Abstract
Schizophrenia and bipolar disorder are among the leading causes of disability, morbidity, and mortality worldwide. In addition to being serious mental illness, these disorders are associated with considerable systemic physiological dysfunction, including chronic inflammation and elevated oxidative stress. The advent of sophisticated sequencing techniques has led to a growing interest in the potential role of gut microbiota in human health and disease. Advances in this area have transformed our understanding of a number of medical conditions and have generated a new perspective suggesting that gut microbiota might be involved in the development and maintenance of brain / mental health. Animal models have demonstrated strong though indirect evidence for a contributory role of intestinal microbiota in psychiatric symptomatology and have linked the microbiome with neuropsychiatric conditions. We present a systematic review of clinical studies of microbiome in schizophrenia and bipolar disorder. The published literature has a number of limitations; however, the investigations suggest that these disorders are associated with reduced microbial diversity and show global community differences compared to non-psychiatric comparison samples. In some reports, specific microbial taxa were associated with clinical disease characteristics, including physical health, depressive and psychotic symptoms, and sleep, but little information on the functional potential of those community changes. Studies also suggest increased intestinal inflammation and permeability, which may be among principal mechanism by which microbial dysbiosis impacts systemic physiological functioning. We highlight gaps in the current literature and implications for diagnosis and therapeutic interventions, and outline future directions for microbiome research in psychiatry.
Keywords: Psychosis, depression, gut, bacteria, microbes, inflammation, oxidative stress
Severe mental illnesses (SMI), mainly schizophrenia and bipolar disorder (BD), are a leading global cause of disability (Whiteford et al., 2013) and rank among the most substantial causes of death worldwide (Walker et al., 2015). Compared with the general population, people with these psychiatric disorders have higher rates of chronic medical conditions and die younger (Brown, 1997; Cuijpers and Smit, 2002; Harris and Barraclough, 1998; Roshanaei-Moghaddam and Katon, 2009; Saha et al., 2007). Excess deaths in these groups are not primarily from mental disorders themselves or suicide, but due to metabolic and cardiovascular diseases, cancers, and other chronic diseases (Casey et al., 2009; De Hert et al., 2009; Hennekens et al., 2005; Katon, 2003; Kupfer, 2005; Tsuang and Woolson, 1978). Even more concerning is the fact that the gap in longevity between people with schizophrenia and general population has increased 37% since the 1970s (Lee et al., in press). Despite the enormous burden of SMI, the underlying mechanisms associated with disease pathogenesis and progression are still not fully understood. The potential role of intestinal microbiota in the etiology of various human diseases has attracted considerable attention during the last decade. However, no article to our knowledge has systematically reviewed all the available studies of the microbiome in human, clinical populations of schizophrenia and BD. We highlight gaps in our knowledge, potential implications for diagnosis and therapeutic interventions, and outline future directions for microbiome research in psychiatry.
I. Why Microbiome?
The microbiome is a dynamic ecological community of microorganisms and their genes, including mainly bacteria, but also archaea, microbial eukaryotes, fungi, and viruses that inhabit the human body. For decades, the importance of the human microbiome remained elusive, due to technical challenges in studying unculturable microorganisms (Pace, 1997; Qin et al., 2010; Sogin et al., 1972; Woese and Fox, 1977). Only with the advent of high-throughput sequencing techniques has it become apparent that the microbiome is a rich and diverse ecosystem with implications for human health and disease (Human Microbiome Project, 2012; Knight et al., 2017). Humans, on average, harbor at least as many bacteria as the “human” cells in our bodies (Sender et al., 2016). Of the many distinct environments inhabited by microbes (e.g., skin, mouth, upper gastrointestinal tract), the distal large intestine has the greatest microbial biomass, with 1,000+ unique bacterial species and between 2 million and 20 million unique genes, which dwarf the human genome (20,000 genes) by more than 100:1 (Costello et al., 2009; Human Microbiome Project, 2012; Qin et al., 2010). Unlike the human genome, which is fixed from birth, the microbiome is a dynamic environment that is highly variable over time (Caporaso et al., 2011) and can be shaped by development (from birth through old age) (Dominguez-Bello et al., 2010; Koenig et al., 2011; Yatsunenko et al., 2012) and in response to intrinsic (e.g., immune system) and extrinsic (e.g., diet, exposure to drugs/medications, or physical geography) environmental factors. Thus, the microbiome is potentially more easily modifiable than human genome.
The microbiome has emerged as the “new” biomarker of human health. It is critical in maintaining host physiology, particularly in developing and shaping the immune system (Duerkop et al., 2009; Forsythe and Bienenstock, 2010). The human microbiome has been shown to have a pivotal role across a range of medical conditions including gastrointestinal (GI) disorders, such as inflammatory bowel disease (Kostic et al., 2014), obesity and metabolic diseases (Bouter et al., 2017; Hartstra et al., 2015), cancer (Schwabe and Jobin, 2013), and chronic pulmonary diseases (Budden et al., 2017; O’Dwyer et al., 2016), to name a few. Parallels can be drawn between these medical disorders and SMI. Gut (Severance et al., 2015) and metabolic (De Hert et al., 2009; Hennekens et al., 2005) dysfunction is highly prevalent in SMI, and cardiovascular, cerebrovascular, and digestive diseases rank as the top three leading causes of natural death in schizophrenia (Saha et al., 2007). Thus, schizophrenia and BD are not just severe mental illnesses but also severe physical illnesses (Jeste et al., 2011). As microbial colonization of the gut is critical for the development of the immune system (Round and Mazmanian, 2009), imbalance and/or depletion of the intestinal ecosystem may alter immune responses (Kamada et al., 2013) and contribute to systemic physiological dysfunctions, including elevated inflammation and oxidative stress, seen in these disorders (Berk et al., 2011; Flatow et al., 2013; Kirkpatrick and Miller, 2013). Therefore, microbiome research may contribute to a greater understanding of the pathogenesis and treatment of chronic mental illnesses.
II. Preclinical Studies of the Microbiome in Neuropsychiatric Disorders
In this ever-expanding field, researchers are now investigating how the intestinal microbiota influence distal sites, particularly the brain. In psychiatric disorders, the gut microbiome has been of particular interest because it plays a significant role in brain function and behavior (Diaz Heijtz et al., 2011), which has led to coining of the term “gut-brain axis.” The mechanisms by which peripheral intestinal microorganisms are linked to emotional and cognitive functions of the brain are not fully understood, but they are thought to include the vagus nerve, gut hormone signaling, the immune system, tryptophan metabolism, and microbial metabolites such as short-chain fatty acids (Cryan and Dinan, 2012). Investigations using germ-free animals have been critical in allowing direct assessment of the microbiome’s impact on different aspects of behavior relevant to psychiatric disorders, including depression and anxiety. It is beyond the scope of this article to review all the preclinical articles relevant to brain and behavior; these have been more comprehensively reviewed elsewhere (Cryan and Dinan, 2012). However, we highlight some of the landmark studies that have shaped our understanding of the gut-brain axis.
Sudo and colleagues (2004) first demonstrated that the intestinal microbiota could modulate the hypothalamus-pituitary-adrenal (HPA) axis. In their study, germ-free mice exposed to mild stress displayed elevated adrenocorticotrophic hormone and corticosterone release compared to control mice with normal gut microflora. This stress response could be fully reversed by reconstitution with Bifidobacterium infantis and partially reversed by colonization with fecal matter from control mice. Subsequent studies have also shown that germ-free mice have reduced anxiety-like behavior and altered levels of neurotransmitters and neurotrophic factors in the brain (Clarke et al., 2013; Crumeyrolle-Arias et al., 2014; Heijtza et al., 2011; Neufeld et al., 2011). In an innovative translational study, Zheng and colleagues (2016) incorporated both preclinical and clinical samples to assess how gut microbiota physiologically influence psychobehavioral characteristics and determine whether alterations of gut microbiota may have a causal role in depression-like behavior. The investigators demonstrated that germ-free mice display decreased depression-like behavior, and that patients diagnosed with major depressive disorder (MDD) have microbiota compositions that were significantly different from non-psychiatric comparison subjects, characterized by changes in the relative abundance of Firmicutes, Actinobacteria, and Bacteroidetes. Furthermore, fecal microbiota transplantation (FMT) of germ-free animals with the microflora derived from MDD patients resulted in increased depression-like behaviors compared to mice that had received FMT from healthy control individuals. Similar results were replicated in an independent study in which FMT from MDD patients to germ-free rats induced behavioral and physiological features characteristic of depression in the recipient animals, including anhedonia, anxiety-like behaviors, and alterations in tryptophan metabolism (Kelly et al., 2016). These experiments provide compelling evidence that the gut microbiota can physiologically induce depression- and anxiety-like behavior in animals.
An emerging body of research is also beginning to link the intestinal microbiota with neurodevelopmental and neurodegenerative disorders. Studies have demonstrated differences in the gut microbial composition of children with autism spectrum disorders compared to typically developing children (Ding et al., 2017). In a notable study using a maternal immune activation (MIA) mouse model that is known to display features of autism, Hsiao et al. (2013) demonstrated that certain microbial shifts in the gut led to subsequent onset of behavioral changes consistent with the clinical picture of autism. Alongside microbial dysbiosis, defects in intestinal permeability and elevated inflammatory cytokines were also observed. Treatment with the commensal bacterium Bacteroides fragilis, which had been previously demonstrated to restore GI pathology through immunomodulatory effects in mouse models of colitis (Mazmanian et al., 2008), reversed the physiological, neurological, metabolic, and immunological abnormalities. Importantly, findings from this study suggest leaky gut and related elevations in pro-inflammatory cytokines as potential mechanisms for intestinal dysbiosis and by which commensal bacteria may improve GI and behavioral abnormalities in autism. On the other end of the developmental spectrum, patients with Parkinson’s disease (PD) display altered microbiomes (Hasegawa et al., 2015; Keshavarzian et al., 2015; Scheperjans et al., 2015), and the microbiome has been shown to promote progression to PD in genetically susceptible individuals. Using mice that overexpress α-synuclein, a neuropathological protein relevant to Parkinsonian movement disorders, Sampson and colleagues (2016) demonstrated that germ-free strains of these animals do not display characteristic motor deficits, microglial activation, and α-synuclein pathology characteristic of PD. Remarkably, colonization of α-synuclein-overexpressing mice with the microflora from patients with PD enhances motor dysfunction in these animals, compared to microbiota transplants from healthy human donors.
One study investigated the gut microbiota in a putative partial rat model of schizophrenia. Jorgensen and colleagues (2015) observed that sub-chronic phencyclidine treatment impaired novel object recognition and increased locomotor sensitivity—the type of neurobehavioral impairment that is consistent with schizophrenia, and altered the gut microbiota composition of treated animals. Furthermore, the microbiota profiles correlated with object recognition memory, suggesting that pathological gut microbiome may contribute to cognitive impairment.
Together, these preclinical studies indicate that changes in the gut microbiome impact mental and neurological health. Nevertheless, questions that need to be further addressed are whether dysbiosis is disease-specific and whether it is secondary to altered neural regulation of gut functions and/or if primary gut aberrations impact brain development and function.
III. A Systematic Review of Clinical Studies of the Microbiome in Schizophrenia and Bipolar Disorder
We conducted a systematic review of studies of the microbiome in patients with schizophrenia and BD. We searched PubMed, PsycINFO, and Embase for articles published before May 3, 2017 using the following search string: microbiome AND (schizophrenia OR psychosis OR bipolar OR severe mental illness). We considered studies that utilized high-throughput sequencing methods to characterize microorganisms in individuals clinically diagnosed with schizophrenia, schizoaffective disorder, BD, or related psychotic disorders.
Review Process
Our database search yielded 123 articles. The titles and abstracts of all articles were screened and, of these, 19 met inclusion criteria for further review. Three additional articles were obtained from the references of identified papers. In total, 22 full-text articles were assessed for eligibility, and 5 met the above-mentioned criteria for review.
Because so few studies have been published on microbiome in schizophrenia and BD, we also considered investigations that did not use high-throughput sequencing but indirectly measured other biomarkers that may reflect organisms within the gut microbiome and/or suggest alterations to the intestinal environment. These included studies of markers of microbial translocation (i.e., the passage of indigenous gut microbes from the gastrointestinal tract to extra-intestinal sites, such as the bloodstream). Based on this expansion of the inclusion criteria, five additional studies reviewed. Their inclusion provides additional context for the microbiome studies and implicate a potential mechanism by which the gut microbiome may contribute to systemic immunological dysfunction. The PRISMA flow chart depicting information through different stages of the systematic review is shown in Figure 1.
Figure 1.
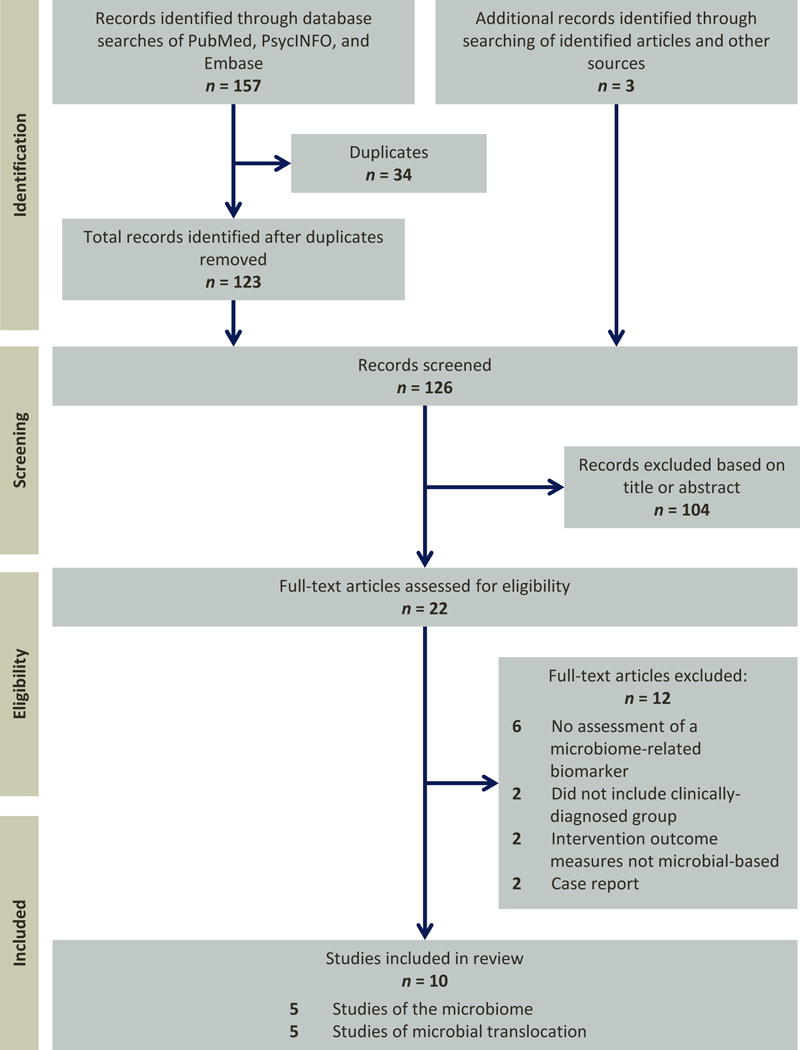
PRISMA flow diagram for selection of published articles for review.
Characteristics of Reviewed Studies
Study characteristics and summary statistics for the 10 reviewed studies are presented in Table 1. Overall, the mean sample size for clinical samples across studies was 103, with mean age of 35.7 years and mean gender ratio (M/F) of 1.3. Five investigations used high-throughput sequencing technology to characterize participants’ microbiota. Of these, three were investigations of the gut microbiome through fecal sampling, and two examined oropharyngeal microbiome by swabbing the posterior pharynx. Four studies investigated exposure to fungal pathogens by measuring serum levels of immunoglobulin G antibodies, and one study quantified serological protein markers of bacterial translocation. In terms of clinical populations examined, four investigations included individuals with schizophrenia and/or schizoaffective disorder, three studies assessed individuals with BD, and three articles focused on a mixed sample of individuals with schizophrenia and BD.
Table 1.
Summary of Study Characteristics for Reports in Review
| Clinical Sample | Comparison Sample | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Sample Characteristics | Mean | Median | Range | Mean | Median | Range |
| Studies of the Microbiome | ||||||
|
| ||||||
| Number of Participants | 49.2 | 41.0 | 16–115 | 39.6 | 33.0 | 16–69 |
| Mean Age (years) | 39.2 | 39.2 | 26-50 | 38.7 | 34.3 | 28–52 |
| Gender Ratio (M/F) | 1.1 | 1.3 | 0.35–1.9 | 0.94 | 1.0 | 0.44–1.4 |
|
| ||||||
| Studies of the Microbial Translocation | ||||||
|
| ||||||
| Number of Participants | 124.8 | 78.0 | 30–270 | 119.6 | 70.5 | 26–277 |
| Mean Age (years) | 34.2 | 34.6 | 22–45 | 35.1 | 33.3 | 29–48 |
| Gender Ratio (M/F) | 1.4 | 1.4 | 0.4–3.2 | 1.2 | 0.77 | 0.4–4.7 |
F = females; M = males
We highlighted findings related to global community diversity, taxonomic differences, and clinical and disease-related characteristics that may be associated with microbiome alterations in SMI. Summary information for these studies is provided in Table 2.
Table 2.
Studies of the microbiome in patients with schizophrenia and bipolar disorder
| Publication | Sample Size | Mean Age | Gender | Location | Diversity | Taxonomic Differences1 | Association with clinical features | Limitations |
|---|---|---|---|---|---|---|---|---|
| Castro-Nallar et al., 2015 | SZ: 16 NC: 16 |
SZ: 34.7 (SD=4.8) NC: 34.3 (SD=10.1) |
SZ: 9M:7F NC: 9M:7F |
Oral | Alpha diversity: SZ ↓ richness (Observed, Chaol, ACE, Fisher); ↑ evenness (Simpson, Inverse Simpson). Groups equivalent in species richness after accounting for species evenness (Shannon Index) Beta diversity: different between SZ and NC; grouping may be influenced, in part, by cigarette smoking (Jensen-Shannon distance) |
Phyla: Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria dominated both groups; SZ ↑ Ascomycota and Firmicutes; NC ↑ Bacteroidetes and Actinobacteria Species: SZ ↑ Lactobacillus gasseri, Catenibacterium mitsuokai, Eubacterium hallii, Candida dubliniensis, Lactobacillus salivarius, Bifidobacterium pseudocatenulatum, Streptococcus gordonii, Streptococcus thermophilus, Streptococcus sp. (oral taxon 071), and Bifidobacterium breve; ↓ Neisseria, Haemophilus, and Capnocytophaga |
N/A | – Small sample size – All NCs were non-smokers, which might confound the effects of SZ from those of smoking on microbiome composition – Did not examine differential relationship of community diversity/specific taxa to demographic or clinical/disease-specific features between groups |
| Evans et al., 2017 | BD: 115 NC: 64 |
BD: 50.2 (SD=12.8) NC: 48.6 (SD=16.6) |
BD: 32M:83F NC: 24M:40F |
Fecal | Beta diversity: different between BD and NC (Yue and Clayton distance). | BD ↓ Faecalibacterium, an unclassified member from the Ruminococcaceae family | Faecalibacterium associated with ↑ physical health, ↓ depression, ↑ sleep quality; Anaerostipes and Ruminococcaceae associated with ↑ physical health, Enterobacteriaceae associated with ↓ physical health. | – Did not report alpha diversity – Requires further examination of associations with disease-specific features (e.g., duration of illness, number of mood episodes) – Did not quantify the amount of or investigate the effects of medication use among BD – Used mean scores on self-report scales rather than assessment(s) most temporally related to time of stool collection |
| Flowers et al., 2017 | BD on AP treatment: 46 BD off AP treatment: 69 |
BD on AP treatment: 46.0 (SD=12.0) BD off AP treatment: 51.7 (SD=13.5) |
BD on AP treatment: 12M:34F BD off AP treatment: 21M:48F |
Fecal | Alpha diversity: AP-treated women ↓ diversity (Simpson Diversity Index); no differences between BD men. Beta diversity: different between BD medication groups (Yue and Clayton distance). OTUs identified for differing directions of the medication groups: Lachnospiraceae, Alistipes, and Akkermansia. |
AP-treated patients ↑ Lachnospiraceae Non-AP-treated patients ↑ Akkermansia and Sutterella |
Akkermansia ↓ in non-obese AP-treated patients. | – Further examination of relationship with duration of illness or other indicators of disease or symptom severity – Further investigation of relationship of comorbid medical conditions or other metabolic biomarkers on microbiome, given the relationship between atypical APs and metabolic disease – No information regarding diet, which is an important environmental factor that drives gut microbial composition and as atypical APs could increase appetite |
| Schwartz, et al., 2017 | FEP: 28 NC: 16 |
FEP: 25.9 (SD=5.5) NC: 27.1 (SD=6.0) |
FEP: 16M:12F NC: 8M:8F |
Fecal | N/A | Families: FEP ↑ Lactobacillaceae, Halothiobacillaceae, Brucellaceae and Micrococcineae, ↓ Veillonellaceae Genera: FEP ↑ Lactobacillus, Tropheryma, Halothiobacillus, Saccharophagus, Ochrobactrum, Deferribacter and Halorubrum, ↓ Anabaena, Nitrosospira, and Gallionella |
Lachnospiraceae, Bacteroides spp., Lactobacillus correlated with ↑ psychotic symptoms. Lachnospiraceae, Bacteroides spp., and predominant bacteria [identified by the authors Lachnospiraceae (Eubacterium rectale group), Ruminococcaceae (Clostridium leptum group), Bacteroides spp., Atopobium group, bifidobacteria, Lactobacillus-group (genera Lactobacillus, Leuconostoc, Pediococcus, and Weissella)] associated with ↑ negative symptoms. Lactobacillus correlated with ↑ positive symptoms. Ruminococcaceae, Bacteroides spp., Lactobacillus, and predominant bacteria associated with ↓ GAF. Duration of AP treatment not correlated with bacteria. Subgroup of FEP with greatest differences in composition from NCs showed ↑ negative symptoms and ↓ GAF but not with positive symptoms. Microbiota clustering associated with ↑ remission at 12-month follow-up; 70% FEP that clustered with NCs showed remission, compared to only 28% of patients with “abnormal,” even after controlling for baseline GAF. |
– Small sample size – No community-level characteristics reported (alpha and beta-diversity) – Model predicting remission only used top 5 families rather than the entire population – More specific information about/examination of the impact of AP medication use – Further examination of relationship with metabolic and inflammatory biomarkers collected – No information regarding diet |
| Yolken et al., 2015 | SZ: 41 NC: 33 |
SZ: 39.2 (SD=9.9) NC: 30.9 (SD=8.8) |
SZ: 27M:14F NC: 19M:14F |
Oral | N/A | SZ ↑ Lactobacillus phage φ adh, after adjusting for age, gender, race, maternal education, BMI, and cigarette smoking. | Lactobacillus phage φ adh marginally associated with ↑ positive symptoms and ↑ comorbid immunological disease. SZ not receiving valproate had greater Lactobacillus phage compared to those on the medication; no other significant associations with other medications, including AP, were found. Levels not associated with negative symptoms, length of illness, number of hospitalizations, AP medications, or other medical condition. | – Did not characterize within- or between-group diversity of the virome/phageome – While cigarette smoking was adjusted for in the model, there was only a single NC subject who was a smoker. |
↑↓ arrows indicate increase or decrease in relative abundance, when referring to taxonomic differences
ACE = Abundance-based Coverage Estimator; AOS = age of onset; AP = antipsychotic medication; ASCA = Anti-Saccharomyces cerevisiae; BD = bipolar disorder; BMI = body mass index; DOI = duration of illness; F = female; FEP = first-episode patients; GAF = Global Assessment of Functioning; GI = gastrointestinal; NC = non-psychiatric comparison group; M = male; SD = standard deviation; SZ = schizophrenia;
Clinical Studies of the Microbiome in Schizophrenia
Only one study has investigated gut microbiome in schizophrenia and related psychotic disorders. Schwartz and colleagues (2017) observed that patients with first-episode psychosis exhibited an altered taxonomic signature compared to non-psychiatric comparison subjects (NCs), with a relatively increased abundance of the families Lactobacillaceae, Halothiobacillaceae, Brucellaceae, and Micrococcineae, and decreased Veillonellaceae. Particularly, Lactobacillaceae were overrepresented among the taxa that were most strongly increased in patients. Importantly, gut microbial composition was associated with severity of psychotic symptoms and global functioning in patients at the time of hospitalization for first-episode psychosis. Notably, first-episode patients who showed the greatest abnormalities in microbiota composition from NCs at the time of hospitalization showed lower rate of disease remission at one-year follow-up (28% compared to 70% who clustered within the distribution of NCs). The model to find this separation was constructed from five families that were most significantly different between groups, as identified by LEfSe method (Segata et al., 2011) using partial least squares regression, and was robust to potential confounders such as baseline level of global functioning, level of physical activity, body mass index, duration of antipsychotic treatment, and food intake.
Two studies investigated the oropharyngeal microbiome. Patients with schizophrenia showed decreased oral microbial biodiversity (Castro-Nallar et al., 2015), known as alpha diversity or species richness and evenness in ecological terms, which is a measure of the total number and distribution of species in a community. It is commonly observed that high diversity is the hallmark of a healthy microbiome (Yatsunenko et al., 2012). Additionally, the overall microbial composition of schizophrenia patients was significantly different from NCs, although the latter finding might have been confounded by increased cigarette smoking in the schizophrenia group (Castro-Nallar et al., 2015). Patients with schizophrenia had higher relative proportions of Firmicutes in comparison to NCs, who had higher proportions of Bacteroidetes and Actinobacteria. At the genus level, lactic acid bacteria (Lactobacillus and Bifidobacterium) were relatively more abundant in schizophrenia, particularly species Lactobacillus gasseri. Notably, lactic acid bacteria are often considered health-promoting and anti-inflammatory, and are commonly found in the composition of probiotics (Naidu et al., 1999). These taxa have been shown to enhance immunity following supplementation (Schiffrin et al., 1997; Sheih et al., 2001). Their relatively increased presence in this population is surprising given that schizophrenia and BD have been associated with chronic inflammatory states (Berk et al., 2011; Kirkpatrick and Miller, 2013). Conversely, another study observed elevated levels of Lactobacillus phage phiadh in patients with schizophrenia, which is a bacteriophage that preferentially infects the bacteria Lactobacillus gasseri; this study also observed a positive association between colonization with Lactobacillus phage and co-morbid immunological diseases (Yolken et al., 2015). These conflicting results may be due to differences between populations, as schizophrenia is a very heterogeneous disease, or underscore the complexity of community relationships within the microbiota. They may also highlight the current challenges of microbiome data analysis, where the risk of false positive discovery can be difficult to avoid due to considerable between- and within-subject variability at the phylogenetic level in the human microbiota over time (Human Microbiome Project Consortium, 2012). However, the functional composition (i.e., functional potential of the gene content of the metagenome) of the gut microbiota is much more stable, and may, therefore, provide greater insight into the role of the microbiome in disease than taxonomic profiling (Human Microbiome Project Consortium, 2012). Indeed, Castro-Nallar and colleagues (2015) characterized the functionality of the oral microbiota and found that pathways associated with schizophrenia were related to metabolite transport systems, while those associated with NCs were related to energy metabolism.
Clinical Studies of the Microbiome in Bipolar Disorder
Global gut microbial community differences have also been observed in BD, with decreased fractional representation of Faecalibacterium and an unclassified member from the Ruminococcaceae family, both from the phylum Firmicutes (Evans et al., 2016). Among these patients, increased Faecalibacterium was associated with improved physical health, reduced depressive symptoms, and better sleep. Notably, Ruminococcaceae and Faecalibacterium have also been observed to be relatively decreased in the gut microbiome of patients with MDD, and levels of Faecalibacterium were negatively associated with depressive symptoms (Jiang et al., 2015). This finding is consistent with literature in several other medical conditions and disease states—including IBD (Schwiertz et al., 2010; Sokol et al., 2009), colorectal cancer (Balamurugan et al., 2008), and diabetes (Furet et al., 2010; Zhang et al., 2013), all of which express lower gut Faecalibacterium—suggesting that increased abundance of this genus is associated with better health. A second study from the same group also demonstrated that atypical antipsychotic treatment was associated with reduced gut biodiversity, particularly in female patients (Flowers et al., 2016). Patients on atypical antipsychotic treatment showed specific taxonomic shifts, with relatively increased levels of Lachnospiraceae, while non-treated individuals had preferentially higher levels of Akkermansia.
Intestinal Inflammation in Schizophrenia and Bipolar Disorder
Several clinical studies have also investigated blood-based biomarkers of microbial translocation (Table 3). Patients with schizophrenia and BD exhibited higher serum antibody levels to fungal pathogens Saccharomyces cerevisiae and Candida albicans (Severance et al., 2012; Severance et al., 2016; Severance et al., 2014) and soluble CD14 (sCD14)(Severance et al., 2013), a protein marker of bacterial translocation. When considering the impact of psychotropic medications on these biomarkers, levels of antibodies to Saccharomyces cerevisiae were higher in first-episode psychosis patients who were antipsychotic-naïve compared to those receiving antipsychotic treatment (Severance et al., 2012). Otherwise, antibodies to C. albicans, sCD14, and lipopolysaccharide binding protein did not differ among patients receiving different types of psychiatric medication (including lithium, valproate, or antipsychotics) (Severance et al., 2016; Severance et al., 2013; Severance et al., 2014).
Table 3.
Studies of microbial translocation in patients with schizophrenia and bipolar disorder
| Publication | Sample Size | Mean Age | Gender | Biomarker | Differences between SZ/BD and comparison groups | Association with clinical features | Limitations |
|---|---|---|---|---|---|---|---|
| Severance et al., 2012 |
Cohort #1 SZ recent: 67 SZ non-recent: 193 NC: 207 |
SZ recent: 22.3 (SD=5.32) SZ non-recent: 42.0 (SD=11.8) NC: 32.1 (SD=11.5) |
SZ recent: 51M:16F SZ nonrecent: 114M:79F NC: 56M:151F |
Serum anti-Saccharomyces cerevisiae IgG antibodies (ASCA) | SZ recent-onset and SZ non-recent onset ↑ ASCA compared to NCs. Within SZ non-recent, SZ females ↑ ASCA compared to NC females; levels between SZ males and NC males did not differ. In SZ recent-onset, SZ males ↑ ASCA compared to NC males; no differences between SZ and NC females. | ASCA were not related to age, race, or other infectious disease antibody levels (Toxoplasma gondii, EBV, HSV-1, influenza B) in any group. ASCA correlated with ↑ anti-casein and ↑ anti-gluten IgG in SZ non-recent-onset, and ↑ anti-gluten IgG in SZ recent-onset. | – Many of the patterns were sex-specific, but full interpretations of these associations are limited by the low number of women in the recent-onset SZ group and in both groups of cohort 2 – Further examination of relationship with DOI or other indicators of disease or symptom severity and clinical features – Cohort 1 and 2 were recruited with different study designs and thus have different exclusion criteria (e.g., vary in terms of inclusion of immunological variables such as a history of immune disorders, recent infection and use of anti-inflammatory agents or other immune-modulatory drugs) |
|
Cohort #2 SZ AP-treated: 63 SZ AP-naïve: 40 |
SZ AP-treated: 28.3 (SD=9.6) SZ AP-naïve: 29.7 (SD=9.3) |
SZ AP-treated: 52M:11F SZ AP-naïve: 27M:13F |
Serum anti-Saccharomyces cerevisiae IgG antibodies (ASCA) |
AP-naïve ↑ ASCA; only AP-naïve males showed ↑ ASCA. | ASCA antibodies were not related to age, race, or other infectious disease antibody levels (Toxoplasma gondii, Eps EBV, HSV-1, influenza B) in any group. ASCA correlated with ↑ anti-casein and ↑ anti-gluten IgG in AP-naïve females. | ||
| Severance et al., 2013 |
Cohort #1 SZ:141 BD: 75 NC: 78 |
SZ: 40.4 (SD=10.7) BD: 35.1 (SD=12.1) NC: 34.4 (SD=1.4) |
SZ: 85M:56F BD: 23M:52F NC: 22M:56F |
Serum soluble CD14 (sCD14), lipopolysacchar ide binding protein (LBP) | SZ and BD ↑ sCD14. sCD14 seropositivity was associated with ↑ odds ratio for SZ and trend for BD. LBP levels and LBP seropositivity not different among SZ, BD, and NCs. Between patient groups, BD l LBP compared to SZ. | SZ females ↑ sCD14 and LBP compared to SZ males. NC females ↑ LBP levels than NC males. LBP correlated with ↑ BMI in SZ. sCD14 and LBP correlated with ↑ CRP in SZ but not in BD or NC. Neither sCD14 nor LBP associated with smoking status, casein or gluten antibodies, or celiac disease-associated anti-tTG IgG antibodies in SZ, but anti-tTG antibodies was significantly correlated with sCD14 in BD. | – Blood draws were not standardized nor taken under fasting conditions, which may not significantly impact IgG antibodies but may affect other immune or inflammatory markers – No available BMI data for NCs – Cohort 1 and 2 were recruited with different study designs and thus have different exclusion criteria (see above) – Further examination of relationship with DOI or other indicators of disease or symptom severity and clinical features |
|
Cohort #2 SZ AP-treated: 38 SZ AP-naïve: 78 |
SZ AP-treated: 36.4 (SD=14.2) SZ AP-naïve: 29.9 (SD=9.7) |
SZ AP-treated: 18M:20F SZ AP-naïve: 47M:31F |
Serum soluble CD14 (sCD14), lipopolysacchar ide binding protein (LBP) | sCD14 and LBP no different between AP-treated and AP-naïve groups. | sCD14 and LBP correlated with ↑ gluten antibodies in SZ AP-naïve. No sex differences observed between groups. | ||
| Severance et al., 2014 | BD with psychosis: 38 BD without psychosis: 226 NC: 207 |
BD with psychosis: 26.7 (SD=8.1) BD without psychosis: 37.8 (SD=12.6) NC: 32.1 (SD=11.5) |
BD with psychosis: 12M:26F BD without psychosis: 68M:158F NC: 56M:151F |
Serum anti-Saccharomyces cerevisiae IgG antibodies (ASCA) | Both BD groups ↑ ASCA than NCs. ASCA seropositivity was associated with ↑ odds ratio for BD with psychosis and without psychosis, compared to NCs. | ASCA was not associated with age, cognitive scores, symptom severity scores on the RBANS, PANSS, YMRS, or HAM-D assessments, type of psychiatric medication (lithium, valproate, or AP), or history of past and lifetime psychotic features. ASCA correlated with ↑ casein and gluten IgG in BD groups, and ↑ measles IgG and T. gondii in BD females. | Blood draws were not standardized nor taken under fasting conditions, which may not significantly impact IgG antibodies but may affect other immune or inflammatory markers Small sample size of BD with recent onset of psychosis group relative to other groups |
| Severance et al., 2016 |
Cohort #1 SZ:261 BD: 270 NC: 277 |
SZ: 37.7 (SD=13.7) BD: 34.1 (SD=13.2) NC: 32.0 (SD=11.3) |
SZ: 161M:100F BD: 77M:193F NC: 108M:169F |
Serum anti-Candida albicans IgG antibodies | C. albicans IgG did not differ among groups. When sex was considered, SZ and BD males ↑ antibodies compared to NC male. C. albicans antibodies not different between SZ and NC females. | C. albicans associated with ↑ homelessness in BD males. Seropositive SZ females had ↓ cognition than seronegative SZ females and female NCs. Seropositive SZ females and BD had ↓ memory. C. albicans were associated with ↑ GI conditions in SZ males and BD females. | – Further information on immunosuppression and collection of data on other inflammatory biomarkers – Further examination of relationship with DOI or other indicators of disease or symptom severity and clinical features – Cohort 1 and 2 were recruited with different study designs and thus have different exclusion criteria (see above) |
|
Cohort #2 SZ AP-treated: 61 SZ AP-naive: 78 |
SZ AP-treated: 36.5 (SD=13.2) SZ AP-naive: 30.3 (SD=10.6) |
SZ AP-treated: 30M:31F SZ AP-naïve: 44M:34F |
Serum anti-Candida albicans IgG antibodies | No differences in C. albicans IgG antibodies between AP-naive vs. AP-treated; no differences between genders in either groups. | N/A | ||
| Severance et al., 2017 | SZ probiotic: 30 SZ placebo: 26 |
SZ probiotic: 44.7 (SD=11.4) SZ placebo: 48.1 (SD=9.6) |
SZ probiotic: 22M:8F SZ placebo: 15M:11F |
Serum anti-Candida albicans IgG antibodies and anti-Saccharomyces cerevisiae IgG antibodies (ASCA) | C. albicans IgG antibodies ↓ with probiotic treatment, compared to placebo treatment, only in SZ males. No treatment effect in SZ females. No treatment effect on S. cerevisiae IgG antibodies. | C. albicans seropositivity not related to age, race, smoking, BMI, or maternal education. C. albicans IgG ↑ in patients reporting GI conditions and females reporting genitourinary conditions. C. albicans IgG levels not associated with dietary intake of calories, carbohydrates, fats, fiber, protein, sodium and sugar. Change in PANSS positive scores over time ↑ in C. albicans seronegative males in the probiotic group compared to those who were seropositive. In the treatment group, difficulty with bowel movements was not different between seropositive and seronegative individuals, and not different from seronegative individuals in the placebo group. | – Small sample sizes and exploratory nature of analyses; may be unmeasured demographic, lifestyle and medication factors contributing to the effects – Further inclusion of NC treatment and placebo group – Further examination of cognitive measure and whether probiotic treatment improved cognition, given previous results (2016) – Analyses were not intent-to-treat, thus potentially introducing bias; no information about those who dropped out, why, and if significantly different between treatment groups |
anti-tTG = anti-transglutaminase antibodies; AOS = age of onset; AP = antipsychotic medication; ASCA = Anti-Saccharomyces cerevisiae; BD = bipolar disorder; BMI = body mass index; C. albicans = Candida albicans; CRP = C-reactive protein; DOI = duration of illness; EBV = Epstein–Barr virus; F = female; FEP = first-episode patients; GAF = Global Assessment of Functioning; GI = gastrointestinal; HAM-D = Hamilton Rating Scale for Depression; NC = non-psychiatric comparison group; IgG = immunoglobulin G; LBP = lipopolysaccharide binding protein; M = male; PANSS = Positive and Negative Syndrome Scale; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; S. cerevisiae = Saccharomyces cerevisiae; sCD14 = soluble CD14; SD = standard deviation; SZ = schizophrenia; T. gondii = Toxoplasma gondii; YMRS = Young Mania Rating Scale
Elevation of these serological biomarkers suggests increased permeability of the intestinal lumen or “leaky gut” through the process of microbial translocation, and indicates intestinal inflammation (Dickerson et al., 2017). If the GI barrier is compromised, increased levels of gut-dwelling commensal enteric microbes are exposed to systemic circulation. This process may be a resultant mechanism by which intestinal dysbiosis impacts systemic physiological functioning in SMI. As previously mentioned, Hsiao and colleagues’ (2013) found that microbial dysbiosis led to intestinal permeability and elevated cytokine levels in a preclinical model of autism. Consequently, the gut may become a source of autointoxication. Depletion or dysbiosis of microbes that promote development of the immune system may be at the root of a chronic inflammatory state in SMI, which can impact central nervous system functioning (Dickerson et al., 2007; Frodl and Amico, 2014; Pedersen et al., 2008). Indeed, memory performance was significantly reduced in women with schizophrenia and BD who were seropositive for Candida albicans, compared to their respective seronegative counterparts (Severance et al., 2016). The observation that probiotic treatment was found to reduce levels of these inflammatory indices (Severance et al., 2017) further suggests that alterations in the gut microbiome may modulate immune responses and infers that microbial therapeutics could be a potential intervention strategy.
IV. Conclusions and Perspectives
Schizophrenia and BD are heterogeneous diseases with multiple plausible pathophysiological contributors. Abundant evidence points to the immune system as an important factor in the pathogenesis and developmental trajectories of these disorders (Anderson et al., 2013; Benros et al., 2014; Berk et al., 2011; Watanabe et al., 2010). The gut is the largest immune system in the body. Microbiota colonization of the gut early in life is crucial for the optimal development and function of the immune system (Round and Mazmanian, 2009), and dysbiosis of the intestinal ecosystem may alter immune responses (Kamada et al., 2013). Consequently, chronic inflammation, oxidative stress, and other physiological dysfunctions that have been implicated in schizophrenia and BD, are proposed to be, at least in part, associated with changes in the microbiome. A greater understanding of the microbiome not only offers insight into how microbial dynamics may be associated with these physiological alterations in SMI, but also presents exciting avenues for future therapies.
Microbiome research within clinical mental health is nascent, and only a limited number of studies have been conducted to date. Altered microbiota have been observed in other psychiatric populations, including MDD (Aizawa et al., 2016; Jiang et al., 2015; Lin et al., 2017; Naseribafrouei et al., 2014), autism spectrum disorders (Rosenfeld, 2015), and anorexia nervosa (Carr et al., 2016). Still, there are considerable gaps in our knowledge, and the limitations of the current state of research must be highlighted. There were only five studies of the microbiome in SMI. Several papers were from the same group of investigators—and presumably, overlapping cohort of participants—thus restricting the scope of the review. Most of these findings are still preliminary, as they were based on small sample sizes, and results are inconsistent across studies and populations. Often the directionality of specific taxa is mixed. For example, Lactobacillus and Bifidobacterium levels have been reported to be lower in MDD (Aizawa et al., 2016), which is contrary to the finding of increased abundances of these bacteria in schizophrenia (Schwarz et al., 2017). On the other hand, children with autism also show increased Lactobacillus compared to NCs (Tomova et al., 2015). The inconsistencies across investigations may be attributable partly to heterogeneity among the mental illnesses themselves and partly to the compositionality problem inherent to microbiome investigations (Friedman and Alm, 2012; Morton et al., 2017). Microbiome data are compositional, meaning that they are relative and, on their own, carry no information about absolute abundances. Thus, over-interpretation of increased versus decreased abundances of specific species may lead to conflicting and erroneous conclusions. With this in mind, novel techniques to study changes in microbial composition are being developed (Morton et al., 2017). They acknowledge that changes in microbial abundance make sense only in the context of other microbes.
Another major issue when comparing results across studies of the microbiome is replicability. Based on what is known from other diseases (Forslund et al., 2015; Walters et al., 2014), technical differences (e.g., DNA extraction methods, PCR primers, sequencing platforms, taxonomy databases) can produce systematic biases that can obscure biologically meaningful compositional differences (Lozupone et al., 2013; Walters et al., 2014). This is especially true when the effects of a biological parameter are expected to be subtle, making replication across investigations challenging. Because the currently available studies in SMI used different sequencing methods (16S rRNA vs. shotgun metagenomic sequencing), sampled from different regions (oral vs. gut), and studied different patient cohorts (e.g., first-episode, chronic, medicated vs. unmedicated), it is not surprising that results were not replicated. Relatedly, because of these differences, it was not feasible to compare results across studies. Consequently, given these current challenges, the extent of conclusions that can be drawn from available findings at this time is limited. Finally, as with any review, publication biases might have influenced a predominance of positive results.
Nevertheless, the growing accumulation of studies suggesting altered microbiomes in human neuropsychiatric populations supports the concept of the gut-brain axis and a role of the microbiome not only in physical health and disease but also in mental and cognitive functioning. The available literature suggests that certain characteristics of microbiome may be associated with specific clinical features of schizophrenia and BD, including severity of mood and psychiatric symptoms (negative symptoms, depression, sleep), physical health and disease, psychiatric medication use, and overall global functioning (Evans et al., 2016; Flowers et al., 2016; Schwarz et al., 2017; Yolken et al., 2015), and that gut microbial composition may modulate treatment response and disease remission (Schwarz et al., 2017). This latter finding presents an exciting possibility that the microbiome could be an early indicator of individuals at higher risk for disease progression and an important trait marker that can help identify at-risk individuals who may benefit most from strategies to prevent further decline. Though no study has yet examined the relationship between the microbiota and cognition in schizophrenia or BD, seropositivity for C. albicans was associated with reduced memory abilities and overall cognition (Severance et al., 2016). Both animal and human studies in other populations have demonstrated relationships between gut microbiota and specific domains of cognition, including learning/memory, processing speed, sustained attention, and executive function (Bajaj et al., 2012; Gareau et al., 2011), all of which are impaired in schizophrenia and BD (Bearden et al., 2001; Nuechterlein et al., 2004). It would be interesting to differentiate microbial associations of cognitive impairment compared to psychopathology in SMI.
The clinical implications of these data are considerable. Microbiome research holds promise for predicting clinical prognosis and for therapeutics in SMI. In addition to serving as an important prognostic indicator, it may also be a viable therapeutic target to modulate immune response and other physiological functions in addition to psychiatric symptoms. Present-day treatment of schizophrenia and BD includes the use of antipsychotics and mood stabilizers, which may improve some psychiatric symptoms, but are plagued by side effects (Honig et al., 1999; Leucht et al., 2009). Identification of associations between these disorders and the gut microbiome may pave the way for development of novel diagnostic tools and “whole body” therapeutics that improve not only mental/cognitive health and wellbeing but also physical health and longevity. By including the microbiome as a part of the evaluation process, future clinical psychiatry could be in a better position to move away from phenomenology-based characterizations of mental illnesses, to precision medicine data-driven diagnostics and therapeutic recommendations. In addition, modulation of the gut microbiota may be a tractable strategy for developing novel treatments for schizophrenia. Addition of microbial treatments (e.g., probioticss) to the therapeutic armamentarium would not entail either major side effects or high costs (Gilbert et al., 2016). Both probiotics (live microorganisms that when administered in adequate amounts confer a health benefit on the host; Hill et al., 2014) and prebiotics (nondigestible food components that are selectively fermented by intestinal microflora that are associated with health and wellbeing; Gibson et al., 2004) are intended to regulate the microbiome. An alternative approach is to completely restore a dysbiotic microbial community through administration of a complete, complex microbiota from a healthy donor through fecal microbiota transplantation (Bakken et al., 2011; Kassam et al., 2013; Weingarden et al., 2015), which has been successful in the treatment of relapsing Clostridium difficile infection (Shahinas et al., 2012; van Nood et al., 2013).
Future Directions
The published studies are important for laying the groundwork for future investigations. Additional investigations are needed to replicate existing findings and elucidate the role of the microbiome in the development, presentation, and progression of major psychiatric disorders as well as its relationship with other clinical factors and biological markers. There are a number of limitations in the current state of our knowledge that future research should aim to address.
With one exception (Evans et al., 2016), most studies of the microbiome in severe psychiatric illness thus far have included limited sample sizes. Given the variability among samples and power needed for high-throughput sequencing analyses (Debelius et al., 2016; Goodrich et al., 2014), investigations involving larger sample sizes are needed. Larger samples will also allow for more complex models that can account for factors—other than disease state and relevant variables of interest, such as age, sex, diet, or medication—that might impact microbial composition.
Better demographic and phenotypic characterization of clinical samples is needed. Aside from age and gender, most studies did not provide further phenotypic description of the clinical samples. Other important socio-demographic and clinical variables to consider include educational attainment, socioeconomic status, and residential status including homelessness. Of particular relevance are measures that indicate level of disease severity, such as age of disease onset, duration of illness, number of hospitalizations, and current and/or cumulative antipsychotic medication exposure, which might be related to greater physiological decline due to wear and tear on the body from factors associated with having a mental illness (Nguyen et al., 2017).
To further elucidate the functional role of the microbiome in SMI, it is imperative that investigations include careful clinical assessment of participants, including mood and psychotic symptoms (e.g., depression, mania, positive and negative psychotic symptoms, sleep), neurocognition (e.g., memory, processing speed, executive functions), psychosocial functioning (e.g., functional capacity, stress, social support), physical characteristics (e.g., body mass index), medical morbidity (e.g., cumulative rating of physical illness and severity, cardiovascular disease risk), and lifestyle behaviors (e.g., diet/nutrition, smoking, substance use, sedentary behavior, and physical activity). Including matched samples of NCs on various lifestyle or physical morbidity variables could help disentangle the impact of these factors on the microbiome from the mental illness itself.
Studies of the microbiome should also include assessment of other biological factors related to the gut-brain axis and/or known to be altered in SMI. These are exemplified by, but are not limited to, pro-inflammatory cytokines and chemokines, measures of oxidative stress, mitochondrial dysfunction, and metabolic dysregulation. Understanding the relationship of the microbiome to other markers of physiological abnormalities may help identify mechanisms by which the gut ecosystem impacts the brain and physical morbidity.
It is hypothesized that schizophrenia and BD are associated with accelerated biological aging with greater age-related deficits and steeper age-related declines (Berk et al., 2011; Kirkpatrick et al., 2008; Nguyen et al., 2017). Both disorders have been characterized by chronic, low-grade inflammation, suggesting a process of immunosenescence or “inflammaging” (Franceschi and Campisi, 2014), and the gut microbiome may be an important mechanism involved in this process. To shed greater light on the issue of accelerated biological aging, studies should investigate the differential relationship of age and impact of aging on gut microbial composition in mental illness groups compared to NCs.
The problem of compositionality underlines the importance of having a baseline reference point for a microbial community and how it changes with mental illness. Longitudinal studies can capture within-subject variability and will further our understanding of the temporal variations of microbial composition. This may be particularly relevant after an intervention or experimental challenge. For example, microbial composition could be measured before and after probiotic supplementation to determine target engagement of the probiotic compound and ascertain whether changes in the outcome measures of interest were driven by changes in the underlying mechanism. Moreover, longitudinal sampling during different mood (e.g., mania vs. depression) or symptom (e.g., during active psychosis vs. remission) states would help elucidate whether changes in the microbiota are trait or state related.
The field of microbiome research is evolving. The goal of this overview was not to draw definitive conclusions about the microbiome in SMI but to summarize current knowledge, discuss limitations and shortcomings in existing research, and present recommendations and future directions for research. We aimed to stimulate interest in the microbiome as an important component of clinical mental health research, especially in SMI, where there are limited treatment options. Advances in sequencing technologies and data/statistical analyses provide an exciting opportunity to further elucidate the mechanisms underlying altered developmental trajectories in SMI, especially with aging. The emerging literature provides initial exciting evidence suggesting that the gut microbiome is altered in these disorders and, importantly, is associated with clinical features of mental illness. Further rigorous investigations in this area are needed, as microbiome research holds promise for precision medicine through data-driven diagnostics and cost-effective therapeutic strategies (e.g., probiotic or prebiotic supplementation, dietary manipulation, FMT). Strategies for achieving and maintaining healthy microbiome may lead to numerous potential benefits, including improved physical and mental health, cognition, and consequently, everyday functioning.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by National Institute of Mental Health (grant numbers 2R01 MH094151-06 and 5T32 MH019934-24 to DVJ), the Desert-Pacific Mental Illness Research, Education, and Clinical Center (TTN, LTE), the Department of Veterans Affairs Office of Academic Affiliations (TTN), UC San Diego Stein Institute for Research on Aging, and UC San Diego Center for Microbiome Innovation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, Ota M, Koga N, Hattori K, Kunugi H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. Journal of affective disorders. 2016;202:254–257. doi: 10.1016/j.jad.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Anderson G, Berk M, Dodd S, Bechter K, Altamura AC, Dell’osso B, Kanba S, Monji A, Fatemi SH, Buckley P, Debnath M, Das UN, Meyer U, Muller N, Kanchanatawan B, Maes M. Immuno-inflammatory, oxidative and nitrosative stress, and neuroprogressive pathways in the etiology, course and treatment of schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2013;42:1–4. doi: 10.1016/j.pnpbp.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. American journal of physiology. Gastrointestinal and liver physiology. 2012;302(1):G168–175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, Moore TA, Russell G, Surawicz C, Transplantation FM. Treating Clostridium difficile Infection With Fecal Microbiota Transplantation. Clin Gastroenterol H. 2011;9(12):1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan R, Rajendiran E, George S, Samuel GV, Ramakrishna BS. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J Gastroen Hepatol. 2008;23(8):1298–1303. doi: 10.1111/j.1440-1746.2008.05490.x. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar disorders. 2001;3(3):106–150. doi: 10.1034/j.1399-5618.2001.030302.x. discussion 151-103. [DOI] [PubMed] [Google Scholar]
- Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune diseases and psychosis. Biological psychiatry. 2014;75(4):300–306. doi: 10.1016/j.biopsych.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yucel M, Gama CS, Dodd S, Dean B, Magalhaes PV, Amminger P, McGorry P, Malhi GS. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience and biobehavioral reviews. 2011;35(3):804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology. 2017;152(7):1671–1678. doi: 10.1053/j.gastro.2016.12.048. [DOI] [PubMed] [Google Scholar]
- Brown S. Excess mortality of schizophrenia. A meta-analysis. The British journal of psychiatry : the journal of mental science. 1997;171:502–508. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- Budden KF, Gellatly SL, Wood DLA, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombaugh J, Knights D, Gajer P, Ravel J, Fierer N, Gordon JI, Knight R. Moving pictures of the human microbiome. Genome biology. 2011;12(5):R50. doi: 10.1186/gb-2011-12-5-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J, Kleiman SC, Bulik CM, Bulik-Sullivan EC, Carroll IM. Can attention to the intestinal microbiota improve understanding and treatment of anorexia nervosa? Expert Rev Gastroent. 2016;10(5):565–569. doi: 10.1586/17474124.2016.1166953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DE, Hansen TE, Meyer J, Nasrallah H. Excessive mortality and morbidity associated with schizophrenia. In: Meyer JM, Nasrallah HA, editors. Medical illness and schizophrenia. American Psychiatric Press, Inc; Washington, D.C: 2009. pp. 17–35. [Google Scholar]
- Castro-Nallar E, Bendall ML, Perez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, Schroeder JR, Yolken RH, Crandall KA. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3:e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Dauge V, Naudon L, Rabot S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi: 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature reviews. Neuroscience. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. Journal of affective disorders. 2002;72(3):227–236. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Moller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC) European psychiatry : the journal of the Association of European Psychiatrists. 2009;24(6):412–424. doi: 10.1016/j.eurpsy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Debelius J, Song SJ, Vazquez-Baeza Y, Xu ZZ, Gonzalez A, Knight R. Tiny microbes, enormous impacts: what matters in gut microbiome studies? Genome biology. 2016;17(1):217. doi: 10.1186/s13059-016-1086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain, behavior, and immunity. 2017;62:46–52. doi: 10.1016/j.bbi.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophrenia research. 2007;93(1–3):261–265. doi: 10.1016/j.schres.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Ding HT, Taur Y, Walkup JT. Gut Microbiota and Autism: Key Concepts and Findings. J Autism Dev Disord. 2017;47(2):480–489. doi: 10.1007/s10803-016-2960-9. [DOI] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerkop BA, Vaishnava S, Hooper LV. Immune Responses to the Microbiota at the Intestinal Mucosal Surface. Immunity. 2009;31(3):368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Evans SJ, Bassis CM, Hein R, Assari S, Flowers SA, Kelly MB, Young VB, Ellingrod VE, McInnis MG. The gut microbiome composition associates with bipolar disorder and illness severity. Journal of psychiatric research. 2016;87:23–29. doi: 10.1016/j.jpsychires.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biological psychiatry. 2013;74(6):400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers SA, Evans SJ, Ward KM, McInnis MG, Ellingrod VL. Interaction between Atypical Antipsychotics and the Gut Microbiome in a Bipolar Disease Cohort. Pharmacotherapy. 2016 doi: 10.1002/phar.1890. Epub 30 December 2016. [DOI] [PubMed] [Google Scholar]
- Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, Arumugam M, Kristiansen K, Voigt AY, Vestergaard H, Hercog R, Costea PI, Kultima JR, Li J, Jorgensen T, Levenez F, Dore J, Nielsen HB, Brunak S, Raes J, Hansen T, Wang J, Ehrlich SD, Bork P, Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe P, Bienenstock J. Immunomodulation by Commensal and Probiotic Bacteria. Immunol Invest. 2010;39(4–5):429–448. doi: 10.3109/08820131003667978. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The journals of gerontology. Series A, Biological sciences and medical sciences. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Friedman J, Alm EJ. Inferring correlation networks from genomic survey data. PLoS computational biology. 2012;8(9):e1002687. doi: 10.1371/journal.pcbi.1002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Progress in neuro-psychopharmacology & biological psychiatry. 2014;48:295–303. doi: 10.1016/j.pnpbp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Dore J, Henegar C, Rizkalla S, Clement K. Differential Adaptation of Human Gut Microbiota to Bariatric Surgery-Induced Weight Loss Links With Metabolic and Low-Grade Inflammation Markers. Diabetes. 2010;59(12):3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17(2):259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, Jansson JK, Dorrestein PC, Knight R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535(7610):94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- Goodrich JK, Di Rienzi SC, Poole AC, Koren O, Walters WA, Caporaso JG, Knight R, Ley RE. Conducting a microbiome study. Cell. 2014;158(2):250–262. doi: 10.1016/j.cell.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EC, Barraclough B. Excess mortality of mental disorder. The British journal of psychiatry: the journal of mental science. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- Hartstra AV, Bouter KEC, Backhed F, Nieuwdorp M. Insights Into the Role of the Microbiome in Obesity and Type 2 Diabetes. Diabetes Care. 2015;38(1):159–165. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, Shibata A, Fujisawa Y, Minato T, Okamoto A, Ohno K, Hirayama M. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PloS one. 2015;10(11) doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijtza RD, Wang SG, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. American heart journal. 2005;150(6):1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastro Hepat. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Honig A, Arts BM, Ponds RW, Riedel WJ. Lithium induced cognitive side-effects in bipolar disorder: a qualitative analysis and implications for daily practice. International clinical psychopharmacology. 1999;14(3):167–171. [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophrenia bulletin. 2011;37(3):451–455. doi: 10.1093/schbul/sbr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain, behavior, and immunity. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Jorgensen BP, Krych L, Pedersen TB, Plath N, Redrobe JP, Hansen AK, Nielsen DS, Pedersen CS, Larsen C, Sorensen DB. Investigating the long-term effect of subchronic phencyclidine-treatment on novel object recognition and the association between the gut microbiota and behavior in the animal model of schizophrenia. Physiology & behavior. 2015;141:32–39. doi: 10.1016/j.physbeh.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nature reviews. Immunology. 2013;13(5):321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Kassam Z, Lee CH, Yuan YH, Hunt RH. Fecal Microbiota Transplantation for Clostridium difficile Infection: Systematic Review and Meta-Analysis. Am J Gastroenterol. 2013;108(4):500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biological psychiatry. 2003;54(3):216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, Brien CO, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of psychiatric research. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic Bacterial Composition in Parkinson’s Disease. Movement Disord. 2015;30(10):1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophrenia bulletin. 2008;34(6):1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophrenia bulletin. 2013;39(6):1174–1179. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R, Callewaert C, Marotz C, Hyde ER, Debelius JW, McDonald D, Sogin ML. The Microbiome and Human Biology. Annual review of genomics and human genetics. 2017 doi: 10.1146/annurev-genom-083115-022438. [DOI] [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Xavier RJ, Gevers D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterology. 2014;146(6):1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ. The increasing medical burden in bipolar disorder. Jama. 2005;293(20):2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- Lee EE, Liu J, Tu X, Palmer BW, Eyler LT, Jeste DV. A widening longevity gap between people with schizophrenia and general population: A literature review and call for action. Schizophrenia research. doi: 10.1016/j.schres.2017.09.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- Lin P, Ding B, Feng C, Yin S, Zhang T, Qi X, Lv H, Guo X, Dong K, Zhu Y, Li Q. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. Journal of affective disorders. 2017;207:300–304. doi: 10.1016/j.jad.2016.09.051. [DOI] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh J, Gonzalez A, Ackermann G, Wendel D, Vazquez-Baeza Y, Jansson JK, Gordon JI, Knight R. Meta-analyses of studies of the human microbiota. Genome research. 2013;23(10):1704–1714. doi: 10.1101/gr.151803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Morton JT, Sanders J, Quinn RA, McDonald D, Gonzalez A, Vazquez-Baeza Y, Navas-Molina JA, Song SJ, Metcalf JL, Hyde ER, Lladser M, Dorrestein PC, Knight R. Balance Trees Reveal Microbial Niche Differentiation. mSystems. 2017;2(1) doi: 10.1128/mSystems.00162-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu AS, Bidlack WR, Clemens RA. Probiotic spectra of lactic acid bacteria (LAB) Crit Rev Food Sci. 1999;39(1):13–126. doi: 10.1080/10408699991279187. [DOI] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroent Motil. 2014;26(8):1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroent Motil. 2011;23(3) doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Eyler LT, Jeste DV. Systemic Biomarkers of Accelerated Aging in Schizophrenia: A Critical Review and Future Directions. Schizophrenia bulletin. 2017 doi: 10.1093/schbul/sbx069. Epub ahead of print [4 July 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia research. 2004;72(1):29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- O’Dwyer DN, Dickson RP, Moore BB. The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J Immunol. 2016;196(12):4839–4847. doi: 10.4049/jimmunol.1600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace NR. A molecular view of microbial diversity and the biosphere. Science. 1997;276(5313):734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Diedrich M, Kaestner F, Koelkebeck K, Ohrmann P, Ponath G, Kipp F, Abel S, Siegmund A, Suslow T, von Eiff C, Arolt V, Rothermundt M. Memory impairment correlates with increased S100B serum concentrations in patients with chronic schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32(8):1789–1792. doi: 10.1016/j.pnpbp.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Meta HITC, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS. Microbiome Disturbances and Autism Spectrum Disorders. Drug Metab Dispos. 2015;43(10):1557–1571. doi: 10.1124/dmd.115.063826. [DOI] [PubMed] [Google Scholar]
- Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatric services. 2009;60(2):147–156. doi: 10.1176/ps.2009.60.2.147. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiome shapes intestinal immune responses during health and disease. Nature reviews. Immunology. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Archives of general psychiatry. 2007;64(10):1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167(6):1469–+. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Movement Disord. 2015;30(3):350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- Schiffrin EJ, Brassart D, Servin AL, Rochat F, Donnet-Hughes A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: criteria for strain selection. The American journal of clinical nutrition. 1997;66(2):515S–520S. doi: 10.1093/ajcn/66.2.515S. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E, Maukonen J, Hyytiainen T, Kieseppa T, Oresic M, Sabunciyan S, Mantere O, Saarela M, Yolken R, Suvisaari J. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophrenia research. 2017 doi: 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Schwiertz A, Jacobi M, Frick JS, Richter M, Rusch K, Kohler H. Microbiota in Pediatric Inflammatory Bowel Disease. J Pediatr-Us. 2010;157(2):240–U294. doi: 10.1016/j.jpeds.2010.02.046. [DOI] [PubMed] [Google Scholar]
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome biology. 2011;12(6) doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS biology. 2016;14(8):e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophrenia research. 2012;138(1):48–53. doi: 10.1016/j.schres.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, Adamos MB, Sweeney KM, Origoni AE, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ schizophrenia. 2016;2:16018. doi: 10.1038/npjschz.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CLG, Adamos MB, Sweeney KM, Origoni AE, Khushalani S, Dickerson FB, Yolken RH. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain, behavior, and immunity. 2017;62:41–45. doi: 10.1016/j.bbi.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophrenia research. 2013;148(1–3):130–137. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Yang S, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Alaedini A, Dickerson FB, Yolken RH. Seroreactive marker for inflammatory bowel disease and associations with antibodies to dietary proteins in bipolar disorder. Bipolar disorders. 2014;16(3):230–240. doi: 10.1111/bdi.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Current psychiatry reports. 2015;17(5):27. doi: 10.1007/s11920-015-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahinas D, Silverman M, Sittler T, Chiu C, Kim P, Allen-Vercoe E, Weese S, Wong A, Low DE, Pillai DR. Toward an Understanding of Changes in Diversity Associated with Fecal Microbiome Transplantation Based on 16S rRNA Gene Deep Sequencing. mBio. 2012;3(5) doi: 10.1128/mBio.00338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheih YH, Chiang BL, Wang LH, Liao CK, Gill HS. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. Journal of the American College of Nutrition. 2001;20(2 Suppl):149–156. doi: 10.1080/07315724.2001.10719027. [DOI] [PubMed] [Google Scholar]
- Sogin SJ, Sogin ML, Woese CR. Phylogenetic measurement in procaryotes by primary structural characterization. J Mol Evol. 1972;1(2):173–184. doi: 10.1007/BF01659163. [DOI] [PubMed] [Google Scholar]
- Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier L, Beaugerie L, Cosnes J, Corthier G, Marteau P, Dore J. Low Counts of Faecalibacterium prausnitzii in Colitis Microbiota. Inflamm Bowel Dis. 2009;15(8):1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. Journal of Physiology. 2004;558(1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiology & behavior. 2015;138:179–187. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Woolson RF. Excess mortality in schizophrenia and affective disorders: Do suicides and accidental deaths solely account for this excess? Archives of general psychiatry. 1978;35(10):1181–1185. doi: 10.1001/archpsyc.1978.01770340031002. [DOI] [PubMed] [Google Scholar]
- van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, Speelman P, Dijkgraaf MGW, Keller JJ. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. New Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA psychiatry. 2015;72(4):334–341. doi: 10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS letters. 2014;588(22):4223–4233. doi: 10.1016/j.febslet.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry and clinical neurosciences. 2010;64(3):217–230. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- Weingarden A, Gonzalez A, Vazquez-Baeza Y, Weiss S, Humphry G, Berg-Lyons D, Knights D, Unno T, Bobr A, Kang J, Khoruts A, Knight R, Sadowsky MJ. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome. 2015;3:10. doi: 10.1186/s40168-015-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Woese CR, Fox GE. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proceedings of the National Academy of Sciences of the United States of America. 1977;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, Origoni A, Katsafanas E, Schweinfurth LA, Savage CL, Banis M, Khushalani S, Dickerson FB. Metagenomic Sequencing Indicates That the Oropharyngeal Phageome of Individuals With Schizophrenia Differs From That of Controls. Schizophrenia bulletin. 2015;41(5):1153–1161. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Shen DQ, Fang ZW, Jie ZY, Qiu XM, Zhang CF, Chen YL, Ji LN. Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance. PloS one. 2013;8(8) doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Molecular psychiatry. 2016;21(6):786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


