Summary
Temperature is a key environmental variable influencing plant growth and survival. Protection against high temperature stress in eukaryotes is coordinated by heat shock factors (HSFs), transcription factors that activate the expression of protective chaperones such as HEAT SHOCK PROTEIN 70 (HSP70); however, the pathway by which temperature is sensed and integrated with other environmental signals into adaptive responses is not well understood. Plants are exposed to considerable diurnal variation in temperature, and we have found that there is diurnal variation in thermotolerance in Arabidopsis thaliana, with maximal thermotolerance coinciding with higher HSP70 expression during the day. In a forward genetic screen, we identified a key role for the chloroplast in controlling this response, suggesting that light-induced chloroplast signaling plays a key role. Consistent with this, we are able to globally activate binding of HSFA1a to its targets by altering redox status in planta independently of a heat shock.
Keywords: Arabidopsis, thermotolerance, HSP70, diurnal, chloroplast, plastoquinone, HSF, light
Graphical Abstract
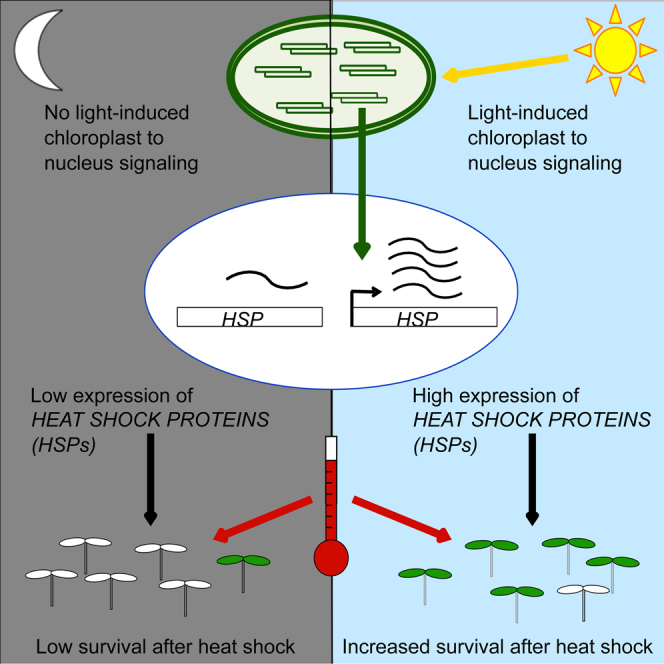
Highlights
-
•
There is a diurnal pattern of basal thermotolerance in Arabidopsis
-
•
Thermotolerance correlates with diurnal expression patterns of heat-associated genes
-
•
Chloroplast mutants have greater heat shock gene expression and thermotolerance
-
•
A chloroplast generated light signal gates HSFA1 and heat shock gene expression
Plants are most resilient to heat stress during the day, a response controlled by HSFA1 transcription factors activating heat shock genes. Dickinson et al. find that perturbations of chloroplast electron transport affect heat shock gene expression. They show that HSFA1 activity is gated by a light-dependent chloroplast signal.
Introduction
Temperature has a major role in plant growth and survival and therefore affects crop productivity. For example, wheat yields are predicted to decrease by ∼6% for every 1°C rise in global temperature (Asseng et al., 2014). High temperatures induce the expression of protective chaperones and modulate growth responses. Key players in the heat protection response are transcription factors of the HEAT SHOCK FACTOR A1 (HSFA1) family, which trigger the depletion of repressive H2A.Z-nucleosomes and target gene expression (Cortijo et al., 2017, Kumar and Wigge, 2010, Liu et al., 2011), however, the pathways that activate the HSFA1 class TFs and how these perceive temperature and integrate it with other environmental signals are not fully understood. Plants are exposed to considerable diurnal temperature variation and have evolved pathways to anticipate likely future conditions. For example, the cold response pathway is gated by the circadian clock, enabling the degree of responsiveness to be controlled in the context of the environment (Dodd et al., 2006, Lee and Thomashow, 2012) and genes promoting elongation growth and flowering in response to warm, non-stressful, temperature are induced during the night via thermosensory phytochromes (Jung et al., 2016, Legris et al., 2016). A diurnal pattern of thermotolerance has been reported in numerous plant species, including cereals (Laude, 1939), spinach (Li and Guy, 2001), plants using crassulacean acid metabolism (CAM) (Kappen and Lösch, 1984), and black spruce (Colombo et al., 1995). In this study, we find a diurnal pattern of thermotolerance in Arabidopsis, which correlates with the expression of HSP70 over a diurnal cycle. A forward genetic screen with an HSP70-LUCIFERASE reporter line revealed a central role for light-induced chloroplast signaling in gating the level of response to high temperature that accounts for diurnal variation in thermotolerance.
Results and Discussion
Thermotolerance Varies Diurnally and Correlates with the Expression of HSPs
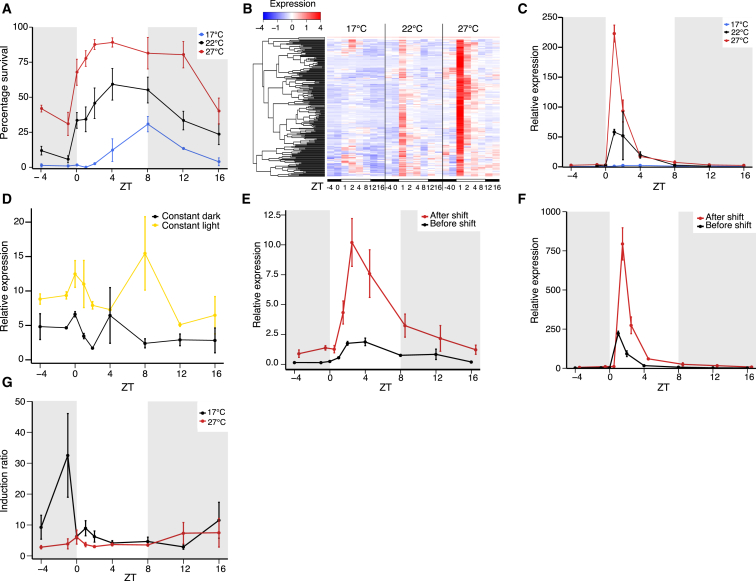
To investigate whether the time of day gates thermotolerance in Arabidopsis, plants were grown for 7 days at constant 17°C, 22°C, or 27°C and survival after a heat treatment applied at nine points over a 24-hr time course was measured. We found that Arabidopsis has a diurnal pattern of thermotolerance (Figures 1A and S1A), consistent with reports from other plant species (Colombo et al., 1995, Kappen and Lösch, 1984, Laude, 1939, Li and Guy, 2001). The lowest levels of survival occur shortly before dawn and greatest survival occurs during the light period. The growth temperature affects the levels and patterns of thermotolerance, with higher growth temperatures resulting in increased thermotolerance occurring earlier in the day compared to lower temperatures (Figure 1A).
Figure 1.
A Diurnal Pattern of Basal Thermotolerance Correlates with a Diurnal Pattern of Heat-Associated Gene Expression
(A) Basal thermotolerance of WT seedlings over a diurnal time course. Error bars are ±SEM (n = 3), where 81 seedlings were scored per time point and temperature for three independent experiments.
(B) Heatmap of the expression of genes in cluster 13-1-1. Transcriptomes shown from plants grown in short days (8 hr light, 16 hr dark) at constant 17°C, 22°C, or 27°C. Black bars below heatmap indicate samples taken in the dark (ZT−4, ZT0, ZT12, and ZT16) and white bars indicate samples taken in the light (ZT1, ZT2, ZT4, and ZT8). In heat maps, low to high expression (Z scores) is shown as blue to red.
(C–F) Expression of HSP70 in WT seedlings assayed by qRT-PCR.
(C) Plants grown at 17°C, 22°C, and 27°C. Error bars are ± the range of two measurements (n = 2) or SEM (n = 3 to 4).
(D) Seedlings entrained in short days at 27°C and shifted to constant light or constant dark at ZT8 of the seventh day after germination. Plants were sampled starting at ZT−4 on the eighth day. Error bars are ± the range of two measurements.
(E and F) Before and after a 30-min shift to 45°C in seedlings grown at 17°C (E) and 27°C (F).
(G) Induction ratios (expression after shift/expression before shift) of HSP70 expression after the temperature shifts in (F) and (G). Error bars are ±SEM (n = 3).
To identify gene expression changes underlying the diurnal pattern of thermotolerance, we analyzed transcriptomes of plants grown at constant 17°C, 22°C, and 27°C over a 24-hr time course. Genes were clustered based on expression patterns and a prominent cluster (cluster 13-1-1) showing a temperature responsive morning peak of expression was identified (Figure 1B). This morning cluster is strongly enriched for genes associated with the response to heat, high light, and oxidative stress (Table S2), including a number of HEAT SHOCK PROTEINS (HSPs) required for thermotolerance, such as HSP101 (Queitsch et al., 2000), consistent with this cluster contributing to the diurnal pattern of thermotolerance. The promoters of genes in the morning cluster show enrichment for HSF binding sites known as heat shock elements (HSE) (Schöffl et al., 1998) (Table S3), suggesting that the temperature responsiveness of this cluster is HSF-dependent. Because HSP70 expression is an indicator of temperature perception status (Kumar and Wigge, 2010) and is representative of the morning cluster (Figures 1C and S1B), we further analyzed HSP70 to understand how the expression of this cluster of genes is controlled.
To test whether the diurnal pattern of HSP70 expression is controlled by the circadian clock, plants were entrained in short days at constant 27°C before shifting to constant light or constant dark. Compared to plants grown at constant 27°C in short days (Figure 1C) there was an absence of the morning peak of HSP70 expression in both constant light and constant dark (Figure 1D) indicating that the diurnal expression pattern is not primarily controlled by the circadian clock, consistent with work performed in spinach (Li and Guy, 2001). The morning peak was present, albeit substantially lower, in a CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) overexpressing line (CCA1:OX) (Figure S1C), which has an arrhythmic clock (Wang and Tobin, 1998). The lower morning peak in CCA1:OX may be the result of an indirect effect of altered reactive oxygen species (ROS) signaling in CCA1:OX (Lai et al., 2012). Supporting the idea that light regulation is dominant over circadian regulation of HSP70 expression, the morning peak of LUCIFERASE activity in a line containing an HSP70-LUCIFERASE (HSP70-LUC) reporter (Kumar and Wigge, 2010) occurs 1 hr after lights come on when the night is extended by up to 4 hr (Figure S1D). The morning peak of HSP70 expression is lost in both constant light and constant dark (Figure 1D) suggesting that it is dependent on the perception of the dark to light transition. The diurnal pattern of HSP70 expression was present in cryptochrome and phytochrome null mutants and plants with a constitutively active phytochrome (cry1/2, phyABCDE, and phyYHB) (Jung et al., 2016) (Figures S1C and S1E) indicating that the morning peak is independent of the main blue (cryptochrome) and red/far red (phytochrome) photoreceptors, although the involvement of other photoreceptors has not been ruled out.
Because there is a diurnal pattern of HSP70 expression at constant temperature (Figures 1C and S1B), we investigated whether the time of day also affects the induction of HSP70 by heat. HSP70 expression was assayed in plants grown at 17°C or 27°C and shifted to 45°C for 30 min, replicating the conditions of the thermotolerance assays. Levels of HSP70 expression after a shift had a similar pattern to HSP70 expression at constant temperature (Figures 1E and 1F) and this correlated with the diurnal patterns of thermotolerance (Figure 1A). The induction of HSP70 by heat was quantified as the expression after shift divided by the expression before shift. Although there was large variability in HSP70 expression levels between time points and growth conditions, induction ratios were similar (Figure 1G). The induction of HSP70 expression in plants grown at 17°C and shifted at Zeitgeber time (ZT)−1 was higher, but this likely reflects experimental noise as the expression levels before the shift at ZT−1 are so low they are hard to measure accurately. There were also no large differences between induction ratios of plants grown at constant 17°C and 27°C, despite large differences in expression levels (Figures 1E–1G). The data are consistent with a model where light gates the magnitude of the response to high temperature, with expression after a temperature shift being a multiple of the expression before a shift. The expression of HSP70 is very low in the dark, and consequently its expression at higher temperature is also minimal. By comparison, in the light a higher baseline expression of HSP70 coincides with a proportionately higher expression level following induction. In this way, light can be thought as “priming” the heat shock response.
Nuclear Genes Encoding Chloroplast Proteins Are Necessary for Correct HSP70 Expression during the Day, and This Correlates with Thermotolerance
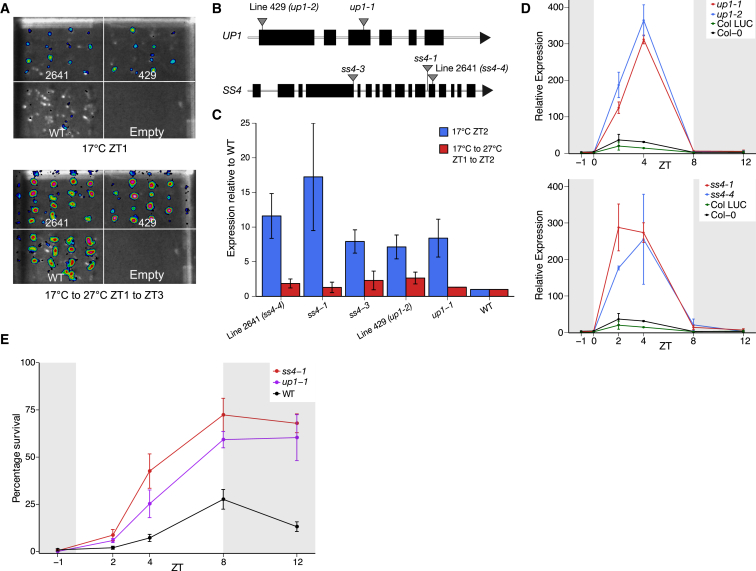
To investigate how the diurnal pattern of thermotolerance might be controlled, we screened for mutants with altered HSP70 expression in the morning using the HSP70-LUC reporter line. Two mutant lines, 429 and 2641, were identified as having increased LUC activity in the morning at 17°C compared to wild-type (WT) (Figure 2A). The causal mutation in line 429 was mapped to a stop codon in a gene encoding an unknown protein found to be localized in the thylakoid membrane (AT5G08540) (Reiland et al., 2011, Simm et al., 2013) and the causal mutation in line 2641 was mapped to a stop codon in STARCH SYNTHASE 4 (SS4) (Figure 2B; Supplemental Experimental Procedures). A T-DNA insertion in the unknown protein (up1-1) shows increased HSP70 expression compared to WT, similar to line 429, and two independent ss4 T-DNA mutants (ss4-1 [Roldán et al., 2007] and ss4-3 [Crumpton-Taylor et al., 2013]) show increased HSP70 expression compared to WT, similar to line 2641 (Figure 2C). Complementation crosses confirmed that the HSP70-LUC mis-expression phenotypes in lines 429 and 2641 were caused by loss of UP1 and SS4 function respectively as F1 plants of crosses between the EMS and T-DNA mutants fail to rescue the LUC activity phenotypes of the EMS lines, whereas crosses to WT do rescue (Figures S2A and S2B).
Figure 2.
Mutations in Genes Encoding Chloroplast-Localized Proteins Cause Increased HSP70 Expression and Increased Thermotolerance
(A) False color image of LUCIFERASE activity of seedlings imaged at 17°C ZT1 and after a shift from 17°C to 27°C from ZT1 to ZT3. Two mutant lines, 2641 and 429, and WT (col-0 containing pHSP70::LUC), are shown from the same plate.
(B) Schematics of UP1 and SS4 with the position of mutations shown. Line 429 (up1-2) has a C to T substitution on chromosome (chr) V at locus 2763941 mutating Gln10 to a stop codon. Line 2641 (ss4-4) has a C to T substitution on chr IV at locus 10086122 mutating Gln872 to a stop codon.
(C and D) HSP70 expression assayed by qRT-PCR.
(C) Mutants at 17°C ZT2 and after a shift from 17°C to 27°C from ZT1 to ZT2. Expression shown relative to WT sampled from the same plate. Error bars are ± the range of two measurements (n = 2) or SEM (n = 3 to 4).
(D) up1 and ss4 mutants over a time course at 17°C in short days sampled at ZT−1, ZT0, ZT2, ZT4, ZT8, and ZT12. Error bars are ± the range of two measurements (n = 2) or SEM (n = 3).
(E) Thermotolerance of up1-1, ss4-1, and WT with plants grown at 17°C and treated at ZT−1, ZT2, ZT4, ZT8, and ZT12. Error bars are ±SEM (n = 3).
The up1 and ss4 mutants have increased HSP70 expression at 17°C ZT2 (Figure 2C). To investigate whether increased HSP70 expression in these mutants is specific to a particular time of day, HSP70 expression was assayed from ZT−1 to ZT12. This time course showed that the up1 and ss4 mutants have greatly increased HSP70 expression specifically during the day (Figure 2D). In WT plants, the diurnal pattern of HSP70 expression correlates with basal thermotolerance (Figures 1A and 1C). Similarly, increased HSP70 expression during the day in up1 and ss4 (Figure 2D) correlates with increased thermotolerance in these mutants (Figures 2E and S2C).
The diurnal pattern of HSP70 expression is light-dependent but broadly circadian clock and photoreceptor independent (Figures 1D and S1C–S1E) necessitating the involvement of another pathway to generate the morning peak of HSP70 expression and therefore increase thermotolerance in response to light. As well as being the site of photosynthesis, the chloroplast is an environmental sensor and can transmit signals to effect nuclear gene expression through retrograde signaling (Chan et al., 2016), including expression of an HSP70 gene in Chlamydomonas (Kropat et al., 1997). Both UP1 and SS4 encode chloroplast-localized proteins suggesting that the chloroplast is involved in generating the morning peak of HSP70 expression. This is supported by the observation that the morning peak of HSP70 expression is absent in light-exposed roots (Figure S2D) and that mutations in other genes encoding chloroplast-localized proteins, including maltose exporter1 (mex1) (Niittylä et al., 2004) and clpc1 (Sjögren et al., 2014) were identified in the screen for HSP70-LUC mis-expression and these mutants also have increased HSP70 expression specifically during the day (Figure S2E).
Increased Expression of Heat-Associated Genes in ss4 Is Associated with the Light Reactions of Photosynthesis
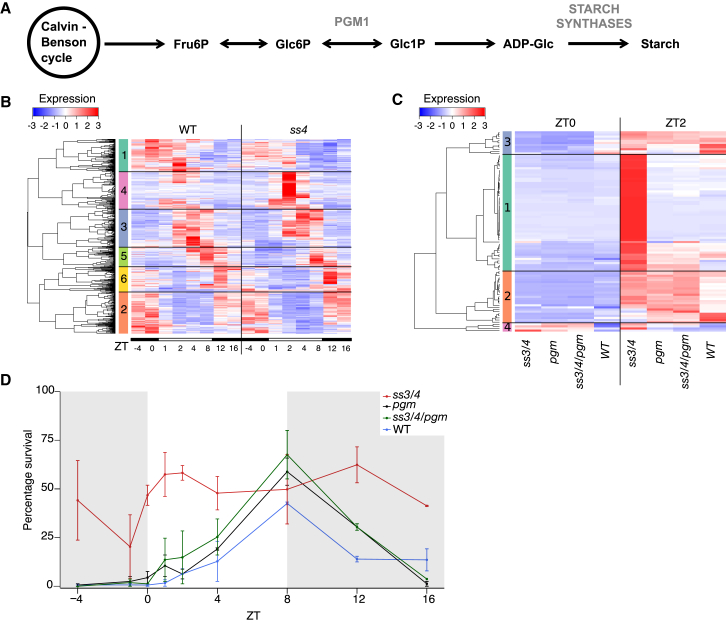
As the function of SS4 is well-characterized (Roldán et al., 2007, Crumpton-Taylor et al., 2013, Ragel et al., 2013), we used it to further characterize how chloroplast signaling affects diurnal patterns of heat-associated gene expression and thermotolerance. SS4 is a soluble starch synthase that catalyzes the addition of glucose from ADP-glucose to amylopectin chains (Streb and Zeeman, 2012) (Figure 3A). There are four soluble starch synthases in Arabidopsis and ss4 is the only single mutant with a clear phenotype, likely because of its involvement in initiating starch granule synthesis (Crumpton-Taylor et al., 2013).
Figure 3.
Increased Expression of Heat-Associated Genes in ss4 Is Caused by Alterations to the Light Reactions of Photosynthesis
(A) Simplified schematic of the starch synthesis pathway.
(B) Heatmap of the expression of genes differentially expressed between ss4-1 and WT at any sampled time-point. Transcriptomes are shown from plants grown in short days at 17°C. Black bars underneath heatmap show samples taken in the dark (ZT−4, ZT0, ZT12, and ZT16) and white bars show samples taken in the light (ZT1, ZT2, ZT4, and ZT8). Replicate shown in Figure S3A.
(C) Heatmap of expression of cluster 4 from (B) in ss3/4, pgm, ss3/4/pgm, and WT (col-0) at 17°C ZT0 and ZT2. Replicate shown in Figure S3C. (B and C) Clusters shown on the left of heat maps and low to high expression (Z scores) is shown as blue to red.
(D) Basal thermotolerance of ss3/4, pgm, ss3/4/pgm, and WT (col-0) grown at 17°C in short days and treated at ZT−4, ZT−1, ZT0, ZT1, ZT2, ZT4, ZT8, ZT12, and ZT16. Error bars are the range of two measurements.
Expression of HSP70 in ss4 at 17°C shows the strongest difference to WT in the morning (Figure 2D), suggesting that the ss4 mutant may affect the regulation of the morning cluster of genes (Figure 1B). To confirm this, we analyzed the ss4 transcriptome over a diurnal time course at 17°C. Differentially expressed genes between ss4 and WT were defined using DESeq2 (Love et al., 2014) and clustered based on expression patterns (Figures 3B and S3A). A cluster of genes (cluster 4) showing increased expression in ss4 in the morning was strongly enriched for heat-associated genes (Table S2). This cluster overlapped with the cluster of morning peak genes (Figure S3B), and HSEs were enriched in the promoters of genes in this cluster (Table S3).
Defective starch synthesis leads to over-accumulation of ADP-glucose in ss4 and ss3/4 mutants (Ragel et al., 2013). This disrupts the photosynthetic electron transport (PET) chain as the over-accumulation of ADP-glucose limits the availability of ADP for the chloroplast ATP-synthase. This substrate-limitation results in decreased proton eflux from the thylakoid lumen through ATP synthase and thus an over-acidified lumen (Sharkey and Vanderveer, 1989, Takizawa et al., 2008). As a consequence, plastoquinol re-oxidation at the cytochrome b6f complex is slowed down (“photosynthetic control”) and non-photochemical quenching of excitation energy in the PSII antenna bed is strongly induced, something that has been suggested to initiate retrograde signaling cascades (Rott et al., 2011).
The ss4 mutation affects both the carbon and light reactions of photosynthesis (Ragel et al., 2013) and causes increased expression of heat-associated genes in response to light (Figure 3B; Table S2). To determine whether the overexpression of heat-associated genes in ss4 is associated with the carbon or light reactions of photosynthesis, we assessed the expression of cluster 4 (Figure 3B) in the transcriptomes of ss3/4, plastidal phosphoglucomutase (pgm), ss3/4/pgm, and WT at ZT0 and ZT2 at 17°C. Four clusters were defined and the genes in the largest cluster (cluster 4-1) were strongly overexpressed in ss3/4 compared to WT in the morning (ZT2). This overexpression was absent in pgm and ss3/4/pgm (Figures 3C and S3C). Cluster 4-1 is strongly enriched for heat-associated genes and for genes with HSEs in their promoters (Table S3). pgm has very low levels of starch and increased levels of soluble sugars compared to WT (Ragel et al., 2013, Streb et al., 2009), therefore, effects on the carbon reactions do not cause increased expression of heat-associated genes as these genes are expressed similarly to WT in pgm (Figures 3C and S3C). Phenotypes associated with the light reactions such as photochemical efficiency, quantum yield of photosystem two, and an over-reduction of the plastoquinone (PQ) pool, are strongly affected in ss4 and ss3/4 compared to pgm (Ragel et al., 2013) suggesting that the mis-expression of cluster 4-1 in ss4 and ss3/4 is caused by alterations to the light reactions. Interestingly, the overexpression of cluster 4-1 is rescued in an ss3/4/pgm mutant, in which the over-accumulation of ADP-glucose in ss3/4 is suppressed (Ragel et al., 2013), suggesting that the over-accumulation of ADP-glucose and the subsequent effects on the light reactions are the cause of the overexpression of heat-associated genes in ss4 and ss3/4. The expression patterns of heat-associated genes in ss3/4, pgm, and ss3/4/pgm correlate with thermotolerance (Figures 3D and S3D) consistent with the central role of the light-induced morning cluster in determining thermotolerance.
The Morning Peak of Heat-Associated Gene Expression Is Associated with a Reduction of the PQ Pool
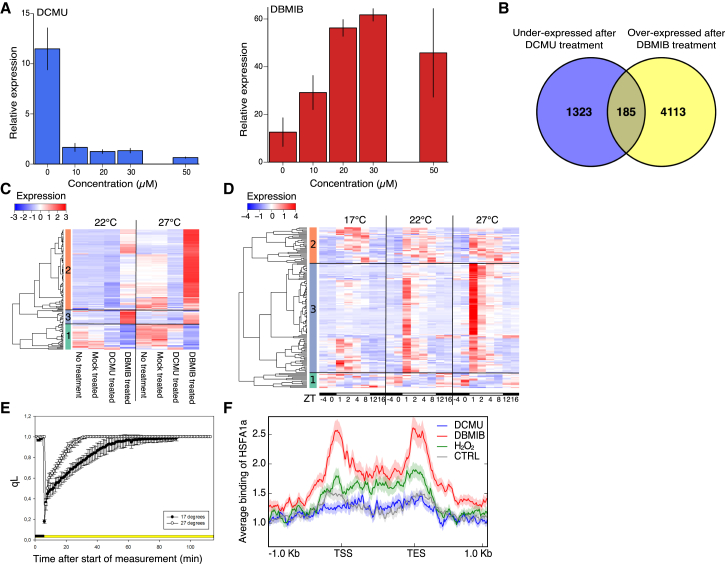
Diurnal patterns of heat-associated gene expression and thermotolerance are affected by the light reactions of photosynthesis and the redox state of the PQ pool has been shown to affect the expression of heat-associated genes in response to excess light (Jung et al., 2013). Because of this and the observation that the PQ pool is more reduced in ss4 than WT, and that this over-reduction is rescued in ss3/4/pgm (calculated using data from Ragel et al. [2013]; Supplemental Experimental Procedures), we investigated whether the redox state of the PQ pool affects HSP70 expression. The redox state of the PQ pool can be modified experimentally by the application of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB). DCMU prevents plastoquinol binding to QB and thus blocks electron transfer to the PQ pool, resulting in a more oxidized PQ pool (Pfalz et al., 2012). DBMIB prevents the PQ pool from transferring electrons to the cytochrome b6f (cyt-b6f) complex, resulting in a reduced PQ pool (Pfalz et al., 2012). We see that the morning peak of HSP70 expression is abolished by DCMU, while it is increased by DBMIB, indicating that a reduced PQ pool is required for HSP70 induction in the morning (Figure 4A).
Figure 4.
The Redox State of the Plastoquinone Pool Is Associated with the Expression of the Morning Cluster of Heat-Associated Genes
(A) HSP70 expression assayed by qRT-PCR after DCMU and DBMIB treatment of seedlings grown at 22°C in short days. Plants treated at ZT−1 and sampled at ZT1. Error bars are ±SEM (n = 3).
(B) Overlaps of genes under-expressed (Log2 fold change <−0.5) after DCMU (30 μM) and overexpressed (Log2 fold change >0.5) after DBMIB (50 μM) treatments compared to a mock treatment at 22°C, treated at ZT−1 and sampled at ZT1.
(C) Expression of cluster 13-1-1 (from Figure 1C) in transcriptomes of untreated, mock-treated, DCMU, and DBMIB-treated seedlings at 22°C and 27°C ZT1 after treatment at ZT−1. Replicate shown in Figure S3E.
(D) Expression of the genes under-expressed after DCMU treatment and overexpressed after DBMIB treatment (overlap from B) in WT over a 24-hr time course (same as used for Figure 1C) at 17°C, 22°C, and 27°C. Black bars below heatmap indicate samples taken in the dark (ZT−4, ZT0, ZT12, and ZT16) and white bars indicate samples taken in the light (ZT1, ZT2, ZT4, and ZT8). (C and D) Clusters are shown on the left of heatmap and low to high expression (Z scores) is shown as blue to red.
(E) qL over dawn at 17°C and 27°C. Error bars are ±SD (n = 4).
(F) Average binding of HSFA1a to genes from the intersection of Figure 4B after treatment at ZT−1 with DCMU (30 μM), DBMIB (50 μM), H2O2 (5 mM), and a mock ethanol treatment. Plants sampled at ZT1. Shading represents SE. Replicate shown in Figure S3F.
As DCMU and DBMIB treatments oppositely affect HSP70 expression, we examined which genes were affected in a similar way, as the expression of these genes may also be affected by the redox state of the PQ pool. At 22°C DBMIB treatment resulted in the overexpression of 4,298 genes and DCMU treatment resulted in the under-expression of 1,508 genes (Figure 4B). Despite the chemical treatments resulting in large changes to the transcriptome, only a small set of genes were both overexpressed after DBMIB and under-expressed after DCMU treatment (Figure 4B). This set of genes was strongly enriched for genes associated with responses to heat (Table S2) and for genes with HSEs in their promoters (Table S3) suggesting that the redox state of the PQ pool affects the morning peak of heat-associated gene expression. This is supported by the observation that the expression of the majority of genes in the morning cluster (Cluster 13-1-1 from Figure 1B) are increased by DBMIB treatment and decreased by DCMU treatment (Figure 4C and S3E). Similarly, the majority of genes that are oppositely affected by DCMU and DBMIB treatment have a morning peak of expression in untreated samples (Figure 4D).
As a reduced PQ pool is associated with the morning peak of heat-associated gene expression, we predicted that the PQ pool would be reduced by light in the morning. As expected the PQ pool was fully oxidized during the night, based on an indirect measure of the redox state of the PQ pool, qL (Kramer et al., 2004). With the onset of light qL decreased and then rose throughout the first hour of the day (Figure 4E), reflecting a reduction of the PQ pool by light and a subsequent re-oxidation when the Calvin-Benson cycle became light-activated. qL values decreased to a lower level at 17°C compared to 27°C and the amount of time until qL returned to one was slower at 17°C compared to 27°C, likely due to a slower induction of the Calvin-Benson cycle (Figure 4E). This indicates that the PQ pool becomes more reduced by light at lower temperatures; therefore, the level of reduction of the PQ pool does not appear to transmit temperature information in the ambient range. In Chlamydomonas, it has been proposed that light and temperature signals are integrated independently via Mg-protoporphyrin IX (MgProto) and HSFs, respectively (von Gromoff et al., 2006), however, the role of MgProto in retrograde signaling has been questioned (Moulin et al., 2008, Mochizuki et al., 2008). Because HSFs have previously been shown to respond to redox status (Jung et al., 2013, Miller and Mittler, 2006, Volkov et al., 2006), we investigated whether they might integrate both light and heat signals for morning cluster gene expression in Arabidopsis. The HSFA1 clade of HSFs have been shown to be essential for the early transcriptional response to heat (Liu et al., 2011), so we investigated the binding of HSFA1a to genes overexpressed after DBMIB treatment and decreased after DCMU treatment (intersection of Figure 4B) by chromatin immunoprecipitation sequencing (ChIP-seq). Interestingly, we observe a strong induction of HSFA1a binding to these genes in response to DBMIB at 22°C, while DCMU prevents the binding of HSFA1a to these genes (Figures 4F and S3F). Taken together, these results are consistent with a model where HSFA1a is able to integrate both the light and temperature signals, with the light signal being either PQ redox state or associated H2O2. Direct H2O2 transfer from the chloroplast to nucleus has been shown to be involved in high light signaling and was repressed by DCMU treatment (Exposito-Rodriguez et al., 2017). As H2O2 can activate HSFs (Volkov et al., 2006) and cause increased HSP70 expression (Figure S3G), a model where H2O2 is produced in the chloroplast at the onset of light and transferred to the nucleus to activate the expression of heat-associated genes is an attractive model for generating diurnal patterns of heat-associated gene expression and thermotolerance.
Conclusions
We find a diurnal pattern of thermotolerance in Arabidopsis that is a consequence of a gating effect of light-induced chloroplast signaling on the expression levels of heat-associated genes such as HSP70, accounting for a long-standing observation that plants have a differential ability to withstand heat stress in the day and night (Laude, 1939).
Under constant temperature conditions, the expression of morning peak genes such as HSP70 is repressed into the afternoon (Figures 1B and 1C), likely because of repressive interactions of HSP70 and HSP90 with HSFA1s (Ohama et al., 2016) resulting in a negative feedback loop. The sharpness of the morning peak may also be influenced by growth chambers, with plants being subjected to a sudden change from darkness to full light, while plants in the field will experience a more gradual rise in light levels. Protective chaperones such as HSP70 have ATPase activity, and at high expression levels, can represent a significant energy demand. Drosophila cells overexpressing HSP70 show greatly attenuated growth (Feder et al., 1992), and constitutive activation of heat-associated gene expression in Arabidopsis likewise greatly reduces growth (Ogawa et al., 2007). It may therefore be advantageous to limit heat-associated gene expression to the daytime, when temperature stress is most probable.
Experimental Procedures
Plant Material and Growth Conditions
Growth conditions were often specific to the experiment performed and these are described in the Supplemental Experimental Procedures.
For details of mutant lines used in this study see the Supplemental Experimental Procedures and Table S1.
Thermotolerance Assays
A thermotolerance assay was developed based on a previously described protocol (Silva-Correia et al., 2014). After 7 days of growth, plates were floated in a water bath preheated to 45°C for 30 min. Survival was defined as the ability to produce new green leaves.
Gene Expression Analysis
For qRT-PCR assays, RNA was extracted using a previously described phenol:chloroform extraction method (Box et al., 2011). Transcript levels were quantified using SybrGreen (Roche) and samples assayed with technical triplicates and on a LightCycler 480 (Roche).
Identifying Candidate Mutants and Mapping Causal Genes
A previously described fusion between the promoter of HSP70 and LUCIFERASE (pHSP70::LUC) (Kumar and Wigge, 2010) was used to screen EMS mutagenized Col-0 plants. LUC activity was imaged using a Photek HRPCS218 camera. Plates were imaged at 17°C at ZT1 and shifted to 27°C for 2 hr and imaged again at ZT3. Causal genes in were mapped by sequencing F2 plants displaying mutant phenotypes after outcrossing to Landsberg erecta.
Transcriptomics
The MagMax RNA extraction kit (Ambion) was used to extract RNA from 20–25, 8-day-old seedlings and libraries prepared using the Illumina TruSeq stranded kit. Sequencing was performed on a NextSeq500 (Illumina) and an in-house RNA sequencing (RNA-seq) processing pipeline was used to process sequence data.
ChIP-Seq
Chromatin was extracted from 1 g of cross-linked plant material, fragmented by sonication and ChIP was performed using anti-FLAG M2 magnetic beads (Sigma, M8823) coupled to a 1/1 mix of protein-A and protein-G Dynabeads (Life Technologies, 10001D and 10003D). ChIP-seq libraries were prepared using a TruSeq ChIP Library kit (Illumina) and sequenced on a NextSeq 500 (Illumina).
Chlorophyll Fluorescence
The Maxi version of the Imaging-PAM M-series (Walz) was used to determine chlorophyll-a fluorescence parameters of intact plants. A custom-made version of the 3010-GWK1 gas exchange chamber (Walz) was used to control humidity and temperature.
Acknowledgments
We thank Ángel Mérida, Sam Zeeman, and Benoit Landrien for Arabidopsis strains. We thank members of the Wigge laboratory for feedback and discussions. P.J.D. was supported by a Gatsby PhD Studentship (PTDC.GAAB). This work was supported by a grant from the Biotechnology and Biological Sciences Research Council (BB/I013350/1). The P.A.W. laboratory is supported by a Fellowship from the Gatsby Foundation (GAT3273/GLB).
Author Contributions
P.J.D. conceived and performed experiments and wrote the manuscript. M.K., C.M., S.J.Y., G.A., and K.E.J. performed experiments. H.L. and V.C. used bioinformatics to analyze results, and M.A.S. and R.B. contributed experiments and analysis. P.A.W. conceived of the project and contributed to writing the manuscript and analyzing the data.
Declaration of Interests
The authors declare no competing interests.
Published: February 13, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and four tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.01.054.
Data and Software Availability
The accession number for the raw and processed data from the RNA sequencing experiments reported in this paper is GEO: GSE96041.
Supplemental Information
Two Gene Ontology (GO) enrichment tools, GOrilla and AgriGO, were used for enrichment analysis and all genes that passed the filters used when calculating Z-scores were used as background. The top five most enriched GO terms from each analysis are shown.
Promoter motif enrichment was performed using HOMER2. Sequences 1 kb upstream of the TSS were obtained from TAIR and these were used to search for known motifs. Sequences 1 kb upstream of all genes that passed the filters used when calculating Z-scores were used as background sequences. The top five most enriched motifs are shown.
References
- Asseng S., Ewert F., Martre P., Rötter R., Lobell D., Cammarano D., Kimball B.A., Ottman M., Wall G., White J. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2014;5:143–147. [Google Scholar]
- Box M.S., Coustham V., Dean C., Mylne J.S. Protocol: a simple phenol-based method for 96-well extraction of high quality RNA from Arabidopsis. Plant Methods. 2011;7:7. doi: 10.1186/1746-4811-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.X., Phua S.Y., Crisp P., McQuinn R., Pogson B.J. Learning the languages of the chloroplast: retrograde signaling and beyond. Annu. Rev. Plant Biol. 2016;67:25–53. doi: 10.1146/annurev-arplant-043015-111854. [DOI] [PubMed] [Google Scholar]
- Colombo S.J., Timmer V.R., Colclough M.L., Blumwald E. Diurnal variation in heat tolerance and heat shock protein expression in black spruce (Piceamariana) Can. J. For. Res. 1995;25:369–375. [Google Scholar]
- Cortijo S., Charoensawan V., Brestovitsky A., Buning R., Ravarani C., Rhodes D., van Noort J., Jaeger K.E., Wigge P.A. Transcriptional regulation of the ambient temperature response by H2A.Z nucleosomes and HSF1 transcription factors in Arabidopsis. Mol. Plant. 2017;10:1258–1273. doi: 10.1016/j.molp.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton-Taylor M., Pike M., Lu K.J., Hylton C.M., Feil R., Eicke S., Lunn J.E., Zeeman S.C., Smith A.M. Starch synthase 4 is essential for coordination of starch granule formation with chloroplast division during Arabidopsis leaf expansion. New Phytol. 2013;200:1064–1075. doi: 10.1111/nph.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd A.N., Jakobsen M.K., Baker A.J., Telzerow A., Hou S.W., Laplaze L., Barrot L., Poethig R.S., Haseloff J., Webb A.A.R. Time of day modulates low-temperature Ca signals in Arabidopsis. Plant J. 2006;48:962–973. doi: 10.1111/j.1365-313X.2006.02933.x. [DOI] [PubMed] [Google Scholar]
- Exposito-Rodriguez M., Laissue P.P., Yvon-Durocher G., Smirnoff N., Mullineaux P.M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 2017;8:49. doi: 10.1038/s41467-017-00074-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder J.H., Rossi J.M., Solomon J., Solomon N., Lindquist S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Dev. 1992;6:1402–1413. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- Jung H.S., Crisp P.A., Estavillo G.M., Cole B., Hong F., Mockler T.C., Pogson B.J., Chory J. Subset of heat-shock transcription factors required for the early response of Arabidopsis to excess light. Proc. Natl. Acad. Sci. USA. 2013;110:14474–14479. doi: 10.1073/pnas.1311632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.H., Domijan M., Klose C., Biswas S., Ezer D., Gao M., Khattak A.K., Box M.S., Charoensawan V., Cortijo S. Phytochromes function as thermosensors in Arabidopsis. Science. 2016;354:886–889. doi: 10.1126/science.aaf6005. [DOI] [PubMed] [Google Scholar]
- Kappen L., Lösch R. Diurnal patterns of heat tolerance in relation to CAM. Z. Pflanzenphysiol. 1984;114:87–96. [Google Scholar]
- Kramer D.M., Johnson G., Kiirats O., Edwards G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004;79:209–218. doi: 10.1023/B:PRES.0000015391.99477.0d. [DOI] [PubMed] [Google Scholar]
- Kropat J., Oster U., Rüdiger W., Beck C.F. Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc. Natl. Acad. Sci. USA. 1997;94:14168–14172. doi: 10.1073/pnas.94.25.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.V., Wigge P.A. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell. 2010;140:136–147. doi: 10.1016/j.cell.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Lai A.G., Doherty C.J., Mueller-Roeber B., Kay S.A., Schippers J.H.M., Dijkwel P.P. CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc. Natl. Acad. Sci. USA. 2012;109:17129–17134. doi: 10.1073/pnas.1209148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude H.H. Diurnal cycle of heat resistance in plants. Science. 1939;89:556–557. doi: 10.1126/science.89.2320.556-a. [DOI] [PubMed] [Google Scholar]
- Lee C.M., Thomashow M.F. Photoperiodic regulation of the C-repeat binding factor (CBF) cold acclimation pathway and freezing tolerance in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2012;109:15054–15059. doi: 10.1073/pnas.1211295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legris M., Klose C., Burgie E.S., Rojas C.C.R., Neme M., Hiltbrunner A., Wigge P.A., Schäfer E., Vierstra R.D., Casal J.J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science. 2016;354:897–900. doi: 10.1126/science.aaf5656. [DOI] [PubMed] [Google Scholar]
- Li Q.B., Guy C.L. Evidence for non-circadian light/dark-regulated expression of Hsp70s in spinach leaves. Plant Physiol. 2001;125:1633–1642. doi: 10.1104/pp.125.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.C., Liao H.T., Charng Y.Y. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 2011;34:738–751. doi: 10.1111/j.1365-3040.2011.02278.x. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Mittler R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 2006;98:279–288. doi: 10.1093/aob/mcl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N., Tanaka R., Tanaka A., Masuda T., Nagatani A. The steady-state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2008;105:15184–15189. doi: 10.1073/pnas.0803245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin M., McCormac A.C., Terry M.J., Smith A.G. Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc. Natl. Acad. Sci. USA. 2008;105:15178–15183. doi: 10.1073/pnas.0803054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niittylä T., Messerli G., Trevisan M., Chen J., Smith A.M., Zeeman S.C. A previously unknown maltose transporter essential for starch degradation in leaves. Science. 2004;303:87–89. doi: 10.1126/science.1091811. [DOI] [PubMed] [Google Scholar]
- Ogawa D., Yamaguchi K., Nishiuchi T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J. Exp. Bot. 2007;58:3373–3383. doi: 10.1093/jxb/erm184. [DOI] [PubMed] [Google Scholar]
- Ohama N., Kusakabe K., Mizoi J., Zhao H., Kidokoro S., Koizumi S., Takahashi F., Ishida T., Yanagisawa S., Shinozaki K. The transcriptional cascade in the heat stress response of Arabidopsis is strictly regulated at the level of transcription factor expression. Plant Cell. 2016;28:181–201. doi: 10.1105/tpc.15.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz J., Liebers M., Hirth M., Grübler B., Holtzegel U., Schröter Y., Dietzel L., Pfannschmidt T. Environmental control of plant nuclear gene expression by chloroplast redox signals. Front. Plant Sci. 2012;3:257. doi: 10.3389/fpls.2012.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queitsch C., Hong S.W., Vierling E., Lindquist S. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell. 2000;12:479–492. doi: 10.1105/tpc.12.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragel P., Streb S., Feil R., Sahrawy M., Annunziata M.G., Lunn J.E., Zeeman S., Mérida Á. Loss of starch granule initiation has a deleterious effect on the growth of Arabidopsis plants due to an accumulation of ADP-glucose. Plant Physiol. 2013;163:75–85. doi: 10.1104/pp.113.223420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiland S., Finazzi G., Endler A., Willig A., Baerenfaller K., Grossmann J., Gerrits B., Rutishauser D., Gruissem W., Rochaix J.D., Baginsky S. Comparative phosphoproteome profiling reveals a function of the STN8 kinase in fine-tuning of cyclic electron flow (CEF) Proc. Natl. Acad. Sci. USA. 2011;108:12955–12960. doi: 10.1073/pnas.1104734108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán I., Wattebled F., Mercedes Lucas M., Delvallé D., Planchot V., Jiménez S., Pérez R., Ball S., D’Hulst C., Mérida A. The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J. 2007;49:492–504. doi: 10.1111/j.1365-313X.2006.02968.x. [DOI] [PubMed] [Google Scholar]
- Rott M., Martins N.F., Thiele W., Lein W., Bock R., Kramer D.M., Schöttler M.A. ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell. 2011;23:304–321. doi: 10.1105/tpc.110.079111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Prändl R., Reindl A. Regulation of the heat-shock response. Plant Physiol. 1998;117:1135–1141. doi: 10.1104/pp.117.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T.D., Vanderveer P.J. Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol. 1989;91:679–684. doi: 10.1104/pp.91.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Correia J., Freitas S., Tavares R.M., Lino-Neto T., Azevedo H. Phenotypic analysis of the Arabidopsis heat stress response during germination and early seedling development. Plant Methods. 2014;10:7. doi: 10.1186/1746-4811-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simm S., Papasotiriou D.G., Ibrahim M., Leisegang M.S., Müller B., Schorge T., Karas M., Mirus O., Sommer M.S., Schleiff E. Defining the core proteome of the chloroplast envelope membranes. Front. Plant Sci. 2013;4:11. doi: 10.3389/fpls.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren L.L.E., Tanabe N., Lymperopoulos P., Khan N.Z., Rodermel S.R., Aronsson H., Clarke A.K. Quantitative analysis of the chloroplast molecular chaperone ClpC/Hsp93 in Arabidopsis reveals new insights into its localization, interaction with the Clp proteolytic core, and functional importance. J. Biol. Chem. 2014;289:11318–11330. doi: 10.1074/jbc.M113.534552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb S., Zeeman S.C. Starch metabolism in Arabidopsis. Arabidopsis Book. 2012;10:e0160. doi: 10.1199/tab.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb S., Egli B., Eicke S., Zeeman S.C. The debate on the pathway of starch synthesis: a closer look at low-starch mutants lacking plastidial phosphoglucomutase supports the chloroplast-localized pathway. Plant Physiol. 2009;151:1769–1772. doi: 10.1104/pp.109.144931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa K., Kanazawa A., Kramer D.M. Depletion of stromal P(i) induces high ‘energy-dependent’ antenna exciton quenching (q(E)) by decreasing proton conductivity at CF(O)-CF(1) ATP synthase. Plant Cell Environ. 2008;31:235–243. doi: 10.1111/j.1365-3040.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- Volkov R.A., Panchuk I.I., Mullineaux P.M., Schöffl F. Heat stress-induced H(2)O (2) is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 2006;61:733–746. doi: 10.1007/s11103-006-0045-4. [DOI] [PubMed] [Google Scholar]
- von Gromoff E.D., Schroda M., Oster U., Beck C.F. Identification of a plastid response element that acts as an enhancer within the Chlamydomonas HSP70A promoter. Nucleic Acids Res. 2006;34:4767–4779. doi: 10.1093/nar/gkl602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.Y., Tobin E.M. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell. 1998;93:1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two Gene Ontology (GO) enrichment tools, GOrilla and AgriGO, were used for enrichment analysis and all genes that passed the filters used when calculating Z-scores were used as background. The top five most enriched GO terms from each analysis are shown.
Promoter motif enrichment was performed using HOMER2. Sequences 1 kb upstream of the TSS were obtained from TAIR and these were used to search for known motifs. Sequences 1 kb upstream of all genes that passed the filters used when calculating Z-scores were used as background sequences. The top five most enriched motifs are shown.