Abstract
Acute respiratory distress syndrome (ARDS) is a fatal clinical condition that can be caused by pulmonary and non-pulmonary diseases. Oxidative stress and inflammation play key roles in the development of ARDS. In this study, we investigated whether ferulic acid (FA), an anti-oxidant, was beneficial for prophylaxis of ARDS. We established an ARDS rat model using lipopolysaccharide (LPS) administration. Lung injury was assessed by lung wet/dry ratio and broncho-alveolar lavage fluid (BALF) analysis. Hematoxylin and eosin staining was performed to evaluate the histological changes of the lungs. Enzyme-linked immunosorbent assay (ELISA) and immunoblotting were performed to detect proteins in BALF and lung tissue, respectively. Pulmonary function was determined by testing the oxygen level in BALF. FA pretreatment significantly alleviated LPS-induced pulmonary histological changes. FA reversed LPS-induced changes of lung wet/dry ratio, total protein in BALF, P(A-a)O2, and PaO2/FiO2. In addition, LPS dramatically up-regulated the secretion of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and IL-10 in BALF (P < 0.01). However, pretreatment of FA significantly improved LPS-induced inflammation. We found that FA indeed reduced oxidative stress in the lungs by testing malondialdehyde level, myeloperoxidase level, and total anti-oxidant capacity. We also proved that FA inactivated multiple mitogen-activated protein kinase signaling pathways in the lungs. In conclusion, FA alleviated LPS-induced ARDS through its anti-inflammatory and anti-oxidant activities.
Keywords: animal model, inflammation, lung injury, reactive oxygen species, respiratory distress syndrome
Introduction
Acute respiratory distress syndrome (ARDS) is a severe clinical disorder that is caused by increased permeability of alveolar epithelial cells and pulmonary capillary endothelial cells, leading to consequent pulmonary edema and impairment of oxygenation.1 The physiopathology of ARDS is complicated. Oxidative stress and inflammatory response are two key players in ARDS development.2,3 As a common etiology, infection by bacteria or viruses can lead to local immune response including recruitment and activation of neutrophils and macrophages. Immune reaction is the main cause of lung injury. These immune cells release a great amount of cytokines and reactive oxygen species (ROS), further resulting in a cascade of up-regulation of inflammation and oxidative stress.2 The crosstalk between pro-inflammatory cytokines and ROS is complicated and interference of such cascade may help block the development of ARDS. Many studies have tried anti-inflammation and anti-oxidant strategies to treat or prevent ARDS. However, as potent anti-inflammatory drugs, the clinical role of corticosteroids is still under consideration.4 A number of other anti-inflammatory agents and anti-oxidants also failed to show improved outcomes in ARDS patients.5 Therefore, new agents for testing anti-inflammation/anti-oxidant theory in ARDS development and for potential treatment of this intractable disease are urgently needed.
Ferulic acid (FA) is extracted from natural plants and has various biological activities including anti-oxidant and anti-inflammation.6 Therefore, FA has been proven to be effective in many disease models such as depression, diabetes, ulcerative colitis, Alzheimer’s disease, epilepsy, and hepatotoxicity.7–12 FA was previously reported to be able to enhance nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) and up-regulate super oxide dismutase (SOD) expression.13,14 Meanwhile, FA could inactivate nicotinamide adenine dinucleotide phosphate, reduced (NADPH) oxidase, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and cyclooxygenase-2 (COX-2) and inhibit expression of several pro-inflammatory cytokines including interleukin (IL)-1β and IL-6.9,15 However, whether FA shows benefits in ARDS protection is currently unknown. Here, we established an ARDS rat model using lipopolysaccharides (LPS) administration and tested the effects of pretreatment of FA in histological changes and pulmonary functions. We also evaluated the efficacy of FA in reducing oxidative stress and inflammation in our ARDS model.
Materials and methods
Establishment of the rat model
Male Wistar rats weighing 200 ± 25 g were purchased from Animal Center in Hebei Medical University. The ARDS rat model was established according to the methods as previously described.16 Briefly, rats were anesthetized with pentobarbital and were treated with intratracheal instillation of LPS solution (2 mg/kg diluted in 100 μL normal saline; Sigma-Aldrich, St. Louis, MO, USA) for 24 h. Control rats were treated with an equal volume of vehicle. In FA-treated groups, FA (50 mg/kg diluted in 100 μL normal saline; Sigma-Aldrich) was intraperitoneally injected once per day for 30 consecutive days before LPS administration. The protocol of this study was reviewed and approved by the Ethics Committee of Xingtai People’s Hospital of Hebei Province. All cultural conditions and procedures of animals complied with the Guidelines for the Care and Use of Laboratory Animals.
Hematoxylin and eosin staining and pathological scoring
Pathology of the lungs was analyzed as previously described.17 Briefly, the lungs were harvested 24 h after LPS administration and were fixed with formalin solution. The dehydrated samples were embedded in paraffin and were cut into 5-μm-thick slides. Hematoxylin and eosin (H&E) staining was applied to the slides, which were then reviewed by an experienced pathologist. The pathological scores were evaluated according to the severity of five parameters including inflammation, edema, hemorrhage, atelectasis, and formation of hyaline membrane. The pathological changes of each parameter were scored from 0 (normal) to 4 (severe injury) under a microscope, and the final score of each sample was calculated by adding scores of all the five parameters.
Lung wet/dry ratio assessment
Lung wet weight was acquired by immediate weighing of the lungs after rats were sacrificed. The lungs were then washed with normal saline for three times to thoroughly remove the blood and were baked at 60°C for 72 h. The dry weight of each sample was acquired by weighing the baked lungs. The wet/dry ratio was calculated as the wet weight divided by the dry weight.16
Broncho-alveolar lavage fluid analysis
The broncho-alveolar lavage fluid (BALF) was harvested from each rat 24 h post LPS treatment. In brief, normal saline was used to wash the left lung for three times through a tracheal cannula. The flushing fluid was collected as much as possible. Cells and cell debris in the BALF were removed by centrifugation. Concentrations of IL-1β, IL-6, IL-10, and tumor necrosis factor (TNF)-α were determined using enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Arterial blood gas analysis
The arterial blood gas analysis was performed as previously described.17 Immediately after the rats’ arterial blood was drawn from carotid arteries, the partial pressure of oxygen in arterial blood (PaO2) and the fraction of inspired oxygen (FiO2) were determined using an automatic blood gas analyzer (Radiometer, Copenhagen, Denmark). Alveolar-arterial oxygen tension difference [P(A-a)O2] was calculated using the standard alveolar gas equation, and PaO2/FiO2 ratio was calculated as PaO2 divided by FiO2.
Oxidative stress evaluation
The oxidative stress of lung tissue was reflected by the malondialdehyde (MDA) level, total anti-oxidant capacity (TAOC), and myeloperoxidase (MPO) level as previously reported.18 The lungs in each rat were harvested as mentioned earlier, and tissue lysates were obtained. Levels of MDA and MPO were determined using ELISA kits (R&D Systems). TAOC was assessed using a colorimetric assay kit (BioVision Inc., Milpitas, CA, USA).
Immunoblotting
The homogenated tissue samples were lysed using radio immunoprecipitation assay (RIPA) lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with proteinase cocktail (Roche, Basel, Swiss) for total protein extraction.16 Totally, 40 μg proteins were loaded for electrophoresis. The proteins were then transferred to a nitrocellulose blotting membrane and were blocked with 5% non-fat milk in room temperature for 1 h. The following indicated primary antibodies were used to incubate the membrane overnight at 4°C: anti-phosphorylated-c-Jun N-terminal kinases (JNK), anti-JNK1/2, anti-phosphorylated-extracellular signal-regulated kinases (ERK), anti-ERK, anti-phosphorylated-p38, anti-p38, and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (all from Cell Signaling Technology, Danvers, MA, USA). The membrane was then incubated with anti-rabbit or anti-mouse secondary antibodies at room temperature for 1 h (Cell Signaling Technology). Enhanced chemiluminescence (Thermo Fisher Scientific) was used to detect the bands. The photodensity of bands was assessed using Image J (NIH, Bethesda, MD, USA), and the levels of phosphorylated protein were normalized to those of corresponding total proteins.
Statistical analysis
Data were presented as the mean ± standard deviation (SD). All statistical analyses were conducted using two-tailed Student’s t-test. P values less than 0.05 were considered statistically significant.
Results
FA improves pulmonary pathological changes in LPS-induced ARDS rats
To investigate the preventive role of FA in ARDS, we first pretreated the rats with FA once daily for 30 days. Then, the rats were challenged with LPS and were subjected to analyses after 24 h (Figure 1(a)). According to our previous pilot studies that tried different doses of FA, FA with a dose of 50 mg/kg showed significant effects without noticeable toxicities (data not shown). Here, we again confirmed that 50 mg/kg FA alleviated LPS-induced pathological changes in the lungs. H&E staining showed that LPS led to significant pulmonary inflammation and hyaline membrane formation, both of which were greatly improved in rats with prophylactic use of FA (Figure 1(b)). Histological score reflects the overall lung injury, and as expected, ARDS group showed a dramatic increase in the histological score. However, FA pretreatment significantly reduced the histological score (Figure 1(c)). These findings suggested that FA was able to improve ARDS-associated lung injury in the rat model.
Figure 1.
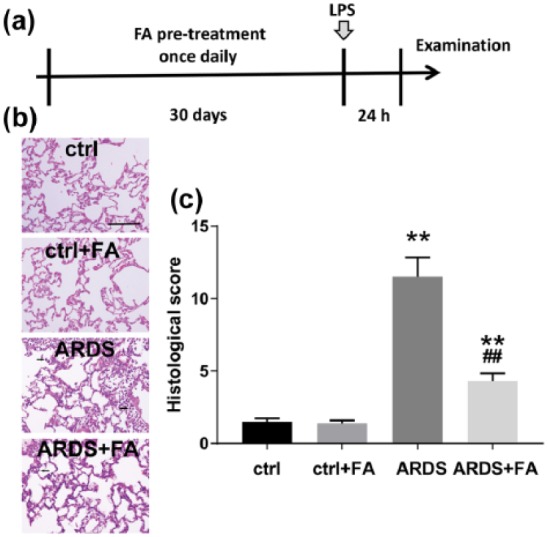
Ferulic acid (FA) alleviates histological changes of the lungs in lipopolysaccharide (LPS)-induced rats. (a) Schematic diagram of the experimental design in this study. Rats were pretreated with FA (50 mg/kg) for consecutive 30 days once per day by intraperitoneal injection and were then subjected to LPS administration (2 mg/kg). After 24 h, the rats were sacrificed for the following examination. FA pretreatment caused significant histological changes in acute respiratory distress syndrome (ARDS) rats, as evidenced by hematoxylin and eosin (H&E) staining in the lung tissues (b) and histological scoring (c).
N = 8. Data are presented as mean ± SD. **P < 0.01 compared to the control group (ctrl); ##P < 0.01 compared to the ARDS group. Scale bar, 50 μm. Black arrows indicate samples of abnormality compared to control.
FA rescues lung injury in LPS-treated rats
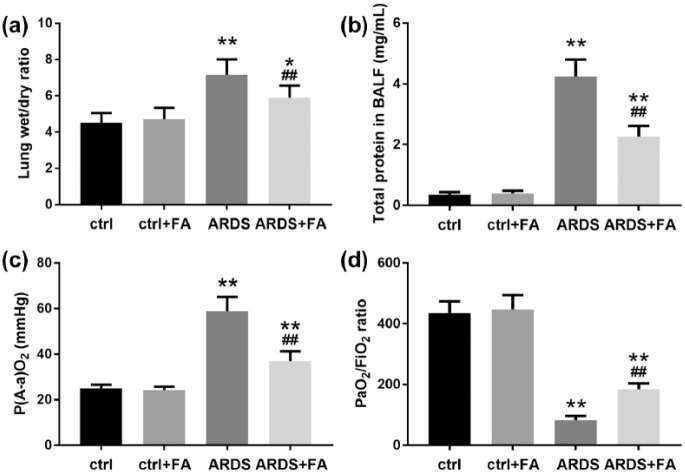
We further tested whether FA pretreatment improved pulmonary function by detecting four common parameters. Since increased permeability of the pulmonary capillary endothelium and alveolar epithelium results in pulmonary edema that can be evaluated by wet/dry ratio of the lung weights, we tested and found that LPS alone increased lung wet/dry ratio by around 50% (Figure 2(a)), suggesting severe pulmonary edema. FA pretreatment significantly reduced lung wet/dry ratio, though it was still higher compared to that of the control rats. Similar results were observed regarding the total proteins in BALF (Figure 2(b)). Furthermore, we assessed the blood oxygenation in the rats. Obviously, in the ARDS group, P(A-a)O2 increased; while in the presence of FA, P(A-a)O2 deceased to a large extent (Figure 2(c)). Consistently, PaO2/FiO2 in rats of ARDS group decreased greatly and was partially rescued by FA (Figure 2(d)). These findings suggested that LPS administration impaired oxygen diffusion, which could be improved by FA pretreatment.
Figure 2.
Pretreatment of FA effectively protects against lung injury in LPS-induced ARDS. Lung injury was evaluated by measuring (a) lung wet/dry ratio, (b) total protein level, (c) P(A-a)O2, and (d) PaO2/FiO2 ratio in broncho-alveolar lavage fluid, which was collected 24 h following LPS administration.
N = 8. Data are presented as mean ± SD. *P < 0.05; **P < 0.01 compared to the control group (ctrl); ##P < 0.01 compared to the ARDS group.
FA mitigates local inflammation in the lungs
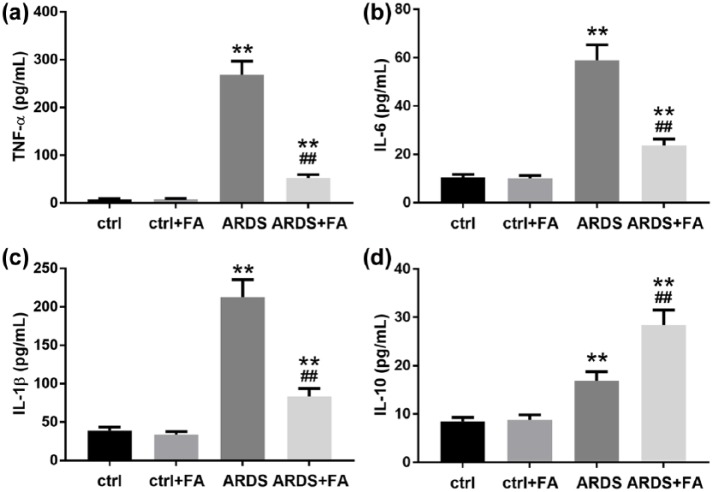
LPS is a potent inflammation inducer and can extensively enhance inflammation, which plays critical roles in ARDS development. We thus evaluated whether FA pretreatment diminished the pro-inflammatory effects of LPS in our rat model. Undoubtedly, the levels of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 in BALF increased dramatically; however, FA pretreatment significantly limited the secretion of these cytokines (Figure 3(a)–(c)). LPS also slightly increased the secretion of IL-10 probably due to a feedback effect induced by an acute inflammation (Figure 3(d)). FA further enhanced IL-10 level in the rats’ BALF, displaying an anti-inflammatory effect. These results together showed that long-term pretreatment of FA could limit acute inflammation caused by LPS.
Figure 3.
Pretreatment of FA relieves pulmonary inflammatory responses in LPS-induced ARDS. BALF was collected 24 h after LPS administration. Pro-inflammatory cytokines including (a) TNF-α, (b) IL-6, and (c) IL-1β and anti-inflammatory cytokine (d) IL-10 were determined by ELISA.
N = 8. Data are presented as mean ± SD. **P < 0.01 compared to the control group (ctrl); ##P < 0.01 compared to the ARDS group.
FA shows anti-oxidant effects in our ARDS rat model
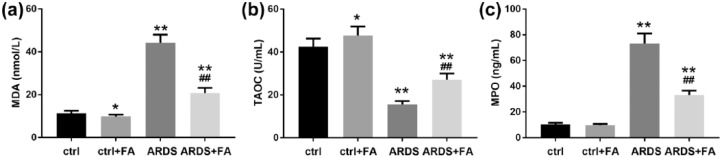
To test whether FA exerted anti-oxidant activity in preventing ARDS, we detected three common indicators of oxidative stress. MDA is an indicator of lipid peroxidation,19 which was found up-regulated in the lungs of our ARDS rats and this up-regulation was limited in the presence of FA (Figure 4(a)). In parallel, the reduced TAOC caused by LPS challenge was largely recovered when FA was pretreated before LPS administration (Figure 4(b)). Noticeably, this anti-oxidant activity of FA could be observed even in rats without LPS challenge, though the effects looked mild (Figure 4(a) and (b)). In addition, we detected the level of MPO, a key regulator of oxidative stress in pro-inflammatory cells,20 and it turned out that ARDS rats showed an MPO level of approximately six times higher than that of the control rats (Figure 4(c)). Altogether, these results confirmed that oxidative stress increased during ARDS development and could be suppressed by FA.
Figure 4.
Pretreatment of FA attenuates oxidative stress in LPS-induced ARDS rats. Oxidative stress was characterized by measuring the (a) malondialdehyde (MDA) level, (b) total anti-oxidant capacity (TAOC), and (c) myeloperoxidase (MPO) level in the lungs.
N = 8. Data are presented as mean ± SD. *P < 0.05; **P < 0.01 compared to the control group (ctrl); ##P < 0.01 compared to the ARDS group.
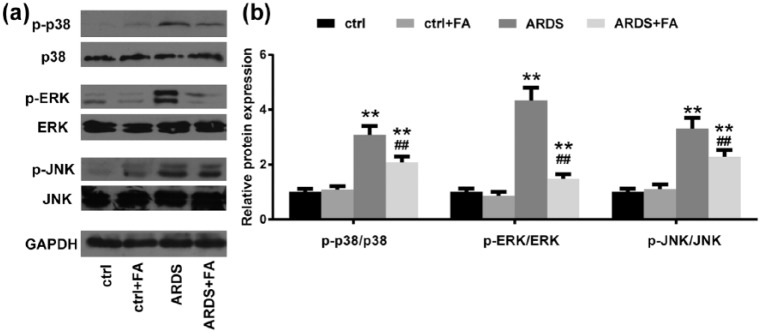
FA down-regulates mitogen-activated protein kinase signaling in LPS-treated rats
Oxidative stress contributes to lung injury during ARDS development,21 and mitogen-activated protein kinase (MAPK) signaling pathways play a key role in mediating the effects of oxidative stress.22 Given that FA is a potent anti-oxidant, we investigated whether FA functioned by blocking MAPK signaling. In healthy conditions, FA had a minimal effect in the phosphorylation of p38, ERK, and JNK (Figure 5(a) and (b)). In ARDS conditions, however, all the three kinases were dramatically up-regulated, indicating enhanced activation of MAPK signaling. When FA was applied to the ARDS rats in advance, the three kinases, especially ERK, were found less phosphorylated, suggesting that pretreatment of FA limited activation of MAPK signaling in the LPS-induced ARDS rat model.
Figure 5.
FA down-regulates mitogen-activated protein kinase (MAPK) signaling pathways in rats with LPS administration. (a and b) MAPK signaling pathway-related proteins (phosphorylated and total proteins of p38, ERK and JNK) were analyzed by immunoblotting. GAPDH was employed as the loading control.
N = 8. Data are presented as mean ± SD. *P < 0.05; **P < 0.01 compared to the control group (ctrl); ##P < 0.01 compared to the ARDS group.
Discussion
In clinic, ARDS is very intractable once developed and only support treatments such as mechanical ventilation and anti-thromboembolism are used.23 Although people now know that various factors such as inflammation and ROS are involved in ARDS development, few strategies have been successfully developed to limit its progression. This leads to high mortality and morbidity in patients with primary or secondary ARDS. Many studies have been focused on the enhanced oxidative stress and inflammatory response during ARDS development.24 In the last century, anti-oxidants N-acetylcysteine and procysteine were found to shorten the duration of acute lung injury.25 Supplement of vitamin C was also reported helpful in ARDS treatment.26 As a well-established anti-oxidant, FA was proven to be not only beneficial for improvement of pulmonary function but also helpful for recovery of histological changes of the lungs. We thus believe that FA and FA analogues may be promising agents for ARDS management in the future.
Up to date, FA has been shown effective in a variety of disease models with up-regulated oxidative stress because of distinct causes.9,12–15 However, no study had used FA for the purpose of ARDS prevention or treatment. LPS is commonly used to induce ARDS in animal models and is capable of inducing oxidative stress,27 and the etiology and physiopathology of LPS-induced ARDS are very similar to those in human patients. We thus hypothesized that FA may be useful in ARDS management. Since most studies in this field tried preventive effects of certain materials, we also tested the prophylactic role of FA in this study. As expected, FA pretreatment reserved the anti-oxidant activity in the injured lungs. Although subtle changes in MDA and TAOC levels were also observed in the absence of LPS, the potential of FA was significantly evoked when LPS existed. From this view, FA may be safely used in patients with a high risk of ARDS to provide anti-oxidant reserve. Thus, the prophylactic use of FA looks reasonable in such clinical scenarios.
Besides the anti-oxidative activity of FA, we also detected a weakened inflammatory response in the lungs. The decreased levels of TNF-α, IL-1β, and IL-6, together with the increased level of IL-10, suggested an immunomodulating effect of FA. Since the expression and secretion of these four cytokines are differently regulated (e.g. inflammasome is required for IL-1β but not other cytokines), it is not likely that FA directly modulates the expression of them at the same time. Therefore, we hypothesized that FA influences an upstream target that functions as an immunomodulator and finally inhibits inflammation. Studies performed in other disease models suggested that NF-κB and nucleotide binding and oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) were regulated by FA and showed very similar alterations of TNF-α, IL-1β, and IL-6.28 It is thus possible that FA also regulates NF-κB and NLRP3 in conditions of ARDS; however, this needs further investigations.
We further found that FA could be a potent MAPK inhibitor, showing significant inactivation of three main kinases in the signaling pathways. Intriguingly, it was previously reported that FA could activate p38 MAPK signaling in an ischemia-reperfusion rat model,29 while other studies supported that FA inhibited p38 MAPK signaling in neurons and microglia.30,31 Opposite effects of FA in ERK were also reported in lymphocytes in the presence or absence of radiation activation.32,33 These discrepancies indicate that the role of FA is highly dependent on disease models and suggest complicated effects of FA. Given the complicated influences caused by FA in our rat model, it can be speculated that FA may be a multi-target chemical. Therefore, the application of FA needs caution.
This study has some limitations. First, we only tested the efficacy of FA as a pretreatment. Whether FA has treatment effect when ARDS is already developed is unknown. Unfortunately, the initiation of ARDS is obscure and difficult to observe clinically.34 When signs and symptoms can be detected, it is usually hard to stop the development of ARDS. It can be foreseen that the treatment effect of FA may be not as good as the prophylactic use of FA. Second, we pretreated the rats with FA for 30 days, which is relatively a long term. The minimal duration that guarantees the efficacy of FA is currently unknown. Third, we used LPS, which is from gram-negative bacteria, to establish an ARDS rat model. We thus are unaware of whether FA is also effective in virus-induced ARDS, which is also a common clinical scenario. In addition, we only showed the possible efficacy of FA without investigating its targets and the detailed mechanisms by which FA functioned as an MAPK inhibitor. However, given the efficacy of FA in our model, further study is valuable to understand its molecular mechanisms in preventing ARDS.
We here demonstrate that FA can ameliorate ARDS-related lung injury through its anti-oxidant effect by interfering MAPK signaling pathways in a LPS-induced ARDS rat model. Mechanically, FA pretreatment can reduce inflammation and ROS level in the lungs. As far as we know, this is the first study showing that pretreatment of FA is able to restrain ARDS development and shed light on it clinical use. Other ARDS animal models are required to verify our conclusions, and the efficacy of FA in existing ARDS needs to be studied.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yalou Jiao  https://orcid.org/0000-0001-9478-2952
https://orcid.org/0000-0001-9478-2952
References
- 1. Koh Y. (2014) Update in acute respiratory distress syndrome. Journal of Intensive Care 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chow CW, Herrera Abreu MT, Suzuki T, et al. (2003) Oxidative stress and acute lung injury. American Journal of Respiratory Cell and Molecular Biology 29: 427–431. [DOI] [PubMed] [Google Scholar]
- 3. Crimi E, Slutsky AS. (2004) Inflammation and the acute respiratory distress syndrome. Best Practice & Research Clinical Anaesthesiology 18: 477–492. [DOI] [PubMed] [Google Scholar]
- 4. Khilnani GC, Hadda V. (2011) Corticosteroids and ARDS: A review of treatment and prevention evidence. Lung India 28: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adhikari N, Burns KE, Meade MO. (2004) Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database of System Reviews 18: CD004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar N, Pruthi V. (2014) Potential applications of ferulic acid from natural sources. Biotechnology Reports 4: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeni ALB, Camargo A, Dalmagro AP. (2017) Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice. Steroids 125: 131–136. [DOI] [PubMed] [Google Scholar]
- 8. Thyagaraju BM, Muralidhara (2008) Ferulic acid supplements abrogate oxidative impairments in liver and testis in the streptozotocin-diabetic rat. Zoological Science 25: 854–860. [DOI] [PubMed] [Google Scholar]
- 9. Sadar SS, Vyawahare NS, Bodhankar SL. (2016) Ferulic acid ameliorates TNBS-induced ulcerative colitis through modulation of cytokines, oxidative stress, iNOs, COX-2, and apoptosis in laboratory rats. EXCLI Journal 15: 482–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sultana R, Ravagna A, Mohmmad-Abdul H, et al. (2005) Ferulic acid ethyl ester protects neurons against amyloid beta-peptide(1–42)-induced oxidative stress and neurotoxicity: Relationship to antioxidant activity. Journal of Neurochemistry 92: 749–758. [DOI] [PubMed] [Google Scholar]
- 11. Hassanzadeh P, Arbabi E, Atyabi F, et al. (2017) Ferulic acid exhibits antiepileptogenic effect and prevents oxidative stress and cognitive impairment in the kindling model of epilepsy. Life Sciences 179: 9–14. [DOI] [PubMed] [Google Scholar]
- 12. Panneerselvam L, Subbiah K, Arumugam A, et al. (2013) Ferulic acid modulates fluoride-induced oxidative hepatotoxicity in male Wistar rats. Biological Trace Element Research 151: 85–91. [DOI] [PubMed] [Google Scholar]
- 13. Das U, Manna K, Khan A, et al. (2017) Ferulic acid (FA) abrogates gamma-radiation induced oxidative stress and DNA damage by up-regulating nuclear translocation of Nrf2 and activation of NHEJ pathway. Free Radical Research 51: 47–63. [DOI] [PubMed] [Google Scholar]
- 14. Das U, Biswas S, Sengupta A, et al. (2016) Ferulic acid (FA) abrogates ionizing radiation-induced oxidative damage in murine spleen. International Journal of Radiation Biology 92: 806–818. [DOI] [PubMed] [Google Scholar]
- 15. Cao YJ, Zhang YM, Qi JP, et al. (2015) Ferulic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NADPH oxidase and NF-kappaB pathway. International Immunopharmacology 28: 1018–1025. [DOI] [PubMed] [Google Scholar]
- 16. Lang S, Li L, Wang X, et al. (2017) CXCL10/IP-10 neutralization can ameliorate lipopolysaccharide-induced acute respiratory distress syndrome in rats. PLoS ONE 12: e0169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pan L, Yao DC, Yu YZ, et al. (2016) Necrostatin-1 protects against oleic acid-induced acute respiratory distress syndrome in rats. Biochemical and Biophysical Research Communications 478: 1602–1608. [DOI] [PubMed] [Google Scholar]
- 18. Zhu WW, Kong GQ, Ma MM, et al. (2016) Short communication: Camel milk ameliorates inflammatory responses and oxidative stress and downregulates mitogen-activated protein kinase signaling pathways in lipopolysaccharide-induced acute respiratory distress syndrome in rats. Journal of Dairy Science 99: 53–56. [DOI] [PubMed] [Google Scholar]
- 19. Del Rio D, Stewart AJ, Pellegrini N. (2005) A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutrition, Metabolism & Cardiovascular Diseases 15: 316–328. [DOI] [PubMed] [Google Scholar]
- 20. Nakazato T, Sagawa M, Yamato K, et al. (2007) Myeloperoxidase is a key regulator of oxidative stress mediated apoptosis in myeloid leukemic cells. Clinical Cancer Research 13: 5436–5445. [DOI] [PubMed] [Google Scholar]
- 21. Tasaka S, Amaya F, Hashimoto S, et al. (2008) Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxidants & Redox Signalling 10: 739–753. [DOI] [PubMed] [Google Scholar]
- 22. Lv H, Yu Z, Zheng Y, et al. (2016) Isovitexin exerts anti-inflammatory and anti-oxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-kappaB and activating HO-1/Nrf2 pathways. International Journal of Biological Sciences 12: 72–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silversides JA, Ferguson ND. (2013) Clinical review: Acute respiratory distress syndrome—Clinical ventilator management and adjunct therapy. Critical Care 17: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han S, Mallampalli RK. (2015) The acute respiratory distress syndrome: From mechanism to translation. Journal of Immunology 194: 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernard GR, Wheeler AP, Arons MM, et al. (1997) A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The antioxidant in ARDS study group. Chest 112: 164–172. [DOI] [PubMed] [Google Scholar]
- 26. Fowler Iii AA, Kim C, Lepler L, et al. (2017) Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World Journal of Critical Care Medicine 6: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sabarirajan J, Vijayaraj P, Nachiappan V. (2010) Induction of acute respiratory distress syndrome in rats by lipopolysaccharide and its effect on oxidative stress and antioxidant status in lung. Indian Journal of Biochemistry and Biophysics 47: 278–284. [PubMed] [Google Scholar]
- 28. Liu YM, Shen JD, Xu LP, et al. (2017) Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. International Immunopharmacology 45: 128–134. [DOI] [PubMed] [Google Scholar]
- 29. Cheng CY, Tang NY, Kao ST, et al. (2016) Ferulic acid administered at various time points protects against cerebral infarction by activating p38 MAPK/p90RSK/CREB/Bcl-2 anti-apoptotic signaling in the subacute phase of cerebral ischemia-reperfusion injury in rats. PLoS ONE 11: e0155748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin WC, Peng YF, Hou CW. (2015) Ferulic acid protects PC12 neurons against hypoxia by inhibiting the p-MAPKs and COX-2 pathways. Iranian Journal of Basic Medical Sciences 18: 478–484. [PMC free article] [PubMed] [Google Scholar]
- 31. Koshiguchi M, Komazaki H, Hirai S, et al. (2017) Ferulic acid suppresses expression of tryptophan metabolic key enzyme indoleamine 2, 3-dioxygenase via NFkappaB and p38 MAPK in lipopolysaccharide-stimulated microglial cells. Bioscience, Biotechnology and Biochemistry 81: 966–971. [DOI] [PubMed] [Google Scholar]
- 32. Ma ZC, Hong Q, Wang YG, et al. (2011) Ferulic acid induces heme oxygenase-1 via activation of ERK and Nrf2. Drug Discoveries & Therapeutics 5: 299–305. [DOI] [PubMed] [Google Scholar]
- 33. Ma ZC, Hong Q, Wang YG, et al. (2011) Ferulic acid protects lymphocytes from radiation-predisposed oxidative stress through extracellular regulated kinase. International Journal of Radiation Biology 87: 130–140. [DOI] [PubMed] [Google Scholar]
- 34. Laffey JG, Talmor D. (2013) Predicting the development of acute respiratory distress syndrome: Searching for the “Troponin of ARDS.” American Journal of Respiratory and Critical Care Medicine 187: 671–672. [DOI] [PubMed] [Google Scholar]