Abstract
Recently, significant progress has been made in ART for the treatment of male infertility. However, current ART has failed to help infertile patients with non-obstructive azoospermia, unless donor sperm is used. In fact, most couples wish to have their own genetically related child. Human induced pluripotent stem cells (hiPSCs) can be generated from patients’ somatic cells and in vitro derivation of functional germ cells from patient-specific iPSCs may provide new therapeutic strategies for infertile couples. The overall developmental dynamics of human primordial germ cells are similar to that in mice, but accumulating evidence suggests that there are crucial differences between human and mouse PGC specification. Unlike mouse iPSCs (miPSCs) in naive state, hiPSCs exhibit a primed pluripotency which possess less potential for the germ cell fate. Based on research in mice, male germ cells at different stages have been derived from hiPSCs with different protocols, including spontaneous differentiation, overexpression of germ cell regulators, addition of cytokines, co-culture with gonadal cells in vitro and xeno-transplantation. The aim of this review is to summarize the current advances in derivation of male germ cells from hiPSCs and raise the perspectives of hiPSCs in medical application for male infertility, as well as in basic research for male germ cell development.
Keywords: induced pluripotent stem cells, embryonic stem cells, male infertility, primordial germ cells, germ cell differentiation, reproductive medicine, gene editing, extracellular vesicles
Introduction
Infertility is a global public health concern with a high prevalence in couples at reproductive age (Inhorn and Patrizio, 2015). Male infertility accounts for approximately half of all cases of infertility, and non-obstructive azoospermia is the most severe form (Miyamoto et al., 2015). Besides genetic factors, azoospermia also occurs due to injuries, exposure to toxicants, immune-suppressive and anticancer treatments (Skakkebaek et al., 2001; Bhartiya et al., 2014). However, a large proportion of infertile males are diagnosed as idiopathic with unknown causes, reflecting poor understanding of the mechanisms regulating spermatogenesis and sperm function in humans (Ferlin et al., 2007).
Recently, significant progress has been made in ART for the treatment of infertility. However, current ART has been unable to help the infertile couples who lack functional gametes, unless donor gametes were used. In fact, most couples wish to have their own genetically related child (Ishii, 2014). With the rapid development of stem cell technology, the possibility to derive artificial gametes from human pluripotent stem cells may provide new therapeutic strategies for infertile couples.
Embryonic stem cells (ESCs) can differentiate into male germ-like cells in vitro, but they are genetically unrelated to the patients, and the sources of human ESCs (hESCs) are limited and accompanied by ethical issues about destruction of embryos (Devolder, 2010; Hou et al., 2014). The ectopic expression of four transcription factors (OCT4, SOX2, KLF4 and MYC) leads to the reprogramming of somatic cells to induced pluripotent stem cells (iPSCs) which resemble ESCs in morphology, pluripotency marker expression and differentiation ability (Park et al., 2008; Takahashi and Yamanaka, 2006). To some extent, human iPSCs (hiPSCs) are superior to hESCs for reproductive medicine application because there are few ethical issues and the sources are abundant. Furthermore, hiPSCs can be generated from patients’ somatic cells and are immuno-compatible for auto-transplantation. So the generation of patient-specific spermatozoa from hiPSCs will provide the foundation for future treatment of male infertility. However, hiPSCs may not faithfully recapitulate the characteristics of hESCs at both genetic and epigenetic levels (Bock, et al., 2011; Doi, et al., 2009). Especially, hiPSCs are reported to keep some epigenetic marks of the donor cell type from which they were reprogrammed (Kim, et al., 2011).
All in all, the discovery of hiPSCs may not only lead to clinical approaches addressing infertility resulting from defects in gametogenesis but also provide an opportunity to investigate the molecular mechanism of human germ cell development. In this review, we summarize the current advances in derivation of male germ cells from hiPSCs and raise perspectives of hiPSCs in medical application for the treatment of male infertility, as well as for basic research into the mechanisms of human germ cell development.
Discussion
Specification of human male germ line cells
Primordial germ cells (PGCs) are founder cells of the germ line and are specified during early embryonic development in mammals. Mouse PGC (mPGC) specification has been studied extensively, which provides a valuable model for mammalian development. Briefly, mPGC specification is initiated by bone morphogenetic protein (BMP) and WNT signals from extra-embryonic tissues which induce the expression of PGC fate regulator genes in a few germ line competent cells of the early post-implantation embryo (Saitou et al., 2005; Weber et al., 2010; Yamaji et al., 2008). The mPGCs at the base of the allantois begin to migrate and colonize the genital ridge, accompanied with genome-wide epigenetic reprogramming to erase imprints and other somatic epigenetic memories (Lawson and Hage, 1994; Tang et al., 2016). Post-migration PGCs start sex-specific development and the male germ cells undergo mitotic arrest, indicating the end of the PGC stage of germ line development (McLaren, 2003).
The overall developmental dynamics of human PGCs (hPGCs) are similar to that in mice, but crucial differences exist. Human PGCs express several lineage regulatory genes that are absent in mPGCs, such as trophectoderm regulator TEA domain transcription factor 4 (TEAD4) and endoderm regulator SRY-box 17 (SOX17); also hPGCs lack the core pluripotency gene SOX2 but express naive pluripotency factors transcription factor CP2 like 1 (TFCP2L1) and Kruppel like factor 4 (KLF4) (de Jong et al., 2008; Perrett et al., 2008; Tang et al., 2015). Intriguingly, SOX17 is a critical specifier of hPGC fate but is dispensable for mPGC specification (Hara et al., 2009; Irie et al., 2015). WNT signals induce the expression of eomesodermin (EOMES) to activate SOX17 for human PGC-like cells (PGCLCs) specification (Kojima, et al., 2017). Knockdown of SOX17 induces the repression of PR/SET domain 1 (PRDM1) expression, indicating that PRDM1 acts downstream of SOX17 (Irie et al., 2015). Additionally, it is known that a tripartite transcription factor network of PRDM1, PRDM14, and transcription factor AP-2 gamma (TFAP2C) represses the somatic fate and promotes the PGC specification in mice (Magnusdottir et al., 2013). However, studies suggest that PRDM14 has a less prominent role in hPGC development, and more research is needed to verify the role of PRDM14 in human germ line development (Guo et al., 2015; Sugawa et al., 2015). Thus, SOX17 and PRDM1 contribute to the human germ cell development, whereas the role of TFAP2C and PRDM14 in hPGC specification remains to be fully addressed.
After colonizing the developing gonad in the genital ridge, hPGCs are known as gonocytes (Stukenborg et al., 2010). Through crosstalk with surrounding somatic cells, gonocytes enter the male germ cell development path and become spermatogonia postnatally; thereafter, spermatogonia undergo mitotic proliferation until puberty, when meiosis is initiated to form final spermatozoa (Manku and Culty, 2015; Stukenborg et al., 2014).
Different pluripotency state between human and mouse iPSCs
Recently, two developmentally and functionally distinct types of pluripotency have been defined: the naive state and the primed state (Nichols and Smith, 2009; Petkova et al., 2014). First, cells in the naive state are competent to form blastocyst chimeras; the presence of two active X chromosomes is an epigenetic signature of naive pluripotecy; naive cells express KLF2 and KLF4 in addition to core pluripotency factors, and naive markers like reduced expression 1 (REX1, officially known as ZFP42), nuclear receptor subfamily 0 group B member 1 (NR0B1) and fibroblast growth factor 4 (FGF4); the two types of pluripotent cells also respond differently to signal molecules, such as leukemia inhibitory factor/signal transducer and activator of transcription 3 (LIF/STAT3) and fibroblast growth factor/extracellular regulated kinase (FGF/ERK); more specifically, the differentiation potential of primed pluripotent cells into PGCs and mature germ cells is drastically different from that of naive pluripotent cells (Arabadjiev et al., 2012; De Los Angeles et al., 2012; Nichols and Smith, 2009, 2011).
Rodent ESCs established from pre-implantation blastocyst could be in a naive state, and rodent ESCs procured from the post-implantation epiblast could be in a primed state preparing for differentiation; however, hESCs only had one primed state (Najm et al., 2011; Nichols and Smith, 2011; Tesar et al., 2007). Mouse iPSCs (miPSCs) present a naive state of pluripotency similar to mouse ESCs (mESCs) derived from the inner cell mass, whereas hiPSCs are considered to show a primed state of pluripotency that resembles the post-implantation epiblast (Nichols and Smith, 2009; Theunissen et al., 2016, 2014). In light of the disparate pluripotency states in miPSCs and hiPSCs, it may be misleading to translate the results achieved in rodent models to human research directly. However, Gafni et al. (2013) established the four-inhibitor-containing culture medium (4i medium) that could facilitate the derivation of naive hiPSCs.
Derivation of male germ line cells from hiPSCs
Murine studies have provided substantial insight into the development of male germ cells from iPSCs both in vitro and in vivo (Cai et al., 2013; Imamura et al., 2010; Li et al., 2014b; Yang et al., 2012; Zhu et al., 2012). Hayashi et al. (2011) made the remarkable finding that PGCLCs could be obtained from mESCs and miPSCs. The PGCLCs could be differentiated into spermatozoa in vivo, resulting in the birth of healthy offspring via intracytoplasmic sperm injection. Zhou et al. (2016) reported the generation of haploid male gametes from mESCs that could produce viable and fertile offspring. Notably PGCLCs derived from different mouse iPS cell lines exhibited different efficiency for spermatogenesis in vivo and some of the offspring died prematurely (Hayashi et al., 2011).
In spite of progress in mice, differentiation of hiPSCs to male germ cells still presents a significant challenge. Unlike miPSCs in naive state, hiPSCs exhibit a primed pluripotency with less potential for the germ cell fate (Hayashi and Surani, 2009; Nichols and Smith, 2009). Therefore, it may not be surprising that the success rate of germ cell derivation from hiPSCs is much lower than that from miPSCs.
Based on research in mice, male germ cell induction from hiPSCs has been attempted with different protocols, including spontaneous differentiation, overexpression of germ cell regulators, addition of cytokines, co-culture with gonadal cells in vitro and xeno-transplantation (Table I). Park et al. (2009) reported the first successful attempt to create PGCLCs from hiPSCs by co-culturing with human fetal gonadal stromal cells and showed that the erasure of the genetic imprint did not initiate efficiently in PGCLCs. BMP signaling is demonstrated to be conserved for both human and mouse germ cell induction, and the addition of BMPs could induce germ cell differentiation from hiPSCs. By combining BMP addition with overexpression of members of the deleted in azoospermia (DAZ) gene family, hiPSCs formed meiotic cells with extensive synaptonemal complexes and post-meiotic haploid cells with a similar pattern of acrosin staining as observed in human spermatids (Panula et al., 2011). Furthermore, Medrano et al. found intrinsic germ cell translational, rather than transcriptional factors could drive germ line formation from hiPSCs in vitro. With overexpression of VASA (officially known as DDX4) and/or deleted in azoospermia like (DAZL) (RNA-binding proteins), hiPSCs differentiated to PGCLCs and progression through meiosis was enhanced. They also found that ectopic expression of VASA resulted in recapitulation of some aspects of germ line reprogramming at the H19 locus (Medrano et al., 2012). Unlike aforementioned studies with genetic manipulation, Eguizabal et al. (2011) and Easley et al. (2012) demonstrated that post-meiotic haploid cells could also be obtained from hiPSCs without the overexpression of germ line specific factors. Eguizabal et al. achieved complete differentiation of hiPSCs derived from different origins (keratinocytes and cord blood) and both genetic sexes into post-meiotic cells in vitro using a 3-step differentiation protocol. However, there was an imprinting re-establishment that was not complete in the differentiated cells. Easley et al. showed that hiPSCs could differentiate directly into post-meiotic, spermatid-like cells under standardized mouse spermatogonial stem cell (SSC) culture conditions. The haploid cells presented similar DNA methylation patterns to human sperm both on paternally and maternally imprinted genes (imprinted maternally expressed transcript (non-protein coding) (H19) and insulin like growth factor 2 (IGF2)).
Table I.
The in vitro differentiation potential of human iPSCs into male germ cells.
| Authors | Donor cells | Methods | Reporters | Isolation strategy | RNA markers | Protein markers | Results | Genetic and epigenetic analysis | |
|---|---|---|---|---|---|---|---|---|---|
| Park et al. (2009) | Dermal fibroblasts | Co-culture with human fetal gonadal cells | SSEA1+/cKIT+/VASA+ and PLAP+/SSEA1+/VASA+ | VASA, PRDM1, DPPA3, and DAZL | cKIT and VASA | PGCLCs | Incomplete imprint erasure | ||
| Panula et al. (2011) | Fetal- and adult-derived fibroblasts | BMP-induced culture and overexpression of the DAZ gene family | VASA:GFP reporter | VASA:GFP+ | VASA, IFITM1, PELOTA, PRDM1A, GCNF, STELLAR, and DMC1 | VASA, DAZL, SCP3, CENP-A and Acrosin | Meiotic cells and haploid cell | DNA content analysis, and FISH | |
| Eguizabal et al. (2011) | Keratinocytes and cord blood | 3-step methods (RA, FRSK, LIF, R115866) | CD9+/CD49f++/CD90−/SSEA-4− | VASA and Stra8 | VASA, SCP3, γ-H2AX and Acrosin | Haploid gamete-like cells | DNA content analysis, FISH, and incomplete imprinting re-establishment | ||
| Easley et al. (2012) | Foreskin fibroblast | Standardized mouse SSC culture conditions | Isolation for haploid cells | VASA, DAZL, CXCR4, PIWIL1, and PLZF | VASA, DAZL, UTF1, CDH1, RET, GFRα1, PIWIL1, HIWI, SCP3, TP1, protamine 1 and Acrosin | Haploid spermatogenic cells | DNA content analysis, FISH, and similar parent imprints | ||
| Medrano et al. (2012) | Fetal- and adult-derived fibroblasts | Overexpression of VASA and/or DAZL and spontaneous differentiation | VASA:GFP reporter | VASA:GFP+ | VASA, IFITM1, DAZL, PRDM1A, GCNF, GDF3, cKIT, PELOTA, SCP3, MLH1, DMC1, GDF9, and ZP4 | VASA, CENP-A, SCP3 and Acrosin | Meiotic cells | DNA content analysis, FISH, and recapitulation of epigenetic reprogramming at the H19 locus | |
| Durruthy-Durruthy et al. (2014) | Dermal fibroblasts | Ectopic expression of VASA | BMP4 treatment in vivo | NANOS3, VASA, and DPPA3 | VASA, DAZ, DAZL, DPPA3, UTF1 and GFRα1 | PGCLCs, and pre-meiotic germ cells | Epigenetic transition from 5-mc to 5-hmc | ||
| Xeno- transplantation | |||||||||
| Ramathal et al. (2014) | Dermal fibroblasts from azoospermic and fertile men |
|
VASA:GFP reporter | VASA:GFP+ | VASA, PRDM1, PRDM14, DAZL, STELLA, IFITM3, and NANOS3 | VASA, DAZL, STELLA, PLZF, UTF1 and DAZ | PGCLCs, and gonocyte-like cells | Global DNA demethylation | |
| Irie et al. (2015) | Somatic cells from a fragile X male patient and normal female | BMP2 or BMP4, LIF, SCF, EGF, and ROCK inhibitor | NANOS3- mCherry reporter | NANOS3+/TNAP+ | NANOS3, BLIMP1, TFAP2C, SOX17, STELLA, OCT4, and PRDM14 | PGCLCs | |||
| Sugawa et al. (2015) | BMP4, ActA, bFGF, LIF | TRA-1–81+/cKIT+ | BLIMP1, STELLA, cKIT, STELLA, NANOS3, and TEX13B | BLIMP1 and STELLA | PGCLCs | Global progress of epigenetic reprogramming | |||
| Sasaki et al. (2015) | Dermal fibroblasts and PMBCs | Activin A, CHIR99021, BMP4, SCF, EGF, LIF | BLIMP1-2 A -tdTomato and TFAP2C-2 A -EGFP reporters | BLIMP1+/TFAP2C+ and EpCAM+/INTEGRINα6+ | BLIMP1, TFAP2C, NANOS3, DPPA3, DDX4, and DAZL | BLIMP1, TFAP2C and SOX17 | PGCLCs | Avoiding of somatic program and epigenetic reprogramming | |
It is important to point out that the gonadal environment in vivo is required for definitive and successful meiosis. However, transplantation of iPSCs or iPSC-derived cells into human testis is limited by ethical and safety issues. Thus, another significant method for male germ cell differentiation is xeno-transplantation of iPSCs into murine or even primate testis to evaluate their differentiation potential for germ line cells. In order to make use of the gonadal niche to promote human germ line formation in vivo, Durruthy-Durruthy et al. transplanted hiPSCs directly into the seminiferous tubules of germ cell-depleted immunodeficient mice. The transplanted iPSCs migrated to the basement membrane of the seminiferous tubule and 8 weeks after transplantation, the differentiated cells expressed PGC and pre-meiotic germ cell markers (Durruthy-Durruthy et al., 2014). Interestingly, they found that iPSCs produced with different factors (addition of VASA to OSKM (OCT4, SOX2, KLF4 and MYC) or OSKM only) revealed divergent fates after xeno-transplantation. In contrast to OSKM cells, OSKMV-reprogrammed iPSCs showed greater germ cell forming potential and did not form tumors, while OSKM cells remained outside the seminiferous tubule proliferated extensively and formed tumors. Using the same method, the authors also transplanted iPSCs derived from azoospermic and fertile men to murine seminiferous tubules. Human iPSCs with azoospermia factor deletions produced significantly fewer germ cell-like cells in vivo with distinct defects in gene expression. The results indicate that xeno-transplantation of hiPSCs directs germ cell differentiation in a manner dependent on donor genetic background (Ramathal et al., 2014). Theoretically, iPSCs from male infertility patients with genetic defects could be genetically corrected, differentiated into PGCLCs or spermatogonia in vitro, and then transplanted back into the patient’s seminiferous tubules for therapeutic purpose. Moreover, xeno-transplantation of iPSCs may also serve as tools for genetic research of human germ cell development in vivo (Fig. 1).
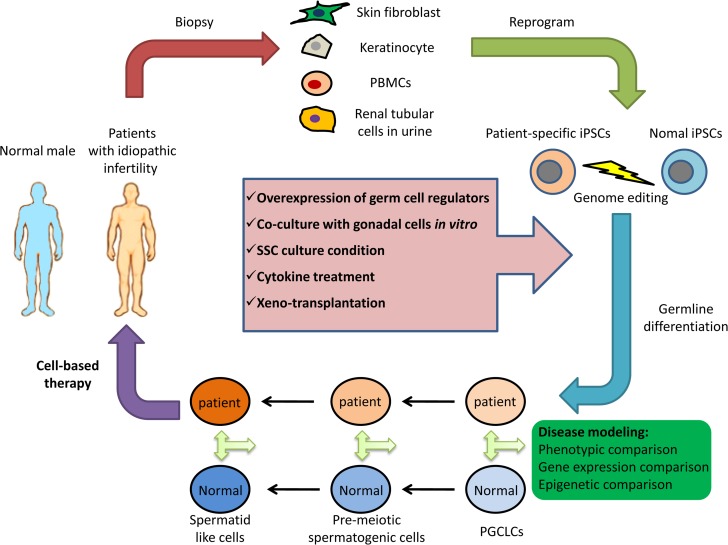
Figure 1.
Derivation and application of patient-specific induced pluripotent stem cells (iPSCs) in male infertility. Different types of somatic cells derived from patients with idiopathic infertility are reprogrammed into iPSCs and then differentiated into male germ cells by multiple methods. If necessary, iPSCs with known genetic defects may be corrected by genome editing technology. These cells can be used for in vitro disease modeling, regeneration research and cell-based therapy. In disease modeling, comparison between patients- and normal derived cells potentially provides novel clues to the underlying mechanisms for idiopathic male infertility, which may further lead to the development of therapeutic strategies. PBMCs, peripheral blood mononuclear cells; SSC, spermatogonial stem cell; PGCLCs, human primordial germ cells-like cells.
Recently, several groups utilized the embryoid body differentiation strategy to achieve in vitro induction of human PGCLCs from iPSCs in response to cytokines resembling those released by early extra-embryonic tissues and involved in critical germ cell fate regulation pathways. Irie et al. (2015) reported that human PGCLCs specification could be induced efficiently and directly in hiPSCs that were maintained in the 4i medium. Furthermore, Sasaki et al. (2015) showed that hiPSCs in primed state could differentiate into incipient mesoderm-like cells by stimulation with Activin A and a WNT signaling agonist (CHIR99021), and then generated PGCLCs in response to growth factors, robustly yielding B-lymphocyte-induced maturation protein 1 (BLIMP1, officially known as PRDM1) and TFAP2C activated cells. Importantly, the authors also demonstrated that transcriptomes of the obtained PGCLCs were similar to PGCs isolated from non-human primates. With different cytokine combinations, Sugawa et al. (2015) also described a defined and stepwise differentiation system for inducing pre-migratory PGCLCs from hiPSCs. Moreover, the PGCLCs they generated showed epigenetic reprogramming that was globally similar to PGCs in vivo.
Taken together, these studies indicate that human male germ cells can be derived from hiPSCs, although most of the differentiated cells remained at the early stages, like PGCLCs. Therefore, development of in vitro conditions that enable robust differentiation of human PGCLCs towards later stages will be necessary.
Prospectives of hiPSCs in male infertility
Reproductive medicine applications
The process of generating male gametes from patient-specific iPSCs could provide better in vitro disease models for male infertility. Comparison of patient-derived hiPSCs with normal hiPSCs for their germ line differentiation abilities may help identify abnormalities and decipher the molecular mechanisms of idiopathic male infertility involved in differentiation and maturation of human gametes. Furthermore, differentiation of male germ cells from hiPSCs would be an invaluable tool to explore the specification of human germ line development, including transcriptional networks, signaling pathways and epigenetic reprogramming. In contrast to studies in mice, studies of human germ line development are limited mainly due to inaccessibility of germ cells during early embryo development and lack of suitable experimental systems (Durruthy-Durruthy et al., 2014). So it is necessary to reconstitute human germ line development in vitro, which could possibly be achieved by differentiation of hiPSCs.
In addition, the establishment of functional male gametes from autologous iPSCs could benefit azoospermia patients who lack mature sperm in the testes. However, the limited publications about haploid spermatid indicate that the derivation of functional gametes from hiPSCs is still an immature technology and insufficient for clinical therapies (Nikolic et al., 2016). Moreover, germ cells go through dramatic epigenetic reprogramming during development (Tang et al., 2016). The evaluation of epigenetic status during germ cell differentiation from hiPSCs is informative and is not fully evaluated in most published studies.
Reconstitution of spermatogenesis niche
The differentiation of male germ cells in vivo depends on a niche composed of spermatogenic and somatic cells. It is important to reconstitute the spermatogenic niche to support differentiation of iPSCs into functional germ cells. However, testing whether human PGCLCs can form spermatozoa in vivo is not feasible for ethical reasons and tumorigenicity after transplantation into human testis. Instead, xeno-transplantation of hiPSCs and PGCLCs into murine seminiferous tubules could provide a somatic environment to promote human germ line formation in vivo (Durruthy-Durruthy et al., 2014; Ramathal et al., 2014).
Additionally, it was demonstrated that co-culture with neonatal testicular somatic cells and sequential exposure to morphogens and sex hormones could promote mESC-derived PGCLCs to recapitulate complete male gametogenesis in vitro (Zhou et al., 2016). Given the limited availability of fetal and neonatal human testicular biopsies, few studies have used the co-culture method to examine human germ cell development. Recently, a 3D testicular cell culture has been established to mimic the testis environment in vivo (Yokonishi et al., 2013). The single testicular cells of neonatal mice formed aggregates in suspension culture and then were transferred to the surface of agarose gel with a gas-liquid interphase culture method. A tubular architecture gradually developed during the following 2 weeks. Furthermore, they also mixed spermatogonial stem cells (SSCs) with the testicular cell suspension and found the incorporation of SSCs in the reconstructed tubules. 3D cultures of hiPSCs and PGCLCs with testicular cells using biomaterials and bioactive factors will offer a new avenue for in vitro germ cell differentiation (Galdon et al., 2016; Sadri-Ardekani and Atala, 2015).
Genome editing in hiPSCs
Male infertility is a multi-factorial disease with at least 15% of cases attributed to genetic disorders (Krausz et al., 2015). Mutations carried by germ cells can lead to disorders in the offspring, and natural selection prevents the transmission of mutations by causing infertility, but this protective mechanism may be overcome by ART (Ferlin et al., 2007). By using optimized gametes for ART, one could obtain healthy offspring without genetic disorders. In vitro derivation of germ cells from patient-specific iPSCs combined with genetic defect correction may provide valuable insights into the targeted treatments for infertility.
The rapid development of novel genome editing technologies, especially with the Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein-9 nuclease (CRISPR/Cas9) system, could realize the full potential of hiPSCs in both basic research and therapies for male infertility. In the past few years, there has been a spike of interest in genome editing in hESCs and hiPSCs, due to their potential in modeling and correcting a variety of genetic diseases (Cherry and Daley, 2013; Soldner and Jaenisch, 2012). For male infertility caused by genetic anomalies, genome editing technologies could be used to target and modify the gene of interest in patient-specific iPSCs before they are differentiated and introduced to patients (Li et al., 2014a,b). Other than cell and gene therapy, genome editing technologies can also facilitate basic research by generating knock-in reporters under the control of regulatory elements of germ cells to better visualize key stages during germ line development from iPSCs in vitro (Irie et al., 2015; Sasaki et al., 2015).
Genome editing technologies will continue to develop for clinical applications, offering hope to infertile male patients with genetic disorders, but also raising ethical arguments, long-term safety issues and even unpredictable impacts on humans. In this regard, future use of genome editing in the clinic requires extra long-term evaluation of the safety of cells that have undergone genome editing.
Human iPSCs-derived extracellular vesicles and male infertility
Male infertility is a common iatrogenic effect of clinical treatment for cancer, including radiotherapy and high-dose chemotherapy, which can severely damage the male gonad leading to spermatogenic failures (Jahnukainen and Stukenborg, 2012; Martin-du Pan and Campana, 1993). Emergence of iPSC-derived germ cells presents a valuable opportunity to replenish autologous germ cells for male infertility patients. Nevertheless, the therapeutic application of iPSCs and their differentiated derivates are limited by their tumorigenicity (Lee et al., 2013). Extracellular vesicles (EVs) are membrane-bound vesicles carrying regulatory molecules, such as microRNAs, proteins and lipids. They may mediate intercellular communications, contributing to cell proliferation and differentiation (Machtinger et al., 2016). It has been demonstrated that exosomes and microvesicles secreted by iPSCs are very effective transmitters of cytoprotective signals to cardiomyocytes in the setting of myocardial ischemia/reperfusion (Wang et al., 2015). Spermatogenesis is a complex process highly dependent on intercellular communications among germ cells, Sertoli cells and Leydig cells. These intercellular communications could be closely related to the biological functions of EVs. So it is hypothesized that iPSC-derived exosomes and microvesicles could transmit cytoprotective signals to the injured spermatogenic microenvironment caused by anticancer treatment and promote the recovery of testicular spermatogenic function without the risk of tumorigenicity.
Conclusion
Although controversial, hiPSCs have tremendous potential for biological and therapeutic applications for male infertility. Recent advances in generation of male germ cells from miPSCs not only hold great promise for the establishment of in vitro human spermatogenesis models, but also provide insights into the mechanism of hPGC specification and human spermatogenesis regulation. Based on these advances, it is conceivable that hiPSCs will have more therapeutic implications for male infertility in combination with genome editing technology and EVs research in the near future. However, the molecular mechanisms underlying human male germ cell development are still poorly understood. More comprehensive understanding of human germ cell development would be of great value for the application of hiPSCs in reproductive medicine and basic research.
Authors’ roles
F.F. designed the review, performed the literature research and drafted the article. Z.L.L. designed the review, performed revisions and critically discussed the complete article. Q.Z. performed revisions and critically discussed the complete article. C.L.X. and H.G.L. designed the review, supervised and revised it critically for important intellectual content.
Funding
National Science & Technology Pillar Program during the 12th 5-Year Plan Period, China (No. 2012BAI32B03) and National Natural Science Foundation of China (No. 81370755).
Conflict of interest
The authors report no financial or other conflict of interest relevant to the subject of this article.
Acknowledgements
We would like to express our gratitude to Dr Elizabeth Corbin for helping with the revision.
References
- Arabadjiev B, Petkova R, Momchilova A, Chakarov S, Pankov R. Of mice and men—differential mechanisms of maintaining the undifferentiated state in mESC and hESC. Biodiscovery 2012;3:1. [Google Scholar]
- Bhartiya D, Hinduja I, Patel H, Bhilawadikar R. Making gametes from pluripotent stem cells—a promising role for very small embryonic-like stem cells. Reprod Biol Endocrinol 2014;12:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, Ziller M, Croft GF, Amoroso MW, Oakley DH et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 2011;144:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Xia X, Wang L, Liu Y, He Z, Guo Q, Xu C. In vitro and in vivo differentiation of induced pluripotent stem cells into male germ cells. Biochem Biophys Res Commun 2013;433:286–291. [DOI] [PubMed] [Google Scholar]
- Cherry AB, Daley GQ. Reprogrammed cells for disease modeling and regenerative medicine. Annu Rev Med 2013;64:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J, Stoop H, Gillis AJ, van Gurp RJ, van de Geijn GJ, Boer M, Hersmus R, Saunders PT, Anderson RA, Oosterhuis JW et al. Differential expression of SOX17 and SOX2 in germ cells and stem cells has biological and clinical implications. J Pathol 2008;215:21–30. [DOI] [PubMed] [Google Scholar]
- De Los Angeles A, Loh YH, Tesar PJ, Daley GQ. Accessing naive human pluripotency. Curr Opin Genet Dev 2012;22:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devolder K. Complicity in stem cell research: the case of induced pluripotent stem cells. Hum Reprod 2010;25:2175–2180. [DOI] [PubMed] [Google Scholar]
- Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, Herb B, Ladd-Acosta C, Rho J, Loewer S et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet 2009;41:1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durruthy-Durruthy J, Ramathal C, Sukhwani M, Fang F, Cui J, Orwig KE, Reijo Pera RA. Fate of induced pluripotent stem cells following transplantation to murine seminiferous tubules. Hum Mol Genet 2014;23:3071–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley CAT, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE et al. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep 2012;2:440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguizabal C, Montserrat N, Vassena R, Barragan M, Garreta E, Garcia-Quevedo L, Vidal F, Giorgetti A, Veiga A, Izpisua Belmonte JC. Complete meiosis from human induced pluripotent stem cells. Stem Cells 2011;29:1186–1195. [DOI] [PubMed] [Google Scholar]
- Ferlin A, Raicu F, Gatta V, Zuccarello D, Palka G, Foresta C. Male infertility: role of genetic background. Reprod Biomed Online 2007;14:734–745. [DOI] [PubMed] [Google Scholar]
- Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013;504:282–286. [DOI] [PubMed] [Google Scholar]
- Galdon G, Atala A, Sadri-Ardekani H. In vitro spermatogenesis: how far from clinical application? Curr Urol Rep 2016;17:49. [DOI] [PubMed] [Google Scholar]
- Guo F, Yan L, Guo H, Li L, Hu B, Zhao Y, Yong J, Hu Y, Wang X, Wei Y et al. The transcriptome and DNA methylome landscapes of human primordial germ cells. Cell 2015;161:1437–1452. [DOI] [PubMed] [Google Scholar]
- Hara K, Kanai-Azuma M, Uemura M, Shitara H, Taya C, Yonekawa H, Kawakami H, Tsunekawa N, Kurohmaru M, Kanai Y. Evidence for crucial role of hindgut expansion in directing proper migration of primordial germ cells in mouse early embryogenesis. Dev Biol 2009;330:427–439. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011;146:519–532. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Surani MA. Self-renewing epiblast stem cells exhibit continual delineation of germ cells with epigenetic reprogramming in vitro. Development 2009;136:3549–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Yang S, Yang H, Liu Y, Liu Y, Hai Y, Chen Z, Guo Y, Gong Y, Gao WQ et al. Generation of male differentiated germ cells from various types of stem cells. Reproduction 2014;147:R179–R188. [DOI] [PubMed] [Google Scholar]
- Imamura M, Aoi T, Tokumasu A, Mise N, Abe K, Yamanaka S, Noce T. Induction of primordial germ cells from mouse induced pluripotent stem cells derived from adult hepatocytes. Mol Reprod Dev 2010;77:802–811. [DOI] [PubMed] [Google Scholar]
- Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update 2015;21:411–426. [DOI] [PubMed] [Google Scholar]
- Irie N, Weinberger L, Tang WW, Kobayashi T, Viukov S, Manor YS, Dietmann S, Hanna JH, Surani MA. SOX17 is a critical specifier of human primordial germ cell fate. Cell 2015;160:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T. Potential impact of human mitochondrial replacement on global policy regarding germline gene modification. Reprod Biomed Online 2014;29:150–155. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Stukenborg JB. Clinical review: present and future prospects of male fertility preservation for children and adolescents. J Clin Endocrinol Metab 2012;97:4341–4351. [DOI] [PubMed] [Google Scholar]
- Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Huo H, Loh YH, Aryee MJ, Lensch MW et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol 2011;29:1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Sasaki K, Yokobayashi S, Sakai Y, Nakamura T, Yabuta Y, Nakaki F, Nagaoka S, Woltjen K, Hotta A et al. Evolutionarily distinctive transcriptional and signaling programs drive human germ cell lineage specification from pluripotent stem cells. Cell Stem Cell 2017;21:517–532 e515. [DOI] [PubMed] [Google Scholar]
- Krausz C, Escamilla AR, Chianese C. Genetics of male infertility: from research to clinic. Reproduction 2015;150:R159–R174. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Hage WJ. Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Found Symp 1994;182:68–84. discussion 84. 91. [DOI] [PubMed] [Google Scholar]
- Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med 2013;19:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Suzuki K, Kim NY, Liu GH, Izpisua Belmonte JC. A cut above the rest: targeted genome editing technologies in human pluripotent stem cells. J Biol Chem 2014. a;289:4594–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang X, Feng X, Liao S, Zhang D, Cui X, Gao F, Han C. Generation of male germ cells from mouse induced pluripotent stem cells in vitro. Stem Cell Res 2014. b;12:517–530. [DOI] [PubMed] [Google Scholar]
- Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update 2016;22:182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusdottir E, Dietmann S, Murakami K, Gunesdogan U, Tang F, Bao S, Diamanti E, Lao K, Gottgens B, Azim Surani M. A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat Cell Biol 2013;15:905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manku G, Culty M. Mammalian gonocyte and spermatogonia differentiation: recent advances and remaining challenges. Reproduction 2015;149:R139–R157. [DOI] [PubMed] [Google Scholar]
- Martin-du Pan RC, Campana A. Physiopathology of spermatogenic arrest. Fertil Steril 1993;60:937–946. [DOI] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol 2003;262:1–15. [DOI] [PubMed] [Google Scholar]
- Medrano JV, Ramathal C, Nguyen HN, Simon C, Reijo Pera RA. Divergent RNA-binding proteins, DAZL and VASA, induce meiotic progression in human germ cells derived in vitro. Stem Cells 2012;30:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Minase G, Okabe K, Ueda H, Sengoku K. Male infertility and its genetic causes. J Obstet Gynaecol Res 2015;41:1501–1505. [DOI] [PubMed] [Google Scholar]
- Najm FJ, Chenoweth JG, Anderson PD, Nadeau JH, Redline RW, McKay RD, Tesar PJ. Isolation of epiblast stem cells from preimplantation mouse embryos. Cell Stem Cell 2011;8:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell 2009;4:487–492. [DOI] [PubMed] [Google Scholar]
- Nichols J, Smith A. The origin and identity of embryonic stem cells. Development 2011;138:3–8. [DOI] [PubMed] [Google Scholar]
- Nikolic A, Volarevic V, Armstrong L, Lako M, Stojkovic M. Primordial germ cells: current knowledge and perspectives. Stem Cells Int 2016;2016:1741072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula S, Medrano JV, Kee K, Bergstrom R, Nguyen HN, Byers B, Wilson KD, Wu JC, Simon C, Hovatta O et al. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet 2011;20:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Galic Z, Conway AE, Lindgren A, van Handel BJ, Magnusson M, Richter L, Teitell MA, Mikkola HK, Lowry WE et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells 2009;27:783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008;451:141–146. [DOI] [PubMed] [Google Scholar]
- Perrett RM, Turnpenny L, Eckert JJ, O’Shea M, Sonne SB, Cameron IT, Wilson DI, Rajpert-De Meyts E, Hanley NA. The early human germ cell lineage does not express SOX2 during in vivo development or upon in vitro culture. Biol Reprod 2008;78:852–858. [DOI] [PubMed] [Google Scholar]
- Petkova R, Arabadjiev B, Chakarov S, Pankov R. Current state of the opportunities for derivation of germ-like cells from pluripotent stem cells: are you a man, or a mouse? Biotechnol Biotechnol Equip 2014;28:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramathal C, Durruthy-Durruthy J, Sukhwani M, Arakaki JE, Turek PJ, Orwig KE, Reijo Pera RA. Fate of iPSCs derived from azoospermic and fertile men following xenotransplantation to murine seminiferous tubules. Cell Rep 2014;7:1284–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Atala A. Regenerative medicine for the treatment of reproductive system disorders: current and potential options. Adv Drug Deliv Rev 2015;82:145–152. [DOI] [PubMed] [Google Scholar]
- Saitou M, Payer B, O’Carroll D, Ohinata Y, Surani MA. Blimp1 and the emergence of the germ line during development in the mouse. Cell Cycle 2005;4:1736–1740. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Yokobayashi S, Nakamura T, Okamoto I, Yabuta Y, Kurimoto K, Ohta H, Moritoki Y, Iwatani C, Tsuchiya H et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 2015;17:178–194. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001;16:972–978. [DOI] [PubMed] [Google Scholar]
- Soldner F, Jaenisch R. Medicine. iPSC disease modeling. Science 2012;338:1155–1156. [DOI] [PubMed] [Google Scholar]
- Stukenborg JB, Colon E, Soder O. Ontogenesis of testis development and function in humans. Sex Dev 2010;4:199–212. [DOI] [PubMed] [Google Scholar]
- Stukenborg JB, Kjartansdottir KR, Reda A, Colon E, Albersmeier JP, Soder O. Male germ cell development in humans. Horm Res Paediatr 2014;81:2–12. [DOI] [PubMed] [Google Scholar]
- Sugawa F, Arauzo-Bravo MJ, Yoon J, Kim KP, Aramaki S, Wu G, Stehling M, Psathaki OE, Hubner K, Scholer HR. Human primordial germ cell commitment in vitro associates with a unique PRDM14 expression profile. EMBO J 2015;34:1009–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- Tang WW, Dietmann S, Irie N, Leitch HG, Floros VI, Bradshaw CR, Hackett JA, Chinnery PF, Surani MA. A unique gene regulatory network resets the human germline epigenome for development. Cell 2015;161:1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WW, Kobayashi T, Irie N, Dietmann S, Surani MA. Specification and epigenetic programming of the human germ line. Nat Rev Genet 2016;17:585–600. [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007;448:196–199. [DOI] [PubMed] [Google Scholar]
- Theunissen TW, Friedli M, He Y, Planet E, O’Neil RC, Markoulaki S, Pontis J, Wang H, Iouranova A, Imbeault M et al. Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell 2016;19:502–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 2014;15:471–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 2015;192:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S, Eckert D, Nettersheim D, Gillis AJ, Schafer S, Kuckenberg P, Ehlermann J, Werling U, Biermann K, Looijenga LH et al. Critical function of AP-2 gamma/TCFAP2C in mouse embryonic germ cell maintenance. Biol Reprod 2010;82:214–223. [DOI] [PubMed] [Google Scholar]
- Yamaji M, Seki Y, Kurimoto K, Yabuta Y, Yuasa M, Shigeta M, Yamanaka K, Ohinata Y, Saitou M. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat Genet 2008;40:1016–1022. [DOI] [PubMed] [Google Scholar]
- Yang S, Bo J, Hu H, Guo X, Tian R, Sun C, Zhu Y, Li P, Liu P, Zou S et al. Derivation of male germ cells from induced pluripotent stem cells in vitro and in reconstituted seminiferous tubules. Cell Prolif 2012;45:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokonishi T, Sato T, Katagiri K, Komeya M, Kubota Y, Ogawa T. In vitro reconstruction of mouse seminiferous tubules supporting germ cell differentiation. Biol Reprod 2013;89:15. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang M, Yuan Y, Wang X, Fu R, Wan H, Xie M, Liu M, Guo X, Zheng Y et al. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell 2016;18:330–340. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hu HL, Li P, Yang S, Zhang W, Ding H, Tian RH, Ning Y, Zhang LL, Guo XZ et al. Generation of male germ cells from induced pluripotent stem cells (iPS cells): an in vitro and in vivo study. Asian J Androl 2012;14:574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]