Abstract
Coping strategies have been associated with differential stress responsivity, perhaps providing a valuable neurobiological marker for susceptibility to the emergence of depressogenic symptoms or vulnerability to other anxiety-related disorders. Rats profiled with a flexible coping phenotype, for example, exhibit increased neurobiological markers of emotional regulation compared to active and passive copers (Bardi et al., 2012; Lambert et al., 2014). In the current study, responses of male and female rats to prediction errors in a spatial foraging task (dry land maze; DLM) were examined after animals were exposed to chronic unpredictable stress (CUS). Brains were processed following the DLM training/assessment for fos-activation patterns and several measures of neuroplasticity in relevant areas. Behavioral responses observed during both the CUS and DLM phases of testing suggested that males and females employ different means of gathering information such as increased ambulatory exploration in males and rear responses in females. Fecal samples collected during baseline and following CUS swim exposure revealed higher corticosterone (CORT) in active copers, whereas flexible copers had higher dehydroepiandrosterone (DHEA) and DHEA/CORT ratios, both indications of enhanced emotional regulation. Focusing on the neural analysis, flexible copers exhibited fewer fos-immunoreactive cells in the basolateral amygdala and a trend toward lower activation in the insula while encountering the prediction error associated with the DLM probe trial. Coping profiles also differentially influenced markers of neuroplasticity; specifically, flexible copers exhibited higher levels nestin-immunoreactivity (ir). Further, less hippocampal glucocorticoid receptor-ir was observed in the flexible copers than the active and passive copers. In sum, flexible coping rats exhibited evidence of emotional resilience as indicated by several neurobiological measures; however, despite increased rates of depression and related symptoms reported in human females, sex effects weren’t as pervasive as coping strategy profiles in the analysis of neurobiological markers employed in the current study.
Keywords: Coping, resilience, stress, uncertainty, depression
1. Introduction
It has been proposed that the lack of reliable biological predictive markers of psychiatric illness contributes to the dearth of effective therapies and rising prevalence rates (Kapur et al, 2012). In contrast, biomarkers for medical conditions such as cardiovascular disease have led to timely preventative measures and a corresponding reduction in associated deaths (Zethelius et al., 2008). Focusing on depression, it is noteworthy that hypersecretion of cortisol has been associated with up to 50% of patients diagnosed with major depression disorder (MDD), although individual differences abound (Kendler et al., 1999; Strickland et al., 2002). Consequently, various distortions of the hypothalamic-pituitary-adrenal (HPA) axis may provide valuable information about emerging emotional disorders (Charney, 2003).
Because stress and anxiety are closely associated with emotional disorders, effective coping strategies provide an opportunity for animals to gain emotional control in unpredictable, stressful environments (McEwen et al., 2015). Although HPA activation is necessary for survival, especially in the context of acute stressors, hypersecretion of glucocorticoids for extended durations leads to disrupted cellular functioning and eventual physiological dysfunction (Mackin and Young, 2004). In the brain, excessive cortisol levels have been linked to atrophic effects in the hippocampus, a brain area compromised in MDD (Feder et al., 2009). Consequently, coping strategies that regulate excessive HPA activation may serve as an important buffer against the emergence of MDD and anxiety disorders (Compare et al., 2014; Gaffey et al., 2016).
Animals exhibiting active coping strategies in the presence of environmental stressors generally exhibit lower glucocorticoid responses than animals exhibiting more passive coping responses (Lu et al., 2009). Thus, specific coping strategies may lead to emotional resilience by enhancing survival with minimal allostatic load (Yehuda et al., 2006; Lambert et al., 2014). Adding to the complexity of the stress response is the secretion of dehydroepiandrosterone (DHEA) which has been described as having anticorticosteroid effects in the brain (Feder et al., 2009). High plasma DHEA sulphate/cortisol levels were found in individuals participating in challenging military survival training and exhibiting both optimal performance and emotional resilience (Morgan et al., 2004). Further, male veterans with PTSD exhibiting the most improvement in symptoms had higher plasma levels of DHEA (Yehuda et al., 2006). Focusing on rodents, in the Flinder-sensitive line of rats known for their susceptibility to depressive responses, lower DHEA levels were observed in brain areas associated with depression such as the amygdala, prefrontal cortex and nucleus accumbens (Genud et al., 2009). Thus, it is important to consider both corticosteroid and DHEA levels when determining potential vulnerability and resilience to MDD symptom emergence in various stressful situations (Bardi et al., 2010).
Contrary to Selye’s suggestion that the stress response is generalized and non-specific (Selye, 1936), evidence suggests that individual differences exist (Koolhaas et al., 1999; Ouwehand et al., 2008). These individual differences open the door for an exploration of biomarker-determined resilience subtypes that may be valuable in the prevention and treatment of psychiatric illness (Kapur et al., 2012). For example, effective coping strategies facilitate neurobiological adaptations to situations that threaten an animal’s fitness (Wechsler, 1995). Extending from research conducted on piglets determining passive and active responses to being restrained on their backs for one minute (Koolhaas et al., 1999), this technique has been adapted for recently weaned rats. Accordingly, rats are gently restrained on their backs for one minute during which time the number of escape attempts are recorded. One week later, the assessment is repeated to determine consistently passive (few attempts) or active (greater number of attempts) coping styles; however, animals exhibiting variability by switching coping strategies (regardless of direction) are categorized as flexible (or variable) copers (Lambert, 2006). Using this technique to profile coping strategies in rats, flexible copers have been found to exhibit significantly more Neuropeptide Y (NPY)-immunoreactive cells, associated with emotional resilience, in the basolateral amygdala and bed nucleus of the stria terminalis than the other coping groups following exposure to chronic unpredictable stress (Hawley et al., 2010). In another study, following a cognitive training program with no chronic stress exposure, flexible rats exhibited higher levels of NPY- immunoreactive cells in the CA1 and CA3 hippocampal subfields than their passive and active counterparts (Bardi et al., 2012). When exposed to the activity-stress paradigm, in which animals are housed in running wheels and fed one hour per day prompting excessive spontaneous levels of running, the flexible copers exhibited lower fecal corticoisteroid metabolites than the other coping groups (Lambert et al., 2006).
The introduction of uncertainty in the form of prediction errors, in which a discrepancy between a predicted and observed outcome is experienced, provides an opportunity to observe an animals’ response flexibility in a non- or moderately-threatening context (Bubic et al., 2010; Robinson et al., 2012; Steinberg et al., 2013). As the animal effectively updates relevant response-outcome contingency probabilities to determine the appropriate response in this novel situation, an enhanced sense of control over the uncertainty-induced stress is achieved (Moore et al., 2009). Referring back to the classic learned helplessness models assessing dogs’ responses in threatening contexts, the presence of perceived controllability in uncertain situations has been associated with the development of emotional resilience against the emergence of depressive symptoms such as behavioral inhibition (Overmier and Seligman, 1967; Abramson et al., 1978; Gladstone and Parker, 2006). When presented with a prediction error in a spatial task, for example, recent research suggests that contingency-trained animals exhibited more targeted search strategies than their noncontingent counterparts. Regardless of training, animals profiled as flexible copers exhibited enhanced evidence of neuroplasticity (i.e., doublecortin-immunoreactivity in the dentate gyrus), potentially associated with the cognitive training, when compared to their noncontingent-trained counterparts (Lambert et al., 2014). Disruptions of neuroplasticity, critical for neuronal adaptation in changing environmental landscapes, have been associated with the onset of mood disorders (Pittenger and Duman, 2008; Czeh and Simon, 2005).
In addition to neural plasticity, specific brain areas have been implicated in an individual’s response to prediction errors and uncertainty. The anterior cingulate and medial prefrontal cortical areas have been implicated in the detection of environmental parameters associated with the prediction error (Ragozzino and Rozman, 2007; Rushworth and Behrens, 2008; Matsumoto and Tanaka, 2004; Alexander and Brown, 2011). Additionally, the insular cortex has been associated with processing the negative consequences associated with prediction errors–likely motivating the animal to avoid the prediction error in the future (Endepols et al., 2010) as well as promoting adaptive decisions in the uncertainty context (Rebola et al., 2012). Another cortical area, the retrosplenial cortex, is involved in the initiation of behavioral shifts necessary to complete tasks involving the balancing of emotional processing when completing demanding cognitive tasks (Vann et al., 2009). Finally, the lateral habenula has been implicated in behavioral suppression following exposure to uncertain situations (Li et al., 2011). Although behavioral suppression can be an adaptive response in the presence of a threatening stimulus, this response is considered to be a risk factor in children for subsequent development of depression and related anxiety disorders (Chao et al., 2010). Interestingly, heightened activity of the lateral habenula has been observed in patients diagnosed with depression (Savitz et al., 2011; Henn, 2012). Thus, when a prediction error is encountered, a network of various brain areas converge to facilitate the individual’s accurate assessment of the changing contingencies so that an alternative adaptive response is generated. This contingency-correcting network of brain areas necessary for flexible coping, working in the context of stress-modulating neurobiological factors such as amygdala and HPA activation, is likely critical in the determination of adaptive versus maladaptive responses in various stressful and threatening contexts (Yang et al., 2010; Lambert et al., 2014).
In the current study, the influence of specific coping strategies was examined in the rats’ responses to prediction errors in the dry land maze probe test; however, prior to training and testing in this task, all animals were exposed to chronic unpredictable stress to heighten HPA activation and susceptibility to depressive symptoms. Due to reported sex differences in susceptibility to depression with females experiencing the disorder at nearly twice the rates as males (Kessler, 2003; Nolen-Hoeksema, 2001; Ryba & Hopko, 2012), both males and females were assessed. Relevant behavioral responses were observed throughout the chronic unpredictable stress exposure, dry land spatial task and subsequent probe trial. Throughout the experimental manipulations, HPA activation was assessed via corticosteroid and DHEA levels. Following the behavioral assessments, activation of various brain areas was investigated as well as the presence of markers of neuroplasticity. Based on prior research in our laboratory, it was hypothesized that flexible copers would exhibit adaptive responses (e.g., higher DHEA/CORT ratios, increased hippocampal neuroplasticity and more strategic behavioral responses to prediction errors); further, due to sex-specific differences in emotional responsivity observed in past research, sex differences were expected to emerge in certain components of the dependent variables.
2. Materials and Methods
2.1. Subjects
Forty male and forty female Long-Evans rats were ordered from Harlan Tekland (Madison WI USA) and arrived at 21–23 days of age. At the time of arrival they were housed five animals per cage (48 × 26 × 21 cm) with corncob bedding and food and water provided ad libitum. A 12 h: 12 h light dark schedule was maintained with lights on at 8:00 am. Following 17 days of habituation to the laboratory, animals were double-housed with a novel cage-mate with the same coping profile (active, passive or flexible; see below for coping profile assessment). At that time males and females were yoked to maintain equal numbers of each sex and coping strategy being tested at the same time throughout all experimental phases (prompted by the necessity of assessing females at consistent times during the estrous cycle).
2.2. Coping Profile Assessment
Two days following arrival to the laboratory, coping profile assessments were conducted in the colony room between the times of 1:30 and 3:30 pm with each session videotaped for subsequent confirmation of behavior. During the assessment, each animal was gently restrained on its back for 1 min so that the number of escape attempts (or wiggles) could be quantified (see Hawley et al., 2010; Lambert et al., 2014). Seven days later the same assessment was conducted; however, the animals were tested in a different order than the first assessment to avoid any confounding sequencing effects. Once the two sessions were observed and scored, the number of escape attempts for each session was used as the criterion score for the determination of coping profiles. Considering that the greatest number of escape attempts for each session was 10 responses in the first assessment, animals with fewer than 6 responses were categorized in the passive coping group and those with 6 or more responses were categorized as active copers. If the response number remained in the same category in the second assessment one week later then its final placement was in that respective category. However, if the animal switched from one category to another (in either direction), it was classified as a flexible coper. The most representative animals were selected from the 40 animals in each sex so that each group consisted of 8 animals with the lowest and highest escape attempts (passive copers and active copers, respectively); accordingly, the flexible copers with the largest differences in escape attempts were assigned to the flexible coping groups. Following the coping profile assessment, the animals designated as active copers responded with an average of 7.4 escape attempts, the passive copers exhibited 3.6 attempts and the flexible copers’ score changed on the average of approximately 5 responses from the first to the second assessment (see Table 1). Animals were pair- housed with a cage mate of the same coping profile. Males and females were also housed in separate rooms. In total, 48 animals, 24 male and 24 female were used in the current study. Animals were maintained in accordance with the Randolph-Macon College Institutional Animal Care and Use Committee.
Table 1.
Escape attempts characterizing the three coping profile assignments.
| Coping Category | Average Escape Movements | Range of Escape Movements |
|---|---|---|
| Passive (n=16) | 3.6 | 2-5 |
| Active (n=16) | 7.4 | 5-10 |
| Flexible (n=16) | 4.6 (represents change in responses from test 1 to test 2) | 3-6 (range representing change from Test 1 to Test 2) |
2.3 Chronic Unpredictable Stress (CUS)
Two weeks after being pair-housed with new cage mates, 24 animals with equal numbers from each group represented (Phase I) were moved into a new colony room for CUS exposure. One week later, the remaining animals from all groups (Phase II) commenced CUS; the animals were run in two phases in order to stagger the times for extensive cognitive training following CUS exposure. Stressors utilized in the CUS paradigm represented two threat categories, those that were considered moderate environmental stressors presenting no immediate survival threat (damp bedding, sour water, strobe light, white noise, tilted cages) and those categorized as more severe environmental stressors that were more likely to be associated with immediate survival (tail clip simulating being bitten by another animal, forced swim, cat predator odor, fox predator odor, predator noise). One stressor from each category was presented at random times each day for a total of 10 days. The duration and pairing of stressors varied daily to increase unpredictability; however, each stressor was presented twice during CUS. Prior to the swim stressor, animals were exposed to a toy block for five minutes to generate an association between the block and the water exposure. Throughout the CUS exposure, behavioral responses to fox odor, swim stress and a third exposure to the toy block (now as a conditioned fear stimulus) were videotaped for further behavioral assessment. All animals were weighed at various points during CUS and subsequently evaluated using previously determined growth rates to ensure they did not lose more than 10% of their expected body weight during this active growth phase.
2.4. Dry Land Maze (DLM) Training and Problem Solving Assessment
Five days following the last day of CUS, animals commenced habituation training for the DLM task (see Franssen et al., 2011; Bardi et al., 2013). During these five days, animals were given cereal treats (i.e., Froot Loops® cereal pieces) to reduce neophobia to the food reward used during DLM training. Each day, three hours prior to DLM training, food was removed to enhance food motivation. A circular apparatus measuring 124.5 cm in diameter and 40.5 cm in height was used for training and testing. Eight plastic wells (2 cm in diameter and 1 cm in height) were secured on the bottom of the arena positioned equidistantly along the periphery. During habituation day 1, all 8 wells were baited with one-half of a cereal piece. Animals were run in a random order to avoid a treatment order bias and were given 6 min to locate and consume the food rewards. On days 2 and 3 of habituation training, four wells (every other one) and two wells (two of the four) were baited, respectively, for the 6 min trials. On the following day, animals were exposed to the Acquisition Trial which consisted of only one of the two previously baited wells being baited for the testing and probe trial assessment. Throughout training, if all rewards were consumed prior to the six minute cap, the rat was removed from the apparatus. For the three subsequent test days, the same single well was baited and each animal was exposed to two test trials (maximum of 6 minutes each trial) each day, with an inter-trial interval of one minute. Throughout testing, animals were placed in varying start positions throughout the test trials; however, the start positions were consistent for all animals.
Following training/testing each day, animals were removed from cages for females to be vaginally smeared and males to receive similar handling. To determine stage of the estrus cycle, females were gently restrained by the experimenters so that calcium alginate swabs with a diameter of approximately 0.025cm (Puritan Medical Products, #25801A50, Fisher Scientific #22-029-501; Waltham, MA, USA) soaked in sterile saline could be inserted no more than 0.25cm into the vagina, slid along the vaginal wall and then rolled onto a microscope slide. Males were also removed from their cages and handled in a similar fashion so that a sterile saline soaked swab was pressed to the penile area to best mimic the protocol performed on the female animals. Cells on slides were stained with cressyl violet and analyzed for vaginal cytology (McLean et al., 2012). Only females in the diestrus stage of the estrus cycle were assessed in the probe trial. The vaginal cells of females not in diestrus were evaluated daily until diestrus was reached. As previously stated, each female was yoked to a male to control for equal numbers of males and females in the specific groups being assessed in the DLM on any given day.
On the day following the third day of testing or a day or two later when the female was in diestrus, animals were exposed to the probe trial to assess reactions to prediction errors by being placed in the DLM arena for 5 min with no reward in the previously baited well. Each animal’s behavior was videotaped for subsequent behavioral analysis of the following behaviors: latency to approach the previously baited well; time spent in proximity to the baited well; rearing in specific zones of the arena; investigation of other previously baited wells and freezing responses. Additionally, a microsequencing analysis of behavioral sequences and transitions for both movements and grooming were conducted so that distractions or interruptions of sequenced behavioral responses, previously associated with heightened anxiety, could be assessed (Bardi et al., 2011). Thus, the number of interrupted grooming sequences (either an incomplete chain or a chain diverging from the typical cephalocaudal structure), the number of stereotypical movements, and the number of behavioral transitions (e.g., from rearing to exploring) were scored.
2.5 Physiological Responses
To assess physiological responses to the behavioral tests, CORT and DHEA metabolized in excreta were assessed using fresh fecal samples (see Bardi et al., 2010 for parametric validation of times used in fecal sample collection). During CUS, samples were taken between 9:00 pm and 10:00 pm following the first forced swim task and the second fox predator odor. A baseline sample was taken four days following the completion of CUS exposure. For fecal collection, rats were briefly isolated (no more than 10 min for each animal was necessary to collect samples) and samples (0.1 g each) were collected from each animal and frozen unmixed in sealed containers at −70°C until assaying.
2.6. Assay Validation
Endocrinological assays were validated using standard methods described in previous research in our laboratory (Bardi et al., 2010). Briefly, prior to extraction, previously collected fecal samples were thawed at room temperature and placed in a glass tube with 1 ml of 100% methanol. The contents of the tube were then mixed via vortex (Vortex Genie 2, Scientific Industries, Inc.) for approximately 30 s. Next, the tube was centrifuged for 15 min at 1000 × g. Using a transfer pipette, the sample was transferred to a 13 × 100 mm glass test tube. The final step of extraction procedures was to dilute the sample in MeOH (concentration 1:20) in an EIA buffer. Assay procedures were carried out using materials and protocols provided by an Enzyme ImmunoAssay (EIA) kit (Assay Designs, Anne Arbor, Michigan). Correlate-EIA sample readings were completed using an automated micro-plate reader (BioTek, Winooski, VT, model Synergy) and the Gen5 software (BioTek, Winooski, VT, version 2.04.11). Readings were assessed at a wavelength of 405λ with correction at 490λ. Log-logit transformations of the data were analyzed by least-squared regression analysis. Accuracy was demonstrated at each standard curve point (n = 6, range 12 to 5000 pg/mL): accuracy was 98 ± 3% for CORT and 93 ± 6 % for DHEA. Quality control pools were assayed in triplicate on each plate, with the following results: CORT (high) 7.4% coefficients of variation (cv); CORT (low) 6.6 % cv; DHEA (high) 5.4% cv; DHEA (low) 6.1% cv.
2.7. Histological Preparation
Sixty minutes following the 5-min probe trial to assess the response to a prediction error, animals were anesthetized and perfused to detect fos-immunoreactivity as well as other relevant neural markers. Specifically, individual animals were exposed to 1 mL of Halothane liquid (Sigma-Aldrich; St. Louis MO) until respiratory rate slowed and were subsequently given an intraperitoneal injection of 0.2 mL sodium pentabarbitol at an overdose of 50 mg/Kg and transcardially perfused at 40 mL/min using a MasterFlex L/S perfusion pump initially with 100 mL phosphate-buffered saline solution, then with 200 mL 4% paraformaldehyde. Following extraction, brains were post-fixed in 4% paraformaldehyde overnight at 4°C, then transferred to a 10% sucrose solution for 24 h at 4°C followed by a 20% sucrose solution at 4°C ending with 30% sucrose solution at 4°C until the time of sectioning. Brains were subsequently sectioned at −25°C using a HM525 Microm cryostat (purchased via Thermo-Fisher Scientific) at the appropriate Bregma position for each targeted brain (based on Paxinos and Watson, 2007). For all sections, every 7th section was used to avoid double-counting cells in serial sections and to provide enough tissue for all the histological protocols.
Six free-floating sections (40 μm thickness) were collected through the following brain areas: nucleus accumbens (core and shell), anterior cingulate cortex and lateral septum, as well as sections containing the lateral habenula; hippocampus (dentate gyrus, CA1, CA2, and CA3); basolateral amygdala; and cortex [insular, restrosplenial, and piriform areas (the piriform cortex was assessed due to projections to both the limbic and cortical structures; (Johnson, Liig, Behan & Haberly, 2000)]. For fos-immunoreactivity assessment, following a 10 min incubation in sodium citrate at 90°C, brains were immersed in 0.1% hydrogen peroxide, sections were then blocked for 30 min in 10% normal goat serum (Vector, Burlingame, CA) in PBST (0.3% Triton-X, Spectrum Chemical, New Brunswick, NJ, USA). Sections were subsequently incubated in c-fos primary antibody (1:10,000; Immunostar, Inc., Hudson, WI, USA) overnight at 4°C followed by 90 minute exposure to goat anti-rabbit secondary antibody (Vector) at 1:500, and further processed with a standard Vectastain ABC kit (modified with Bovine Triton-X PBS; Vector). A similar protocol was used for BDNF (primary 1:1000; Abcam, Inc., Cambridge, MA USA and secondary 1:250; Vector), Ki67 (primary 1:400 and secondary 1:200; Vector), nestin (primary antibody dilution of 1:4000; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA USA) and secondary dilution of 1:200; Vector), glucocorticoid receptors (GR) (Santa Cruz Biotechnology, Inc. Dallas, TX, USA, primary 1:500 and secondary 1:250; Vector) and neuropeptide Y (NPY) (Immunostar, primary 1:10,000 and secondary 1:200; Vector) to assess neurotrophic activity, neuronal restructuring (Hendrickson, Rao, Demerdash & Kalil, 2011) and neuronal stress reaction, respectively. All sections were visualized with DAB peroxidase substrate and then cleared through a series of 70, 95, and 100% EtOH and Citrisolv (Fisher Scientific, Fair Lawn, NJ, USA) washes then coverslipped using Permount (Fisher Scientific).
For fluorescent immunocytochemistry, sections were incubated in 0.3% sodium citrate solution for 10 min in a 90°C water bath. Following three 5-min PBS washes, sections were exposed to 0.1% hydrogen peroxide in PBS for 30 min, and then blocked for 60 min in 10% NGS in PBST with BSA (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). After blocking, sections were incubated overnight at 4°C in a 1:200 dilution of rabbit anti-DCX (Abcam). Following three PBST washes, sections were exposed to the secondary antibody, Alexa-488(green) fluorescence (Jackson) anti-rabbit antibody, at a dilution of 1:200 (Abcam). Sections were counterstained with DAPI (0.01%; Abcam) for 1 h in the dark and coverslipped.
2.8 Neural Quantification
Prior to being quantified, all slides were recoded so that experimenters would be blind to experimental conditions. A Zeiss Axioskop light microscope (Carl Zeiss, Oberkochen, Germany) and Neurolucida software (Microbrightfield, Inc., Williston, VT, USA) were used to quantify fos-immunoreactive cells in the nucleus accumbens core and shell, lateral septum, anterior cingulate cortex, basolateral amygdala, insula, CA1 and piriform cortex; BDNF-immunoreactive cells in the CA1, CA3 and anterior cingulate cortex; and GR-immunoreactive cells in CA1, CA3 and basolateral amygdala (at 40× magnification with a 300 × 300 μm grid). In these areas, cells were marked with a computer-generated colored symbol and quantified for each section.
For the remaining markers, a BA400 light microscope (Motic, Richmond, BC, Canada) was used for the standard neuroquantification of Ki67-immunoreactive cells in the dentate gyrus (DG), nestin immunoreactive cells in DG and NPY immunoreactive cells in the basolateral amygdala (using a 135 × 135 μm area at 40× magnification) as well as for fluorescent neuroquantification for DCX and DAPI in the DG and DAPI in the basolateral amygdala (135 × 135 μm at 40× magnification). In all cases, quantification was accomplished using light-thresholding software (Bioquant Life Sciences, Nashville, TN, USA) so that the proportion of positively stained tissue to nonstained tissue could be determined for each visual field. For the determination of nestin-immunoreactive cells, the nestin-immunoreactive blood vessels were subtracted from the visual field so that only pyramidal neuronal cells were quantified.
2.9. Statistical Analysis
A two-way General Linear Model (GLM) analysis was used to determine the effects of sex (females and males) and coping profile (active, passive and flexible). The α-value was set at 0.05. Following the GLM analyses, Tukey post-hoc tests were conducted to further elucidate relationships among dependent variables. Correlation among dependent variables was tested using Pearson’s r. We used eta squared (η2) and Cohen’s-d to provide measures of effect size. Eta-squared indicates the percentage of variance in the dependent variable (DV) that is attributable to a particular independent variable (IV), and it is calculated as η2 = SSbetween/SStotal. Cohen’s-d indicates the size of the difference (effect size) between two means in standard deviation units for each pairwise comparison of interest, using the formula δ=μ2 − μ1/σ, where δ is the population parameter of Cohen’s-d, and where it is assumed that σ1 = σ2 = σ, i.e., homogeneous population variances, and μ1 is the mean of the respective population.
To assess the independent associations among selected variables, a multi-dimensional scaling (MDS) analysis was used. MDS is a data reduction technique used to reveal the similarities among variables and individual cases in a set of data (Kruskal and Wish, 1978). Distances between variables were derived looking at partial correlations (i.e., proximities) among variables, which were subsequently used to create a matrix of distance could be displayed graphically. The closer two or more variables are on the map, the more highly correlated they are, while the farther apart they are, the less correlated they are. In order to arrange the variable into a map sensitive to each individual contribution, a limited lack of fit between the data and the model is inevitable. This lack of fit is known as the s-stress. The values of s-stress range from 0 (perfect fit) to 1 (worst possible fit). Thus, the aim of MDS is to find a map of the variables that minimizes the s-stress. The number of dimensions in a map is linked to the number of latent underlying factors in the dataset, similarly to other procedures like factor analysis. As a consequence, the optimal number of dimensions to represent the data is dependent on several factors: (1) the number of variables in the model, (2) the lack of fit (s-stress value), given the number of dimensions, (3) an index of fit of the model (r2-value), and (4) interpretability of the dimensions (Manly, 1994). Typically, r2-values of 0.8 or higher are considered acceptable.
3. Results
3.1 Behavioral Results
CUS Behaviors
The total duration of floating (or immobility) during the CUS swim test did not differ significantly by sex (F1,41=1.47, N=47, p=0.23; η2=0.03) or by coping (F2,41=0.42, N=47, p=0.66; η2=0.02). Latency to float data, however, exhibited a trend toward significance by sex: females were quicker to float than males (F1,41=3.19, N=47, p=0.081; η2=0.06). Latency to float was not significantly different by coping (F2,41=1.08, N=47, p=0.35; η2=0.02). The number of diving attempts was significantly higher in males (F1,41=5.36; N=47, p=0.026; η2=0.08) and also varied significantly by coping (F2,41=3.85, N=47, p=0.029; η2=0.12); Tukey post hoc tests indicated that flexible copers exhibited a significantly higher number of diving attempts than both active and passive copers (p=0.01; Cohen’s d=0.70). A sex × coping significant interaction (F2,41=5.58, N=47, p=0.007; η2=0.17), however, indicated that, as seen in Figure 1A, flexible male rats exhibited the highest number of dives (Tukey post hoc test: p=0.03; Cohen’s d=1.86).
Figure 1. Chronic Stress Behaviors.
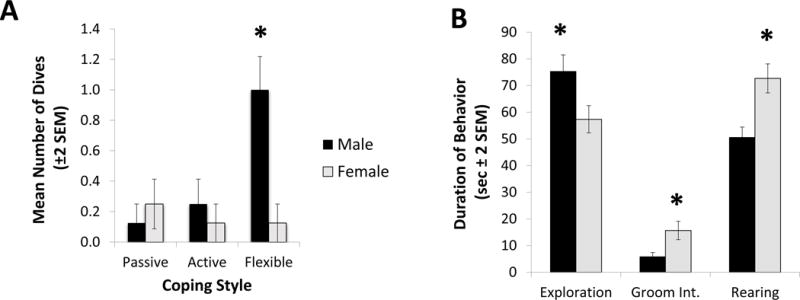
During chronic stress exposure, significant effects of coping and gender were observed in the swim task and conditioned stimulus task. (A) Although a main effect of sex was observed in the frequency of dives recorded during the swim task (F1,41=5.36; N=47, p=0.026; η2=0.08), a significant sex × coping interaction effect was also observed (F2,41=5.58, N=47, p=0.007; η2=0.17). Specifically, in the flexible coping group the males dove more than the females whereas no sex-based differences were observed in the comparable active and passive coping groups. (*) Indicates the significant post-hoc effect of male flexible copers (Tukey post hoc test: p=0.03; Cohen’s d=1.86). (B) The variable of sex significantly affected several behaviors recorded when the rats were exposed to the conditioned fear stimulus (block). Specifically, males explored the cage away from the block/conditioned stimulus longer than females (F1,41=5.24, N=47, p=0.027; η2=0.10). Additionally, males exhibited less grooming interruption than females (F1,41=5.65, N=47, p=0.022; η2=0.11). Females displayed a significantly higher rate of rearing near the block stimulus (F1,41=11.73, N=47, p=0.001; η2=0.20). (*) Indicates the significant main effect of sex in each of the three behaviors.
During the exposure to fox urine in the CUS phase of the study, no significant effects of sex or coping were observed in all behavioral measures (approaching, sniffing, burying, and digging) by sex and coping (all p-values > 0.1).
In the conditioned fear assessment during CUS, males explored the cage away from the block/conditioned stimulus longer than females (F1,41=5.24, N=47, p=0.027; η2=0.10 - see Figure 1B). Additionally, males exhibited less grooming interruption than females (F1,41=5.65, N=47, p=0.022; η2=0.11 - see Figure 1B). Females displayed a significantly higher rate of rearing near the block stimulus (F1,41=11.73, N=47, p=0.001; η2=0.20 - also depicted in Figure 1B). No differences between males and females were found in the rate of interactions with the block stimulus (F1,41=2.01; p=0.16; η2=0.02). Additionally, no effects of coping were found during the conditioned fear test (all p-values > 0.1); further, no significant interaction between sex and coping was observed (all p-values > 0.1).
DLM Behaviors
During acquisition day of DLM assessment, there were no significant main effects or sex by coping interactions in the frequency of approaching and eating the sweet cereal reward. Focusing on the duration of time spent in proximity to the previously baited well in the DLM probe test, no significant differences were found as well (all p-values > 0.1). However, males spent more time exploring than females (F1,41=4.65 N=47, p=.037; η2=0.09). Females, on the other hand, spent more time rearing than males during the probe test (F1,41=5.76, N=47, p=.021; η2=0.10). Further, a sex per coping interaction effect was found in relation to rearing behavior (F2,41=3.25 N=47, p=.049; η2=0.11); whereas active males tended to exhibit a longer duration of rearing than their passive and flexible coping counterparts, active females tended to spend less time in a rearing response than both passive and flexible females (Figure 2A). A sex per coping interaction effect was also found in relation to grooming interrupted (F2,41=3.38, N=47, p=.044; η2=0.13 - see Figure 2B); whereas flexible males tended to show grooming interruption more often, active females displayed more grooming interruption. No additional coping effects were observed for behaviors observed during the probe test (all p-values > 0.1).
Figure 2. Probe Trial Behaviors.
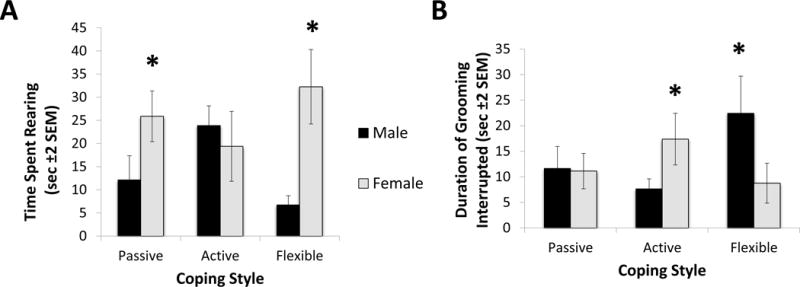
During the probe test, sex and coping affected rear responses and interrupted grooming bouts. (A) In addition to a main effect of sex observed in rearing behavior indicating that females spent more time rearing than males (F1,41=5.76, N=47, p=.021; η2=0.10), a sex × coping interaction effect was also found (F2,41=3.25 N=47, p=.049; η2=0.11); specifically, whereas no effect of sex was observed in active and passive coping groups, flexible females exhibited a higher duration of rearing than flexible males. (*) Indicates the significant difference between passive males and females and between flexible males and females. (B) During the probe test, a sex × coping interaction effect was found for grooming interrupted, associated with heightened anxiety (F2,41=3.38, N=47, p=.044; η2=0.13). Whereas flexible males exhibited a longer duration of grooming interruption, in females active copers exhibited a significant higher number of interrupted grooming. (*) Indicates the significant difference between active males and females and between flexible males and females.
3.2 Endocrine Results
Corticosterone metabolites increased significantly between the baseline levels collected at the beginning phases of the experimental procedures and after the second day of the swim test (F1,41=24.82, N=47, p<0.001; η2=0.37). An interaction effect between time and coping revealed that active copers increased their corticosterone metabolite levels significantly more than the other two groups (F2,41=4.02, N=47, p=0.025; η2=0.21 - Tukey post hoc test: p=0.03; Cohen’s d=0.56 - see Figure 3A). No significant difference in the corticosterone metabolite levels was observed between females and males (F1,41=1.71; p=0.19; η2=0.05), whereas a significant effect of coping was found (F2,41=6.28, N=47, p=0.04; η2=0.08), as well as a significant sex × coping effect (F2,41=4.53, N=47, p=0.017; η2=0.25 - see Figure 2B). Post hoc tests revealed that, while in females the differences among coping styles was not significant, active coper males exhibited the highest levels of cortisol metabolites (Tukey post hoc test: p=0.04; Cohen’s d=0.55).
Figure 3. Stress-Related Endocrine Measures.
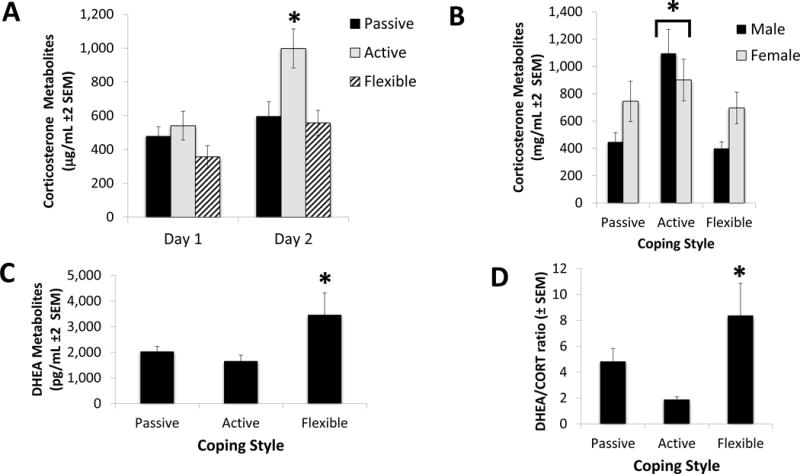
Coping profile categorization influenced corticosterone and DHEA levels across the swim task exposures; additionally a coping × stress effect was observed in the corticosterone data. (A) Corticosterone levels increased significantly from the baseline collection to after the forced swim task (F1,41=24.82, N=47, p<0.001; η2=0.37). Active copers, in particular, showed an elevated corticosterone activation following the swim stress (F2,41=4.02, N=47, p=0.025; η2=0.21 - indicated in the figure by *). (B) Corticosterone levels after the forced swim task were affected by coping (F2,41=6.28, N=47, p=0.04; η2=0.08) and by the interaction coping × sex (F2,41=4.53, N=47, p=0.017; η2=0.25). While the differences among the coping styles of females was not significant, male active copers had corticosterone levels more than double than both passive and flexible copers (indicated in the figure by *). (C) Flexible copers had significantly higher DHEA levels after the forced swim test than both active and passive copers (F2,41=3.42, N=47, p=0.04; η2=0.13). (D) Flexible copers had a significantly higher DHEA/Corticosterone ratio after the forced swim test than both active and passive copers (F2,41=4.38, N=47, p=0.019; η2=0.24).
DHEA metabolites increased significantly between the baseline levels and after the second day of the swim test during CUS exposure (F1,41=6.53, N=47, p=0.014; η2=0.13). No interaction effects between time and coping or between time and sex were found. Additionally, no significant difference in the DHEA metabolite levels was found between females and males (p=0.98; η2=0.001). Whereas a significant effect of coping was found (F2,41=3.42, N=47, p=0.04; η2=0.13 - see Figure 3C), no significant interaction effect between sex and coping was observed (p=0.20; η2=0.02). Post hoc tests revealed that DHEA metabolite levels were higher in flexible copers.
DHEA/corticosterone ratios also indicated a significant effect of coping (F2,41=4.38, N=47, p=0.019; η2=0.24 - see Figure 3D), but not of sex (p=0.88; η2=0.001) or the interaction coping × sex (p=0.52; η2=0.02). Tukey post hoc tests revealed that flexible copers had the highest ratio; also, they revealed that passive copers had a significantly higher ratio than active copers (p=0.02; Cohen’s d=1.12; and p=0.03, Cohen’s d=0.73 - respectively).
3.3 Neural Results
A significant coping effect was observed in the fos-immunoreactive (ir) cells quantified in the basolateral amygdala (F2,41=3.68, N=47, p=0.034; η2=0.10 - see Figure 4A), and a trend toward significance was shown in the insular cortex (F2,41=2.61; p=0.08; η2=0.06). Specifically, flexible copers had significantly lower levels of fos-ir in those two areas. No significant effects of sex or of the interaction between sex and coping were observed.
Figure 4. Neural Immunoreactive Cells.
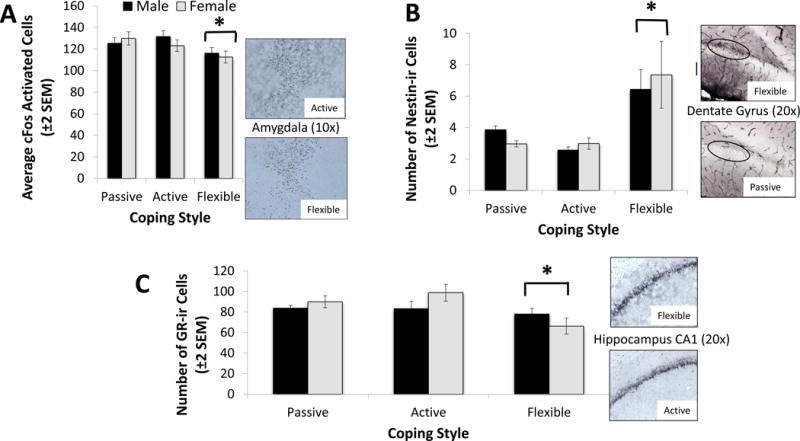
The coping profile significantly affected neuroplasticity markers in three brain areas: the amygdala, dentate gyrus and hippocampus CA1 and CA3 areas. (A) The average number of c-Fos immunoreactive cells (c-Fos-ir) in the amydgala was significantly lower in flexible copers, as indicated by (*) (F2,41=3.68, N=47, p=0.034; η2=0.10). No significant effect of sex was observed. (B) The average number of nestin- immunoreactive cells (Nestin-ir) in the dentate gyrus was significantly higher in flexible copers (F2,41=9.28, N=47, p<0.001; η2=0.33). No significant effect of sex was observed. (*) Indicates the significant difference between flexible copers and the other two groups. (C) The average number of glucocorticoid-receptors (GR-ir) in both the CA1 and CA3 areas were significantly lower in flexible copers CA1: F2,41=4.53 N=47, p=0.017; η2=0.15 - CA3: F2,41=6.51, N=47, p=0.003; η2=0.23). No significant effect of sex was observed. (*) Indicates the significant difference between flexible copers and the other two groups. Note: Since there were no significant differences between the active and passive copers, only one group was selected to compare to the flexible copers.
Coping style had a significant effect on the number of nestin-ir cells in the dentate gyrus (F2,41=9.28, N=47, p<0.001; η2=0.33 - see Figure 4B). Post-hoc tests indicated that flexible copers had a significantly higher number of nestin-ir cells than the other two groups (p<0.001; Cohen’s d=1.41). No significant effects of sex or of the interaction between sex and coping were observed.
The expression of glucocorticoid-receptors (GR) was also influenced by coping mechanisms in both the CA1 and CA3 areas (CA1: F2,41=4.53 N=47, p=0.017; η2=0.15 - CA3: F2,41=6.51, N=47, p=0.003; η2=0.23 - see Figure 4C for CA1 data). Tukey post hoc tests revealed that flexible copers exhibited the lowest levels of GR-ir in both hippocampal areas (p = 0.02; Cohen’s d=0.66; and p=0.01; Cohen’s d=1.20 - respectively). No significant effects of sex or of the interaction between sex and coping were observed in regard to the expression of glucocorticoid-receptors.
All other neural measures (DCX, Ki67, BDNF, NPY) did not yield significant effects by sex, coping, and their interaction effect (all p-values > 0.1). See Table 2 for significant and nonsignificant neural findings.
Table 2.
Histological antibodies used for immunocytochemistry investigation of neural markers in various brain areas and relevant significance.
| Stain | Brain area | *Significance |
|---|---|---|
| cFos | Amygdala | p<0.05 (coping effect) |
| cFos | Insular cortex | p<0.09 (coping effect) |
| Nestin | Dentate Gyrus | p<0.05 (coping effect) |
| Glucocorticoid receptor | CA1, CA3 | P<0.05 (coping effect) |
| cFos | CA1, piriform cortex, cingulate cortex, lateral septum, nucleus accumbens core and shell | p>0.05 |
| BDNF | CA1, CA3, anterior cingulate cortex | p>0.05 |
| K-I67 | Dentate gyrus | p>0.05 |
| Doublecortin | Dentate gyrus | p>0.05 |
| NPY | Amygdala | p>0.05 |
| GR | Amygdala | p>0.05 |
Light gray indicates areas found to be statistically significant or a nonsignificant trend; darker gray indicates no significance.
3.4 Integrative Multidimensional Scaling Map
To take into account the multivariate association among measures assessing different systems (neural, endocrine, and behavioral measures), multidimentional scaling (MDS) analyses was utilized to assess several different treatment combinations.
The best MDS model for coping generated the separation of groups as depicted in Figure 5. The Kruskal’s stress index determined a stress value equal to 0.07, indicating a good fit between the dimensions and the mapped distances. The R2 value designated that 95.3% of the variance was explained by the data. Based on the two dimensions, the integrative effect of coping on behavioral and neuroendocrine responses were divided into two major clusters, the left and right quadrants in the figure. Flexible animals were mostly clustered in the right quadrants of the figures, indicating an independent association with higher DHEA/corticosterone ratios, higher nestin-ir cells, and a bolder approach to the swimming task (high number of dives). The duration of rearing behavior overlapped with both clusters, confirming the univariate associations with the interaction effect coping × sex. On the left quadrants of the figure, the distinction between active and passive animals was less defined, and a couple of subjects (both active copers) were mapped outside both circles of influences. In the upper left quadrant, passive animals were associated with fos-ir in the amygdala, higher frequency of grooming interrupted, and heightened immunoreactivity of glucocorticoid receptors in the CA1. Active animals, in the lower left quadrants, were associated with fos-ir in the CA1.
Figure 5. Multidimensional Scaling Analysis.
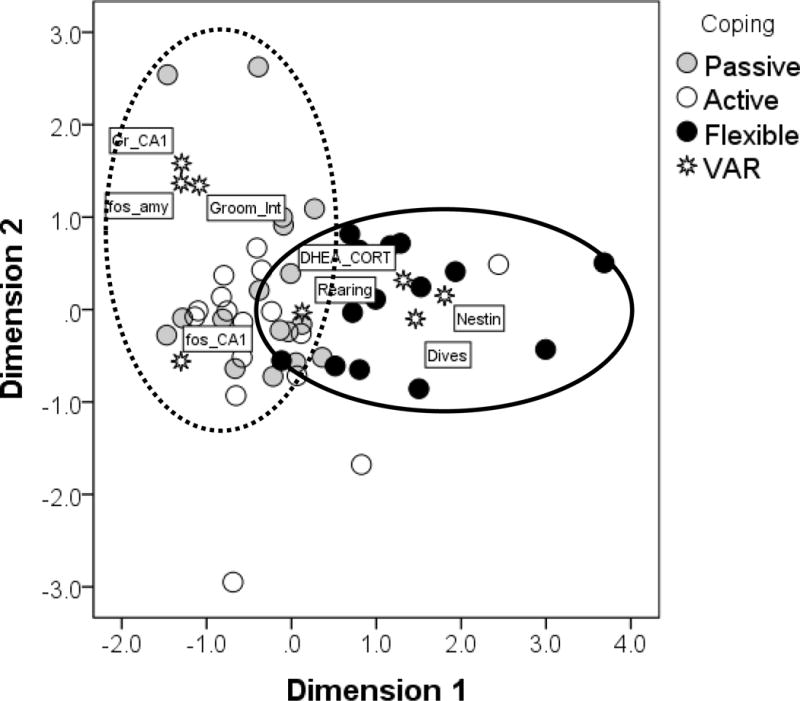
The multidimensional scaling (MDS) generated a map of associations among the variables selected (DHEA_CORT = DHEA/corticosterone ratio; Dives = number of dives in the swim task; Nestin = Nestin-ir cells; Rearing = Duration of rearing behavior; Groom-Int = Frequency of grooming interrupted; fos_CA1 = fos-ir cells in the CA1; fos_amy = fos-ir cells in the amygdala; Gr_CA1 = Glucocorticoids receptors in the CA1). This map was able to discriminate between flexible and consistent (both passive and active) copers, as indicated by the circles. Flexible copers were associated with higher DHEA/CORT ratios, Nestin-ir, duration of rearing, and number of dives. A couple of subjects (both active copers) were outliers outside of both areas of influence.
An acceptable model fitting the association with the dependent variables and sex did not emerge from the data collected in the current study. The best model that was generated was unsatisfactory (Kruskal’s stress index = 0.26; R2 = 63%). More importantly, the variables entered in the model failed to generate a clear separation between males and females.
4. Discussion
The results of the current study corroborate past findings suggesting that specific coping strategies influence stress responsivity (Shouten and Wiegant, 1997; Cavigelli and McClintock, 2003; Hawley et al., 2010). Specifically, flexible coping strategies have been associated with indicants of emotional resilience such as lower HPA-reactivity and increased neuroplasticity (Hawley et al., 2010, Lambert et al., 2014). Additionally, behavioral flexibility has been associated with involvement of the prefrontal cortex and dopaminergic and serotonergic activity (Coppens et al., 2010). In the current study, animals were exposed to chronic stress to activate the HPA axis prior to assessing responses to prediction errors in the DLM probe test. Chronic activation of the stress response provided an opportunity to assess responses in the DLM and subsequent probe trial following an extended time of heightened HPA activity. Whereas past research had not identified coping strategies as having an impact on responses to uncertainty in the DLM probe trial (Lambert et al., 2014), following the exposure to unpredictable chronic stress in the current study, coping strategies were influential in the prediction error/probe trial response assessments. Specifically, flexible copers exhibited less fos activation in the amygdala and insula than the more consistently responding active and passive copers. Further, although not specifically tied to the probe trial, heightened nestin-ir in the dentate gyrus was observed in the flexible copers following the cognitive training. Throughout the chronic stress exposure, flexible copers also responded with higher DHEA/corticosterone levels, accompanied by higher diving rates in the males. Flexible copers also had lower levels of glucocorticoid receptor immunoreactivity following chronic stress and cognitive training and testing. Because decreased fear activation, increased neuroplasticity, and higher DHEA levels have been associated with emotional resilience (Charney, 2003; McEwen et al., 2015), the flexible coping rats are viewed as exhibiting enhanced resilience in the current study. Thus, prior association with chronic unpredictable stress exacerbated the beneficial effects of utilizing a more varied, context-dependent coping strategy. Considering that our research thus far has focused on young adult animals, more research is necessary to determine the longer-term stability of the coping profile categorizations in middle and later adulthood. Additionally, it would be interesting to examine comparable stress responses in animals that do not neatly profile into the diverse coping profile categories investigated in the current study.
Providing an additional level of analysis, the multidimensional scaling analysis provided further support of the qualitatively different responses in the three coping groups. Specifically, the flexible coping rats were most strongly associated with higher rates of diving in the swim task, higher DHEA/CORT ratio levels during the CUS assessments, and increased nestin-ir following stress exposure and subsequent cognitive training. In contrast, there was no significant statistical separation of the active and passive coping groups and, focusing on the active-passive merged groups as a single “consistent” coping group, these rats were strongly associated with increased evidence of interrupted grooming sequences during CUS exposure, increased rates of fos-ir in the amygdala during the probe/prediction-error task and, following stress exposure and subsequent cognitive training, increased glucocorticoid receptor immunoreactivity in CA1 and CA3 areas of the hippocampus. Thus, converging evidence from both the MDS and the traditional GLM findings suggests that animals profiled as flexible copers exhibited more resilient responses to stress and uncertainty (e.g., increased behavioral activation, diminished HPA activity and enhanced neuroplasticity) than observed in the rats profiled as active and passive copers. Interestingly, recent research investigating the effects of DHEA in humans indicated increased connectivity among brain areas associated with enhanced emotional regulation (Sripada et al., 2013); hence, the DHEA response in flexible animals deserves further investigation as a key mechanism in resilience against depressogenic symptoms.
Although uncertainty in a foraging task appears to be less threatening than many stressors that have been introduced to laboratory animals, environmental conditions leading to uncertainty have been associated with heightened stress responses. After establishing the components of classical conditioning, Ivan Pavlov demonstrated how stressful uncertain conditions can be when he exposed dogs to ambiguous stimuli which prompted the animals to be uncertain about which stimulus was associated with shock. In this situation, the animals appeared extremely agitated, a condition he described as experimental neurosis (described in Windholz, 1990). Further, when human subjects are given a choice between uncertainty about future shock delivery and predicted immediate shock, they prefer imminent immediate shocks, exhibiting an aversion to uncertainty about the future delivery of shocks (Badia et al., 1966). Thus, cognitive uncertainty can generate a similar stress response as other threatening stimuli (Greco and Roger, 2003); further, it has been proposed that a low threshold to uncertainty is a reliable cognitive marker of depression susceptibility (Liao and Wei, 2011). If so, the identification of markers of enhanced tolerance of uncertainty, as well as effective and unbiased processing of safety and danger cues, will be informative in the understanding of developing treatment approaches to combat symptoms of anxiety and depression (Lohr et al., 2007; Grupe and Nitschke, 2013; Liao and Wei, 2011). In the current study, flexible coping strategies were associated with less stress responsiveness to prediction errors and uncertainty than observed in active and passive coping strategies (Lambert et al., 2014; Lambert et al., 2006).
In situations in which uncertainty disrupts an animals’ ability to effectively avoid threats in the environment, it represents a risk factor for the emergence of subsequent various anxiety disorders (Grupe and Nitschke, 2013). Because flexible copers exhibited a constellation of responses associated with less disruptive HPA activation in the form of higher DHEA/CORT ratios, less fear as indicated by decreased fos-ir in the amygdala and enhanced information processing by exhibiting increased rears, it appears that the flexible copers exhibited effective strategies in the face of uncertainty. Further, increased nestin-ir in the dentate gyrus following cognitive training suggests that the flexible copers may be better prepared to process new information about the environment, an ability that also diminishes uncertainty. Considering that individual differences in coping styles may provide a valuable model of genetic susceptibility to stress-related anxiety disorders, these results are interesting when considered in the context of a recent proposal that both chronic stress responsiveness and genetic susceptibility modify excitatory synaptic connections among specific components of the emotional brain circuitry (e.g., PFC and nucleus accumbens) associated with emotional disorders (Thompson et al., 2015). In accordance with the recently generated Research Domain Criteria by the NIMH as a constructive framework for research associated with psychiatric illness, the flexible copers’ responses in the current study can be viewed as impacting both the negative and positive valences, as well as the cognitive construct, included in that document. Specifically, flexible coping animals exhibited less evidence of fear and stress (negative valence systems) and more evidence of motivational responses (positive valence systems), as well as the potential for increased processing/learning (cognitive systems; NIMH; Insel, 2014).
Focusing on sex differences, males and females were only observed to be different in the behavioral tasks. In the probe trial of the DLM maze, males exhibited more general exploratory behavior while females exhibited more rears toward the center of the maze. Similar sex differences were observed in the conditioned fear test. Thus, it appeared that females and males may be gathering information in different ways, rearing in the females vs. exploratory ambulation across the cage in the males. Although the rodent literature generally reports a trend for females to have higher HPA reactivity accompanied by an enhanced susceptibility of female humans to develop adverse mental health symptoms (Bourke et al., 2012; Bangasser, 2013), no main effect of sex was observed in the stress endocrinological measures (i.e., including corticosterone, DHEA and glucocorticoid receptors) in the current study. Additionally, since many of the HPA-related effects have been found to be situation specific with age, estrus cycle and intensity of stressor influencing the results, more research is necessary in this area. It would be interesting, for example, to examine the responsivity of females in each stage of the estrous cycles.
Using various anxiety/conflict tests such as the elevated plus maze and the light-dark box, females have performed in a manner associated with less anxiety (Donner and Lowry, 2013). As previously described, in the probe trial, with estrous cycle controlled, a task that exposes the animals to a less anxiety-provoking context but assesses the animals’ response to uncertainty, sex differences were less apparent in the current study. In sum, the behavioral responses, although different between the sexes, did not clearly point to one sex exhibiting a more adaptive strategy—which is supported by the lack of sex differences observed in the endocrine and neural data. Although these findings corroborate prior research with animal models, the current findings are at odds with epidemiological research pointing to a preponderance of depressive disorders in human females (Piccinelli and Wilkinson, 2000). As previously mentioned, additional research focusing on more specific categories of symptoms and responses may resolve the incongruences between the human and nonhuman animal models in this area.
In summary, the coping profiles explored in the current study represent an interesting opportunity to explore individual differences in stress responsiveness and potential resilience to the emergence of depressogenic symptoms. Further, with the building evidence that intolerance to uncertainty represents an underlying relevant construct for all anxiety disorders (Carleton et al., 2012; Boswell et al., 2013), the probe trial of the Dry Land Maze cognitive assessment task is a valuable model to investigate key neurobiological elements of this important psychological construct. Thus, further exploration of the effects of variable and consistent coping strategies in response to both certain and uncertain threatening conditions may yield important information necessary for targeting behavioral and neurobiological markers of psychiatric illness, especially related to anxiety and mood disorders. As common denominators for these clusters of disorders are under investigation, the results of the current study point to relevant factors that are potentially transdiagnostic across mood and anxiety disorders (Carleton et al., 2012). Further, focusing on individual differences may facilitate the identification of precision-treatment approaches that may enhance the disappointing efficacy rates observed with pharmacological therapies (Andrews et al., 2012; Insel, 2014). In sum, because, compared to other medical conditions, biomarkers of psychiatric illness have not been sufficiently identified (Deacon, 2013), additional research is necessary to determine markers of specific psychiatric illnesses so that precautionary measures can be implemented to deter the likelihood that a mental illness, with all of its accompanying personal costs, will emerge (Insel and Quirion, 2005).
Highlights.
-
~
Coping strategies differentially affect stress responsivity
-
~
Flexible coping responses are associated with heightened resilience against the onset of depressogenic symptoms
-
~
Coping strategies are associated with mitigated stress responses and enhanced neuroplasticity in the current study
-
~
Despite sex differences in emotional responses in humans, they were not apparent in the neurobiological responses observed in the current study
Acknowledgments
This work was supported by NIMH award 1R15H101698-01A1 to Kelly Lambert.
References
- Abramson LY, Seligman MEP, Teasdale JP. Learned Helplessness in Humans: Critique and Reformulation. J Abnorm Psychol. 1978;87:49–74. [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW, Thomson JA, Amstadter A, Neale MC. Primum non nocere: an evolutionary analysis of whether antidepressants do more harm than good. Front Psychol. 2012;24 doi: 10.3389/fpsyg.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia P, McBane B, Suter S, Lewis P. Preference behavior in an immediate versus variable delayed shock situation with and without a warning signal. J Exper Psychol 24. 1966;72:847–852. [Google Scholar]
- Bangasser DA. Sex differences in stress-related receptors: “micro” differences with “macro” implications for mood and anxiety disorders. Biol Sex Diff. 2013;4:2. doi: 10.1186/2042-6410-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi M, Hampton JE, Lambert KG. Fecal dehydroepiandrosterone (DHEA) immunoreactivity as a noninvasive index of functional DHEA activity in male laboratory rats. Comp Med. 2010;60:455–460. [PMC free article] [PubMed] [Google Scholar]
- Bardi M, Rhone AP, Franssen CL, Hampton JE, Shea E, Hyer MM, Huber J, Lambert KG. Behavioral training and predisposed coping strategies interact to influence resilience in male Long-Evans rats: Implications for depression. Stress. 2012;15:306–317. doi: 10.3109/10253890.2011.623739. [DOI] [PubMed] [Google Scholar]
- Bardi M, True M, Franssen CL, Kaufman C, Rzucidlo A, Lambert KG. Effort-based reward (EBR) training enhances neurobiological efficiency in a problem-solving task: Insights for depression therapies. Brain Res. 2013;1490:101–110. doi: 10.1016/j.brainres.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Bardi M, Franssen CL, Hampton JE, Shea EA, Fanean AP, Lambert KG. Paternal experience and stress responses in the California mouse (Peromyscus californicus) Comp Med. 2011;60:20–30. [PMC free article] [PubMed] [Google Scholar]
- Boswell JF, Thompson-Hollands J, Farchione TJ, Barlow DH. Intolerance of uncertainty: A common factor in the treatment of emotional disorders. J Clin Psychol. 2013;69 doi: 10.1002/jelp.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: Adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012;62:210–218. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubic A, Cramon DY, Schubotz RI. Prediction, cognition and the brain. Front Hum Neuro. 2010 doi: 10.3389/fnhum.2010.00025. http://dx.doi.org/10.3389/frhum.2010.00025. [DOI] [PMC free article] [PubMed]
- Carleton RN, Mulvogue MK, Thibodeau MA, Mccabe RE, Antony MM, Asmundson GJG. Increasingly certain about uncertainty: Intolerance of uncertainty across anxiety and depression. J Anxiety Disord. 2012;26:468–479. doi: 10.1016/j.janxdis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. PNAS. 2003;100:16131–6. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Z, Roseboom PH, Nanda SA, Lane JC, Speers JM, Kalin NH. Anxiety-related behavioral inhibition in rats: A model to examine mechanisms underlying the risk to develop stress-related psychopathology. Genes Brain and Behav. 2010;9:974–094. doi: 10.1111/j.1601-183X.2010.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compare A, Zarbo C, Shonin E, Van Gordon W, Marconi M. Emotional regulation and depression: A potential mediator between heart and mind. Cardiovasc Psychiatry Neurol. 2014 doi: 10.1155/2014/324374. http//.dx.doi.org/10.1155/2014/324374. [DOI] [PMC free article] [PubMed]
- Coppens CM, de Boer SF, Koolhaas JM. Coping styles and behavioural flexibility: towards underlying mechanisms. Phil Trans R Soc B. 2010;365:4021–4028. doi: 10.1098/rstb.2010.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS. The psychobiology of resilience and vulnerability to anxiety disorders: implications for prevention and treatment. Dialogues Clin Neuro. 2003;5:207–221. doi: 10.31887/DCNS.2003.5.3/dcharney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Simon M. Neuroplasticity and Depression. Psychiatria Hungarica. 2005;20:4–17. [PubMed] [Google Scholar]
- Deacon B. The biomedical model of mental disorder: A critical analysis of its validity, utility, and effects on psychotherapy research. Clin Psychol Rev. 2013;33:846–861. doi: 10.1016/j.cpr.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Donner NC, Lowry CA. Sex differences in anxiety and emotional behavior. Eur J Physiol. 2013;465:601–626. doi: 10.1007/s00424-013-1271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endepols H, Sommer S, Backes H, Wiedermann D, Garf R, Hauber W. Effort-based decision making in the rat: An [18F] Fluorodeoxyglucose micro positron emission tomography study. J Neurosci. 2010;30:9708–9714. doi: 10.1523/JNEUROSCI.1202-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen CL, Bardi M, Shea EA, Hampton JE, Franssen RA, Kinsley CH, Lambert KG. Fatherhood enhances learning and associated neural responsiveness. J Neuroendocrin. 2011;36:2589–2602. doi: 10.1111/j.1365-2826.2011.02225.x. [DOI] [PubMed] [Google Scholar]
- Gaffey AE, Bergeman CS, Clark LA, Wirth MM. Aging and the HPA axis: Stress and resilience in older adults. Neurosci Biobehav Rev. 2016;68:928–945. doi: 10.1016/j.neubiorev.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genud R, Merenlender A, Gispan-Herman I, Maayan R, Weizman A, Yadid G. DHEA lessens depressive-like behavior via GABA-ergic modulation of the mesolimbic system. Neuropsychopharmacology. 2009;34:577–584. doi: 10.1038/npp.2008.46. [DOI] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB. Is behavioral inhibition a risk factor for depression? J Affect Disorders. 2006;95:85–94. doi: 10.1016/j.jad.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Greco V, Roger D. Uncertainty, stress and health. Pers Indiv Differ. 2003;34:1057–1068. [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley DF, Bardi M, Everette AM, Higgins TJ, Tu KM, Kinsley CH, Lambert KG. Neurobiological constituents of active, passive and variable coping strategies in rats: Integration of regional brain neuropeptide Y levels and cardiovascular responses. Stress. 2010;13:172–183. doi: 10.3109/10253890903144621. [DOI] [PubMed] [Google Scholar]
- Henderickson ML, Rao AJ, Demerdash ONA, Kalil RE. Expression of nestin by neural cells in the adult rat and human brain. Plos One. 2011;6:e18535. doi: 10.1371/journal.pone.0018535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn FA. Circuits, cells and synapses: Toward a new target for deep brain stimulation and depression. Neuropsychopharm Rev. 2012;37:307–308. doi: 10.1038/npp.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Quirion R. Psychiatry as a clinical neuroscience discipline. JAMA. 2005;294:2221–2224. doi: 10.1001/jama.294.17.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T. The NIMH research domain criteria (RDoC) project: Precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17:1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disorders. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Begt BJ, Van Reenen CG, Hopsger H, De Jong IC, Ruix MAW, Blokhuis JJ. Coping styles in animals: Current status in behavior and stress physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Kruskal JB, Wish M. Multidimensional Scaling. CA: Beverly Hills: Sage Publications; 1978. [Google Scholar]
- Johnson PM, Liig KR, Behan M, Haberly LB. New features of connections in pyriform cortex visualized by intracellular injections of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in the sensory system. J Neurosci. 2000;20:6974–85. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert KG. Rising rates of depression in today’s society: Consideration for the roles of effort-based rewards and enhanced resilience in day to day functioning. Neurosci Biobehav Rev. 2006;30:497–510. doi: 10.1016/j.neubiorev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Hyer MM, Rzucidlo AA, Bergeron T, Landis T, Bardi M. Contingency-based emotional resilience: effort-based reward training and flexible coping lead to adaptive responses to uncertainty in male rats. Front Behav Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert KG, Tu K, Everette A, Love G, McNamara I, Bardi M, Kinsley CH. Resilience in Children. Vol. 1094. New York City: New York Academy of Sciences; 2006. Explorations of coping strategies, learned persistence, and resilience in Long-Evans rats: Innate versus acquired characteristics; pp. 319–324. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiationonto habenula neurons in learned helplessness model of depression. Nature. 2011;470:535–39. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao KYS, Wei M. Intolerance of uncertainty, depression, and anxiety: the moderating and mediating roles of rumination. J Clin Psychol. 2011;67:1220–1239. doi: 10.1002/jclp.20846. [DOI] [PubMed] [Google Scholar]
- Lohr JM, Olatunji BO, Sawchuk CN. A functional analysis of danger and safety signals in anxiety disorders. Clin Psychol Rev. 2007;27:114–126. doi: 10.1016/j.cpr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Lu A, Steiner MA, Vogl AM, Walser SM, Abeitner M, Refogo D, Ekker M, Rubenstein jL, Stalla GK, Singewald N, Holsboer R, Wotjak CT, Wurst CT, Wurst W, Deussing JM. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Molecular Psychiatr. 2009;13:1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- Mackin P, Young AH. The role of cortisol and depression : Exploring new opportunities for treatments. Psychiatr Times. 2004 http://www.psychiatrictimes.com/cme/content/article/10168/47266.
- Manly BF. Multivariate Statistical Methods. Chapnell & Hall; London: 1994. [Google Scholar]
- Matsumoto K, Tanaka K. The role of the medial prefrontal cortex in achieving goals. Curr Opin Neurobiol. 2004;14:178–185. doi: 10.1016/j.conb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gray JD, Nasca C. Recognizing resilience: Learning from the effects of stress on the brain. Neurobiol Stress. 2015;1:1–11. doi: 10.1016/j.ynstr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JW, Lagnado D, Deal DC, Haggard P. Feelings of control: Contingency determines experience of action. Cognition. 2009;110:279–283. doi: 10.1016/j.cognition.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Rasmussen A, PIetrzak RH, Coric V, Southwick SM. Relationships among plasma dehydroepiandrosteone and dehydroepiandrosterone sulfate, cortisol, symptoms of dissociation, and objective performance in humans exposed to underwater navigation stress. Biol Psychiatr. 2009;66:334–340. doi: 10.1016/j.biopsych.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in depression. Current Directions in Psychol Sci. 2001;10:173–176. [Google Scholar]
- Ouwehand C, de Ridder DTD, Bensing JM. Individual differences in the use of proactive coping strategies by middle-aged and older adults. Pers Indiv Diff. 2008;45:28–33. [Google Scholar]
- Overmier JB, Seligman MEP. Effects of inescapable shock upon subsequent escape and avoidance responding. J Comp Physiol Psychol. 1967;63:28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th. Academic Press; New York, NY: 2007. [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. Brit J Psychiat. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, Depression, and Neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Rozman S. The effect of rat anterior inactivation in cognitive flexibility. Behav Neurosci. 2007;121:698–706. doi: 10.1037/0735-7044.121.4.698. [DOI] [PubMed] [Google Scholar]
- Rebola J, Castelhano J, Ferreira C, Castelo-Branco M. Functional parcellation of the operculo-insular cortex in perceptual decision-making: an fMRI study. Neuropsychologia. 2012;50:3693–3701. doi: 10.1016/j.neuropsychologia.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Overstreet C, Letkiewicz A, Grillon C. Depressed mood enhances anxiety to unpredictable threat. Psychol Med. 2012;42:1397–1407. doi: 10.1017/S0033291711002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens T. Choice, Uncertainty, and value in prefrontal and cingulate cortex. Nat Neuro. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Ryba MM, Hopko DR. Gender differences in depression: Assessing mediational effects of overt behaviors and environmental reward through daily diary monitoring. Depress Res Treat. 2012 doi: 10.1155/2012/865679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Nugent AC, Bogers W, Roiser JP, Bain Ee, Neumeister A, Zarate CA, Manji HK, Cannon DM, Marrett S, Henn F, Charney DS, Drevets WC. Habenula volume in bipolar disorder and major depressive disorder: a high-resolution magnetic resonance imaging study. Biol Psychiatry. 2011;69:336–343. doi: 10.1016/j.biopsych.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten WG, Wiegant VM. Individual responses to acute and chronic stress in pigs. Acta Phsyiologica Scandinavica Supplementum. 1997;640:640–88. [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse noxious agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rajaram N, Garfindel SN, Abelson JL, Liberzon I. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology. 2013;38:1798–1807. doi: 10.1038/npp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–73. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland PL, Deakin JRW, Percival C, Dixon J, Gater RA, Goldberg DP. The bio-social origins of depression in the community. Interactions between social adversity, cortisol and serotonin neurotransmission. Brit J Psychiat. 2002;180:168–173. doi: 10.1192/bjp.180.2.168. [DOI] [PubMed] [Google Scholar]
- Strunk W, Jr, White EB. The Elements of Style. fourth. Longman; New York: 2000. [Google Scholar]
- Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015;38:279–294. doi: 10.1016/j.tins.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SP, Aggleton JP, Maquire EA. What does the retrosplenial cortex do? Nat Rev Neuro. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Wechsler B. Coping and coping strategies: a behavioral view. Appl Anim Behav Sci. 1995;43:123–143. [Google Scholar]
- Windholz G. Pavlov, psychoanalysis and neurosis. Pavlov J Biol Sci. 1990;25:48–53. doi: 10.1007/BF02964603. [DOI] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, Bischoff-Grethe A, Lansing AE, Brown G, Strigo IA, Wu J, Paulus MP. Adolescents with major depression demonstrate increased amygdala activation. J Am Acad Child Adolesc Psychiatry. 2010;49:42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Braind SR, Colier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatrica Scandinavica. 2006;114:187–193. doi: 10.1111/j.1600-0447.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge, Arnlov J. Use of multiple biomarkers to imrove the prediction of death from cardiovascular causes. New Engl J Med. 2008;358:2107–16. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]


