Summary
The evolution of broadly neutralizing antibodies (bNAbs) in spontaneous controllers of HIV appears in the setting of low but persistent viral replication, in the absence of significant viral diversity, but in the presence of a unique inflammatory signature.
Keywords: neutralizing antibodies, inflammation, HIV vaccine.
Abstract
Background.
Understanding the mechanism(s) by which broadly neutralizing antibodies (bNAbs) emerge naturally following infection is crucial for the development of a protective vaccine against human immunodeficiency virus (HIV). Although previous studies have implicated high viremia and associated immune activation as potential drivers for the development of bNAbs, here we sought to unlink the effect of these 2 parameters by evaluating the key inflammatory predictors of bNAb development in HIV-infected individuals who spontaneously control HIV in the absence of antiretroviral therapy (“controllers”).
Methods.
The breadth of antibody-mediated neutralization against 11 tier 2 or 3 viruses was assessed in 163 clade B spontaneous controllers of HIV. Plasma levels of 17 cytokines were screened in the same set of subjects. The relationship of the inflammatory signature was assessed in the context of viral blips or viral RNA levels in peripheral blood or gastrointestinal biopsies from aviremic controllers (<50 copies RNA/mL) and in the context of viral sequence diversity analysis in the plasma of viremic controllers (<50–2000 copies RNA/mL).
Results.
A unique inflammatory profile, including high plasma levels of CXCL13, sCD40L, IP10, RANTES, and TNFα, was observed in HIV controllers who developed bNAbs. Interestingly, viral load and tissue viremia, but not intermittent viral blips, were associated with these cytokine profiles. However, viral diversity was not significantly associated with increased breadth in controllers.
Conclusion.
These results suggest that low antigenic diversity in the setting of a unique inflammatory profile associated with antigen persistence may be linked to the evolution of neutralizing antibody breadth.
The development of a protective vaccine against human immunodeficiency virus (HIV) will likely require the induction of cross-reactive broadly neutralizing antibodies (bNAbs). Over the past decade, there has been a rapid increase in the discovery of a large number of novel bNAbs [1–3] that have highlighted that a limited number of sites of neutralizing vulnerability exist on the surface of the HIV viral envelope. However, despite the identification of these key immunological targets on the virus, the mechanism(s) by which bNAbs naturally emerge is still unclear.
Roughly 10–30% of chronically infected individuals develop neutralizing antibody breadth but only years after infection [4–9]. Key to the evolution of bNAbs is the selection of highly mutated and unusual B cell receptors (BCR) [10, 11]. Recent studies point to a critical role for rapid viral diversification in driving the evolution of these unusual BCR clonal repertoires [12–14]. By contrast, population-based studies, using cross-sectional cohorts, point to an association between high viremia [4], low CD4+ T cell counts [15], and infection-associated immune activation [5], including high levels of the germinal center (GC)- recruiting chemokine CXCL13 [16, 17] as drivers of the development of cross-neutralizing activity. Thus, although it is possible that a rapidly evolving antigen is critical for B-cell diversification, it is also plausible that changes in CD4 T-cell help, B-cell retention within the GC, and/or the adjuvanting activity of specific inflammatory cytokine profiles may be equally critical for the selection of the most effective B-cell responses. However, although viral load, CD4 count, and inflammation have all been linked to the development of bNAbs, the contribution of these parameters individually to the evolution of bNAbs is incompletely understood.
Although the majority of natural history studies on bNAb evolution have focused on cohorts of untreated individuals with persistent viremia, more recent studies have shown that even subjects who spontaneously control viremia to low levels, otherwise known as HIV controllers [18–20], also develop broad neutralizing activity. Moreover, a number of recent potent bNAbs have been isolated from these HIV controllers [2, 7, 21], suggesting a role for additional factors, beyond high viremia, such as partial B-cell restoration [22], that may be crucial for the development of neutralizing antibody breadth.
Thus, here we speculated that immunological signatures associated with the development of bNAbs, beyond high viremia, could be defined in HIV controllers and may provide critical insights for the specific immunological signals that may be required for the evolution of these potent humoral immune responses. This study highlights the existence of a unique inflammatory signature in HIV controllers, in the setting of persisting, rather than highly diverse, HIV viral antigenemia, enriched among individuals that evolve neutralizing antibody breadth.
MATERIAL AND METHODS
Study Subjects
A total of 163 controllers were recruited for this study including both elite controllers (ECs), who spontaneously control viral replication to undetectable levels (<75 copies/mL with CD4 = 353–1813 cells/mm3) and viremic controllers (VCs) with detectable but low viral loads (20–1658 copies of RNA/mL and CD4 = 172–1794 cells/mm3). All subjects were infected with HIV-1 for over 10 years. All subjects signed informed consent, and the study was approved by the MGH Institutional Review Board.
Neutralization Assay
HIV-1 neutralization breadth was assessed using the Tzm-bl cell–based pseudovirus neutralization assay, as described [34], against a panel of Env-pseudoviruses derived from 9 Clade B Tier 2 viruses: AC10.0.29*, RHPA4259.7*, THRO4156.18*, REJO4541.67*, WITO4160.33*, TRO.11*, SC422661.8*, QH0692.42*, CAAN5342.A2#, and 2 Tier 3 viruses: PVO.4* and TRJO4551.58*. Neutralization was defined as at least 50% inhibition of infection at a 1:20 dilution. Relative neutralization breadth was defined by the percentage of the 11 isolates neutralized by each plasma sample. All samples that showed reactivity to the murine leukemia virus–pseudotyped virion controls were excluded, and only samples showing activity 3 times over the murine leukemia virus-pseudotyped virion controls were deemed positive and included in the analyses.
Cytokine Levels
Plasma levels of 17 cytokines including IP10, sCD40L, tumor necrosis factor α (TNFα), MIP1α, MIP1β, regulated on activation, normal T cell expressed and secreted (RANTES), interleukin 4 (IL4), IL5, IL6, IL8, IL10, IL13, IL12p70, IL17, interferon γ (IFNγ), granulocyte-macrophage colony-stimulating factor (GMCSF), and IL1β were measured by luminex (Millipore) and analyzed on a Bio-plex 200 (Bio-Rad Laboratories). CXCL13 plasma levels were determined by enzyme-linked immunosorbent assay (ELISA) (R and D systems).
Detecting and Quantifying HIV Viral Load by qRT-PCR
Pinch biopsies of intestinal tissue were mechanically homogenized. RNA was extracted (Qiagen) and quantitative reverse transcription polymerase chain reaction(qRT-PCR) was performed (Agilent Technologies) using the HIV-1 gag SK462 (AGTTGGAGGACATCAAGCAGCCATGCAAAT) and SK431 (TGCTATGTCACTTCCCCTTGGTTCTCT) primers. Relative HIV RNA copy numbers (viral load [VL]) were normalized to levels of ribosomal S9 (ribs9) protein by qRT-PCR (forward: AAGGCCGCCCGGGAACTGCTGAC, reverse: ACCACCTGCTTGCGGACCCTGATA). Average relative gut viral load was calculated as the mean viral load across each measured gut compartment (transverse colon, terminal ileum, and duodenum). The limit of detection (10–7 relative copies) was used for compartments in which no HIV RNA was detected.
Viral Diversity Analysis
The entire envelope gene from 18 representative patients was amplified using single genome amplification (SGA) as described previously [35]. Briefly, viral RNA was isolated from blood plasma and subjected to cDNA synthesis with gene specific priming. Newly synthesized cDNA was diluted to single copy and then amplified by nested PCR. Entire amplicons were sequenced using Sanger-based sequencing. In total, 365 sequences were obtained from 18 patients (range 5–30). The mean pairwise diversity measurements were obtained from DIVEIN [36]. Phylogenetic trees were generated with the neighbor joining method. All sequences were deposited in GenBank.
Statistical Analysis and Partial Least Squares Discriminant Analysis
An ANOVA with a post hoc Tukey’s test was used to analyze cytokines/chemokines differences between groups. Spearman’s correlations were used to examine the inter-relatedness of different parameters. P values less than .05 were considered significant. Partial least squares discriminant analysis (PLSDA) [37, 38] was used to determine multivariate cytokine/chemokine profiles that best distinguished between HIV controllers who developed bNAbs versus those who did not, as previously described [38]. Cross-validation was performed by iteratively excluding subsets of data (in groups of 10%) before model generation, then testing model performance using excluded data. After initial generation of a model with all measured cytokines, the variable importance in the projection (VIP) score of each cytokine was used to select cytokines that contributed most to cohort classification.
RESULTS
HIV Controllers Evolve Neutralizing Antibody Breadth
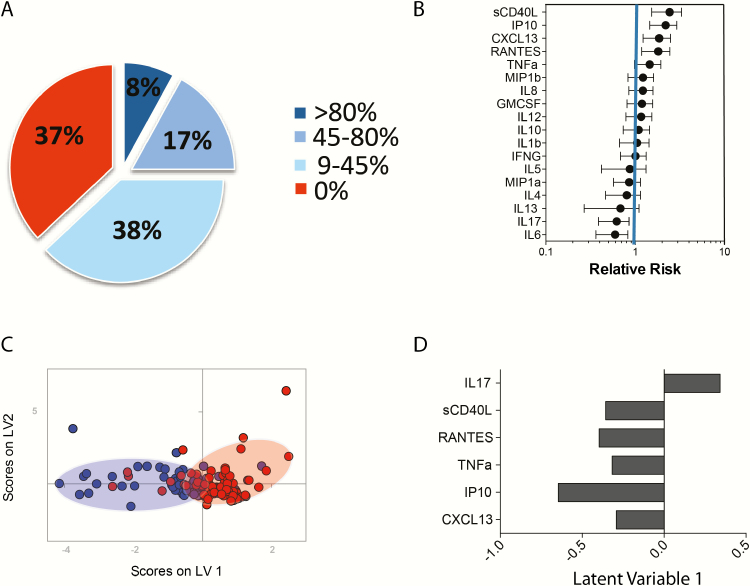
Previous studies have linked high viremia, low CD4 counts, immune activation, and high plasma levels of CXCL13 to the evolution of neutralizing antibody breadth [4, 5, 15, 16]. However, given that these features are all highly inter-related, it has been difficult to differentiate the effects of the virus from unique inflammatory signatures that may be key to B-cell activation. Yet many of the most potent bNAbs have been cloned from individuals with low viremia [2, 7, 20], arguing that neutralizing antibody activity can evolve in the setting of low viral replication. Thus, here we aimed to determine whether unique inflammatory signatures were enriched among spontaneous controllers of HIV [23]. The breadth of neutralization was screened in 163 clade B-infected controllers, infected for over 10 years, using a panel of 11 clade B tier 2 and 3 viruses. Approximately 25% of HIV controllers neutralized at least 5 tier 2 viruses (Figure 1A), with 8% of the controllers able to neutralize 80% of viruses. Conversely, 37% of this controller cohort did not elicit any tier 2 neutralizing antibodies and were therefore classified as “non-neutralizers” (Figure 1A). These data illustrate that despite low-to-undetectable plasma viremia, a significant proportion of controllers generate neutralizing antibody breadth, with similar frequencies of neutralizers among controllers as has been observed in cohorts of viremic patient populations [4].
Figure 1.
An inflammatory signature associates with the development of neutralizing antibody breadth in controllers. (A) The pie chart depicts the distribution of neutralizing antibody breadth against a panel of 11 Tier 2 and Tier 3 viruses in our cohort. (B) The whisker plots represents the “relative risk” or contribution of 18 individual cytokines to separate out subjects that possess neutralizing antibody breadth (relative risk > 1) compared to those that do not exhibit any neutralizing antibody breadth (relative risk < 1). (C) Partial least squares discriminant analysis (PLSDA) illustrates that a multivariate cytokine signature can differentiate controllers who develop neutralizing breadth (blue dots) and controllers who do not (red dots) with 69% classification accuracy and 66% cross-validation accuracy, respectively. Cross-validation was performed via the iterative exclusion of subsets of data (in groups of 10%) before model generation, then testing model performance using excluded data. (D) The box plot represents the top 6 cytokines that separate controllers with neutralizing antibody breadth (left) from those without breadth (right) along latent variable 1 (LV1) in the multivariate profile. Specifically, the direction of the column depicts the subject group in which the cytokine is most elevated, as a mirror of the PLSDA dot plot, where high levels of sCD40L, RANTES, TNFα, IP10, and CXCL13 are enriched in subjects with neutralizing antibody breadth, and IL-17 is enriched in subjects that do not generate neutralizing antibody breadth. Abbreviations: IL, interleukin; RANTES, regulated on activation, normal T cell expressed and secreted; TNFα, tumor necrosis factor α.
A Unique Inflammatory Signature Associates with the Development of Neutralizing Breadth in Controllers
Beyond associations with high viremia, the evolution of neutralizing antibody activity has also been linked to enhanced immune activation [5]. Thus, we next examined whether specific inflammatory profiles existed among controllers who developed bNAbs in the absence of high antigenemia. The levels of 18 serum cytokines involved in T-cell/B-cell communication, including cytokines, chemokines, and secreted ligands, were analyzed among controllers who neutralized more than 45% of viruses and those that did not demonstrate any neutralizing activity. High plasma levels of soluble CD40 ligand (sCD40L), chemokine (C-X-C) ligand 10 (CXCL10 or IP10), CXCL13, chemokine (C-C motif) ligand 5 (also CCL5 or RANTES), and TNFα were each individually associated with the emergence of neutralizing antibody breadth in controllers in univariate analyses (Figure 1B). Conversely, cytokines typically involved in T- or B-cell activation (e.g., IL4, IL6, and IL13) were associated with a lower likelihood of inducing neutralizing breadth (Figure 1B). Given that cytokines are frequently induced in a coordinated manner, we next aimed to define the multivariate biomarker profile that best distinguished controllers who develop significant neutralizing breadth versus those who do not. Partial least squares discriminant analysis (PLSDA) revealed that CD40L, RANTES, TNF-α, IP-10, and CXCL13 classified controllers who develop neutralizing breadth with nearly 70% accuracy, and this profile was significantly more powerful as a predictor than viremia and/or CD4 counts (Figure 1C, 1D). Moreover, the multivariate analysis also identified the same cytokines as independent predictors of the evolution of neutralization breadth (Figure 1D). Although this profile was unable to perfectly classify individuals who developed neutralization breadth, the selected subjects exhibited a range of neutralization breadth profiles, suggesting that even among this highly heterogeneous group of individuals, this combination of cytokines was more likely to be produced in individuals with neutralizing breadth compared to those with limited to no neutralizing breadth.
Viremia in Blood and Tissues Associates with the Development of Neutralization Breadth
Recent studies suggest that HIV viral RNA itself may drive particular cytokine profiles, including CXCL13 and IP-10, via toll like receptor signaling [17]. Thus, we next sought to examine whether low-level viremia may induce differential inflammatory profiles that may be conducive to the development of bNAbs in controllers. In fact, viral loads in controllers who exhibited detectable levels of viremia were significantly correlated with plasma levels of CXCL13, IP10, and sCD40L (r = 0.27 and P = .0094, r = 0.22 and P = .0318, r = 0.27 and P = .0084, respectively; Figure 2A) suggesting that even low antigenemia may be sufficient to drive persistent levels of cytokines.
Figure 2.
Cytokines, linked to neutralizing antibody breadth, are associated with plasma viral loads. The correlation plots depict the relationship between the concentration (pg/mL) of CXCL13, IP10, TNFα, RANTES, and sCD40L in controllers with detectable plasma viremia (n = 190). P values less than .05 were considered significant. Abbreviations: RANTES, regulated on activation, normal T cell expressed and secreted; TNFα, tumor necrosis factor α.
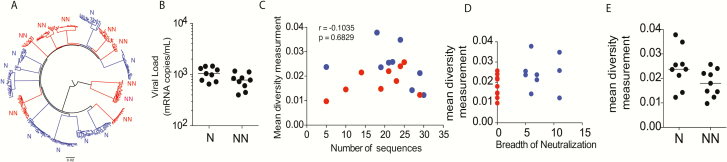
Interestingly, among the controllers, a subset of individuals termed “Elite controllers” (ECs) had undetectable viremia. Yet 20% of ECs, with undetectable viremia, still exhibited neutralization breadth (Figure 3A). Importantly, CD4 counts, which are typically inversely correlated with viremia in progressive cohorts of subjects with active viral replication [24], were also inversely correlated with the existence of greater neutralizing antibody breadth in ECs (Figure 3B), arguing that the virus may still be active outside of the circulation where it may continue to deplete CD4+ T cells and drive continued antigen-exposure and immune activation. Thus, we sought to determine whether ECs that develop neutralizing antibody activity possessed evidence of more active virus in tissues, associated with enhanced inflammation, both via the analysis of transient loss of viral control (blips) as well as gastrointestinal biopsies. Interestingly, although the number of viral blips did not differ between the ECs who developed breadth and those who did not (Figure 3C), viral loads were amplified in at least 1 sampled tissue (peripheral blood mononuclear cells [PBMCs], duodenum, ileum, and colon) in all the ECs with neutralization breadth but was rarely observed in biopsies from ECs who did not evolve breadth (Figure 3D). Moreover, neutralization breadth in ECs with neutralizing breadth correlated with RNA copy levels across all tissue biopsies (Figure 3E; P = .03) but was also predicted by the level of viral RNA in circulating T cells (Figure 3F; P = .0099), suggesting that the circulating cellular reservoir may mark the tissue reservoir, whose persistence may be linked to the evolution of neutralizing antibody breadth. These data highlight the critical nature of viral persistence as a critical driver of these unique cytokine profiles that are enriched even among spontaneous HIV controllers that elicit neutralizing antibody breadth.
Figure 3.
Viremia in tissues associates with the development of neutralizing antibody breadth. (A) The pie chart represents the frequency of aviremic controllers (Elite controllers - ECs) who are able to neutralize >45% of tier 2/3 viruses (neutralizer -N = blue) compared to those who neutralize no viruses (non-neutralizing –NN = red). (B) The correlation plots depict the relationship between neutralizing antibody breadth and CD4 counts among the ECs. (C) The dot plot illustrates that no difference exists in the number of documented viral blips between ECs who develop neutralizing antibody breadth (N) and those who do not (NN). (D) The dot plot shows the level of viral RNA detected by qPCR in tissue biopsies collected from PBMCs, duodenum, ileum and colon in ECs with neutralization breadth (N) versus those without neutralizing breadth (NN) where each color represents an individual patient. (E) The correlation plot depicts the relationship between HIV viral RNA in tissue biopsies and neutralization breadth in ECs where each color represents an individual patient and each dot shape represents a distinct tissue. (F) The correlation plot shows the relationship between HIV viral RNA in PBMCs only and neutralization breadth in the ECs with colors representing different subjects. Abbreviations: EC, elite controller; HIV, human immunodeficiency virus; PBMC, peripheral blood mononuclear cell; qPCR, quantitative polymerase chain reaction.
Viral Diversity is not Associated with the Evolution of Neutralizing Antibody Breadth
Rapid and/or extensive viral diversification [12, 25] has been linked to the evolution of neutralizing antibody breadth in several case studies. Similarly, high viremia, which is associated with greater viral diversity, has also been linked to the evolution of bNAbs in larger cohorts of HIV-infected subjects [13]. Thus, we examined whether viral diversity, in addition to elevated antigen levels, was key to the evolution of neutralization breadth among a group of 18 controllers. Single genome amplification was performed to generate a total of 365 whole viral envelope sequences from the plasma of 9 viremic controllers who developed neutralizing antibody breadth (n = 195 envelope sequences) and 9 viremic controllers who were matched for plasma viral load levels but did not develop neutralization breadth (n = 170 envelope sequences) (Figure 4A). Similar numbers of envelopes were amplified from each group and individual to minimize sampling bias. Interestingly, only marginal differences (P = .16, Figure 4B) in mean envelope diversity were observed among subjects who developed bNAbs compared to those who did not (Figure 4D and 4E). These data suggest that the presence of persistent antigenemia, rather than extensive plasma viral diversity, in the setting of a unique inflammatory profile, are key features associated with the development of neutralizing antibody breadth among controllers.
Figure 4.
High viral diversity is not associated with neutralizing antibody breath in controllers. (A) The phylogenetic tree represents the diversity of HIV viral envelopes sequenced in the plasma of 9 controllers with neutralizing antibody breadth (N = blue) compared to 9 controllers without neutralizing antibody breadth (NN = red) with detectable plasma viral loads. (B) The dot plot shows limited differences in plasma viral loads among the 2 groups. (C) The correlation plot illustrates the lack of a relationship between the observed mean viral diversity and the number of sequenced viruses across the neutralizers (blue) or non-neutralizers (red), suggesting that sampling differences did not account for observed relationships. (D) The correlation plot depicts the relationship between the breadth of neutralization and the mean viral diversity among all 18 VCs, half with bNAbs (blue circles) and half without (red circles). (E) The dot plot represents the mean viral diversity among the 9 subjects who possessed bNAbs (N) and the 9 individuals who did not (NN). A nonparametric Mann-Whitney was used to determine whether there were any differences between groups and Spearman’s rank correlations were used to examine relationships between features. P values less than .05 were considered significant. Abbreviations: HIV, human immunodeficiency virus; VC, viremic controller.
DISCUSSION
Understanding the mechanism(s) by which bNAbs arise naturally may provide the key for the rational development of a vaccine able to provide protection from HIV infection. The data presented here demonstrate that a subset of spontaneous controllers of HIV evolve neutralizing antibody breadth [4–9] despite the presence of low viral set-points. Moreover, neutralizing breadth in HIV controllers appears to evolve in the setting of low but persistent viral replication, in the absence of significant viral diversity, but in the presence of a unique cytokine profile. These data have implications for next generation immunization strategies, suggesting that modulation of cytokine profiles, for example, through the use of specific adjuvants coupled to stabilized trimers or sequential immunization strategies, may support the evolution of neutralization breadth.
Whether the observed cytokine profiles contribute to the evolution of neutralization breadth or are a simple biomarker of persisting viral replication within specific tissues is unclear. However, the cytokine signature observed in HIV controllers who evolve bNAbs represents a unique set of biomarkers that are intimately and centrally linked to B-cell activation and germinal center (GC) formation [26–28]. Yet, these cytokines may also mark persistent viral replication within GCs, an established viral sanctuary [29]. Thus, given that CXCL13 is readily produced within the GC [30], has been linked to the evolution of bNAbs [16], and is induced in a TLR7/8-dependent manner by HIV viral RNA [17], it is plausible that persisting levels of CXCL13 may act as a biomarker of a sustained antiviral response within the GC as well as drive the continued recruitment or retention of B cells in GCs. Conversely, although previous reports have pointed to CXCL13 as a critical biomarker for B-cell selection and the evolution of neutralizing antibody breadth [16], CXCL13 alone was insufficient to predict bNAb activity in our cohort of subjects with low viremia (Figure 1). Instead a cocktail of cytokines, many of which were correlated with viral levels in the blood (Figure 2), were required to segregate subjects with neutralizing breadth. Because distinct adjuvants have been shown to selectively drive unique cytokine profiles, including TLR7 agonists that mimic viral RNA activation [17], it is plausible that future custom adjuvanting approaches coupled to novel sustained release vaccine vehicles [31] could be coupled to emerging antigen-design strategies to selectively drive and maintain B-cell activation for continued B-cell selection and affinity maturation.
Despite the fact that emerging data suggest that the HIV viral reservoir selectively persists within GCs in the animal model of controllers [32], not all human controllers generate bNAbs, suggesting that maintaining a GC reservoir is not sufficient to drive bNAb evolution. Thus, our data suggest that vaccine strategies able to “depot” and retain a small number of viral antigens within GCs, linked to the persistent induction of “effective” cytokine profiles via the use of TLR7/8 adjuvants (that mimic viral RNA) may represent one approach for the elicitation of protective antibodies.
Contrary to previous studies highlighting the critical nature of viral diversity in the evolution of bNAbs, here, we observed only a marginal difference in mean viral diversity across VCs who develop significant neutralizing antibody breadth compared to those who do not. However, whether the controllers who evolve neutralization breadth were infected with unique viral sequences that enabled the recruitment of particular germline BCRs or whether these individuals harbor viruses that diversified rapidly [12, 13] or potentially possess greater viral diversity within their GCs where the virus may replicate predominantly [29] is unclear. Importantly, the envelope sequences sampled among the groups did not exhibit unique features and no peculiar germline selection profile was observed in our patient population (data not shown). Thus, in the context of previous reports suggesting the importance of viral diversity for the evolution of breadth [12, 13], here, we can postulate that: (1) early diversity, but not persistent diversity, may be critical to initiate the correct linages required for the evolution of breadth; (2) blood diversity may not represent tissue diversity that may be essential for driving the evolution of breadth; or (3) diversity may not be required to induce breadth, but that persisting antigen in the setting of a competent GC may be sufficient to drive effective broadening of the response. Although the first 2 hypotheses are currently being tested in the context of sequential vaccination strategies, aimed at introducing antigenic diversity to focus the immune response on conserved sites that may provide breadth of recognition [33], slow-release vaccine strategies, coupled to distinct adjuvants, may additionally contribute to improved GC persistence and quality addressed under the final hypothesis. Thus, future efforts aimed at combining both diversity and persistent inflammation strategies may mimic the events associated with bNAb evolution in the setting of low antigenemia, seen in controllers and take advantage of both the emerging viral and host signatures associated with the evolution of neutralizing antibody breadth.
Collectively, our results provide evidence that neutralizing antibody breadth may be achievable in the absence of high levels of viremia and viral diversity. Instead, low concentrations of a limited pool of antigens, linked to persistent GC activation and a unique cytokine profile are predictive of the development of neutralization breadth. Although persistent viremia may be necessary for the production of the cytokines/chemokines associated with B-cell selection and GC formation, specific adjuvants, alone or in combination, that drive similar inflammatory profiles may promote the development of neutralization breadth in the setting of persistent vaccine immunogens, providing a novel means by which next generation vaccines may elicit neutralization breadth.
Notes
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States. The contents of this manuscript are the responsibility of the authors and do not necessarily reflect the views of USAID or the US Government.
Financial support. This work was supported by the Bill and Melinda Gates Foundation CAVD (OPP1066973: Development of broadly neutralizing antibodies in HIV infection and following immunization and OPP1032817: Leveraging Antibody Effector Function), the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E, R01 AI080289, R01 A102660-01 and R37 AI080289-06A1 and the Ragon Institute of MGH, MIT, and Harvard. This work was partially funded by IAVI with the generous support of USAID, Ministry of Foreign Affairs of the Netherlands, and the Bill and Melinda Gates Foundation; a full list of IAVI donors is available at www.iavi.org.
Potential Conflicts of Interest. F. P. is an employee of Novartis Institutes for Biomedical Research; however, work completed in this manuscript occurred prior to her employment at NIBR. All other authors have no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Corti D, Langedijk JP, Hinz A, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One 2010; 5:e8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walker LM, Phogat SK, Chan-Hui PY, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 2009; 326:285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010; 329:856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog 2011; 7:e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sather DN, Armann J, Ching LK, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol 2009; 83:757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gray ES, Madiga MC, Moore PL, et al. Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region. J Virol 2009; 83:11265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simek MD, Rida W, Priddy FH, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol 2009; 83:7337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Gils MJ, Euler Z, Schweighardt B, Wrin T, Schuitemaker H. Prevalence of cross-reactive HIV-1-neutralizing activity in HIV-1-infected patients with rapid or slow disease progression. AIDS 2009; 23:2405–14. [DOI] [PubMed] [Google Scholar]
- 9. Doria-Rose NA, Klein RM, Daniels MG, et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol 2010; 84:1631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walker LM, Huber M, Doores KJ, et al. ; Protocol G Principal Investigators. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011; 477:466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mouquet H, Scheid JF, Zoller MJ, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature 2010; 467:591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doria-Rose NA, Schramm CA, Gorman J, et al. ; NISC Comparative Sequencing Program. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 2014; 509:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liao HX, Lynch R, Zhou T, et al. ; NISC Comparative Sequencing Program. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 2013; 496:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kong L, Ju B, Chen Y, et al. Key gp120 glycans pose roadblocks to the rapid development of VRC01-class antibodies in an HIV-1-infected Chinese donor. Immunity 2016; 44:939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Euler Z, van Gils MJ, Bunnik EM, et al. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. J Infect Dis 2010; 201:1045–53. [DOI] [PubMed] [Google Scholar]
- 16. Havenar-Daughton C, Lindqvist M, Heit A, et al. ; IAVI Protocol C Principal Investigators. CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci U S A 2016; 113:2702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen KW, Dugast AS, Alter G, McElrath MJ, Stamatatos L. HIV-1 single-stranded RNA induces CXCL13 secretion in human monocytes via TLR7 activation and plasmacytoid dendritic cell-derived type I IFN. J Immunol 2015; 194:2769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Medina-Ramírez M, Sánchez-Merino V, Sánchez-Palomino S, et al. Broadly cross-neutralizing antibodies in HIV-1 patients with undetectable viremia. J Virol 2011; 85:5804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gach JS, Achenbach CJ, Chromikova V, et al. HIV-1 specific antibody titers and neutralization among chronically infected patients on long-term suppressive antiretroviral therapy (ART): a cross-sectional study. PLoS One 2014; 9:e85371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheid JF, Mouquet H, Feldhahn N, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 2009; 458:636–40. [DOI] [PubMed] [Google Scholar]
- 21. Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med 2009; 15:866–70. [DOI] [PubMed] [Google Scholar]
- 22. Ferreira CB, Merino-Mansilla A, Llano A, et al. Evolution of broadly cross-reactive HIV-1-neutralizing activity: therapy-associated decline, positive association with detectable viremia, and partial restoration of B-cell subpopulations. J Virol 2013; 87:12227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis 2008; 197:563–71. [DOI] [PubMed] [Google Scholar]
- 24. Arnaout RA, Lloyd AL, O’Brien TR, Goedert JJ, Leonard JM, Nowak MA. A simple relationship between viral load and survival time in HIV-1 infection. Proc Natl Acad Sci U S A 1999; 96:11549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhiman JN, Anthony C, Doria-Rose NA, et al. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat Med 2015; 21:1332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol 2004; 172:3422–7. [DOI] [PubMed] [Google Scholar]
- 27. Zheng B, Ozen Z, Zhang X, et al. CXCL13 neutralization reduces the severity of collagen-induced arthritis. Arthritis Rheum 2005; 52:620–6. [DOI] [PubMed] [Google Scholar]
- 28. Mountz JD, Wang JH, Xie S, Hsu HC. Cytokine regulation of B-cell migratory behavior favors formation of germinal centers in autoimmune disease. Discov Med 2011; 11:76–85. [PMC free article] [PubMed] [Google Scholar]
- 29. Horiike M, Iwami S, Kodama M, et al. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology 2012; 423:107–18. [DOI] [PubMed] [Google Scholar]
- 30. Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med 1998; 187:655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bobbala S, Tamboli V, McDowell A, Mitra AK, Hook S. Novel injectable pentablock copolymer based thermoresponsive hydrogels for sustained release vaccines. AAPS J 2016; 18:261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fukazawa Y, Lum R, Okoye AA, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015; 21:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Escolano A, Steichen JM, Dosenovic P, et al. Sequential immunization elicits broadly neutralizing anti-HIV-1 antibodies in Ig knockin mice. Cell 2016; 166:1445–1458.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature 2003; 422:307–12. [DOI] [PubMed] [Google Scholar]
- 35. Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 2008; 105:7552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deng W, Maust BS, Nickle DC, et al. DIVEIN: a web server to analyze phylogenies, sequence divergence, diversity, and informative sites. Biotechniques 2010; 48:405–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lau KS, Juchheim AM, Cavaliere KR, Philips SR, Lauffenburger DA, Haigis KM. In vivo systems analysis identifies spatial and temporal aspects of the modulation of TNF-α-induced apoptosis and proliferation by MAPKs. Sci Signal 2011; 4:ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simmons RP, Scully EP, Groden EE, et al. HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. AIDS 2013; 27:2505–17. [DOI] [PMC free article] [PubMed] [Google Scholar]