Patients with burn injuries are at high risk for infections with multidrug-resistant organisms. The risk of infection with multidrug-resistant organisms increases with burn center length of stay. A multidisciplinary approach that includes an infectious diseases specialist is recommended.
Keywords: burn, multidrug resistance, pneumonia, mortality, trauma
Abstract
Patients who are admitted to the hospital after sustaining a large burn injury are at high risk for developing hospital-associated infections. If patients survive the initial 72 hours after a burn injury, infections are the most common cause of death. Ventilator-associated pneumonia is the most important infection in this patient population. The risk of infections caused by multidrug-resistant bacterial pathogens increases with hospital length of stay in burn patients. In the first days of the postburn hospitalization, more susceptible, Gram-positive organisms predominate, whereas later more resistant Gram-negative organisms are found. These findings impact the choice of empiric antibiotics in critically ill burn patients. A proactive infection control approach is essential in burn units. Furthermore, a multidisciplinary approach to burn patients with a team that includes an infectious disease specialist and a pharmacist in addition to the burn surgeon is highly recommended.
Burn injury is a frequent source of morbidity and mortality in the United States. In 2016, approximately 486000 patients received medical care for burn injuries of whom approximately 40000 required hospitalization. Importantly, 3275 patient deaths were attributed to burn injuries [1]. Burn injury results in a state of immune system dysregulation that predisposes patients to infection. The most obvious effect is the loss of the natural cutaneous barrier. Beyond this is a more complex interplay of pro- and anti-inflammatory signals that result in dysregulation of the innate and adaptive immune responses [2]. Furthermore, inhalation injury, endotracheal intubation, central venous access, arterial lines, urinary catheters, and prolonged hospitalization all contribute to increased risk of infection in burn patients [3].
With advancements in burn care over the last 50 years, infection is now the leading cause of death after extensive burn injuries. Multiple studies over the last decade have shown that 42%–65% of deaths in burn victims are attributable to infection [4–7]. In addition, burn patients with infections have more than twice the mortality rate of uninfected patients [8]. The prevalence of multidrug-resistant (MDR) bacteria in burn centers may result in the empiric selection of antibiotics that target MDR bacteria, thus propagating a vicious cycle of increased antimicrobial resistance. A multidisciplinary approach involving an infectious diseases physician and pharmacist working in collaboration with the burn surgeon may optimize care for this complex patient population.
COMMON INFECTIOUS SYNDROMES IN BURN PATIENTS
Infection is by far the most frequent complication encountered by patients with burn injuries. The 2016 National Burn Repository Report found that 7 of the 10 most frequent complications occurring in the burn patient were of an infectious etiology, with pneumonia, urinary tract infection (UTI), and cellulitis topping the list [1]. Recognizing the limitations of data collated by the National Burn Repository (voluntary reporting from burn centers with no standardized definitions for infections), respiratory tract infections are most frequently reported. Contributing factors include the presence of inhalation injury in some patients and the frequent need for prolonged mechanical ventilation. From 2006 to 2016, pneumonia occurred in 5.4% of all patients presenting with fire/flame burns [1]. Urinary tract infection was the second most frequently reported infectious complication in burn patients; a UTI complicated the hospital course of 3.4% of patients with fire/flame burns. This risk is likely associated with prolonged hospitalization and the frequent need for Foley catheters. Burn wound infection, septicemia, bacteremia, and miscellaneous other infections are among the other most frequent complications reported.
TIMELINE OF BACTERIAL INFECTION IN BURN PATIENTS
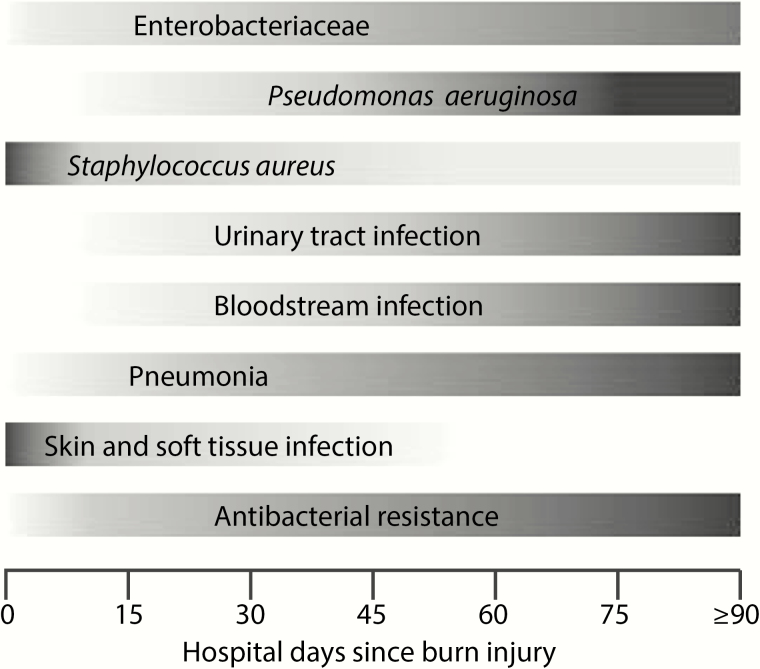
The pattern of hospital-associated infections (HAIs) in patients with burn injuries follows a relatively predictable timeline (Figure 1). Not surprisingly, skin and soft tissue infections occur earlier during hospitalization, generally during the first week. In contrast, pneumonia, bloodstream infections, and urinary tract infections tend to occur later in the hospitalization, each with a median onset >30 days after admission [9]. Several studies have shown that the length of hospitalization after a burn injury is associated with the types of bacterial species that are isolated from patients. In a retrospective study of 125 burn patients admitted to a Canadian burn center (2010–2013), the association between time since admission and the distribution of Gram-negative isolates from clinical cultures was described. Of note, the main change was seen in the contribution of Pseudomonas aeruginosa. Within the first 7 days of admission, P. aeruginosa was rare, constituting only 8% of all Gram-negative isolates. After 28 days of hospitalization, this increased to 55%. A sharp decline meanwhile was seen for Haemophilus influenzae; from 36% within 7 days to virtually absent after 7 days [10]. A similar increase associated with longer length of stay and the incidence of positive cultures with P. aeruginosa was seen in a single-center study of 5524 burn patients admitted from 2004 to 2013 [9]. In that same study, Gram-positive organisms tended to occur earlier during hospitalization as compared with Gram-negative bacteria. For instance, the median time from admission to first positive culture was 3 days (interquartile range [IQR], 2–8 days) for Staphylococcus aureus versus 18 days (IQR, 9–36 days) for P. aeruginosa [9].
Figure 1.
Timeline of common infections and pathogens after burn injury [9, 10]. Shown is the relative incidence over time of various common hospital-associated infections as well as common pathogens. Darker shading indicates increased relative incidence.
ANTIMICROBIAL-RESISTANT BACTERIAL PATHOGENS IN BURN PATIENTS
Pathogens of specific concern in the burn population include MDR strains of P. aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia and methicillin resistant S. aureus (MRSA). Outbreaks with carbapenem-resistant Enterobacteriaceae in burn units have also been described [11]. A single-center study from 2008 to 2012 reported rates of multidrug resistance in bacteria causing HAIs, as defined by the Centers for Disease Control and Prevention, of 33.8%, 90.8%, 21.1%, and 82% in Pseudomonas spp., A. baumannii, S. maltophilia, and S. aureus, respectively [12]. Focusing on respiratory isolates, a comparison of rates of multidrug resistance within intensive care units found 41% of burn intensive care unit respiratory isolates were MDR, whereas only 14% of isolates from other intensive care units in the same hospital were MDR [13]. A 2007 study in Miami revealed similarly alarming rates of MDR Acinetobacter spp., with 87% being both imipenem resistant and MDR [14]. Researchers from the military burn center at the Brooke Army Medical Center in Texas reported less dramatic but quite significant rates of MDR pathogens: 15% of Pseudomonas spp., 53% of Acinetobacter spp., and 34% of S. aureus [15].
RISK FACTORS FOR ACQUISITION OF MULTIDRUG-RESISTANT BACTERIA IN BURN PATIENTS
Although length of hospital stay in patients admitted for burn injury is obviously associated with various clinical characteristics such as burn size and the presence of inhalational injury, burn center length of stay is also a major risk factor for infection with MDR bacteria. For instance, in the above-mentioned Canadian study of 125 patients, 6% of bacterial species isolated during the first 7 days were MDR as compared with 44% after 28 days of hospitalization [10]. Furthermore, in the previously cited study of >5000 burn patients, the rates of MDR Gram-negative bacteria increased sharply during hospitalization [9]. From the first week of admission to week 4 or later, rate of Enterobacteriaceae per 1000 patient-days increased from 0.04 to 0.82 for carbapenem-resistant Enterobacteriaceae, 0.26 to 0.46 for extended-spectrum β-lactamase-producing Enterobacteriaceae, and 0.52 to 2.61 for fluoroquinolone-resistant Enterobacteriaceae. The rate of MDR Pseudomonas spp. similarly increased from 0.04 per 1000 patient-days in the first week to 1.85 per 1000 patient-days in the 4th week and later of admission [9].
In addition, risk factors that have been described in other populations for the acquisition of MDR organisms have also been reported for the burn population [16, 17]. Most important among these are previous antibiotic exposure and the use of invasive medical devices such as endotracheal tubes and urinary catheters.
PREVENTION OF MULTIDRUG-RESISTANT BACTERIAL INFECTIONS IN BURN PATIENTS
Infection prevention through a number of different strategies has been integral in the improvement of outcomes in patients with burn injuries (Table 1). As a consequence of prolonged hospitalizations and frequent invasive procedures, burn patients are at high risk for nosocomial infections. Infection control procedures such as hand hygiene, contact isolation, and environmental cleaning/disinfection are vital to reducing incidence of HAIs [18–20]. Multiple studies have shown the benefit of infection control strategies in preventing the spread of MDR organisms in burn patients [21, 22]. However, shared resources that are used in the care of multiple patients such as the hydrotherapy room (also known as the “tank room”) complicate the implementation of strict infection control measures. In a sustained outbreak of MDR P. aeruginosa in a Swiss burn unit, a cluster of 23 infected patients who were cared for over 3 years shared the same P. aeruginosa genotype (DLST 1–18). This genotype was also recovered from 2 hydrotherapy rooms in which 19 of the 23 infected patients had been treated, and the outbreak was controlled by instituting environmental cleaning/disinfection procedures aimed primarily at the hydrotherapy room [23].
Table 1.
Prevention and Management of Multidrug-Resistant Bacterial Infections in Burn Injury
| Strategies based on evidence-based clinical practice guidelines | |
|---|---|
| Generally accepted | 1. Hand hygiene protocols [18, 34] 2. Strict environmental cleaning/disinfection protocols [20, 34, 55, 56] 3. Daily evaluation for invasive device need and early removal [19, 26] 4. Prompt removal of catheters colonized with biofilm-producing pathogens in the setting of infection [48, 49] 5. Creation of a local burn unit antibiogram to assist with empirical antibiotics [50, 51] 6. Contact isolation of patients known to have multidrug-resistant organisms [56] 7. Involvement of an antimicrobial stewardship program [34, 51, 52] 8. Early excision and graft of full thickness burns when feasible [34, 57] 9. Use of topical antimicrobial agents for burn wounds in conjunction with debridement [31, 35, 58] |
| Remain controversial | 10. Adding US Food and Drug Administration–approved inhaled antibiotics to systemic antibiotics for ventilator-associated pneumonia due to Gram-negative organism susceptible only to aminoglycosides and polymyxins [59, 60] 11. Avoiding prophylactic systemic antibiotics for inhalation injury or large burn wounds [32–34] 12. Avoiding routine central venous catheter changes [26–29] |
| Strategies based on clinical studies | |
| Generally accepted | 13. Engagement of hospital epidemiology for infection prevention [61] 14. Involvement of infectious diseases specialist [62–64] 15. Performing bronchoscopy to assist with diagnosis of ventilator-associated pneumonia in patients with inhalation injury [36] |
| Remain controversial | 16. Use of procalcitonin to guide antibiotic duration in sepsis [45, 46] 17. Avoid universal glove and gown use in intensive care units [22, 65, 66] 18. Avoid routine bronchoscopy in burn patients without inhalational injury to assist in diagnosis of pneumonia [37, 38] 19. Routine surveillance cultures of burn patients to detect asymptomatic colonization with multidrug-resistant organisms [24, 25, 67–69] 20. Using the modified Marshall multiple organ dysfunction scoring system after postburn day 3 to recognize sepsis-related organ dysfunction in burn patients [43] 21. Use of perioperative antibiotics for excision and grafting procedures [32, 35] |
| Strategies based on case series and expert opinion | |
| Generally accepted | 22. Obtaining wound swab or biopsy cultures in septic patients [39–41] 23. Involvement of experienced pharmacy personnel to assist with optimizing antimicrobial dosing for burn patients [53, 54] 24. Cohorting patients with multidrug-resistant organisms during an outbreak [56] 25. Ward closure aid in management of difficult to control outbreaks [70, 71] |
| Remain controversial | 26. Defining sepsis as documented infection plus 3 of 6 triggers (hyper/hypoglycemia, tachycardia, tachypnea, thrombocytopenia, hyperglycemia, and inability to tolerate enteral feeds) [43] 27. Avoiding routine quantitative wound cultures in stable patients [40, 41] |
A variety of practices have been used for screening for MDR organisms in burn units, including weekly surveillance for MRSA and vancomycin-resistant Enterococci and outbreak-guided surveillance [11, 24]. An approach of thrice weekly endotracheal aspirate surveillance cultures in patients with inhalation injury was reported to predict MDR organisms in subsequent ventilator-associated pneumonia (VAP) with a sensitivity of 83% and specificity of 96% [25]. Although more data are still needed to define whether active surveillance cultures should be instituted routinely in burn centers, the high rates of MDR organisms in burn units warrant careful consideration of the costs, risks, and benefits of various screening approaches.
Management of intravascular catheters in the burn population is a controversial topic. Although guidelines from the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America recommend that central venous catheters should not be routinely replaced, most burn units use this practice, based on a scarcity of data in the burn population [26, 27]. A single center study performed in 2000–2001 compared patients (n = 17) in whom central venous catheters were replaced every 4th day with historical controls (n = 38), who underwent every 3rd day routine replacement [28]. The number of catheter-related bloodstream infections (defined as those bacteremias in which the causative organism was also grown from a catheter segment culture) increased from 0.18 per patient in the every 3rd day historical control group to 1.18 per patient in the every 4th day group [28]. Another study looked at increasing time between line exchanges from every 48 hours to every 72 hours and found no increase in rates of line-related infections [29]. Based on these limited studies, many burn units perform routine line exchanges every 72 hours. Clearly, a multicenter, randomized controlled trial is needed to determine whether any routine changes are indicated in this population and, if so, to determine the appropriate interval between line exchanges.
From a surgical perspective, early excision of burn wounds and grafting of full thickness burns has been found to significantly decrease rates of mortality and may also reduce the incidence of infection [30]. In addition, meticulous wound care focusing on removal of devitalized tissue should be performed as a routine part of the care of patients with burn wounds. Topical antimicrobials such as mafenide and silver sulfadiazine, in combination with early excision, have been associated with a decline in the incidence of sepsis due to burn wound infections [31]. Prophylactic systemic antibiotics to prevent infection have not been shown to be efficacious in multiple studies in burn patients. A Cochrane review in 2013 evaluated 36 studies and concluded there was not sufficient evidence to recommend their usage [32]. However, a retrospective Japanese study in 2016 found that using either a first-generation cephalosporin or ampicillin/sulbactam as prophylaxis improved 28-day mortality in patients with severe burns who required mechanical ventilation [33]. If confirmed in other independent studies, these results raise the possibility that prophylaxis may be useful in a subset of burn patients. Currently, however, systemic prophylactic antibiotics are not recommended by the International Society for Burn Injury [34]. Data regarding the utility of routine perioperative antibiotics in burn management are also inconclusive, but experts suggest that the use of perioperative antibiotics for excision and grafting procedures may be considered [35].
DIAGNOSIS OF MULTIDRUG-RESISTANT BACTERIAL INFECTIONS IN BURN PATIENTS
As in other critically ill populations, the main challenge in the diagnosis of MDR bacterial infection in burn patients is making the distinction between infection and colonization (Table 1). Colonization tends to precede infection, and often a clear transition point from one state into the next is not clinically apparent. Patients who undergo prolonged mechanical ventilation, which is quite common after a large burn, will inevitably develop respiratory tract colonization as well as endotracheal or tracheostomy tube colonization. Similarly, urinary bacterial colonization is almost universal in the setting of long-term in-dwelling uretheral catheterization. Unfortunately, most patients with large burns are critically ill and are unable to provide clinical information, which is so crucial in diagnosing infection.
Both inhalation injury and acute respiratory distress syndrome secondary to burn injury complicate the diagnosis of VAP. An analysis of National Burn Repository data found that among patients with inhalation injury and pneumonia, those who underwent bronchoscopy, compared with patients who did not, had an 18% reduced risk of death [36]. However, the benefit of preforming bronchoscopy in all burn patients to diagnosis pneumonia is less clear [37]. Detecting an MDR organism causing VAP with bronchoscopy at time of diagnosis or after 4 days of treatment may assist in deciding on the duration of antimicrobial therapy, allowing less virulent organisms to be treated with a shorter duration of antibiotics [38].
Burn cellulitis and invasive burn wound infection can be difficult to distinguish from noninfectious burn erythema. Tissue biopsy for histology, which is often not performed due to labor and cost, remains the gold standard for diagnosis of invasive wound infection [39]. Methods for semiquantitative surface swab cultures and quantitative tissue biopsy culture have been well-described for burn patients, and historically high bacterial counts have been used to define infection in some studies [39]. Some experts recommend performing routine infection surveillance of burn wounds using swab cultures for excised burns and areas of skin too thin to biopsy and biopsy for tissue below eschars [39]. However, one study team demonstrated that a single quantitative swab or biopsy may not be representative of all pathogens involved. The same investigators found that neither quantitative bacterial counts from swabs nor biopsies at the time of excision or dressing change were able to predict graft loss, bacteremia within 1 hour of wound manipulation, or clinical failure (defined as need for antibiotics within 72 hours, new appearance of fever, rigors, hypotension, or graft loss) [40, 41]. Based on such findings, swab cultures and biopsies should probably be limited to patients with changes in wound appearance or signs of systemic infection to avoid missing a source of infection, especially one caused by an MDR organism.
Recognizing sepsis in the burn population is also challenging because other systemic indicators of infection such as fever, hypotension, and elevated peripheral blood white blood cell count are quite common in uninfected burn patients [42]. In 2007, the American Burn Association Consensus Conference to Define Sepsis and Infection in Burns defined sepsis as a documented infection plus 3 of 6 triggers (hyper/hypothermia, tachycardia, tachypnea, thrombocytopenia, hyperglycemia, and inability to tolerate enteral feeding). The committee promoted the modified Marshall multiple organ dysfunction scoring system (after day 3 postburn) as the best tool for recognizing sepsis-related organ dysfunction in burn patients [43]. However, because no criteria for sepsis have performed satisfactorily in clinical studies of burn patients, the search continues 10 years later for the best strategy to diagnosis sepsis in this population [44].
The measurement of procalcitonin may show promise in diagnosis of sepsis in burn patients, although its exact role remains to be determined [45]. The introduction of a procalcitonin-based antibiotic algorithm resulted in antibiotic therapy being discontinued 5 days earlier, on average, in a small observational study [46]. Given the high incidence of MDR bacterial infections, rapid diagnostics that indicate the presence or absence of MDR phenotype have great potential in the burn population. These may be used as screening tools for MDR bacterial carriage, as well as for rapid diagnosis of MDR bacterial infection.
TREATMENT OF MULTIDRUG-RESISTANT BACTERIAL INFECTIONS IN BURN PATIENTS
Once the decision is made to treat a burn patient with suspected or confirmed MDR bacterial infection, a number of specific issues should be taken into account. As in all infected patients, timely source control when feasible is crucial. For example, in patients with burn wound cellulitis and deeper skin and soft tissue infections, excision of the burn eschar will usually lead to rapid resolution of the infection [47]. Similarly, the prompt removal of infected catheters, especially infection with biofilm-producing pathogens, is recommended to improve outcomes [48, 49].
Knowledge of the local burn unit antibiogram, which may be quite different from the rest of the hospital, is essential for the optimization of empirical antibiotics [50]. At the same time, involvement of an antimicrobial stewardship program (ASP) is highly recommended to limit antibiotic exposure in patients when antibiotics are not necessary and therefore prevent future infection with MDR bacteria [34, 51]. The results of a recent systematic review of inpatient ASPs suggest that ASPs can improve prescribing and institutional resistance patterns without a large negative impact on patient outcomes [52]. The International Society for Burn Injury practice guidelines recommend that burn centers develop, implement, and monitor a local ASP, which will allow for investigation of patient and microbial resistance outcomes of ASPs specifically in burn populations [34].
Antibiotic dosing in patients with large burn injuries is complicated by a hyperdynamic state that often results in increased renal clearance of commonly used antibiotics [53, 54]. This hyperdynamic state displays high intrapatient and interpatient variability; therefore, patients may require higher than usual or more frequent antibiotic doses. Cota et al reviewed available data and modeling to derive evidence-based dosing for 15 antibiotics for patients with ≥20% total body surface area‑ burn and normal renal function after 48 hours of admission [53]. Clearly, inclusion of a dedicated pharmacist in the multidisciplinary burn team is advisable.
Similar to other populations, a number of questions regarding MDR bacterial infection treatment remain unanswered in burn patients. These include the impact of combination therapy on outcomes as well as on subsequent resistance development. Also, the role of newer agents directed at MDR bacteria such as novel cephalosporins, cephalosporin/β-lactamase inhibitor combinations, long-acting anti-MRSA antibiotics, and others, remains to be determined in the burn population.
CONCLUSIONS
Patients who have sustained a large burn injury are at risk for HAIs. With increasing duration of hospitalization, they are at increasing risk of MDR bacterial infections. The high prevalence of MDR bacteria in burn units is likely a consequence of several factors, including high antibiotic pressures, high colonization pressures, need for intensive medical and surgical therapy, and a vulnerable, immunocompromised patient population. Prevention of spread of MDR bacteria in this population similarly needs to consist of a multipronged approach that includes hand hygiene, antibacterial stewardship, optimization of surgical interventions, thoughtful use of medical devices, and environmental control. Involvement of an infectious diseases specialist in this process as well as in the day-to-day care of these complex patients is highly recommended.
Notes
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of General Medical Sciences (grant 5T32GM008450-23 to C. G. H.), the National Center for Advancing Translational Sciences, NIH (grant KL2TR001109 to A. M. L.), and by the National Center for Advancing Translational Sciences, NIH (grant UL1TR001111 to D. v. D.).
Potential conflicts of interest. D.v.D. has served as a consultant for Allergan, Achaogen, Shionogi, Tetraphase, Sanofi-Pasteur, Medimmune and Astellas, and has received research funding from Steris Inc. and Scynexis. A. M. L. has served as a consultant for Destum Partners and KPB Biosciences and received research funding from GlaxoSmithKline. D. J. W. has served on the advisory board for Pfizer and Merck. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. National Burn Repository 2016 Report. Chicago, IL: American Burn Association; 2016. [Google Scholar]
- 2. Neely CJ, Kartchner LB, Mendoza AE et al. . Flagellin treatment prevents increased susceptibility to systemic bacterial infection after injury by inhibiting anti-inflammatory IL-10+ IL-12- neutrophil polarization. PLoS One 2014; 9:e85623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schultz L, Walker SA, Elligsen M et al. . Identification of predictors of early infection in acute burn patients. Burns 2013; 39:1355–66. [DOI] [PubMed] [Google Scholar]
- 4. Krishnan P, Frew Q, Green A, Martin R, Dziewulski P. Cause of death and correlation with autopsy findings in burns patients. Burns 2013; 39:583–8. [DOI] [PubMed] [Google Scholar]
- 5. Keen EF 3rd, Robinson BJ, Hospenthal DR et al. . Incidence and bacteriology of burn infections at a military burn center. Burns 2010; 36:461–8. [DOI] [PubMed] [Google Scholar]
- 6. Bloemsma GC, Dokter J, Boxma H, Oen IM. Mortality and causes of death in a burn centre. Burns 2008; 34:1103–7. [DOI] [PubMed] [Google Scholar]
- 7. Sharma BR, Harish D, Singh VP, Bangar S. Septicemia as a cause of death in burns: an autopsy study. Burns 2006; 32:545–9. [DOI] [PubMed] [Google Scholar]
- 8. Alp E, Coruh A, Gunay GK, Yontar Y, Doganay M. Risk factors for nosocomial infection and mortality in burn patients: 10 years of experience at a university hospital. J Burn Care Res 2012; 33:379–85. [DOI] [PubMed] [Google Scholar]
- 9. van Duin D, Strassle PD, DiBiase LM et al. . Timeline of health care-associated infections and pathogens after burn injuries. Am J Infect Control 2016; 44:1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wanis M, Walker SA, Daneman N et al. . Impact of hospital length of stay on the distribution of Gram negative bacteria and likelihood of isolating a resistant organism in a Canadian burn center. Burns 2016; 42:104–11. [DOI] [PubMed] [Google Scholar]
- 11. Kanamori H, Parobek CM, Juliano JJ et al. . A prolonged outbreak of KPC-3-producing Enterobacter cloacae and Klebsiella pneumoniae driven by multiple mechanisms of resistance transmission at a large academic burn center. Antimicrob Agents Chemother 2017; 61:pii:e01516-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber DJ, van Duin D, DiBiase LM et al. . Healthcare-associated infections among patients in a large burn intensive care unit: incidence and pathogens, 2008–2012. Infect Control Hosp Epidemiol 2014; 35:1304–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lachiewicz AM, van Duin D, DiBiase LM et al. . Rates of hospital-associated respiratory infections and associated pathogens in a regional burn center, 2008–2012. Infect Control Hosp Epidemiol 2015; 36:601–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trottier V, Segura PG, Namias N, King D, Pizano LR, Schulman CI. Outcomes of Acinetobacter baumannii infection in critically ill burned patients. J Burn Care Res 2007; 28:248–54. [DOI] [PubMed] [Google Scholar]
- 15. Keen EF 3rd, Robinson BJ, Hospenthal DR et al. . Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns 2010; 36:819–25. [DOI] [PubMed] [Google Scholar]
- 16. Cavalcante Rde S, Canet P, Fortaleza CM. Risk factors for the acquisition of imipenem-resistant Acinetobacter baumannii in a burn unit: an appraisal of the effect of colonization pressure. Scand J Infect Dis 2014; 46:593–8. [DOI] [PubMed] [Google Scholar]
- 17. Wibbenmeyer L, Williams I, Ward M et al. . Risk factors for acquiring vancomycin-resistant Enterococcus and methicillin-resistant Staphylococcus aureus on a burn surgery step-down unit. J Burn Care Res 2010; 31:269–79. [DOI] [PubMed] [Google Scholar]
- 18. Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep 2002; 51:1–45; quiz CE41-44. [PubMed] [Google Scholar]
- 19. Yokoe DS, Anderson DJ, Berenholtz SM et al. ; Society for Healthcare Epidemiology of America (SHEA) A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol 2014; 35:967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sehulster L, Chinn RY; CDC; HICPAC Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52:1–42. [PubMed] [Google Scholar]
- 21. Simor AE, Lee M, Vearncombe M et al. . An outbreak due to multiresistant Acinetobacter baumannii in a burn unit: risk factors for acquisition and management. Infect Control Hosp Epidemiol 2002; 23:261–7. [DOI] [PubMed] [Google Scholar]
- 22. van Duin D, Jones SW, Dibiase L et al. . Reduction in central line-associated bloodstream infections in patients with burns. Infect Control Hosp Epidemiol 2014; 35:1066–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tissot F, Blanc DS, Basset P et al. . New genotyping method discovers sustained nosocomial Pseudomonas aeruginosa outbreak in an intensive care burn unit. J Hosp Infect 2016; 94:2–7. [DOI] [PubMed] [Google Scholar]
- 24. Wibbenmeyer L, Appelgate D, Williams I et al. . Effectiveness of universal screening for vancomycin-resistant enterococcus and methicillin-resistant Staphylococcus aureus on admission to a burn-trauma step-down unit. J Burn Care Res 2009; 30:648–56. [DOI] [PubMed] [Google Scholar]
- 25. Brusselaers N, Logie D, Vogelaers D, Monstrey S, Blot S. Burns, inhalation injury and ventilator-associated pneumonia: value of routine surveillance cultures. Burns 2012; 38:364–70. [DOI] [PubMed] [Google Scholar]
- 26. O’Grady NP, Alexander M, Burns LA et al. ; Healthcare Infection Control Practices Advisory Committee (HICPAC) Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011; 52:e162–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sood G, Heath D, Adams K et al. . Survey of central line-associated bloodstream infection prevention practices across American Burn Association–certified adult burn units. Infect Control Hosp Epidemiol 2013; 34:439–40. [DOI] [PubMed] [Google Scholar]
- 28. King B, Schulman CI, Pepe A, Pappas P, Varas R, Namias N. Timing of central venous catheter exchange and frequency of bacteremia in burn patients. J Burn Care Res 2007; 28:859–60. [DOI] [PubMed] [Google Scholar]
- 29. Kagan RJ, Neely AN, Rieman MT et al. . A performance improvement initiative to determine the impact of increasing the time interval between changing centrally placed intravascular catheters. J Burn Care Res 2014; 35:143–7. [DOI] [PubMed] [Google Scholar]
- 30. Norbury W, Herndon DN, Tanksley J, Jeschke MG, Finnerty CC. Infection in burns. Surg Infect (Larchmt) 2016; 17:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown TP, Cancio LC, McManus AT, Mason AD Jr. Survival benefit conferred by topical antimicrobial preparations in burn patients: a historical perspective. J Trauma 2004; 56:863–6. [DOI] [PubMed] [Google Scholar]
- 32. Barajas-Nava LA, Lopez-Alcalde J, Roque i Figuls M, Sola I, Bonfill Cosp X. Antibiotic prophylaxis for preventing burn wound infection. Cochrane Database Syst Rev 2013; 6:CD008738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tagami T, Matsui H, Fushimi K, Yasunaga H. Prophylactic antibiotics may improve outcome in patients with severe burns requiring mechanical ventilation: propensity score analysis of a Japanese Nationwide Database. Clin Infect Dis 2016; 62:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Isbi Practice Guidelines Committee. ISBI practice guidelines for burn care. Burns 2016; 42:953–1021. [DOI] [PubMed] [Google Scholar]
- 35. D’Avignon LC, Chung KK, Saffle JR, Renz EM, Cancio LC; Prevention of Combat-Related Infections Guidelines Panel Prevention of infections associated with combat-related burn injuries. J Trauma 2011; 71:S282–9. [DOI] [PubMed] [Google Scholar]
- 36. Carr JA, Phillips BD, Bowling WM. The utility of bronchoscopy after inhalation injury complicated by pneumonia in burn patients: results from the National Burn Repository. J Burn Care Res 2009; 30:967–74. [DOI] [PubMed] [Google Scholar]
- 37. Wahl WL, Franklin GA, Brandt MM et al. . Does bronchoalveolar lavage enhance our ability to treat ventilator-associated pneumonia in a trauma-burn intensive care unit? J Trauma 2003; 54:633–8; discussion 638–639. [DOI] [PubMed] [Google Scholar]
- 38. Wahl WL, Taddonio MA, Arbabi S, Hemmila MR. Duration of antibiotic therapy for ventilator-associated pneumonia in burn patients. J Burn Care Res 2009; 30:801–6. [DOI] [PubMed] [Google Scholar]
- 39. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev 2006; 19:403–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Steer JA, Papini RP, Wilson AP, McGrouther DA, Parkhouse N. Quantitative microbiology in the management of burn patients. II. Relationship between bacterial counts obtained by burn wound biopsy culture and surface alginate swab culture, with clinical outcome following burn surgery and change of dressings. Burns 1996; 22:177–81. [DOI] [PubMed] [Google Scholar]
- 41. Steer JA, Papini RP, Wilson AP, McGrouther DA, Parkhouse N. Quantitative microbiology in the management of burn patients. I. Correlation between quantitative and qualitative burn wound biopsy culture and surface alginate swab culture. Burns 1996; 22:173–6. [DOI] [PubMed] [Google Scholar]
- 42. Lavrentieva A, Kontakiotis T, Lazaridis L et al. . Inflammatory markers in patients with severe burn injury. What is the best indicator of sepsis? Burns 2007; 33:189–94. [DOI] [PubMed] [Google Scholar]
- 43. Greenhalgh DG, Saffle JR, Holmes JH 4th et al. . American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res 2007; 28:776–90. [DOI] [PubMed] [Google Scholar]
- 44. Greenhalgh DG. Defining sepsis in burn patients: still a long way to go. J Burn Care Res 2017; doi: 10.1097/BCR.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 45. Cabral L, Afreixo V, Almeida L, Paiva JA. The use of procalcitonin (PCT) for diagnosis of sepsis in burn patients: a meta-analysis. PLoS One 2016; 11:e0168475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lavrentieva A, Kontou P, Soulountsi V, Kioumis J, Chrysou O, Bitzani M. Implementation of a procalcitonin-guided algorithm for antibiotic therapy in the burn intensive care unit. Ann Burns Fire Disasters 2015; 28:163–70. [PMC free article] [PubMed] [Google Scholar]
- 47. Pruitt BA Jr, McManus AT, Kim SH, Goodwin CW. Burn wound infections: current status. World J Surg 1998; 22:135–45. [DOI] [PubMed] [Google Scholar]
- 48. Mermel LA, Allon M, Bouza E et al. . Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Raz R, Schiller D, Nicolle LE. Chronic indwelling catheter replacement before antimicrobial therapy for symptomatic urinary tract infection. J Urol 2000; 164:1254–8. [PubMed] [Google Scholar]
- 50. Binkley S, Fishman NO, LaRosa LA et al. . Comparison of unit-specific and hospital-wide antibiograms: potential implications for selection of empirical antimicrobial therapy. Infect Control Hosp Epidemiol 2006; 27:682–7. [DOI] [PubMed] [Google Scholar]
- 51. Barlam TF, Cosgrove SE, Abbo LM et al. . Implementing an Antibiotic Stewardship Program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wagner B, Filice GA, Drekonja D et al. . Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect Control Hosp Epidemiol 2014; 35:1209–28. [DOI] [PubMed] [Google Scholar]
- 53. Cota JM, FakhriRavari A, Rowan MP, Chung KK, Murray CK, Akers KS. Intravenous antibiotic and antifungal agent pharmacokinetic-pharmacodynamic dosing in adults with severe burn injury. Clin Ther 2016; 38:2016–31. [DOI] [PubMed] [Google Scholar]
- 54. Jamal JA, Economou CJ, Lipman J, Roberts JA. Improving antibiotic dosing in special situations in the ICU: burns, renal replacement therapy and extracorporeal membrane oxygenation. Curr Opin Crit Care 2012; 18:460–71. [DOI] [PubMed] [Google Scholar]
- 55. Rutala WA, Weber DJ. Disinfection and sterilization: an overview. Am J Infect Control 2013; 41:S2–5. [DOI] [PubMed] [Google Scholar]
- 56. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control 2007; 35(10 suppl 2): S165–93. [DOI] [PubMed] [Google Scholar]
- 57. Barret JP, Herndon DN. Effects of burn wound excision on bacterial colonization and invasion. Plast Reconstr Surg 2003; 111:744–50; discussion 751–2. [DOI] [PubMed] [Google Scholar]
- 58. Hospenthal DR, Murray CK, Andersen RC et al. ; Infectious Diseases Society of America; Surgical Infection Society Guidelines for the prevention of infections associated with combat-related injuries: 2011 update: endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J Trauma 2011; 71:S210–34. [DOI] [PubMed] [Google Scholar]
- 59. Kalil AC, Metersky ML, Klompas M et al. . Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Daniels LM, Juliano J, Marx A, Weber DJ. Inhaled antibiotics for hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis 2017; 64:386–7. [DOI] [PubMed] [Google Scholar]
- 61. Haley RW, Culver DH, White JW et al. . The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol 1985; 121:182–205. [DOI] [PubMed] [Google Scholar]
- 62. Jeng JC, Shoham S. Leveraging the unique expertise of our clinical colleagues: a real-world example for collaborative harnessing of the National Burn Repository. J Burn Care Res 2008; 29:704–5. [DOI] [PubMed] [Google Scholar]
- 63. Schmitt S, McQuillen DP, Nahass R et al. . Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis 2014; 58:22–8. [DOI] [PubMed] [Google Scholar]
- 64. Vogel M, Schmitz RP, Hagel S et al. . Infectious disease consultation for Staphylococcus aureus bacteremia—a systematic review and meta-analysis. J Infect 2016; 72:19–28. [DOI] [PubMed] [Google Scholar]
- 65. Harris AD, Pineles L, Belton B et al. ; Benefits of Universal Glove and Gown (BUGG) Investigators Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013; 310:1571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McManus AT, Mason AD Jr, McManus WF, Pruitt BA Jr. A decade of reduced Gram-negative infections and mortality associated with improved isolation of burned patients. Arch Surg 1994; 129:1306–9. [DOI] [PubMed] [Google Scholar]
- 67. Hacek DM, Paule SM, Thomson RB Jr, Robicsek A, Peterson LR. Implementation of a universal admission surveillance and decolonization program for methicillin-resistant staphylococcus aureus (MRSA) reduces the number of MRSA and total number of S. aureus isolates reported by the clinical laboratory. J Clin Microbiol 2009; 47:3749–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huskins WC, Huckabee CM, O’Grady NP et al. ; STAR*ICU Trial Investigators Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med 2011; 364:1407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huang SS, Yokoe DS, Hinrichsen VL et al. . Impact of routine intensive care unit surveillance cultures and resultant barrier precautions on hospital-wide methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2006; 43:971–8. [DOI] [PubMed] [Google Scholar]
- 70. Wong H, Eso K, Ip A et al. . Use of ward closure to control outbreaks among hospitalized patients in acute care settings: a systematic review. Syst Rev 2015; 4:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Girerd-Genessay I, Bénet T, Vanhems P. Multidrug-resistant bacterial outbreaks in burn units: a synthesis of the literature according to the ORION statement. J Burn Care Res 2016; 37:172–80. [DOI] [PubMed] [Google Scholar]