Abstract
The plasminogen activation (PA) system is best known for its role in fibrinolysis. However, it has also been shown to regulate many non-fibrinolytic functions in the central nervous system (CNS). In particular, tissue-type plasminogen activator (tPA) is reported to have pleiotropic activities in the CNS, regulating events such as neuronal plasticity, excitotoxicity and cerebrovascular barrier integrity, whereas urokinase-type plasminogen activator (uPA) is mainly associated with tissue remodeling and cell migration. It has been suggested that the role tPA plays in controlling barrier integrity may provide a unifying mechanism for the reported diverse, and often opposing, functions ascribed to tPA in the CNS. Here we will review the possibility that the pleiotropic effects reported for tPA in physiologic and pathologic processes in the CNS may be a consequence of its role in the neurovascular unit in regulation of cerebrovascular responses and subsequently parenchymal homeostasis. We propose that this might offer an explanation for the ongoing debate regarding the neurotoxic versus neuroprotective roles of tPA.
Keywords: tPA, PDGF-CC, PDGFRα, PAI-1, Neuroserpin, Blood-brain barrier
INTRODUCTION
In the vascular lumen the main function of the serine protease tissue-type plasminogen activator (tPA) is activation of the zymogen plasminogen to plasmin, and subsequent degradation of fibrin clots1. Because of its role in fibrinolysis, tPA is used as a thrombolytic agent for treatment of ischemic stroke, although its use is greatly limited due to concerns for hemorrhagic complications and the requirement that it is administered within a few hours of onset of symptoms2. The mechanism by which thrombolytic tPA might lead to hemorrhagic transformation of ischemic stroke is not completely understood, but it appears to be due to unique activities of tPA in the central nervous system (CNS), beyond its well established role in fibrinolysis. These unique CNS activities of tPA have over the past two decades been reported to include many diverse processes such as neuronal development3, neuronal plasticity4, axonal regeneration5,6, neurovascular coupling7, excitotoxicity8–10, neuroprotection11, microglial activation/inflammation12,13, and blood-brain barrier (BBB) control14–16. In addition, these pleiotropic effects of tPA in the CNS have also been proposed to be mediated via multiple potential substrates and receptors17,18. How tPA mediates such diverse functions in the CNS is still a matter of debate but several hypotheses have been suggested, including tPA concentration and tPA conformation (single chain tPA versus two chain tPA) (reviewed in19). Recently we proposed an alternative explanation, suggesting that the neurovascular events regulated by tPA might provide a unifying pathway for many of the pleiotropic effects of tPA20. We reasoned that, instead of tPA directly performing all these diverse functions in the CNS, it might be that tPA’s role in regulating cerebrovascular integrity, and thereby parenchymal homeostasis, could indirectly affect events such as neuronal signaling pathways and excitotoxicity through loss of precise control of the extracellular environment. In this review we will summarize what is known about the role of tPA in the CNS during physiology and disease in light of this cerebrovascular regulatory theory.
THE NEUROVASCULAR UNIT AND THE BBB
The cerebrovascular bed is unique in that normal CNS function requires a highly regulated extracellular environment to keep the concentrations of most molecules within a very narrow range21. Cerebrovascular homeostasis is maintained by the BBB, which forms a mechanical and functional barrier between the systemic circulation and the CNS that tightly controls trafficking of substances between the blood and the CNS22. The barrier properties are established by the brain endothelial cells, but it is widely recognized that the other cells in the neurovascular unit (NVU), including perivascular astrocytes in particular, as well as vascular mural cells (vascular smooth muscle cells and pericytes) and neurons, all work together in a coordinated way to regulate the extracellular environment of the brain parenchyma (Figure 1)20,21,23,24. Thus, the interaction between neurons, astrocytes and endothelial cells plays a central role coupling energy supply and changes in the CNS extracellular environment with neuronal activity25–28.
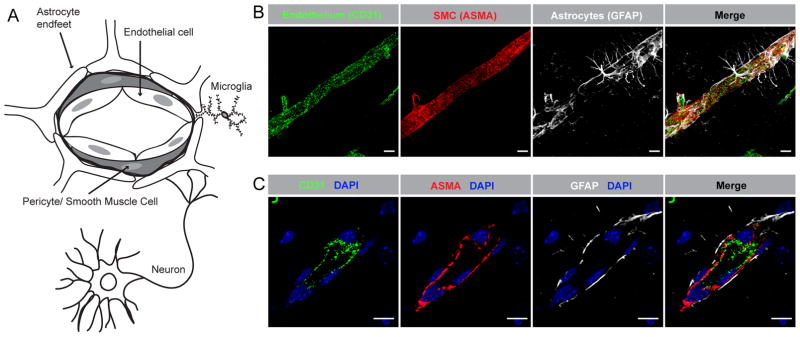
Figure 1.
The neurovascular unit (NVU). A) Schematic illustration of the structure and cellular components of the NVU. B) Confocal microscopy image showing the NVU in the naïve adult wild type murine brain. The perivascular astrocytic endfeet (white) completely ensheath the vascular smooth muscle cell (SMC) layer (red) and the endothelial tube (green). C) High magnification confocal image showing the order of cells in the NVU, with the endothelial cells (green) closest to the vessel lumen and the SMCs (red) tightly wrapping around the endothelial tube. Immunofluorescent staining of endothelial cells with anti-CD31 antibodies (CD31), SMC with anti-ASMA antibodies and perivascular astrocytes with anti-GFAP antibodies. Cell nuclei were visualized with DAPI (blue). The images in B display maximum intensity projections generated from confocal Z-stacks (25 μm). Scale bars in B and C 10 μm.
IS tPA-MEDIATED REGULATION OF NEUROVASCULAR RESPONSES THE INITIAL EVENT DIRECTING LATER RESPONSES IN THE CNS?
It is well established that cerebrovascular responses to neuronal activity are critical for maintaining parenchymal homeostasis through three closely linked and related effects: 1) neurovascular coupling, which refers to increases in cerebral blood flow in response to neuronal activity; 2) neurobarrier coupling, which refers to changes in transport or the movement of molecules across the BBB; and 3) neurometabolic coupling, which refers to changes in local metabolic factors, such as glucose and lactate, in response to neuronal activity (reviewed in28). The reports showing that tPA promotes neurovascular7, neurobarrier14, and possibly neurometabolic coupling29 thus suggest that under physiologic conditions tPA is released into the NVU in response to neuronal activity where it exerts these neurovascular effects to accommodate the increased energy demand incurred by the increased firing rates occurring during routine processing in the neocortex. However, during pathologic conditions this is exaggerated leading to disproportionate opening of the BBB, which results in extravasation of harmful blood-borne substances into the CNS and the subsequent loss of the tight regulation of the CNS extracellular environment. Based on this we hypothesize that the neurovascular events regulated by tPA could provide a unifying pathway for many of the pleiotropic effects of tPA in the CNS. For example, studies have suggested that a primary role of tPA in the CNS is direct regulation of neuronal activity through its action on the N-methyl-D-aspartate (NMDA)-receptor and neuronal calcium signaling10,30,31. However, since the concentration of glutamate is 25–200-fold higher in plasma than in the CNS extracellular fluids32, then it is conceivable that tPA-induced changes in BBB permeability could overwhelm the astrocyte-mediated glutamate shuttle resulting in the buildup of extracellular glutamate33 which in turn, could promote dysregulation of the NMDA signaling pathways and lead to excitotoxicity9. Thus, we suggest a possible common pathway for tPA permitting modulation of CNS function through the regulation of the neurovascular unit.
PHYSIOLOGIC ROLES OF tPA IN THE CNS
Our understanding about the physiologic role of tPA in the CNS is still very limited and most of our knowledge is based on studies utilizing genetically modified mice in various experimental models of disease as well as in vitro systems. However, in recent years significant efforts and advancements have been made to delineate the function and mechanism of action of tPA in the CNS (summarized in table 1).
Table 1.
Physiologic roles of tPA in the CNS
| Biologic process | Method | Effect | References | |
|---|---|---|---|---|
| CNS development | Survival | Genetic deletion studies | Non essential role of tPA during embryonic development | 69,71 |
| Neuronal migration | Ex vivo studies of tPA−/− mice and controls using cerebellar slice technology | Perturbed migration of cerebellar granule neurons in tPA−/− mice | 75 | |
| Neuronal remodeling | In vitro studies of neuroblastoma clonal cell lines and neural progenitor cell | tPA is released in the neuronal growth cone where it might affect neurite outgrowth and remodeling | 73,74 | |
| Cerebrovascular development | Immunohistochemical and confocal microscopy studies in tPA−/− and control murine brains | Reduced number of vascular smooth-muscle cell covered, large diameter vessels and increased junctional localization of tight junction protein ZO1 in tPA−/− mice | 76 | |
| Cerebroventricular development | In vivo MRI and immunohistochemical/confocal microscopy studies in tPA−/− and control mice | Mild cerebral ventricular malformations and distorted ependymal lining in tPA−/− mice | 76 | |
| Adult CNS physiology | Learning and memory | Expression analysis and experimental in vivo models of learning/memory in tPA−/−, tPA overexpressor and control mice | Learning and memory is significantly decreased in tPA−/− mice whereas overexpression of tPA facilitates learning | 4,36,80,81,82,83,85,86 |
| Neurovascular coupling |
In vivo models of whisker evoked neuronal activation in tPA−/− mice. Immunohistochemical/confocal microscopy studies and in vitro/in vivo effects of tPA on blood vessel tone |
Neurovascular coupling is perturbed in tPA−/− mice. tPA is expressed in vasoactive interneurons and might regulate vascular tone. |
7,20,93 | |
| Neurometabolic effects | In vitro neuronal cultures | tPA increase neuronal uptake of glucose | 29,95 | |
| Vascular permeability | In vitro and in vivo studies | tPA increase permeability of the BBB through interaction with the NVU. | 14,15,16,99,135 |
Expression, regulation and downstream mediators
Expression
tPA is widely expressed in the CNS, but until recently little has been reported on its cellular and subcellular distribution in vivo in healthy brain. Among the highest expression of tPA in the murine CNS is seen within the vascular endothelial cells34, however it is believed that this source of tPA is nearly exclusively released into the blood stream upon stimuli and not into the parenchyma of the CNS35. High expression of tPA in the murine brain has also been reported in the cortex, amygdala and the mossy fibers of the hippocampus36,37, in a variety of different cells including astrocytes38, microglia12, and neurons39. This expression pattern was confirmed by studies in transgenic mice expressing a LacZ reporter gene under the human tPA promoter showing remarkable tPA promoter directed expression to the hippocampus, dentate gyrus and to various layers within the cortex40. Whilst the expression in astrocytes and microglia cells in the healthy murine brain has been questioned by some researchers41, the neuronal expression has been confirmed by many studies in mice12,20,37,41 and in humans42. A recent study claims that the neuronal expression of tPA is restricted to a subset of excitatory neurons in mice and rats (after blockage of axo-dendritic transport)41, however this is not supported by our own findings indicating that tPA is also expressed in a subset of perivascular interneurons in the naïve murine CNS20. Nevertheless, the neuronal expression of tPA and the fact that it has been shown to be released in an activity-dependent manner via exocytosis43,44, in combination with the reports that tPA can be found in close proximity to arterioles on the brain parenchymal side of the cerebral vessels (Figure 2A)15,20,37, has led to the suggestion that tPA is involved in regulation of cerebrovascular responses in physiologic settings (discussed in further detail below)7. This is supported by our recent findings that the perivascular neurons expressing tPA are VIP (vasoactive intestinal peptide)-positive interneurons, which are known to regulate vascular responses such as vascular tone22,45,46.
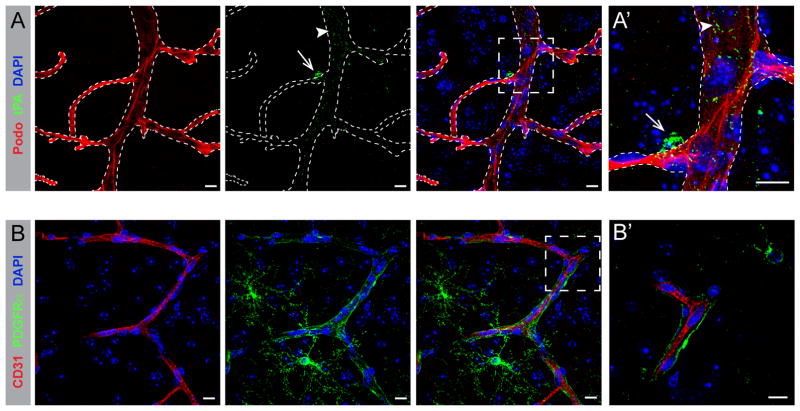
Figure 2.
Perivascular expression of tPA and PDGFRα in the NVU. A, A′) Confocal microscopy image showing expression of tPA (green) in the NVU of the naïve wild type murine brain. tPA is expressed as two distinct pools in the NVU; one within the endothelial cells (arrowheads) and another on the abluminal side of the vessels (arrows). Vessels were visualized by immunofluorescent staining using the endothelial cell marker podocalyxin (red, Podo). B, B′) Confocal image showing expression of PDGFRα (green) ensheathing (arrows) the vessel (red) in the NVU of the naïve wild type murine brain. Vessels were visualized by immunofluorescent staining using the endothelial cell markers in A, A′) podocalyxin (Podo) and in B, B′) CD31. Cell nuclei were visualized with DAPI (blue). The images display maximum intensity projections generated from confocal Z-stacks (A, A′ = 17 μm; B = 20 μm; B′ = 4 μm). Scale bars, 10 μm.
Regulation
The main inhibitor of tPA activity in the blood is plasminogen activator inhibitor 1 (PAI-1)1, however, this serine protease inhibitor (serpin) is only weakly expressed in the healthy murine20,47 and human48,49 brain. It was therefore considered unlikely that PAI-1 is a major regulator of tPA activity in the CNS, at least in regulation of physiologic events. Nevertheless, in pathologic conditions, such as cerebral ischemia and traumatic brain injury (TBI), PAI-1 has been shown to play a role in regulation of tPA activity, which is further supported by the increased levels of PAI-1 found in the CNS during pathologic conditions50,51. The identification of another member of the serpin family, neuroserpin, that is largely specific for the CNS48,49,52–54 led to the hypothesis that this is the physiologic relevant inhibitor regulating tPA activity in the brain55. This notion was supported by expression analysis and biochemical evidence showing strong inhibition of tPA by neuroserpin and considerably less efficient inhibition of other serine proteases52,54. However, it was not until recently that we were able to demonstrate that neuroserpin was not only blocking tPA activity in vitro, it was also a relevant regulator of tPA activity in the murine CNS20. Interestingly, confocal microscopy studies revealed that perivascular neuroserpin is expressed in specific somatostatin-positive interneurons, which, like tPA-expressing VIP-positive interneurons, are known to regulate cerebrovascular responses22,45,46. It has been postulated that neuroserpin may also have other protease targets56 and non-inhibitory functions57 in vivo although this requires further exploration (reviewed in58).
Downstream mediators
The mechanism by which tPA exerts its actions in the CNS is controversial. Both plasminogen-dependent and plasminogen-independent pathways have been postulated to act through several potential downstream mediators in the CNS including matrix metalloprotease 9 (MMP-9)59, activated protein C (APC)60,61, neurotrophic factors (pro-NGF and pro-BDNF)62,63 and platelet-derived growth factor CC (PDGF-CC)15,64. tPA is also known to bind and/or activate a set of receptors such as low density lipoprotein receptor-related protein (LRP)65,66, NMDA-receptor10, annexin-II67, and epidermal growth factor receptors (EGFRs)68. These downstream mediators and co-receptors of tPA have been shown to control a wide array of biological functions in the CNS during normal physiology and in disease models, including neurotoxic and neuroprotective roles as well as regulation of cerebrovascular permeability (discussed in further detail below).
Function in the CNS
To investigate the role of tPA in the CNS many studies have utilized mice where the gene for tPA, Plat, has been deleted69. These mice have been said to express the non-proteolytic domains of tPA with the potential to influence experimental outcomes70, however there is no experimental evidence in the literature supporting this claim. It has also been reported that the existing strain of tPA deficient mice (tPA−/−) do retain significant segments of 129 DNA associated with the Plat allele, even after extensive backcrossing onto C57BL/6J background71. This study also showed that this region of chromosome 8 carries a significant number of mutations unique to the 129 strain that co-segregate with the Plat allele, including several potential null mutations. This may confound the interpretation of experiments performed utilizing the original tPA−/− mice69, and is why a novel “passenger mutation”-free tPA deficient mouse strain (tPA−/− NIH) was generated as a useful community resource for further exploration of tPA function in physiology and disease71.
CNS development
Very little is known about the role of tPA in CNS development, although genetic deletion studies indicate that tPA does not play an essential role during embryonic development69,71. Expression studies in the developing mouse CNS have shown that tPA is synthesized by a variety of neuronal cells, with the highest expression reported in areas of extensive neuronal migration and tissue remodeling72. Based on these findings it has been hypothesized that tPA facilitates neuronal migration or neurite outgrowth through degradation of cell-cell or cell-matrix adhesions. Since then a number of studies, mainly in vitro cell culture experiments but also some in vivo studies, have been performed supporting a role for tPA in neuronal remodeling and migration during CNS development. For example, tPA was found to be released at the neuronal growth cone73, and to mediate neurite outgrowth and remodeling74. Further it was shown that migration of cerebellar granule neurons is perturbed in tPA−/− mice75. With regard to the BBB hypothesis it is tempting to speculate that increased metabolic demand during neuronal migration might lead to tPA-mediated changes in neurovascular, and/or neurometabolic, coupling responses.
The tPA−/− mice were recently reported to display congenital brain defects including abnormal cerebrovascularization indicating a previously unrecognized role of tPA in cerebrovascular development76. Interestingly, the tPA−/− mice were also found to display mild cerebral ventricular malformations, a feature previously associated with ablation of PDGF-C77, thereby providing a potential in vivo link between tPA and PDGF signaling in CNS development. However, considering the novel report of the “passenger-mutations” in these tPA−/− mice it will be interesting to determine whether these congenital cerebrovascular and ventricular abnormalities are a true effect of tPA ablation also in the novel tPA−/− NIH strain71. If so, it will be interesting to determine whether this is a plasminogen-dependent or plasminogen-independent process through studies in plasminogen deficient mice. It is interesting in this regard that obstructive hydrocephalus, associated with cerebroventricular enlargement, has been reported to be associated with plasminogen deficiency in humans78. It should be noted that these congenital defects might have unforeseen effects on the experimental outcome and thus needs to be taken into account when interpreting data achieved utilizing genetically modified mice.
Adult CNS physiology
Learning and memory
The idea that tPA is involved in the process of learning and memory came from the findings that tPA was highly expressed in the mossy fibers of the hippocampus and that neuronal activity induced mRNA expression of tPA in hippocampal pyramidal neurons during late phase long-term potentiation (L-LTP)36. L-LTP is a well-studied model system of learning79 and has been shown to be significantly decreased in tPA−/− mice80,81. In addition, tPA has been implicated in other models of increased neuronal activity including models of learning4,82,83, visual cortex plasticity84 and memory85. Studies using transgenic mice over-expressing tPA86 or intrahippocampal infusion of tPA82 confirms that tPA facilitates L-LTP and learning.
The underlying mechanism by which tPA might facilitate L-LTP has been shown to be mediated by LRP87, potentially via plasmin-mediated cleavage of proBDNF to the mature form of BDNF63. Interestingly though, L-LTP has been shown to be associated with increased BBB permeability88 and the large pyramidal neurons within the hippocampus are known to have particularly high metabolic demand, thus making them especially sensitive to damage from a variety of environmental and biological insults89. Thus, in line with our hypothesis, it might be that tPA-induced changes in the NVU and the subsequent increase in cerebrovascular permeability during learning, through an LRP-dependent process, leads to altered parenchymal homeostasis which in turn promotes cleavage of proBDNF.
Neurovascular and neurometabolic coupling
Local cerebral blood flow increases rapidly in response to neuronal activity, a phenomenon termed functional hyperemia or neurovascular coupling. This is believed to be critical for the maintenance of substrate and energy supply to the activated neurons as well as for clearance of metabolic by-products22. Although the process of neurovascular coupling is still not completely understood, perivascular astrocytes and various vasoactive mediators, including tPA7, have been proposed to be of importance22,90. The prospect that tPA has a role in normal neurovascular coupling, as suggested by Park et al. utilizing tPA−/− mice in a model of whisker evoked neuronal activation7, is supported by several lines of evidence. First, coupling of local neuronal activity to local blood flow has been shown to be controlled to a large extent by penetrating arterioles and tPA has been shown to primarily be associated with arterioles in the CNS (Figure 2A)15,20,91. Second, our recent data illustrate perivascular tPA to be expressed by VIP-positive interneurons, whereas its inhibitor neuroserpin was found in somatostatin-positive interneurons20. This is interesting since VIP-expressing ‘vasomotor’ interneurons have been reported to induce dilation of local microvessels, while somatostatin-expressing neurons induce contraction22,45,46, suggesting that neuroserpin/tPA may form a regulatory circuit that regulates cerebrovascular responses. Third, tPA has been reported to reduce vessel reactivity to increased luminal pressure and vasoactive mediators, suggesting that tPA may be involved in regulating vascular tone92,93, and finally, systemic delivery of tPA at low concentrations can directly reduce cerebral vascular resistance and systemic blood pressure93. However, given the recent observations that tPA−/− mice display a decreased number of large diameter ASMA-positive vessels76, and that neurovascular coupling was found to take place exclusively at ASMA-positive, SMC-covered, arterioles94, it might be that the cerebrovascular rearrangements associated with tPA deficiency may explain the attenuated neurovascular coupling response in these mice7. This warrants further investigation.
In addition, tPA has been implicated in neurometabolic coupling29,95, which refers to changes in local metabolic factors, such as glucose and lactate, in response to neuronal activity28,96. Using in vitro neuronal cultures under conditions of oxygen-glucose deprivation it was shown that tPA, via a plasminogen-independent mechanism, increase neuronal uptake of glucose through induction of the glucose transporter GLUT3. Although intriguing, and in line with the idea that tPA facilitates neuronal function through coupling energy supply to demand, this needs to be confirmed in vivo, in non-pathologic conditions.
Vascular permeability
The first indications that tPA plays a role in the regulation of vascular permeability came from animal studies of embolic stroke, where thrombolytic treatment with tPA was associated with evidence of increased vascular permeability97,98. Since then the association of tPA with the NVU and its correlation with BBB regulation has been well established, although the mechanism underlying this capacity of tPA remains controversial18. Some reports have suggested a plasmin-independent role for tPA14,15 whereas others have reported tPA-mediated plasmin generation as crucial for tPA activity on the BBB16,99. Nevertheless, tPA’s action on the BBB has been shown to require interaction with the CNS side of the NVU, as tPA administered intravenously in unchallenged wild type mice does not elicit opening of the BBB whereas tPA administered on the CNS side of the NVU does15.
Our previous data show that platelet-derived growth factor CC (PDGF-CC) is acting directly downstream of tPA in the CNS where it regulates BBB integrity through PDGF receptor α (PDGFRα) signaling on perivascular astrocytes (Figure 2B)15. PDGF-CC is expressed as a latent factor that is cleaved by tPA in a plasmin-independent manner to generate active PDGF-CC capable of binding PDGFRα64. Injection of active PDGF-CC protein into the CSF of mice was reported, like tPA, to rapidly increase BBB permeability and neutralizing antibodies against PDGF-CC inhibited tPA-induced opening of the BBB15. No gross changes of vascular structures were reported within this time frame, even though the extent of Evans Blue extravasation into the brain parenchyma was significant, suggesting that the activation of PDGF-CC/PDGFRα may represent a regulated physiological process that controls the BBB. The activation of PDGF-CC by tPA depends on interactions between the kringle-2 domain of the protease with the two domains in the PDGF-C polypeptide chain100 as well as on binding to the co-receptor LRP15.
This presumably ensures correct positioning of tPA and PDGF-CC enabling activation in close proximity to the PDGFRα receptor in the NVU and stimulation of increased cerebrovascular permeability. This is supported by the findings that desmoteplase, vampire bat-derived plasminogen activator, which lacks the kringle-2 domain101, and thus presumably interaction with PDGF-CC, has been shown to have no effect on inducing BBB permeability16. Taken together we propose that in physiologic conditions tPA is released in response to neuronal activity into the perivascular space where it activates PDGF-CC, and subsequently PDGFRα signaling on perivascular astrocytes, through interaction with LRP, which leads to cerebrovascular changes associated with neuronal activity (Figure 3A)18.
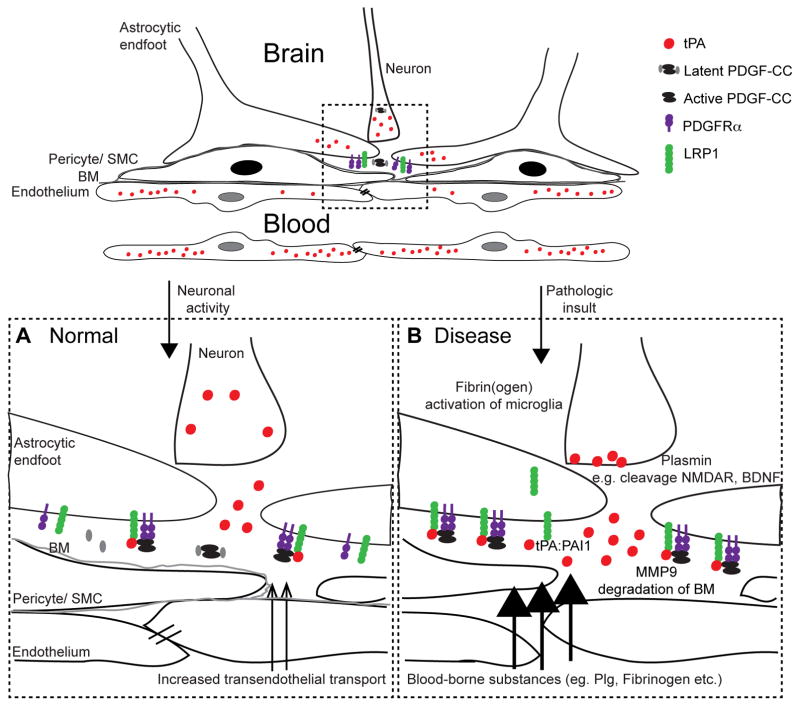
Figure 3.
tPA-mediated regulation of BBB integrity. A) Under physiologic conditions tPA is released by activated neurons into the perivascular space where it activates PDGF-CC, and subsequently PDGFRα signaling on perivascular astrocytes, through interaction with LRP, which leads to cerebrovascular changes associated with neuronal activity. B) In pathologic conditions initial increase in BBB permeability is mediated by proteolytic active tPA, potentially in response to the increased energy and metabolic demand in neurons following insult. Excessive signaling via either active tPA or via tPA:PAI-1 complex formation and LDLR signaling, leads to further opening of the barrier and subsequent extravasation of blood-borne substances from the vascular space into the brain parenchyma. Following intravenous treatment with thrombolytic tPA it is plausible that exogenous tPA enters the brain through the breached BBB thereby exacerbating the PDGF-CC/PDGFRα signaling in the NVU.
It should be noted that the action of tPA on BBB regulation has also been reported to be mediated by plasmin and downstream mediators other than PDGF-CC, including plasmin-induced truncation of MCP116,99. In the case with MCP1 it was found that BBB integrity was compromised much later than that induced by PDGF-CC. Prolonged opening of the barrier will lead to changes in parenchymal homeostasis where blood-borne substances such as plasmin can enter the CSF and in turn cleave MCP1. In support of this hypothesis are our findings that early after barrier breach induced by PDGF-CC, no gross changes of vascular structures were noted15, whereas MCP1-induced opening was associated with disruption of tight junction proteins (occludin and ZO-1) and reorganization of the actin cytoskeleton99.
Further, it has been postulated that the controversy regarding mechanisms mediating the role of tPA in BBB regulation may, at least in part, be due to the concentration of tPA70. Through a number of elaborate calculations and assumptions the overall concentration of tPA in the brain three hours after an ischemic event has been proposed to be ~1 nM, and even lower during physiologic conditions70. Therefore, since the amounts of tPA often used in experimental settings could potentially result in tPA concentrations that are higher than this, it has been suggested that these potentially supraphysiologic concentrations might lead to non-physiologic observations70. However, the actual endogenous concentration of tPA in the NVU is not known, and may in fact be significantly higher than the 1nM suggested above. Indeed, the release of tPA from activated neurons and subsequent binding to co-factors such as LRP, could generate very high local concentrations of tPA in the NVU which could be underestimated when evaluating gross brain tissue extracts.
PATHOPHYSIOLOGIC ROLES OF tPA IN THE CNS
It was quickly recognized that the physiologic roles of tPA in the CNS may also be involved in pathophysiological events observed in several neurological diseases including cerebral ischemia, head trauma and seizures58. This led to a large number of in vitro and in vivo studies, but the role of tPA in pathology is still intensely debated and the literature is conflicted. The main controversy concerns the potential neuroprotective or neurotoxic functions of tPA in the CNS and what downstream mediators facilitates the response. Here we discuss how these controversies might be explained by the early effect of tPA on cerebrovascular permeability. Interestingly, the role of tPA in BBB regulation highlights a commonality in neurovascular signaling events between diverse neurologic disorders and may potentially represent a previously overlooked therapeutic target for seemingly unrelated brain diseases.
Ischemic stroke
Stroke is a leading cause of adult morbidity and mortality102. The most common form of stroke is ischemic stroke, which occurs when there is an abrupt interruption of blood flow to the brain. The finding that tPA specifically binds to, and is stimulated by fibrin103,104 led to the hypothesis that clot lysis (thrombolysis) using tPA would facilitate a localized activation of plasmin at the site of occlusion. This was successfully demonstrated early on105 and today tPA is the only approved thrombolytic drug for treatment of acute ischemic stroke2. However, early animal studies advised against indiscriminate use of thrombolytic tPA in ischemic stroke as it was reported to mediate neuronal damage or cerebral haemorrhage8,106–109. Consistent with these preclinical studies, thrombolysis with tPA in ischemic stroke patients carries a significant risk of intracerebral hemorrhage2,110–113, and due in part to this increased risk of hemorrhagic conversion it is estimated that only 5–7% of ischemic stroke patients receive intravenous tPA, with another 1–2% receiving intra-arterial therapy2,114–119. The mechanism by which thrombolytic tPA might lead to increased hemorrhagic transformation is not completely understood, but it appears to be due in part to unique activities of tPA in the CNS. This is supported by the findings that tPA activity rapidly increases in ischemic tissue in experimental models of ischemic stroke14,107,120.
One of the first studies demonstrating that tPA can negatively affect outcome in an experimental model of middle cerebral artery occlusion (MCAO) showed that tPA−/− mice had significantly smaller cerebral infarcts than wild type mice, and that intravenous administration of tPA to tPA−/− mice increased infarct volume to levels comparable to wild type controls107. In support of this it has been shown that neuroserpin, the primary inhibitor of tPA in the CNS20, provided neuronal protection and reduced infarct volume during MCAO120,121. Based on these studies, it was concluded that tPA has neurotoxic effects in the ischemic brain. It should however be noted that a number of conflicting studies have been published showing that loss of tPA is associated with increased lesion volume122, and conversely, that increased levels of tPA, through intravenous administration of tPA to wild type mice or transgenic overexpression of tPA in neurons of mice (T4), decreased infarct volume following MCAO29. This proposed a neuroprotective role of tPA during MCAO and the mechanism of action was ascribed to tPA-induced increase in GLUT3 expression followed by increased glucose uptake to meet the increased metabolic demand of cerebral cortical neurons. In line with this hypothesis it was reported that the T4 transgenic mice that neuronally overexpress tPA displayed significant upregulation of GLUT3 protein expression in ischemic brain tissue and higher glucose uptake after MCAO29. However, it is somewhat difficult to reconcile these data since GLUT3 expression and glucose uptake was found to be decreased in wild type mice after MCAO, despite an increased endogenous release of tPA during ischemia14,107,120. Thus, it is possible that, similar to the tPA−/− mice, overexpression of tPA in the neurons of T4 mice is associated with some congenital cerebrovascular anomaly that has yet to be discovered.
The mechanism by which tPA exerts neurotoxic effects has been postulated to be mediated through plasmin-independent cleavage of the NR1 subunit in NMDA-receptor followed by enhanced signaling10. However, the direct cleavage of NR1 has later been questioned by other research groups who have suggested that the NMDA-receptor is not a direct target of tPA proteolysis, although plasmin was found to be able to mediate cleavage of the NMDA-receptor123,124. Instead, as noted above an alternative mechanism has emerged, suggesting that tPA-mediated changes in cerebrovascular permeability might be the underlying cause of the neurotoxic effects reported for tPA in the CNS. This was based on the discoveries that tPA increases permeability of the BBB both in rodents14,98 and in humans125 following ischemic stroke. This is particularly interesting since BBB dysfunction is a hallmark of many neurologic diseases, including ischemic stroke126 and it has been proposed that pathologic disruption of the barrier will lead to extravasation of blood-borne molecules such as fibrin(ogen) into the brain parenchyma where it can trigger different cellular and molecular responses including a delayed inflammatory response127.
In one study14 it was demonstrated that tPA−/− mice were protected from early loss of BBB integrity after MCAO. This study further showed that the tPA-induced opening of the BBB was dependent on the proteolytic activity of tPA, but was independent of plasmin, thus indicating the existence of another tPA substrate. Since MMP-9 had been implicated as a downstream mediator of tPA in the CNS59 and MMP-9 deletion in mice had been shown to reduce BBB permeability and infarct volume 24h after stroke128, this was initially thought to be responsible for the effect of tPA on the BBB. However, MMP-9 deficient mice were found to display BBB dysfunction similar to wild type mice early (6h) after MCAO14. Furthermore, depletion of circulating leukocytes was found to completely block the rise in MMP-9 activity within the first 24h after ischemic stroke129, suggesting that the increased MMP-9 activity seen after ischemic stroke is likely due to infiltrating leukocytes. This is consistent with the findings that MMP-9 is not expressed in either neurons or astrocytes in the first 24h after ischemic stroke130. Finally, it was also shown that treatment with recombinant tPA after transient middle cerebral artery occlusion exacerbates BBB disruption 24 hours later in both wild-type and MMP-9 knockout mice131. Collectively these data suggest that MMP-9-mediated events occur on the luminal side of the NVU and are not involved in the early effects on BBB permeability mediated by tPA after ischemic stroke.
The findings that tPA regulation of the BBB is mediated through plasmin-independent catalysis of PDGF-CC and subsequent activation of PDGFRα on perivascular astrocytes (discussed above), and that blocking this pathway, either by neutralizing antibodies against PDGF-CC or with the PDGFRα inhibitor imatinib, significantly reduced BBB permeability and hemorrhagic complications associated with thrombolytic tPA treatment suggested a potential treatment strategy to reduce the complications associated with thrombolytic tPA by inhibiting PDGFRα signaling15. Support for the potential role of PDGF-CC in thrombolysis associated complications in humans was provided by a study showing that PDGF-CC levels are increased in the plasma of ischemic stroke patients after thrombolytic tPA treatment and higher levels of PDGF-CC were associated with an increased risk of hemorrhagic transformation132. On the basis of these findings a randomized controlled clinical trial has been conducted at the Karolinska University Hospital to assess the possibility of using imatinib as an adjuvant therapy with thrombolytic tPA and the outcome of this study is currently being evaluated (Istrokepilot, EudraCT Number: 2010-019014-25).
It is thus possible that decreased energy and metabolic supply to neurons during ischemia leads to increased release of tPA into the NVU, where it, through activation of PDGF-CC/PDGFRα signaling, leads to increased BBB permeability (Figure 3B). However, excessive signaling leads to further opening of the barrier and subsequent extravasation of blood-borne substances from the vascular space into the brain parenchyma. Following intravenous treatment with thrombolytic tPA it is plausible that exogenous tPA enters the brain through the breached BBB thereby exacerbating the PDGF-CC/PDGFRα signaling in the NVU. This might eventually cause complete disruption of the BBB consequently resulting in hemorrhagic complications. It should be noted that thrombolytic therapy with tPA in patients with pulmonary embolism, deep vein thrombosis or myocardial infarction also carries a ~1 to 1.5 % risk of symptomatic intracerebral hemorrhage133,134, suggesting that even without cerebral ischemia exogenous tPA can induce BBB opening and promote hemorrhage. This is also supported by the fact that intravenous administered tPA can cross the intact barrier and slightly increase BBB permeability in naïve rats135.
Interestingly, tPA-catalyzed activation of PDGF-CC/PDGFRα signaling can also induce BBB permeability and contribute to disease progression in a number of other experimental models of neurologic disease including TBI136, seizures20 and amyotrophic lateral sclerosis (ALS)137 (discussed below).
Traumatic brain injury (TBI)
The broadening role of tPA in regulation of BBB permeability in ischemic stroke raised the question of its role in other CNS pathologies. In the case of TBI BBB disruption is a serious consequence which plays a major role in the promotion of cerebral edema and increase in intracranial pressure; two devastating clinical manifestations of TBI that contribute to the high level of mortality and morbidity post injury, and for which there are scant therapeutic options138. Although ischemic stroke and TBI have vastly different initiating stimuli, there was a clear rationale to explore the role of tPA in the promotion of BBB permeability following TBI.
Endogenous tPA, as well as most other components of the plasminogen activating system including plasminogen and PAI-1, are not only expressed in various compartments of the brain, they can be induced following various stimuli, including glutamate analogues51,139,140. As levels of excitotoxic amino acids are known to increase in the damaged brain following TBI141, it stood to reason that this would promote increases in the levels of endogenous tPA following TBI and that this in turn would influence outcome and recovery. Initial support for a detrimental role for tPA post-TBI was provided in a study in 2001 where it was shown that tPA deficient mice had less edema and improved recovery in a model of TBI109. It was also shown in separate publications that administration of tPA to pigs subjected to TBI resulted in an increase in brain water content142. A later publication from the same group linked this effect of tPA to increased vasodilatation and activation of mitogen activated protein kinase143. Although none of these studies specifically investigated tPA mediated changes in BBB permeability following TBI, these results were certainly consistent with this possibility.
Direct evidence that levels of tPA activity were indeed upregulated following TBI, and was having a major influence on BBB permeability following TBI, was provided in 2011 and 2012. Sashindranath et al. used a modified amidolytic assay to show that endogenous level of tPA activity were transiently increased in the cortex following TBI144. tPA activity increased ~25% within 1h of TBI and this increase was maintained at 3h post injury but reduced to pre-injury levels by 24h. A subsequent study from the same group compared the extent of BBB permeability in wild type and tPA−/− mice following TBI using the Evan’s Blue brain extravasation assay145. Wild type mice were shown to have marked increase in BBB permeability when assessed 3h post-TBI, however this was not seen in tPA−/− mice, thereby implicating endogenous tPA in this process. Importantly, tPA−/− mice also had improved neurological outcome following TBI. On the other hand, transgenic mice with neuronal overexpression of tPA (T4 mice, described above) displayed enhanced BBB permeability and a more severe neurological phenotype post-TBI. Hence, tPA expression was causally linked with BBB permeability following TBI, with the absence of tPA being protective, and increased levels being deleterious in TBI, reminiscent of the results seen in earlier studies in mouse models of ischemic stroke.
An interesting observation arising from this study concerned the mechanism by which tPA was promoting BBB permeability following TBI. It was assumed that tPA was promoting parenchymal extravasation following TBI via its proteolytic capacity, either via plasmin generation or via different substrates (i.e. PDGF-CC). It was anticipated that inhibition of endogenous tPA would recapitulate the protective phenotype seen when tPA−/− mice subjected to TBI. The approach used by these authors was to inhibit local tPA activity within the lesion of the brain immediately following TBI by stereotactic injection of PAI-1. This approach indeed inhibited levels of endogenous tPA activity greater than 90% as determined using an amidolytic assay145. However, in stark contrast to expectations, the blocking effect of PAI-1 against tPA actually resulted in an increase in extravasation 3h post injury. This puzzling result was eventually explained when the authors investigated the downstream consequences of complexes formed between tPA and PAI-1. It has been known for decades that tPA:PAI-1 complexes (and indeed many other serpin:protease inhibitor complexes) are cleared from plasma and the extravascular space by LDL receptors146,147. It had also been reported that LDL receptors could modulate activity of integrins and receptor tyrosine kinases that in turn can initiate intracellular signaling148. Speculation therefore arose that the introduction of PAI-1 into the damaged brain following TBI, despite blocking endogenous tPA activity, was initiating an unanticipated signal through LDL receptors via the formation of tPA:PAI-1 complexes. Support for this notion was provided from a number of lines of evidence; first, co-injection of the LDL receptor antagonist, RAP with PAI-1 blocked the increase in extravasation; second, stereotactic injection of PAI-1 into tPA−/− mice had no effect at promoting extravasation post-TBI; third, co-injection of a PAI-1 mutant that poorly reacts with LDL receptors, failed to reproduce the effect, despite it being able to effectively inhibit tPA activity. Finally, injection of pre-formed tPA:PAI-1 complexes into the lesioned area of tPA−/− mice following TBI caused a significant increase in albumin extravasation.
It is possible that the discrepancy of this study, demonstrating that tPA-mediated opening of the BBB in TBI is through proteolytic inactive tPA in form of a tPA:PAI-1 complex145, and the previous in vivo and in vitro studies of tPA-mediated BBB regulation discussed above, showing that opening of the barrier requires proteolytic active tPA14,16 via activation of PDGF-CC signaling15, might be explained by temporally distinct mechanisms regulating BBB integrity in TBI. To address this a recent study was conducted where imatinib, an inhibitor of PDGF-CC signaling, was evaluated in a mouse model of TBI136. It was shown that inhibition of PDGF signaling in TBI significantly attenuated the extent of neuronal injury and BBB opening when evaluated 24h post injury136. Of further note in this study was the finding of PDGF-CC in the cerebrospinal fluid of patients with TBI, with PDGF-CC levels positively correlating with injury severity.
Hence, although at first glance it appears that tPA modulates BBB permeability via two different mechanisms in TBI (i.e. active tPA or inactive tPA:PAI-1 complexes) it may as well be that these differences relate to a temporal order of events. We speculate that the initial increase in BBB permeability during TBI is mediated by proteolytic active tPA, potentially in response to the increased energy and metabolic demand in neurons following insult, and that excessive signaling leads to further opening of the barrier and subsequent increase in PAI-1 levels (through upregulated expression in response to the injury as well as increased extravasation from the vascular space into the brain parenchyma). This in turn leads to formation of tPA:PAI-1 complexes that can exacerbate opening of the BBB, eventually causing disruption of the BBB and leakage of harmful blood-borne substances into the brain after TBI (Figure 3). It remains to be determined whether tPA:PAI-1 complex-mediated increase in BBB permeability also occurs in ischemic stroke, but this is worth investigating. Nonetheless, whatever the mechanism these findings have provided novel opportunities to minimize BBB opening following TBI and in doing so reduce the devastating complications of cerebral edema and the rise in intracranial pressure, thus demonstrating clear translational possibilities of these findings.
Seizures
The first evidence that tPA is important in the development of seizures came from studies in mice showing that tPA expression is increased early after seizures36 and that tPA ablation leads to a higher threshold for seizures8,149, whereas neuronal overexpression leads to a lower seizure threshold150. The relationship between tPA and seizures in humans is less well understood, but a recent study has described a positive correlation between increased serum tPA levels and epilepsy severity in children with idiopathic and intractable epilepsies151. The mechanism by which tPA affects seizures is not fully established, but tPA has been suggested to act directly on neuronal cells by cleavage of the NMDA-receptor10, or indirectly by altering cerebrovascular permeability20. The latter is supported by the fact that impaired integrity of the BBB is a well-known feature of seizures, although it is debated whether impaired BBB function is just a consequence of seizure activity or a contributor to seizure progression (reviewed in126). However, since 30% of individuals with seizures fail to respond to existing treatments152, recent studies have begun to consider the cerebrovasculature as a potential avenue for therapeutic intervention20,153–156.
The seminal observation illustrating that tPA−/− mice are resistant to excitotoxin-induced neuronal death and seizures after intrahippocampal injection of excitotoxins, including the glutamate analog kanic acid (KA), led to the conclusion that, in pathologic conditions, tPA has neurotoxic properties8. Using a different experimental paradigm, where seizures were induced by injection of KA into the amygdala, other researchers were later able to confirm these results149. The fact that KA was not injected directly into the hippocampus allowed these researchers to dissect KA-induced, from seizure-induced, hippocampal neuronal death, yet genetic deficiency of tPA was associated with slower progression of KA-induced seizure activity throughout the limbic system and a decrease in seizures-induced hippocampal cell death.
To study the effect of tPA on excitotoxin-induced neuronal death without the confounding effect of seizures, a group of researchers injected NMDA, another glutamate analog, into the striatum of mice followed by intravenous treatment with tPA157. The advantage of this model is that the injection of NMDA into the striatum does not induce seizures. Using this approach, these investigators found that intravenous administration of tPA increases the volume of the necrotic lesion caused by the injection of NMDA157. Interestingly, glutamate has been shown to induce BBB permeability158 suggesting that following NMDA injection, the BBB may be more permeable thus enabling intravenous tPA to extravasate into the brain parenchyma and further disrupt the barrier integrity.
In line with this a recent study illustrated that tPA regulated seizure progression primarily through control of the neurovascular unit and BBB integrity, and not through direct effects of tPA on neuronal activity20. This was supported by multiple independent experimental results, including the observation that increasing BBB permeability in seizure-resistant tPA−/− mice dramatically enhanced the rate of seizure progression, while interventions that maintain BBB integrity delayed seizure propagation. In addition, the comparison of in vivo EEG recordings to ex vivo hippocampal electrophysiological recordings demonstrates that the phenotypic differences in seizure progression noticed between wild type (intermediate), tPA−/− (protected), and neuroserpin deficient mice (enhanced) in vivo, were absent in ex vivo studies where BBB regulation is no longer important for maintenance of the extracellular environment. As seen in ischemic stroke and TBI, the effect of tPA on BBB permeability in seizures was illustrated to be facilitated, at least in part, through PDGF-CC induced activation of PDGFRα signaling in perivascular astrocytes, which is of particular interest since earlier reports have suggested astrocytes to play a central role in the pathology of seizures155,159.
CONCLUDING REMARKS
It is well appreciated that tPA is controlling unique functions within the CNS distinct from its role in fibrinolysis, although the downstream targets mediating these functions and the suggested outcome ascribed to tPA activity in the CNS are many and intensely debated. Here we summarize the existing evidence in support of the hypothesis that the neurovascular events regulated by tPA might provide a unifying pathway for many of the pleiotropic effects of tPA in the CNS.
ESSENTIALS.
Tissue-type plasminogen activator (tPA) is highly expressed in the CNS
The role of tPA in the CNS is very different from its role in fibrinolysis within the vascular space
tPA is reported to have many pleiotropic activities in the CNS, mediated through a number of various downstream substrates
Here we propose that the neurovascular events regulated by tPA might provide a unifying pathway for many of the pleiotropic effects of tPA in the CNS
Acknowledgments
The authors would like to acknowledge grant support from the following agencies: the Swedish Governmental Agency for Innovation Systems, Grant 2011- 03503 (LF), the Swedish Research Council, Grant 2012-1853 (LF), the National Institutes of Health Grants HL055374 and NS079639 (DAL), the National Health and Medical Research Foundation of Australia Grant ID #104576 and 1045755 (RLM).
References
- 1.Collen D. Ham-Wasserman lecture: role of the plasminogen system in fibrin-homeostasis and tissue remodeling. Hematology (Am Soc Hematol Educ Program) 2001:1–9. doi: 10.1182/asheducation-2001.1.1. [DOI] [PubMed] [Google Scholar]
- 2.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995 Dec 14;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Yu L, Gu X, et al. Tissue plasminogen activator regulates Purkinje neuron development and survival. Proceedings of the National Academy of Sciences of the United States of America. 2013 Jun 25;110(26):E2410–2419. doi: 10.1073/pnas.1305010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeds NW, Basham ME, Ferguson JE. Absence of tissue plasminogen activator gene or activity impairs mouse cerebellar motor learning. J Neurosci. 2003 Aug 13;23(19):7368–7375. doi: 10.1523/JNEUROSCI.23-19-07368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minor K, Phillips J, Seeds NW. Tissue plasminogen activator promotes axonal outgrowth on CNS myelin after conditioned injury. J Neurochem. 2009 May;109(3):706–715. doi: 10.1111/j.1471-4159.2009.05977.x. [DOI] [PubMed] [Google Scholar]
- 6.Seeds NW, Siconolfi LB, Haffke SP. Neuronal extracellular proteases facilitate cell migration, axonal growth, and pathfinding. Cell Tissue Res. 1997 Nov;290(2):367–370. doi: 10.1007/s004410050942. [DOI] [PubMed] [Google Scholar]
- 7.Park L, Gallo EF, Anrather J, et al. Key role of tissue plasminogen activator in neurovascular coupling. Proceedings of the National Academy of Sciences. 2008 Jan 22;105(3):1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995 Sep 28;377(6547):340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- 9.Tsirka SE, Rogove AD, Strickland S. Neuronal cell death and tPA. Nature. 1996 Nov 14;384(6605):123–124. doi: 10.1038/384123b0. [DOI] [PubMed] [Google Scholar]
- 10.Nicole O, Docagne F, Ali C, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001 Jan;7(1):59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 11.Echeverry R, Wu J, Haile WB, Guzman J, Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. The Journal of Clinical Investigation. 2010 Jun;120(6):2194–2205. doi: 10.1172/JCI41722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997 Jan 15;17(2):543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogove AD, Tsirka SE. Neurotoxic responses by microglia elicited by excitotoxic injury in the mouse hippocampus. Current biology: CB. 1998 Jan 1;8(1):19–25. doi: 10.1016/s0960-9822(98)70016-8. [DOI] [PubMed] [Google Scholar]
- 14.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003 Nov;112(10):1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su EJ, Fredriksson L, Geyer M, et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008 Jul;14(7):731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niego B, Freeman R, Puschmann TB, Turnley AM, Medcalf RL. t-PA-specific modulation of a human blood-brain barrier model involves plasmin-mediated activation of the Rho kinase pathway in astrocytes. Blood. 2012 May 17;119(20):4752–4761. doi: 10.1182/blood-2011-07-369512. [DOI] [PubMed] [Google Scholar]
- 17.Lemarchant S, Docagne F, Emery E, Vivien D, Ali C, Rubio M. tPA in the injured central nervous system: different scenarios starring the same actor? Neuropharmacology. 2012 Feb;62(2):749–756. doi: 10.1016/j.neuropharm.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Su EJ, Fredriksson L, Schielke GP, Eriksson U, Lawrence DA. Tissue plasminogen activator-mediated PDGF signaling and neurovascular coupling in stroke. J Thromb Haemost. 2009 Jul;7(Suppl 1):155–158. doi: 10.1111/j.1538-7836.2009.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chevilley A, Lesept F, Lenoir S, Ali C, Parcq J, Vivien D. Impacts of tissue-type plasminogen activator (tPA) on neuronal survival. Front Cell Neurosci. 2015;9:415. doi: 10.3389/fncel.2015.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredriksson L, Stevenson TK, Su EJ, et al. Identification of a neurovascular signaling pathway regulating seizures in mice. Annals of Clinical and Translational Neurology. 2015;2(7):722–738. doi: 10.1002/acn3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005 Jun;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 22.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004 May;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 23.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010 Nov 25;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 24.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006 Jan;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 25.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007 Nov;10(11):1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 26.Parri R, Crunelli V. An astrocyte bridge from synapse to blood flow. Nat Neurosci. 2003 Jan;6(1):5–6. doi: 10.1038/nn0103-5. [DOI] [PubMed] [Google Scholar]
- 27.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proceedings of the National Academy of Sciences of the United States of America. 1994 Oct 25;91(22):10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leybaert L, Cabooter L, Braet K. Calcium signal communication between glial and vascular brain cells. Acta Neurol Belg. 2004 Jun;104(2):51–56. [PubMed] [Google Scholar]
- 29.Wu F, Wu J, Nicholson AD, et al. Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012 Jul 18;32(29):9848–9858. doi: 10.1523/JNEUROSCI.1241-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawlak R, Melchor JP, Matys T, Skrzypiec AE, Strickland S. Ethanol-withdrawal seizures are controlled by tissue plasminogen activator via modulation of NR2B-containing NMDA receptors. Proc Natl Acad Sci U S A. 2005 Jan 11;102(2):443–448. doi: 10.1073/pnas.0406454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parcq J, Bertrand T, Montagne A, et al. Unveiling an exceptional zymogen: the single-chain form of tPA is a selective activator of NMDA receptor-dependent signaling and neurotoxicity. Cell Death Differ. 2012 Dec;19(12):1983–1991. doi: 10.1038/cdd.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr. 2009 Sep;90(3):867S–874S. doi: 10.3945/ajcn.2009.27462BB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stobart JL, Anderson CM. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front Cell Neurosci. 2013;7:38. doi: 10.3389/fncel.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zlokovic BV, Wang L, Sun N, et al. Expression of tissue plasminogen activator in cerebral capillaries: possible fibrinolytic function of the blood-brain barrier. Neurosurgery. 1995 Nov;37(5):955–961. doi: 10.1227/00006123-199511000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Huber D, Cramer EM, Kaufmann JE, et al. Tissue-type plasminogen activator (t-PA) is stored in Weibel-Palade bodies in human endothelial cells both in vitro and in vivo. Blood. 2002 May 15;99(10):3637–3645. doi: 10.1182/blood.v99.10.3637. [DOI] [PubMed] [Google Scholar]
- 36.Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993 Feb 4;361(6411):453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- 37.Salles FJ, Strickland S. Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002 Mar 15;22(6):2125–2134. doi: 10.1523/JNEUROSCI.22-06-02125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Docagne F, Nicole O, Marti HH, MacKenzie ET, Buisson A, Vivien D. Transforming growth factor-beta1 as a regulator of the serpins/t-PA axis in cerebral ischemia. Faseb J. 1999 Aug;13(11):1315–1324. doi: 10.1096/fasebj.13.11.1315. [DOI] [PubMed] [Google Scholar]
- 39.Sappino AP, Madani R, Huarte J, et al. Extracellular proteolysis in the adult murine brain. The Journal of Clinical Investigation. 1993;92(2):679–685. doi: 10.1172/JCI116637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu H, Schleuning WD, Michl M, Liberatore G, Tan SS, Medcalf RL. Control elements between −9.5 and −3.0 kb in the human tissue-type plasminogen activator gene promoter direct spatial and inducible expression to the murine brain. Eur J Neurosci. 2001 Sep;14(5):799–808. doi: 10.1046/j.0953-816x.2001.01700.x. [DOI] [PubMed] [Google Scholar]
- 41.Louessard M, Lacroix A, Martineau M, et al. Tissue Plasminogen Activator Expression Is Restricted to Subsets of Excitatory Pyramidal Glutamatergic Neurons. Mol Neurobiol. 2015 Sep 16; doi: 10.1007/s12035-015-9432-7. [DOI] [PubMed] [Google Scholar]
- 42.Teesalu T, Kulla A, Simisker A, et al. Tissue plasminogen activator and neuroserpin are widely expressed in the human central nervous system. Thrombosis and haemostasis. 2004 Aug;92(2):358–368. doi: 10.1160/TH02-12-0310. [DOI] [PubMed] [Google Scholar]
- 43.Gualandris A, Jones TE, Strickland S, Tsirka SE. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996 Apr 1;16(7):2220–2225. doi: 10.1523/JNEUROSCI.16-07-02220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lochner JE, Honigman LS, Grant WF, et al. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol. 2006 May;66(6):564–577. doi: 10.1002/neu.20250. [DOI] [PubMed] [Google Scholar]
- 45.Cauli B, Tong XK, Rancillac A, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004 Oct 13;24(41):8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006 Mar;100(3):1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 47.Sawdey MS, Loskutoff DJ. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. The Journal of Clinical Investigation. 1991 Oct;88(4):1346–1353. doi: 10.1172/JCI115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrimpf SP, Bleiker AJ, Brecevic L, et al. Human neuroserpin (PI12): cDNA cloning and chromosomal localization to 3q26. Genomics. 1997 Feb 15;40(1):55–62. doi: 10.1006/geno.1996.4514. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto M, Sawaya R, Mohanam S, et al. Expression and cellular localization of messenger RNA for plasminogen activator inhibitor type 1 in human astrocytomas in vivo. Cancer research. 1994 Jul 1;54(13):3329–3332. [PubMed] [Google Scholar]
- 50.Kietzmann T, Roth U, Jungermann K. Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxia-inducible factor-1 in rat hepatocytes. Blood. 1999 Dec 15;94(12):4177–4185. [PubMed] [Google Scholar]
- 51.Masos T, Miskin R. mRNAs encoding urokinase-type plasminogen activator and plasminogen activator inhibitor-1 are elevated in the mouse brain following kainate-mediated excitation. Brain Res Mol Brain Res. 1997 Jul;47(1–2):157–169. doi: 10.1016/s0169-328x(97)00040-5. [DOI] [PubMed] [Google Scholar]
- 52.Osterwalder T, Cinelli P, Baici A, et al. The axonally secreted serine proteinase inhibitor, neuroserpin, inhibits plasminogen activators and plasmin but not thrombin. The Journal of biological chemistry. 1998 Jan 23;273(4):2312–2321. doi: 10.1074/jbc.273.4.2312. [DOI] [PubMed] [Google Scholar]
- 53.Osterwalder T, Contartese J, Stoeckli ET, Kuhn TB, Sonderegger P. Neuroserpin, an axonally secreted serine protease inhibitor. The EMBO journal. 1996 Jun 17;15(12):2944–2953. [PMC free article] [PubMed] [Google Scholar]
- 54.Hastings GA, Coleman TA, Haudenschild CC, et al. Neuroserpin, a brain-associated inhibitor of tissue plasminogen activator is localized primarily in neurons. Implications for the regulation of motor learning and neuronal survival. The Journal of biological chemistry. 1997 Dec 26;272(52):33062–33067. doi: 10.1074/jbc.272.52.33062. [DOI] [PubMed] [Google Scholar]
- 55.Yepes M, Lawrence DA. Neuroserpin: a selective inhibitor of tissue-type plasminogen activator in the central nervous system. Thromb Haemost. 2004 Mar;91(3):457–464. doi: 10.1160/TH03-12-0766. [DOI] [PubMed] [Google Scholar]
- 56.Wu J, Echeverry R, Guzman J, Yepes M. Neuroserpin protects neurons from ischemia-induced plasmin-mediated cell death independently of tissue-type plasminogen activator inhibition. The American journal of pathology. 2010 Nov;177(5):2576–2584. doi: 10.2353/ajpath.2010.100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee TW, Coates LC, Birch NP. Neuroserpin regulates N-cadherin-mediated cell adhesion independently of its activity as an inhibitor of tissue plasminogen activator. Journal of neuroscience research. 2008 May 1;86(6):1243–1253. doi: 10.1002/jnr.21592. [DOI] [PubMed] [Google Scholar]
- 58.Lee TW, Tsang VW, Birch NP. Physiological and pathological roles of tissue plasminogen activator and its inhibitor neuroserpin in the nervous system. Front Cell Neurosci. 2015;9:396. doi: 10.3389/fncel.2015.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003 Oct;9(10):1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 60.Cheng T, Petraglia AL, Li Z, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nature medicine. 2006 Nov;12(11):1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 61.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood. 2015 May 7;125(19):2898–2907. doi: 10.1182/blood-2015-02-355974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proceedings of the National Academy of Sciences of the United States of America. 2006 Apr 25;103(17):6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pang PT, Teng HK, Zaitsev E, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004 Oct 15;306(5695):487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 64.Fredriksson L, Li H, Fieber C, Li X, Eriksson U. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 2004 Sep 29;23(19):3793–3802. doi: 10.1038/sj.emboj.7600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bu G, Williams S, Strickland DK, Schwartz AL. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herz J. LRP: a bright beacon at the blood-brain barrier. J Clin Invest. 2003 Nov;112(10):1483–1485. doi: 10.1172/JCI20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siao CJ, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J Neurosci. 2002 May 1;22(9):3352–3358. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Correa F, Gauberti M, Parcq J, et al. Tissue plasminogen activator prevents white matter damage following stroke. The Journal of experimental medicine. 2011 Jun 6;208(6):1229–1242. doi: 10.1084/jem.20101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carmeliet P, Schoonjans L, Kieckens L, et al. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994 Mar 31;368(6470):419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 70.Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the central nervous system. Front Cell Neurosci. 2015;9:304. doi: 10.3389/fncel.2015.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szabo R, Samson AL, Lawrence DA, Medcalf RL, Bugge TH. Passenger mutations and aberrant gene expression in congenic tissue plasminogen activator-deficient mouse strains. Journal of thrombosis and haemostasis: JTH. 2016 Apr 15; doi: 10.1111/jth.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Friedman GC, Seeds NW. Tissue plasminogen activator expression in the embryonic nervous system. Brain Res Dev Brain Res. 1994 Aug 12;81(1):41–49. doi: 10.1016/0165-3806(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 73.Krystosek A, Seeds NW. Plasminogen activator release at the neuronal growth cone. Science. 1981 Sep 25;213(4515):1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- 74.Lee SH, Ko HM, Kwon KJ, et al. tPA regulates neurite outgrowth by phosphorylation of LRP5/6 in neural progenitor cells. Mol Neurobiol. 2014 Feb;49(1):199–215. doi: 10.1007/s12035-013-8511-x. [DOI] [PubMed] [Google Scholar]
- 75.Seeds NW, Basham ME, Haffke SP. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proceedings of the National Academy of Sciences of the United States of America. 1999 Nov 23;96(24):14118–14123. doi: 10.1073/pnas.96.24.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stefanitsch C, Lawrence A-LE, Olverling A, Nilsson I, Fredriksson L. tPA deficiency in mice leads to rearrangement in the cerebrovascular tree and cerebroventricular malformations. Front Cell Neurosci. 2015 Nov 30;2015:9. doi: 10.3389/fncel.2015.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fredriksson L, Nilsson I, Su EJ, et al. Platelet-derived growth factor C deficiency in C57BL/6 mice leads to abnormal cerebral vascularization, loss of neuroependymal integrity, and ventricular abnormalities. The American journal of pathology. 2012 Mar;180(3):1136–1144. doi: 10.1016/j.ajpath.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chai F, Coates H. Otolaryngological manifestations of ligneous conjunctivitis. Int J Pediatr Otorhinolaryngol. 2003 Feb;67(2):189–194. doi: 10.1016/s0165-5876(02)00365-8. [DOI] [PubMed] [Google Scholar]
- 79.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001 Nov 2;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 80.Frey U, Muller M, Kuhl D. A different form of long-lasting potentiation revealed in tissue plasminogen activator mutant mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996 Mar 15;16(6):2057–2063. doi: 10.1523/JNEUROSCI.16-06-02057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang YY, Bach ME, Lipp HP, et al. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proceedings of the National Academy of Sciences of the United States of America. 1996 Aug 6;93(16):8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pawlak R, Nagai N, Urano T, et al. Rapid, specific and active site-catalyzed effect of tissue-plasminogen activator on hippocampus-dependent learning in mice. Neuroscience. 2002;113(4):995–1001. doi: 10.1016/s0306-4522(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 83.Seeds NW, Williams BL, Bickford PC. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995 Dec 22;270(5244):1992–1994. doi: 10.1126/science.270.5244.1992. [DOI] [PubMed] [Google Scholar]
- 84.Muller CM, Griesinger CB. Tissue plasminogen activator mediates reverse occlusion plasticity in visual cortex. Nat Neurosci. 1998 May;1(1):47–53. doi: 10.1038/248. [DOI] [PubMed] [Google Scholar]
- 85.Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998 Oct;21(4):813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- 86.Madani R, Hulo S, Toni N, et al. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. The EMBO journal. 1999 Jun 1;18(11):3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhuo M, Holtzman DM, Li Y, et al. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000 Jan 15;20(2):542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blau CW, Cowley TR, O’Sullivan J, et al. The age-related deficit in LTP is associated with changes in perfusion and blood-brain barrier permeability. Neurobiol Aging. 2012 May;33(5):1005 e1023–1035. doi: 10.1016/j.neurobiolaging.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 89.Hsu TM, Kanoski SE. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Front Aging Neurosci. 2014;6:88. doi: 10.3389/fnagi.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takano T, Tian GF, Peng W, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006 Feb;9(2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 91.Levin EG, del Zoppo GJ. Localization of tissue plasminogen activator in the endothelium of a limited number of vessels. The American journal of pathology. 1994 May;144(5):855–861. [PMC free article] [PubMed] [Google Scholar]
- 92.Cipolla MJ, Lessov N, Clark WM, Haley EC., Jr Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke; a journal of cerebral circulation. 2000 Apr;31(4):940–945. doi: 10.1161/01.str.31.4.940. [DOI] [PubMed] [Google Scholar]
- 93.Nassar T, Akkawi S, Shina A, et al. In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone. Blood. 2004 Feb 1;103(3):897–902. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- 94.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional Blood Flow in the Normal and Ischemic Brain Is Controlled by Arteriolar Smooth Muscle Cell Contractility and Not by Capillary Pericytes. Neuron. 2015 Jul 1;87(1):95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.An J, Haile WB, Wu F, Torre E, Yepes M. Tissue-type plasminogen activator mediates neuroglial coupling in the central nervous system. Neuroscience. 2014 Jan 17;257:41–48. doi: 10.1016/j.neuroscience.2013.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li B, Freeman RD. Neurometabolic coupling between neural activity, glucose, and lactate in activated visual cortex. J Neurochem. 2015 Nov;135(4):742–754. doi: 10.1111/jnc.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002 Nov;33(11):2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Z, Zhang L, Yepes M, et al. Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation. 2002 Aug 6;106(6):740–745. doi: 10.1161/01.cir.0000023942.10849.41. [DOI] [PubMed] [Google Scholar]
- 99.Yao Y, Tsirka SE. Truncation of monocyte chemoattractant protein 1 by plasmin promotes blood-brain barrier disruption. Journal of cell science. 2011 May 1;124(Pt 9):1486–1495. doi: 10.1242/jcs.082834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fredriksson L, Ehnman M, Fieber C, Eriksson U. Structural requirements for activation of latent platelet-derived growth factor CC by tissue plasminogen activator. J Biol Chem. 2005 Jul 22;280(29):26856–26862. doi: 10.1074/jbc.M503388200. [DOI] [PubMed] [Google Scholar]
- 101.Medcalf RL. Desmoteplase: discovery, insights and opportunities for ischaemic stroke. Br J Pharmacol. 2012 Jan;165(1):75–89. doi: 10.1111/j.1476-5381.2011.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012 Jan 3;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- 104.Verheijen JH, Caspers MP, Chang GT, de Munk GA, Pouwels PH, Enger-Valk BE. Involvement of finger domain and kringle 2 domain of tissue-type plasminogen activator in fibrin binding and stimulation of activity by fibrin. EMBO J. 1986 Dec 20;5(13):3525–3530. doi: 10.1002/j.1460-2075.1986.tb04678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weimar W, Stibbe J, Van Seyen AJ, Billiau A, De Somer P, Collen D. Specific lysis of an iliofemoral thrombus by administration of extrinsic (tissue-type) plasminogen activator. Lancet. 1981;8254:1018–1019. doi: 10.1016/s0140-6736(81)91217-4. [DOI] [PubMed] [Google Scholar]
- 106.Tsirka SE. Clinical implications of the involvement of tPA in neuronal cell death. J Mol Med. 1997 May;75(5):341–347. doi: 10.1007/s001090050119. [DOI] [PubMed] [Google Scholar]
- 107.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998 Feb;4(2):228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 108.del Zoppo GJ. tPA: a neuron buster, too? Nat Med. 1998 Feb;4(2):148–150. doi: 10.1038/nm0298-148. [DOI] [PubMed] [Google Scholar]
- 109.Mori T, Wang X, Kline AE, et al. Reduced cortical injury and edema in tissue plasminogen activator knockout mice after brain trauma. Neuroreport. 2001 Dec 21;12(18):4117–4120. doi: 10.1097/00001756-200112210-00051. [DOI] [PubMed] [Google Scholar]
- 110.Tanne D, Kasner SE, Demchuk AM, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation. 2002 Apr 9;105(14):1679–1685. doi: 10.1161/01.cir.0000012747.53592.6a. [DOI] [PubMed] [Google Scholar]
- 111.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004 Mar 6;363(9411):768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 112.Hill MD, Buchan AM. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. Cmaj. 2005 May 10;172(10):1307–1312. doi: 10.1503/cmaj.1041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24(1):1–10. doi: 10.1159/000103110. [DOI] [PubMed] [Google Scholar]
- 114.Kleindorfer D, de los Rios La Rosa F, Khatri P, Kissela B, Mackey J, Adeoye O. Temporal trends in acute stroke management. Stroke; a journal of cerebral circulation. 2013 Jun;44(6 Suppl 1):S129–131. doi: 10.1161/STROKEAHA.113.001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. Jama. 2015 Apr 14;313(14):1451–1462. doi: 10.1001/jama.2015.3058. [DOI] [PubMed] [Google Scholar]
- 116.Ahmed N, Wahlgren N, Grond M, et al. Implementation and outcome of thrombolysis with alteplase 3–4.5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol. 2010 Sep;9(9):866–874. doi: 10.1016/S1474-4422(10)70165-4. [DOI] [PubMed] [Google Scholar]
- 117.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012 Jun 23;379(9834):2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. Jama. 2013 Jun 19;309(23):2480–2488. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 119.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014 Nov 29;384(9958):1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yepes M, Sandkvist M, Wong MK, et al. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000 Jul 15;96(2):569–576. [PubMed] [Google Scholar]
- 121.Cinelli P, Madani R, Tsuzuki N, et al. Neuroserpin, a neuroprotective factor in focal ischemic stroke. Molecular and cellular neurosciences. 2001 Nov;18(5):443–457. doi: 10.1006/mcne.2001.1028. [DOI] [PubMed] [Google Scholar]
- 122.Tabrizi P, Wang L, Seeds N, et al. Tissue plasminogen activator (tPA) deficiency exacerbates cerebrovascular fibrin deposition and brain injury in a murine stroke model: studies in tPA-deficient mice and wild-type mice on a matched genetic background. Arteriosclerosis, thrombosis, and vascular biology. 1999 Nov;19(11):2801–2806. doi: 10.1161/01.atv.19.11.2801. [DOI] [PubMed] [Google Scholar]
- 123.Matys T, Strickland S. Tissue plasminogen activator and NMDA receptor cleavage. Nat Med. 2003 Apr;9(4):371–372. doi: 10.1038/nm0403-371. author reply 372–373. [DOI] [PubMed] [Google Scholar]
- 124.Samson AL, Nevin ST, Croucher D, et al. Tissue-type plasminogen activator requires a co-receptor to enhance NMDA receptor function. J Neurochem. 2008 Nov;107(4):1091–1101. doi: 10.1111/j.1471-4159.2008.05687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kidwell CS, Latour L, Saver JL, et al. Thrombolytic toxicity: blood brain barrier disruption in human ischemic stroke. Cerebrovasc Dis. 2008;25(4):338–343. doi: 10.1159/000118379. [DOI] [PubMed] [Google Scholar]
- 126.Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012 Jul;32(7):1207–1221. doi: 10.1038/jcbfm.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bardehle S, Rafalski VA, Akassoglou K. Breaking boundaries-coagulation and fibrinolysis at the neurovascular interface. Front Cell Neurosci. 2015;9:354. doi: 10.3389/fncel.2015.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000 Dec;20(12):1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 129.Copin JC, Merlani P, Sugawara T, Chan PH, Gasche Y. Delayed matrix metalloproteinase inhibition reduces intracerebral hemorrhage after embolic stroke in rats. Exp Neurol. 2008 Sep;213(1):196–201. doi: 10.1016/j.expneurol.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao BQ, Wang S, Kim HY, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nature medicine. 2006 Apr;12(4):441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 131.Copin JC, Bengualid DJ, Da Silva RF, Kargiotis O, Schaller K, Gasche Y. Recombinant tissue plasminogen activator induces blood-brain barrier breakdown by a matrix metalloproteinase-9-independent pathway after transient focal cerebral ischemia in mouse. Eur J Neurosci. 2011 Oct;34(7):1085–1092. doi: 10.1111/j.1460-9568.2011.07843.x. [DOI] [PubMed] [Google Scholar]
- 132.Rodriguez-Gonzalez R, Blanco M, Rodriguez-Yanez M, Moldes O, Castillo J, Sobrino T. Platelet derived growth factor-CC isoform is associated with hemorrhagic transformation in ischemic stroke patients treated with tissue plasminogen activator. Atherosclerosis. 2013 Jan;226(1):165–171. doi: 10.1016/j.atherosclerosis.2012.10.072. [DOI] [PubMed] [Google Scholar]
- 133.Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. Jama. 2014 Jun 18;311(23):2414–2421. doi: 10.1001/jama.2014.5990. [DOI] [PubMed] [Google Scholar]
- 134.Gurwitz JH, Gore JM, Goldberg RJ, et al. Risk for intracranial hemorrhage after tissue plasminogen activator treatment for acute myocardial infarction. Participants in the National Registry of Myocardial Infarction 2. Ann Intern Med. 1998 Oct 15;129(8):597–604. doi: 10.7326/0003-4819-129-8-199810150-00002. [DOI] [PubMed] [Google Scholar]
- 135.Turner RJ, Vink R. Combined tissue plasminogen activator and an NK1 tachykinin receptor antagonist: an effective treatment for reperfusion injury following acute ischemic stroke in rats. Neuroscience. 2012 Sep 18;220:1–10. doi: 10.1016/j.neuroscience.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 136.Su EJ, Fredriksson L, Kanzawa M, et al. Imatinib treatment reduces brain injury in a murine model of traumatic brain injury. Front Cell Neurosci. 2015 Oct 7;2015:9. doi: 10.3389/fncel.2015.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lewandowski SA, Nilsson I, Fredriksson L, et al. Presymptomatic activation of the PDGF-CC pathway accelerates onset of ALS neurodegeneration. Acta Neuropathologica. 2015:1–12. doi: 10.1007/s00401-015-1520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nature reviews Neurology. 2010 Jul;6(7):393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999 May 11;99(18):2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 140.Matsuoka Y, Kitamura Y, Taniguchi T. Induction of plasminogen in rat hippocampal pyramidal neurons by kainic acid. Neuroscience Letters. 1998 Aug 14;252(2):119–122. doi: 10.1016/s0304-3940(98)00562-x. [DOI] [PubMed] [Google Scholar]
- 141.Palmer AM, Marion DW, Botscheller ML, Swedlow PE, Styren SD, DeKosky ST. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J Neurochem. 1993 Dec;61(6):2015–2024. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 142.Armstead WM, Nassar T, Akkawi S, et al. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nat Neurosci. 2006 Sep;9(9):1150–1155. doi: 10.1038/nn1757. [DOI] [PubMed] [Google Scholar]
- 143.Armstead WM, Kiessling JW, Riley J, Cines DB, Higazi AA. tPA contributes to impaired NMDA cerebrovasodilation after traumatic brain injury through activation of JNK MAPK. Neurol Res. 2011 Sep;33(7):726–733. doi: 10.1179/016164110X12807570509853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sashindranath M, Samson AL, Downes CE, et al. Compartment- and context-specific changes in tissue-type plasminogen activator (tPA) activity following brain injury and pharmacological stimulation. Laboratory investigation; a journal of technical methods and pathology. 2011 Jul;91(7):1079–1091. doi: 10.1038/labinvest.2011.67. [DOI] [PubMed] [Google Scholar]
- 145.Sashindranath M, Sales E, Daglas M, et al. The tissue-type plasminogen activator-plasminogen activator inhibitor 1 complex promotes neurovascular injury in brain trauma: evidence from mice and humans. Brain: a journal of neurology. 2012 Nov;135(Pt 11):3251–3264. doi: 10.1093/brain/aws178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Orth K, Willnow T, Herz J, Gething MJ, Sambrook J. Low density lipoprotein receptor-related protein is necessary for the internalization of both tissue-type plasminogen activator-inhibitor complexes and free tissue-type plasminogen activator. The Journal of biological chemistry. 1994 Aug 19;269(33):21117–21122. [PubMed] [Google Scholar]
- 147.Ashcom JD, Tiller SE, Dickerson K, Cravens JL, Argraves WS, Strickland DK. The human alpha 2-macroglobulin receptor: identification of a 420-kD cell surface glycoprotein specific for the activated conformation of alpha 2-macroglobulin. The Journal of cell biology. 1990 Apr;110(4):1041–1048. doi: 10.1083/jcb.110.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiological reviews. 2008 Jul;88(3):887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yepes M, Sandkvist M, Coleman TA, et al. Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent. J Clin Invest. 2002 Jun;109(12):1571–1578. doi: 10.1172/JCI14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tan ML, Ng A, Pandher PS, et al. Tissue plasminogen activator does not alter development of acquired epilepsy. Epilepsia. 2012 Nov;53(11):1998–2004. doi: 10.1111/j.1528-1167.2012.03635.x. [DOI] [PubMed] [Google Scholar]
- 151.Hassanien SH, Awadalla MM, Saad AA, Aziz NAA. Tissue plasminogen activator in children with idiopathic and intractable epilepsies. Journal of Pediatric Neurology. 2010 Jan 01;8(2):193–197. [Google Scholar]
- 152.Schuele SU, Luders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008 Jun;7(6):514–524. doi: 10.1016/S1474-4422(08)70108-X. [DOI] [PubMed] [Google Scholar]
- 153.Janigro D. Does leakage of the blood-brain barrier mediate epileptogenesis? Epilepsy currents/American Epilepsy Society. 2007 Jul-Aug;7(4):105–107. doi: 10.1111/j.1535-7511.2007.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marchi N, Angelov L, Masaryk T, et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007 Apr;48(4):732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Friedman A, Kaufer D, Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009 Aug;85(2–3):142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marchi N, Tierney W, Alexopoulos AV, Puvenna V, Granata T, Janigro D. The etiological role of blood-brain barrier dysfunction in seizure disorders. Cardiovasc Psychiatry Neurol. 2011;2011:482415. doi: 10.1155/2011/482415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reddrop C, Moldrich RX, Beart PM, et al. Vampire bat salivary plasminogen activator (desmoteplase) inhibits tissue-type plasminogen activator-induced potentiation of excitotoxic injury. Stroke; a journal of cerebral circulation. 2005 Jun;36(6):1241–1246. doi: 10.1161/01.STR.0000166050.84056.48. [DOI] [PubMed] [Google Scholar]
- 158.Mayhan WG, Didion SP. Glutamate-induced disruption of the blood-brain barrier in rats. Role of nitric oxide. Stroke; a journal of cerebral circulation. 1996 May;27(5):965–969. doi: 10.1161/01.str.27.5.965. discussion 970. [DOI] [PubMed] [Google Scholar]
- 159.Tian GF, Azmi H, Takano T, et al. An astrocytic basis of epilepsy. Nature medicine. 2005 Sep;11(9):973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]