Synopsis
In older adults, pathophysiologic, clinical, and environmental factors all affect the presentation of infections. We explore how age-related changes influence the manifestation and evaluation of infections in this population. Specific topics include immunosenescence, age related organ-specific physiologic changes, and frailty. We also describe clinical factors influencing infection risk and presentation in older adults including temperature regulation, cognitive decline, and malnutrition. Finally, we discuss the influence of the setting in which older adults reside on the clinical evaluation of infection. Understanding the influence of all these changes may facilitate the prevention, early recognition and treatment of infections in older adults.
Keywords: Older adults, immunosenescence, frailty, thermoregulation, malnutrition
Introduction
The topic of human longevity has invited extensive scientific and philosophic debate. Haller, a prominent Swiss physiologist of the 18th century, thought that people ought to live to 200 years. Buffon, an 18th century French naturalist, was of the opinion that when someone did not die from some accident or disease they would reach 90 or 100 years.1 Nobel laureate Élie Metchnikoff, arguably the father of modern immunology and gerontology, found it “impossible to accept the view that the high mortality between the ages of 70 and 75 indicates a natural limit of human life”. In his 1907 book, The Prolongation of Life, he equated aging to a disease process that can be studied and possibly cured until death inevitably settles in from “natural causes”. He suggested that similar to the instinct of sleep, there could be an instinct of death that is neither due to diseases nor accidental, rather the result of age-related physiologic changes. He thought these changes were the result of self-digesting macrophages and poisoning by intestinal microbiota.1 While this theory has been disproved, his contributions to immunology and gerontology were groundbreaking and continue to shape our understanding of infectious processes in older adults.
A testament to the contributions by these pioneers in the field of gerontology, people are living longer such that the number of older adults is rapidly increasing, both in the United States (U.S.) and globally. In 2015, approximately 617 million people were ≥ 65 years, representing 8.5% of the 7.3 billion people worldwide.2 Projections estimate that by 2050, approximately 1.6 billion people will be ≥ 65 years, with the proportion nearly doubling to 16.6% of the total world population. In the U.S. the proportion of people projected to be ≥ 65 years by 2050 will constitute over 20% of our total population.2 Bartels and Naslund famously described this demographic trend as the “silver tsunami”.3 Understanding the process of aging and how it influences the clinical presentation of diseases in general, and infectious diseases in particular, is a necessity for modern practitioners.
Aging changes the risk of and the clinical presentation of infection. This is due to factors intrinsic to individuals fortunate enough to age and to the environment in which they reside. Intrinsic factors include age-related physiologic changes, which can sometimes result in frailty, a pathologic state. Some age-related changes also influence the clinical manifestation of infection, presenting as alterations in temperature regulation, cognitive decline and malnutrition.4 Environmental factors also play a role, particularly those related to the living situation (e.g. nursing home), and the healthcare setting to which older adults present. In combination, these factors make it difficult for healthcare workers to determine whether changes in clinical status are due to infection. This may contribute to a low threshold for prescribing systemic antimicrobials, which in turn increases older adults’ risk for acquiring multi-drug resistant organisms (MDRO) and Clostridium difficile.
Here we review age-related physiologic changes that may progress to frailty; these include both immune and organ-specific changes. We also address clinical factors that influence the manifestation of infections in older adults. Finally, we consider the influence of the external environment on the presentation and evaluation of infections in older adults with consideration of the subjective roles and perspectives of caregivers within different settings.
1. Pathophysiologic factors influencing infection risk and presentation in older adults
With aging, physiologic changes occur that affect the immune system as well as various organ systems. Aging itself is not a disease, yet as time passes, the accumulation of such changes can sometimes lead to a clinical condition in older adults known as frailty. In this section, we discuss these changes and introduce the concept of frailty.
1.1. Age-related immune changes
Gavazzi and Krause describe immunosenescence as “an age-related dysfunction of the immune system which leads to enhanced risk of infection”.5 This phenomenon is an area of active research and encompasses a large body of evidence. Globally, the total number of immune cells does not decrease with aging, but studies demonstrate a functional decline in both innate immunity and adaptive immunity that encompasses cell mediated and humoral immunity.
Changes in innate immunity include reduced phagocytic activity of neutrophils, macrophages, and natural killer cells.5–7 This is accompanied by upregulation of a number of pro-inflammatory cytokines including IL-6, C-reactive protein, tumor-necrosis factor-α (TNFα), and CXC chemokine ligand-10.6,8–10 This increase in cytokine and chemokine production results in a heightened chronic pro-inflammatory state in older adults that may contribute to the development of infection and other diseases (e.g. atherosclerosis, arthritis, diabetes mellitus etc.).11,12 Franceschi et al. referred to this chronic pro-inflammatory state as “inflammaging”,13 which can result in anorexia, nutritional compromise, muscle weakness and weight loss, all of which could be presentations of infection in older adults but also represent characteristics of frailty, as discussed below.5,6 As such, the distinction between the clinical condition of frailty and presentation of infection becomes challenging, particularly for healthcare workers who encounter an individual patient for the first time in an acute care setting. Knowledge of older adults’ clinical baseline is therefore of great benefit when evaluating a suspected infectious process in this population.
One of the changes in cell-mediated immunity is the decline in the proportion of naïve T cells with aging. This occurs as a consequence of thymus involution and an increase in the proportion of circulating memory T cells in the setting of continued antigenic stimulation. This increase in the number of memory T cells is offset by their restricted clonal diversity (Figure).12,14–16 These changes in turn limit the antibody response to foreign antigens due to reduced regulatory control of T cells on B cells. Interestingly, Van Epps et al. recently demonstrated in vitro that although naïve T cells are reduced in number in older adults, they had enhanced functional ability, mainly the CD8+ T cells.17 The clinical relevance of these findings is unclear at this time but this increased functionality may also contribute, along with cytokine upregulation, to “inflammaging”. Some chronic infections also contribute to generalized chronic inflammation and through accumulation of damage to host cells, hasten the aging process.5 The best investigated example is HIV which results in accelerated aging. Gross et al. have demonstrated that HIV-infected individuals on sustained antiretroviral therapy have an epigenetic age about 5 years older than healthy controls.18 Smith et al. proposed that in addition to inflammation caused by HIV, adverse effects of antiretroviral drugs, specifically those that affect the mitochondria, also contribute to the accelerated aging of treated HIV-infected patients.19 Viruses other than HIV, namely CMV and HSV, also appear to correlate with premature immune aging5. Given that most individuals do not receive antiviral therapy for these viruses, the implication is that these viral infections directly contribute to this phenomenon.5
Figure.
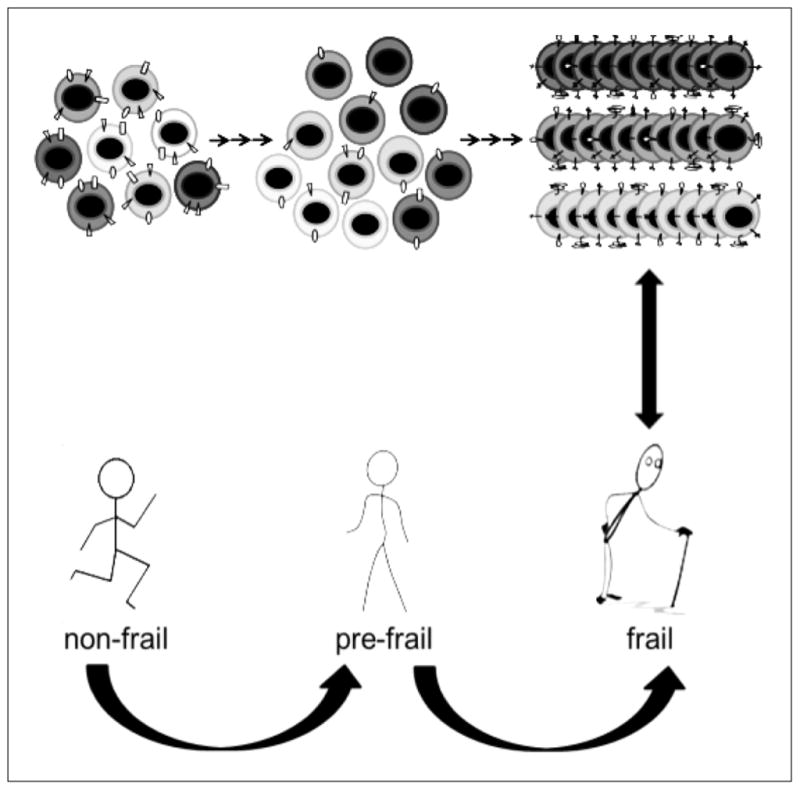
Antigen-specific clonal expansion of T-cells in older adults leads to an increased proportion of replicative senescent cell populations, filling the immunological space and resulting in decreased repertoire diversity.
As a result of the changes to adaptive and innate immune responses, while the incidence of severe infections is higher in older adults, the protective effect elicited by vaccines is lower.12 This is the case for influenza,20,21 hepatitis B,22 and pneumococcal vaccines,23 supporting the idea that vaccination in older adults is associated with modest clinical effectiveness.
Finally, it is important to appreciate that immunosenescence and chronic inflammation are gradual, relentless processes. Their clinical impact may not be fully apparent until progression to frailty. Coupled with other factors such as comorbidities and declining functional status, frailty results in increased morbidity and mortality, including from infection.
1.2. Age related organ-specific physiologic changes
In addition to immunosenescence, aging also causes physiologic changes that affect nearly every organ system, independently of existing co-morbidities and disease. This process is the result of lifelong accumulation of molecular and cellular damage caused by a number of mechanisms regulated by a complex maintenance and repair network.6,24 Described in Table 1, these changes include structural transformations, altered anatomy, and decreased function in multiple physiologic systems, as well as loss of feedforward and feedback mechanisms between interacting systems.11,25 The resulting constellation of physiologic changes results in progressive homeostatic dysregulation and may contribute to vulnerability to infections.6,25
Table 1.
Age-related organ-specific physiologic changes that increase the risk of infection and affect the clinical presentation of infectious syndromesa
| Organ system | Physiologic changes |
|---|---|
| Urinary | - Mechanical changes: reduction in bladder capacity, uninhibited contractions, decreased urinary flow rate and post-void residual volume - Urothelial change: enhanced bacterial adherence - Bladder prolapse in women and prostatic disease in men increase urinary stasis - Diminished estrogen in post-menopausal women |
| Pulmonary | - Blunting of cough and other reflexes that protect airway - Decreased mucociliary clearance, lung elasticity, chest compliance and respiratory muscle strength - Decreased immunoglobulin in respiratory secretion |
| Skin and soft tissue | - Loss of subcutaneous tissue - Loss of collagen from the dermis and slower wound healing - Reduction in the size of blood vessels in the dermis impairs delivery of immune cells - Dry skin resulting from diminished water-binding capacity of the stratum corneum - Flattened dermal-epidermal junctions and reduced dermal-epidermal adhesion - |
| Gastrointestinal | - Decreased saliva production and alterations in antimicrobial proteins in the saliva - Decreased tongue strength and slower swallowing - Decreased gastric acidity (mucosal gastric atrophy, proton pump inhibitors, surgery) - Decreased intestinal motility - Modifications of resident intestinal flora (protective Bifidobacteria and anaerobes decrease, Enterobacteriaceae increase) - Slow recovery of the gut microbiome following antimicrobial use in older adults |
| Central nervous system | - Structural and functional changes to microglial cells (resident immune cell population of the CNSb and CNS equivalents of macrophages) |
| Endocrine system | - Gradual rise in cortisol release with age and increased catabolism with resultant anorexia, weight loss, reduced energy expenditure, and decreased muscle mass (all components of frailty) |
| Musculoskeletal system | - Sarcopenia (loss of skeletal muscle mass, often catabolism-induced following acute disease events) leads to decreased strength and functionality |
Sources: Gavazzi and Krause (2002), Htwe et al. (2007), Amella (2006), Clegg et al. (2013), Norman (2009), and Cruz-Jentoft et al. (2010)
CNS; Central Nervous System
An example is the increased risk of pneumonia in older adults. The strength of respiratory muscles, compliance of the chest wall, and static elastic recoil all progressively decline with aging, making the lungs less resistant to environmental insults such as infectious agents and pollutants.26,27 Older adults are also at an increased risk of aspiration pneumonia. Aging is associated with a higher incidence of dysphagia which can lead to aspiration as a result of misdirection of oropharyngeal secretions with a high bacterial load or gastric material into the lower respiratory tract.11 Decreased respiratory muscle strength and cough reflexes increase the risk of aspiration.
Skin and skin structure infections offer a second example of organ-specific changes contributing to an increase risk of infection. By age 70, about 70% of people have at least one underlying skin problem, such as keratoses, dermatitides, pressure sores etc. Physiologic changes that occur with age contribute to skin fragility. Reduction in the size of blood vessels in the dermis impairs delivery of immune cells, and loss of collagen from the dermis results in thinner skin. Beyond these physiologic changes, older adults may also have pathologic conditions such as edema or trauma that further impair the integrity of the skin. All of these changes place older adults at a higher risk for skin and skin structure infections.28
In summary, consideration of how age-related organ-specific physiologic changes may alter the clinical presentation of infections may not only improve early recognition of infection in older adults, but may also help to discriminate conditions and changes not related to infection.11,29
1.3. Frailty
Frailty affects 13% to 28% of older adults and up to one-third of those 80 years and older.30,31 Fried et al. defined a frequently adopted phenotypic definition of frailty as the presence of three or more of the following readily identifiable characteristics: unintended weight loss, exhaustion, weakness, slow gait speed and low physical activity.29,32 Other clinical features include falls, delirium, and fluctuating disability, all of which may overlap with presentation of infections in the older adults. While several definitions of frailty exist, the criteria proposed by Fried et al., which are based on phenotypic features, are the most feasible to assess clinically. That said, frailty extends beyond an apparent physical phenotype to include functional and cognitive statuses of the individual, as well as the setting in which they evolve or present.29 Additionally, continuous low intensity inflammatory processes, detectable by moderately elevated levels of inflammatory cytokines, may also contribute to frailty.10,11,30
Differentiation of the physiologic changes that accompany aging, the clinical entity of frailty, and the baseline clinical features of individual subjects may help improve recognition of manifestations of infection in older adults. Notwithstanding the aging process and the resulting decline in various organ systems, these systems are resilient and do not immediately fail, owing to the gradual nature of that decline and to redundancy that provides for significant physiologic reserve.6 This reserve is unfortunately not inexhaustible and a threshold is eventually reached beyond which vulnerability for subsequent morbidity and mortality becomes significant, triggered even by minor stressors.6,29 Fried et al. showed that the likelihood of frailty increases nonlinearly in relationship to the number of abnormal physiologic systems, and that the number of abnormal systems is more predictive of frailty than any of the individual affected systems.25 This aggregate loss results in frailty which in turn is associated with increased morbidity and mortality.
2. Clinical factors influencing infection risk and presentation in older adults
2.1. Temperature regulation
The evidence suggesting a lower baseline temperature and fever suppression in older adults, albeit mitigated by concerns over measurement difficulties, is unanimous in showing that mean body temperature decreases with age.33–36 A recent analysis of cross-sectional data from more than 18,000 adults shows a difference of 0.3°F between the oldest and youngest groups after controlling for sex, body mass index and white blood cell count.37 This is commensurate with the adage, “the older, the colder”.38
Fever, or at least an elevation of temperature over baseline, occurs in most cases of infection in older adults.33 In a landmark study of mostly male veteran nursing home residents, Castle et al. showed that a single temperature reading of 101°F (38.3°C) had a sensitivity of 40% for predicting infection, compared to 70% when lowered to 100°F (37.8°C), while maintaining a specificity of 90%.39 Studies of specific syndromes confirm this observation and show a blunted fever response in older compared to younger adults in the contexts of bloodstream infections, endocarditis, meningitis, intraabdominal infection, nosocomial infections, and tuberculosis.34 It is important to recognize that a robust fever of 38.3°C or higher in a geriatric patient is indicative of a serious infection and needs to be promptly addressed.40
The definition of fever is relative and depends in part on the population for which it is intended (Box 1), making its recognition challenging for caregivers.41,42 One of the criteria offered by the Infectious Disease Society of America is an increase in temperature of >2°F (1.1°C) over the baseline temperature4. The relative temperature change indicative of infection will vary among individuals. Accordingly, knowledge of an older adult’s baseline temperature, such as might be available in electronic health records, may help caregivers ascertain if a change in temperature truly indicates a fever.
Box 1. Fever definitions by the Infectious Diseases Society of America (IDSA) 4,42.
Fever in patients with neutropenia (one of the following)
Oral temperature measurement of >101°F (38.3°C)
Temperature of >100.4°F (38.0°C) sustained over a one-hour period.
Fever in older adult residents of long-term care facilities (one of the following)
Single oral temperature >100°F (>37.8°C)
Repeated oral temperatures >99°F (>37.2°C) or rectal temperatures >99.5°F (>37.5°C)
An increase in temperature of >2°F (1.1°C) over the baseline temperature.
Reasons for decreased body temperature in older adults reflect thermoregulatory changes in cutaneous vasomotor and sudomotor responses. Other physiologic mechanisms responsible for temperature variations in older adults remain unclear.33,36 Pervious studies indicate that they experience alterations in the circadian temperature rhythms, with flatter and earlier phased rhythms.43,44 In the context of infection, animal data shows that quantitative and qualitative abnormalities occur in the production and response to peripheral endogenous mediators, such as IL-1, IL-6 and TNFα, induced by bacterial products, such as lipopolysaccharide.33,45–47 Furthermore, peripheral endogenous mediators are unable to cross the blood-brain barrier to exert their effect on the central nervous system,33,46 resulting in a blunted fever response in older adults.
Finally, some evidence suggests that lower body temperature might confer a survival advantage in humans. Analysis from the Baltimore Longitudinal Study of Aging over 25 years of follow up showed that men with body temperatures below the median had significantly higher survival rates than those with body temperatures above the median temperature.37,48 Nonetheless, mounting a fever in the context of infection is part of overall health defense and the absence of fever in response to a serious infection is a poor prognostic sign.34,49,50
2.2 Cognitive decline
Cognitive decline in older adults encompasses a clinical spectrum ranging from mild cognitive impairment to overt dementia. Memory deficits but intact activities of daily living and preserved general cognitive function characterize mild cognitive impairment. Overt dementia is manifested by progressive deterioration in cognitive ability and in capacity for independent living.51,52 Here we will focus on overt dementia because infections are often exceptionally difficult to ascertain in this population.
The global burden of dementia in adults 60 years of age or older is estimated at 5% to 7% in most world regions and at 6.8% in the U.S.52 In 2010, the U.S. had the second largest estimated number of people living with dementia (3.9 million), surpassed only by China (5.4 million). Nursing home residents with advanced dementia have significant functional impairment and may be effectively mute. In a retrospective study of 148 hospitalized older adults with pneumonia, Johnson et al. compared nonspecific presenting symptoms, with the exception of delirium, between older (>65 years) and younger adults (<65 years). When subjects with dementia were excluded, non-specific symptoms (e.g. generalized weakness, decreased appetite, falls, and delirium) were similar in both groups.53 They found that the presence of dementia, not age, explained the difference in the clinical presentation of pneumonia in their cohort.
Estimating the exact contribution of dementia to the clinical presentation of infection in older adults is difficult. Misdiagnosis of infection, frequent and inappropriate antimicrobial use are common in older adults, particularly nursing home residents, contributing to rising antimicrobial resistance in this setting.54,55 In a prospective cohort of 362 nursing home residents with advanced dementia, Mitchell et al. showed that 66% of residents experienced at least one suspected infection over 12 months.55 They defined advanced dementia in this study as a nurse-measured Global Deterioration Scale (GDS) score of 7/7 (manifested as profound memory deficits, severely curtailed verbal ability with command of <5 words, incontinence, and inability to walk). The most commonly observed infections in these residents were respiratory tract infections, followed by urinary tract and skin infections. Over 90% of skin infections met treatment criteria compared to only 19% of urinary tract infections.55 The authors attributed this discrepancy to the fact that objective changes support the diagnosis of skin infections while subjective symptoms obtained from nursing homes residents or observed from healthcare workers may trigger a concern for a urinary tract infection.
Just as cognitive decline may influence presentation of infection, chronic infection and inflammation are linked to cognitive decline.56–60 As discussed earlier, chronic infections such as HIV contribute to the aging process through chronic inflammation. Infection causes immune activation in the form of cytokines, chemokines, adhesion molecules and matrix metalloproteinases that in turn activate microvascular endothelial cells, facilitating vascular leukocyte adhesion and dissolving the basement membrane. Vessel leakage, microbleeds and inflammatory progression of cerebrovascular lesions ensue, precipitating cognitive decline.56 Support for this theory comes from the Honolulu Aging Study that linked elevated C-reactive protein to all cause dementia.59 A subset of the Northern Manhattan Study also suggests that the accumulation of infectious burden (measured as an index based on serum titers of antibodies against five common viruses and bacteria) correlates with cognitive decline.56,60 Other clinical data suggest an association between hospitalization with pneumonia and increased risk of dementia,57 as well as an association between Helicobacter pylori infection and dementia.58
2.3. Malnutrition
After retiring in his country estate in 44 B.C., Roman philosopher and politician Marcus Cicero wrote a short treatise on the subject of old age, De Senectute, which offers the earliest known account of malnutrition of aging.61 Malnutrition affects 20% to 30% of older adults and is more prevalent in institutionalized and hospitalized individuals.5,62,63 It is the result of the interplay of multiple factors: socioeconomic, psychologic (e.g., depression, stress) and biologic (e.g., decreased senses of smell and taste, dental problems, increased pro-inflammatory cytokines associated with excess catabolism, alterations in the production of appetite-regulating peptides and hormones).62–64 Dehydration is also a common problem in older adults, specifically during a febrile illness. Reasons include poor oral intake and impaired vasopressin responses in older adults, manifested as decreased thirst.4 Polypharmacy may also be a factor leading to malnutrition and dehydration in older adults.
Malnutrition contributes to immune dysregulation in older adults and results in increased susceptibility to infections. It is also a significant predictor of mortality in this group.65,66 For example, in a study of 188 nursing home residents with physical and cognitive impairment, urinary tract infection during the preceding year was independently associated with poor nutritional status.67 Similar to the association between frailty and infection, there is a two-way association between malnutrition and infection, as well as between dehydration and infection. In contrast to frailty, however, age-related immune and organ-specific changes that occur in older adults, malnutrition and dehydration both respond to intervention.5 For example, Langkamp-Henken et al. studied EXP, an experimental nutritional formula, and found that it enhanced immune function in nursing home residents older than 65 years, indicated by increased influenza vaccine response and lymphocyte activation, less fever, and fewer newly prescribed antimicrobials compared to those consuming a standard ready-to-drink nutritional supplement.66
On the other end of the spectrum, obesity is also problematic in older adults. Along with diabetes, it is a recognized cause of accelerated aging triggered by increased inflammation resulting from macrophage infiltration of the adipose tissue, which in turn increases cytokine levels (e.g., TNFα and IL-6). That said, obesity in older adults is a controversial topic. Some data suggests that it correlates with an increased risk of morbidity in older adults while other studies found that it may confer a protective effect in older adults.68
In summary, recognizing and addressing nutritional concerns may help mitigate some complications in older adults, while recognizing that only some aspects of poor oral intake may be remediable.
3. The influence of the living environment on infection risk and presentation in older adults
Older adults reside in a variety of settings (e.g., home, nursing homes, assisted living facilities) and may also come to medical attention in a number of different settings (e.g., physician’s office, nursing homes, emergency departments or hospitals). Currently in the U.S., approximately 1.4 million people reside in 15,600 nursing homes.69 Despite ongoing efforts to help people “age in place”,70 as our population ages, the need for post-acute and long-term care will continue to increase.
Nursing home residents share dining, recreation and therapeutic facilities. They are also highly dependent upon healthcare workers for assistance with activities of daily living (ADLs), namely bathing, toileting, dressing, eating and mobility.69 Hospitals are closely linked to nursing homes with frequent patient transfers between the two types of facilities and as many opportunities for the spread of MDROs and C. difficile from one setting to the other. During hospitalization, older adults may acquire drug-resistant pathogens and, upon transfer to a nursing home, become a reservoir and source of transmission to others.
In addition, inappropriate antimicrobial use has been shown to be a significant problem in nursing homes with as much as 25–75% of antimicrobial prescriptions in nursing homes being inappropriate.71 The magnitude of the antimicrobial resistance problem in nursing homes is potentially substantial knowing that by 2030, 70 million U.S. residents will be aged 65 years or older and that 3.5% are nursing home residents.71 Fear of infection in long-term care residents with cognitive decline may promote injudicious antimicrobial use, MDRO selection, and C. difficile infection. In the Mitchell et al. study, most nursing home residents with suspected infections received antimicrobials (72%) and the cumulative incidence of acquisition of MDRO acquisition was 48%; acquisition was independently associated with exposure to antimicrobials.55 Other contributing factors include lack of time and reimbursement, and the fact that nursing home practitioners are often family or internal medicine physicians without specialty training in geriatrics or infectious diseases. As such their expertise in addressing complicated infections in the absence of further support might be limited.4,71,72 Antimicrobial stewardship programs in nursing homes, made mandatory by the Centers for Medicare and Medicaid (CMS) starting 2017, might aid practitioners and help reduce antimicrobial misuse and the prevalence of MDROs.73
In an attempt to assist in the diagnosis of infection and the initiation of antimicrobials in residents of long term care facility (LTCF), the Society for Healthcare Epidemiology of America (SHEA) convened a consensus conference that established minimum criteria for initiating antimicrobials in this population. The conference elaborated criteria for the most common infectious syndromes in residents of LTCF, namely respiratory tract infections, urinary tract infections, skin and soft tissue infections, and fever where the focus of infection is unknown.54 These criteria, commonly referred to as the Loeb minimum criteria and detailed in Table 2, can facilitate the diagnosis of infection in LTCF residents as well as in older adults with advanced dementia.
Table 2.
Loeb minimum criteria for initiating antibiotics in residents of long-term care facilities for selected infectious syndromesa
| Infection/ Site | Criteria | Notes |
|---|---|---|
| Urine tract infection in resident without catheter | Acute dysuria alone or Fever (>37.9°C [100°F] or 1.5°C [2.4°F] increase above baseline temperature) and at least one of the following: suprapubic pain, gross hematuria, costovertebral angle tenderness, or new or worsening urgency, frequency or urinary incontinence. |
- Foul smelling or cloudy urine is not a valid indication for initiating antibiotics - Asymptomatic bacteriuria should not be treated with antibiotics |
| Urine tract infection in resident with catheter | Presence of at least one of the following:
|
|
| Respiratory Infections in the febrile patients | If temperature >38.9°C [102°F]:
|
- Epidemiologic setting (e.g., influenza outbreak) in interpreting clinical features is essential - Fever and the sudden onset of new pleuritic chest pain are an indication for transfer to hospital for diagnostic testing to rule out a pulmonary embolus. - Congestive heart failure must be considered within the differential diagnosis of residents with acute respiratory symptoms and signs |
| Respiratory Infections in the afebrile patients | Afebrile residents with COPDb
|
|
| Skin and Soft-Tissue Infections | New or increasing purulent drainage at a wound, skin, or soft-tissue site or At least two of the following:
|
- Erythema alone is not adequate as a minimum criterion for initiating antibiotics - Deeper infections, such as olecranon bursitis, may present with similar symptoms - Thromboembolic disease should be considered with an erythematous or swollen leg - Gout can be mistaken for cellulitis |
Source: Loeb et al. (2001)
COPD: Chronic Obstructive Pulmonary Disease
Caregivers are important in establishing a baseline description of the clinical status of long-term care residents under their care. It is therefore important, during history-taking, to obtain a detailed account of baseline clinical features from caregivers, including patients’ relatives and friends. This includes knowledge of residents’ baseline frailty, temperature as well as their cognitive, functional and psychological status. A good understanding of these baseline parameters will significantly aid discerning clinical changes that might be related to infection or other new stressors. This contributes, in turn, to improving diagnostic accuracy which supports reducing inappropriate and unnecessary antimicrobial use.
While it is possible for caregivers in nursing homes to observe and understand the baseline clinical features of their residents, this is not usually possible for caregivers in other settings. Older adults account for 12% to 24% of all emergency department (ED) visits with pneumonia (25%), urinary tract infection (22%), and sepsis and bacteremia (18%) representing the most frequently cited infections.74 They also visit the ED more frequently than younger adults, arrive more often by ambulance, have a higher level of medical acuity, higher rates of test use, longer ED stays, higher rates of hospital admission, and more serious medical illnesses.74 Absent detailed communication with nursing home staff or other caregivers accompanying older adults, ED personnel have no reference while evaluating geriatric patients. As a result, these patients are more likely to be misdiagnosed., and more frequently discharged with unrecognized health problems.74,75 A common scenario is an older adult with a change in mental status who receives treatment for a urinary tract infection based on the results of a urinalysis. This may not only expose them to unnecessary antimicrobials but also overlook more common and treatable causes for a mental status change, such as dehydration, pain, recent change in medications, or sleep deprivation.
While several risk assessment and screening tools are available for use by ED personnel in caring for older adults, there is a need for more guidance in optimizing the evaluation of older adults in the ED setting. Comprehensive geriatric evaluation of older adults in the ED by trained specialized nurses or interdisciplinary teams is effective. Coupled with risk assessment and screening tools, it helps in detecting geriatric syndromes and other missed diagnoses, increasing community referrals, and avoiding hospital admission on the ED index visit.74,75
Conclusion
We have summarized pathophysiologic, clinical, and environmental influences on the presentation of infections in older adults. Healthcare professionals should understand those changes and the specific features that affect presentation of illness in older adults, especially at a time when the increasing geriatric population is referred to as the “geriatric demographic imperative”76 or the “silver tsunami”3. Pope John XXIII once joked: “Men are like wine - some turn to vinegar, but the best improve with age.”77 Recognition of age-related changes as well as their treatable effects will aid in the prevention, early diagnosis and treatment of infections and other illnesses, ultimately improving clinical outcomes in this population.
Key points.
Age-related physiologic changes affect several organ systems and contribute to increased vulnerability to infections.
With aging comes immunosenescence, affecting both the adaptive and innate immune systems and contributing to an increased risk of infection.
Older adults experience a reduced febrile response caused by altered thermoregulation and a decrease in mean body temperature.
Addressing malnutrition and dehydration among older adults may reduce their risk of infection.
Knowledge of debilitated older adults’ functional and cognitive baseline may support early recognition of infection and discernment of conditions not related to infection.
Acknowledgments
The authors gratefully acknowledge Puja Van Epps for providing the Figure for this manuscript. Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers R01AI100560, R01AI063517, R21AI114508, and R01AI072219 to RAB. This study was supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, Award Numbers 1I01BX001974 to RAB, and PPO 16-118-1 to RLPJ from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10 to RAB and RLPJ. The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Veterans Affairs, National Institutes of Health or the United States Government.
Footnotes
Disclosure statement:
NEC has no conflicts of interest.
RAB: AstraZeneca: Grant Investigator/recipient, Merck: Grant Investigator/recipient
RLPJ: Pfizer: Grant investigator/recipient. Steris: Grant investigator/recipient
References
- 1.Metchnikoff E, Mitchell PC. The prolongation of life; optimistic studies. London, New York: W. Heinemann; G.P. Putnam’s Sons; 1907. [Google Scholar]
- 2.He Wan, Goodkind Daniel, Kowal Paul. An Aging World: 2015. Washington, DC: U.S. Census Bureau; 2016. [Accessed 6 February, 2017]. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p95-16-1.pdf. [Google Scholar]
- 3.Bartels SJ, Naslund JA. The underside of the silver tsunami--older adults and mental health care. N Engl J Med. 2013;368(6):493–496. doi: 10.1056/NEJMp1211456. [DOI] [PubMed] [Google Scholar]
- 4.High KP, Bradley SF, Gravenstein S, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Society of America. Journal of the American Geriatrics Society. 2009;57(3):375–394. doi: 10.1111/j.1532-5415.2009.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavazzi G, Krause K-H. Ageing and infection. The Lancet Infectious Diseases. 2002;2(11):659–666. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 6.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, Sambhara S. Challenges for vaccination in the elderly. Immun Ageing. 2007;4:9. doi: 10.1186/1742-4933-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu T, Walston JD, Yang H, et al. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009;130(3):161–166. doi: 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu T, Yang H, Walston JD, Fedarko NS, Leng SX. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine. 2009;46(3):319–324. doi: 10.1016/j.cyto.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133(6):456–466. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Heppner HJ, Sieber C, Walger P, Bahrmann P, Singler K. Infections in the elderly. Critical care clinics. 2013;29(3):757–774. doi: 10.1016/j.ccc.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243–284. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- 13.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 14.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464(7288):520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273(5271):70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 16.Herndler-Brandstetter D, Landgraf K, Tzankov A, et al. The impact of aging on memory T cell phenotype and function in the human bone marrow. J Leukoc Biol. 2012;91(2):197–205. doi: 10.1189/jlb.0611299. [DOI] [PubMed] [Google Scholar]
- 17.Van Epps P, Banks R, Aung H, Betts MR, Canaday DH. Age-related differences in polyfunctional T cell responses. Immun Ageing. 2014;11:14. doi: 10.1186/1742-4933-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross AM, Jaeger PA, Kreisberg JF, et al. Methylome-wide Analysis of Chronic HIV Infection Reveals Five-Year Increase in Biological Age and Epigenetic Targeting of HLA. Mol Cell. 2016;62(2):157–168. doi: 10.1016/j.molcel.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith RL, de Boer R, Brul S, Budovskaya Y, van Spek H. Premature and accelerated aging: HIV or HAART? Front Genet. 2012;3:328. doi: 10.3389/fgene.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine. 2010;28(38):6145–6151. doi: 10.1016/j.vaccine.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172(6):3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg C, Bovin NV, Bram LV, et al. Age is an important determinant in humoral and T cell responses to immunization with hepatitis B surface antigen. Hum Vaccin Immunother. 2013;9(7):1466–1476. doi: 10.4161/hv.24480. [DOI] [PubMed] [Google Scholar]
- 23.Ridda I, Macintyre CR, Lindley R, et al. Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009;27(10):1628–1636. doi: 10.1016/j.vaccine.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120(4):437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64(10):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angulo J, El Assar M, Rodriguez-Manas L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol Aspects Med. 2016;50:1–32. doi: 10.1016/j.mam.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Janssens J-P, Krause K-H. Pneumonia in the very old. The Lancet Infectious Diseases. 2004;4(2):112–124. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- 28.Anderson DJ, Kaye KS. Skin and soft tissue infections in older adults. Clinics in geriatric medicine. 2007;23(3):595–613. vii. doi: 10.1016/j.cger.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clinics in geriatric medicine. 2011;27(1):79–87. doi: 10.1016/j.cger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theou O, Cann L, Blodgett J, Wallace LM, Brothers TD, Rockwood K. Modifications to the frailty phenotype criteria: Systematic review of the current literature and investigation of 262 frailty phenotypes in the Survey of Health, Ageing, and Retirement in Europe. Ageing Res Rev. 2015;21:78–94. doi: 10.1016/j.arr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 33.Norman DC. Fever in the elderly. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;31(1):148–151. doi: 10.1086/313896. [DOI] [PubMed] [Google Scholar]
- 34.Yoshikawa T, Norman D. Infectious disease in the aging: a clinical handbook. Springer Science & Business Media; 2009. [Google Scholar]
- 35.Kenney WL, Munce TA. Invited review: aging and human temperature regulation. J Appl Physiol (1985) 2003;95(6):2598–2603. doi: 10.1152/japplphysiol.00202.2003. [DOI] [PubMed] [Google Scholar]
- 36.Blatteis CM. Age-dependent changes in temperature regulation - a mini review. Gerontology. 2012;58(4):289–295. doi: 10.1159/000333148. [DOI] [PubMed] [Google Scholar]
- 37.Waalen J, Buxbaum JN. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66(5):487–492. doi: 10.1093/gerona/glr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castle SC, Norman DC, Yeh M, Miller D, Yoshikawa TT. Fever response in elderly nursing home residents: are the older truly colder? Journal of the American Geriatrics Society. 1991;39(9):853–857. doi: 10.1111/j.1532-5415.1991.tb04450.x. [DOI] [PubMed] [Google Scholar]
- 39.Castle SC, Yeh M, Toledo S, Yoshikawa T, Norman DC. Lowering the temperature criterion improves detection of infections in nursing home residents. Aging Immunol Infect Dis. 1993;4:67–76. [Google Scholar]
- 40.Keating HJ, 3rd, Klimek JJ, Levine DS, Kiernan FJ. Effect of aging on the clinical significance of fever in ambulatory adult patients. Journal of the American Geriatrics Society. 1984;32(4):282–287. doi: 10.1111/j.1532-5415.1984.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 41.O’Grady NP, Barie PS, Bartlett JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330–1349. doi: 10.1097/CCM.0b013e318169eda9. [DOI] [PubMed] [Google Scholar]
- 42.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(4):427–431. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 43.Vitiello MV, Smallwood RG, Avery DH, Pascualy RA, Martin DC, Prinz PN. Circadian temperature rhythms in young adult and aged men. Neurobiol Aging. 1986;7(2):97–100. doi: 10.1016/0197-4580(86)90146-6. [DOI] [PubMed] [Google Scholar]
- 44.Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC. Chronobiology of aging: temperature, sleep-wake rhythms and entrainment. Neurobiol Aging. 1982;3(4):299–309. doi: 10.1016/0197-4580(82)90018-5. [DOI] [PubMed] [Google Scholar]
- 45.Blatteis CM. A personal recollection: 60 years in thermoregulation. Temperature (Austin) 2016;3(1):1–7. doi: 10.1080/23328940.2016.1148524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plata-Salaman CR, Peloso E, Satinoff E. Interleukin-1beta-induced fever in young and old Long-Evans rats. Am J Physiol. 1998;275(5 Pt 2):R1633–1638. doi: 10.1152/ajpregu.1998.275.5.R1633. [DOI] [PubMed] [Google Scholar]
- 47.Scarpace PJ, Borst SE, Bender BS. The association of E. coli peritonitis with an impaired and delayed fever response in senescent rats. J Gerontol. 1992;47(4):B142–145. doi: 10.1093/geronj/47.4.b142. [DOI] [PubMed] [Google Scholar]
- 48.Roth GS, Lane MA, Ingram DK, et al. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297(5582):811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 49.Weinstein MP, Murphy JR, Reller LB, Lichtenstein KA. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. II. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis. 1983;5(1):54–70. doi: 10.1093/clinids/5.1.54. [DOI] [PubMed] [Google Scholar]
- 50.Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. The adaptive value of fever. Infectious disease clinics of North America. 1996;10(1):1–20. doi: 10.1016/s0891-5520(05)70282-8. [DOI] [PubMed] [Google Scholar]
- 51.Burns A, Zaudig M. Mild cognitive impairment in older people. Lancet. 2002;360(9349):1963–1965. doi: 10.1016/S0140-6736(02)11920-9. [DOI] [PubMed] [Google Scholar]
- 52.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Johnson JC, Jayadevappa R, Baccash PD, Taylor L. Nonspecific presentation of pneumonia in hospitalized older people: age effect or dementia? Journal of the American Geriatrics Society. 2000;48(10):1316–1320. doi: 10.1111/j.1532-5415.2000.tb02607.x. [DOI] [PubMed] [Google Scholar]
- 54.Loeb M, Bentley DW, Bradley S, et al. Development of minimum criteria for the initiation of antibiotics in residents of long-term-care facilities: results of a consensus conference. Infect Control Hosp Epidemiol. 2001;22(2):120–124. doi: 10.1086/501875. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell SL, Shaffer ML, Loeb MB, et al. Infection management and multidrug-resistant organisms in nursing home residents with advanced dementia. JAMA Intern Med. 2014;174(10):1660–1667. doi: 10.1001/jamainternmed.2014.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swardfager W, Black SE. Dementia: A link between microbial infection and cognition? Nat Rev Neurol. 2013;9(6):301–302. doi: 10.1038/nrneurol.2013.93. [DOI] [PubMed] [Google Scholar]
- 57.Tate JA, Snitz BE, Alvarez KA, et al. Infection hospitalization increases risk of dementia in the elderly. Crit Care Med. 2014;42(5):1037–1046. doi: 10.1097/CCM.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang WS, Yang TY, Shen WC, Lin CL, Lin MC, Kao CH. Association between Helicobacter pylori infection and dementia. J Clin Neurosci. 2014;21(8):1355–1358. doi: 10.1016/j.jocn.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52(2):168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 60.Katan M, Moon YP, Paik MC, Sacco RL, Wright CB, Elkind MS. Infectious burden and cognitive function: the Northern Manhattan Study. Neurology. 2013;80(13):1209–1215. doi: 10.1212/WNL.0b013e3182896e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cicero MT, Freeman P, Cicero MT, Cicero MT. How to grow old : ancient wisdom for the second half of life. Princeton: Princeton University Press; 2016. [Google Scholar]
- 62.Sanford AM. Anorexia of aging and its role for frailty. Curr Opin Clin Nutr Metab Care. 2017;20(1):54–60. doi: 10.1097/MCO.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 63.Donini LM, Poggiogalle E, Piredda M, et al. Anorexia and eating patterns in the elderly. PLoS One. 2013;8(5):e63539. doi: 10.1371/journal.pone.0063539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Htwe TH, Mushtaq A, Robinson SB, Rosher RB, Khardori N. Infection in the elderly. Infectious disease clinics of North America. 2007;21(3):711–743. ix. doi: 10.1016/j.idc.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Lilamand M, Kelaiditi E, Demougeot L, Rolland Y, Vellas B, Cesari M. The Mini Nutritional Assessment-Short Form and mortality in nursing home residents--results from the INCUR study. J Nutr Health Aging. 2015;19(4):383–388. doi: 10.1007/s12603-014-0533-1. [DOI] [PubMed] [Google Scholar]
- 66.Langkamp-Henken B, Wood SM, Herlinger-Garcia KA, et al. Nutritional formula improved immune profiles of seniors living in nursing homes. Journal of the American Geriatrics Society. 2006;54(12):1861–1870. doi: 10.1111/j.1532-5415.2006.00982.x. [DOI] [PubMed] [Google Scholar]
- 67.Carlsson M, Haglin L, Rosendahl E, Gustafson Y. Poor nutritional status is associated with urinary tract infection among older people living in residential care facilities. J Nutr Health Aging. 2013;17(2):186–191. doi: 10.1007/s12603-012-0087-z. [DOI] [PubMed] [Google Scholar]
- 68.Vaughan KL, Mattison JA. Obesity and Aging in Humans and Nonhuman Primates: A Mini-Review. Gerontology. 2016;62(6):611–617. doi: 10.1159/000445800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-Term Care Providers and services users in the United States: data from the National Study of Long-Term Care Providers, 2013–2014. Vital Health Stat 3. 2016;(38):x–xii. 1–105. [PubMed] [Google Scholar]
- 70.United States Congress. [Accessed 6 February, 2017];Older Americans Act Amendments of 2006. 2006 http://www.aoa.acl.gov/AoA_Programs/OAA/oaa_full.asp-_Toc153957728.
- 71.Jump RL, Olds DM, Jury LA, et al. Specialty care delivery: bringing infectious disease expertise to the residents of a Veterans Affairs long-term care facility. J Am Geriatr Soc. 2013;61(5):782–787. doi: 10.1111/jgs.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caprio TV, Karuza J, Katz PR. Profile of physicians in the nursing home: time perception and barriers to optimal medical practice. J Am Med Dir Assoc. 2009;10(2):93–97. doi: 10.1016/j.jamda.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 73.United States Department of Health and Human Services. Centers for Medicare and Medicaid Services Survey and Certification Group 2016/2017 Nursing Home Action Plan. 2016. [Google Scholar]
- 74.Samaras N, Chevalley T, Samaras D, Gold G. Older patients in the emergency department: a review. Annals of emergency medicine. 2010;56(3):261–269. doi: 10.1016/j.annemergmed.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 75.Aminzadeh F, Dalziel WB. Older adults in the emergency department: A systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Annals of emergency medicine. 2002;39(3):238–247. doi: 10.1067/mem.2002.121523. [DOI] [PubMed] [Google Scholar]
- 76.Crossley KB, Peterson PK. Infections in the elderly. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1996;22(2):209–215. doi: 10.1093/clinids/22.2.209. [DOI] [PubMed] [Google Scholar]
- 77.Bingham J. [Accessed 6 February, 2017];Pope Francis: ‘like good wine we get better with age’. 2013 http://www.telegraph.co.uk/news/worldnews/the-pope/9932391/Pope-Francis-like-good-wine-we-get-better-with-age.html.


