Abstract
Background/Aim:
The association between sarcopenia and nonalcoholic fatty liver disease (NAFLD) has been suggested by recent epidemiological studies, although the results have been inconsistent. This meta-analysis was conducted to summarize all available data and estimate the risk of NAFLD among patients with sarcopenia.
Materials and Methods:
A comprehensive literature review was conducted using MEDLINE and EMBASE databases through November 2016 to identify all studies that compared the risk of NAFLD among patients with sarcopenia versus those without sarcopenia. Effect estimates from each study were extracted and combined using the random-effect, generic inverse variance method of DerSimonian and Laird.
Results:
Five cross-sectional studies with 27,804 participants met the eligibility criteria and were included in the meta-analysis. The risk of NAFLD in patients with sarcopenia was significantly higher than those without sarcopenia with the pooled odds ratio of 1.54 (95% confidence interval, 1.05–2.26). The statistical heterogeneity was high with an I2of 83%.
Conclusions:
A significantly increased risk of NAFLD among patients with sarcopenia was observed in this study.
Keywords: Low muscle mass, meta-analysis, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, sarcopenia
INTRODUCTION
Sarcopenia is a condition characterized by loss of muscle mass, power, strength, and performance.[1,2] Low muscle mass is defined as a decrease in appendicular muscle mass of two standard deviations below the mean for healthy adults.[3] An epidemiologic study estimated that sarcopenia may affect up to 57% of men and 60% of women over the age of 80 years.[4] Sarcopenia is associated with increased mortality, functional impairment, disability, and fall.[5] The etiology of sarcopenia is usually multifactorial in nature, including chronic inflammation, insulin resistance, nutritional deficiencies, and endocrine abnormality.[5]
Nonalcoholic fatty liver disease (NAFLD) is a condition characterized by abnormal fat accumulation in the liver without a history of significant alcohol consumption. It has been increasingly known as the liver manifestation of metabolic syndrome.[6] Prevalence of NAFLD is increasing worldwide as a result of the obesity epidemic.[6,7] Risk factors of NAFLD include metabolic syndrome components, hyperuricemia, lack of sleep, and sedentary lifestyle/physical inactivity.[8,9,10,11]
Interestingly, NAFLD and sarcopenia share several abnormal pathophysiologic processes such as insulin resistance and chronic inflammation. In fact, previous epidemiologic studies have suggested an association between sarcopenia and the development of NAFLD even though the results were inconsistent.[12,13,14,15,16,17,18] This systematic review and meta-analysis was conducted to summarize all available evidence with the aim to better characterize this relationship.
MATERIALS AND METHODS
Information sources and search strategy
A systematic literature search was conducted using EMBASE and MEDLINE databases from inception to November 2016 to identify all original studies that investigated the association between sarcopenia and NAFLD. The systematic literature review was independently conducted by three investigators (K.W., P.P., and P.U.) using the search strategy that included the terms for “sarcopenia” and “nonalcoholic fatty liver disease,” as described in online supplementary data 1 (55KB, pdf) . A manual search for additional potentially relevant studies using references of the included articles was also performed. No language limitation was applied. This study was conducted in accordance with the PRISMA (Preferred reporting Items for Systematic Reviews and Meta-Analysis) statement which is provided as online supplementary data 2 (65.3KB, pdf) .
Selection criteria
Eligible studies were case-control, cross-sectional, or cohort studies that investigated the association between sarcopenia and NAFLD. They had to provide the effect estimates [odds ratios (OR), relative risks (RR), hazard ratios (HR), or standardized incidence ratio (SIR)] with 95% confidence intervals (CI). Inclusion was not restricted by study size. When more than one article using the same database/cohort was available, the study with the most comprehensive data/analyses was included.
Retrieved articles were independently reviewed for their eligibility by the same three investigators. Discrepancy was resolved by conference with all investigators. Newcastle-Ottawa quality assessment scale was used to appraise the quality of study in three areas including the recruitment of cases and controls, the comparability between the two groups, and the ascertainment of the outcome of interest for cohort study and the exposure for case-control study.[19] The modified Newcastle–Ottawa scale, as described by Herzog et al., was used for this cross-sectional study.[20]
Data abstraction
A structured data collection form was used to extract the following data from each study: title of the study, publication year, name of the first author, year of the study, country where the study was conducted, number of participants, demographic data of participants, defitinion of sarcopenia, methods used to identify and verify sarcopenia and NAFLD, adjusted effect estimates with 95% CI, and covariates that were adjusted in the multivariable analysis.
To ensure accuracy, this data extraction process was independently performed by two investigators (K.W. and P.P.) and was reviewed by the senior investigator (P.U.).
Statistical analysis
Data analysis was performed using the Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom). Adjusted point estimates from each study were combined by the generic inverse variance method of DerSimonian and Laird, which assigned the weight of each study in the pooled analysis based on its variance.[21] As the outcome interest was relatively uncommon, we planned to use RR and HR of cohort study as an estimate for OR to calcualte the pooled effect estimates with OR of case-control study and cross-sectional study. In light of the high likelihood of between-study variance because of different study designs, populations, and definition of hyperuricemia, random-effect model was used. Cochran's Q-test and I2statistic were used to determine the between-study heterogeneity. A value of I2of 0-25% represents insignificant heterogeneity, 26-50% represents low heterogeneity, 51-75% represents moderate heterogeneity, and more than 75% represents high heterogeneity.[22]
RESULTS
Six hundred and one potentially eligible articles were identified using our search strategy (203 articles from Medline and 398 articles from EMBASE). After the exclusion of duplicated 201 articles, 400 articles underwent title and abstract review. Three hundred and seventy-five articles were excluded at this stage because they were case reports, correspondences, review articles, in-vitro studies, animal studies, or interventional studies, leaving 25 articles for full-text review. Fifteen of them were excluded after the full-length review as they did not report the outcome of interest whereas three articles were excluded since they were descriptive studies without comparative analysis. Seven studies met the eligibility criteria. However, three studies utilized the same database.[16,17,23] To avoid duplication of participants, the most comprehensive study was included in the analysis.[16] Finally, five cross-sectional studies with 27,804 participants were included in the meta-analysis.[12,13,15,16,18] The literature retrieval, review, and selection process are shown in Figure 1. The characteristics and quality assessment of the studies are presented in Table 1. It should be noted that the inter-rater agreement for the quality assessment using the Newcastle-Ottawa scale was high with the kappa statistics of 0.80.
Figure 1.
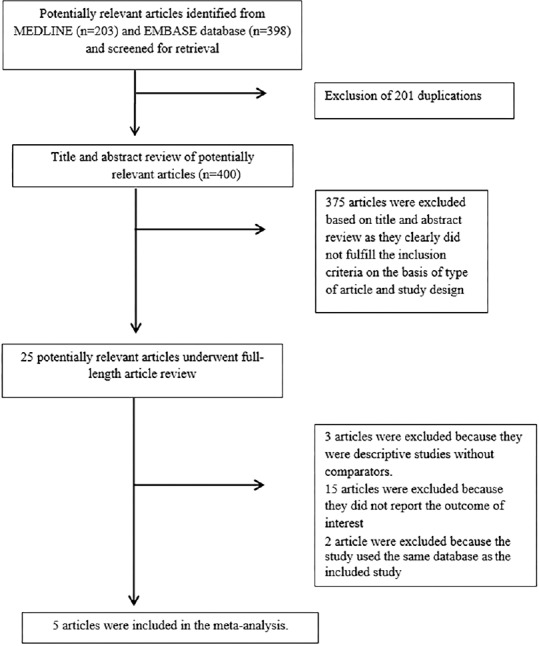
Literature review process
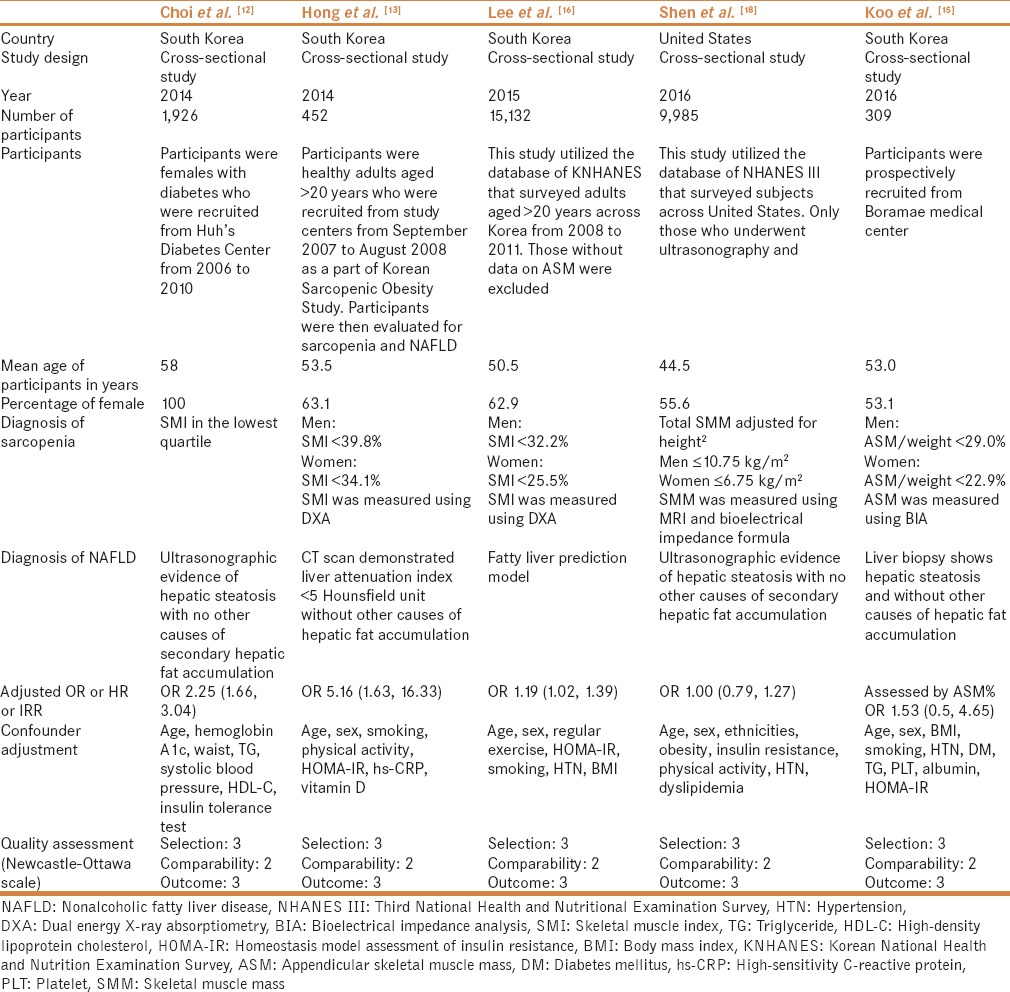
Table 1.
Characteristics of the studies
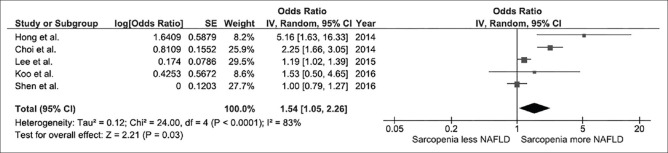
We found a significantly increased risk of NAFLD among individuals with sarcopenia with the pooled OR of 1.54 (95% CI, 1.05-2.26), as shown in Figure 2. The heterogeneity between studies of the overall analysis was high with an I2of 83%.
Figure 2.
Forest plot
Evaluation for publication bias
Funnel plot was used to evaluate publication bias [Figure 3]. The graph is asymmetric and suggests that publication bias in favor of positive studies might have been present.
Figure 3.
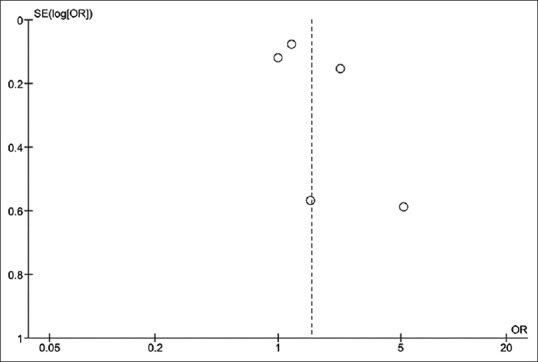
Funnel plot
DISCUSSION
To the best of our knowledge, this is the first systematic review and meta-analysis that summarized all available studies regarding the association between sarcopenia and NAFLD. We found an approximately 1.5-fold increased risk of NAFLD among patients with sarcopenia compared to those without sarcopenia.
The exact pathogenesis of this association is yet to be elucidated. There are several possible explanations.
First, skeletal muscle is a major insulin-responsive target organ.[23,24,25] Thus, loss of skeletal muscle will promote insulin resistance and reduce energy expenditures, which could eventually lead to metabolic syndrome and NAFLD.[26] Interestingly, studies have also demonstrated that decreased insulin sensitivity (or impairment in insulin signaling) can promote skeletal muscle loss via reduction of the synthesis of muscle protein and mitochondrial dysfunction,[27] causing the vicious cycle between insulin insensitivity and sarcopenia.
Second, myokines secreted by skeletal muscles such as irisin, interleukin-6, myostatin, adipocytokines and adiponectin are involved in the regulation of glucose and fatty acid metabolism in the liver.[28,29,30,31,32] The imbalance of myokine levels associated with loss of muscle mass could possibly lead to disturbance of glucose and fatty acid metabolism and abnormal hepatic fat accumulation.
Third, it is also possible that the relationship between sarcopenia and NAFLD is not causal. The apparent association could be a result of the same underlying factors that could predispose patients to both NAFLD and sarcopenia. For example, it has been demonstrated that oxidative stress and proinflammatory cytokines of chronic inflammation, such as tumor necrotic factor-α and c-reactive protein, could promote catabolic state, resulting in loss of skeletal muscle.[27,33] Similarly, chronic inflammation plays a vital role in the pathogenesis of NAFLD (two-hit hypothesis).[34] Prolonged deficiency of vitamin D has been shown to be associated with a reduction in type 2 muscle fibers.[35] Vitamin D deficiency is also associated with an increased risk of NAFLD, possibly due to increased inflammatory cytokines.[36,37]
The systematic literature review process of this study was comprehensive and the quality of included studies was high as reflected by the high Newcastle-Ottawa scores. However, we acknowledge that this study had some limitations and the results should be interpreted with caution.
First, the methods used to diagnose sarcopenia varied across studies. Only three studies used dual energy X-ray absorptiometry which is considered as the gold standard for muscle mass measurement.[12,13,16] Other studies used bioelectrical impedance analysis to estimate the skeletal muscle mass which may have a lower accuracy.[15,18] Second, all the included studies were cross-sectional in nature. Therefore, the temporal relationship between sarcopenia and NAFLD could not be established. Third, the statistical heterogeneity of this meta-analysis was high. We believe that the difference in underlying populations as well as the methods used to diagnose NAFLD and sarcopenia was responsible for this between-study variation. Fourth, publication bias in favor of positive study may have been present in this meta-analysis.
CONCLUSION
This study demonstrated a significantly increased risk of NAFLD among patients with sarcopenia. However, it is unclear whether this association is causal or is a result of shared predisposing factors.
Disclosure
The authors have no commercial associations that might be a conflict of interest about this article. No funding support was received for this article.
Authors' contributions
All authors had access to the data and a role in writing the manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen I. The epidemiology of sarcopenia. Clin Geriatr Med. 2011;27:355–63. doi: 10.1016/j.cger.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–36. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 4.Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20-93 yr. J Appl Physiol. 1997;83:1581–7. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 5.Janssen I. Influence of sarcopenia on the development of physical disability: The Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 7.Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin Liver Dis. 2016;20:205–14. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Byrne CD, Targher G. NAFLD: A multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Wijarnpreecha K, Panjawatanan P, Lekuthai N, Thongprayoon C, Cheungpasitporn W, Ungprasert P. Hyperuricemia and Risk of Nonalcoholic Fatty Liver Disease: A Meta-analysis. Liver Int. 2017;37:906–18. doi: 10.1111/liv.13329. [DOI] [PubMed] [Google Scholar]
- 10.Wijarnpreecha K, Thongprayoon C, Edmonds PJ, Cheungpasitporn W. Associations of sugar- and artificially sweetened soda with nonalcoholic fatty liver disease: A systematic review and meta-analysis. QJM. 2016;109:461–6. doi: 10.1093/qjmed/hcv172. [DOI] [PubMed] [Google Scholar]
- 11.Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Short sleep duration and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:1802–7. doi: 10.1111/jgh.13391. [DOI] [PubMed] [Google Scholar]
- 12.Choi YJ, KS K, Kwak JJ, Park SW, Lee EJ, Huh KB. Age-related skeletal muscle loss as an independent predictor of NAFLD risk in Korean women with type 2 diabetes. Diabetes Res Clin Pract. 2014;106(1 Suppl):S162–3. [Google Scholar]
- 13.Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772–8. doi: 10.1002/hep.26716. [DOI] [PubMed] [Google Scholar]
- 14.Kim W. KBK, Joo S.K., Kim J.H., Park S.C. Sarcopenia is an independent risk factor for biopsyproven non-alcoholic steatohepatitis. J Hepatol. 2016;64:S502. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123–31. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011) J Hepatol. 2015;63:486–93. doi: 10.1016/j.jhep.2015.02.051. [DOI] [PubMed] [Google Scholar]
- 17.Lee YH, Kim SU, Song K, Park JY, Kim do Y, Ahn SH, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011) Hepatology. 2016;63:776–86. doi: 10.1002/hep.28376. [DOI] [PubMed] [Google Scholar]
- 18.Shen H. LS. Association between sarcopenia and prevalence of nonalcoholic fatty liver disease: A cross-sectional study from the third national health and nutrition examination survey. Gastroenterology. 2016;150:S1143–S4. doi: 10.1111/apt.12944. [DOI] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SW, Youm Y, Lee WJ, Choi W, Chu SH, Park YR, et al. Appendicular skeletal muscle mass and insulin resistance in an elderly korean population: The korean social life, health and aging project-health examination cohort. Diabetes Metab J. 2015;39:37–45. doi: 10.4093/dmj.2015.39.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, et al. Sarcopenic obesity: Prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA) Diabetes Care. 2010;33:1652–4. doi: 10.2337/dc10-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skov-Jensen C, Skovbro M, Flint A, Helge JW, Dela F. Contraction-mediated glucose uptake is increased in men with impaired glucose tolerance. Appl Physiol Nutr Metab. 2007;32:115–24. doi: 10.1139/h06-098. [DOI] [PubMed] [Google Scholar]
- 26.Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: The Korean sarcopenic obesity study. Int J Obesity. 2009;33:885–92. doi: 10.1038/ijo.2009.130. [DOI] [PubMed] [Google Scholar]
- 27.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–29. doi: 10.1016/S2213-8587(14)70034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merli M, Dasarathy S. Sarcopenia in non-alcoholic fatty liver disease: Targeting the real culprit? J Hepatol. 2015;63:309–11. doi: 10.1016/j.jhep.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Miller AM, Wang H, Bertola A, Park O, Horiguchi N, Ki SH, et al. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology. 2011;54:846–56. doi: 10.1002/hep.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nature Rev Endocrinol. 2012;8:457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 32.Zhang HJ, Zhang XF, Ma ZM, Pan LL, Chen Z, Han HW, et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol. 2013;59:557–62. doi: 10.1016/j.jhep.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 33.Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Models Mech. 2013;6:25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day CP, James OF. Steatohepatitis: A tale of two “hits”? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 35.Sanders KM, Scott D, Ebeling PR. Vitamin D deficiency and its role in muscle-bone interactions in the elderly. Curr Osteoporosis Rep. 2014;12:74–81. doi: 10.1007/s11914-014-0193-4. [DOI] [PubMed] [Google Scholar]
- 36.Nelson JE, Roth CL, Wilson LA, Yates KP, Aouizerat B, Morgan-Stevenson V, et al. Vitamin D Deficiency Is Associated With Increased Risk of Non-alcoholic Steatohepatitis in Adults With Non-alcoholic Fatty Liver Disease: Possible Role for MAPK and NF-kappaB? Am J Gastroenterol. 2016;111:852–63. doi: 10.1038/ajg.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park D, Kwon H, Oh SW, Joh HK, Hwang SS, Park JH, et al. Is Vitamin D an Independent Risk Factor of Nonalcoholic Fatty Liver Disease.: A Cross-Sectional Study of the Healthy Population? J Korean Med Sci. 2017;32:95–101. doi: 10.3346/jkms.2017.32.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.