.55,56 This was not considered in the initial solubility data. To account for this possibility, in this study, the solubility of l-glutamic acid was determined by measuring the concentration of both l-glutamic acid and l-pyroglutamic acid in the same sample by using the UPLC method.53
1.3. Incomplete Solubility Data of Amino Acids
Data on the solubility of l-asparagine, l-aspartic acid, l-glutamine, l-histidine, and l-leucine in water and water and ethanol mixtures were published in peer reviewed journals, but did not include data in ethanol solutions.32,35 The solubilities of these α-amino acids were measured for this work ethanol using the UPLC method.
Data on the solubility of glycine in water, water–ethanol mixtures, and ethanol are conflicting. Reports show the solubility of glycine in water to be 4.25 and 2.733 g/100 mL. For this reason, the solubility of glycine in water, water–ethanol mixtures, and ethanol was remeasured.
No published data could be found on the solubility of l-alanine, l-arginine, l-lysine, l-proline, and l-cysteine in water–ethanol mixtures and ethanol. Their solubility in these systems was measured and reported here.
2. Experimental Section
The α-amino acids that were used in this article were purchased from Sigma-Aldrich. These α-amino acids were at least 99% pure. Table 1 lists the supplier and purity of the chemicals used in this work.
Table 1. Description of Chemicals and Solvents Used.
| chemical name | source | mole fraction purity | purification method |
|---|---|---|---|
| glycine | Sigma-Aldrich | 1.00 | none |
| l-alanine | Sigma-Aldrich | 0.98 | none |
| l-arginine | Sigma-Aldrich | 0.98 | none |
| l-asparagine | Sigma-Aldrich | 1.00 | none |
| l-aspartic acid | Sigma-Aldrich | 0.99 | none |
| l-cysteine | Sigma-Aldrich | 0.97 | none |
| l-glutamic acid | Sigma-Aldrich | 0.99 | none |
| l-glutamine | Sigma-Aldrich | 0.99 | none |
| l-histidine | Sigma-Aldrich | 0.99 | none |
| l-leucine | Sigma-Aldrich | 1.00 | none |
| l-lysine | Sigma-Aldrich | 0.97 | none |
| l-methionine | Sigma-Aldrich | 0.98 | none |
| l-phenylalanine | Sigma-Aldrich | 0.98 | none |
| l-proline | Sigma-Aldrich | 0.99 | none |
| l-tryptophan | Sigma-Aldrich | 0.98 | none |
| l-tyrosine | Sigma-Aldrich | 0.98 | none |
| ethanol | Sigma-Aldrich | >0.99 | none |
For l-arginine, l-lysine, l-proline, l-methionine, l-cysteine, l-glutamic acid, and l-phenylalanine in water–ethanol mixtures, excess amounts of these α-amino acids were added to 15.0 mL Greiner tubes in duplicate. Then 0.0%, 25.0%, 50.0%, 75.0%, and 100.0% ethanol (g/g) solutions in water were added to the Greiner tubes and sealed. The tubes were mixed and added to a jacketed shaking water bath set to 298.15 K and 0.1 MPa and left to mix at 80 rpm until they had reached equilibrium. Both the samples and the water in the water bath were continuously monitored. The amino acids were said to have reached equilibrium when successive measurements, 24 h apart, yielded a concentration within the variation of the balance. All measurements were performed in duplicate. The solubility of glycine, l-asparagine, l-aspartic acid, l-glutamine, l-histidine and l-leucine was measured in ethanol using the same procedure.
The amino acids with low solubilities produced results below the detection limits of the balance. For this reason, these samples were analyzed by UPLC. For l-glutamic acid, all systems were measured with the UPLC as it enabled simultaneous measurement of l-pyroglutamic acid.
The UPLC method is based on automated precolumn derivatization in the injection needle of the amino acid in an autosampler using o-phthalaldehyde (OPA) reagent in combination with 9-fluorenylmethyl chloroformate (FMOC) that enables the amino acids to fluoresce. Separation was achieved with a Dionex RSLC system using an Acquity UPLC BEH C18 reversed-phase column. Sample analysis was performed with an UltiMate 3000 Rapid Separation pump and autosampler. Derivatized amino acids were detected at 263 nm (FMOC derivative of l-proline) and 338 nm (OPA derivatives of the other amino acids).
For the samples that were measured using the gravimetric analytical technique, approximately 3 g of each solution was filtered through a sterile 0.45 μm Minisart filter. Then, the filtered sample was added to a predried and preweighed drying tin and weighed again to ±0.0001 g using a AB204 analytical balance from Mettler Toledo. All samples were filtered and weighed in duplicate. The samples were put in a drying oven set at 315.15 K and 0.1 MPa for 5 days and weighed again; 24 h later, the dry samples were weighed once more. This procedure was repeated until the weights were within the error range of the analytical balance, and the sample was assumed to be at equilibrium.
Additionally, samples of l-cysteine were measured on a LCQ Fleet Ion Trap mass spectrometer from Thermo Scientific. This was done to ensure there was undetectable l-cystine formation.
The saturated mole fraction solubility of all amino acids was calculated by eq 1, while the mole fraction composition of the solvent mixture was calculated by eq 2:
| 1 |
| 2 |
where m1, m2, and m3 are the mass of the amino acid, ethanol, and water and M1, M2, and M3 are the molecular mass of the amino acid, ethanol, and water.
3. Results
3.1. Review of Amino Acid Solubility Data and Methodologies
All results from the analyses in this work are shown in Table 2. The method by which the samples were measure is also shown in Table 2.
Table 2. Experimental Mole Fraction Solubilities, x1, of Amino Acids, the Ethanol Solvent Mole Fraction, x2, the Method of Detection, and the Relative Uncertainty at 298.15 K and p = 0.1 MPaa.
| amino acid | method | 102·x1 | x2 | 104·ur | amino acid | method | 102·x1 | x2 | 104·ur |
|---|---|---|---|---|---|---|---|---|---|
| Glycine | UPLC | 0.0046 | 1.0000 | 0.000 | l-lysine | gravimetric | 14.6733 | 0.1153 | 2.3774 |
| l-alanine | UPLC | 1.3635 | 0.0891 | 9.893 | l-lysine | gravimetric | 5.0789 | 0.2811 | 23.98 |
| l-alanine | UPLC | 0.7857 | 0.2068 | 1.155 | l-lysine | gravimetric | 2.4035 | 0.5398 | 1.583 |
| l-alanine | UPLC | 0.4204 | 0.3697 | 0.5582 | l-lysine | gravimetric | 0.6180 | 1.0000 | 1.319 |
| l-alanine | UPLC | 0.1013 | 0.6100 | 0.3112 | l-methionine | gravimetric | 0.6768 | 0.0000 | 1.991 |
| l-alanine | UPLC | 0.0282 | 1.0000 | 0.0357 | l-methionine | gravimetric | 0.3135 | 0.1153 | 0.0467 |
| l-arginine | gravimetric | 1.8614 | 0.0000 | 3.237 | l-methionine | gravimetric | 0.2014 | 0.2811 | 0.2762 |
| l-arginine | gravimetric | 1.3238 | 0.1153 | 4.896 | l-methionine | gravimetric | 0.0859 | 0.5398 | 0.4284 |
| l-arginine | gravimetric | 0.6303 | 0.2811 | 3.554 | l-methionine | gravimetric | 0.0057 | 1.0000 | 0.0534 |
| l-arginine | gravimetric | 0.1292 | 0.5398 | 1.116 | l-phenylalanine | gravimetric | 0.1994 | 0.1153 | 0.5595 |
| l-arginine | gravimetric | 0.0143 | 1.0000 | 0.1584 | l-phenylalanine | gravimetric | 0.1994 | 0.2811 | 0.4120 |
| l-asparagine | UPLC | 0.0002 | 1.0000 | 0.0008 | l-phenylalanine | gravimetric | 0.1478 | 0.5398 | 0.0982 |
| l-aspartic acid | UPLC | 0.0002 | 1.0000 | 0.0006 | l-phenylalanine | gravimetric | 0.0118 | 1.0000 | 0.0196 |
| l-cysteine | gravimetric | 1.2992 | 0.0000 | 0.3806 | l-proline | gravimetric | 21.6583 | 0.1153 | 13.4861 |
| l-cysteine | gravimetric | 0.4652 | 0.1153 | 3.8438 | l-proline | gravimetric | 15.5698 | 0.2811 | 50.27 |
| l-cysteine | gravimetric | 0.2546 | 0.2811 | 0.0204 | l-proline | gravimetric | 13.9836 | 0.5398 | 38.49 |
| l-cysteine | gravimetric | 0.1318 | 0.5398 | 1.0616 | l-proline | gravimetric | 0.5176 | 1.0000 | 43.34 |
| l-cysteine | gravimetric | 0.0113 | 1.0000 | 0.2332 | l-tryptophan | gravimetric | 0.1034 | 0.0000 | 1.846 |
| l-glutamic acid | UPLC | 0.1000 | 0.0000 | 0.0334 | l-tryptophan | gravimetric | 0.1047 | 0.1153 | 0.1578 |
| l-glutamic acid | UPLC | 0.0434 | 0.0891 | 0.1152 | l-tryptophan | gravimetric | 0.1691 | 0.2811 | 0.5306 |
| l-glutamic acid | UPLC | 0.0273 | 0.2068 | 0.2070 | l-tryptophan | gravimetric | 0.1250 | 0.5398 | 0.2640 |
| l-glutamic acid | UPLC | 0.0225 | 0.3697 | 0.1621 | l-tryptophan | gravimetric | 0.0243 | 1.0000 | 0.4692 |
| l-glutamic acid | UPLC | 0.0183 | 0.6100 | 0.1588 | l-tyrosine | gravimetric | 0.0035 | 0.0000 | 0.0678 |
| l-glutamic acid | UPLC | 0.0009 | 1.0000 | 0.0017 | l-tyrosine | gravimetric | 0.0031 | 0.1153 | 0.0176 |
| l-glutamine | UPLC | 0.0001 | 1.0000 | 0.0002 | l-tyrosine | gravimetric | 0.0015 | 0.2811 | 0.0660 |
| l-histidine | UPLC | 0.0004 | 1.0000 | 0.0014 | l-tyrosine | gravimetric | 0.0020 | 0.5398 | 0.0521 |
| l-leucine | UPLC | 0.0095 | 1.0000 | 0.0003 | l-tyrosine | gravimetric | 0.0031 | 1.0000 | 0.4375 |
Standard uncertainties u are u(T) = 0.5 K, u(P) = 5 kPa, and ur(x) = 0.15.
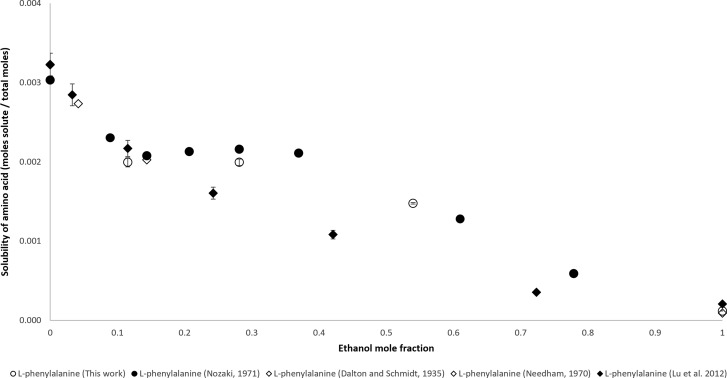
Solubility data of l-phenylalanine comes from four literature sources. The initial solubility measurement in water was conducted by Dalton and Schmidt (1935), then in water and water–ethanol mixtures by Needham (1970), Nozaki et al. (1971), and Lu et al. (2012). In this article, an additional set of data was collected in water–ethanol mixtures. All data was collected by the gravimetric method except for Dalton and Schmidt, who used the dissolution method. The data of Needham, Dalton and Schmidt, Nozaki et al. (2012), and the experimental data collected for this article are similar, as can be seen in Figure 1. After an initial decrease in solubility, as ethanol mole fraction increases from 0 to 0.100 mole fraction, the solubility of l-phenylalanine is greater between an ethanol mole fraction of 0.100 and 0.400 than below 0.100. The solubility of l-phenylalanine decreases again at an ethanol mole fraction above 0.400. The exception to this is Lu et al. (2012), who did not measure an increase in solubility between 0.100 and 0.400.
Figure 1.
Mole fraction solubility of l-phenylalanine.
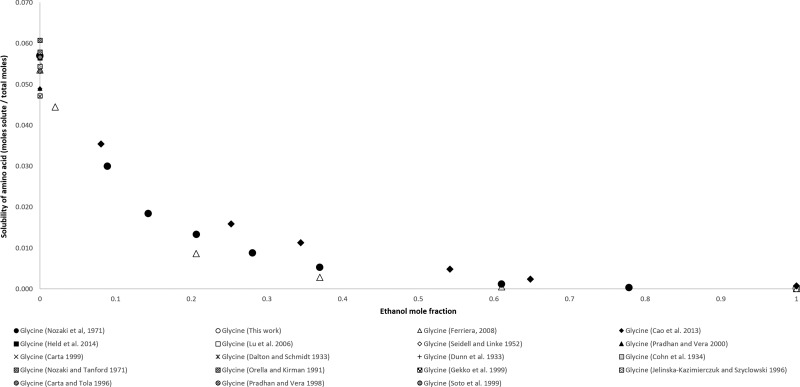
In Figure 2, the concentration of glycine is plotted against the mole fraction of ethanol from 0.000 to 1.000. Several authors have measured the solubility of glycine in water. Cao et al. (2013), Ferreira (2008), Nozaki et al. (1971), and this work have measured the solubilty of glycine in various binary solutions of ethanol and water and in ethanol. Solubilities reported by Nozaki et al. (1971) are higher than those of Ferreira (2008), while the solubilities measured by Cao et al. (2013) are the highest reported. At a solvent mole fraction of 1.000 ethanol, the solubilities reported by Ferreira (2008), using the ninhydrin method were within the standard deviations measured by this work, using the UPLC method. These were 4.59 × 10–5 and 5.52 × 10–5 respectively. Cao et al. (2013), using the gravimetric method, reported a solubility mole fraction of 0.0007.
Figure 2.
Mole fraction solubility of glycine.
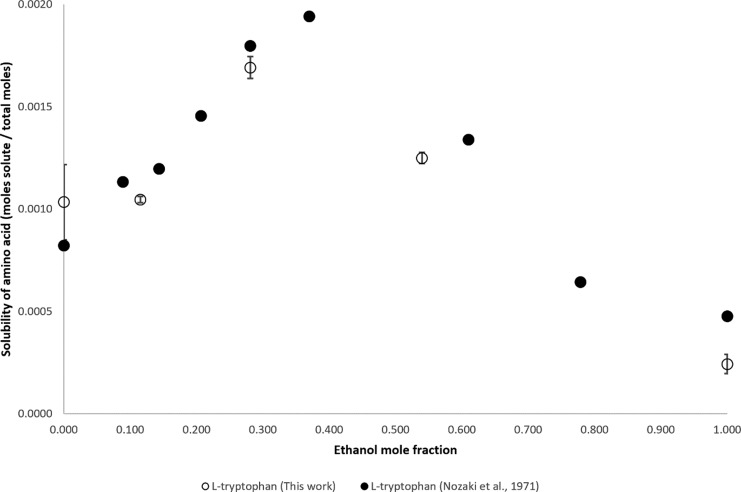
The solubility measured by Nozaki et al. (1971) and our own measurements of l-tryptophan are shown in Figure 3. Except for that in water, at all mole fractions of ethanol, the solubility measured by Nozaki et al. (1971) was higher than the new data reported in this article. The solubility of l-tryptophan peaks between ethanol mole fractions of 0.281 and 0.540. The highest solubility of l-tryptophan was measured by Nozaki et al. at an ethanol mole fraction of 0.371.
Figure 3.
Mole fraction solubility of l-tryptophan.
3.2. Impact of Amino Acid Conversions on Their Solubilities
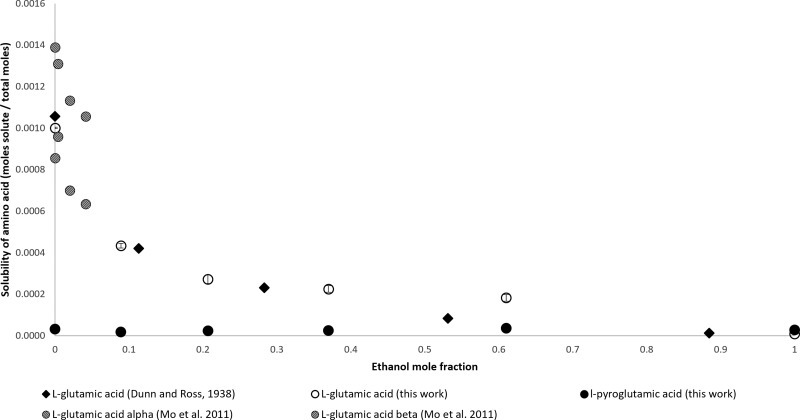
It has been shown that l-glutamic acid can form l-pyroglutamic acid in solution.53 Previous solubility studies did not take this into consideration. In Figure 4, the solubility of l-glutamic acid from Dunn and Ross, 1938, who used the gravimetric method of dissolution, is compared to the simultaneous measurement of l-glutamic acid and l-pyroglutamic acid in this work. Furthermore, the work of Mo et al. shows that the form of the crystal influences the solubility of the α-amino acid. The β-crystal forms follow the solubility data from Dunn and Ross, 1938, and this work closely. At pure water, the solubility reported by Dunn and Ross is approximately equal to the combined l-glutamic acid and l-pyroglutamic acid solubility collected experimentally in this work. At higher ethanol mole fraction, the difference between the data presented by Dunn and Ross and this work increases. The solubilities reported by Dunn and Ross fall below the standard deviation of those in this report at ethanol mole fractions above 0.370. Models on the solubility of amino acids in water and ethanol mixtures53 show that the data generated in this work, shown in Figure 4, fit better than the data by Dunn and Ross, 1938. Note should be taken that the measurements of l-pyroglutamic acid in Figure 4 are not at maximum solubility.
Figure 4.
Mole fraction solubility of l-glutamic acid.
While the l-cysteine trials were kept in a low oxygen environment, there is still the possibility that oxidation to l-cystine took place, which in turn could affect the solubility of l-cysteine. Therefore, the l-cysteine (molar mass = 121.16 g/mol) solubility trials were checked by mass spectrometer in that negligible amounts of l-cystine (molar mass = 240.3 g/mol) were formed. To accommodate for any build up on the detector of the mass spectrometer, the detector was cleaned before each measurement. In both measurements, only trace amounts of l-cystine were found, Figure S1, leading to the conclusion that the trace amounts of l-cystine do not affect the solubility data presented of l-cysteine in this work. The measured solubility data is presented in Figure 7 and discussed in the subsequent section.
Figure 7.
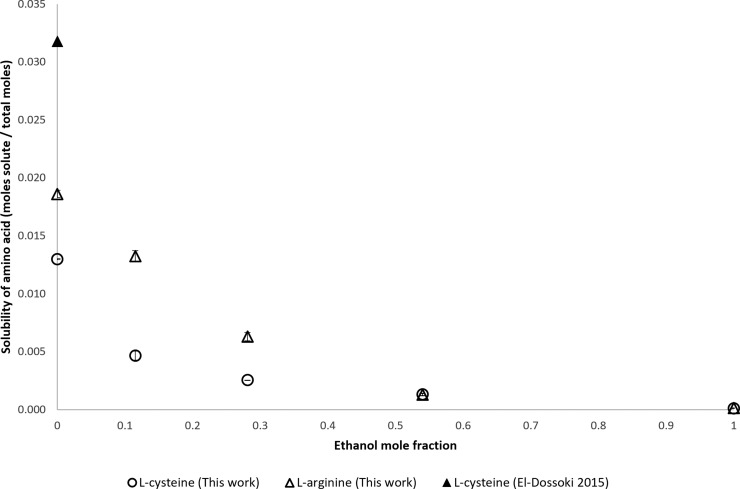
Mole fraction solubility of l-cysteine and l-arginine.
3.3. Incomplete Solubility Data of Amino Acids
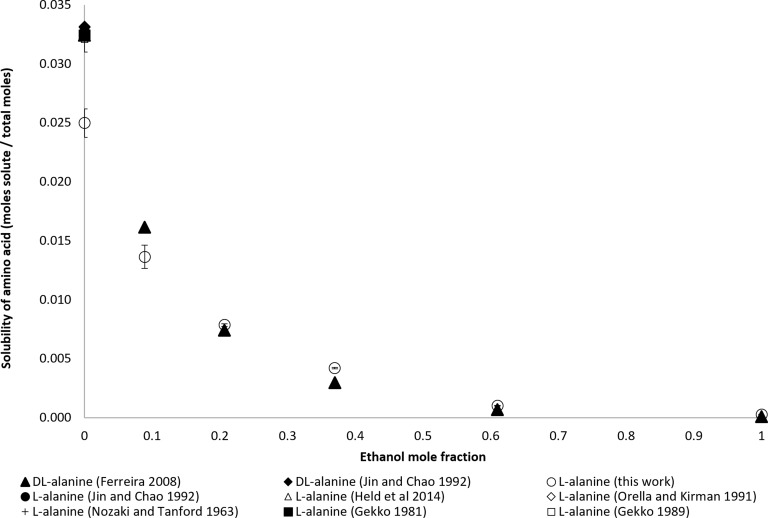
Previous work has published the solubility of dl-alanine but not l-alanine in various ethanol mole fractions. Furthermore, the reported solubilities of l-alanine in water vary widely. In Figure 5, the solubilities of the published dl-alanine, l-alanine, and the newly measured solubilities of l-alanine are compared. In water, more dl-alanine dissolved than l-alanine for all reported data. It is unclear from the literature what the individual fractions of d-alanine and l-alanine are in the dl-alanine mixture. Measured as a mixture, the dl-alanine measurements are only slightly more soluble than l-alanine alone at 0.00 and 0.100 ethanol mole fraction. This gives evidence that the chiral forms have a negative impact on the other’s solubility. At 0.200 ethanol mole fraction and higher, l-alanine has a higher solubility than the dl-alanine mixture. The solubility data of l-alanine in water, water–ethanol mixtures, and ethanol measured for this work were measured using the UPLC.
Figure 5.
Mole fraction solubility of l-alanine.
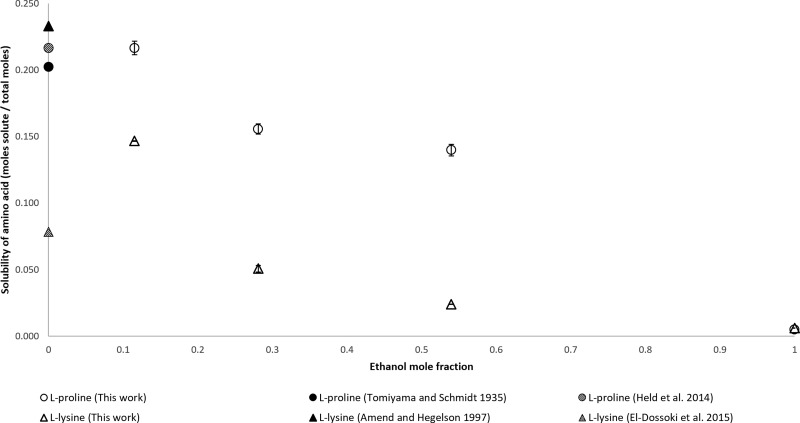
The results for l-proline and l-lysine are shown in Figure 6. Both α-amino acids have similar solubility ranges. Their solubilities are lower at higher ethanol mole fractions. The l-proline solubilities in water reported by Held et al. are similar to those reported by Tomiyama and Schmidt. The solubility of l-proline in water reported by El-Dossoki is much lower than the solubility of l-proline reported by Amend and Hegelson. Furthermore, the solubility of l-proline in water reported by El-Dossoki is lower than the solubility of l-proline measured for this work at 0.100 ethanol mole fraction.
Figure 6.
Mole fraction solubility of l-proline and l-lysine.
The solubilities of l-cysteine and l-arginine are shown in Figure 7. Also, both l-cysteine and l-arginine have similar solubility ranges, but the solubility of l-arginine decreases faster than that of l-cysteine as the ethanol mole fraction increases. The solubility of l-cysteine in water that was reported by El-Dossoki and El-Damarany is higher than the solubility measured for this work. El-Dossoki and El-Damarany do not report that their measurements were taken in a sealed, oxygen-poor environment. This could account for elevated experimentally measured solubilty due to the formation of the dimer cystine.
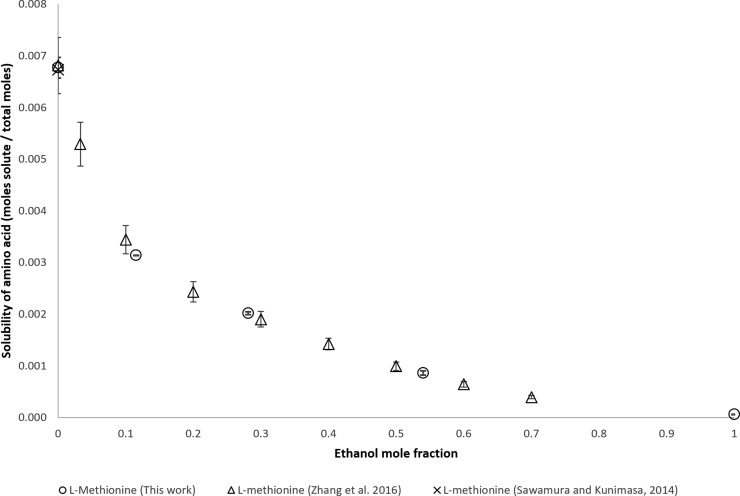
The solubility of l-methionine is shown in Figure 8 and is the α-amino acid with the lowest solubility of the α-amino acids for which new data are being presented. The measurements of Zhang et al., Sawamura and Kunimasa, and this work are similar.
Figure 8.
Mole fraction solubility of l-methionine.
4. Discussion
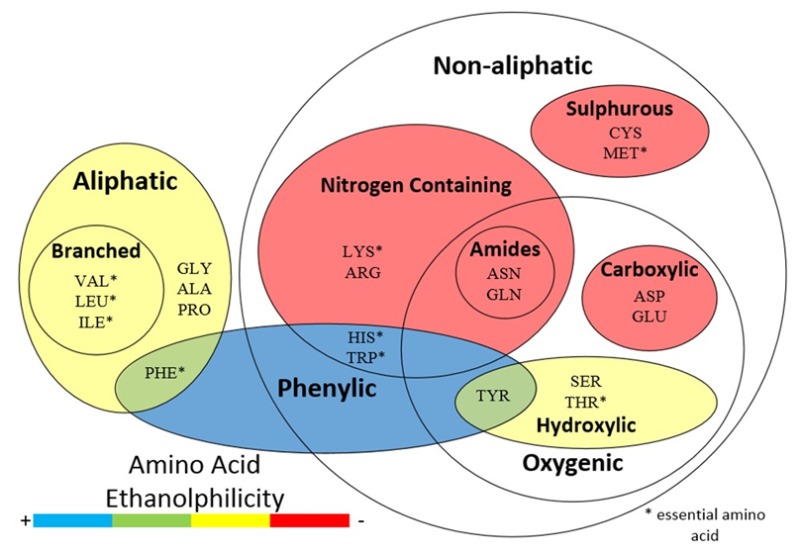
The influence of ethanol on the solubility of amino acids is not the same for all amino acids. Most amino acids have a lower solubility when their solvent is at a higher ethanol mole fraction. All amino acids have a loss in solubility above a mole fraction of 0.5. The change in solubility is not the same for all amino acids in the range of 0–0.5 mole fraction ethanol. This difference between the amino acids is most pronounced at ethanol mole fractions around 0.2. The effect of ethanol on the solubility of amino acids can be characterized by the groups found in their side chains.
Five amino acids have a ring in the side chain. These amino acids are l-tryptophan, l-tyrosine, l-proline, l-phenylalanine, and l-histidine. These rings include either phenyl, pyrrolidine, or imidazole. The amino acids with rings in the side chains had the least decrease in solubility as ethanol is added. The average decrease in solubility of these amino acids at an ethanol fraction of 0.2 was only −3.8%. In the case of l-tryptophan, the solubility was even increased by 105.6%. We hypothesize that the rings of these amino acids are ethanolphilic, while the amino and carboxylic groups on these amino acids are ethanolphobic. Moderate ethanol fractions between 0.2 and 0.4 increase the solubility of these amino acids. The water and ethanol molecules arrange themselves at the respective groups of the molecule, creating a lattice around the amino acids. Higher ethanol mole fractions lower the solubility of these amino acids, because the ethanol molecules surround the amino acid molecule and disrupt the water molecules surrounding the amino and carboxylic groups on the amino acid molecule.
The aliphatic amino acids, l-phenylalanine, l-isoleucine, l-leucine, l-alanine, l-methionine, and l-valine, show initially a low to medium decrease in solubility at an ethanol mole fraction of approximately 0.2. The decreases range from −31.0% to −71.6%. The aliphatic amino acids showed an average decrease of −54.8% solubility. l-Phenylalanine is both aliphatic and phenylic and shows a lower decrease in solubility, −33.9%, than the average for the aliphatic group in this range. This decrease could be possibly mitigated by the phenyl ring.
The hydroxyl containing amino acids, l-tyrosine, l-serine, and l-threonine, show a medium decrease in solubility. Together, l-serine and l-threonine have an average decrease of −68.5% at ethanol mole fraction levels around 0.2. l-Tyrosine, which is both hydroxylic and phenylic, has only a low decrease in solubility −1.3%. Here, as in the case of l-phenylalanine, the decrease in solubility is mitigated by the phenyl ring.
The amide containing amino acids, l-glutamine and l-asparagine, show a high decrease in solubility of −75.1 and −77.4%, respectively, at ethanol mole fraction around 0.2. The average for amide containing amino acids increases slightly to −72.9% when l-arginine is added to this group. l-Arginine contains an amide group and is positively charged; it is added to this group.
A high decrease in solubility is seen in the charged amino acids l-glutamic acid, l-aspartic acid, and l-lysine. The average decrease at an ethanol mole fraction of 0.2 was −78.1%. The lower decrease in solubility of the charged amino acids l-histidine (−66.1%) and l-arginine (−66.1%) seemed to be mitigated by either their ring-containing side chain, imidazole, or amide, respectively.
l-Cysteine is the only amino acid that contains sulfur. It has the highest decrease in solubility at ethanol mole fraction of 0.2. The decrease was −80.4%. l-Methionine also contains a sulfur molecule but is normally considered aliphatic. The l-methionine solubility decrease is −70.2%.
Glycine, containing no side chain, had the largest decrease in solubility. Glycine solubility decrease by −83.9% at an ethanol mole fraction around 0.2.
5. Conclusion
For most α-amino acids, the gravimetric, ninhydrin, and UPLC methods produced similar solubility data. Exceptions to this are α-amino acids that convert to other forms (l-glutamic acid and l-cysteine) and α-amino acids at very low solute concentrations (e.g., in pure ethanol).
The two α-amino acids that are the exceptions in the previous paragraph are l-glutamic acid, which has been shown to convert to l-pyroglutamic acid, and l-cysteine, which can convert to the dimer cystine. Therefore, all amino acids that have a possibility to convert to other amino acids in solution should be analyzed by a technique that takes this into account. The UPLC technique used in this work shows reliable results.
At low concentrations (e.g., α-amino acids in pure ethanol) using gravimetric analytical techniques to measure amino acid solubility is not always reliable. However, the UPLC method used in this work was reliable at low concentrations. The data produced by the UPLC were also within the variation of the data published by Ferreira, 2008. Ferreira used the ninhydrin method of analysis. While the ninhydrin method produced data consistent to data in this work for several α-amino acids, it is not able to detect and differentiate multiple amino acids in solution, as does the UPLC method.
Most data points of several amino acids by Nozaki et al. (1971) and the data for Cao et al. for glycine were higher when compared to those in this work and those in the work of Ferreira (2008), Dalton and Schmidt (1933), and Needham (1970). A possible explanation for these results includes, but is not limited to, the samples being measured when the solutions were oversaturated or when dissolved from a crystal of another shape (e.g., α-crystal versus β-crystal) as shown by Mo et al.
This work gives a new more complete look at the solubility of all 20 proteinogenic α-amino acids. The new data published in this work doubles the peer-reviewed data on α-amino acid solubility in water, water/ethanol, and ethanol systems. This data is the first published data in ethanol and ethanol/water systems for l-alanine, l-proline, l-arginine, l-cysteine and l-lysine solubility. Furthermore, this work gives the first data for the solubility of l-asparagine, l-glutamine, l-histidine and l-leucine in pure ethanol.
Lastly, the side chain of an amino acid has an effect on the solubility of that amino acid when ethanol is added. This is shown at ethanol mole fractions around 0.2. Side chains containing rings show the least decrease in solubility when water is replaced by a water–ethanol mixture due to the ethanolphilic properties of these rings. This is followed in descending order by the aliphatic amino acids, hydroxyl containing amino acids, amide containing amino acids, charged amino acids, sulfur containing amino acids, and the amino acid with no side chain. Amino acids with side chains of two characteristics, such as l-tyrosine, which is both phenylic and containing a hydroxyl group, show a decrease in solubility in between both of their groups.
Acknowledgments
M.E.B. acknowledges the public-private partnership ‘Kleinschalige Bioraffinage’ that is supported by the Dutch Top Sector Agri & Food.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jced.7b00486.
Mass spectrophotometer results from the l-cysteine trial (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Scott E.; Peter F.; Sanders J. Biomass in the Manufacture of Industrial Products – The use of Proteins and Amino Acids. Appl. Microbiol. Biotechnol. 2007, 75, 751–762. 10.1007/s00253-007-0932-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammens T. M.; Franssen M. C. R.; Scott E. L.; Sanders J. P. M. Availability of protein-derived amino acids as feedstock for the production of bio-based chemicals. Biomass Bioenergy 2012, 44, 168–181. 10.1016/j.biombioe.2012.04.021. [DOI] [Google Scholar]
- Lammens T. M., Bio-based Industrial Chemicals from Glutamic Acid. Ph.D. Thesis, Wageningen University Press, Wageningen, The Netherlands, 2011. [Google Scholar]
- Lammens T. M.; Potting J.; Sanders J. P. M.; De Boer I. J. Environmental comparison of biobased chemicals from glutamic acid with their petrochemical equivalents. Environ. Sci. Technol. 2011, 45, 8521–8. 10.1021/es201869e. [DOI] [PubMed] [Google Scholar]
- Jin X. Z.; Chao K.-C. Solubility of four amino acids in water and of four pairs of amino acids in their water solutions. J. Chem. Eng. Data 1992, 37, 199–203. 10.1021/je00006a016. [DOI] [Google Scholar]
- Matsuo H.; Suzuki Y.; Sawamura S. Solubility of -amino acids in water under high pressure: glycine, -alanine, -valine, -leucine, and - isoleucine. Fluid Phase Equilib. 2002, 200, 227–237. 10.1016/S0378-3812(02)00029-8. [DOI] [Google Scholar]
- Held C.; Reschke T.; Muller R.; Kunz W.; Sadowski G. Measuring and modeling aqueous electrolyte/amino-acid solutions with ePC-SAFT. J. Chem. Thermodyn. 2014, 68, 1–12. 10.1016/j.jct.2013.08.018. [DOI] [Google Scholar]
- El-Dossoki F.; El-Damarany M. Solvation of Basic and Neutral Amino Acids in Aqueous Electrolytic Solutions: Measurements and Modeling. J. Chem. Eng. Data 2015, 60, 2989–2999. 10.1021/acs.jced.5b00393. [DOI] [Google Scholar]
- Sawamura S.; Kunimasa N. High-Pressure Solubility of L-Methionine in Water. J. Solution Chem. 2014, 43, 1810–1815. 10.1007/s10953-014-0242-8. [DOI] [Google Scholar]
- Lu J.; Lin Q.; Rohani S.; Li Z. Solubility of L-Phenylalanine Anhydrous and Monohydrate Forms: Experimental Measurements and Predictions. J. Chem. Eng. Data 2012, 57, 1492–1498. 10.1021/je201354k. [DOI] [Google Scholar]
- Lu J.; Wang X.-J.; Yang X.; Ching C.-B. Solubilities of Glycine and Its Oligopeptides in Aqueous Solutions. J. Chem. Eng. Data 2006, 51, 1593–1596. 10.1021/je0600754. [DOI] [Google Scholar]
- Mo Y.; Dang L.; Wei H. Solubility of alpha-form and beta-form of L-glutamic acid in different aqueous solvent mixtures. Fluid Phase Equilib. 2011, 300, 105–109. 10.1016/j.fluid.2010.10.020. [DOI] [Google Scholar]
- Zhou X.; Fan J.; Li N.; Du Z.; Ying H.; Wu J.; Xiong J.; Bai J. Solubility of L-phenylalanine in water and different binary mixtures from 288.15 to 318.15 K. Fluid Phase Equilib. 2012, 316, 26–33. 10.1016/j.fluid.2011.08.029. [DOI] [Google Scholar]
- Seidell A.; Linke W. F.. Solubility of Inorganic and Organic Compounds, 3rd ed.; Van Nostrand Co.: New York, 1952. [Google Scholar]
- Pradhan A. A.; Vera J. H. Effect of acids and bases on the solubility of amino acids. Fluid Phase Equilib. 1998, 152, 121–132. 10.1016/S0378-3812(98)00387-2. [DOI] [Google Scholar]
- Carta R.; Tola G. Solubilities of L-cystine, L-tyrosine, L-leucine, and glycine in aqueous solutions at various pHs and NaCl concentrations. J. Chem. Eng. Data 1996, 41, 414–417. 10.1021/je9501853. [DOI] [Google Scholar]
- Orella C. J.; Kirwan D. J. Correlation of amino acid solubilities in aqueous aliphatic alcohol solutions. Ind. Eng. Chem. Res. 1991, 30, 1040–1045. 10.1021/ie00053a028. [DOI] [Google Scholar]
- Gekko K. Mechanism of polyol-induced protein stabilization: solubility of amino acids and diglycine in aqueous polyol solutions. J. Biochem. 1981, 90, 1633–1641. 10.1093/oxfordjournals.jbchem.a133638. [DOI] [PubMed] [Google Scholar]
- Gekko K.; Idota Y. Acid Solubility and Protein Stability in Aqueous malitol Solutions Agric. Agric. Biol. Chem. 1989, 53, 89–90. 10.1271/bbb1961.53.89. [DOI] [Google Scholar]
- Nozaki Y.; Tanford C. T. The Solubility of Amino Acids and Related Compounds in Aqueous Urea Solutions. J. Biol. Chem. 1963, 238, 4074–4081. [PubMed] [Google Scholar]
- Nozaki Y.; Tanford C. T. The solubility of amino acids and related compounds in aqueous ethylene glycol solutions. J. Biol. Chem. 1965, 240, 3568–3573. [PubMed] [Google Scholar]
- Dalton J. B.; Schmidt C. L. A. The solubilities of certain amino acids in water, the densities of their solutions at twenty-five degrees, and the calculated hears of solution and partial molal volumes. J. Biol. Chem. 1933, 103, 549–578. [Google Scholar]
- Dunn M. S.; Ross F. J.; Read L. S. The solubility of the amino acids in water. J. Biol. Chem. 1933, 103, 579–595. [Google Scholar]
- Cohn E. J.; McMeekin T. J.; Edsall J. T.; Weare J. H. Studies in the Physical Chemistry of Amino Acids, Peptides and Related Substances. II. The Solubility of α-Amino Acids in Water and in Alcohol—Water Mixtures. J. Am. Chem. Soc. 1934, 56, 2270–2282. 10.1021/ja01326a019. [DOI] [Google Scholar]
- Gekko K.; Ohmae E.; Kameyama K.; Takagi T. Acetonitrile-protein interactions: amino acid solubility and preferential solvation. Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol. 1998, 1387, 195–205. 10.1016/S0167-4838(98)00121-6. [DOI] [PubMed] [Google Scholar]
- Jeliñska-Kazimierczuk M.; Szycłowski J. Solubility of α-amino acids in water under high pressure: glycine, l-alanine, l-valine, l-leucine, and l-isoleucine. J. Solution Chem. 1996, 25, 1175–1184. 10.1007/BF00972645. [DOI] [Google Scholar]
- Carta R.; Tola G. Solubilities of l-Cystine, l-Tyrosine, l-Leucine, and Glycine in Aqueous Solutions at Various pHs and NaCl Concentrations. J. Chem. Eng. Data 1996, 41, 414–417. 10.1021/je9501853. [DOI] [Google Scholar]
- Soto A.; Arce A.; Khoshkbarchi M. K.; Vera J. H. Measurements and modelling of the solubility of a mixture of two amino acids in aqueous solutions. Fluid Phase Equilib. 1999, 158–160, 893–901. 10.1016/S0378-3812(99)00087-4. [DOI] [Google Scholar]
- Hlynka I. Thesis, California Institute of Technology, Pasadena, CA, 1939. [Google Scholar]
- Stoddard M. P.; Dunn M. S. Quantitative investigations of amino acids and peptides. J. Biol. Chem. 1942, 142, 329–343. [Google Scholar]
- Carta R. Solubilities of l-Cystine, l-Tyrosine, l-Leucine, and Glycine in Their Water Solutions. J. Chem. Thermodyn. 1998, 30, 379–387. 10.1006/jcht.1997.0313. [DOI] [Google Scholar]
- McMeekin T. L.; Cohn E. J.; Weare J. H. Studies in the Physical Chemistry of Amino Acids, Peptides and Related Substances. III. The Solubility of Derivatives of the Amino Acids in Alcohol—Water Mixtures. J. Am. Chem. Soc. 1935, 57, 626–633. 10.1021/ja01307a010. [DOI] [Google Scholar]
- Dunn M. S.; Ross F. J. Quantitative Investigations of Amino Acids and Peptides: IV. The Solubilities of the Amino Acids in Water-Ethyl Alcohol Mixtures. J. Biol. Chem. 1938, 125, 309–332. [Google Scholar]
- Needham T. E.; Paruta A. N.; Gerraugh Rj Solubility of Amino Acids in Pure Solvent Systems. J. Pharm. Sci. 1971, 60, 565. 10.1002/jps.2600600410. [DOI] [PubMed] [Google Scholar]
- Nozaki Y.; Tanford C. The Solubility of Amino Acids and Two Glycine Peptides in Aqueous Ethanol and Dioxane Solutions: Establishment of a hydrophobicity scale. J. Biol. Chem. 1971, 246, 2211–2217. [PubMed] [Google Scholar]
- Conio G.; Curletto L.; Patrone E. Temperature coefficient of solubility of some clycyl peptides in water-ethanol mixtures. J. Biol. Chem. 1973, 248, 5448–5450. [PubMed] [Google Scholar]
- Cao Z.; Hu Y.; Li J.; Kai Y.; Yang W. Solubility of glycine in binary system of ethanol + water solvent mixtures: Experimental data and thermodynamic modeling. Fluid Phase Equilib. 2013, 360, 156–160. 10.1016/j.fluid.2013.09.013. [DOI] [Google Scholar]
- Zhang T.; Li Z.; Wang Y.; Li C.; Yu B.; Zheng X.; Jiang L.; Gong J. Determination and correlation of solubility and thermodynamic properties of L-methionine in binary solvents of water + (methanol, ethanol, acetone). J. Chem. Thermodyn. 2016, 96, 82–92. 10.1016/j.jct.2015.12.022. [DOI] [Google Scholar]
- Yu Q.; Ma X.; Xu L. Solubility, dissolution enthalpy and entropy of L-Glutamine in mixed solvents of ethanol+water and acetone+water. Thermochim. Acta 2013, 558, 6–9. 10.1016/j.tca.2013.02.007. [DOI] [Google Scholar]
- Liu Y.; Wang Y.; Liu Y.; Xu S.; Chen M.; Du S.; Gong J. Solubility of L-histidine in different aqueous binary solvent mixtures from 283.15 to 318.15 K with experimental measurement and thermodynamic modelling. J. Chem. Thermodyn. 2017, 105, 1–14. 10.1016/j.jct.2016.09.039. [DOI] [Google Scholar]
- Gude M. T.; Meuwissen H. H. J.; van der Wielen L. A. M.; Luyben K. C. A. M. Partition Coefficients and Solubilities of α-Amino Acids in Aqueous 1-Butanol Solutions. Ind. Eng. Chem. Res. 1996, 35, 4700–4712. 10.1021/ie960031w. [DOI] [Google Scholar]
- vanBerlo M.; Gude M. T.; vanderWielen L. A. M.; Luyben K. Partition coefficients and solubilities of glycine in the ternary solvent system 1-butanol plus ethanol plus water. Ind. Eng. Chem. Res. 1997, 36, 2474–2482. 10.1021/ie960762w. [DOI] [Google Scholar]
- Gude M. T.; van der Wielen L. A. M.; Luyben K. C. A. M. Phase behavior of α-amino acids in multicomponent aqueous alkanol solutions. Fluid Phase Equilib. 1996, 116, 110–117. 10.1016/0378-3812(95)02878-1. [DOI] [Google Scholar]
- Widyarani; Bowden N. A.; Sanders J. P. M.; Kolfschoten R. C.; Bruins M. E. Fractional precipitation of amino acids from agro-industrial residues using ethanol. Ind. Eng. Chem. Res. 2016, 55, 7462–7472. 10.1021/acs.iecr.6b00054. [DOI] [Google Scholar]
- Ferreira L. A.; Macedo E. A.; Pinho S. P. Solubility of amino acids and diglycine in aqueous–alkanol solutions. Chem. Eng. Sci. 2004, 59, 3117–3124. 10.1016/j.ces.2004.05.001. [DOI] [Google Scholar]
- Ferreiraa L. A.; Pinho S. P.; Macedo E. A. Solubility of L-serine, L-threonine and L-isoleucine in aqueous aliphatic alcohol solutions. Fluid Phase Equilib. 2008, 270, 1–9. 10.1016/j.fluid.2008.05.008. [DOI] [Google Scholar]
- Fuchs D.; Fischer J.; Tumakaka F.; Sadowski G. Solubility of amino acids: influence of the pH value and the addition of alcoholic cosolvents on aqueous solubility. Ind. Eng. Chem. Res. 2006, 45, 6578–6584. 10.1021/ie0602097. [DOI] [Google Scholar]
- Grosse Daldrup J. B.; Held C.; Ruether F.; Schembecker G.; Sadowski G. Measurement and Modeling Solubility of Aqueous Multisolute Amino-Acid Solutions. Ind. Eng. Chem. Res. 2010, 49, 1395–1401. 10.1021/ie900913c. [DOI] [Google Scholar]
- Held C.; Sadowski G.; Carneiro A.; Rodriguez O.; Macedo E. A. Modeling thermodynamic properties of aqueous single-solute and multi-solute sugar solutions with PC-SAFT. AIChE J. 2013, 59, 4794–4805. 10.1002/aic.14212. [DOI] [Google Scholar]
- Ji P.; Zou J.; Feng W. Effect of alcohol on the solubility of amino acid in water. J. Mol. Catal. B: Enzym. 2009, 56, 185–188. 10.1016/j.molcatb.2008.06.008. [DOI] [Google Scholar]
- Zhou X.; Fan J.; Li N.; Du Z.; Ying H.; Wu J.; Xiong J.; Bai J. Solubility of L-phenylalanine in water and different binary mixtures from 288.15 to 318.15 K. Fluid Phase Equilib. 2012, 316, 26–33. 10.1016/j.fluid.2011.08.029. [DOI] [Google Scholar]
- Bowden N. A.; Sevillano D. M.; Sanders J. P. M.; Bruins M. E. Modelling the effects of ethanol on the solubility of the proteinogenic amino acids with the NRTL, Gide and Jouyban-Acree models. Fluid Phase Equilib. 2018, 459, 158. 10.1016/j.fluid.2017.11.036. [DOI] [Google Scholar]
- Meussen B.; van Zeeland A. T.; Bruins M. E.; Sanders J. P. M. A Fast and Accurate UPLC Method for Analysis of Proteinogenic Amino Acids. Food Anal. Methods 2014, 7, 1047–1055. 10.1007/s12161-013-9712-7. [DOI] [Google Scholar]
- Kendall E. C.; Nord F. F. Reversible Oxidation-Reduction Systems of Cysteine-Cystine and Reduced and Oxidized Glutathione. J. Biol. Chem. 1926, 69, 295–337. [Google Scholar]
- Kumar A.; Bachhawat A. K. Pyroglutamic acid: throwing light on a lightly studied metabolite. Curr. Sci. 2012, 102, 288–297. [Google Scholar]
- Haitinger L. Vorlauge Mittheilung uber Glutaminsaure und Pyrrol. Monatsh. Chem. 1882, 3, 228–229. 10.1007/BF01516801. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.