Abstract
Introduction
Glioblastoma (GBM) is the most aggressive malignant brain cancer in adults, and its poor prognosis and resistance to the existing standard of care require the development of innovative therapeutic modalities. The local delivery of stem cells as therapeutic carriers against glioma has produced encouraging results, but encounters obstacles with regards to the repeatability and invasiveness of administration. Intranasal delivery of therapeutic stem cells could overcome these obstacles, among others, as a noninvasive and easily repeatable mode of administration.
Areas covered
This review describes nasal anatomy, routes of stem cell migration, and factors affecting stem cell delivery to hard-to-reach tumors. Furthermore, this review discusses the molecular mechanisms underlying stem cell migration following delivery, as well as possible stem cell effector functions to be considered in combination with intranasal delivery.
Expert opinion
Further research is necessary to elucidate the dynamics of stem cell effector functions in the context of intranasal delivery and optimize their therapeutic potency. Nonetheless, the technique represents a promising tool against brain cancer and has the potential to be expanded for use against other brain pathologies.
Keywords: Glioblastoma, malignant glioma, stem cells, intranasal delivery, CNS delivery
1. Introduction
Glioblastoma (GBM) or malignant glioma is the most prevalent primary malignant brain tumor in humans and carries an extremely poor prognosis. Despite aggressive standard of care therapies, such as surgical resection, radiation, and chemotherapy, median survival remains less than 15 months and less than 5% of patients survive 5 years after diagnosis [1]. One of the biggest challenges in the field of brain tumor therapy is the presence of the blood brain barrier (BBB), which limits the efficacy of therapeutic delivery. Thus, there is an urgent need for next-generation therapies that can either bypass or efficiently cross the BBB and specifically deliver therapy to the site of tumor.
The use of stem cells as therapeutic carriers against central nervous system (CNS) tumors has been well established. Stem cells of various lineages are attractive modes of delivery due to their versatility as therapy vehicles, their ability to cross the BBB, and their inherent migratory capacity to sites of brain trauma, including ischemia, traumatic brain injury, neurodegeneration, and brain tumors [2–4]. The main types of stem cells being evaluated for therapies in the CNS are neural stem cells (NSCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells. Thus far, NSCs have been favored for use in clinical trials: a completed Phase I clinical trial investigated the use of genetically modified NSCs that conferred prodrug activation (NCT01172964), and three other clinical trials employing NSC-mediated therapy are currently recruiting participants (NCT03072134, NCT02015819, NCT02192359). Stem-cell carriers could be particularly tailored to GBM treatment because of their ability to migrate to the tumor site, allowing for targeted therapy. Therefore, stem-cell-based therapies for GBM and other brain tumors warrant thorough scientific exploration in order to move toward clinical trials in the future.
Intranasal delivery (IND) has the advantage of bypassing the BBB entirely, and a wide range of therapeutics can reach the brain directly from the nasal passages through various mechanisms [5–7]. IND also benefits from being easily repeatable and noninvasive. In this review, we focus on relevant nasal anatomy, routes of stem-cell migration, and factors affecting stem-cell delivery to hard-to-reach tumors. Afterward, we summarize the current research on anti-glioma therapies utilizing therapeutic stem cells and an intranasal route of delivery.
2. IND of cell-based agents for glioma
Brain malignancies present a unique challenge to the success of stem-cell therapies as opposed to other cancer types, as the BBB protects the brain and prevents the majority of therapeutics from reaching the brain parenchyma. Often, the concentration of the drug that reaches the tumor site may be greatly reduced from what is administered. Moreover, increased dosing increases the possibility of systemic toxicity. Stem-cell-based therapies may be a viable solution to these obstacles, as stem cells are inherently tumor tropic and can enhance targeted therapeutic delivery.
Moreover, an ideal therapy would cross or bypass the BBB and efficiently reach the tumor site with limited off-target effects and systemic toxicity. IND of therapeutic agents has recently shown promise in preclinical and clinical settings because of its ability to bypass the BBB [6,7]. Furthermore, IND of therapeutic stem cells, as opposed to systemic injection, allows practitioners to avoid problems associated with first-pass clearance. Upon intravenous infusion, it has been shown that the majority of MSCs and NSCs become entrapped in microvasculature of the pulmonary circulation, in part due to their larger size [8]. Moreover, studies show that MSCs injected intravenously move from the lung to the liver, spleen, kidney, and bone marrow within 48 h [9]. Utilizing IND for stem-cell-based therapeutics may help improve their efficacy against glioma, by directing migration from the nasal cavity to the tumor site.
3. Normal intranasal anatomy
3.1. Nasal anatomy of mouse and human
The primary features of nasal anatomy in the mouse and human affecting IND are the gross structure of the nasal cavity and the nature of the cells that form the primary barrier of the respiratory tract. Differences in nasal anatomy between the laboratory mouse and human require further consideration, especially for translation of IND into clinical trials. For example, mice are obligate nose-breathers and have a nasal structure optimized for olfaction, while human nasal structure is more optimized for respiration and protection of the respiratory system [10]. Moreover, after entering the nostrils, air enters the nasal vestibule and passes through the nasal valve, a narrow passage leading to the main nasal chamber. The nasal chamber of both species is composed of nasal turbinates, intricate bony structures that support the nasal epithelium and increase the surface area of the nasal passage. Murine nasal turbinates are more prevalent, and the mouse has approximately five times the epithelial surface area relative to humans [11]. This could lead to the increased uptake of IND therapies in the mouse compared to the human; thus, downstream dosing studies have to be species specific. Differences between the mouse and human in nasal anatomy and absorption of therapy through the nasal epithelium have so far not been elucidated; however, they are crucial for translational research.
4. Stem-cell migration pathways
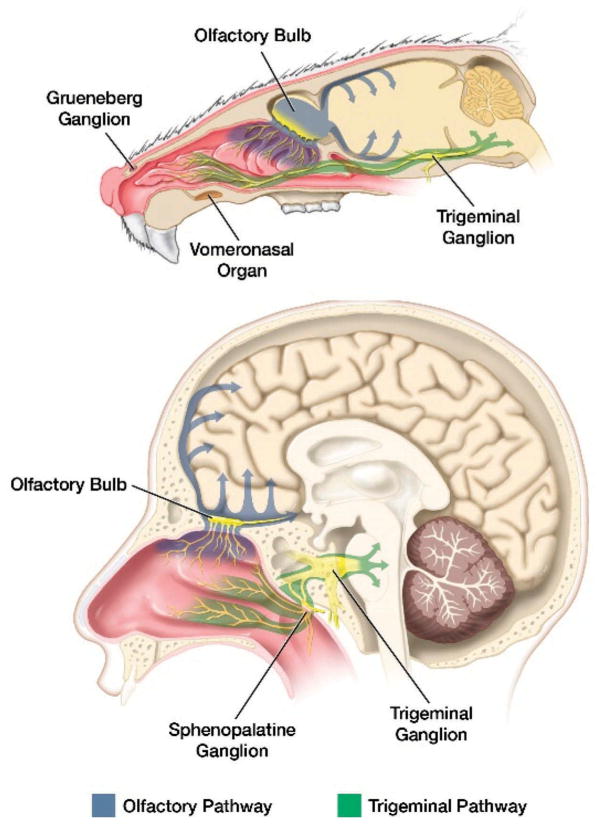
After IND, stem cells may follow several pathways to the tumor, as shown in Figure 1. The route of migration might depend on the type of stem cells used for therapy. The first barrier intranasally delivered stem cells encounter is the epithelium of the nasal cavity, which is well vascularized and innervated [10]. After penetration, the therapeutic stem cells migrate to the brain; we investigate two possible routes of migration below.
Figure 1.
Comparative sagittal views of mouse and human heads demonstrate potential routes of stem cell entry into the brain from the nasal cavity.
Blue arrows indicate the olfactory route, whereas green arrows indicate the trigeminal nerve path of entry of stem cells to different regions of brain. The trigeminal and olfactory nerves are shown in yellow. Full color available online.
4.1. Migration by olfactory nerves
The nasal epithelium is innervated by the olfactory nerves, which can be utilized by cell-based therapies to rapidly gain access to the brain after IND across the cribriform plate and into mitral cells of the olfactory bulbs. From the olfactory bulbs, afferent neurons carry olfactory signals to the olfactory tract, piriform cortex, amygdala, and hypothalamus. Specialized myelinating glia (olfactory ensheathing cells) form cerebrospinal fluid (CSF)-filled cavities along the olfactory nerves, which are thought to play a role in extracellular trafficking into the brain after IND [12]. The majority of studies on IND found that the delivery of agents occurred within a short time period (5–30 min), suggesting the roles of extracellular transport and bulk flow mechanisms [13–17]. From the olfactory bulb, stem cells can either migrate directly into the frontal lobe or to caudal portions of the brain via CSF flow [18]. The olfactory pathways have been shown to be a large component of IND therapy, with our lab and others showing that stem cells delivered intranasally can be visualized in the olfactory bulb and frontal lobe with MRI [13,19]. As the olfactory epithelium is significantly larger in mouse, it is likely that this pathway plays a larger role in cell migration in the murine model than in humans.
4.2. Migration by trigeminal nerve
The mandibular and maxillary segment of the trigeminal nerve innervates the olfactory region in the distal nasal cavity, terminating in the spinal trigeminal nuclei in the pons. Intranasally delivered therapeutic agents enter the brain through this route, suggesting that stem cells may also [6,20–22]; however, this requires further investigation.
5. Molecular mechanisms of stem-cell migration
The success of stem-cell-based therapies greatly depends on their ability to migrate toward and distribute throughout the tumor [23–25]. Thus, optimization of stem-cell-based therapies requires the understanding of molecular mechanisms responsible for stem-cell migration to brain tumors. Several studies have shown that stem-cell tumor-tropism occurs along a chemotactic gradient, and several soluble factor receptors have been identified that influence stem-cell migration in a tumor model [26–30]. Additionally, there is evidence that the stem-cell interactions with astrocytes, neurons, and the extracellular matrix (ECM) could affect stem-cell migration following IND [31–34]. Although this review does not intend to cover the complexity of all migratory mechanisms utilized by stem cells in the brain, we highlight several of them in the section below.
5.1. Chemotactic gradients can affect stem-cell migration to brain tumors after IND
The vascular endothelial growth factor receptor has been found by several studies to be necessary to induce stem-cell migration in the brain and implicates the hypoxic microenvironment of the tumor [26,27]. The CXC-motif chemokine receptor 4 (CXCR4) has also been found to effect stem-cell migration to malignancies in the brain [26,35]. Expression of CXCL12, the associated ligand for CXCR4, has been shown to be controlled by hypoxia-specific transcription, and increased expression of CXCL12 resulted in enhanced stem-cell migration [28,36]. Finally, brain malignancies secrete macrophage chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8), TGF-β1, and neurotrophin-3 (NT-3), which recruit MSCs to the tumor through their respective chemotactic axes [30,35]. MCP-1, in addition to CXCR4, played an important role in MSC migration to gliomas in vitro, an effect that diminished with neutralizing antibodies [35]. Ligands IL-8, TGF-β1, and NT-3 also had significant, dose-dependent effects in recruiting MSCs toward gliomas when incubated with MSCs separately [30]. Together, these data suggest that the tumor secretes a wide array of chemoattractive and angiogenic cytokines that actively recruit stem cells to the site of the tumor. Further investigation of these gradients may be useful to enhance the efficacy of stem cell-based, intranasally delivered therapies.
5.2. Haptotactic interactions promote stem-cell tumor tropism
Stem-cell tumor tropism has also been shown to be dependent on haptotactic cell-to-cell contacts and interactions with the ECM governed by integrins, selectins, and other cellular adhesion molecules.
Stem cells interact with vascular endothelial cells as they travel through circulation, with a loose adhesion to selectins and glycoproteins resembling that of disseminated lymphocytes [31]. Furthermore, like other tissue-homing cells, stem cells rely on interactions between cellular adhesion molecules to arrest on the surface of endothelial cells. Similar mechanisms to those found in the vasculature and lymphatics could be involved in stem-cell homing to brain malignancies after IND, including those involving, for example, the integrin α4 subunit of VCAM-1 and very-late antigen 4 [32]. Increasing evidence also suggests an important role for the ECM in stem-cell migration through extracellular spaces in the brain parenchyma, with the implication of numerous ECM components in brain malignancies [34]. Thus, the interactions of selectins, glycoproteins, cellular adhesion molecules, and ECM components are vital to the migratory ability of stem cells, and their engineering can be used to enhance the therapeutic efficacy of these treatments.
6. Effector functions of therapeutic stem cells
Stem cells have been used to carry prodrug activators, deliver viral vectors and nanoparticles (NPs), as well as produce antibodies [37]. By using tumor-tropic stem cells as a vehicle for therapeutic agents, there is the potential to overcome challenges posed by the in vivo environment on the therapeutic vector alone, including poor dissemination and absorption, toxicity, a short half-life, elimination by the immune system, and a lack of target specificity [23,25,38]. The inherent ability of stem cells to migrate to the tumor may offer benefits when delivered intranasally that not conferred by other therapeutic vectors, namely viruses or NPs, without further modification. However, both viral [39–41] and NP [42] systems have demonstrated therapeutic benefit against glioma when delivered via the intranasal route. While there is limited literature available directly comparing the various techniques, we have demonstrated the significant survival benefit to irradiated mice after delivering oncolytic virus in NSCs cultured in hypoxic conditions in comparison to oncolytic viruses alone [5]. In the future, the scope of cross-comparison experiments should be expanded in order to determine the most efficient strategy of therapeutic delivery.
Before examining each effector function, it is worth noting that the underlying dynamics and mechanisms of each must be further investigated in the context of IND in order to optimize therapeutic benefit. Stem cells that are genetically engineered, whether it be to express prodrug activators, antibodies, or antiproliferative agents, must be followed after IND to chronicle the rate of accumulation in tumors and establish a timeline for therapeutic delivery. A table summarizing therapeutic effector functions is below. As seem in Table 1, we summarized the representative examples and further discussed in details the effector functions stem cells in context of GBM and other cancers.
Table 1.
Preclinical evaluation of stem cells as therapeutic carriers for brain malignancies.
| Effector function | Stem-cell type | Examples |
|---|---|---|
| Prodrug activators | NSCs | Conversion of prodrug 5-FC to 5-FU [43] |
| Conversion of CPT-prodrug to topoisomerase I inhibitor [44] | ||
| Delivery of thymidine kinase (HSVtk) to activate ganciclovir [45,46] | ||
| MSCs | HSVtk/ganciclovir system [47] | |
| Viral vectors | NSCs | CRAd-S-pk7 [48,49] |
| MSCs | CRAd-CXCR4-5/3 [50] | |
| Δ24-RGD [51] | ||
| oHSV [52] | ||
| MV [53–55] | ||
| Nanoparticles | NSCs | Magnetic spinning disk nanoparticles (SD) [56] |
| MSCs | Silica nanorattle-doxorubicin anchored by a nanoparticulate patch system [57] | |
| MIAMI | Cytotoxic Fc-diOH [58,59] | |
| Immunostimulatory agents | MSCs | IFN-β [25], IFN-β co-expressed with IL18 [29] |
| MSCs | IFN-β [60] | |
| Antiproliferative and proapoptotic agents | MSCs | BMP4 [61,62] |
| TRAIL and EGFR-targeted nanobody [63] | ||
| NSCs | Gas1 [64] | |
| Thrombospondin (TSP-1) [65] | ||
| PEX [66] | ||
| Both MSCs and NSCs | TRAIL [67–69] | |
| Antibody based | MSCs | scFvs for EGFRvIII [70] |
| Bispecific αCEA/αCD3 diabody [71] | ||
| NSCs | Anti-HER2 for brain metastases of HER2-overexpressing breast cancer [72] |
5-FC: 5-Fluorocytosine; 5-FU: 5-flurouracil; NSCs: neural stem cells; o-HSV: oncolytic herpes simplex virus; MS: measles virus; MIAMI: marrow-isolated adult multilineage inducible; BMP4: bone morphogenetic protein 4; Gas1: growth arrest specific-1.
6.1. Prodrug activators
Prodrugs often suffer from incomplete absorption and delivery and poor site specificity when delivered systematically. Their utilization can be enhanced through the use of stem cells as a producer synthesizer of the relevant enzymes needed to biologically transform prodrug components into active therapeutics, increasing the concentration of therapeutic drug in the tumor. The cytosine deaminase (CD)-expressing clonal human NSC line HB1.F3.CD, shown to enhance local conversion of prodrug 5-fluorocytosine to the chemotherapeutic 5-flurouracil [43], and the herpex simplex virus thymidine kinase (HSVtk)/ganciclovir system [45,46] are examples of prodrug therapies delivered with stem cells.
6.2. Viral vectors
Oncolytic virotherapy, or the employment of genetically engineered viruses to induce apoptosis, is a promising approach for treating brain malignancies. Stem cells are well poised to overcome the limitations that direct injection into the CNS is highly invasive and naked viral vectors elicit an immune response, currently posed by viral vectors [73]. Examples of viral vectors delivered by stem cells include Δ24-RGD loaded into bone marrow-derived MSCs [51], CRAd-S-pK7 loaded into NSCs [48,49], CRAd-CXCR4-Ad5/3 loaded into MSCs [50], oncolytic herpes simplex virus loaded into MSCs [52], and the measles virus (MV) loaded into MSCs [53–55]. Currently, a clinical trial investigating the application of MV-loaded MSCs is underway in patients with ovarian cancer (NCT02068794).
6.3. Nanoparticles
NPs offer a vector for the transportation of therapeutics to GBM, protecting them from degradation, preventing their rejection at the BBB, improving their biodistribution, supporting innovative imaging techniques [74,75]. They are commonly composed of polymers and lipids and can carry multiple therapeutic agents, including drugs [76–78], DNA [79,80], and radionuclides [81]. Furthermore, NPs themselves can behave as magnetic and thermal therapeutic agents [82] by rotating to mechanically induce apoptosis, responding to ultrasound to deliver drugs, or producing heat in response to alternating magnetic fields [83]. NPs have been incorporated into stem cells spontaneously, through passive transport, or through active transport [84–87]. They have also been used to manipulate MSCs to produce bone morphogenetic protein 4 (BMP4), thus avoiding the use of viral engineering in the delivery of antiproliferative agents [88]. Examples of stem-cell-based NP therapeutic systems against GBM include the silica nanorattle-doxorubicin nanoparticulate patch system in MSCs [57], the use of cytotoxic Fc-diOH-loaded MIAMI cells [59], and magnetic spinning disk NPs (SD)-loaded NSCs [56]. Loading stem cells with NPs may protect them from removal by the mononuclear phagocyte system, enhance distribution within the tumor, and allow researchers to circumvent the need to engineer a NP to be tumor tropic.
6.4. Immunostimulatory agents
GBM takes advantage of both the immune-privileged environment of the brain and the general ability of cancer to evade the endogenous immune system to proliferate aggressively and recur [89]. One class of immunostimulatory agents is composed of interferons (IFN), signaling proteins that activate various cells of the immune system and include cytotoxic T cells, natural killer cells, and dendritic cells [90]. While there are many distinct IFN, IFN-β has been the most successful therapeutic for the solid tumor environment of glioma, shown to disrupt the vasculature niche of glioma stem cells and induce apoptosis in solid tumors [91–94]. The use of both MSCs and NSCs as delivery mechanisms for IFN-β has been shown by multiple groups to show promise as a therapeutic [25,60]. IFN-β used in combination with IL18 promoted glioma cell apoptosis, the production of cytokines, and the infiltration of CD4+ and CD8+ in comparison to the secretion of either IL18 or IFN-β alone [29]. These promising results open the door to further studies on the combinational therapies that target the heterogeneous nature of glioma.
6.5. Antiproliferative and proapoptotic agents
Antiproliferative agents refer to a wide range of substances that control the proliferation, migration, and aggressiveness of cancer cells. Naked antiproliferative agents are often nonspecific toward tumors and therefore require the development for tumor-specific delivery methods. BMPs [61,62], growth arrest-specific-1 [64], thrombospondin (TSP-1) [65], the naturally occurring fragment of human metalloproteinase-2 PEX [66], and TNF-related apoptosis-inducing ligand [63,67–69] have been investigated as potential targets utilizing stem cells as carriers to GBM.
6.6. Antibody-based therapy
Antibody-based brain tumor therapy takes advantage of antigens expressed on the surface of cancerous cells as potential targets and has been effective in the treatment of patients with chronic lymphocytic leukemia, lymphomas, and HER2-overexpressing breast cancers [95–97]. However, with regards to the treatment of solid brain malignancies, antibodies have been found to be too large to bypass the BBB and have difficulty distributing evenly within the tumor mass. Furthermore, while receptors used by antibody-based therapy like HER2 may be overexpressed in aberrant cancer cells, they may also be found on normal cells, raising significant concerns about off-target toxicity. To overcome these obstacles, the development of stem cells that express small single-chain antibody (scFv) on their surface has been pursued. Antibodies targeting cancer-exclusive genetic mutation EGFRvIII [70,98] and HER2 [72] have been previously investigated for stem-cell-based GBM treatment; bispecific α-carcinoembryonic antigen (αCEA)/αCD3 diabody-secreting MSCs have been investigated for the treatment of colon cancer [71]. The viability of antibodies from genetically modified stem cells must be evaluated in the context of time and explored further in order for a successful transition into the clinic.
7. Experimental considerations for IND in preclinical studies
Many considerations should be given when developing stem-cell-based therapies for IND to the brain tumor. Unlike systemic therapies, for which the exact location of delivery does not affect the efficacy of therapy (i.e. intravenous), intranasal administration may elicit different results based on small variations in the orientation of the skull [99] and the depth of administration within the nasal cavity [100]. Although it has not been directly demonstrated, results may also be affected by the volume of therapeutic delivered. The fast clearance of therapeutic agents observed following IND should be taken into account when developing preclinical experiments [5,13,44]. Additionally, how stem cells utilize the olfactory and trigeminal nerve, the vasculature, and the lymphatic pathways for brain entry remains to be delineated [6,20,22,101–103]. Brain malignancies are extremely diverse in terms of their molecular characteristics and location in the brain; the molecular and migratory characteristics of the different stem-cell types will determine their selection as a therapeutic carrier.
8. Considerations for translating IND to the clinic
Experimental variables, such as the administration technique, vehicle volume, and cell type, must also be carefully considered in IND’s eventual translation to human patients in the clinic. This review has already discussed differences in murine and human nasal anatomy, as it pertains to the development of the IND of stem cells carrying anti-glioma treatment. Moreover, many technical considerations for the IND of drugs must be investigated: the angle of the head during administration, depth, and volume of dosage all have a considerable effect on the efficiency of treatment, as well as the addition of mucoadhesives or permeation enhancers [104–107]. Additionally, the inherent pharmacokinetic and dynamic properties of any therapeutic, especially cell-based therapies, are of concern in translational research.
In preclinical models, mice are often anaesthetized and held in a supine position, which results in direct contact between the administered substance and the nasal epithelium. In preclinical trials with nonhuman primates and in human clinical trials, several positions have been attempted, such as supine with the head laying off the table (Mybargs position) or such ‘praying to mecca’ positions, with the head down and forward [108]. However, both of these positions are uncomfortable for the patient, and alternatives must be explored.
The depth of administration also has a large effect on the accessibility of the nasal epithelium and the corresponding treatment efficiency. While the two positions noted above achieve good contact with the nasal epithelium, other approaches have also been attempted, such as delivering smaller, more localized doses directly to the nasal epithelium by breathing tubes or catheters [109]. These techniques also can limit the volume of dosage and reduce the risk of respiratory distress, which favors a repeated dosing schedule in preclinical, nonhuman primate, and human studies [110,111].
The kinetic and dynamic properties of stem-cell-based therapies applied intranasally have not been thoroughly studied, although preliminary work in the area suggests that various aspects of therapy affect retention and therapeutic efficiency. The availability of the nasal epithelium is of primary concern during dosing, but the migration, retention, and longevity of these cells in the brain often correlate with their ability to target and reduce intracranial malignancies.
9. Imaging to track intranasally delivered stem cells
Different methods of imaging may be utilized to track the migratory behavior of intranasally delivered stem cells in the skull and elucidate the dynamics of therapeutic delivery. Fluorescent microscopy, [H3]-thymidine, and near-infrared live imaging have previously been utilized to track MSCs following IND; florescent microscopy has also been used to image NSCs following IND [2,16,44,112,113].
Currently, researchers rely on magnetic resonance imaging (MRI) as the clinically relevant method of tracking stem cells in the brain. MRI was used to follow MSCs loaded with super-paramagnetic iron oxide NPs (SPIOs) or micron-size paramagnetic iron oxides and NSCs loaded with SPIOs [114]. However, due to its reliance on iron to generate the images, the utility of MRI is restricted by false-positive signaling from macrophage engulfment of imaging agents, small hemorrhages, or iron deposits in degenerating brains [115–118].
One promising development in imaging technology is the use of SPECT radiotracers, specifically 111Indium (111In) or Technetium 99mTc. SPECT imaging overcomes many of the challenges posed by MRI, bypassing the false-positive signaling, and provides enhanced spatial resolution and sensitivity. Our group has previously conjugated 111In to a mesoporous silica NP (MSN), which effectively migrates toward xenografted glioma following local and systematic injection, allowing for the dynamic in vivo SPECT imaging of NSCs [119]. The development of SPECT imaging represents a clinically relevant improvement on imaging technologies that may help further anti-glioma therapeutics.
10. Conclusion
Treatment of brain malignancies stands to be improved with the implementation of noninvasive IND of stem-cell-based therapeutics. The literature supports that stem-cell-based delivery of therapeutics notably improved the efficacy of the treatment in comparison to the delivery of the naked therapeutic. In combination with IND, stem-cell-based therapy could be a potent tool in the treatment of GBM, as IND harnesses the direct pathways between nasal epithelium to the brain and bypasses the BBB. The application of IND is furthermore promising for broader applications in the future, including for the treatment of brain metastases and lower grade tumors. It is especially fitting for the latter, as these malignancies typically have a more intact BBB and require treatments that circumvent it [120]. While more research needs to be done investigating the use of specific pathways and optimizing treatment based on the location of the tumor, this minimally invasive and repeatable delivery method already offers solutions to common problems in the treatment of malignancies in the brain.
11. Expert opinion
The IND of stem-cell-based therapies allows for a promising array of diverse treatment opportunities for glioma, considering the flexibility of stem cells to employ a wide variety of effector functions. The road to a cure for GBM is not simple, as it is a pervasive and persistent disease, heterogeneous both within the tumor and among patients; what may be needed are combinative therapies that take advantage of weaknesses in each specific tumor microenvironment. As the cancer evolves in an individual patient, it is important that the therapy evolves with it, and intranasal stem-cell delivery offers the necessary flexibility and repeatability. IND also offers the benefit of avoiding the first-pass effect associated with the systemic delivery of therapeutic stem cells. In comparison to systemic delivery, which is hindered by the BBB, and local delivery, which is dependent on advancements in catheter technology or the ability to operate on the tumor, an intranasal route stands out as a revolutionary possibility to tackle these problems [121,122].
Due to the novelty of the approach, it is anticipated that investigators run into challenges that require troubleshooting and examination. For example, the differences in anatomy between mice and human nasal epithelia may pose a challenge upon translation from preclinical to clinical studies. These differences must be fully investigated prior to patient trials in order to ensure the success of the novel delivery modality. Moreover, further research opportunities are presented in the investigation of the migratory pathways; while the olfactory nerve has been studied more in depth than the trigeminal nerve, it will be necessary to more deeply investigate each route in both mice and humans.
Furthermore, IND procedures may need to be optimized in order to increase stem-cell migration and their survival in the tumor milieu. The time frame and mechanisms of therapeutic effect of each stem-cell effector function must also be more deeply investigated before translation from preclinical to clinical studies. To these ends, investigators have genetically engineered stem cells to overexpress survival genes and cytokines or have administered the stem cells after a dose of hyaluronidase [16,123]. While more exploration into the specific pathways of IND, the behavior of stem cells at the site of delivery, and the efficacy and applicability of the novel delivery method for the various effector functions is necessary, this innovation stands to revolutionize cancer treatment. IND of stem-cell-based therapeutics takes advantage of stem cells’ natural tropism toward glioma and their capacity to administer multiple therapeutic modalities in a repeatable and minimally invasive course. Once the modality is fully understood, it may be used in the treatment of different brain malignancies or other brain disorders [2,15,31,124–126].
Article highlights.
Delivery of stem cells through the intranasal route has the potential to overcome many of the challenges of repeated CNS drug delivery currently faced, as it is a non-invasive and easily repeatable technique.
Differences in nasal anatomy between mouse and human exist and are highlighted.
Possible routes of migration for stem cells carrying therapeutics are explored, including pathways along the olfactory and trigeminal nerves.
Both mesenchymal and neural stem cells have a natural tropism towards glioblastoma, which makes them an appealing modality for the delivery of an array of therapeutics for malignant brain tumors.
Considerations for work that prepares intranasal delivery of stem cell-based therapy into the clinic are investigated.
This box summarizes key points contained in the article.
Acknowledgments
The authors would like to extend their gratitude to their medical illustrator, Michael Gallagher, for his contribution to this manuscript.
Funding
This paper was funded by the U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, R01CA122930 (MSL) and NIH R01NS087990 (MSL, IVB) and K08NS092895 (MD).
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005 Mar 10;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.van Velthoven CT, Kavelaars A, van Bel F, et al. Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr Res. 2010 Nov;68(5):419–422. doi: 10.1203/PDR.0b013e3181f1c289. [DOI] [PubMed] [Google Scholar]
- 3.Sun J, Wei ZZ, Gu X, et al. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp Neurol. 2015;272:78–87. doi: 10.1016/j.expneurol.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Lunn JS, Sakowski SA, Hur J, et al. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011 Sep;70(3):353–361. doi: 10.1002/ana.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Dey M, Yu D, Kanojia D, et al. Intranasal oncolytic virotherapy with CXCR4-enhanced stem cells extends survival in mouse model of glioma. Stem Cell Reports. 2016 Sep 13;7(3):471–482. doi: 10.1016/j.stemcr.2016.07.024. The authors of this paper further support intranasal stem cell delivery as a viable treatment option in their exploration of the CXCR4/SDF-1 signaling axis to optimize OV-carrying NSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorne RG, Pronk GJ, Padmanabhan V, et al. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Dhanda DS, Wh F, Ii, Leopold D, et al. Approaches for drug deposition in the human olfactory epithelium. Drug Delivery Technol. 2005;5:64–72. [Google Scholar]
- 8.Schrepfer S, Deuse T, Reichenspurner H, et al. Stem cell transplantation: the lung barrier. Transplant Proc. 2007 Mar;39(2):573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Dennis JE, Muzic RF, et al. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 10.Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicologic Pathology. 2006;34(3):252–269. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- 11.Treuting PM, Dintzis SM, Montine KS. Comparative anatomy and histology a mouse and human atlas introduction. In: Frevert CW, Liggitt D, editors. Comparative anatomy and histology: a mouse and human atlas. Cambridge, (MA): Academic Press; 2011. pp. 1–6. [Google Scholar]
- 12.Adams DR. Olfactory and non-olfactory epithelia in the nasal cavity of the mouse, Peromyscus. Am J Anat. 1972 Jan;133(1):37–49. doi: 10.1002/aja.1001330104. [DOI] [PubMed] [Google Scholar]
- 13•.Balyasnikova IV, Prasol MS, Ferguson SD, et al. Intranasal delivery of mesenchymal stem cells significantly extends survival of irradiated mice with experimental brain tumors. Mol Ther. 2014 Jan;22(1):140–148. doi: 10.1038/mt.2013.199. The results published in this study establish therapeutic relevance of intranasally delivered MSCs toward glioma xenografts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks WA, During MJ, Niehoff ML. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9–39) after intranasal administration. J Pharmacol Exp Ther. 2004 May;309(2):469–475. doi: 10.1124/jpet.103.063222. [DOI] [PubMed] [Google Scholar]
- 15.Danielyan L, Beer-Hammer S, Stolzing A, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014;23(Suppl 1):S123–39. doi: 10.3727/096368914X684970. [DOI] [PubMed] [Google Scholar]
- 16•.Danielyan L, Schafer R, von Ameln-Mayerhofer A, et al. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009 Jun;88(6):315–324. doi: 10.1016/j.ejcb.2009.02.001. Authors demonstrate that intranasally delivered MSCs are capable of migrating to the brain from the nasal cavity. [DOI] [PubMed] [Google Scholar]
- 17.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010 Apr;99(4):1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 18.Lochhead JJ, Wolak DJ, Pizzo ME, et al. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab. 2015 Mar;35(3):371–381. doi: 10.1038/jcbfm.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Reitz M, Demestre M, Sedlacik J, et al. Intranasal delivery of neural stem/progenitor cells: a noninvasive passage to target intracerebral glioma. Stem Cells Transl Med. 2012 Dec;1(12):866–873. doi: 10.5966/sctm.2012-0045. The authors of this paper show that neural stem/progenitor cells (NSPCs) migrate rapidly to and accumulate specifically in intracranial glioma xenografts, establishing IND as a feasible treatment platform. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhuria SV, Hanson LR, Frey WH., 2nd Novel vasoconstrictor formulation to enhance intranasal targeting of neuropeptide therapeutics to the central nervous system. J Pharmacol Exp Ther. 2009 Jan;328(1):312–320. doi: 10.1124/jpet.108.145565. [DOI] [PubMed] [Google Scholar]
- 21.Ross TM, Zuckermann RN, Reinhard C, et al. Intranasal administration delivers peptoids to the rat central nervous system. Neurosci Lett. 2008 Jul 4;439(1):30–33. doi: 10.1016/j.neulet.2008.04.097. [DOI] [PubMed] [Google Scholar]
- 22.Thorne RG, Hanson LR, Ross TM, et al. Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience. 2008 Mar 27;152(3):785–797. doi: 10.1016/j.neuroscience.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bexell D, Gunnarsson S, Tormin A, et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009 Jan;17(1):183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005 Apr 15;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 26.Zhao D, Najbauer J, Garcia E, et al. Neural stem cell tropism to glioma: critical role of tumor hypoxia. Molecular Cancer Res. 2008 Dec;6(12):1819–1829. doi: 10.1158/1541-7786.MCR-08-0146. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt NO, Przylecki W, Yang W, et al. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia (New York, NY) 2005 Jun;7(6):623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004 Aug;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 29.Xu G, Guo Y, Seng Z, et al. Bone marrow-derived mesenchymal stem cells co-expressing interleukin-18 and interferon-beta exhibit potent antitumor effect against intracranial glioma in rats. Oncol Rep. 2015 Oct;34(4):1915–1922. doi: 10.3892/or.2015.4174. [DOI] [PubMed] [Google Scholar]
- 30.Birnbaum T, Roider J, Schankin CJ, et al. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007 Jul;83(3):241–247. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- 31.Merzaban JS, Imitola J, Starossom SC, et al. Cell surface glycan engineering of neural stem cells augments neurotropism and improves recovery in a murine model of multiple sclerosis. Glycobiology. 2015 Dec;25(12):1392–1409. doi: 10.1093/glycob/cwv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma JC, Cheng P, Hu Y, et al. Integrin alpha4 is involved in the regulation of glioma-induced motility of bone marrow mesenchymal stem cells. Oncol Rep. 2015 Aug;34(2):779–786. doi: 10.3892/or.2015.4012. [DOI] [PubMed] [Google Scholar]
- 33.Lee SY, Kim JM, Cho SY, et al. TIMP-1 modulates chemotaxis of human neural stem cells through CD63 and integrin signalling. Biochem J. 2014 May 1;459(3):565–576. doi: 10.1042/BJ20131119. [DOI] [PubMed] [Google Scholar]
- 34.Ziu M, Schmidt NO, Cargioli TG, et al. Glioma-produced extracellular matrix influences brain tumor tropism of human neural stem cells. J Neurooncol. 2006 Sep;79(2):125–133. doi: 10.1007/s11060-006-9121-5. [DOI] [PubMed] [Google Scholar]
- 35.Xu F, Shi J, Yu B, et al. Chemokines mediate mesenchymal stem cell migration toward gliomas in vitro. Oncol Rep. 2010 Jun;23(6):1561–1567. doi: 10.3892/or_00000796. [DOI] [PubMed] [Google Scholar]
- 36.McGrath KE, Koniski AD, Maltby KM, et al. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Developmental Biology. 1999 Sep 15;213(2):442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 37.Roth JC, Curiel DT, Pereboeva L. Cell vehicle targeting strategies. Gene Ther. 2008 May;15(10):716–729. doi: 10.1038/gt.2008.38. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed AU, Rolle CE, Tyler MA, et al. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol Ther. 2010 Oct;18(10):1846–1856. doi: 10.1038/mt.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozduman K, Wollmann G, Piepmeier JM, et al. Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J Neurosci. 2008 Feb 20;28(8):1882–1893. doi: 10.1523/JNEUROSCI.4905-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiprianova I, Thomas N, Ayache A, et al. Regression of glioma in rat models by intranasal application of parvovirus h-1. Clin Cancer Res. 2011 Aug 15;17(16):5333–5342. doi: 10.1158/1078-0432.CCR-10-3124. [DOI] [PubMed] [Google Scholar]
- 41.Bongiorno EK, Garcia SA, Sauma S, et al. Type 1 immune mechanisms driven by the response to infection with attenuated rabies virus result in changes in the immune bias of the tumor micro-environment and necrosis of mouse GL261 brain tumors. J Immunol. 2017 Jun 01;198(11):4513–4523. doi: 10.4049/jimmunol.1601444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Woensel M, Mathivet T, Wauthoz N, et al. Sensitization of glioblastoma tumor micro-environment to chemo- and immunotherapy by Galectin-1 intranasal knock-down strategy. Sci Rep. 2017 Apr 27;7(1):1217. doi: 10.1038/s41598-017-01279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aboody KS, Najbauer J, Metz MZ, et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med. 2013 May 8;5(184):184ra59. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Gutova M, Shahmanyan D, Oganesyan D, et al. Intranasal delivery of therapeutic neural stem cells to target intracerebral glioma. Enliven: J Stem Cells Regen Med. 2015 Apr 15;1(1):1–7. Authors demonstrate that NSCs delivered intranasally specifically migrate toward tumors, and mice treated with NSCs genetically modified to express prodrug-activating carboxylesterase show increased survival. [Google Scholar]
- 45.Uhl M, Weiler M, Wick W, et al. Migratory neural stem cells for improved thymidine kinase-based gene therapy of malignant gliomas. Biochem Biophys Res Commun. 2005 Mar 4;328(1):125–129. doi: 10.1016/j.bbrc.2004.12.164. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Gao Y, Tokuyama T, et al. Genetically engineered neural stem cells migrate and suppress glioma cell growth at distant intracranial sites. Cancer Lett. 2007 Jun 28;251(2):220–227. doi: 10.1016/j.canlet.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 47.Bak XY, Lam DH, Yang J, et al. Human embryonic stem cell-derived mesenchymal stem cells as cellular delivery vehicles for prodrug gene therapy of glioblastoma. Hum Gene Ther. 2011 Nov;22(11):1365–1377. doi: 10.1089/hum.2010.212. [DOI] [PubMed] [Google Scholar]
- 48.Tyler MA, Ulasov IV, Sonabend AM, et al. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009 Feb;16(2):262–278. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morshed RA, Gutova M, Juliano J, et al. Analysis of glioblastoma tumor coverage by oncolytic virus-loaded neural stem cells using MRI-based tracking and histological reconstruction. Cancer Gene Ther. 2015 Jan;22(1):55–61. doi: 10.1038/cgt.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonabend AM, Ulasov IV, Tyler MA, et al. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008 Mar;26(3):831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 51.Yong RL, Shinojima N, Fueyo J, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009 Dec 1;69(23):8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duebgen M, Martinez-Quintanilla J, Tamura K, et al. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. J Natl Cancer Inst. 2014 Jun;106(6):dju090. doi: 10.1093/jnci/dju090. [DOI] [PubMed] [Google Scholar]
- 53.Ong HT, Federspiel MJ, Guo CM, et al. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J Hepatol. 2013 Nov;59(5):999–1006. doi: 10.1016/j.jhep.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mader EK, Butler G, Dowdy SC, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J Transl Med. 2013;11:20. doi: 10.1186/1479-5876-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castleton A, Dey A, Beaton B, et al. Human mesenchymal stromal cells deliver systemic oncolytic measles virus to treat acute lymphoblastic leukemia in the presence of humoral immunity. Blood. 2014 Feb 27;123(9):1327–1335. doi: 10.1182/blood-2013-09-528851. [DOI] [PubMed] [Google Scholar]
- 56.Muroski ME, Morshed RA, Cheng Y, et al. Controlled payload release by magnetic field triggered neural stem cell destruction for malignant glioma treatment. PLoS One. 2016;11(1):e0145129. doi: 10.1371/journal.pone.0145129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L, Guan Y, Liu H, et al. Silica nanorattle-doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano. 2011 Sep 27;5(9):7462–7470. doi: 10.1021/nn202399w. [DOI] [PubMed] [Google Scholar]
- 58.D’Ippolito G, Howard GA, Roos BA, et al. Isolation and characterization of marrow-isolated adult multilineage inducible (MIAMI) cells. Exp Hematol. 2006 Nov;34(11):1608–1610. doi: 10.1016/j.exphem.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 59.Roger M, Clavreul A, Huynh NT, et al. Ferrociphenol lipid nanocapsule delivery by mesenchymal stromal cells in brain tumor therapy. Int J Pharm. 2012 Feb 14;423(1):63–68. doi: 10.1016/j.ijpharm.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 60.Lee DH, Ahn Y, Kim SU, et al. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin Cancer Res. 2009 Aug 1;15(15):4925–4934. doi: 10.1158/1078-0432.CCR-08-3076. [DOI] [PubMed] [Google Scholar]
- 61.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006 Dec 7;444(7120):761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 62.Li Q, Wijesekera O, Salas SJ, et al. Mesenchymal stem cells from human fat engineered to secrete BMP4 are nononcogenic, suppress brain cancer, and prolong survival. Clin Cancer Res. 2014 May 1;20(9):2375–2387. doi: 10.1158/1078-0432.CCR-13-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu Y, Bassoff N, Reinshagen C, et al. Bi-specific molecule against EGFR and death receptors simultaneously targets proliferation and death pathways in tumors. Sci Rep. 2017 Jun 01;7(1):2602. doi: 10.1038/s41598-017-02483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez-Ornelas A, Vergara P, Segovia J. Neural stem cells producing an inducible and soluble form of Gas1 target and inhibit intracranial glioma growth. Cytotherapy. 2014 Jul;16(7):1011–1023. doi: 10.1016/j.jcyt.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 65.van Eekelen M, Sasportas LS, Kasmieh R, et al. Human stem cells expressing novel TSP-1 variant have anti-angiogenic effect on brain tumors. Oncogene. 2010 Jun 3;29(22):3185–3195. doi: 10.1038/onc.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim SK, Cargioli TG, Machluf M, et al. PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin Cancer Res. 2005 Aug 15;11(16):5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- 67.Ehtesham M, Kabos P, Gutierrez MA, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002 Dec 15;62(24):7170–7174. [PubMed] [Google Scholar]
- 68.Kim SM, Lim JY, Park SI, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008 Dec 1;68(23):9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 69.Shah K, Bureau E, Kim DE, et al. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol. 2005 Jan;57(1):34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 70.Balyasnikova IV, Franco-Gou R, Mathis JM, et al. Genetic modification of mesenchymal stem cells to express a single-chain antibody against EGFRvIII on the cell surface. J Tissue Eng Regen Med. 2010 Jun;4(4):247–258. doi: 10.1002/term.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Compte M, Cuesta AM, Sanchez-Martin D, et al. Tumor immunotherapy using gene-modified human mesenchymal stem cells loaded into synthetic extracellular matrix scaffolds. Stem Cells. 2009 Mar;27(3):753–760. doi: 10.1634/stemcells.2008-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kanojia D, Balyasnikova IV, Morshed RA, et al. Neural Stem cells secreting anti-HER2 antibody improve survival in a preclinical model of HER2 overexpressing breast cancer brain metastases. Stem Cells. 2015 Oct;33(10):2985–2994. doi: 10.1002/stem.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim J, Hall RR, Lesniak MS, et al. Stem cell-based cell carrier for targeted oncolytic virotherapy: translational opportunity and open questions. Viruses. 2015 Dec;7(12):6200–6217. doi: 10.3390/v7122921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rome C, Gravier J, Morille M, et al. Near-infrared optical imaging of nucleic acid nanocarriers in vivo. Methods Mol Biol. 2013;948:49–65. doi: 10.1007/978-1-62703-140-0_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.David S, Passirani C, Carmoy N, et al. DNA nanocarriers for systemic administration: characterization and in vivo bioimaging in healthy mice. Mol Ther Nucleic Acids. 2013;2:e64. doi: 10.1038/mtna.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang C, Wang K, Stephen ZR, et al. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl Mater Interfaces. 2015 Apr 1;7(12):6674–6682. doi: 10.1021/am5092165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goel S, Chen F, Hong H, et al. VEGF(1)(2, 1)-conjugated mesoporous silica nanoparticle: a tumor targeted drug delivery system. ACS Appl Mater Interfaces. 2014 Dec 10;6(23):21677–21685. doi: 10.1021/am506849p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010 Jan 1;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Morille M, Montier T, Legras P, et al. Long-circulating DNA lipid nanocapsules as new vector for passive tumor targeting. Biomaterials. 2010 Jan;31(2):321–329. doi: 10.1016/j.biomaterials.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 80.Guerrero-Cazares H, Tzeng SY, Young NP, et al. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano. 2014 May 27;8(5):5141–5153. doi: 10.1021/nn501197v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang FY, Lee TW, Chang CH, et al. Evaluation of 188Re-labeled PEGylated nanoliposome as a radionuclide therapeutic agent in an orthotopic glioma-bearing rat model. Int J Nanomedicine. 2015;10:463–473. doi: 10.2147/IJN.S75955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dreaden EC, Austin LA, Mackey MA, et al. Size matters: gold nanoparticles in targeted cancer drug delivery. Ther Deliv. 2012 Apr;3(4):457–478. doi: 10.4155/tde.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li M, Deng H, Peng H, et al. Functional nanoparticles in targeting glioma diagnosis and therapies. J Nanosci Nanotechnol. 2014 Jan;14(1):415–432. doi: 10.1166/jnn.2014.8757. [DOI] [PubMed] [Google Scholar]
- 84.Ferreira E, Potier E, Logeart-Avramoglou D, et al. Optimization of a gene electrotransfer method for mesenchymal stem cell transfection. Gene Ther. 2008 Apr;15(7):537–544. doi: 10.1038/gt.2008.9. [DOI] [PubMed] [Google Scholar]
- 85.Musyanovych A, Schmitz-Wienke J, Mailander V, et al. Preparation of biodegradable polymer nanoparticles by miniemulsion technique and their cell interactions. Macromol Biosci. 2008 Feb 11;8(2):127–139. doi: 10.1002/mabi.200700241. [DOI] [PubMed] [Google Scholar]
- 86.Lorenz MR, Holzapfel V, Musyanovych A, et al. Uptake of functionalized, fluorescent-labeled polymeric particles in different cell lines and stem cells. Biomaterials. 2006 May;27(14):2820–2828. doi: 10.1016/j.biomaterials.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 87.Huang X, Zhang F, Wang H, et al. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials. 2013 Feb;34(7):1772–1780. doi: 10.1016/j.biomaterials.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mangraviti A, Tzeng SY, Gullotti D, et al. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials. 2016;100:53–66. doi: 10.1016/j.biomaterials.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jackson C, Ruzevick J, Phallen J, et al. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011;2011:732413. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang BX, Rahbar R, Fish EN. Interferon: current status and future prospects in cancer therapy. J Interferon Cytokine Res. 2011 Jul;31(7):545–552. doi: 10.1089/jir.2010.0158. [DOI] [PubMed] [Google Scholar]
- 91.Williams RF, Sims TL, Tracey L, et al. Maturation of tumor vasculature by interferon-beta disrupts the vascular niche of glioma stem cells. Anticancer Res. 2010 Sep;30(9):3301–3308. [PubMed] [Google Scholar]
- 92.Li Y, Zhu H, Zeng X, et al. Suppression of autophagy enhanced growth inhibition and apoptosis of interferon-beta in human glioma cells. Mol Neurobiol. 2013 Jun;47(3):1000–1010. doi: 10.1007/s12035-013-8403-0. [DOI] [PubMed] [Google Scholar]
- 93.Guo Y, Wang G, Gao WW, et al. Induction of apoptosis in glioma cells and upregulation of Fas expression using the human interferon-beta gene. Asian Pac J Cancer Prev. 2012;13(6):2837–2840. doi: 10.7314/apjcp.2012.13.6.2837. [DOI] [PubMed] [Google Scholar]
- 94.Happold C, Roth P, Silginer M, et al. Interferon-beta induces loss of spherogenicity and overcomes therapy resistance of glioblastoma stem cells. Mol Cancer Ther. 2014 Apr;13(4):948–961. doi: 10.1158/1535-7163.MCT-13-0772. [DOI] [PubMed] [Google Scholar]
- 95.Baselga J. Treatment of HER2-overexpressing breast cancer. Ann Oncol. 2010 Oct;21(Suppl 7):vii36–40. doi: 10.1093/annonc/mdq421. [DOI] [PubMed] [Google Scholar]
- 96.Morris JC, Waldmann TA. Antibody-based therapy of leukaemia. Expert Rev Mol Med. 2009;11:e29. doi: 10.1017/S1462399409001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Criscitiello C, Curigliano G. Immunotherapeutics for breast cancer. Curr Opin Oncol. 2013 Nov;25(6):602–608. doi: 10.1097/CCO.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 98.Balyasnikova IV, Ferguson SD, Sengupta S, et al. Mesenchymal stem cells modified with a single-chain antibody against EGFRvIII successfully inhibit the growth of human xenograft malignant glioma. PLoS One. 2010;5(3):e9750. doi: 10.1371/journal.pone.0009750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van den Berg MP, Romeijn SG, Verhoef JC, et al. Serial cerebrospinal fluid sampling in a rat model to study drug uptake from the nasal cavity. J Neurosci Methods. 2002 Apr 30;116(1):99–107. doi: 10.1016/s0165-0270(02)00033-x. [DOI] [PubMed] [Google Scholar]
- 100.Charlton ST, Davis SS, Illum L. Nasal administration of an angiotensin antagonist in the rat model: effect of bioadhesive formulations on the distribution of drugs to the systemic and central nervous systems. Int J Pharm. 2007 Jun 29;338(1–2):94–103. doi: 10.1016/j.ijpharm.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 101.Walter BA, Valera VA, Takahashi S, et al. Evidence of antibody production in the rat cervical lymph nodes after antigen administration into the cerebrospinal fluid. Arch Histol Cytol. 2006 Mar;69(1):37–47. doi: 10.1679/aohc.69.37. [DOI] [PubMed] [Google Scholar]
- 102.Walter BA, Valera VA, Takahashi S, et al. The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol Appl Neurobiol. 2006 Aug;32(4):388–396. doi: 10.1111/j.1365-2990.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- 103.Liu H, Ni Z, Chen Y, et al. Olfactory route for cerebrospinal fluid drainage into the cervical lymphatic system in a rabbit experimental model. Neural Regen Res. 2012 Apr 05;7(10):766–771. doi: 10.3969/j.issn.1673-5374.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jogani VV, Shah PJ, Mishra P, et al. Intranasal mucoadhesive micro-emulsion of tacrine to improve brain targeting. Alzheimer Dis Assoc Disord. 2008 Apr-Jun;22(2):116–124. doi: 10.1097/WAD.0b013e318157205b. [DOI] [PubMed] [Google Scholar]
- 105.Kumar M, Misra A, Mishra AK, et al. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. Journal of Drug Targeting. 2008 Dec;16(10):806–814. doi: 10.1080/10611860802476504. [DOI] [PubMed] [Google Scholar]
- 106.Dufes C, Olivier JC, Gaillard F, et al. Brain delivery of vasoactive intestinal peptide (VIP) following nasal administration to rats. Int J Pharm. 2003 Apr 14;255(1–2):87–97. doi: 10.1016/s0378-5173(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 107.Chen SC, Eiting K, Cui K, et al. Therapeutic utility of a novel tight junction modulating peptide for enhancing intranasal drug delivery. J Pharm Sci. 2006 Jun;95(6):1364–1371. doi: 10.1002/jps.20510. [DOI] [PubMed] [Google Scholar]
- 108.Raghavan U, Logan BM. New method for the effective instillation of nasal drops. J Laryngol Otol. 2000 Jun;114(6):456–459. doi: 10.1258/0022215001905832. [DOI] [PubMed] [Google Scholar]
- 109.Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res. 2013 Feb;3(1):42–62. doi: 10.1007/s13346-012-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van den Berg MP, Merkus P, Romeijn SG, et al. Hydroxocobalamin uptake into the cerebrospinal fluid after nasal and intravenous delivery in rats and humans. Journal of Drug Targeting. 2003 Jul;11(6):325–331. doi: 10.1080/10611860310001640075. [DOI] [PubMed] [Google Scholar]
- 111.van den Berg MP, Verhoef JC, Romeijn SG, et al. Uptake of estradiol or progesterone into the CSF following intranasal and intravenous delivery in rats. Eur J Pharm Biopharm. 2004 Jul;58(1):131–135. doi: 10.1016/j.ejpb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 112.Danielyan L, Schafer R, von Ameln-Mayerhofer A, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011 Feb;14(1):3–16. doi: 10.1089/rej.2010.1130. [DOI] [PubMed] [Google Scholar]
- 113.Bossolasco P, Cova L, Levandis G, et al. Noninvasive near-infrared live imaging of human adult mesenchymal stem cells transplanted in a rodent model of Parkinson’s disease. Int J Nanomedicine. 2012;7:435–447. doi: 10.2147/IJN.S27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gutova M, Frank JA, D’Apuzzo M, et al. Magnetic resonance imaging tracking of ferumoxytol-labeled human neural stem cells: studies leading to clinical use. Stem Cells Transl Med. 2013 Oct;2(10):766–775. doi: 10.5966/sctm.2013-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gu E, Chen WY, Gu J, et al. Molecular imaging of stem cells: tracking survival, biodistribution, tumorigenicity, and immunogenicity. Theranostics. 2012;2(4):335–345. doi: 10.7150/thno.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kurpisz M, Czepczynski R, Grygielska B, et al. Bone marrow stem cell imaging after intracoronary administration. Int J Cardiol. 2007 Oct 1;121(2):194–195. doi: 10.1016/j.ijcard.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 117.Pawelczyk E, Arbab AS, Chaudhry A, et al. In vitro model of bromo-deoxyuridine or iron oxide nanoparticle uptake by activated macrophages from labeled stem cells: implications for cellular therapy. Stem Cells. 2008 May;26(5):1366–1375. doi: 10.1634/stemcells.2007-0707. [DOI] [PubMed] [Google Scholar]
- 118.Brass SD, Chen NK, Mulkern RV, et al. Magnetic resonance imaging of iron deposition in neurological disorders. Top Magn Reson Imaging. 2006 Feb;17(1):31–40. doi: 10.1097/01.rmr.0000245459.82782.e4. [DOI] [PubMed] [Google Scholar]
- 119.Cheng SH, Yu D, Tsai HM, et al. Dynamic in vivo SPECT imaging of neural stem cells functionalized with radiolabeled nanoparticles for tracking of glioblastoma. J Nucl Med. 2016 Feb;57(2):279–284. doi: 10.2967/jnumed.115.163006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nduom EK, Yang C, Merrill MJ, et al. Characterization of the blood-brain barrier of metastatic and primary malignant neoplasms. J Neurosurg. 2013 Aug;119(2):427–433. doi: 10.3171/2013.3.JNS122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009 Oct;9(10):1519–1527. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Larsen JM, Martin DR, Byrne ME. Recent advances in delivery through the blood-brain barrier. Curr Top Med Chem. 2014;14(9):1148–1160. doi: 10.2174/1568026614666140329230311. [DOI] [PubMed] [Google Scholar]
- 123.Li YH, Feng L, Zhang GX, et al. Intranasal delivery of stem cells as therapy for central nervous system disease. Exp Mol Pathol. 2015 Apr;98(2):145–151. doi: 10.1016/j.yexmp.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 124.Linares GR, Chiu CT, Scheuing L, et al. Preconditioning mesenchymal stem cells with the mood stabilizers lithium and valproic acid enhances therapeutic efficacy in a mouse model of Huntington’s disease. Exp Neurol. 2016;281:81–92. doi: 10.1016/j.expneurol.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 125.Wei ZZ, Gu X, Ferdinand A, et al. Intranasal delivery of bone marrow mesenchymal stem cells improved neurovascular regeneration and rescued neuropsychiatric deficits after neonatal stroke in rats. Cell Transplant. 2015;24(3):391–402. doi: 10.3727/096368915X686887. [DOI] [PubMed] [Google Scholar]
- 126.Liao W, Pham V, Liu L, et al. Mesenchymal stem cells engineered to express selectin ligands and IL-10 exert enhanced therapeutic efficacy in murine experimental autoimmune encephalomyelitis. Biomaterials. 2016;77:87–97. doi: 10.1016/j.biomaterials.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]