Abstract
Background:
There is insufficient evidence to support the effectiveness of multidisciplinary rehabilitation on the health-related quality of life (HRQoL) of MS patients.
Objectives:
To evaluate the longer term effectiveness of inpatient multidisciplinary rehabilitation on the HRQoL of MS patients.
Methods:
The study was a two-hospital, pragmatic, randomized controlled trial with a 6-month follow-up. Patients aged 18–65 years with MS and Expanded Disability Status Scale scores ≤7.5 were randomly assigned (1:1) to 4 weeks of inpatient multidisciplinary rehabilitation (20 days of scheduled rehabilitation) or 6 months on a wait list. The outcome measures were Functional Assessment in Multiple Sclerosis (FAMS), Multiple Sclerosis Impact Scale-29 (MSIS-29), EQ-5D-5L and 15D.
Results:
We randomized 213 patients to the wait-list control group and 214 patients to the treatment group. Trends in favour of the treatment group were observed across all measures. However, the difference was significant in only two of the six measures. The treatment effect was −2.7 (95% CI: −5.6 to (−0.1)), p = 0.046) for the MSIS-29 Psychological and 0.017 (95% CI: 0.005–0.030, p = 0.008) for the 15D. FAMS, which we used to calculate the sample size, was not significant.
Conclusion:
The results indicated that inpatient multidisciplinary rehabilitation is effective in improving the HRQoL of MS patients after 6 months.
Keywords: Multidisciplinary rehabilitation, rehabilitation, multiple sclerosis, quality of life, inpatient, randomized controlled trial, pragmatic clinical trial
Introduction
Despite recent advances in medical management, MS remains a chronic, progressive neuroinflammatory and neurodegenerative disease with increasing symptom development and functional loss over time.1 This progressive nature has severe implications for the individual on many levels,2 leading to ongoing declines in quality of life (QoL).3 The prevalence of poor health-related QoL (HRQoL) is high among MS patients;4 thus, improving or at least preserving the QoL of these patients is imperative. Therefore, multidisciplinary rehabilitation (MDR) remains a cornerstone of MS treatment to maintain the best possible QoL in MS patients.3
Rehabilitation has a long history and currently serves as a health strategy that, based on the World Health Organization (WHO)’s integrative model of Functioning, Disability and Health (ICF),5 focuses on achieving and maintaining optimal functioning.6 MS rehabilitation applies and integrates different approaches to strengthen the patients’ mastery of the disease, to preserve independence and self-reliance and to address future symptoms and requirements to enhance HRQoL,3,6,7 which is the subjective perspective on health status,6,8 consistent with the Danish White Paper on Rehabilitation.9 The few randomized controlled trials (RCTs) conducted on MDR have shown improved overall activity and participation according to the ICF7,10–12 and some limited but insufficient evidence to improve HRQoL.10,12 Comparisons of the results are difficult because the studies used different MS populations, rehabilitation types and intensities and outcome measures.
A Cochrane review13 concluded that future research should focus on improving the methodological and scientific rigour of clinical trials. Further research into appropriate outcome measures of HRQoL and the optimal intensity, frequency, cost and longer term effectiveness of MDR are warranted. In trying to address these issues, we have conducted a pragmatic study of inpatient MDR in MS patients. In the present article, which is the first in a series of articles on the study, we present the HRQoL results from a controlled 6-month follow-up.
Patients and methods
Study design
This study is reported in accordance with the CONSORT 2010 statement14 and CONSORT Extension for Randomized Trials of Non-pharmacologic Treatment.15 It was a pragmatic, two-hospital clinical trial with a semi-crossover hybrid design, with a controlled outpatient 6-month follow-up and an uncontrolled outpatient 12-month follow-up. Many other measures in addition to HRQoL were undertaken and they will be reported in subsequent articles, as well as the results from the 12-month follow-up. For details of the study design, see the published protocol.16
The Danish Research Ethics Committee approved the protocol (ref. no. 1-01-83-0002-07).16 The Danish Data Authority granted permission to collect and store the required project information (ref. no. 2011-41-6751). The study was registered at www.controlled-trials.com (http://www.controlled-trials.com/ISRCTN05245917).
Participants
We recruited study participants from all patients referred to 4 weeks of inpatient MDR from March 2012 to June 2014. The inclusion criteria were as follows: age between 18 and 65 years, diagnosis of MS, Expanded Disability Status Scale (EDSS) score ≤7.5, and familiarity with the use of a personal computer. The exclusion criteria were as follows: within 3 months of relapse; less than 6 months from diagnosis; participation in inpatient rehabilitation within 6 months; cognition subscale score (EDSS Functional System) >2 or cognitive limitations or any other illness that could impede study participation. The screening neurologist made the final decision on inclusion and written informed consent was obtained from all participants.
The study occurred at the Danish MS Hospitals in Ry and Haslev, which offer inpatient MDR for MS patients. They have 36 and 42 beds with annual admissions of approximately 500 and 600 patients, respectively. They operate as one unit with one director.
Randomization and masking
Patients were randomly assigned (1:1) to either the treatment group or the wait-list control group using a computer-generated minimization sequence17 to ensure balance between the groups for expected potential prognostic factors. Factors for minimization were sex, age, MS type, time since the first symptom, time since diagnosis, EDSS score, and first referral. The neurologists enrolled the patients, and the researchers performed the randomization and assigned the patients to groups. The allocation sequence was concealed until the moment of assignment. Because of the nature of the study, blinding of the patients and MS specialists (professionals performing the MDR) was not possible. There were no assessors because all outcome measures were self-completed questionnaires.
Wait-list control group
Wait-list control group patients were assigned to a wait list for 6 months. However, they were hospitalized more quickly than those on the general wait list. The general expected waiting time from referral to admission was approximately 1 year at that time. During the wait, patients were not precluded from participating in local community-based training or services, including physiotherapy and occupational therapy. They were also regularly seen by their neurologists at the MS Clinics. Wait-list control patients were admitted shortly after reassessment at the 6-month follow-up. After discharge, the treatment patients had access to the same community-based options. It would have been unethical to restrict patients’ access to local practice and MS Clinics. We excluded and admitted the wait-list control patients who were in acute need of MDR.
The multidisciplinary rehabilitation programme
Within 2 weeks after randomization, the treatment patients received a comprehensive inpatient MDR programme in line with the NICE guidelines.18 It was organized as 4 weeks of continuous hospitalization with 20 days of scheduled rehabilitation. Patients were free to return home for the weekend or remain in the unit. The MDR programme was MS specialized according to the principles charted by the Danish Health Authority.19 A case manager and a team of MS specialists were assigned to every patient based upon personal needs and goals.
In total, 177 MS specialists, including neurologists, neuropsychologists, clinical psychologists, social workers, occupational therapists, physiotherapists, nutritional therapists, dieticians, nurses and nursing assistants, provided the MDR treatment. The MS specialists included 160 females and 17 males, with a median practice experience at the MS hospitals of 8 years (range: 1–32). The case managers were experienced physiotherapists, occupational therapists or nurses with specialized MS knowledge. The majority were certified coaches and oversaw the effort of each patient’s team and progress towards predetermined goals. The neurologist served as a consultant for the patient and the supervisor of the team. Symptomatic drug therapy was managed in accordance with consensus guidelines20 and the neurologist’s judgement. The MDR programme was individualized, holistic and balanced, with input from the different disciplines depending on the patient’s main focus area, such as energy and fatigue, cognitive function, physical function or mental resilience.
The MDR programme consisted of a mean of approximately 3.5 hours of therapy per day (range: 1.9–6.9), including consultation with the neurologist, individual and group-based physiotherapy and occupational therapy, sessions with neuropsychologists and psychologists and lessons on different topics either group-based or individual discussions. Physiotherapy and occupational therapy combined with supervised self-directed exercise constituted a mean of 2 hours of interrupted sessions per day (range: 0.4–5.2). See the Supplementary Material for a more detailed description of the different activities of the MDR programme. No continuation or repetition of the MDR programme was available after discharge.
Measurements
Assessments and reassessments of the patients occurred at baseline, discharge (treatment group), 2 months after baseline (treatment group) and 6 months after baseline. We used Danish versions of the validated HRQoL measures. The primary outcome was the change in MS-specific HRQoL at 6 months from baseline, measured by the Functional Assessment of Multiple Sclerosis questionnaire (FAMS)21 and the Multiple Sclerosis Impact Scale-29 version 2 (MSIS-29).22 The secondary outcome was the change in generic HRQoL at 6 months from baseline, measured by the EQ-5D-5L23 and 15D questionnaires.24
All patients answered the FAMS, MSIS-29, EQ-5D-5L and 15D at baseline and again 6 months later (6-month follow-up). Additionally, the treatment group completed the FAMS, MSIS-29, and EQ-VAS at discharge. The FAMS was completed again 1 month after discharge (2-month follow-up). We assigned patients with a unique study identification number and password, providing access to a web-based data collection system25 where the patients completed the online questionnaires at home.
Statistical analysis
The sample size calculation was based on published FAMS data26 and indicated an optimal sample size of 210 in each group (5-point difference, standard deviation = 20, significance level = 5%, power = 90%, expected maximum attrition rate = 20%). Descriptive statistics were calculated to describe the groups in terms of baseline demographics, disease characteristics and outcome scores.
We used linear mixed-effects models to estimate for all outcomes: (1) adjusted Least Squares (LS) means at baseline, (2) LS mean changes from baseline to discharge and at the 2- and 6-month follow-ups, and (3) the treatment effect at the 6-month follow-up. Group, baseline score, minimization factors and the use of immunomodulatory therapy were modelled as fixed effects, and the time of randomization and hospital (and individual in case 2) were modelled as random effects.
We analysed data using the Intention-To-Treat (ITT) principle with multiple imputation by random forests on bootstrapped data. For all estimates, we obtained bootstrap means and 95% confidence intervals (CIs). The p values were calculated by comparing the estimated t-value to the bootstrap t-value distribution under the null hypothesis of no treatment effect. An Exploratory Factor Analysis (EFA) was conducted to identify possible lower dimensional latent factors underlying the correlated outcomes, and the identified factors were added to the set of outcomes (see Supplementary Material).
In a post hoc analysis, changes in scores at 6 months were categorized as improved, unchanged or deteriorated. The results were analysed using chi-squared statistics. We used the statistical software R version 3.3.2.27
Results
Patients
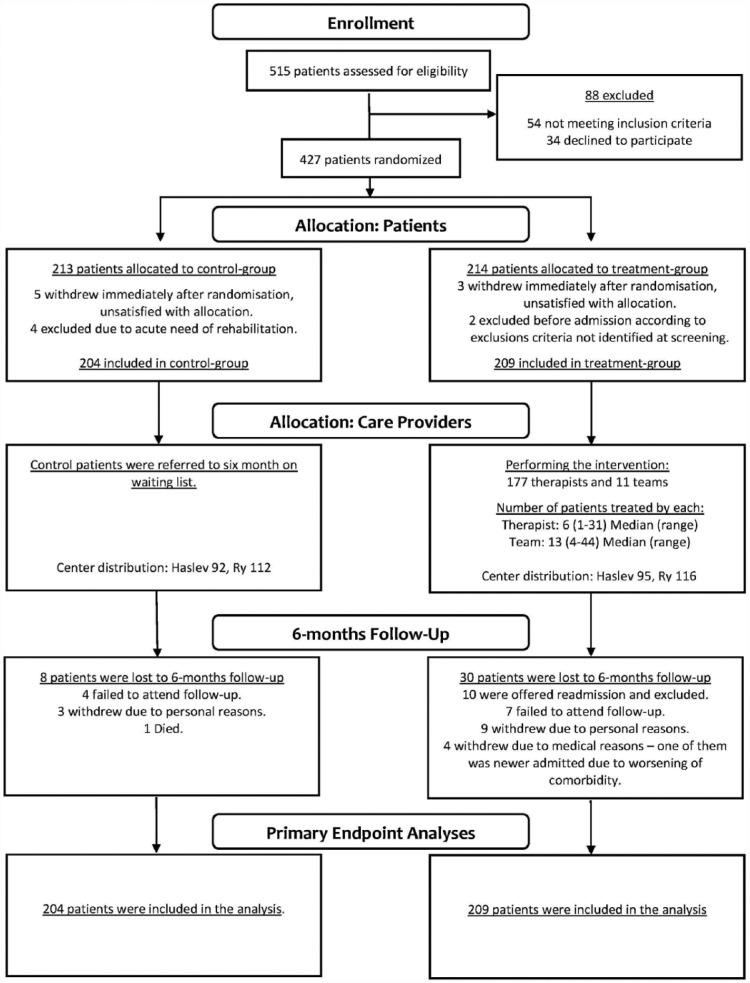
From 13 March 2012 to 7 May 2014, we screened 515 patients for eligibility. In all, 54 patients met the exclusion criteria and 34 patients declined to participate. In total, 427 patients were included. Figure 1 shows the trial profile. We randomized 213 patients to the wait-list control group and 214 patients to the treatment group. Baseline demographics, disease characteristics and unadjusted outcome scores are shown in Table 1.
Figure 1.
Trial profile.
Table 1.
Baseline statistics.
| Control | Treatment | ||
|---|---|---|---|
| No. of patients | 213 | 214 | |
| No. of dropouts | Dropout group 1a | 9 | 5 |
| Dropout group 2b | 8 | 30 | |
| No. of complete cases | 196 | 179 | |
| Location | Haslev | 97 | 98 |
| Ry | 116 | 116 | |
| Age | Years | 51 (44, 56) | 51 (44, 58) |
| Sex | Female | 146 | 145 |
| Male | 67 | 69 | |
| MS type | RR | 85 | 89 |
| SP | 92 | 90 | |
| PP | 36 | 35 | |
| EDSS | Range: 0–10 | 4.5 (3.5, 6.5) | 5 (3.5, 6.5) |
| Time since diagnosis | Years | 7 (3, 14) | 9 (4, 15) |
| Time since first symptoms | Years | 14 (8, 21) | 15 (9, 23) |
| Immuno-treatment | No | 98 | 101 |
| Yes | 115 | 113 | |
| Outcome measures | n = 203c | n = 211 | |
| FAMS Total | Score 0–176 | 114 (96, 132) | 115 (98, 134) |
| MSIS-29 Physical | Score 0–100d | 38 (27, 54) | 40 (25, 53) |
| MSIS-29 Psychological | Score 0–100d | 30 (15, 44) | 33 (19, 44) |
| 15D Index | Score 0.106–1.000 | 0.77 (0.72, 0.83) | 0.77 (0.71, 0.83) |
| EQ-5D-5L Index | Score: −0.624 to 1.000 | 0.69 (0.61, 0.79) | 0.70 (0.62, 0.76) |
| EQ-VASe | Score: 0–100 | 65 (50, 80) | 60 (50, 75) |
MS: multiple sclerosis; EDSS: Expanded Disability Status Scale; FAMS: Functional Assessment in Multiple Sclerosis; MSIS: Multiple Sclerosis Impact Scale-29; EQ-VAS: EuroQol–visual analogue scales; EQ-5D-5L: EuroQoL 5 Dimensions 5 Levels.
Continuous data are presented as median (IQR: 50% interquartile range).
Withdrew or were excluded immediately after randomization or before admission.
Lost to 6-month follow-up.
One patient did not complete the web-based questionnaires at baseline.
Higher scores indicate more impact.
n = 165 and 173.
After randomization, 14 patients withdrew or were excluded from the study, including 9 from the wait-list control group and 5 from the treatment group. In eight cases, the reason for withdrawal was dissatisfaction with the allocation. We excluded 4 patients from the wait-list control group because they were in acute need of rehabilitation. Additionally, we excluded two patients in the treatment group before admission due to severe cognitive difficulties, leaving 204 patients in the wait-list control group and 209 patients in the treatment group for the ITT analysis.
One patient in the treatment group was never admitted due to a worsening comorbidity. In total, 208 patients were admitted, with a median length of stay of 19 days (range: 7–20 days). However, only 48% of the admitted patients received the full 20 days of MDR offered, and 30% received ≤18 days.
Dropouts and missing data
Missing data, the number of dropouts and reasons for dropping out were not equally distributed between the wait-list control group and the treatment group; 8 patients were lost to 6-month follow-up in the wait-list control group, including 4 who failed to attend the 6-month follow-up, 3 who withdrew due to personal reasons, and 1 who died. A total of 30 patients were lost to 6-month follow-up in the treatment group, including 7 who failed to attend the 6-month follow-up, 9 who withdrew due to personal reasons, 4 who withdrew due to medical reasons and 10 patients who were readmitted. Readmission was offered when treatment was greatly hindered due to personal issues or illness. Due to unforeseen technical issues with the web-based data collection software, the EQ-VAS was not employed until 6 months after study initiation. This issue affected every patient included during the first 6 months; thus, the first 80 patients included did not complete the EQ-VAS.
Changes in scores
At the 6-month follow-up, all measured outcome scores suggested a positive trend between treatment and HRQoL, as shown in Table 2. The changes were significant for the MSIS-29 Psychological (p = 0.046) and 15D (p = 0.008). The treatment effect observed with the MSIS-29 Psychological was estimated to be −2.7 (95% CI: −5.6 to (−0.1)) and that of the 15D was 0.017 (95% CI: 0.005–0.030). There were no other significant differences between the groups, including the other primary outcome FAMS, although all mean changes at the 6-month follow-up favoured the treatment group.
Table 2.
Estimated mean outcomes.
| Outcome measures | n | Adjusted means |
Baseline–adjusted mean changes |
Mean differences between groups at 6-month follow-up |
|||
|---|---|---|---|---|---|---|---|
| Baseline |
Discharge |
2 months |
6 months |
ITT analysis |
|||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | p value | ||
| FAMSa (Score 0–176) | |||||||
| Intervention | 209 | 115.9 (111.0, 120.8) | 12.6 (10.0, 15.2) | 3.0 (0.5, 5.3) | 0.6 (–2.1, 3.5) | 1.6 (–1.4, 4.7) | 0.232 |
| Control | 204 | 115.1 (110.0, 120.4) | –1.0 (–3.5, 1.6) | Ref. | |||
| MSIS-29 Physicala (Score 0–1001) | |||||||
| Intervention | 209 | 40.8 (37.0, 44.6) | –12.0 (–14.1, –10.2) | –0.2 (–3.0, 2.3) | –0.6 (–3.0, 1.8) | 0.640 | |
| Control | 204 | 41.0 (37.1, 45.1) | 0.4 (–2.1, 2.8) | Ref. | |||
| MSIS-29 Psychologicala (Score 0–100b) | |||||||
| Intervention | 209 | 31.8 (27.9, 35.2) | –9.7 (–11.6, –7.9) | –1.2 (–3.8, 1.2) | –2.7 (–5.6, –0.1) | 0.046 | |
| Control | 204 | 29.8 (25.7, 33.3) | 1.5 (–0.7, 3.6) | Ref. | |||
| 15D Index (Score 0.106–1.000) | |||||||
| Intervention | 209 | 0.771 (0.754, 0.789) | 0.011 (–0.007, 0.027) | 0.017 (0.005, 0.030) | 0.008 | ||
| Control | 204 | 0.777 (0.762, 0.794) | –0.006 (–0.023, 0.008) | Ref. | |||
| EQ-5D-5L Index (Score −0.624 to −1.000) | |||||||
| Intervention | 209 | 0.638 (0.606, 0.669) | –0.032 (–0.070, 0.001) | 0.006 (–0.015, 0.028) | 0.596 | ||
| Control | 204 | 0.638 (0.605, 0.669) | –0.038 (–0.075, –0.002) | Ref. | |||
| EQ-VAS (Score 0–100) | |||||||
| Intervention | 173 | 60.4 (56.7, 64.1) | 9.7 (7.1, 12.4) | 3.8 (–0.1, 7.1) | 2.5 (–1.1, 5.9) | 0.112 | |
| Control | 165 | 62.9 (58.9, 66.6) | 1.2 (–2.5, 4.8) | Ref. | |||
| Factor 1 | |||||||
| Intervention | 209 | 0.064 (–0.147, 0.264) | 0.063 (–0.044, 0.187) | 0.116 (0.003, 0.229) | 0.039 | ||
| Control | 204 | 0.130 (–0.068, 0.339) | –0.053 (–0.145, 0.059) | Ref. | |||
| Factor 2 | |||||||
| Intervention | 209 | –0.161 (–0.308, –0.010) | –0.053 (–0.186, 0.063) | 0.035 (–0.058, 0.139) | 0.473 | ||
| Control | 204 | –0.189 (–0.342, –0.037) | –0.089 (–0.206, 0.030) | Ref. | |||
CI: confidence interval; FAMS: Functional Assessment in Multiple Sclerosis; ITT: Intention-To-Treat; MSIS-29: Multiple Sclerosis Impact Scale-29; EQ-VAS: EuroQol–visual analogue scales; EQ-5D-5L: EuroQoL 5 Dimensions 5 Levels.
Primary outcome.
Higher score indicates greater impact.
The EFA identified two latent factors that accounted for 67.5% of the variation in the scores at baseline, with high loadings of the MSIS-29 Psychological (−0.91) and FAMS (0.77) on factor 1 and the EQ-5D-5L (0.70) and MSIS-29 Physical (−0.65) on factor 2 (see Supplementary Material). In contrast, the 15D showed comparable loadings on both factors (0.58 and 0.59, respectively). Factor 1 showed a significant treatment effect (p = 0.039), but factor 2 did not (p = 0.473).
Within-group changes were not tested, but the statistics showed a general deteriorating trend among the wait-list control patients during the study period. The mean changes in scores at the 6-month follow-up were worse in five of six HRQoL measures. In contrast, the treatment patients seemed to improve their HRQoL substantially from the MDR programme as the mean changes on all four HRQoL measures (FAMS, MSIS-29 Physical, MSIS-29 Psychological, and EQ-VAS) at discharge were quite large. Despite a clear decline from discharge, the MDR treatment still affected HRQoL at the 6-month follow-up, as the mean changes in scores were better than baseline in five of six HRQoL measures.
The results from the post hoc analysis showed that HRQoL was unchanged or improved at the 6-month follow-up in a significantly greater proportion of the treatment group than the wait-list control group for three of six HRQoL measures, and a fourth measure demonstrated a nonsignificant trend (Table 3).
Table 3.
Categorical changes in outcomes from baseline to 6 months (number, %).
| Outcome measures | Control group (n = 195a) |
Intervention group (n = 179b) |
p valuec | ||||
|---|---|---|---|---|---|---|---|
| Deteriorated | Unchanged | Improved | Deteriorated | Unchanged | Improved | ||
| FAMS Total | 121 (62.1) | 3 (1.5) | 71 (36.4) | 93 (52.0) | 3 (1.7) | 83 (46.4) | 0.048 |
| MSIS-29 Physical | 100 (51.3) | 8 (4.1) | 87 (44.6) | 86 (48.0) | 6 (3.4) | 87 (48.6) | 0.532 |
| MSIS-29 Psychological | 97 (49.7) | 20 (10.3) | 78 (40.0) | 66 (36.9) | 26 (14.5) | 87 (48.6) | 0.012 |
| 15D Index | 106 (54.4) | 2 (1.0) | 87 (44.6) | 77 (43.0) | 1 (0.6) | 101 (56.4) | 0.028 |
| EQ-5D-5L Index | 91 (46.7) | 16 (8.2) | 88 (45.1) | 86 (48.0) | 13 (7.3) | 80 (44.7) | 0.790 |
| EQ-VAS | 66 (41.5) | 16 (10.1) | 77 (48.4) | 48 (31.4) | 24 (15.7) | 81 (52.9) | 0.063 |
FAMS: Functional Assessment in Multiple Sclerosis; MSIS: Multiple Sclerosis Impact Scale-29; EQ-VAS: EuroQol–visual analogue scales; EQ-5D-5L: EuroQoL 5 Dimensions 5 Levels.
EQ-VAS: n = 159.
EQ-VAS: n = 153.
Deteriorated versus unchanged and improved combined.
Discussion
This pragmatic study investigated the effectiveness of 4 weeks of inpatient MDR on HRQoL in a heterogeneous MS patient sample. The ITT findings indicated an overall improvement favouring the intervention group, even though the carry-over effect to the community diminished from discharge to the 6-month follow-up.
In contrast to previous studies,11,28 the MSIS-29 Psychological dimension was significantly better in the intervention group than in the wait-list control group at the 6-month follow-up. The 15D, which has not been previously applied in this type of trial, was also significantly better in the intervention group at the 6-month follow-up. The mean difference in changes of 0.017 in the 15D has been proposed to be of clinical importance.29
The variability in outcomes was large, likely due to the heterogeneity of our sample population, and the observed differences at the 6-month follow-up were relatively small. These factors made it difficult to obtain statistical significance. However, based on the mean changes and confidence intervals, the trend was consistent. Treatment patients seemed to experience better HRQoL across all outcome measures at the 6-month follow-up than the wait-list control patients. Although we cannot exclude a true difference of zero,30 for the nonsignificant differences, most of the plausible values supported a beneficial effect from the MDR programme, and all mean changes were in favour of the treatment group. From the EFA, the high loading of the FAMS on factor 1 suggests that the non-significance of the FAMS may be due to a lack of power and may therefore represent a type II error; supporting that the common positive trend found among the measured scores is a true signal. Therefore, we believe that failure to achieve statistical significance in the other primary outcome FAMS was the result of insufficient statistical power rather than a lack of effectiveness of the MDR programme. However, based on the findings, the MSIS-29 Psychological dimension turned out to be a more potent measure of HRQoL than the FAMS.
Because the findings revealed clear positive effects from the inpatient MDR programme on HRQoL at discharge, continuation and repetition of the MDR programme would have been preferable to enhance the carry-over effect to the community. The decision not to do so has probably affected the results negatively. Despite these limitations, we believe that the study still provides useful information and insights. Pragmatic trials are useful because empirical knowledge, which we build our practice on, should be grounded in trial designs, reflecting the clinical reality that we operate within. As a consequence, pragmatic trials generally yield smaller effects than tightly controlled trials.
However, even small improvements in HRQoL could have an important impact for MS patients, particularly because MS is a progressive disease. Therefore, MDR is also about maintaining HRQoL which was supported by the results from the post hoc analysis. A significantly larger proportion of patients in the treatment group improved or maintained their HRQoL at 6 months, in three of six outcome measures.
Several real-world factors contributed to the nature and limitations of our pragmatic trial. Most notably, many patients needed days off during admission or had to be discharged prematurely for various personal reasons. Therefore, the fact that 30% of the patients in the treatment group received 18 days or less of MDR might have had a negative impact on the results.
Lack of blinding and using a wait-list as a control rather than a sham intervention may have confounded the results for two reasons. The comprehensive support and care provided by the MS specialists could improve outcomes, and the control patients on a wait-list may have been dissatisfied because they had to wait.11 However, the professional care, encouragement, attention, and time provided by MS specialists and the comforting environment are integral to the MDR philosophy and represent important interacting factors. Blinding of study participants is nearly impossible in MDR trials. Even if blinded to trial participation, patients are aware that they are receiving rehabilitation. Another issue with blinding may be the ethical consideration of delaying admission for control patients without their knowledge. Therefore, trying to blind the MS specialists and the patients would have altered the ecology of the MDR programme.31
Selecting appropriate outcome measures is among the most important elements of an MDR trial, perhaps second only to the MDR programme itself. The existing psychometric HRQoL scales cannot fully intercept the impact of MDR on a patient’s capability, mastery of coping strategies and QoL and could likely underestimate the benefit from MDR.32 These limitations is supported by our EFA findings that indicated that only part of the latent content in the outcome measures was significantly affected by treatment. The FAMS and MSIS-29 have previously been used as measures of HRQoL in MDR studies11,12,28 and are recommended for this type of research33. The 15D may be more applicable than the EQ-5D to MS research,34 which was supported by our findings.
From the Danish National Survey of Patient Experiences and various other sources, such as internal patient surveys and dialogue meetings, we know that after receiving MDR, patients reportedly became more fit, more well-informed and ‘dared more greatly’. A frequent patient statement was, ‘What you really rehabilitate is our heart and mind’. We could capture these psychosocial factors and changes more effectively with complementary qualitative approaches or refined measures for open-ended questioning or composite outcomes. Despite some variation, it is reasonable to assume that the MDR programme and the study findings are applicable to some extent to MS patients in general.
In conclusion, the study results indicated the longer-term effectiveness of inpatient MDR on HRQoL in MS patients. Future studies are needed to confirm and strengthen our results and investigate the significance of the response shift phenomenon,11,35 a subject that was not addressed in this work. Further thorough, long-term RCTs on MDR in MS patients are required to improve our understanding of MDR.
Supplementary Material
Acknowledgments
This study was made possible through a non-earmarked inheritance from Johan and Maja Jørgensen to the Danish MS Hospitals. We thank the patients who participated in this study, the staff and volunteers from the MS Society and the two MS hospitals. Their contributions are gratefully acknowledged.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: F.B., M.N., P.T., P.V.R., T.P., B.L. and J.S. declare that they have received no funding.
Contributor Information
Finn Boesen, The Danish MS Hospitals, Haslev, Denmark.
Michael Nørgaard, The Danish MS Hospitals, Haslev, Denmark.
Philipp Trénel, Danish Technological Institute, Aarhus, Denmark.
Peter Vestergaard Rasmussen, Department of Neurology, Aarhus University hospital, Aarhus, Denmark.
Thor Petersen, Department of Neurology, Aarhus University hospital, Aarhus, Denmark.
Brita Løvendahl, The Danish MS Hospitals, Haslev, Denmark.
Jan Sørensen, Centre of Health Economics Research (COHERE), Department of Public Health, University of Southern Denmark, Odense, Denmark/Healthcare Outcome Research Center, Royal College of Surgeons in Ireland, Dublin, Ireland.
References
- 1. Confavreux C. Paper handed out at the teaching course 13: Controversies in the natural history of multiple sclerosis, lecture 37: Emerging concepts from natural history studies: An overview. In: 28th congress of the European Committee for treatment and research in multiple sclerosis, Lyon, 10 October 2012. [Google Scholar]
- 2. Frankel D. MS – neurological rehabilitation. In: Umphred DA. (ed.) Neurological rehabilitation. 4th ed. St. Louis, MO: Mosby, 2001, pp. 595–615. [Google Scholar]
- 3. Kesselring J, Beer S. Symptomatic therapy and neurorehabilitation in multiple sclerosis. Lancet Neurol 2005; 4: 643–652. [DOI] [PubMed] [Google Scholar]
- 4. Mitchell AJ, Benito-León J, González J-MM, et al. Quality of life and its assessment in multiple sclerosis: Integrating physical and psychological components of wellbeing. Lancet Neurol 2005; 4: 556–566. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. International classification of functioning, disability and health (ICF), http://www.who.int/classifications/icf/icf_more/en/ (accessed 3 March 2017).
- 6. Meyer T, Gutenbrunner C, Bickenbach J, et al. Towards a conceptual description of rehabilitation as a health strategy. J Rehabil Med 2011; 43: 765–769. [DOI] [PubMed] [Google Scholar]
- 7. Freeman JA, Langdon DW, Hobart JC, et al. The impact of inpatient rehabilitation on progressive multiple sclerosis. Ann Neurol 1997; 42: 236–244. [DOI] [PubMed] [Google Scholar]
- 8. Wade DT, De Jong BA. Recent advances in rehabilitation. BMJ 2000; 320: 1385–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MarselisborgCentret. Rehabilitation in Denmark: The white paper of the rehabilitation concept. Århus: MarselisborgCentret, 2004. (in Danish). [Google Scholar]
- 10. Craig J, Young CA, Ennis M, et al. A randomised controlled trial comparing rehabilitation against standard therapy in multiple sclerosis patients receiving intravenous steroid treatment. J Neurol Neurosurg Psychiatry 2003; 74: 1225–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan F, Pallant JF, Brand C, et al. Effectiveness of rehabilitation intervention in persons with multiple sclerosis: A randomised controlled trial. J Neurol Neurosurg Psychiatry 2008; 79: 1230–1235. [DOI] [PubMed] [Google Scholar]
- 12. Salhofer-Polanyi S, Windt J, Sumper H, et al. Benefits of inpatient multidisciplinary rehabilitation in multiple sclerosis. Neurorehabilitation 2013; 33: 285–292. [DOI] [PubMed] [Google Scholar]
- 13. Khan F, Turner-Stokes L, Ng L, et al. Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database Syst Rev 2007(2): CD006036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boutron I, Moher D, Altman DG, et al. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: Explanation and elaboration. Ann Intern Med 2008; 148: 295–309. [DOI] [PubMed] [Google Scholar]
- 16. Sørensen J, Lee A, Løvendahl B, et al. Study protocol: To investigate effects of highly specialized rehabilitation for patients with multiple sclerosis. A randomized controlled trial of a personalized, multidisciplinary intervention. BMC Health Serv Res 2012; 12: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evans S, Royston P, Day S. Minim: Allocation by minimisation in clinical trials 2011, https://www-users.york.ac.uk/~mb55/guide/minim.htm (accessed 3 May 2016).
- 18. Multiple sclerosis in adults: Management guidance and guidelines NICE, https://www.nice.org.uk/guidance/cg186?unlid=346205354201732316549 (accessed 7 April 2017).
- 19. Danish Health Authority. Øget faglighed i genoptræning og rehabilitering efter udskrivning fra sygehus. Copenhagen: Sundhedsstyrelsen, 2014. (in Danish). [Google Scholar]
- 20. Henze T, Rieckmann P T, oyka KV. Symptomatic treatment of multiple sclerosis. Multiple Sclerosis Therapy Consensus Group (MSTCG) of the German Multiple Sclerosis Society. Eur Neurol 2006; 56: 78–105. [DOI] [PubMed] [Google Scholar]
- 21. Cella DF, Dineen K, Arnason B, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology 1996; 47: 129–139. [DOI] [PubMed] [Google Scholar]
- 22. Hobart J, Lamping D, Fitzpatrick R, et al. The Multiple Sclerosis Impact Scale (MSIS-29): A new patient-based outcome measure. Brain J Neurol 2001; 124: 962–973. [DOI] [PubMed] [Google Scholar]
- 23. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life 2011; 20: 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sintonen H. The 15D instrument of health-related quality of life: Properties and applications. Ann Med 2001; 33: 328–336. [DOI] [PubMed] [Google Scholar]
- 25.SurveyXact. Aarhus: Rambøll Management Consulting. [Google Scholar]
- 26. Sørensen J, Bay J, Damsgaard T, et al. Validation of the Danish version of functional assessment of multiple sclerosis: A quality of life instrument. Mult Scler Int 2011; 2011: 121530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, http://www.R-project.org/ (2017, accessed 7 April 2017). [Google Scholar]
- 28. Storr L, Sørensen P, Ravnborg M. The efficacy of multidisciplinary rehabilitation in stable multiple sclerosis patients. Mult Scler 2006; 12: 235–242. [DOI] [PubMed] [Google Scholar]
- 29. Alanne S, Roine RP, Räsänen P, et al. Estimating the minimum important change in the 15D scores. Qual Life Res 2014; 24: 599–606. [DOI] [PubMed] [Google Scholar]
- 30. Sainani K. Interpreting ‘null’ results. PM R 2013; 5: 520–523 [DOI] [PubMed] [Google Scholar]
- 31. Ware JH, Hamel MB. Pragmatic trials – guides to better patient care? N Engl J Med 2011; 364: 1685–1687. [DOI] [PubMed] [Google Scholar]
- 32. Edwards SGM, Playford ED, Hobart JC, et al. Comparison of physician outcome measures and patients’ perception of benefits of inpatient neurorehabilitation. BMJ 2002; 324: 1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen ET, Potter K, Allen DD, et al. Selecting rehabilitation outcome measures for people with multiple dclerosis. Int J MS Care 2015; 17: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuspinar A, Finch L, Pickard S, et al. Using existing data to identify candidate items for a health state classification system in multiple sclerosis. Qual Life Res 2013; 23: 1445–1457. [DOI] [PubMed] [Google Scholar]
- 35. Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: A theoretical model. Soc Sci Med 1999; 48: 1507–1515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.