Abstract
Context
Substantial innovation related to cancer prevention and treatment has occurred in recent decades. However, these innovations have often come at a significant cost. Cost-utility analysis provides a useful framework to assess if the benefits from innovation are worth the additional cost. This systematic review on published cost-utility analyses related to cancer care is from 1988 through 2013. Analyses were conducted in 2013–2015.
Evidence acquisition
This review analyzed data from the Tufts Medical Center Cost-Effectiveness Analysis Registry (www.cearegistry.org), a comprehensive registry with detailed information on 4,339 original cost-utility analyses published in the peer-reviewed medical and economic literature through 2013.
Evidence synthesis
There were 721 cancer-related cost-utility analyses published from 1998 through 2013, with roughly 12% of studies focused on primary prevention and 17% focused on secondary prevention. The most often studied cancers were breast cancer (29%); colorectal cancer (11%); and prostate cancer (8%). The median reported incremental cost-effectiveness ratios (in 2014 U.S. dollars) were $25,000 for breast cancer, $24,000 for colorectal cancer, and $34,000 for prostate cancer.
Conclusions
The current evidence indicates that there are many interventions that are cost effective across cancer sites and levels of prevention. However, the results highlight the relatively small number of cancer cost-utility analyses devoted to primary prevention compared with secondary or tertiary prevention.
Recent years have seen a rapid increase in new interventions in cancer prevention, diagnosis, and treatment.1 Although these treatments often improve survival and quality of life,1 in many cases they have also dramatically increased the cost of care. The National Cancer Institute estimates that costs of cancer care will increase by at least 27% from 2010 to 2020 simply due to an aging population.2 The economic impact will be particularly acute in the Medicare program because of the program’s limited ability to control service utilization and the increasing number of beneficiaries due to demographic changes.3 For example, Medicare is legally required to cover cancer drugs that are considered a “medically accepted indication,” which includes not only indications approved by the U.S. Food and Drug Administration but also indications supported by peer-reviewed publications and listed on medical compendia.4 However, across Europe, economic evaluation is used extensively to ensure that adopted treatments provide good value for the money.5 Articles about the rising costs of cancer care have increasingly appeared in the popular press and medical literature.1,5–8 However, the situation is complicated by the fact that some cancer interventions are seen as “low value,”4 whereas others may provide reasonable value by conventional economic benchmarks despite their high costs.
Economic evaluations of healthcare interventions provide a framework for assessing the value of new cancer screening, diagnosis, and treatments and are used to inform reimbursement and coverage decisions directly or indirectly in various countries,5,9–12 including by some payers in the U.S.13–15 Cost-effectiveness analysis (CEA) is a widely used economic evaluation technique to assess the value of healthcare interventions including cancer intervention programs.16–19 CEA describes an intervention’s impact in terms of an incremental cost-effectiveness ratio (ICER), which consists of its incremental costs divided by its incremental health benefits. To allow comparison of the relative efficiency of health-care interventions across a spectrum of conditions such as cancer, benefits are often measured using quality-adjusted life-years (QALYs), and the resulting CEA referred to as a cost-utility analysis (CUA), with a lower ICER corresponding to greater value.20 QALYs capture both changes in the length and quality of life.
Application of CEA in oncology has been described in previous work,16,21,22 summarizing CUAs prior to 2007.8 From 2008 through 2013, the number of cancer-related CUA articles nearly quadrupled, with an increased focus on primary and secondary prevention. In this article, the authors update their previous work, concentrating on specific cancer sites, as well as on treatment and primary and secondary prevention.
Evidence Acquisition
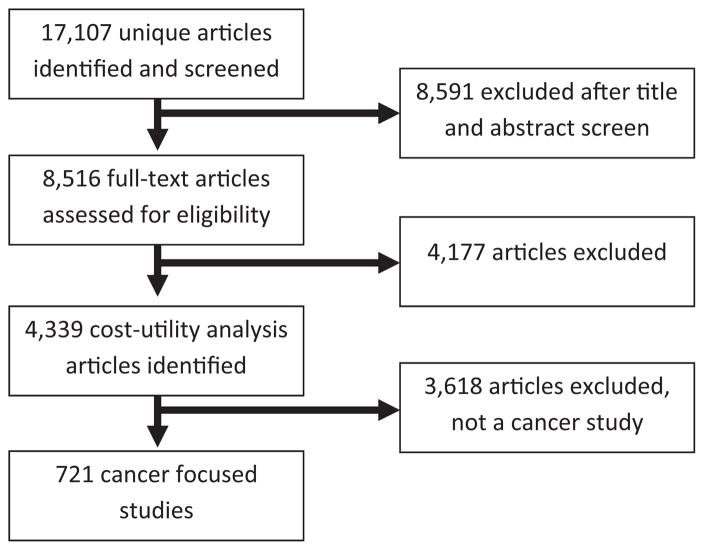
The authors analyzed data from the Tufts Medical Center CEA Registry (www.cearegistry.org), a comprehensive registry with detailed information on 3,243 original CEAs published in the peer-reviewed medical and economic literature from 1988 through 2013. Details of the CEA Registry have been described elsewhere.23 The review started with the first cancer-related CUA study conducted in 1988 and included all studies through 2013, the most recent year the CEA was updated when the study was conducted. The Tufts Medical Center CEA Registry contains original English-language CUA data that were obtained using MEDLINE, which searched for medical subject headings or text keywords such as quality-adjusted, quality-adjusted life year or QALY, and cost-utility analysis. A total of 10,693 unique articles were identified, 4,013 of which were selected for full-text review based on an assessment of titles and abstracts and 4,339 included in the CEA registry (Figure 1). The authors excluded reviews, editorials, methodologic articles, and articles presenting health effects in units other than QALYs. Each article was reviewed with the aid of a standard data auditing form to determine its clarity, completeness, and health economic methodologic quality.8 The quality assessment did not assess the accuracy of the clinical or modeling assumptions but rather if the authors correctly reported their results, stated assumptions, appropriately used utility weights, and characterized uncertainty. The form was developed based on a variety of sources, including the “checklist” for reporting reference-case CUAs recommended by the Panel on Cost-Effectiveness in Health and Medicine as well as other published guidelines.9,20 Two readers, with master’s- or doctoral-degree training in decision analysis and CEA, independently read and audited each article and then convened for a consensus audit to resolve discrepancies. The CEA Registry collected data on a wide variety of elements related to study sponsorship; the intervention and comparators under investigation; and the methods used to estimate and report costs, health effects, preference weights, modeling assumptions, and data limitations. The review included all studies that pertained to prevention, screening, and treatment of cancer, resulting in a total of 721 articles (Figure 1).
Figure 1.
PRISMA article identification and selection process.
The primary outcomes of CUAs are the costs and QALYs. The cost-utility ratio reports the incremental change of the new intervention compared to the status quo. Because the CUAs in this review were conducted in numerous countries using different currencies for a period of >20 years, all non-U.S. currencies were converted into U.S. dollars using the appropriate foreign exchange factor for the relevant year, and all ratios inflated to 2014 U.S. dollars using the Consumer Price Index obtained from the Bureau of Labor Statistics available from www.bls.gov/cpi/data.htm.
The authors grouped studies pertaining to cancer into 18 broad subcategories by the type of cancer treated or prevented: breast; colorectal; cervix; prostate; lung; melanoma; hematologic cancers; gastrointestinal and hepatocellular; ovarian; brain; bladder; kidney; head and neck (the nose, sinuses, lips, oral cavity, salivary glands, throat, or larynx)24; uterus; pancreas; stomach; esophagus; and other cancers. Because some CUAs compared several interventions and included scenarios specific to patient subgroups or settings, a single study may have contributed more than one cost-utility ratio. In these situations, the ratio was weighted inversely according to the number of ratios reported in the article. That is, each ratio was assigned a statistical weight of 1/n, where n was the number of ratios reported in an article.
The reported ratios were classified as most cost effective with values <$50,000, cost effective as $50,000–$100,000, and least cost effective as >$100,000 per QALY using willingness-to-pay threshold levels sometimes used by health economists. These threshold levels, though somewhat arbitrary, are based on conventions sometimes used in the U.S. to determine the cost effectiveness of an intervention.20 Additionally, some ratios report cost savings and increasing health, which health economists refer to as dominant and the authors report as “cost saving,” and other ratios report decreased health with increasing cost, which health economists refer to as dominated and the authors report as “decreased health, increased costs.” Chi-square tests were used to determine if categorical variables were statistically significant. Analyses were conducted in 2013–2015.
Interventions were classified as primary, secondary, or tertiary prevention.25 Primary prevention is defined as the avoidance of the onset of disease by behavior modification or treatment. Examples of primary prevention could include chemoprevention for breast cancer, immunization, health education, and promotion of improved nutrition. Secondary prevention is defined as the avoidance or alleviation of disease by early detection and appropriate management. Examples include early clinical services and population screening to identify disease in asymptomatic people to enable timely treatment. Tertiary prevention is defined as being the treatment of an active disease and may include treatment to reduce complications and progression of established disease, for example, chemotherapy, surgical removal of a tumor, and radiation therapy.
Evidence Synthesis
The authors identified 721 original cancer-related CUAs in the CEA Registry. Appendix A (available online) provides summaries of the articles. The annual average number of cancer-related CUAs has increased markedly over time (Appendix Figure 1, available online). Moreover, the proportion of studies focused on primary and secondary prevention has increased from an average of four studies per year between 1998 and 2006 to 20 studies per year between 2007 and 2011 to 100 studies per year in 2012 and 2013. The top three studied diseases were breast cancer (29% of studies); colorectal cancer (11%); and prostate cancer (8%) (Table 2). Most studies pertained to the U.S. (40% of studies), followed by the United Kingdom (14%); Canada (9%); and the Netherlands (6%) (Table 1). The majority examined interventions for tertiary prevention (i.e., chemotherapy and post-diagnosis interventions, 71%), followed by secondary prevention (i.e., screening, 17%) and primary prevention (i.e., vaccination, 12%). Most published CUAs focused on pharmaceutical interventions (51%), followed by screening strategies (16%) and medical procedures (11%). Approximately 30% of studies were funded by pharmaceutical or device companies; 49% were funded by non-industry sources (i.e., government, foundations, and healthcare organizations); and 21% did not disclose their funding source (Table 1).
Table 2.
Number of Studies and Incremental Cost-Effectiveness Ratios (ICERs) (2014 USD) by Cancer Types and Level of Preventiona
| Cancer type | Overall | Primary prevention | Secondary prevention | Tertiary prevention | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| N | Median ICER | N | Median ICER | N | Median ICER | N | Median ICER | |
| Overall | 721 | 29,000 | 90 | 23,000 | 120 | 34,000 | 511 | 29,000 |
|
| ||||||||
| Breast | 208 | 25,000 | 16 | 48,000 | 31 | 34,000 | 161 | 23,000 |
|
| ||||||||
| Colorectal | 76 | 24,000 | 7 | Cost- saving | 15 | 14,000 | 54 | 26,000 |
|
| ||||||||
| Cervix | 58 | 18,000 | 37 | 16,000 | 18 | 22,000 | 3 | 46,000 |
|
| ||||||||
| Prostate | 59 | 34,000 | 5 | 120,000 | 8 | 85,500 | 46 | 29,000 |
|
| ||||||||
| Lung | 58 | 37,000 | 4 | 17,000 | 8 | 61,000 | 46 | 38,000 |
|
| ||||||||
| Melanoma | 14 | 46,000 | 4 | 27,000 | 2 | 66,500 | 8 | 77,000 |
|
| ||||||||
| Hematologic | 57 | 34,000 | — | — | — | — | 57 | 34,000 |
|
| ||||||||
| Gastrointestinal and hepatocellular | 44 | 44,000 | 5 | 34,000 | 15 | 40,000 | 24 | 47,000 |
|
| ||||||||
| Ovarian | 20 | 26,500 | 2 | Cost-saving | 2 | 15,250 | 16 | 32,500 |
|
| ||||||||
| Brain | 12 | 41,000 | — | — | 1 | 2,400,000 | 11 | 39,000 |
|
| ||||||||
| Bladder | 5 | Cost-saving | — | — | — | — | 5 | Cost-saving |
|
| ||||||||
| Kidney | 15 | 97,000 | — | — | 3 | 41,000 | 12 | 108,500 |
|
| ||||||||
| Head and neck | 29 | 5,200 | 2 | 0 | 4 | 2,250 | 23 | 13,000 |
|
| ||||||||
| Uterus | 1 | Increases costs, decreases health | — | — | — | — | 1 | Increases costs, decreases health |
|
| ||||||||
| Pancreas | 12 | 66,000 | 1 | 20,000 | 1 | 86,000 | 10 | 77,000 |
|
| ||||||||
| Stomach | 1 | 35,000 | 1 | 35,000 | — | — | — | — |
|
| ||||||||
| Esophagus | 7 | 66,500 | — | — | 2 | 84,000 | 5 | 66,500 |
|
| ||||||||
| Other | 45 | 39,000 | 6 | 63,000 | 10 | 38,000 | 29 | 39,000 |
In each study, the estimated incremental cost-utility ratio was compared with the status quo (i.e., an older treatment regime, less effective screening technique, etc.).
Table 1.
Characteristics of the Cancer Cost-Utility Analysis Literature
| Characteristic | Number of studies (total N=721) | Percentage of studies, % |
|---|---|---|
| Country of study | ||
| U.S. | 287 | 40 |
| United Kingdom | 98 | 14 |
| Canada | 67 | 9 |
| Netherlands | 41 | 6 |
| Australia | 19 | 3 |
| Italy | 19 | 3 |
| Sweden | 18 | 2 |
| France | 17 | 2 |
| China | 15 | 2 |
| Other | 140 | 19 |
| Intervention | ||
| Pharmaceuticals | 367 | 51 |
| Screening | 118 | 16 |
| Medical procedure | 82 | 11 |
| Diagnostic | 53 | 7 |
| Other | 62 | 9 |
| Surgery | 39 | 6 |
| Level of prevention | ||
| Primary | 89 | 12 |
| Secondary | 121 | 17 |
| Tertiary | 511 | 71 |
| Study sponsorship | ||
| Non-industry | 349 | 49 |
| Industry | 146 | 30 |
| Not specified | 226 | 21 |
Note: Intervention categories consist of the following: Pharmaceuticals = any drug or biotech product used for medical treatment or prevention; Screening = refers to measures that detect disease (or test for risk factors) before it is symptomatic; Medical Procedure = non-surgical, non-diagnostic procedures; Diagnostic = a method used to determine if and what type of disease is present; Surgery = invasive, cutting involved (e.g., transplantation, although bone marrow transplantation would be a medical procedure, appendectomy).
Overall, the 721 analyses presented 2,053 ICERs (of which 52% CUAs presented more than one ratio). The median reported cost-effectiveness ratios (in 2014 USD) for all cancer-related CUAs were $29,000, which is not significantly different between prevention categories (p=0.11). The median reported cost-effectiveness ratios (in 2014 USD) were $25,000 for breast cancer, $24,000 for colorectal cancer, $18,000 for cervical cancer, $37,000 for lung cancer, and $46,000 for melanoma (Table 2).
The reported cost-effectiveness ratios for screening interventions were generally in ranges conventionally thought of as most cost effective (<$50,000), except for prostate and kidney cancer, which was still found to be cost effective ($50,000–$100,000) (Appendix Table 1). The search identified 118 studies focused on screening interventions. For cancers recommended for screening by the U.S. Preventive Services Task Force at the time these data were collected, the median ICER was $22,000 for breast, $15,000 for colorectal, and $28,000 for cervical. On the other hand, for cancers not recommended for screening at the time these data were collected, the median ICER was $30,000 for lung and $46,000 for melanoma. Few studies have examined the cost effectiveness of screening for melanoma.
The median ICER for interventions on primary cancer prevention programs was $23,000 (Table 2). For specific cancer sites, the median reported cost-effectiveness ratios were $48,000 for breast, cost saving for colorectal, $16,000 for cervical, $17,000 for lung, $120,000 for prostate, and $27,000 for melanoma. The distribution of ICERs by cancer site is presented in Table 3.
Table 3.
Distribution of Incremental Cost-Effectiveness Ratios for All Prevention Strategy Compared by Cancer Site at Different Willingness-to-Pay Thresholdsa
| Cancer type | Total (n) | Increases health, decreases costs, % | <50,000, % | 50,000–100,000, % | >100,000, % | Decreases health, increases costs, % |
|---|---|---|---|---|---|---|
| All prevention stages | ||||||
| Overall | 721 | 15 | 54 | 13 | 15 | 6 |
| Breast | 208 | 12 | 55 | 12 | 17 | 4 |
| Colorectal | 76 | 22 | 43 | 11 | 15 | 8 |
| Cervix | 59 | 5 | 78 | 6 | 8 | 3 |
| Prostate | 59 | 10 | 45 | 13 | 20 | 12 |
| Lung | 58 | 11 | 43 | 17 | 25 | 4 |
| Melanoma | 14 | 60 | 19 | 21 | 0 | 0 |
| Hematologic | 57 | 5 | 56 | 21 | 13 | 5 |
| Gastrointestinal and hepatocellular | 44 | 7 | 48 | 24 | 20 | 2 |
| Ovarian | 20 | 20 | 51 | 14 | 9 | 6 |
| Brain | 12 | 17 | 47 | 3 | 27 | 6 |
| Bladder | 5 | 73 | 27 | 0 | 0 | 0 |
| Kidney | 15 | 23 | 8 | 23 | 36 | 10 |
| Neck | 29 | 24 | 48 | 5 | 9 | 13 |
| Uterus | 1 | 33 | 67 | 0 | 0 | 0 |
| Pancreas | 12 | 17 | 24 | 28 | 20 | 12 |
| Stomach | 1 | 100 | 0 | 0 | 0 | 0 |
| Esophagus | 7 | 21 | 25 | 18 | 18 | 18 |
| Other | 45 | 20 | 37 | 15 | 18 | 10 |
| Primary prevention | ||||||
| Overall | 90 | 26 | 52 | 12 | 7 | 3 |
| Breast | 16 | 6 | 45 | 23 | 20 | 6 |
| Colorectal | 7 | 50 | 34 | 8 | 3 | 5 |
| Cervix | 37 | 5 | 85 | 4 | 5 | 2 |
| Prostate | 5 | 27 | 13 | 60 | 0 | 0 |
| Lung | 4 | 100 | 0 | 0 | 0 | 0 |
| Melanoma | 4 | 91 | 9 | 0 | 0 | 0 |
| Gastrointestinal and hepatocellular | 5 | 61 | 26 | 13 | 0 | 0 |
| Ovarian | 2 | 50 | 25 | 13 | 13 | 0 |
| Neck | 2 | 50 | 50 | 0 | 0 | 0 |
| Stomach | 1 | 100 | 0 | 0 | 0 | 0 |
| Other | 1 | 100 | 0 | 0 | 0 | 0 |
| Secondary prevention | ||||||
| Overall | 120 | 11 | 52 | 14 | 18 | 5 |
| Breast | 31 | 6 | 52 | 15 | 25 | 3 |
| Colorectal | 15 | 8 | 59 | 9 | 11 | 12 |
| Cervix | 18 | 7 | 70 | 10 | 8 | 4 |
| Prostate | 8 | 13 | 31 | 9 | 31 | 16 |
| Lung | 8 | 4 | 40 | 28 | 28 | 0 |
| Melanoma | 2 | 50 | 50 | 0 | 0 | 0 |
| Gastrointestinal and hepatocellular | 15 | 2 | 54 | 19 | 23 | 1 |
| Ovarian | 2 | 88 | 13 | 0 | 0 | 0 |
| Brain | 1 | 60 | 40 | 0 | 0 | 0 |
| Kidney | 3 | 33 | 28 | 11 | 11 | 17 |
| Neck | 4 | 25 | 75 | 0 | 0 | 0 |
| Pancreas | 1 | 50 | 50 | 0 | 0 | 0 |
| Esophagus | 2 | 13 | 38 | 50 | 0 | 0 |
| Other | 10 | 10 | 46 | 13 | 22 | 10 |
| Tertiary prevention | ||||||
| Overall | 511 | 16 | 46 | 15 | 16 | 6 |
| Breast | 161 | 14 | 57 | 10 | 15 | 4 |
| Colorectal | 54 | 22 | 40 | 12 | 17 | 8 |
| Prostate | 3 | 52 | 10 | 38 | 0 | 0 |
| Lung | 46 | 10 | 49 | 14 | 14 | 13 |
| Melanoma | 46 | 13 | 38 | 17 | 27 | 4 |
| Hematologic | 8 | 47 | 20 | 32 | 0 | 0 |
| Gastrointestinal and hepatocellular | 57 | 5 | 56 | 21 | 13 | 5 |
| Ovarian | 24 | 12 | 41 | 26 | 19 | 2 |
| Brain | 16 | 19 | 50 | 16 | 9 | 6 |
| Bladder | 11 | 19 | 52 | 3 | 23 | 3 |
| Kidney | 5 | 73 | 27 | 0 | 0 | 0 |
| Neck | 12 | 21 | 3 | 26 | 42 | 8 |
| Uterus | 23 | 22 | 48 | 7 | 11 | 13 |
| Pancreas | 1 | 33 | 67 | 0 | 0 | 0 |
| Esophagus | 10 | 20 | 13 | 34 | 19 | 14 |
| Other | 5 | 25 | 20 | 25 | 5 | 25 |
In each study, the estimated incremental cost-utility ratio was compared with the status quo (i.e., an older treatment regime, less effective screening technique, etc.). Percentages may not add to 100 because of rounding.
When the distribution of ICERs across all studies was assessed, 69% of ratios were <$50,000 or cost saving, 13% of ratios were between $50,000 and $100,000, and 21% of ratios were >$100,000 or decreased health and increased costs (Table 3). When the distribution of ICERs among primary prevention studies (Table 3) was examined, 78% were <$50,000 or cost saving, 12% were between $50,000 and $100,000, and 11% of ratios were >100,000 or decreased health and increased costs (Table 3). When looking at specific cancer sites among studies focused on primary prevention, the distribution of ICERs was similar across diseases, with the exception of prostate cancer (five studies), where at least half of the studies reported ICERs >$100,000.
There were more CUAs focused on tertiary (n=511) than secondary prevention (n=120) (Table 2). Though median ICERs were somewhat higher for secondary ($34,000) than for tertiary prevention ($29,000) (Table 2), the distribution was similar (Table 3), with 63% of studies reporting ICERs <$50,000 or cost saving, 31% reporting ICERs >$50,000, and 5%–6% reporting decreased health and increased costs.
Discussion
The authors systematically reviewed all published cancer-related CUAs and updated their previous review. Since 2007, the number of cancer-related CUAs has almost quadrupled. This is likely due to payers worldwide (e.g., the National Institute for Health and Care Excellence in the United Kingdom) using data from economic evaluations to inform and support reimbursement decisions.5,9,11,12 However, the focus of studies is similar to prior work.8 Consistent with cancer-related CUAs published before 2008, this study found that researchers have devoted relatively little attention, as measured by publications, to the cost effectiveness of primary prevention compared with secondary or tertiary prevention.8 As seen in work published before 2008, the majority of primary prevention studies were focused on cervical or breast cancer. For secondary prevention, there have been many CUAs focused on breast, colorectal, and cervical cancer, which is perhaps not surprising as these are cancer sites where screening is recommended. By contrast, few CUAs have examined screening for melanoma or lung cancer, areas in which screening are not recommended during our study period. However, in recent years, there has been movement to recommend low-dose computed tomography screening to detect lung cancer for specified group of individuals at high risk.23 CUA studies published since 2013 on lung cancer have been mixed, and have shown that the cost effectiveness of lung cancer screening is very sensitive to the assumptions on the effectiveness of screening.24–26
Numerous CUAs have examined tertiary prevention. Interestingly, the median and distribution of cancer cost-effectiveness ratios are similar to those found in other fields of health care.26–29 Despite the fact that this updated review of cancer-related CUAs included many new expensive cancer drugs (e.g., cetuximab, bevacizumab, sunitinib, sorafenib), the distribution of ICERs has remained relatively constant over time. However, the cost-effectiveness literature may have been subject to publication bias in the sense that researchers and funders may selectively conduct cost-effectiveness studies and under-report unfavorable cost-effectiveness results.27
Limitations
This study has several limitations. First, it excluded CEAs that used measures of health outside of the QALY metric (e.g., cost per life-year gained or cost per 1 year of progression-free survival). Second, it only included English-language peer-reviewed publications indexed in MEDLINE. For example, health technology assessment reports, created by governmental bodies such as the National Institute for Clinical Excellence in the United Kingdom or other health technology assessment agencies, were excluded. However, the findings in some of these reports are complementary to the reviewed studies.5,9,11,12 Third, this study did not assess the merits of clinical or modeling assumptions included in analyses or the quality of the data collected in studies conducted alongside clinical trials. Fourth, environmental, system, and policy interventions were not included in the reviewed studies. Fifth, this study only goes through 2013 and does not capture more recently published studies.
A final point worth emphasizing is that policy-makers using cost-utility information should examine carefully the individual studies and their interventions, comparators, and target populations when making decisions. The results presented here are summary data that provide a portrait of the literature to date. However, individual studies will investigate very different contexts (e.g., a colorectal cancer screening finding may be based on a comparison of colonoscopy versus fecal occult blood testing, whereas a treatment for stomach cancer result might be compared with diet and nutrition counseling). In recognition of the importance of economic evaluation, some countries have begun to use outcomes of economic analyses to inform coverage decisions, which has in turn contributed to restrictions in patients’ access to new and expensive drugs.9,10,12,30,31 Some have also acknowledged the unique circumstances of end-of-life care and have created mechanisms for coverage of cancer drugs or more flexible reimbursement criteria.9,10,12 For example, in Canada or the United Kingdom, cancer drugs have been adopted at higher than average thresholds of cost effectiveness.10,12 However, these higher thresholds appear to apply only to drugs and not to cancer screening.
Conclusions
In summary, the current evidence indicates that there are many interventions that are cost effective across cancer sites and stages of prevention. However, the results highlight the relatively small number of cancer CUAs devoted to primary prevention compared with secondary or tertiary prevention.
Supplementary Material
Acknowledgments
Funding for this study was provided by the Division of Cancer Prevention and Control, CDC. We are grateful to Teja Thorat for help with data collection and analysis and Megan Farquhar for comments on the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
Appendix. Supplementary data
Supplementary data associated with this article can be found at http://dx.doi.org/10.1016/j.amepre.2015.08.009.
Footnotes
No financial disclosures were reported by the authors of this paper.
References
- 1.Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27(23):3868–3874. doi: 10.1200/JCO.2009.23.1183. http://dx.doi.org/10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. http://dx.doi.org/10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lubitz J, Cai L, Kramarow E, Lentzner H. Health, life expectancy, and health care spending among the elderly. N Engl J Med. 2003;349(11):1048–1055. doi: 10.1056/NEJMsa020614. http://dx.doi.org/10.1056/NEJMsa020614. [DOI] [PubMed] [Google Scholar]
- 4.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626–633. doi: 10.1056/NEJMhpr0807774. http://dx.doi.org/10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 5.Drummond MF, Mason AR. European perspective on the costs and cost-effectiveness of cancer therapies. J Clin Oncol. 2007;25(2):191–195. doi: 10.1200/JCO.2006.07.8956. http://dx.doi.org/10.1200/JCO.2006.07.8956. [DOI] [PubMed] [Google Scholar]
- 6.Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst. 2009;101(15):1044–1048. doi: 10.1093/jnci/djp177. http://dx.doi.org/10.1093/jnci/djp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnipper LE, Meropol NJ, Brock DW. Value and cancer care: toward an equitable future. Clin Cancer Res. 2010;16(24):6004–6008. doi: 10.1158/1078-0432.CCR-10-1643. http://dx.doi.org/10.1158/1078-0432.CCR-10-1643. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg D, Earle C, Fang CH, Eldar-Lissai A, Neumann PJ. When is cancer care cost-effective? A systematic overview of cost-utility analyses in oncology. J Natl Cancer Inst. 2010;102(2):82–88. doi: 10.1093/jnci/djp472. http://dx.doi.org/10.1093/jnci/djp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason AR, Drummond MF. Public funding of new cancer drugs: is NICE getting nastier? Eur J Cancer. 2009;45(7):1188–1192. doi: 10.1016/j.ejca.2008.11.040. http://dx.doi.org/10.1016/j.ejca.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 10.Rocchi A, Menon D, Verma S, Miller E. The role of economic evidence in Canadian oncology reimbursement decision-making: to lambda and beyond. Value Health. 2008;11(4):771–783. doi: 10.1111/j.1524-4733.2007.00298.x. http://dx.doi.org/10.1111/j.1524-4733.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 11.Cairns J. Providing guidance to the NHS: The Scottish Medicines Consortium and the National Institute for Clinical Excellence compared. Health Pol. 2006;76(2):134–143. doi: 10.1016/j.healthpol.2005.05.006. http://dx.doi.org/10.1016/j.healthpol.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Raftery JP. Paying for costly pharmaceuticals: regulation of new drugs in Australia, England and New Zealand. Med J Aust. 2008;188(1):26–28. doi: 10.5694/j.1326-5377.2008.tb01500.x. [DOI] [PubMed] [Google Scholar]
- 13.Neumann PJ. Using Cost-Effectiveness Analysis to Improve Health Care: Opportunities and Barriers. New York: Oxford University Press; 2005. p. xii.p. 209. [Google Scholar]
- 14.Neumann PJ, Greenberg D. Is the United States ready for QALYs? Health Aff. 2009;28(5):1366–1371. doi: 10.1377/hlthaff.28.5.1366. http://dx.doi.org/10.1377/hlthaff.28.5.1366. [DOI] [PubMed] [Google Scholar]
- 15.Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost-effectiveness analysis. N Engl J Med. 2005;353(14):1516–1522. doi: 10.1056/NEJMsb050564. http://dx.doi.org/10.1056/NEJMsb050564. [DOI] [PubMed] [Google Scholar]
- 16.Neumann PJ, Thorat T, Shi J, Saret CJ, Cohen JT. The changing face of the cost-utility literature, 1990–2012. Value Health. 2015;18(2):271–277. doi: 10.1016/j.jval.2014.12.002. http://dx.doi.org/10.1016/j.jval.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Amin NP, Sher DJ, Konski AA. Systematic review of the cost effectiveness of radiation therapy for prostate cancer from 2003 to 2013. Appl Health Econ Health Policy. 2014;12(4):391–408. doi: 10.1007/s40258-014-0106-9. http://dx.doi.org/10.1007/s40258-014-0106-9. [DOI] [PubMed] [Google Scholar]
- 18.Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N. A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer. Health Technol Assess. 2001;5(32):1–195. doi: 10.3310/hta5320. http://dx.doi.org/10.3310/hta5320. [DOI] [PubMed] [Google Scholar]
- 19.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(2):96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. http://dx.doi.org/10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Public Health Service Panel on Cost-effectiveness in Health and Medicine, United States. Cost-effectiveness in Health and Medicine: Report to the US Public Health Service. Washington, DC: Office of Disease Prevention and Health Promotion; 1996. p. xviii. [Google Scholar]
- 21.Shih YC, Halpern MT. Economic evaluations of medical care interventions for cancer patients: how, why, and what does it mean? CA Cancer J Clin. 2008;58(4):231–244. doi: 10.3322/ca.2008.0008. http://dx.doi.org/10.3322/CA.2008.0008. [DOI] [PubMed] [Google Scholar]
- 22.Grusenmeyer PA, Wong YN. Interpreting the economic literature in oncology. J Clin Oncol. 2007;25(2):196–202. doi: 10.1200/JCO.2006.09.0738. http://dx.doi.org/10.1200/JCO.2006.09.0738. [DOI] [PubMed] [Google Scholar]
- 23.Neumann PJ, Greenberg D, Olchanski NV, Stone PW, Rosen AB. Growth and quality of the cost-utility literature, 1976–2001. Value Health. 2005;8(1):3–9. doi: 10.1111/j.1524-4733.2005.04010.x. http://dx.doi.org/10.1111/j.1524-4733.2005.04010.x. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute. [Accessed May 16, 2014];Head and neck cancer. 2014 www.cancer.gov/cancertopics/types/head-and-neck.
- 25.Martin EA. Concise Medical Dictionary. 7. Oxford: Oxford University Press; 2007. p. 807. [Google Scholar]
- 26.Wilson AW, Neumann PJ. The cost-effectiveness of biopharmaceuticals: A look at the evidence. MAbs. 2012;4(2):281–288. doi: 10.4161/mabs.4.2.18812. http://dx.doi.org/10.4161/mabs.4.2.18812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell CM, Urbach DR, Ray JG, et al. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332(7543):699–703. doi: 10.1136/bmj.38737.607558.80. http://dx.doi.org/10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen JT, Neumann PJ, Weinstein MC. Does preventive care save money? Health economics and the presidential candidates. N Engl J Med. 2008;358(7):661–663. doi: 10.1056/NEJMp0708558. http://dx.doi.org/10.1056/NEJMp0708558. [DOI] [PubMed] [Google Scholar]
- 29.Dalziel K, Segal L, Mortimer D. Review of Australian health economic evaluation—245 interventions: what can we say about cost effectiveness? Cost Eff Resour Alloc. 2008;6:9. doi: 10.1186/1478-7547-6-9. http://dx.doi.org/10.1186/1478-7547-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrag D. The price tag on progress—chemotherapy for colorectal cancer. N Engl J Med. 2004;351(4):317–319. doi: 10.1056/NEJMp048143. http://dx.doi.org/10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 31.Shemer J. Year 2006 update of the National List of Health Services—an endless process. Isr Med Assoc J. 2006;8(9):646–648. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.