Summary
Although new HPV infections and associated precancers can occur throughout a woman’s lifetime, only a small proportion are acquired in mid-adult women and are vaccine-preventable. Our model-based explorations provide insight into causal HPV infections and highlight the potential limitations of using surrogate endpoints for vaccine efficacy studies of mid-adult women to guide policy decisions for implementation.
Keywords: Human papillomavirus, cervical cancer, simulation modeling
Abstract
Background
Although new human papillomavirus (HPV) infections can occur at all ages, the age at which women acquire their “causal” HPV infection that develops into cervical cancer is poorly understood and practically unobservable. We aimed to estimate the age distribution at which individuals acquired their causal HPV infection in the absence of HPV vaccination or screening to help guide the optimal use of both.
Methods
Using an empirically calibrated mathematical model that simulates the natural history of cervical cancer, we estimated the cumulative number of causal HPV infections by age, stratified by HPV genotype (HPV16 vs. other HPV genotypes), and the direct age-specific reduction in cancer incidence for alternative vaccination initiation scenarios (i.e., age 9–45 years).
Results
Our model projected that among all cervical cancers, 50% and 75% of women acquired their causal HPV infection by ages 20.6 (range: 20.1–21.1) and 30.6 (range: 29.6–31.6) years, respectively. HPV16 infections were acquired at an earlier age. Assuming 95% efficacy against HPV16 and HPV18 infections, the direct reduction in lifetime risk of cervical cancer varied from 55% (53–56%) among women vaccinated at age 9 years to 6% (range: 6–7%) among women vaccinated at age 45 years. Similar patterns were observed for the second-generation vaccine.
Conclusions
Although new HPV infections and precancers can occur throughout a woman’s lifetime, only a small proportion are acquired in mid-adult women and are vaccine-preventable. Our simulations highlight the potential limitations of using surrogate endpoints for vaccine efficacy studies of mid-adult women to guide policy decisions for implementation.
The risk of acquiring cervical human papillomavirus (HPV) infections, causally linked to several cancers and genital warts, peaks shortly after sexual initiation, subsequently declining with age in most women [1]. Following acquisition, high-risk HPV infections may persist and progress to a precancerous lesion, a proportion of which will become invasive cancer over time [2] if not detected and treated in a timely fashion. The peak incidence of cervical cancer occurs generally 20–30 years after the population median age of sexual debut [3], but the age at which women acquire their causal HPV infection, the one that ultimately progresses into invasive cancer, is poorly understood and inherently unobservable. The development of prophylactic HPV vaccines, ideally targeted to young individuals prior to HPV exposure as they do not protect against prevalent vaccine-type HPV infections, allows for strategic combinations of primary and secondary cervical cancer control policies. As the ultimate goal of HPV vaccination is to prevent the burden of cervical cancer (and other HPV-related cancers), understanding the natural history of causal cervical HPV infections helps to quantify the health and economic impact and inform decisions around HPV vaccination and cervical cancer screening policies.
Using existing evidence documenting vaccine protection against persistent HPV infections and precursor lesions in older women [4, 5], several advisory groups have proposed the expanded use of the HPV vaccines to include older age groups. However, how these intermediate endpoints translate to prevention of cervical cancer is not known and unlikely to be observed directly through clinical trials. Furthermore, HPV genotypes targeted by the first-generation vaccines (i.e., HPV16 and HPV18) contribute to a smaller proportion of cancers that develop after age 50 years, resulting in a lower maximum clinical benefit of vaccinating older women [6, 7]. In settings without existing screening programs, the impact of HPV vaccination at older ages on long-term outcomes will inform whether secondary prevention approaches, such as HPV testing, should also be coupled with an HPV vaccination policy. The optimal choice of intervention(s) will be determined by the cumulative proportion of causal infections that have already been acquired at the time of vaccination, and thereby the resulting cancer could only be prevented through screening, diagnosis, and treatment of the antecedent cervical precancer, compared with those that are yet to be acquired and could be prevented via HPV vaccination [8].
Given the long natural history of cervical cancer, disease-simulation modeling is increasingly being used to project long-term epidemiological endpoints and evaluate the cost-effectiveness of new approaches to reduce the global burden of cervical cancer [9]. These models are uniquely positioned to explore the natural history of unobservable transitions, such as the age distribution of the acquisition of causal HPV infections. Our objective was to use a well-documented natural history disease simulation model to explicitly identify the age distribution at which individuals acquire their causal HPV infection in the absence of HPV vaccination or screening in order to help guide the optimal use of both. To enumerate the direct benefits (excluding herd immunity) of extending the target age of HPV vaccination, we evaluated the health benefits associated with later vaccination age on age-specific cervical cancer incidence rates.
METHODS
Analytic Overview and Analysis
We employed an individual-based cervical cancer natural history model that integrates empirical data from the largest prospective and clinical studies of HPV-induced cervical carcinogenesis into a single analytic framework [10]. This model, which is continually updated and refined using emerging empirical data, is well published and has informed cervical cancer prevention policy worldwide [11–16]. We used the model to simulate a cohort of women in the absence of screening or HPV vaccination to project the cumulative number of causal HPV infections by age and HPV genotype (i.e., HPV16- vs. non-HPV16). For each cancer case observed in the model, we identified the age at which the individual women acquired their HPV infection that developed into a clinically detected cancer (i.e., cancers that remained undetected throughout a woman’s lifetime were excluded) (Figure 1). To isolate the direct benefits of extending the vaccine target age on age-specific cancer incidence, we simulated seven scenarios in which the age of HPV vaccination was varied (i.e., ages 9, 12, 18, 25, 30, 35, or 45 years) in the absence of cervical cancer screening. We assumed 95% efficacy against incident vaccine-targeted HPV infections with lifelong durability for both the first-generation bivalent and quadrivalent vaccines that target HPV16 and HPV18 oncogenic genotypes and the second-generation nonavalent vaccine that targets HPV16, 18, 31, 33, 45, 52, and 58 oncogenic genotypes. In sensitivity analysis, we evaluated the impact of 2 HPV vaccine durability scenarios on the lifetime reductions in cervical cancer incidence.
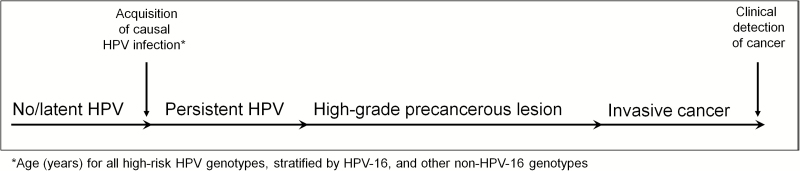
Figure 1.
Natural history schematic of the causal HPV infection conditioned on development of clinically detected cervical cancer. Abbreviation: HPV, human papillomavirus.
Model Overview and Parameterization
Individual women enter the model at age 9 years with a healthy cervix and face monthly probabilities of acquiring HPV (HPV16, 18, 31, 33, 45, 52, and 58, other grouped high-risk types, and grouped low-risk types) and transitioning between HPV-related health states (e.g., normal, HPV infection, cervical intraepithelial neoplasia, grades 2 (CIN2) and 3 (CIN3) and cervical cancer) until death, either from background causes or cervical cancer after its onset. Transitions may be a function of duration (i.e., time since HPV infection or precancer development), HPV genotype, age, and history of HPV infections. Baseline model input parameters were primarily derived from large, prospective cohort studies [17–19], supplemented by data from the published literature and expert opinion, and involved extensive model-fitting to observed data. For the parameters with high uncertainty and variability, we relied on a multiparameter calibration process [10, 20] to maximize correspondence between model outputs and empirical targets from the United States, such as age- and genotype- specific HPV prevalence and HPV genotype distribution in CIN3 and cervical cancer [21, 22]. The development and calibration process of our US individual-based natural history model of cervical carcinogenesis has been previously described [10, 20], validated, and used to analyze US screening and vaccination policy (e.g., Kim et al 2015 [14] and Kim et al 2016 [13]). For the current analysis, we additionally evaluated model performance against important vaccine-relevant outcomes, such as the age-specific frequencies of HPV16 infections (Supplementary Data) in cervical cancer.
To provide a range of our best estimates and compensate for the highly uncertain nature of projecting unobservable outcomes, such as the age of acquiring the causal HPV infection, we conducted all analyses using 50 statistically good-fitting parameter sets to capture the uncertainties in the natural history process [10]. For each parameter set, we calculated the mean of outcomes across the 50 “good-fitting” natural history parameter sets, and uncertainty bounds reflected the lower and upper quintiles across the parameter sets, that is, mean (lower quintile- upper quintile).
RESULTS
Acquisition of Causal Human Papillomavirus Infections
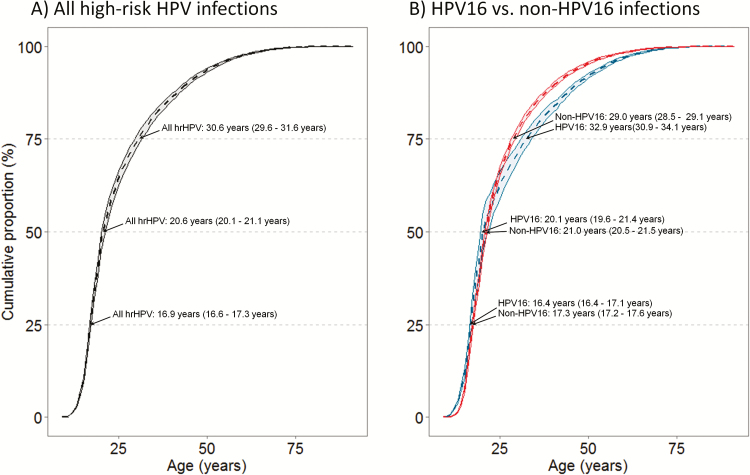
In the absence of primary (i.e., HPV vaccination) or secondary (i.e., screening) prevention, our model projected that among all cervical cancers, 50% and 75% of women acquired their causal HPV infection by ages 20.6 (20.1–21.1) and 30.6 (29.6–31.6) years, respectively (Figure 2, left panel). In contrast, only 10% of women with cervical cancer acquired their causal HPV infections after age 40 years. Importantly, the age distribution varied by HPV genotype (Figure 2, right panel) where causal HPV16 infections were generally acquired at a younger age relative to non-HPV16 infections. For example, we found that 6% (5–19%) more women had already acquired their causal HPV16 infection by age 20 years (i.e., 50% HPV16 vs. 44% non-HPV16 infections), resulting in a 1-year difference in the median age of acquisition of the causal HPV16 infection (i.e., 20.1 vs. 21.0 years). By ages 35 and 45 years, the model projected that 78% (77–80%) and 89% (88–89%) of cancers attributable to vaccine-targeted HPV16 and HPV18 infections would have already been acquired, respectively (results not shown), and therefore not vaccine preventable.
Figure 2.
Cumulative proportion of the age of acquisition of causal HPV infection for (A) all high-risk HPV infections, and (B) HPV16 infections compared to non-HPV16 infections. Dotted line reflects mean across good-fitting parameter sets; shaded area reflect the upper and lower quintiles across the 50 “good-fitting” natural history parameter sets. Abbreviation: HPV, human papillomavirus.
Reduction in Cancer Incidence
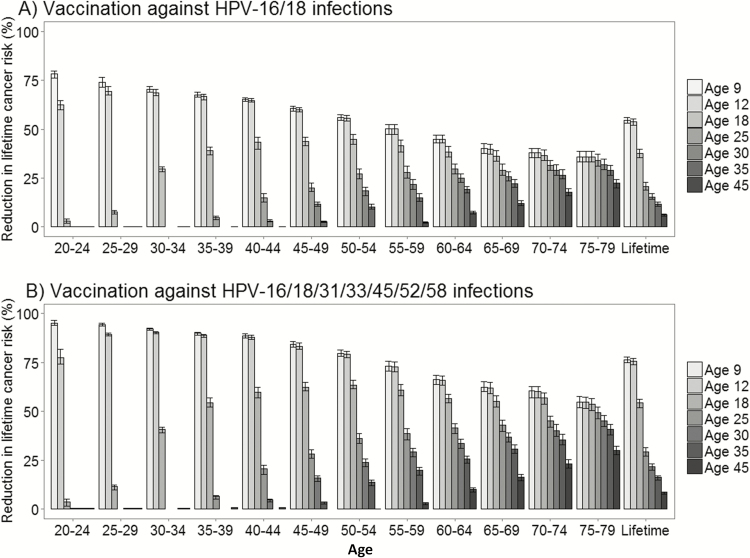
Assuming a 95% lifelong vaccine efficacy against HPV16 and HPV18 infections, the direct vaccination benefits yielded reductions in lifetime risk of cervical cancer that varied from 55% (53–56%) among women vaccinated at age 9 years to 6% (6–7%) among women vaccinated at age 45 years (Figure 3a). For girls vaccinated against HPV16 and HPV18 infections in adolescence, the age-specific reductions in cervical cancer incidence compared to no vaccination decreased as the contribution of vaccine-targeted HPV genotypes also decreased by age. For example, among women vaccinated against HPV16 and HPV18 infections at age 12, the age-specific reductions in cancer incidence peaked at ages 25–29 (69% [68–72%]) and fell to 36% (33–39%) at ages 75–79 years. The direct benefits of HPV vaccination of women age 45 years reduced the projected reductions among women aged 75–79 to 22% (20–24%). The impact of vaccination on age-specific cervical cancer incidence was not observed until 10 to 15 years later following vaccine initiation.
Figure 3.
Reductions in age-specific cervical cancer incidence compared to no vaccination for HPV vaccination ages 9, 12, 18, 25, 30, 35, and 45 years among (A) Females vaccinated against HPV16 and HPV18 infections, and (B) Females vaccinated against HPV16/18/31/35/45/52/58 infections; errors bars reflect the upper and lower bounds across the 50 “good-fitting” natural history parameter sets. Abbreviation: HPV, human papillomavirus.
Similar patterns of direct vaccine benefit were projected for the second-generation nonavalent HPV vaccine (protecting against HPV16/18 infections and 5 additional oncogenic HPV genotypes) but with overall greater benefit across all scenarios (Figure 3b). For example, the reductions in lifetime risk of cervical cancer increased to 76% (75–78%) and to 9% (7–9%) among women who received the nonavalent vaccine at ages 9 and 45 years, respectively. Importantly, initiating HPV vaccination at ages 25, 35, or 45 resulted in a loss of 60%, 70%, and 90% of potential cancer prevention benefit over a lifetime and were similar across the HPV vaccine types.
When we varied the durability of the HPV vaccines to protect against vaccine-targeted HPV types, we found that compared to lifetime protection, vaccine benefits were reduced by approximately 60% and 40% when assuming the vaccines began to wane after 10 and 20 years, respectively (Table 1). Notably, if vaccine protection wanes, increasing vaccination initiation from age 9 to 12 years yielded greater lifetime reductions; however, vaccination initiation after age 12 years consistently resulted in declining benefits.
Table 1.
Sensitivity Analysis Evaluating the Impact of Two HPV Vaccine Durability Scenarios on the Reductions in Lifetime Risk of Cervical Cancer by HPV Vaccine and Age of HPV Vaccination Initiation. Results Reflect the Mean Outcomes Across the 50 “good-fitting” Natural History Parameter Sets, and Uncertainty Bounds Reflect the Lower and Upper Quintiles Across the 50 “good-fitting” Natural History Parameter Sets
| HPV Vaccine and Age of Initiation | Reduction in Lifetime Incidence of Developing Cervical Cancer | ||
|---|---|---|---|
| 10 years of protectiona | 20 years of protectiona | Lifelong protection | |
| HPV-16/18 vaccines | |||
| Age 9 | 22% (19–25%) | 34% (31–36%) | 55% (53–56%) |
| Age 12 | 26% (23–29%) | 36% (34–38%) | 54% (52–55%) |
| Age 18 | 19% (18–20%) | 27% (26–28%) | 38% (36–40%) |
| Age 25 | 10% (9–11%) | 15% (14–16%) | 21% (19–23%) |
| Age 30 | 8% (7–9%) | 12% (11–13%) | 15% (14–17%) |
| Age 35 | 6% (6–7%) | 9% (8–10%) | 12% (10–13%) |
| Age 45 | 4% (4–4%) | 5% (5–6%) | 6% (6–7%) |
| HPV-16/18/31/33/45/52/58 vaccine | |||
| Age 9 | 32% (29–35%) | 49% (47–52%) | 76% (75–78%) |
| Age 12 | 38% (35–41%) | 52% (50–55%) | 76% (74–77%) |
| Age 18 | 30% (29–31%) | 40% (39–41%) | 54% (52–56%) |
| Age 25 | 15% (14–16%) | 21% (20–23%) | 29% (27–31%) |
| Age 30 | 12% (11–12%) | 16% (16–18%) | 22% (20–23%) |
| Age 35 | 9% (8–9%) | 13% (12–14%) | 16% (15–17%) |
| Age 45 | 5% (5–6%) | 7% (7–8%) | 9% (7–9%) |
Abbreviation: HPV, human papillomavirus.
aDuration of vaccine protection assuming 95% efficacy against vaccine-targeted HPV genotypes, followed by complete vaccine waning over a 10-year period.
DISCUSSION
In our analysis, we used a well-documented natural history model of cervical cancer to estimate the age distribution at which women acquire their causal HPV infection, the one that leads to invasive cervical cancer. The natural history of these causal HPV infections are practically unobservable as there is neither a way to identify the causal infection a priori and differentiate from other, benign infections, nor is it possible to follow a cohort of sufficient size and duration to describe this age distribution. Using a model-based approach, we estimated that more than half of high-risk HPV infections that will develop into cancer are acquired by age 21 years, 75% by the age of 31 years, and more than 85% by the age 40 years. To the best of our knowledge, this analysis represents the first attempt to identify the age distribution of the acquisition of the causal HPV infection. Although other model-based analyses implicitly capture the age distribution when evaluating the cost-effectiveness of HPV vaccination catch-up programs (e.g., Kim et al 2008 [23] and Jit et al 2008 [24]), no models have explicitly enumerated the underlying cumulative distribution that informs these projections.
Of note, the median age of acquisition of the causal HPV infection was younger for HPV16 infections compared with non-HPV16 infections, suggesting potential important HPV vaccine-related implications at later vaccination ages. Earlier acquisition of causal HPV16 infections (i.e., age 20 years) relative to other HPV genotypes (i.e., age 21 years) may stem from the higher prevalence of HPV16 infections circulating in the population but also due to the carcinogenicity of HPV16 infections [10]. However, as HPV16 infections continue to contribute to the majority of cervical cancers for women throughout their lifetime, HPV16 is the most important HPV genotype to prevent regardless of age of vaccination. Under all HPV vaccine assumptions we found that the clinical impact of HPV vaccination declined as vaccination age increased, and benefits were generally not observable for at least 10–15 years after vaccine receipt.
Our analysis provides a framework for projecting the impact of HPV vaccination on cancer endpoints, especially for novel vaccinations strategies that are under consideration, such as mid-adult HPV vaccination and a 1-dose HPV vaccination schedule. Clinical trials continue to evaluate the efficacy of vaccination against HPV for mid-adult women (up to ages 45 or 55 years) using surrogate endpoints (e.g., persistent HPV infection, precancer) [4, 5]. As noted [8], the attack rates over the 7 years of follow-up for precancerous lesions in the placebo arm was quite low, even among those who had persistent HPV in the first 4 years after baseline, which was consistent with our analysis that showed that there are very few causal infections acquired in mid-adult women. These clinical trials have prompted proposals for the expanded use of HPV vaccines in older women who were not been initially recommended for routine vaccination. For example, the recently proposed HPV-FASTER protocol involves extending routine HPV vaccination programs to women up to age 50 years [25] to reduce the reliance on screening. The key question, and the focus of subsequent analyses, is whether in limited resource settings a second round of screening or HPV vaccination in mid-adult women is more effective and cost-effective for cervical cancer prevention. Questions also remain about how much additional reassurance HPV vaccination (vs. no vaccination) provides mid-adult women who test negative for a high-risk HPV infection for long-term endpoints such as cancer or death. It is unlikely that clinical trials will be able to quantify the reductions in cervical cancer risk as it is unethical to not intervene, in addition to the economic and logistical requirements of conducting sufficiently large trials followed long enough to measure differences in cancer incidence among prevention strategies.
Although the current 3-dose HPV vaccination schedule for the bivalent and quadrivalent vaccines have been shown to provide protection for at least 10 years [26, 27], the durability of the vaccines beyond this time frame remain uncertain. The aforementioned novel vaccination dosing schedule induces lower antibody titers [28] and therefore may be less likely to provide lifelong immunity. When we varied the duration of vaccine protection we found that if the nonavalent HPV vaccine provided only 20 years of complete protection, the reduction in the lifetime risk of developing cervical cancer reduced by 40% compared with when we assumed lifetime protection against vaccine-targeted HPV types. Waning vaccine protection also shifted the vaccination age at which cancer reductions were greatest, from age 9 years to 12 years; vaccinating older than age 12 years consistently resulted in declining benefits as age of vaccine initiation increased.
Limitations
Although our disease simulation model shows good correspondence to both age- and genotype-specific HPV prevalence, and type distribution in CIN3 and cancer (see Supplementary Figure 1 and Campos et al 2014 [10]), all models are simplifications. In our model, HPV incidence is a function of age and is a proxy for time since sexual initiation within the context of the United States; therefore, our estimates of the age of causal HPV acquisition may not be generalizable to settings with different patterns of sexual initiation and behavior. For example, populations with later peak ages of sexual initiation may be associated with a shift in the age of causal HPV infection acquisition to older ages. However, our findings are broadly applicable to settings with a high peak HPV prevalence that declines to a plateau in older ages. Similarly, we did not capture potential herd immunity benefits of vaccination; rather we estimated the direct benefits of HPV vaccination expected for individuals. Estimating total population benefits are dependent on capturing both direct and indirect vaccine benefits, which can be highly variable across populations. Furthermore, a comprehensive analysis that evaluates alternative HPV-Faster strategies requires a dynamic transmission model to inform the optimal upper age limit to vaccinate women against HPV infections. Although our analysis has implications for the upper age limit for HPV-Faster, we elected to restrict our analysis to focus on the estimation of age of causal HPV infection using a static model and the consequent relative reductions of direct vaccination benefits. Future analyses will need to consider not only the herd immunity benefits from extending the vaccination age to older women but also the other uncertainties and variations by setting (e.g., sexual mixing patterns) that are necessary for an in-depth policy analysis.
Although vaccination in the absence of screening is not currently relevant for the US context, we selected our US-calibrated model to explore these epidemiological questions as model-fitting was informed using high-quality and highly specific data on age- and genotype-specific prevalence, which are not as readily available in other settings. Consistent with a statistical model estimated using Dutch empirical data [29], our disease simulation model reflects duration-based progression and regression probabilities rather than exponential distributions, which avoids overestimating the influence of fast-progressing precancer on cancer incidence.
Policy Implications
Understanding the age distribution of acquired causal HPV infections and the potential loss in cancer prevention as vaccination age increases helps inform decisions regarding HPV vaccination ages and how screening approaches should be integrated contingent on vaccination age. According to our model, only 10–12% of causal HPV infections can be prevented after age 45 years, and therefore screening for persistent HPV or underling precancer will likely be most valuable in these women. Our analysis complements the shift toward risk-based screening and necessitates a risk-based framework that involves knowing the age of HPV vaccination. Our analysis also questions policies that call for vaccination at any age, as new incident infections that progress to cancer are increasing unlikely as women age. Lastly, continuing to perform clinical or model-based analyses that evaluate reductions in surrogate endpoints such as HPV incidence, prevalence, or precancer [30] and using these arguments to justify HPV vaccination of older women [25] fail to capture the most important health outcome of an HPV vaccination policy, that is, to prevent cancer.
CONCLUSIONS
Opportunities to prevent the causal HPV infection decrease as women age, and our model projects that nearly half of women who develop cancer have already acquired their HPV16 infection by age 20 years. Our model-based explorations contribute to the current understanding of the natural history of the causal HPV infection and highlight the limitations of using surrogate endpoints in vaccine efficacy studies of mid-adult women to guide policy decisions for implementation.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Contributions. E. A. B., J. J. K., and P. E. C. contributed to the conception and design of the study. All authors contributed to the analysis and interpretation of data. E. A. B. drafted the manuscript, and all authors participated in critically revising the manuscript for important intellectual content and provided final approval of the version to be published.
Acknowledgments. We thank Cassie Regan (Harvard Center for Health Decision Science) for her support in processing model output.
Financial support. This work was supported by the National Cancer Institute at the National Institutes of Health (grant R01 CA160744; PI Jane Kim). E. A. B. is partially supported by the Norwegian Research Council (grant 238042).
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Moscicki AB, Schiffman M, Burchell A et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine 2012; 30(Suppl 5):F24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCredie MR, Sharples KJ, Paul C et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol 2008; 9:425–34. [DOI] [PubMed] [Google Scholar]
- 3. Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev 2013; 22:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wheeler CM, Skinner SR, Del Rosario-Raymundo MR et al. ; VIVIANE Study Group. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis 2016; 16:1154–68. [DOI] [PubMed] [Google Scholar]
- 5. Castellsagué X, Muñoz N, Pitisuttithum P et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer 2011; 105:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammer A, Rositch A, Qeadan F, Gravitt PE, Blaakaer J. Age-specific prevalence of HPV16/18 genotypes in cervical cancer: a systematic review and meta-analysis. Int J Cancer 2016; 138:2795–803. [DOI] [PubMed] [Google Scholar]
- 7. Saraiya M, Unger ER, Thompson TD et al. ; HPV Typing of Cancers Workgroup. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015; 107:djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castle PE, Burger EA. Age of human papillomavirus vaccination? Lancet Infect Dis 2016; 16:1091–3. [DOI] [PubMed] [Google Scholar]
- 9. Goldie SJ, Goldhaber-Fiebert JD, Garnett GP. Chapter 18: Public health policy for cervical cancer prevention: the role of decision science, economic evaluation, and mathematical modeling. Vaccine 2006; 24(Supplement 3): S155–63. [DOI] [PubMed] [Google Scholar]
- 10. Campos NG, Burger EA, Sy S et al. An updated natural history model of cervical cancer: derivation of model parameters. Am J Epidemiol 2014; 180:545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campos NG, Kim JJ, Castle PE et al. Health and economic impact of HPV 16/18 vaccination and cervical cancer screening in Eastern Africa. Int J Cancer 2012; 130:2672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campos NG, Maza M, Alfaro K et al. The comparative and cost-effectiveness of HPV-based cervical cancer screening algorithms in El Salvador. Int J Cancer 2015; 137:893–902. [DOI] [PubMed] [Google Scholar]
- 13. Kim JJ, Burger EA, Sy S, Campos NG. Optimal cervical cancer screening in women vaccinated against human papillomavirus. J Natl Cancer Inst 2016;109: doi:10.1093/jnci/djw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JJ, Campos NG, Sy S et al. ; New Mexico HPV Pap Registry Steering Committee. Inefficiencies and high-value improvements in US cervical cancer screening practice: a cost-effectiveness analysis. Ann Intern Med 2015; 163:589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burger EA, Ortendahl JD, Sy S, Kristiansen IS, Kim JJ. Cost-effectiveness of cervical cancer screening with primary human papillomavirus testing in Norway. Br J Cancer 2012; 106:1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA 2002; 287:2382–90. [DOI] [PubMed] [Google Scholar]
- 17. Rodríguez AC, Schiffman M, Herrero R et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herrero R, Hildesheim A, Rodríguez AC et al. ; Costa Rica Vaccine Trial (CVT) Group. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine 2008; 26:4795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muñoz N, Méndez F, Posso H et al. ; Instituto Nacional de Cancerologia HPV Study Group. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis 2004; 190:2077–87. [DOI] [PubMed] [Google Scholar]
- 20. Kim JJ, Kuntz KM, Stout NK et al. Multiparameter calibration of a natural history model of cervical cancer. Am J Epidemiol 2007; 166:137–50. [DOI] [PubMed] [Google Scholar]
- 21. Wheeler CM, Hunt WC, Cuzick J et al. ; New Mexico HPV Pap Registry Steering Committee. A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer 2013; 132:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wheeler CM, Hunt WC, Joste NE, Key CR, Quint WG, Castle PE. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst 2009; 101:475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359:821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ 2008; 337:a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bosch FX, Robles C, Díaz M et al. HPV-FASTER: broadening the scope for prevention of HPV-related cancer. Nat Rev Clin Oncol 2016; 13:119–32. [DOI] [PubMed] [Google Scholar]
- 26. Naud PS, Roteli-Martins CM, De Carvalho NS et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine. Hum Vaccin Immunother 2014; 10:2147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferris D, Samakoses R, Block SL et al. Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics 2014; 134:e657–65. [DOI] [PubMed] [Google Scholar]
- 28. Safaeian M, Porras C, Pan Y et al. ; CVT Group. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013; 6:1242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vink MA, Bogaards JA, van Kemenade FJ, de Melker HE, Meijer CJ, Berkhof J. Clinical progression of high-grade cervical intraepithelial neoplasia: estimating the time to preclinical cervical cancer from doubly censored national registry data. Am J Epidemiol 2013; 178:1161–9. [DOI] [PubMed] [Google Scholar]
- 30. Baussano I, Lazzarato F, Ronco G, Dillner J, Franceschi S. Benefits of catch-up in vaccination against human papillomavirus in medium- and low-income countries. Int J Cancer 2013; 133:1876–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.