Abstract
These guidelines are intended for use by healthcare professionals who care for children and adults with suspected or confirmed infectious diarrhea. They are not intended to replace physician judgement regarding specific patients or clinical or public health situations. This document does not provide detailed recommendations on infection prevention and control aspects related to infectious diarrhea.
Keywords: diarrhea, infectious, diagnostics, management, prevention
EXECUTIVE SUMMARY
The following evidence-based guidelines for management of infants, children, adolescents, and adults in the United States with acute or persistent infectious diarrhea were prepared by an expert panel assembled by the Infectious Diseases Society of America (IDSA) and replace guidelines published in 2001 [1]. Public health aspects of diarrhea associated with foodborne and waterborne diarrhea, international travel, antimicrobial agents, immunocompromised hosts, animal exposure, certain sexual practices, healthcare-associated diarrheal infections, and infections acquired in childcare and long-term care facilities will be referred to in these guidelines, but are not covered extensively due to availability of detailed discussions of this information in other publications. For recommendations pertaining to Clostridium difficile, refer to the existing IDSA/Society for Healthcare Epidemiology of America (SHEA) guidelines on C. difficile infections, which are in the process of being updated.
Summarized below are recommendations made in the updated guidelines for diagnosis and management of infectious diarrhea. The Panel followed a process used in development of other IDSA guidelines [2] which included a systematic weighting of the strength of recommendation and quality of evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) [3–7]. A detailed description of the methods, background, and evidence summaries that support each of the recommendations can be found online in the full text of the guidelines.
RECOMMENDATIONS FOR THE DIAGNOSIS AND MANAGEMENT OF INFECTIOUS DIARRHEA
Clinical, Demographic, and Epidemiologic Features
I. In people with diarrhea, which clinical, demographic, or epidemiologic features have diagnostic or management implications? (Tables 1–3)
Table 1.
Modes of Acquisition of Enteric Organisms and Sources of Guidelines
| Mode | Title | URL | Author/Issuing Agency |
|---|---|---|---|
| International travel | Expert Review of the Evidence Base for Prevention of Travelers’ Diarrhea | http://www.ncbi.nlm.nih.gov/pubmed/19538575 | DuPont et al [113] |
| Medical Considerations Before International Travel | http://www.ncbi.nlm.nih.gov/pubmed/27468061 | Freedman et al [207] | |
| The Yellow Book | http://wwwnc.cdc.gov/travel/page/ yellowbook-home-2014 | CDC | |
| Travelers Health | http://wwwnc.cdc.gov/travel | CDC | |
| Immunocompromised hosts | Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents | http://aidsinfo.nih.gov/contentfiles/lvguidelines/ adult_oi.pdf | CDC/NIH/HIVMA/IDSA |
| Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Exposed and HIV-Infected Children | http://aidsinfo.nih.gov/contentfiles/lvguidelines/ oi_guidelines_pediatrics.pdf | CDC/NIH/HIVMA/IDSA | |
| Foodborne and waterborne | Surveillance for Foodborne Disease Outbreaks—United States, 2009–2010 | http://www.cdc.gov/mmwr/preview/mmwrhtml/ mm6203a1.htm?s_cid=mm6203a1_w | CDC |
| Food Safety | http://www.cdc.gov/foodsafety/ | CDC | |
| Healthy Water | http://wwwnc.cdc.gov/healthywater | CDC | |
| Antimicrobial-associated (C. difficile) | Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children 2017 Update (in press) | http://www.jstor.org/stable/10.1086/651706 | IDSA/SHEA |
| 2010 Clinical Practice Guidelines for Clostridium difficile Infection in Adults | https://www.idsociety.org/ Organ_System/#Clostridiumdifficile | IDSA/SHEA | |
| Healthcare-associated | Healthcare-Associated Infections | http://www.cdc.gov/hai/ | CDC |
| Child care settings | Caring for Our Children: National Health and Safety Performance Standards; Guidelines for Early Care and Education Programs | http://nrckids.org. | AAP, APHA, NRC |
| Recommendations for Care of Children in Special Circumstances—Children in Out- of-Home Child Care (pp 132–51) | http://redbook.solutions.aap.org/redbook.aspx | AAP | |
| Managing Infectious Diseases in Child Care and Schools | http://ebooks.aappublications.org/content/managing-infectious-diseases-in-child-care-and-schools- 3rd-edition | AAP | |
| Long-term care settings | Nursing Homes and Assisted Living (Long- term Care Facilities) | http://www.cdc.gov/longtermcare/ | CDC |
| Infection Prevention and Control in the Long-term Care Facility | http://www.shea-online.org/assets/files/position-papers/ic-ltcf97.pdf | SHEA/APIC | |
| Zoonoses | Compendium of Measures to Prevent Disease Associated With Animals in Public Settings | http://www.cdc.gov/mmwr/preview/mmwrhtml/ rr6004a1.htm?s_cid=rr6004a1_w | CDC |
| Exposure to Nontraditional Pets at Home and to Animals in Public Settings: Risks to Children | http://pediatrics.aappublications.org/ content/122/4/876 | Pickering et al [51] | |
| Review of Institute of Medicine and National Research Council Recommendations for One Health Initiative | http://wwwnc.cdc.gov/eid/article/19/12/12-1659_ article.htm | Rubin et al [208] |
Abbreviations: AAP, American Academy of Pediatrics; APHA, American Public Health Association; APIC, Association for Professionals in Infection Control and Epidemiology; CDC, Centers for Disease Control and Prevention; HIV, human immunodeficiency virus; HIVMA, HIV Medicine Association; IDSA, Infectious Diseases Society of America; NIH, National Institutes of Health; NRC, National Resource Center for Health and Safety in Child Care and Early Education; SHEA, Society for Healthcare Epidemiology of America.
Recommendations
1. A detailed clinical and exposure history should be obtained from people with diarrhea, under any circumstances, including when there is a history of similar illness in others (strong, moderate).
2. People with diarrhea who attend or work in child care centers, long-term care facilities, patient care, food service, or recreational water venues (eg, pools and lakes) should follow jurisdictional recommendations for outbreak reporting and infection control (strong, high).
II. In people with fever or bloody diarrhea, which clinical, demographic, or epidemiologic features have diagnostic or management implications? (Tables 1–3)
Recommendations
3. People with fever or bloody diarrhea should be evaluated for enteropathogens for which antimicrobial agents may confer clinical benefit, including Salmonella enterica subspecies, Shigella, and Campylobacter (strong, low).
4. Enteric fever should be considered when a febrile person (with or without diarrhea) has a history of travel to areas in which causative agents are endemic, has had consumed foods prepared by people with recent endemic exposure, or has laboratory exposure to Salmonella enterica subspecies enterica serovar Typhi and Salmonella enterica subspecies enterica serovar Paratyphi (strong, moderate). In this document, Salmonella Typhi represents the more formal and detailed name Salmonella enterica subspecies enterica serovar Typhi, and Salmonella Paratyphi corresponds to the Paratyphi serovar.
III. What clinical, demographic, or epidemiologic features are associated with complications or severe disease? (Tables 2 and 3)
Recommendations
5. People of all ages with acute diarrhea should be evaluated for dehydration, which increases the risk of life-threatening illness and death, especially among the young and older adults (strong, high).
6. When the clinical or epidemic history suggests a possible Shiga toxin–producing organism, diagnostic approaches should be applied that detect Shiga toxin (or the genes that encode them) and distinguish Escherichia coli O157:H7 from other Shiga toxin–producing E. coli (STEC) in stool (strong, moderate). If available, diagnostic approaches that can distinguish between Shiga toxin 1 and Shiga toxin 2, which is typically more potent, could be used (weak, moderate). In addition, Shigella dysenteriae type 1, and, rarely, other pathogens may produce Shiga toxin and should be considered as a cause of hemolytic uremic syndrome (HUS), especially in people with suggestive international travel or personal contact with a traveler (strong, moderate).
7. Clinicians should evaluate people for postinfectious and extraintestinal manifestations associated with enteric infections (strong, moderate) [8].
Diagnostics
IV. Which pathogens should be considered in people presenting with diarrheal illnesses, and which diagnostic tests will aid in organism identification or outbreak investigation?
Recommendations
8. Stool testing should be performed for Salmonella, Shigella, Campylobacter, Yersinia, C. difficile, and STEC in people with diarrhea accompanied by fever, bloody or mucoid stools, severe abdominal cramping or tenderness, or signs of sepsis (strong, moderate). Bloody stools are not an expected manifestation of infection with C. difficile. STEC O157 should be assessed by culture and non-O157 STEC should be detected by Shiga toxin or genomic assays (strong, low). Sorbitol-MacConkey agar or an appropriate chromogenic agar alternative is recommended to screen for O157:H7 STEC; detection of Shiga toxin is needed to detect other STEC serotype (strong, moderate).
9. Blood cultures should be obtained from infants <3 months of age, people of any age with signs of septicemia or when enteric fever is suspected, people with systemic manifestations of infection, people who are immunocompromised, people with certain high-risk conditions such as hemolytic anemia, and people who traveled to or have had contact with travelers from enteric fever–endemic areas with a febrile illness of unknown etiology (strong, moderate).
-
10. Stool testing should be performed under clearly identified circumstances (Table 2) for Salmonella, Shigella, Campylobacter, Yersinia, C. difficile, and STEC in symptomatic hosts (strong, low). Specifically,
a. Test for Yersinia enterocolitica in people with persistent abdominal pain (especially school-aged children with right lower quadrant pain mimicking appendicitis who may have mesenteric adenitis), and in people with fever at epidemiologic risk for yersiniosis, including infants with direct or indirect exposures to raw or undercooked pork products.
b. In addition, test stool specimens for Vibrio species in people with large volume rice water stools or either exposure to salty or brackish waters, consumption of raw or undercooked shellfish, or travel to cholera-endemic regions within 3 days prior to onset of diarrhea.
11. A broader set of bacterial, viral, and parasitic agents should be considered regardless of the presence of fever, bloody or mucoid stools, or other markers of more severe illness in the context of a possible outbreak of diarrheal illness (eg, multiple people with diarrhea who shared a common meal or a sudden rise in observed diarrheal cases). Selection of agents for testing should be based on a combination of host and epidemiologic risk factors and ideally in coordination with public health authorities (strong, moderate).
12. A broad differential diagnosis is recommended in immunocompromised people with diarrhea, especially those with moderate and severe primary or secondary immune deficiencies, for evaluation of stool specimens by culture, viral studies, and examination for parasites (strong, moderate). People with acquired immune deficiency syndrome (AIDS) with persistent diarrhea should undergo additional testing for other organisms including, but not limited to, Cryptosporidium, Cyclospora, Cystoisospora, microsporidia, Mycobacterium avium complex, and cytomegalovirus (strong, moderate).
13. Diagnostic testing is not recommended in most cases of uncomplicated traveler’s diarrhea unless treatment is indicated. Travelers with diarrhea lasting 14 days or longer should be evaluated for intestinal parasitic infections (strong, moderate). Testing for C. difficile should be performed in travelers treated with antimicrobial agent(s) within the preceding 8–12 weeks. In addition, gastrointestinal tract disease including inflammatory bowel disease (IBD) and postinfectious irritable bowel syndrome (IBS) should be considered for evaluation (strong, moderate).
14. Clinical consideration should be included in the interpretation of results of multiple-pathogen nucleic acid amplification tests because these assays detect DNA and not necessarily viable organisms (strong, low).
15. All specimens that test positive for bacterial pathogens by culture-independent diagnostic testing such as antigen-based molecular assays (gastrointestinal tract panels), and for which isolate submission is requested or required under public health reporting rules, should be cultured in the clinical laboratory or at a public health laboratory to ensure that outbreaks of similar organisms are detected and investigated (strong, low). Also, a culture may be required in situations where antimicrobial susceptibility testing results would affect care or public health responses (strong, low).
16. Specimens from people involved in an outbreak of enteric disease should be tested for enteric pathogens per public health department guidance (strong, low).
Table 2.
Exposure or Condition Associated With Pathogens Causing Diarrhea
| Exposure or Condition | Pathogen(s) |
|---|---|
| Foodborne | |
| Foodborne outbreaks in hotels, cruise ships, resorts, restaurants, catered events | Norovirus, nontyphoidal Salmonella, Clostridium perfringens, Bacillus cereus, Staphylococcus aureus, Campylobacter spp, ETEC, STEC, Listeria, Shigella, Cyclospora cayetanensis, Cryptosporidium spp |
| Consumption of unpasteurized milk or dairy products | Salmonella, Campylobacter, Yersinia enterocolitica, S. aureus toxin, Cryptosporidium, and STEC. Listeria is infrequently associated with diarrhea, Brucella (goat milk cheese), Mycobacterium bovis, Coxiella burnetii |
| Consumption of raw or undercooked meat or poultry | STEC (beef), C. perfringens (beef, poultry), Salmonella (poultry), Campylobacter (poultry), Yersinia (pork, chitterlings), S. aureus (poultry), and Trichinella spp (pork, wild game meat) |
| Consumption of fruits or unpasteurized fruit juices, vegetables, leafy greens, and sprouts | STEC, nontyphoidal Salmonella, Cyclospora, Cryptosporidium, norovirus, hepatitis A, and Listeria monocytogenes |
| Consumption of undercooked eggs | Salmonella, Shigella (egg salad) |
| Consumption of raw shellfish | Vibrio species, norovirus, hepatitis A, Plesiomonas |
| Exposure or contact | |
| Swimming in or drinking untreated fresh water | Campylobacter, Cryptosporidium, Giardia, Shigella, Salmonella, STEC, Plesiomonas shigelloides |
| Swimming in recreational water facility with treated water | Cryptosporidium and other potentially waterborne pathogens when disinfectant concentrations are inadequately maintained |
| Healthcare, long-term care, prison exposure, or employment | Norovirus, Clostridium difficile, Shigella, Cryptosporidium, Giardia, STEC, rotavirus |
| Child care center attendance or employment | Rotavirus, Cryptosporidium, Giardia, Shigella, STEC |
| Recent antimicrobial therapy | C. difficile, multidrug-resistant Salmonella |
| Travel to resource-challenged countries | Escherichia coli (enteroaggregative, enterotoxigenic, enteroinvasive), Shigella, Typhi and nontyphoidal Salmonella, Campylobacter, Vibrio cholerae, Entamoeba histolytica, Giardia, Blastocystis, Cyclospora, Cystoisospora, Cryptosporidium |
| Exposure to house pets with diarrhea | Campylobacter, Yersinia |
| Exposure to pig feces in certain parts of the world | Balantidium coli |
| Contact with young poultry or reptiles | Nontyphoidal Salmonella |
| Visiting a farm or petting zoo | STEC, Cryptosporidium, Campylobacter |
| Exposure or condition | |
| Age group | Rotavirus (6–18 months of age), nontyphoidal Salmonella (infants from birth to 3 months of age and adults >50 years with a history of atherosclerosis), Shigella (1–7 years of age), Campylobacter (young adults) |
| Underlying immunocompromising condition | Nontyphoidal Salmonella, Cryptosporidium, Campylobacter, Shigella, Yersinia |
| Hemochromatosis or hemoglobinopathy | Y. enterocolitica, Salmonella |
| AIDS, immunosuppressive therapies | Cryptosporidium, Cyclospora, Cystoisospora, microsporidia, Mycobacterium avium–intercellulare complex, cytomegalovirus |
| Anal-genital, oral-anal, or digital-anal contact | Shigella, Salmonella, Campylobacter, E. histolytica, Giardia lamblia, Cryptosporidium as well as sexually transmitted infections |
Abbreviations: ETEC, enterotoxigenic Escherichia coli; STEC, Shiga toxin–producing Escherichia coli.
V. Which diagnostic tests should be performed when enteric fever or bacteremia is suspected?
Recommendation
17. Culture-independent, including panel-based multiplex molecular diagnostics from stool and blood specimens, and, when indicated, culture-dependent diagnostic testing should be performed when there is a clinical suspicion of enteric fever (diarrhea uncommon) or diarrhea with bacteremia (strong, moderate). Additionally, cultures of bone marrow (particularly valuable if antimicrobial agents have been administered), stool, duodenal fluid, and urine may be beneficial to detect enteric fever (weak, moderate). Serologic tests should not be used to diagnose enteric fever (strong, moderate).
VI. When should testing be performed for Clostridium difficile?
Recommendation
18. Testing may be considered for C. difficile in people >2 years of age who have a history of diarrhea following antimicrobial use and in people with healthcare-associated diarrhea (weak, high). Testing for C. difficile may be considered in people who have persistent diarrhea without an etiology and without recognized risk factors (weak, low). A single diarrheal stool specimen is recommended for detection of toxin or a toxigenic C. difficile strain (eg, nucleic acid amplification testing) (strong, low). Multiple specimens do not increase yield.
VII. What is the optimal specimen (eg, stool, rectal swab, blood) for maximum yield of bacterial, viral, and protozoal organisms (for culture, immunoassay, and molecular testing)? (Table 5)
Recommendation
19. The optimal specimen for laboratory diagnosis of infectious diarrhea is a diarrheal stool sample (ie, a sample that takes the shape of the container). For detection of bacterial infections, if a timely diarrheal stool sample cannot be collected, a rectal swab may be used (weak, low). Molecular techniques generally are more sensitive and less dependent than culture on the quality of specimen. For identification of viral and protozoal agents, and C. difficile toxin, fresh stool is preferred (weak, low).
VIII. What is the clinical relevance of fecal leukocytes or lactoferrin or calprotectin in a person with acute diarrhea?
Recommendation
20. Fecal leukocyte examination and stool lactoferrin detection should not be used to establish the cause of acute infectious diarrhea (strong, moderate). There are insufficient data available to make a recommendation on the value of fecal calprotectin measurement in people with acute infectious diarrhea.
IX. In which clinical scenarios should nonmicrobiologic diagnostic tests be performed (eg, imaging, chemistries, complete blood count, and serology)?
Recommendations
21. Serologic tests are not recommended to establish an etiology of infectious diarrhea or enteric fever (strong, low), but may be considered for people with postdiarrheal HUS in which a stool culture did not yield a Shiga toxin–producing organism (weak, low).
22. A peripheral white blood cell count and differential and serologic assays should not be performed to establish an etiology of diarrhea (strong, low), but may be useful clinically (weak, low).
23. Frequent monitoring of hemoglobin and platelet counts, electrolytes, and blood urea nitrogen and creatinine is recommended to detect hematologic and renal function abnormalities that are early manifestations of HUS and precede renal injury for people with diagnosed E. coli O157 or another STEC infection (especially STEC that produce Shiga toxin 2 or are associated with bloody diarrhea) (strong, high). Examining a peripheral blood smear for the presence of red blood cell fragments is necessary when HUS is suspected (strong, high).
24. Endoscopy or proctoscopic examination should be considered in people with persistent, unexplained diarrhea who have AIDS, in people with certain underlying medical conditions as well as people with acute diarrhea with clinical colitis or proctitis and in people with persistent diarrhea who engage in anal intercourse (strong, low). Duodenal aspirate may be considered in select people for diagnosis of suspected Giardia, Strongyloides, Cystoisospora, or microsporidia infection (weak, low).
25. Imaging (eg, ultrasonography, computed tomography, or magnetic resonance imaging) may be considered to detect aortitis, mycotic aneurysms, signs and symptoms of peritonitis, intra-abdominal free air, toxic megacolon, or extravascular foci of infection in older people with invasive Salmonella enterica or Yersinia infections if there is sustained fever or bacteremia despite adequate antimicrobial therapy or if the patient has underlying atherosclerosis or has recent-onset chest, back, or abdominal pain (weak, low).
X. What follow-up evaluations of stool specimens and nonstool tests should be performed in people with laboratory-confirmed pathogen-specific diarrhea who improve or respond to treatment, and in people who fail to improve or who have persistent diarrhea?
Recommendations
26. Follow-up testing is not recommended in most people for case management following resolution of diarrhea (strong, moderate). Collection and analysis of serial stool specimens using culture-dependent methods for Salmonella enterica subspecies enterica serovar Typhi or Salmonella enterica subspecies enterica serovar Paratyphi, STEC, Shigella, nontyphoidal Salmonella, and other bacterial pathogens are recommended in certain situations by local health authorities following cessation of diarrhea to enable return to child care, employment, or group social activities (strong, moderate). Practitioners should collaborate with local public health authorities to adhere to policies regarding return to settings in which transmission is a consideration (strong, high).
27. A clinical and laboratory reevaluation may be indicated in people who do not respond to an initial course of therapy and should include consideration of noninfectious conditions including lactose intolerance (weak, low).
28. Noninfectious conditions, including IBD and IBS, should be considered as underlying etiologies in people with symptoms lasting 14 or more days and unidentified sources (strong, moderate).
29. Reassessment of fluid and electrolyte balance, nutritional status, and optimal dose and duration of antimicrobial therapy is recommended in people with persistent symptoms (strong, high).
Empiric Management of Infectious Diarrhea
XI. When is empiric antibacterial treatment indicated for children and adults with bloody diarrhea and, if indicated, with what agent?
a. What are modifying conditions that would support antimicrobial treatment of children and adults with bloody diarrhea?
b. In which instances should contacts be treated empirically if the agent is unknown?
Recommendations
-
30. In immunocompetent children and adults, empiric antimicrobial therapy for bloody diarrhea while waiting for results of investigations is not recommended (strong, low), except for the following:
a. Infants <3 months of age with suspicion of a bacterial etiology.
b. Ill immunocompetent people with fever documented in a medical setting, abdominal pain, bloody diarrhea, and bacillary dysentery (frequent scant bloody stools, fever, abdominal cramps, tenesmus) presumptively due to Shigella.
c. People who have recently travelled internationally with body temperatures ≥38.5°C and/or signs of sepsis (weak, low). See https://wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/travelers-diarrhea.
31. The empiric antimicrobial therapy in adults should be either a fluoroquinolone such as ciprofloxacin, or azithromycin, depending on the local susceptibility patterns and travel history (strong, moderate). Empiric therapy for children includes a third-generation cephalosporin for infants <3 months of age and others with neurologic involvement, or azithromycin, depending on local susceptibility patterns and travel history (strong, moderate).
32. Empiric antibacterial treatment should be considered in immunocompromised people with severe illness and bloody diarrhea (strong, low).
33. Asymptomatic contacts of people with bloody diarrhea should not be offered empiric treatment, but should be advised to follow appropriate infection prevention and control measures (strong, moderate).
34. People with clinical features of sepsis who are suspected of having enteric fever should be treated empirically with broad-spectrum antimicrobial therapy after blood, stool, and urine culture collection (strong, low). Antimicrobial therapy should be narrowed when antimicrobial susceptibility testing results become available (strong, high). If an isolate is unavailable and there is a clinical suspicion of enteric fever, antimicrobial choice may be tailored to susceptible patterns from the setting where acquisition occurred (weak, low).
35. Antimicrobial therapy for people with infections attributed to STEC O157 and other STEC that produce Shiga toxin 2 (or if the toxin genotype is unknown) should be avoided (strong, moderate). Antimicrobial therapy for people with infections attributed to other STEC that do not produce Shiga toxin 2 (generally non-O157 STEC) is debatable due to insufficient evidence of benefit or the potential harm associated with some classes of antimicrobial agents (strong, low).
XII. When is empiric treatment indicated for children and adults with acute, prolonged, or persistent watery diarrhea and, if indicated, with what agent?
a. What are modifying conditions that would support empiric antimicrobial treatment of children and adults with watery diarrhea?
b. In which instances, if any, should contacts be treated empirically if the agent is unknown?
Recommendations
36. In most people with acute watery diarrhea and without recent international travel, empiric antimicrobial therapy is not recommended (strong, low). An exception may be made in people who are immunocompromised or young infants who are ill-appearing. Empiric treatment should be avoided in people with persistent watery diarrhea lasting 14 days or more (strong, low).
37. Asymptomatic contacts of people with acute or persistent watery diarrhea should not be offered empiric or preventive therapy, but should be advised to follow appropriate infection prevention and control measures (strong, moderate).
Directed Management of Infectious Diarrhea
XIII. How should treatment be modified when a clinically plausible organism is identified from a diagnostic test?
Recommendation
38. Antimicrobial treatment should be modified or discontinued when a clinically plausible organism is identified (strong, high).
Supportive Treatment
XIV. How should rehydration therapy be administered?
Recommendations
39. Reduced osmolarity oral rehydration solution (ORS) is recommended as the first-line therapy of mild to moderate dehydration in infants, children, and adults with acute diarrhea from any cause (strong, moderate), and in people with mild to moderate dehydration associated with vomiting or severe diarrhea.
40. Nasogastric administration of ORS may be considered in infants, children, and adults with moderate dehydration, who cannot tolerate oral intake, or in children with normal mental status who are too weak or refuse to drink adequately (weak, low).
41. Isotonic intravenous fluids such as lactated Ringer’s and normal saline solution should be administered when there is severe dehydration, shock, or altered mental status and failure of ORS therapy (strong, high) or ileus (strong, moderate). In people with ketonemia, an initial course of intravenous hydration may be needed to enable tolerance of oral rehydration (weak, low).
42. In severe dehydration, intravenous rehydration should be continued until pulse, perfusion, and mental status normalize and the patient awakens, has no risk factors for aspiration, and has no evidence of ileus (strong, low). The remaining deficit can be replaced by using ORS (weak, low). Infants, children, and adults with mild to moderate dehydration should receive ORS until clinical dehydration is corrected (strong, low).
43. Once the patient is rehydrated, maintenance fluids should be administered. Replace ongoing losses in stools from infants, children, and adults with ORS, until diarrhea and vomiting are resolved (strong, low).
XV. When should feeding be initiated following rehydration?
Recommendations
44. Human milk feeding should be continued in infants and children throughout the diarrheal episode (strong, low).
45. Resumption of an age-appropriate usual diet is recommended during or immediately after the rehydration process is completed (strong, low).
Ancillary Management
XVI. What options are available for symptomatic relief, and when should they be offered?
Recommendations
46. Ancillary treatment with antimotility, antinausea, or antiemetic agents can be considered once the patient is adequately hydrated, but their use is not a substitute for fluid and electrolyte therapy (weak, low).
47. Antimotility drugs (eg, loperamide) should not be given to children <18 years of age with acute diarrhea (strong, moderate). Loperamide may be given to immunocompetent adults with acute watery diarrhea (weak, moderate), but should be avoided at any age in suspected or proven cases where toxic megacolon may result in inflammatory diarrhea or diarrhea with fever (strong, low).
48. Antinausea and antiemetic (eg, ondansetron) may be given to facilitate tolerance of oral rehydration in children >4 years of age and in adolescents with acute gastroenteritis associated with vomiting (weak, moderate).
XVII. What is the role of a probiotic or zinc in treatment or prevention of infectious diarrhea in children and adults?
Recommendations
49. Probiotic preparations may be offered to reduce the symptom severity and duration in immunocompetent adults and children with infectious or antimicrobial-associated diarrhea (weak, moderate). Specific recommendations regarding selection of probiotic organism(s), route of delivery, and dosage may be found through literature searches of studies and through guidance from manufacturers.
50. Oral zinc supplementation reduces the duration of diarrhea in children 6 months to 5 years of age who reside in countries with a high prevalence of zinc deficiency or who have signs of malnutrition (strong, moderate).
XVIII. Which asymptomatic people with an identified bacterial organism from stool culture or molecular testing should be treated with an antimicrobial agent?
Recommendations
51. Asymptomatic people who practice hand hygiene and live and work in low-risk settings (do not provide healthcare or child or elderly adult care and are not food service employees) do not need treatment, except asymptomatic people with Salmonella enterica subspecies enterica serovar Typhi in their stool who may be treated empirically to reduce potential for transmission (weak, low). Asymptomatic people who practice hand hygiene and live and work in high-risk settings (provide healthcare or child or elderly adult care and are food service employees) should be treated according to local public health guidance (strong, high).
Prevention
XIX. What strategies, including public health measures, are beneficial in preventing transmission of pathogens associated with infectious diarrhea?
Recommendations
52. Hand hygiene should be performed after using the toilet, changing diapers, before and after preparing food, before eating, after handling garbage or soiled laundry items, and after touching animals or their feces or environments, especially in public settings such as petting zoos (strong, moderate).
53. Infection control measures including use of gloves and gowns, hand hygiene with soap and water, or alcohol-based sanitizers should be followed in the care of people with diarrhea (strong, high). The selection of a hand hygiene product should be based upon a known or suspected pathogen and the environment in which the organism may be transmitted (strong, low). See https://www.cdc.gov/hicpac/2007IP/2007isolationPrecautions.html.
54. Appropriate food safety practices are recommended to avoid cross-contamination of other foods or cooking surfaces and utensils during grocery shopping, food preparation, and storage; ensure that foods containing meats and eggs are cooked and maintained at proper temperatures (strong, moderate).
55. Healthcare providers should direct educational efforts toward all people with diarrhea, but particularly to people with primary and secondary immune deficiencies, pregnant women, parents of young children, and the elderly as they have increased risk of complications from diarrheal disease (strong, low).
56. Ill people with diarrhea should avoid swimming, water-related activities, and sexual contact with other people when symptomatic while adhering to meticulous hand hygiene (strong, low).
XX. What are the relative efficacies and effectiveness of vaccines (rotavirus, typhoid, and cholera) to reduce and prevent transmission of pathogens associated with infectious diarrhea, and when should they be used?
Recommendations
57. Rotavirus vaccine should be administered to all infants without a known contraindication (strong, high).
58. Two typhoid vaccines (oral and injectable) are licensed in the United States but are not recommended routinely. Typhoid vaccination is recommended as an adjunct to hand hygiene and the avoidance of high-risk foods and beverages, for travelers to areas where there is moderate to high risk for exposure to Salmonella enterica subspecies enterica serovar Typhi, people with intimate exposure (eg, household contact) to a documented Salmonella enterica subspecies enterica serovar Typhi chronic carrier, and microbiologists and other laboratory personnel routinely exposed to cultures of Salmonella enterica subspecies enterica serovar Typhi (strong, high). Booster doses are recommended for people who remain at risk (strong, high).
59. A live attenuated cholera vaccine, which is available as a single-dose oral vaccine in the United States, is recommended for adults 18–64 years of age who travel to cholera-affected areas (strong, high). See https://www.cdc.gov/cholera/vaccines.html.
XXI. How does reporting of nationally notifiable organisms identified from stool specimens impact the control and prevention of diarrheal disease in the United States?
Recommendation
60. All diseases listed in the table of National Notifiable Diseases Surveillance System at the national level, including those that cause diarrhea, should be reported to the appropriate state, territorial, or local health department with submission of isolates of certain pathogens (eg, Salmonella, STEC, Shigella, and Listeria) to ensure that control and prevention practices may be implemented (strong, high).
INTRODUCTION AND BACKGROUND
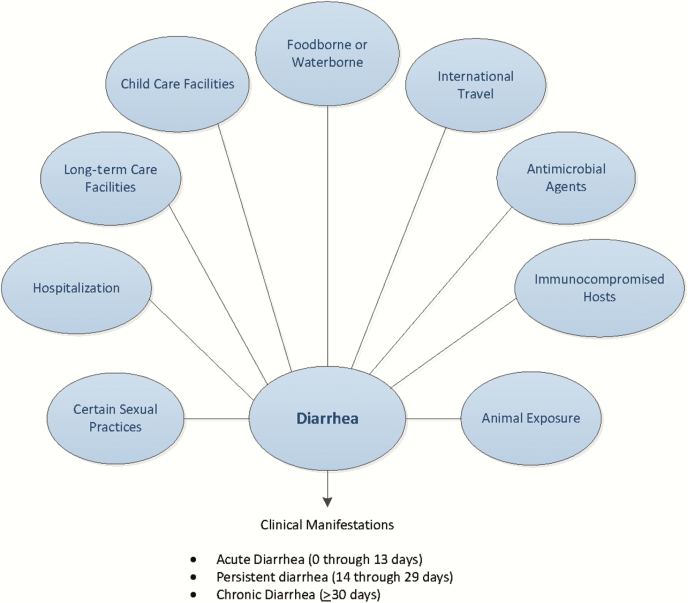
The greatest burden of infectious diarrhea occurs in low- and middle-income countries, where inadequate sanitation and hygiene are prevalent. Nonetheless, economic development also creates opportunities for introduction and transmission of enteric pathogens, including global travel, food importations, mass production and distribution of food, municipal water systems serving large segments of the population, and widespread use of childcare, long-term care, and recreational water facilities. Other risk factors include hospitalization, animal exposures (especially in public venues), and certain sexual practices (Figure 1). Outbreaks attributed to contaminated food and water and contact with infected people and animals continue to occur. Challenge studies involving adult volunteers and epidemiologic studies including those in child care centers show that infections with Cryptosporidium, Entamoeba histolytica, Giardia, norovirus, rotavirus, Shiga toxin–producing E. coli (STEC), and Shigella are spread by low inocula, and result in secondary transmission. As a result, a considerable burden of diarrheal disease occurs in the United States due to a wide variety of endemic and outbreak-associated infections with enteric pathogens that are capable of causing acute and persistent infectious diarrhea in infants, children, adolescents, and adults, sometimes complicated by extraintestinal manifestations.
Figure 1.
Considerations when evaluating people with infectious diarrhea. Modified from Long SS, Pickering LK, Pober CG, eds. Principles and Practice of Pediatric Infectious Diseases, 4th ed. New York: Elsevier Saunders, 2012.
The widening array of recognized enteric pathogens, known epidemiologic risk factors often associated with specific pathogens (Table 2), increasing number of immunosuppressed people in the United States, increasing number and availability of diagnostic methods (Table 5), increasing numbers of isolates resistant to antimicrobial agents, risk of severe illness including hemolytic uremic syndrome (HUS) due to STEC and Guillain-Barré syndrome following Campylobacter infection, and enhanced demand for cost containment sharpen the need for evidence-based clinical and public health guidelines. With the growing availability of multiplex diagnostic panels that can simultaneously detect several enteric pathogens, clinicians can expect to see patients from whom >1 pathogen is detected [9], potentially making selection of therapy with appropriate antimicrobial agents difficult. Research is needed to help interpret the clinical significance of such findings.
Table 5.
Laboratory Diagnostics for Organisms Associated With Infectious Diarrhea
| Etiologic Agent | Diagnostic Procedures | Optimal Specimen |
|---|---|---|
| Clostridium difficile | NAAT | Stool |
| GDH antigen with or without toxin detection followed by cytotoxin or Clostridium difficile toxin or toxigenic C. difficile strain | ||
| Salmonella enterica, Shigella spp, Campylobacter spp | Routine stool enteric pathogen culturea or NAAT | Stool |
| Salmonella enterica serovars Typhi and Paratyphi (enteric fever) | Routine culture | Stool, blood, bone marrow, and duodenal fluid |
| Shiga toxin–producing Escherichia coli | Culture for E. coli O157:H7b and Shiga toxin immunoassay or NAAT for Shiga toxin genes |
Stool |
| Yersinia spp, Plesiomonas spp, Edwardsiella tarda, Staphylococcus aureus, E. coli (enterotoxigenic, enteroinvasive, enteropathogenic, enteroaggregative) | Specialized stool culture or molecular assaysc or NAAT | Stool |
| Clostridium perfringens | Specialized procedure for toxin detectiond | Stool |
| Bacillus cereus, S. aureus | Specialized procedure for toxin detectiond | Food |
| Clostridium botulinum | Mouse lethality assay (performed at a state public health laboratory, or CDC) e,f,g | Serum, stool, gastric contents, vomitus |
|
Entamoeba
histolytica; Blastocystis hominih; Dientamoeba fragilish; Balantidium coli; Giardia lamblia; nematodes (generally not associated with diarrhea) including Ascaris lumbricoides, Strongyloides stercoralisi, Trichuris trichiura, hookworms; cestodes (tapeworms); trematodes (flukes) |
Ova and parasite examination including permanent stained smeari or NAAT | Stool Duodenal fluid for Giardia and Strongyloides |
| E. histolytica |
E. histolytica species-specific immunoassay or NAAT |
Stool |
| G. lamblia j | EIA or NAAT | Stool |
| Cryptosporidium spp [121]j | Direct fluorescent immunoassay, EIA, or NAAT | Stool |
| Cyclospora cayetanensis, Cystoisospora bellik | Modified acid-fast staink performed on concentrated specimen, ultraviolet fluorescence microscopy, or NAAT |
Stool |
| Microsporidia (now classified as a fungus) | Modified trichrome staink performed on concentrated specimen |
Stool |
| Histologic examination with electron microscopic confirmation | Small bowel biopsy | |
| Calicivirus (norovirus, sapovirus)k; enteric adenovirus; enterovirus/ parechovirusk; rotavirus | NAAT | Stool |
| Rotavirus, enteric adenovirus | EIA | Stool |
| Enteric adenovirusl; enterovirus/parechovirus | Viral culture | Stool |
| Cytomegalovirus | Histopathological examination | Biopsy |
| Cytomegalovirus culture | Biopsy |
The field of rapid diagnostic testing is rapidly expanding. We expect that additional diagnostic assays will become available following publication of these guidelines, specifically panel-based molecular diagnostics, including NAAT. Contact the laboratory for instructions regarding container, temperature, and transport guidelines to optimize results.
Abbreviations: CDC, Centers for Disease Control and Prevention; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; NAAT, nucleic acid amplification test.
aRoutine stool culture in most laboratories is designed to detect Salmonella spp, Shigella spp, Campylobacter spp, and E. coli O157 or Shiga toxin–producing E. coli, but this should be confirmed with the testing laboratory.
bIt is recommended that laboratories routinely process all stool specimens submitted for bacterial culture for the presence of Shiga toxin–producing strains of E. coli including O157:H7. However, in some laboratories, O157:H7 testing is performed only by specific request.
cSpecialized cultures or molecular assays may be required to detect these organisms in stool specimens. The laboratory should be notified whenever there is a suspicion of infection due to one of these pathogens.
d Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus are associated with diarrheal syndromes that are toxin mediated. An etiologic diagnosis is made by demonstration of toxin in stool for C. perfringens and demonstration of toxin in food for B. cereus and S. aureus.
eToxin assays are either performed in public health laboratories or referred to laboratories specializing in such assays.
fTesting for Clostridium botulinum toxin is either performed in public health laboratories or referred to laboratories specializing in such testing. The toxin is lethal and special precautions are required for handling. Class A bioterrorism agent and rapid sentinel laboratory reporting schemes must be followed. Immediate notification of a suspected infection to the state health department is mandated.
gImplicated food materials may also be examined for C. botulinum toxin, but most hospital laboratories are not equipped for food analysis.
hThe pathogenicity of Blastocystis hominis and Dientamoeba fragilis remains controversial. In the absence of other pathogens, they may be clinically relevant if symptoms persist. Reporting semiquantitative results (rare, few, many) may help determine significance and is a College of American Pathologists accreditation requirement for participating laboratories.
iDetection of Strongyloides in stool may require the use of Baermann technique or agar plate culture.
j Cryptosporidium and Giardia lamblia testing is often offered and performed together as the primary parasitology examination. Further studies should follow if the epidemiologic setting or clinical manifestations suggest parasitic disease.
kThese stains may not be routinely available.
lEnteric adenoviruses may not be recovered in routine viral culture.
These guidelines will focus on the clinical presentation of acute and persistent diarrhea, with emphasis on infectious etiologies in the industrialized world, specifically the United States, where diagnostic services are widespread, public health systems are in place, and endemic cholera and typhoid fever have long been controlled. For the approach to diagnosis and management of diarrheal illness in resource-challenged settings, refer to the guidelines published by the World Health Organization (WHO) [10]. It is important to note that at the time that this guideline was published, the Clostridium difficile guidelines were still in development and, while every effort was made to ensure that the recommendations were concordant, there may be minor differences.
DISEASE BURDEN AND CLINICAL PRESENTATIONS
The WHO defines diarrhea as passage of 3 or more loose or liquid stools per 24 hours, or more frequently than is normal for an individual person [10]. Frequent passing of formed stools is not diarrhea, nor is passing of loose, “pasty” stools by infants consuming human milk.
Several clinical presentations of infectious diarrhea have been described, each of which has different, albeit overlapping, etiologies, treatments, and outcomes:
1. Acute watery diarrhea (includes cholera) and acute bloody diarrhea (includes dysentery, which manifests as frequent scant stools with blood and mucus) that lasts <7 days [11]. Acute vomiting and/or diarrhea, often referred to as acute gastroenteritis, is a frequent cause of outpatient visits and hospitalizations in the United States.
2. Prolonged diarrhea that lasts 7–13 days.
3. Persistent diarrhea that lasts 14–29 days.
4. Chronic diarrhea that lasts 30 days or longer.
Acute gastroenteritis is a frequent cause of outpatient visits and hospitalizations in the United States, with an estimated annual burden of 179 million outpatient visits, nearly 500000 hospitalizations, and >5000 deaths [12]. Specific data on acute gastroenteritis in adults are sparse, with 1.5% of all hospital discharges coded as gastroenteritis. The lifetime risk of being discharged from the hospital with a diagnosis of gastroenteritis is estimated to be 1 in 8 among adults in the United States [13]. The estimated prevalence of diarrhea among adults the month before interview was 3%–7% with the rate being age-dependent [14]. Disease incidence is highest among children <5 years; however, the percentages of hospitalization and death are highest in persons 65 years or older [15].
The Foodborne Diseases Active Surveillance Network (FoodNet) national surveillance system maintained by Centers for Disease Control and Prevention (CDC) is perhaps the most comprehensive source of data on the pathogen-specific burden of diarrheal disease in the United States. Norovirus and Salmonella enterica subspecies were the leading pathogens among the 24 gastroenteritis pathogens transmissible by food that were assessed. Whereas norovirus (58%) exceeded Salmonella enterica subspecies (11%) as a cause of illness, Salmonella enterica subspecies exceeded norovirus as a cause of hospitalization (35% vs 28%) and death (28% vs 11%). Rotavirus was the most common pathogen among children <5 years before rotavirus vaccine introduction, causing an estimated 3 million annual episodes of acute gastroenteritis, >500000 outpatient visits and 27000 hospitalizations, and about 25 deaths [16–18]. Norovirus has assumed the lead since introduction of rotavirus vaccine, and is associated with nearly 1 million ambulatory care visits and 14000 hospitalizations annually [19, 20]. The most common bacterial pathogens in this age group are Salmonella enterica subspecies (42%), Campylobacter (28%), Shigella (21%), Yersinia (5%), and E. coli O157 (3%) [20]. Together these 5 pathogens caused an estimated 291000 illnesses, 103000 physician visits, 7800 hospitalizations, and 64 deaths yearly. Before introduction of rotavirus vaccine, an average of 369 children aged <5 years died from diarrhea each year; among infants, the risk of death was increased among African Americans and those with prematurity, low birth weight, less maternal education, and low income [21].
Most acute diarrhea episodes in previously healthy, immunocompetent people are of short duration and self-resolving, and are of viral or unknown etiology. Therefore, laboratory investigation generally is not warranted. However, many factors may justify the expense and complexity of laboratory testing including epidemiologic (Table 2) and clinical features (Table 3), which encompass diarrhea in immunocompromised people, noninfectious and extraintestinal manifestations associated with enteric pathogens (Table 4), the potential for results of laboratory investigation to impact management, and suspicion of an outbreak situation.
Table 3.
Clinical Presentations Suggestive of Infectious Diarrhea Etiologies
| Finding | Likely Pathogens |
|---|---|
| Persistent or chronic diarrhea | Cryptosporidium spp, Giardia lamblia, Cyclospora cayetanensis, Cystoisospora belli, and Entamoeba histolytica |
| Visible blood in stool | STEC, Shigella, Salmonella, Campylobacter, Entamoeba histolytica, noncholera Vibrio species, Yersinia, Balantidium coli, Plesiomonas |
| Fever | Not highly discriminatory—viral, bacterial, and parasitic infections can cause fever. In general, higher temperatures are suggestive of bacterial etiology or E. histolytica. Patients infected with STEC usually are not febrile at time of presentation |
| Abdominal pain | STEC, Salmonella, Shigella, Campylobacter, Yersinia, noncholera Vibrio species, Clostridium difficile |
| Severe abdominal pain, often grossly bloody stools (occasionally nonbloody), and minimal or no fever | STEC, Salmonella, Shigella, Campylobacter, and Yersinia enterocolitica |
| Persistent abdominal pain and fever | Y. enterocolitica and Y. pseudotuberculosis; may mimic appendicitis |
| Nausea and vomiting lasting ≤24 hours | Ingestion of Staphylococcus aureus enterotoxin or Bacillus cereus (short-incubation emetic syndrome) |
| Diarrhea and abdominal cramping lasting 1–2 days | Ingestion of Clostridium perfringens or B. cereus (long-incubation emetic syndrome) |
| Vomiting and nonbloody diarrhea lasting 2–3 days or less | Norovirus (low-grade fever usually present during the first 24 hours in 40% if infections) |
| Chronic watery diarrhea, often lasting a year or more | Brainerd diarrhea (etiologic agent has not been identified); postinfectious irritable bowel syndrome |
Abbreviation: STEC, Shiga toxin–producing Escherichia coli.
Table 4.
Postinfectious Manifestations Associated With Enteric Pathogens
| Manifestation | Organism(s) |
|---|---|
| Erythema nodosum | Yersinia, Campylobacter, Salmonella, Shigella |
| Glomerulonephritis | Shigella, Campylobacter, Yersinia |
| Guillain-Barré syndrome | Campylobacter |
| Hemolytic anemia | Campylobacter, Yersinia |
| Hemolytic uremic syndrome | STEC, Shigella dysenteriae serotype 1 |
| Immunoglobulin A nephropathy | Campylobacter |
| Reactive arthritisa | Salmonella, Shigella, Campylobacter, Yersinia, rarely Giardia, and Cyclospora cayetanensis |
| Postinfectious irritable bowel syndrome | Campylobacter, Salmonella, Shigella, STEC, Giardia |
| Meningitis | Listeria, Salmonella (infants ≤3 months of age are at high risk) |
| Intestinal perforation | Salmonella including Salmonella Typhi, Shigella, Campylobacter, Yersinia, Entamoeba histolytica |
| Ekiri syndrome (lethal, toxic encephalopathy) and/or seizure | Shigella |
| Aortitis, osteomyelitis, extravascular deep tissue focus | Salmonella, Yersinia |
Abbreviation: STEC, Shiga toxin–producing Escherichia coli.
aIncludes Reiter syndrome.
The burden of acute gastroenteritis has been reduced since implementation of 2 US Food and Drug Administration (FDA)–licensed rotavirus vaccines, recommended by the Advisory Committee on Immunization Practices (ACIP) in 2006 and 2008 [22]. Clinically significant disease and hospitalization and office visits have been decreased in infants who have received a rotavirus vaccine (direct protection) as well as in adults through community protection of unvaccinated infants and age-ineligible children and adults [23, 24] (indirect, or community protection) living in high- and middle-income countries and reductions in all-cause diarrhea deaths in several middle-income countries.
Reduction of acute infectious diarrhea also can be achieved through general measures, including use of hand hygiene; proper food preparation and storage; avoidance of high-risk foods such as undercooked meat and seafood, unpasteurized milk, and soft cheese made with unpasteurized milk; avoidance of unsafe water; use of infection prevention and control measures in hospitals, childcare, and nursing home settings; appropriate use of antimicrobial agents; and appropriate pet selection and supervision of contact with animals, specifically in public settings. In addition, people with diarrhea should refrain from recreational water activities, food preparation or service, and sexual activities while symptomatic. Specific preventive measures, in addition to routine use of rotavirus vaccine in infants [25], include typhoid and cholera vaccines for travelers when indicated [26].
Highly effective measures are available to prevent and treat diarrheal disease and its complications. Avoiding dehydration by ensuring adequate fluid and electrolyte intake for replacement and maintenance is the mainstay of diarrheal illness management. Increasing resistance to antimicrobial agents and risk of worsening illness (such as diarrhea associated with C. difficile) can result from antimicrobial and antimotility drug use and highlight the need for appropriate use of these interventions.
METHODOLOGY
Panel Composition
A panel of multidisciplinary experts in management of infectious diarrhea in children and adults was convened in 2012. The panel consisted of pediatricians and internists with expertise in clinical medicine, infectious disease, epidemiology, gastroenterology, preventive medicine, nutrition, microbiology, and enteric disease. Panel participants included representatives from the Society for Healthcare Epidemiology of America (SHEA), CDC, and the IDSA Standards and Practice Guidelines Committee (SPGC). The guideline was reviewed and endorsed by SHEA and the Pediatric Infectious Diseases Society. The guideline was also reviewed and approved by the IDSA SPGC and the Board of Directors.
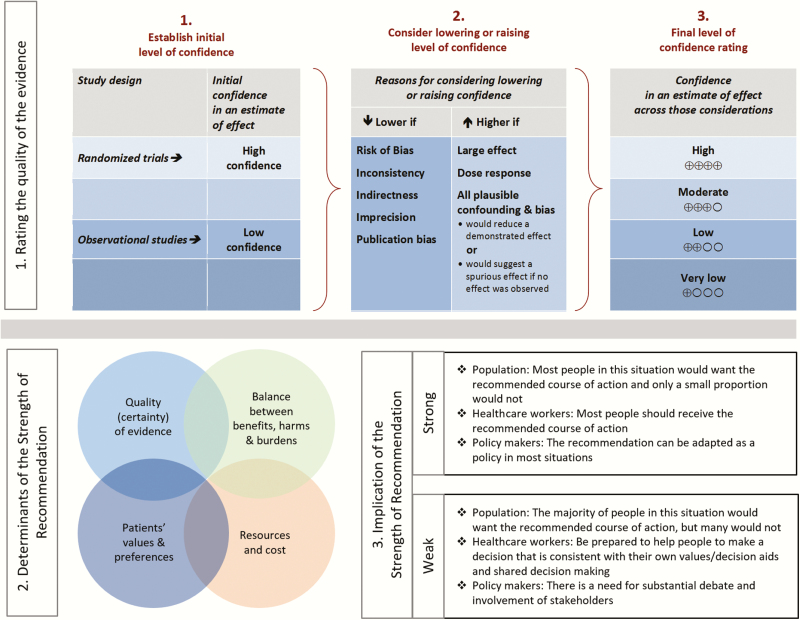
Grading of Recommendations Assessment, Development and Evaluation Approach and Process Overview
The panel applied GRADE to the assessment of quality of evidence and development of recommendations [3–7]. The quality of evidence is categorized as high, moderate, low, or very low; the strength of recommendation is categorized as strong or weak (Figure 2). Key factors that determine the strength of recommendation include quality of evidence, balance between desirable and undesirable effects, and values and preferences. Teleconferences and face-to-face meetings were held in which a list of 21 clinical questions to be addressed in the guidelines was generated, discussed, and prioritized.
Figure 2.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Literature Review, Analysis, and Selection
The panel identified current and valid studies from both the Medline and Embase databases, with a focus on randomized controlled trials (RCTs), allowing for admission of systematic reviews and extant practice guidelines if adequate RCTs and method validation studies for diagnostics did not exist. The search period included 1 January 2000–31 December 2013. Data published after 1 January 2014 also were considered in the final preparation of the manuscript. The search was restricted to English-language articles and largely was confined to US and/or North American sources. English-language studies with European authors also were included for the purpose of determining diagnostic guidelines. For international travel–associated infections, such as enteric fever and cholera, geographic restrictions were not applied. Selected references with relevant updates to practice were included.
Following removal of duplicate and irrelevant studies, the panel based judgments regarding inclusion in the guidelines on evidence demonstrated by the aggregated RCTs and/or the strength of evidence indicated in a systematic review of multiple studies. Articles were evaluated for relevance to each of the focus sections in the guidelines, up to and including: background; clinical presentations; diagnostics; treatment of nonresponders and persistence; management (specific treatment, supportive treatment, empiric treatment, ancillary treatment); epidemiology and surveillance; prevention; and future treatments. Primary key search terms were as follows: acute gastroenteritis, antimotility agents, antimicrobial agents, antiparasitic agents, cholera, C. difficile, colitis, diarrhea/dehydration, dysentery, enteric fever, enteric pathogens, enterocolitis, enzyme immunoassay, gastroenteritis, hand hygiene, management, molecular diagnostics, pseudomembranous enterocolitis, probiotics, rehydration, rotavirus, and STEC.
Guideline and Conflicts of Interest
All panel members complied with IDSA policy on conflict of interests, which requires disclosure of any financial or other interest that might be construed as constituting an actual, potential, or apparent conflict. Members were provided IDSA’s conflicts of interest disclosure statement and asked to identify associations with companies developing products that might be affected by promulgation of the guideline. Information was requested regarding employment, consultancies, stock ownership, honoraria, research funding, expert testimony, speaking engagements, and membership on company advisory committees. Decisions were made on a case-by-case basis as to whether an individual’s role should be limited as a result of a conflict. Potential conflicts of interest are listed in the Notes section.
Future Revision Dates
At annual intervals, the panel chair, SPGC liaison advisor, and SPGC chair will determine the need for guideline revisions by reviewing current literature. If necessary, the entire panel will be reconvened. When appropriate, the panel will recommend revisions to the IDSA SPGC, Board of Directors, and other collaborating organizations for review and approval.
RECOMMENDATIONS FOR THE DIAGNOSIS AND MANAGEMENT OF INFECTIOUS DIARRHEA
Clinical, Demographic, and Epidemiologic Features
I. In people with diarrhea, which clinical, demographic, or epidemiologic features have diagnostic or management implications? (Tables 2–4)
Recommendations
1. A detailed clinical and exposure history should be obtained from people with diarrhea, under any circumstances, including when there is a history of similar illness in others (strong, moderate) (Figure 1).
2. People with diarrhea who attend or work in child care centers, long-term care facilities, patient care, food service, or recreational water venues (eg, pools and lakes) should follow jurisdictional recommendations for outbreak reporting and infection control (strong, high).
Evidence Summary
A broad range of exposures or conditions have been implicated as sources of infections with specific pathogens (Table 2). Exposures or conditions that may suggest certain causes of infectious diarrhea include consumption of shellfish, raw milk, unpasteurized juice, undercooked meats, fish, or eggs, or contaminated fruits or vegetables; exposure to contaminated drinking or recreational water; contact with animals or their feces or environment; recent antimicrobial therapy; international travel; institutional exposure; and anal or oral sexual contact [27–47].
Of great importance are exposures associated with food. In a review of outbreaks of foodborne illness investigated by FoodNet between 2003 and 2008 [48], a specific food vehicle was identified in 232 of 1200 (32%) outbreaks [49]. Outbreaks were most commonly reported to be associated with commercial food preparation; this is likely to reflect that outbreaks associated with a single restaurant or other establishment may be more likely than other outbreaks to be noticed, reported to public health officials, and investigated. Other important exposures implicated in outbreaks include animal contact [41, 50, 51], recreational water exposure [52], and sexual practices [53] (Table 2).
Outbreaks of diarrhea in institutional settings are a substantial public health problem. The National Outbreak Reporting System [54] collects data on waterborne and foodborne disease outbreaks, person-to-person transmitted disease outbreaks, animal contact disease outbreaks, environmental contamination disease outbreaks, and other enteric illness outbreaks. During 2009–2013, the National Outbreak Reporting System reported 10756 acute gastroenteritis outbreaks for which the primary mode of transmission occurred through person-to-person contact, environmental contamination, and unknown modes of transmission. These outbreaks resulted in 356530 reported illnesses, 5394 hospitalizations, and 459 deaths. In 7001 outbreaks where a setting was reported, 70% occurred in long-term care facilities. In contrast, 59% of Shigella-associated outbreaks and 36% of Salmonella-associated outbreaks were identified in childcare facilities. Norovirus was implicated in 84% of 2430 outbreaks where an etiology was suspected or confirmed; bacterial pathogens were identified in a substantial minority [55]. During 2009–2013, norovirus accounted for most deaths and healthcare visits associated with acute gastroenteritis outbreaks. Specific infection control guidelines are recommended for control of norovirus and the extremely chlorine-tolerant Cryptosporidium in institutional settings [56, 57]. Food worker health or hygiene has been identified as a contributing factor in 64% of foodborne outbreaks associated with restaurants in the United States [58].
II. In people with fever or bloody diarrhea, which clinical, demographic, or epidemiologic features have diagnostic or management implications? (Tables 2–4)
Recommendations
3. People with fever or bloody diarrhea should be evaluated for enteropathogens for which antimicrobial agents may confer clinical benefit including Salmonella enterica subspecies, Shigella, and Campylobacter (strong, low).
4. Enteric fever should be considered when a febrile person (with or without diarrhea) has a history of travel to areas in which causative agents are endemic, has consumed foods prepared by people with recent endemic exposure, or has had laboratory exposure to Salmonella enterica subspecies enterica serovar Typhi and Salmonella enterica subspecies enterica serovar Paratyphi (strong, moderate). In this document, Salmonella Typhi represents the more formal and detailed name Salmonella enterica subspecies enterica serovar Typhi, and Salmonella Paratyphi corresponds to the Paratyphi serovar.
Evidence Summary
Although bacterial causes of diarrhea can have similar clinical presentations, they differ with regard to clinical management. For example, whereas antimicrobial agents may be indicated for Campylobacter or Shigella infections, they are not indicated for STEC or for most Salmonella infections.
Identification of bacterial agents can prevent other unnecessary procedures such as colonoscopy, abdominal surgery, or medical treatment for suspected ulcerative colitis. Conversely, negative stool studies for infectious pathogens increase suspicion for noninfectious conditions such as inflammatory bowel disease (IBD).
Salmonella enterica serovar Typhi and Paratyphi A and Paratyphi B cause bacteremic illnesses referred to respectively as typhoid and paratyphoid fever, and collectively as enteric fever. These conditions are characterized by fever that may be associated with headache, lethargy, malaise, and abdominal pain, followed by hepatosplenomegaly and stupor. While the portal of entry is the gastrointestinal tract, diarrhea is an uncommon feature [59].
Typhoid fever incidence is high in parts of South and Southeast Asia, and moderate in Central and South America, Africa, Central and East Asia, and Oceania [60]. Typhoid fever outbreaks in the United States are uncommon and usually associated with foodborne transmission from an asymptomatic carrier [61]. FoodNet data from the period 2004–2009 demonstrated that a history of travel was reported in 68% of patients with Salmonella enterica serovar Typhi and 50% of patients with Salmonella enterica serovar Paratyphi [35]. Typhoid fever may be difficult to distinguish from other febrile conditions in returned travelers, and can present with fever without focus, abdominal pain without diarrhea, or with extraintestinal foci of infection [62].
III. What clinical, demographic, or epidemiologic features are associated with complications or severe disease? (Tables 2–4)
Recommendations
5. People of all ages with acute diarrhea should be evaluated for dehydration, which increases the risk of life-threatening illness and death, especially among the young and older adults (strong, high).
6. When the clinical or epidemic history suggests a possible Shiga toxin–producing organism, diagnostic approaches should be applied that detect Shiga toxin (or the genes that encode them) and distinguish E. coli O157:H7 from other STEC in stool (strong, moderate). If available, diagnostic approaches that can distinguish between Shiga toxin 1 and Shiga toxin 2, which is typically more potent, could be used (weak, moderate). In addition, Shigella dysenteriae type 1 and, rarely, other pathogens may produce Shiga toxin and should be considered as a cause of HUS, especially in people with suggestive international travel or personal contact with a traveler (strong, moderate).
7. Clinicians should evaluate people for postinfectious and extraintestinal manifestations associated with enteric infections (strong, moderate) [8]. (Table 4)
Evidence Summary
Volume depletion is a frequently identified risk factor for diarrhea-related deaths in people of all ages in the United States; other related risk factors include fluid and electrolyte disorders, nontraumatic shock, and acute renal failure [63, 64]. In addition, dehydration at the time of admission among children with postdiarrheal HUS is associated with an increased need for dialysis [65]. Intravenous fluid administered during the diarrhea phase of STEC infections reduces the risk of oligoanuric renal failure among those children who subsequently develop HUS [66].
Although most patients with laboratory-confirmed STEC who develop HUS have bloody diarrhea, approximately 10% do not [67]. In addition to patient reported bloody diarrhea or visibly bloody stool, other factors independently associated with increased risk of STEC O157 infection compared with other enteric infections in patients of all ages include abdominal tenderness and absence of fever at first medical evaluation [68]. Approximately 65% of patients infected with E. coli O157 will have a peripheral white blood cell count >10000 cells/µL [69]. Early identification of STEC infections is important to reduce the risk of complications and the risk of person-to-person transmission. It is important to perform both cultures for STEC O157 and test for Shiga toxin (either in broth cultures, not stool) or the genes that encode this toxin family, because STEC O157 is the most consistently virulent STEC in the United States, and early identification through culture can aid in clinical management and public health control measures. Detection of all other STEC serotypes first requires detection of Shiga toxin (or the genes that encode them) [70].
STEC carrying Shiga toxin 2 (stx2) genes are associated with increased risk of both bloody diarrhea and HUS [71, 72]. In the United States, most STEC stains isolated from patients with HUS are serogroup O157, and are stx2 positive. New multiplex nucleic acid amplification tests (MP-NAATs) that can detect evidence of multiple pathogens and toxins can distinguish between Shiga toxins 1 and 2 and some assays also distinguish E. coli O157. Although clinical laboratories cannot typically differentiate between subtypes of Shiga toxin 2, subtypes 2a, 2c, and 2d are associated with more severe disease [73]. Known postinfectious manifestations of infections with their associated enteric organisms are listed in Table 4. When a clinical syndrome consistent with one of these manifestations is encountered, an exposure history should be obtained along with a diagnostic evaluation and directed management, which may have public health or outbreak evaluation implications. Early identification of particularly virulent STEC infection (eg, STEC O157 and other Shiga toxin 2–producing strains) facilitates rapid implementation of measures in the home that prevent cross-contamination [74].
Diagnostics
IV. Which pathogens should be considered in people presenting with diarrheal illnesses, and which diagnostic tests will aid in organism identification or outbreak investigation?
Recommendations
8. Stool testing should be performed for Salmonella, Shigella, Campylobacter, Yersinia, C. difficile, and STEC in people with diarrhea accompanied by fever, bloody or mucoid stools, severe abdominal cramping or tenderness, or signs of sepsis (strong, moderate). Bloody stools are not an expected manifestation of infection with C. difficile. STEC O157 should be assessed by culture and non-O157 STEC should be detected by Shiga toxin or genomic assays (strong, low). Sorbitol-MacConkey agar or an appropriate chromogenic agar alternative is recommended to screen for O157:H7 STEC; detection of Shiga toxin is needed to detect other STEC serotype (strong, moderate).
9. Blood cultures should be obtained from infants <3 months of age, people of any age with signs of septicemia or when enteric fever is suspected, people with systemic manifestations of infection, people who are immunocompromised, people with certain high-risk conditions such as hemolytic anemia, and people who traveled to or have had contact with travelers from enteric fever–endemic areas with a febrile illness of unknown etiology (strong, moderate).
-
10. Stool testing should be performed under clearly identified circumstances (Table 2) for Salmonella, Shigella, Campylobacter, Yersinia, C. difficile, and STEC in symptomatic hosts (strong, low). Specifically,
a. Test for Yersinia enterocolitica in people with persistent abdominal pain (especially school-aged children with right lower quadrant pain mimicking appendicitis who may have mesenteric adenitis), and in people with fever at epidemiologic risk for yersiniosis, including infants with direct or indirect exposures to raw or undercooked pork products.
b. In addition, test stool specimens for Vibrio species in people with large volume rice water stools or either exposure to salty or brackish waters, consumption of raw or undercooked shellfish, or travel to cholera-endemic regions within 3 days prior to onset of diarrhea.
11. A broader set of bacterial, viral, and parasitic agents should be considered regardless of the presence of fever, bloody or mucoid stools, or other markers of more severe illness in the context of a possible outbreak of diarrheal illness (eg, multiple people with diarrhea who shared a common meal or a sudden rise in observed diarrheal cases). Selection of agents for testing should be based on a combination of host and epidemiologic risk factors and ideally in coordination with public health authorities (strong, moderate).
12. A broad differential diagnosis is recommended in immunocompromised people with diarrhea, especially those with moderate and severe primary or secondary immune deficiencies, for evaluation of stool specimens by culture, viral studies, and examination for parasites (strong, moderate). People with acquired immune deficiency syndrome (AIDS) with persistent diarrhea should undergo additional testing for other organisms including, but not limited to, Cryptosporidium, Cyclospora, Cystoisospora, microsporidia, Mycobacterium avium complex, and cytomegalovirus (CMV) (strong, moderate).
13. Diagnostic testing is not recommended in most cases of uncomplicated traveler’s diarrhea unless treatment is indicated. Travelers with diarrhea lasting 14 days or longer should be evaluated for intestinal parasitic infections (strong, moderate). Testing for C. difficile should be performed in travelers treated with antimicrobial agent(s) within the preceding 8–12 weeks. In addition, gastrointestinal tract disease including IBD and postinfectious irritable bowel syndrome (IBS) should be considered for evaluation (strong, moderate).
14. Clinical consideration should be included in the interpretation of results of MP-NAAT because these assays detect DNA and not necessarily viable organisms (strong, low).
15. All specimens that test positive for bacterial pathogens by culture-independent diagnostic testing such as antigen-based molecular assays (gastrointestinal tract panels) and for which isolate submission is requested or required under public health reporting rules should be cultured in the clinical laboratory or at a public health laboratory to ensure that outbreaks of similar organisms are detected and investigated (strong, low). Also, a culture may be required in situations where antimicrobial susceptibility testing results would affect care or public health responses (strong, low).
16. Specimens from people involved in an outbreak of enteric disease should be tested for enteric pathogens per public health department guidance (strong, low).
Evidence Summary
Determination of the precise cause of diarrhea is not always necessary. Assessment of a stool specimen to determine the cause should be performed on patients at high risk of severe illness and for whom identification of a pathogen would be important for the patient or for public health reasons. As first described in the original IDSA guidelines on management of infectious diarrhea [1], diagnostic algorithms that combine clinical and epidemiologic factors that meet requirements of clinical medicine and public health are needed. Although the majority of diarrheal illnesses are self-limited and identification of the infectious etiology often has little value to these individual patients, for certain infections, an organism-specific diagnosis is important to guiding clinical management. Furthermore, from a public health perspective, an organism-specific diagnosis is valuable for the majority of diarrheal illnesses because identification of an organism facilitates outbreak detection and monitoring of disease trends. Selective testing recommendations below are based on clinical management needs as well as on the efficient use of diagnostic testing to meet the needs of public health surveillance systems.
Identification of bacterial pathogens can be important for both clinical management and public health disease control efforts. However, testing all patients with acute diarrhea for these pathogens would be inefficient. Among adults presenting with diarrhea to emergency departments in the United States, 17% of patients who submitted a stool specimen (as opposed to rectal swab) were found to have a bacterial enteric infection. A bacterial etiology was found in 5%–11% of children seeking care in emergency departments and outpatient settings [75–77]. Restricting testing to patients with bloody stools, fever, or abdominal tenderness can increase the likelihood of identifying a bacterial pathogen [68, 76–79] (Table 5). Risk factors for invasive nontyphoidal Salmonella infection include young and advanced age, impaired immunity due to human immunodeficiency virus (HIV) infection and cytotoxic chemotherapy, malnutrition, hemoglobinopathies, recent malaria, and cirrhosis [80–82]. Other bacterial infections, including Campylobacter [83] and Shigella [44, 84] and Listeria infections are more likely to be severe or recurrent in patients with HIV infection. Aneurysms of the aorta and aortitis can occur in elderly patients with invasive nontyphoidal salmonellosis or yersiniosis [85, 86].
Risk factors for invasive noncholera vibriosis, especially Vibrio vulnificus infections, are chronic liver disease (including cirrhosis, alcoholic liver disease, and hepatitis), iron overload states (hemochromatosis, hemolytic anemia, or chronic renal failure) and other immunocompromising conditions [87, 88].
Yersinia enterocolitica can be associated with an array of clinical presentations including, but not limited to, nonbloody diarrhea, bloody diarrhea, and a febrile pseudoappendicular syndrome that can mimic appendicitis. Invasive yersiniosis in an adult may be associated with the presence of mycotic aneurysms [86]. Foods that have been associated with Y. enterocolitica infections include pork (eg, chitterlings) and dairy products. Higher risk groups in the United States include young African American and Asian children, especially during winter months, as well as diabetics and those with chronic liver disease, malnutrition, or iron-overload states. The higher rates among African American children had been attributable to cross-contamination within the home during preparation of chitterlings, a seasonal dish prepared from pig intestines. However, the high incidence rates in African American children observed in the late 1990s have declined dramatically following preventive health campaigns focusing on avoidance of cross-contamination in the kitchen [89].
Identification and investigation of outbreaks together serve important roles in reducing the burden of diarrheal illnesses by truncating the duration of the outbreak and uncovering the contributing factors that led to the outbreak so that those factors can be addressed to prevent future outbreaks and illnesses. An organism-specific diagnosis usually is necessary for public health control efforts, because clinical factors alone rarely are sufficient to distinguish between etiologic agents. Identifying the etiologic agent(s) from ill people is important for case finding and investigating possible sources of infection. The most common cause of diarrheal disease outbreaks is norovirus, but a broad range of bacterial and parasitic agents have been implicated in outbreaks [90–94]. The specific pathogens for which to test may vary by clinical presentation and exposures. Health departments can provide guidance on testing, and often public health laboratories can assist in testing for agents that exceed the diagnostic capacity of the clinical laboratory.
Immunocompromised people are more likely to experience severe or prolonged illness. Diarrhea in immunocompromised patients may involve a broad spectrum of potential causes, including bacterial, viral, parasitic, and fungal pathogens depending on underlying immune status [95]. In addition, people with HIV-associated immune compromise are at risk for diarrhea due to enteroaggregative E. coli [96–98], Cryptosporidium [99], microsporidia [100–102], Cystoisospora belli (formerly Isospora belli), CMV, and Mycobacterium avium complex (MAC) [95, 103]. Besides stool examination, other investigations may be necessary for the HIV-infected patient, including blood cultures for diagnosis of MAC infection and colonoscopy with biopsy for CMV enteritis. Diarrhea caused by some protozoa (eg, Cryptosporidium, Cyclospora, Cystoisospora) or microsporidia is more likely to be severe, chronic, or relapsing in immunocompromised people, particularly those with impaired cell-mediated immunity, including advanced HIV infection [104]. Because microscopic examination of stool for ova and parasites is unlikely to include testing for Cryptosporidium and Cyclospora, clinicians should specifically request Cryptosporidium and/or Cyclospora testing. Noninfectious etiologies including adverse effects of antiretroviral therapy or chemotherapy also may account for persistent diarrhea in immunocompromised hosts. In some patients with diarrhea lasting ≥30 days, testing for HIV may be appropriate [105].
Chronic and severe norovirus infection has been reported in patients receiving immunosuppression following organ transplantation [106]. Patients who acquire norovirus infection while hospitalized, especially people with immunocompromising conditions and people of advanced age, may be more likely to die. Guidelines for prevention and control of norovirus gastroenteritis outbreaks in healthcare settings have been published [56]. People >90 years of age residing in long-term care facilities have a 20%–30% increased risk of death and hospitalization during norovirus outbreaks [107]. However, diagnosis of norovirus infections largely has been limited to infections occurring as part of outbreaks. Localized outbreaks affecting hospital wards and long-term care facilities may be more likely to be investigated [107–109]. Investigations to assess the endemic rates of norovirus infection are ongoing. Persistent non-vaccine-related and vaccine-related rotavirus diarrhea has been reported in young children with primary immunodeficiency [110, 111], but rotavirus disease and hospitalizations overall have been reduced markedly since licensure of the 2 ACIP-recommended rotavirus vaccines [112].
The majority of traveler’s diarrhea is self-limited, caused by bacterial and, to a lesser extent, viral pathogens, and lasts for <7 days. Most TD is self-treatable with oral rehydration therapy, and, for nonbloody diarrhea in adults, an antimotility agent [113]. Approximately 10% of traveler’s diarrhea is caused by parasitic infections, which can persist for weeks to months, with giardiasis being the most common. Clostridium difficile–associated diarrhea is of increasing concern among travelers with persistent diarrhea, especially travelers with recent antimicrobial agent therapy, either as self-treatment for traveler’s diarrhea or for other indications [114, 115]. The distribution of gastrointestinal tract pathogens varies substantially by region of travel. In a US FoodNet study between 2004 and 2009, the majority of cases of typhoid fever, paratyphoid fever, and Shigella dysenteriae infection among others, were associated with travel [35]. An abnormal d-xylose absorption test indicates the possibility of tropical sprue, which is most common in adults visiting tropical areas for long periods of time.
Multipathogen nucleic acid amplification tests can simultaneously detect viral, parasitic, and bacterial agents, including some pathogens that previously could not be easily detected in the clinical setting such as norovirus, and enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), and enteroaggregative E. coli (EAEC) in less time than traditional methods. The short time to results could reduce inappropriate use of antimicrobial agents to treat infections that do not require antimicrobial therapy and could shorten the time to targeted management and isolation measures for certain infections such as STEC O157. With these assays, it is common to detect the presence of >1 pathogen that may differ with regard to clinical management [116–118]. Furthermore, even a positive result for 1 pathogen should be interpreted in the context of the patient’s clinical presentation, because less is known about the clinical significance of tests that detect nucleic acid as compared with traditional assays that generally detect viable organisms. The importance of detection of multiple pathogens in the same specimen is often unclear; it is unknown if all pathogens detected in the specimen are clinically relevant or if one is more strongly associated with the illness.
Interpretation of results will improve as more data become available regarding the performance of these assays. For at least one assay, current data show that concordance with traditional diagnostic methods may vary by pathogen [118]. Some experts have proposed that these assays may be particularly well suited for making an organism-specific diagnosis in immunocompromised patients [119]. The current FDA-cleared multiplex assays do not quantify the amount of nucleic acid present. Development of quantitative assays may aid in interpretation of results [120].
Guidelines by IDSA and the American Society for Microbiology (ASM) on utilization of the clinical microbiology laboratory describe optimal tests for detection of pathogens, including those causing diarrhea [121]. The complete findings are summarized in Table 5. Since publication of those guidelines, several gastrointestinal panels that detect >20 viral, bacterial, and parasitic enteric organisms have become available. The availability of one of these panels as well as other assays may vary among clinical laboratories, making requisitions unique to the laboratory to which the sample is submitted.
There are important drawbacks to the increasing use of culture-independent diagnostic tests (CIDTs), including enzyme immunoassays and NAATs in the clinical setting. First, replacement of culture by CIDT in clinical laboratories will impede outbreak detection and investigation. Public health has made important strides in detecting, investigating, and controlling outbreaks of enteric illness using molecular subtyping of the infecting bacterial strains in public health laboratories [122]. The net effect of this enhanced surveillance and control has been to prevent thousands of illnesses [123]. Replacement of culture by CIDT without preserving access to isolates will impede detection of dispersed outbreaks, and thus reduce the capacity of public health to control and to prevent them. Second, for the individual, CIDTs do not provide information on antimicrobial susceptibility to guide clinical management. Actions are needed to avoid this negative impact on public health. In the short term, specimens that test positive for a bacterial pathogen by a CIDT for which isolate submission is requested or required under public health reporting rules should be cultured, either at the clinical laboratory or at a public health laboratory. Cultured organisms can be sent to public health laboratories for species identification, serotyping and further subtyping by molecular methods (eg, pulsed-field gel electrophoresis, and, more recently, whole-genome sequencing). Subtyping enables detection of increases in infections caused by a specific strain and also facilitates outbreak investigations by increasing the probability that case-patients included in an investigation are likely to have had a common exposure. In the longer term, culture-independent methods that serve clinical diagnostic needs and are able to provide subtyping information to distinguish strains are needed [124–128].
V. Which diagnostic tests should be performed when enteric fever or bacteremia is suspected?
Recommendation
17. Culture-independent, including panel-based multiplex molecular diagnostics from stool and blood specimens, and, when indicated, culture-dependent diagnostic testing should be performed when there is a clinical suspicion of enteric fever (diarrhea uncommon) or diarrhea with bacteremia (strong, moderate). Additionally, cultures of bone marrow (particularly valuable if antimicrobial agents have been administered), stool, duodenal fluid, and urine may be beneficial to detect enteric fever (weak, moderate). Serologic tests should not be used to diagnose enteric fever (strong, moderate).
Evidence Summary
For the diagnosis of enteric fever, aerobic blood culture has a sensitivity of approximately 50% compared with the more invasive and technically complex acquisition of bone marrow culture [129]. Bone marrow culture is likely to be more sensitive than blood culture for diagnosis of invasive nontyphoidal Salmonella enterica infection [130, 131]. Routine aerobic blood culture is recommended as the routine practical conventional diagnostic and for the initial diagnostic assessment in people with suspected enteric fever or invasive salmonellosis [129–131]. In enteric fever, culture of other samples such as stool, duodenal fluid, and urine may be helpful. Due to poor performance characteristics, serologic tests should not be used for diagnosis of enteric fever. Nucleic acid amplification tests lack sensitivity for detection of Salmonella enterica serovar Typhi in blood, but may be useful for rapid detection and identification of Salmonella enterica serovar Typhi in research settings [129, 130].
Blood culture should be performed in all people with signs of septicemia and when enteric fever is suspected. Blood cultures may be considered in immunocompromised people who are febrile or from whom bacterial pathogens are detected by stool testing. Some clinical laboratories are now using blood culture technology that can identify a pathogen without isolation [132]. In these situations, it is essential to isolate the organism to facilitate antimicrobial susceptibility testing and additional molecular characterization by public health laboratories. As the median magnitude of bacteremia in enteric fever and invasive nontyphoidal Salmonella enterica disease are low at 0.3 and 1.0 colony-forming units/mL of blood, respectively, larger volumes of blood need to be obtained to maximize detection [131]. Two to three 20-mL blood cultures are adequate for detection of bacteremia in adults [133]. Lower volumes may be sufficient for detection in infants and children who have higher magnitudes of bacteremia than adults [131]. Blood cultures may be drawn simultaneously and should be collected prior to administration of antimicrobial agents to maximize sensitivity. Continuously monitored blood culture systems may shorten the time to detection and improve sensitivity compared with manual blood culture methods.
VI. When should testing be performed for Clostridium difficile?
Recommendation
18. Testing may be considered for C. difficile in people >2 years of age who have a history of diarrhea following antimicrobial use and in people with healthcare-associated diarrhea (weak, high). A single diarrheal stool specimen is recommended for detection of toxin or a toxigenic C. difficile strain (eg, NAAT) (strong, low). Multiple specimens do not increase yield.
Evidence Summary
A complete discussion of Clostridium difficile infection (CDI) in adults and children will be addressed in the updated IDSA/SHEA guidelines devoted to this topic [134].
Up to 85% of patients with C. difficile provide a history of exposure to antimicrobial agents within the previous 28 days. Although a wide range of antimicrobial agents has been implicated, the most strongly associated with development of CDI include cephalosporins, β-lactam/β-lactamase inhibitors, clindamycin, and quinolones. However, the strength of association between antibiotic classes and development of CDI may be confounded by hospitalization, use of multiple antibiotic classes, and duration of exposure. There is increasing recognition of community-acquired C. difficile; some strains appear to be genetically distinct from hospital strains.
Children occasionally develop severe C. difficile disease, but this appears to be uncommon, and occurs mostly among older children. Concomitantly with adults, the incidence of infection has increased among hospitalized children, and community-acquired infections with hypervirulent strains have emerged, but the severity of disease has not increased as it has with adults. In the absence of well-controlled studies that take into account the frequency of asymptomatic colonization, it remains uncertain whether these new epidemiologic patterns represent an emerging burden of disease or an increased rate of asymptomatic colonization among children with comorbidities and exposure to factors that alter the intestinal microbiota such as hospitalization, antibiotics, and immunosuppression. The high frequency (up to 70%) of asymptomatic colonization among healthy newborns is another factor that confounds understanding of the epidemiology of CDI in children. These rates gradually fall to adult levels as the microbiota of the lower intestine becomes established by about 2 years of age, but nonetheless render the significance of identifying the organism or toxin in an individual child <2 years of age uncertain.
Clostridium difficile should be considered in patients with diarrhea occurring in hospitals. Studies have found that C. difficile is more prevalent in diarrheal stools obtained >72 hours after admission. Colonization is common in hospitalized patients and residents of long-term care facilities. Because asymptomatic carriage is recognized, patients without diarrhea should not be tested or treated. In the laboratory, this is usually implemented using a rejection policy for formed stool.
A number of different testing assays and algorithms combining different assays are available. Though kits that test for toxin A and B appear to demonstrate poor sensitivity compared with C. difficile cytotoxicity assay (CCA) or toxigenic culture, evidence suggests that patients with positive toxigenic culture and positive CCA have a poorer outcome than those with a negative CCA result. In the future, when it is possible to reconstruct the genome from specimens without first culturing the isolate, molecular subtype–based epidemiology may help in controlling the spread of this organism.
VII. What is the optimal specimen (eg, stool, rectal swab, blood) for maximum yield of bacterial, viral, and protozoal organisms (for culture, immunoassay, and molecular testing)? (Table 5)
Recommendation
19. The optimal specimen for laboratory diagnosis of infectious diarrhea is a diarrheal stool sample (ie, a sample that takes the shape of the container). For detection of bacterial infections, if a timely diarrheal stool sample cannot be collected, a rectal swab may be used (weak, low). Molecular techniques generally are more sensitive and less dependent than culture on the quality of specimen. For identification of viral and protozoal agents, and C. difficile toxin, fresh stool is preferred (weak, low).
Evidence Summary
A diarrheal stool sample provides greater fecal material and is less prone to environmental degradation when compared with a rectal swab. Viral and bacterial infectious agents were more likely to be detected from stool samples (49% of cases in one study) than from rectal swabs (9% of cases) in adults presenting to emergency departments with diarrhea; detection of norovirus, rotavirus, and bacterial pathogens was 4- to 6-fold greater from stools samples than from rectal swabs. For a thorough review on how samples should be collected, stored, and transported, see the IDSA/ASM Guide to Utilization of the Microbiology Laboratory [121]; the recommendations for infectious diarrhea in these previously published guidelines are summarized in Table 5. In general, only a single stool specimen is required. However, culture of additional specimens may increase the sensitivity to detect bacterial pathogens in patients with persistent diarrhea [135].
Clinical laboratories that have adopted newer CIDTs may have different specimen requirements. However, even in these circumstances, collection of a diarrheal stool specimen is important for culture of samples that test positive by CIDT for bacterial pathogens for public health considerations and antimicrobial susceptibility testing, until CIDTs that can serve these functions are available in the clinical setting [128].
VIII. What is the clinical relevance of fecal leukocytes or lactoferrin or calprotectin in a person with acute diarrhea?
Recommendation
20. Fecal leukocyte examination and stool lactoferrin detection should not be used to establish the cause of acute infectious diarrhea (strong, moderate). There are insufficient data available to make a recommendation on the value of fecal calprotectin measurement in people with acute infectious diarrhea.
Evidence Summary
Fecal leukocyte examination may be used to differentiate inflammatory diarrhea from secretory diarrhea, but performs poorly to establish the infectious cause of diarrhea, especially among inpatients [136]. Fecal leukocyte morphology degrades in feces during transport and processing, making accurate recognition and quantitation difficult. In inflammatory diarrhea, fecal leukocytes are intermittently present and unevenly distributed in stool, limiting sensitivity. Lactoferrin has been used as a surrogate marker for fecal leukocytes as it is not degraded during transport and processing [137]. Lactoferrin screening has been proposed as a cost-saving measure to select a subgroup of stool samples with higher pretest probability of being positive for bacterial pathogens by stool culture [137], but is not used commonly in stool processing algorithms by clinical laboratories. Furthermore, lactoferrin also is present in noninfectious IBD, resulting in decreased specificity for infectious inflammatory diarrhea [138, 139]. Lactoferrin is a normal component of human milk and therefore may be present in varying amounts in stools of infants who consume human milk, making assay results difficult to interpret in these infants. Calprotectin is a protein released in large quantities by granulocytes during inflammatory processes. Calprotectin is an established marker of intestinal inflammation used in patients with IBD. There are limited and conflicting reports about the value of measuring fecal calprotectin levels in patients with acute infectious diarrhea. Whereas some studies in children and adults suggest that higher calprotectin levels may suggest bacterial etiologies of diarrhea [140, 141], other studies have not found diagnostic value [142, 143].
IX. In which clinical scenarios should nonmicrobiologic diagnostic tests be performed (eg, imaging, chemistries, complete blood count, and serology)?
Recommendations
21. Serologic tests are not recommended to establish an etiology of infectious diarrhea or enteric fever (strong, low), but may be considered for people with postdiarrheal HUS in which a stool culture did not yield a Shiga toxin–producing organism (weak, low).
22. A peripheral white blood cell count and differential and serologic assays should not be performed to establish an etiology of diarrhea (strong, low), but may be useful clinically (weak, low).
23. Frequent monitoring of hemoglobin and platelet counts, electrolytes, and blood urea nitrogen and creatinine is recommended to detect hematologic and renal function abnormalities that are early manifestations of HUS and precede renal injury for people with diagnosed E. coli O157 or another STEC infection (especially STEC that produce Shiga toxin 2 or are associated with bloody diarrhea) (strong, high). Examining a peripheral blood smear for the presence of red blood cell fragmentation is necessary when HUS is suspected (strong, high).
24. Endoscopy or proctoscopic examination should be considered in people with persistent, unexplained diarrhea who have AIDS, in people with certain underlying conditions as well as people with acute diarrhea with clinical colitis or proctitis and in people with persistent diarrhea who engage in anal intercourse (strong, low). Duodenal aspirates may be considered in select people for diagnosis of suspected Giardia, Strongyloides, Cystoisospora, or microsporidia infection (weak, low).
25. Imaging (eg, ultrasonography, computed tomography, or magnetic resonance imaging) may be considered to detect aortitis, mycotic aneurysms, signs or symptoms of peritonitis, intra-abdominal free air, toxic megacolon, or extravascular foci of infection in older people with invasive Salmonella enterica or Yersinia infections if there is sustained fever or bacteremia despite adequate antimicrobial therapy or if the patient has underlying atherosclerosis or has recent-onset chest, back, or abdominal pain (weak, low).
Evidence Summary
Although not useful in most circumstances, serologic tests can aid in diagnosing an antecedent STEC infection (the CDC has validated testing available for serogroups O157 and O111) among patients with HUS if a Shiga toxin–producing organism has not been identified by stool culture and Shiga toxin testing [67]. Due to poor performance characteristics, serologic tests, such as the Widal test, should not be used for diagnosis of enteric fever [62].
The total white blood cell count and differential may provide suggestion of a bacterial etiology when viral or parasitic etiologies also are being considered. The total white blood cell count and neutrophil count are often increased with invasive bacterial pathogens and the platelet count may be elevated. In situations of bacterial sepsis, the total white blood cell count and platelet count may be lowered compared with normal values for age. Shigellosis can be associated with a leukemoid reaction. A white blood cell count that is within range for age and a lymphocytic predominance may occur with viral etiologies. An increased eosinophil count may occur with parasitic infections that involve a tissue phase. A high total white blood cell count and neutrophil count often occur in patients with STEC O157 infections who subsequently develop HUS [144, 145]. Monocyte predominance may suggest the presence of an intracellular pathogen such as Salmonella [146].
As HUS evolves over time, a single complete blood cell count is not sufficient to define risk. In fact, a near-normal hemoglobin value may suggest dehydration. Patients with a decreasing platelet count trend during days 1–14 of the diarrheal illness are at greater risk of developing HUS. Daily monitoring can stop when the platelet count begins to increase or stabilize in patients with resolved or resolving symptoms. Patients with an increasing creatinine level and blood pressure and signs of volume overload should be monitored closely and should receive care in a center that can manage acute renal failure [147].
Endoscopy with small bowel biopsy is useful for diagnosis of MAC and microsporidiosis. If colitis is suspected, sigmoidoscopy with biopsy of abnormal mucosa may assist in differentiating infectious colitis from inflammatory bowel disease, CMV disease, or C. difficile colitis. Abdominal computed tomography may detect mucosal thickening or other changes related to colitis and is helpful when intestinal disease is considered. Proctoscopic examination may be useful in diagnosing proctitis in patients who have had receptive anal intercourse. Duodenal aspirate has been shown to be useful in the diagnosis of Giardia and Strongyloides infection in patients with recurring diarrhea in whom stool evaluation did not yield an etiology [148, 149].
Although aortitis and aneurysm formation are rare complications of Salmonella and Yersinia diarrhea, they are universally fatal without appropriate medical and surgical treatment. Delays in diagnosis have been associated with poor prognosis [85, 86].
X. What follow-up evaluations of stool specimens and nonstool tests should be performed in people with laboratory-confirmed pathogen-specific diarrhea who improve or respond to treatment, and in people who fail to improve or who have persistent diarrhea?
Recommendations
26. Follow-up testing is not recommended in most people for case management following resolution of diarrhea (strong, moderate). Collection and analysis of serial stool specimens using culture-dependent methods for Salmonella enterica subspecies enterica serovar Typhi or Salmonella enterica subspecies enterica serovar Paratyphi, STEC, Shigella, nontyphoidal Salmonella, and other bacterial pathogens are recommended in certain situations by local health authorities following cessation of diarrhea to enable return to child care, employment, or group social activities (strong, moderate). Practitioners should collaborate with local public health authorities to adhere to policies regarding return to settings in which transmission is a consideration (strong, high).
27. A clinical and laboratory reevaluation may be indicated in people who do not respond to an initial course of therapy and should include consideration of noninfectious conditions, including lactose intolerance (weak, low).
28. Noninfectious conditions, including IBD and post- IBS, should be considered as underlying etiologies in people with symptoms lasting 14 or more days and unidentified sources (strong, moderate).
29. Reassessment of fluid and electrolyte balance, nutritional status, and optimal dose and duration of antimicrobial therapy is recommended in people with persistent symptoms (strong, high) (Figure 1).
Evidence Summary
The usual duration of symptoms of diarrhea with or without medical therapy can be expected to vary by organism, but duration of up to 10–14 days or longer can occur. Persistent carriage is a concern for some etiologic agents, such as Salmonella, STEC, and Shigella, and there are public health concerns stemming from prolonged carriage for people working in food service, child care, group settings, and long-term care facilities. The majority of patients with diarrhea will not have a laboratory diagnosis, so laboratory-based specific recommendations would be of minimal use. Repeat stool cultures are required in certain situations to enable return to employment and group social activities; these requirements may differ by local jurisdiction. When required, repeat testing is best done using traditional culture methods, as CIDTs do not indicate that living organisms are present, and have not been validated as suitable for proof of cure.
All patients should be educated about mode of spread of diarrheal diseases, typically fecal-oral, and warned that they potentially may be infectious to others after symptom resolution and for ensuing weeks to months. Careful hand hygiene should be observed, particularly if the patient is involved in food preparation, child or adult education, or healthcare. Specific situations in which additional follow-up should be considered are listed below.
As jurisdictional and state regulations regarding the number and timing of stool cultures required for return to the child care setting may vary, clinicians are advised to consult their local public health authority for guidance. As an example, 3 negative stool cultures obtained at least 24 hours apart, at least 48 hours after cessation of antimicrobial therapy, and not earlier than 1 month after symptom onset may be required for readmission of children and staff with Salmonella serovar Typhi infection. If any stool culture yields Salmonella Typhi, obtain monthly stool cultures during the subsequent 12 months until at least 3 consecutive stool cultures are without growth of Salmonella Typhi. Negative stool culture results typically are not required for return to childcare settings in children or staff with nontyphoidal Salmonella enterica serovar infections. For STEC, children are excluded from child care until diarrhea resolves, and 2 stool cultures negative for the organism typically are required for readmission [150]. Given the increasing detection of non-O157 STEC infections in recent years, some jurisdictions have begun basing exclusion policies of people with STEC on the observed virulence of the illness and virulence gene profile of the infecting strain. Regular and consistent follow-up of patients recovering from diarrhea-associated HUS is recommended until laboratory and clinical parameters have returned to normal values. Parameters of concern include indicators of renal function, anemia, and thrombocytopenia. There is no consensus for the frequency of follow-up laboratory testing beyond the point that clinical and laboratory resolution is achieved.
In the situation where a pathogen has not been identified, it may be reasonable to reevaluate stool and/or blood if there is evidence of systemic symptoms, for evaluation for a previously undetected pathogen.
If clinical symptoms worsen, there are several possible explanations. If an antimicrobial agent has been given, antibiotic-associated diarrhea (non–C. difficile) should be considered. If the patient is hospitalized or has had healthcare exposure, C. difficile becomes an additional consideration, particularly if there is fever or leukocytosis >20000 cells/μL [151], and stool should be assessed for C. difficile toxin or a toxigenic C. difficile strain (eg, NAAT). Stool also should be submitted for culture and susceptibility to determine the presence of a bacterial etiology. If a bacterial etiology is confirmed and an antimicrobial agent is indicated or has been used, susceptibility testing may reveal whether the worsening symptoms could be due to antimicrobial agent resistance.
Persistent symptoms (>14 days after onset) may last for months or even years and may respond to a similar management strategy. Protozoa (including Cryptosporidium species, Cyclospora cayetanensis, Cystoisospora belli, and Giardia lamblia) and microsporidia are considerations, particularly in an immunocompromised host. Diagnosis of these pathogens (Table 5) optimally is performed via microscopy or antigen detection. Treatment, where possible or advisable, is outlined in Table 6. When assessments for infectious agents do not yield an etiology, consideration of noninfectious illnesses and inflammatory processes should occur [152]. Both IBD and celiac disease are considerations. Postinfectious functional gastrointestinal disorders, including irritable bowel syndrome (PI-IBS), may occur in 3%–10% of adults following bacterial diarrhea. Symptoms attributable to PI-IBS generally resolve within 1 year, but may persist for several years. Assessment and management by a gastroenterologist for these conditions should be considered.
Table 6.
Recommended Antimicrobial Agents by Pathogen
| Indication | First Choice | Alternative | Comments/Considerations |
|---|---|---|---|
| Bacteriaa | |||
| Campylobacter | Azithromycin | Ciprofloxacin | |
| Clostridium difficile | Oral vancomycin | Fidaxomicin | Fidaxomicin not currently recommended for people <18 years of age. Metronidazole is still acceptable treatment for nonsevere CDI in children and as a second-line agent for adults with nonsevere CDI (eg, who cannot obtain vancomycin or fidaxomicin at a reasonable cost). |
| Nontyphoidal Salmonella entericab | Usually not indicated for uncomplicated infection | NA | Antimicrobial therapy should be considered for groups at increased risk for invasive infection: neonates (up to 3 months old), persons >50 years old with suspected atherosclerosis, persons with immunosuppression, cardiac disease (valvular or endovascular), or significant joint disease. If susceptible, treatment with ceftriaxone, ciprofloxacin, TMP-SMX, or amoxicillin. |
| Salmonella enterica Typhi or Paratyphib | Ceftriaxone or ciprofloxacin | Ampicillin or TMP-SMX or azithromycin | |
| Shigella a | Azithromycinc or ciprofloxacina, or ceftriaxone | TMP-SMX or ampicillin if susceptible | Clinicians treating people with shigellosis for whom antibiotic treatment is indicated should avoid prescribing fluoroquinolones if the ciprofloxacin MIC is 0.12 μg/ mL or higher even if the laboratory report identifies the isolate as susceptible. See https://emergency. cdc.gov/han/han00401.asp |
| Vibrio cholerae | Doxycyclined | Ciprofloxacin, azithromycin, or ceftriaxone | |
| Non–Vibrio choleraed | Usually not indicated for noninvasive disease. Single-agent therapy for noninvasive disease if treated. Invasive disease: ceftriaxone plus doxycycline |
Usually not indicated for noninvasive disease. Single-agent therapy for noninvasive disease if treated. Invasive disease: TMP-SMX plus an aminoglycoside |
|
| Yersinia enterocolitica | TMP-SMX | Cefotaxime or ciprofloxacin | |
| Parasites | |||
| Cryptosporidium spp | Nitazoxanide (HIV-uninfected, HIV-infected in combination with effective cART): | Effective cART: Immune reconstitution may lead to microbiologic and clinical response [154, 209, 210] |
NA |
| Cyclospora cayetanensis | TMP-SMX | Nitazoxanide (limited data) | Patients with HIV infection may require higher doses or longer durations of TMP-SMX treatment |
| Giardia lamblia | • Tinidazole Note: Based on data from HIV-uninfected children • Nitazoxanide |
Metronidazole Note: Based on data from HIV- uninfected children |
• Tinidazole is approved in the United States for children aged ≥3 years. It is available in tablets that can be crushed. • Metronidazole has high frequency of gastrointestinal side effects. A pediatric suspension of metronidazole is not commercially available but can be compounded from tablets. Metronidazole is not FDA approved for the treatment of giardiasis. |
| Cystoisospora belli | TMP-SMX | Pyrimethamine Potential second-line alternatives: • Ciprofloxacin • Nitazoxanide |
|
| Trichinella spp | Albendazole | Alternative: mebendazole | • Therapy less effective in late stage of infection, when larvae encapsulate in muscle |
| Fungus | |||
| Microsporidia | For disseminated (not ocular) and intestinal infection attributed to microsporidia other than Enterocytozoon bieneusi or Vittaforma corneae: • Albendazole after initiation of cART and resolution of signs and symptoms For E. bieneusi or V. corneae infections: • Fumagillin recommended for treatment of infections due to E. bieneusi in HIV-infected adults |
NA | Effective cART therapy: • Immune reconstitution may lead to microbiologic and clinical response • Fumagillin for systemic use is unavailable in the United States and data on dosing in children are unavailable. • Consultation with an expert is recommended. |
Abbreviations: cART, combination antiretroviral therapy; CDI, Clostridium difficile infection; FDA, US Food and Drug Administration; HIV, human immunodeficiency virus; MIC, minimum inhibitory concentration; NA, not applicable; TMP-SMX, trimethoprim-sulfamethoxazole.
aFor information on susceptibility patterns in the United States, see the National Antimicrobial Resistance Monitoring System (NARMS; http://www.cdc.gov/narms). Susceptibility testing should be considered when a therapeutic agent is selected.
bIf invasive disease is suspected or confirmed, ceftriaxone is preferred over ciprofloxacin due to increasing resistance to ciprofloxacin.cMost clinical laboratories do not test for azithromycin susceptibility.
dPrimary therapy is aggressive rehydration; antibiotics are adjunctive therapy.
Although definitive studies are lacking, molecular-epidemiologic assessments and outbreak investigations suggest that reinfection with enteric pathogens and possible recurrence of clinical symptoms are more likely to occur among people who reside in crowded settings with impaired access to hand hygiene. Recurrent symptomatic infections are more likely to occur with enteric pathogens with higher rates of infectivity and when cross-protection to infection with other strains does not result from an infection with one strain or serovar.
Assessment of dosing of an antimicrobial agent to ensure that therapeutic levels are or were achieved may be indicated. In some situations, adjunctive therapy such as a probiotic may be beneficial in restoration of dysbiosis due to the pathogen or treatment [153]. The administration of nitazoxanide has resulted in reduction of clinical symptoms in nonresponders and people with persistent symptoms [154, 155]. Nutritional rehabilitation and fluid and electrolyte administration are the mainstays of management, with a preference for enteral administration when tolerated.
Noninfectious etiologies of diarrhea should be considered if an individual with a worsening clinical course remains unresponsive to management. Imaging, including colonoscopy or endoscopy, may be indicated and consultation with a gastroenterologist may be beneficial in directing evaluation in the worsening host.
The persistence of organisms in the gastrointestinal tract as detected by stool assessments varies by organism and host factors. While asymptomatic shedding may result in transmission of an organism from person to person, the more clinically relevant issue relates to an infection that results in clinical symptomatology. In people who are able to practice meticulous hand hygiene and who are not employed in a setting where transmission could result in a severe infection or outbreak, repetitive testing will not result in clinical benefit and will be detrimental in terms of cost and use of limited healthcare resources. When the result(s) of testing will not impact management, follow-up testing should be deferred. However, in situations where treatment failures are more likely to occur or the infecting pathogen has demonstrated multidrug resistance, a test of cure may be beneficial.
Empiric Management of Infectious Diarrhea
XI. When is empiric antibacterial treatment indicated for children and adults with bloody diarrhea and, if indicated, with what agent?
a. What are modifying conditions that would support antimicrobial treatment of children and adults with bloody diarrhea?
b. In which instances should contacts be treated empirically if the agent is unknown?
Recommendations (Table 6)
-
30. In immunocompetent children and adults, empiric antimicrobial therapy for bloody diarrhea while waiting for results of investigations is not recommended (strong, low), except for the following:
a. Infants <3 months of age with suspicion of bacterial etiology.
b. Ill immunocompetent people with fever documented in a medical setting, abdominal pain, bloody diarrhea, and bacillary dysentery (frequent scant bloody stools, fever, abdominal cramps, tenesmus) presumptively due to Shigella.
c. People who have recently traveled internationally with body temperatures ≥38.5°C and/or signs of sepsis (weak, low). See https://wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/travelers-diarrhea.
31. The empiric antimicrobial therapy in adults should be either a fluoroquinolone such as ciprofloxacin, or azithromycin, depending on the local susceptibility patterns and travel history (strong, moderate). Empiric therapy for children includes a third-generation cephalosporin for infants <3 months of age and others with neurologic involvement, or azithromycin, depending on local susceptibility patterns and travel history (strong, moderate).
32. Empiric antibacterial treatment should be considered in immunocompromised people with severe illness and bloody diarrhea (strong, low).
33. Asymptomatic contacts of people with bloody diarrhea should not be offered empiric treatment, but should be advised to follow appropriate infection prevention and control measures (strong, moderate).
34. People with clinical features of sepsis who are suspected of having enteric fever should be treated empirically with broad-spectrum antimicrobial therapy after blood, stool, and urine culture collection (strong, low). Antimicrobial therapy should be narrowed when antimicrobial susceptibility testing results become available (strong, high). If an isolate is unavailable and there is a clinical suspicion of enteric fever, antimicrobial choice may be tailored to susceptible patterns from the setting where acquisition occurred (weak, low).
35. Antimicrobial therapy for people with infections attributed to STEC O157 and other STEC that produce Shiga toxin 2 (or if the toxin genotype is unknown) should be avoided (strong, moderate). Antimicrobial therapy for people with infections attributed to other STEC that do not produce Shiga toxin 2 (generally non-O157 STEC) is debatable due to insufficient evidence of benefit or the potential harm associated with some classes of antimicrobial agents (strong, low).
Evidence Summary
Intestinal perforation and death were more common in case series of patients with typhoid fever in the preantibiotic era (before 1950) than in the antibiotic era (after 1950) [156]. Patients with enteric fever treated early in their clinical courses have better outcomes than patients treated later [157]. Time to loss of fever was longer and case fatality ratio was higher among series of patients receiving supportive treatment only and patients receiving low doses of appropriate antimicrobial therapy compared with patients receiving recommended doses [158].
In adults, as in children, bloody diarrhea can be due to infectious and noninfectious causes. The presence of fever, abdominal pain, or vomiting is more suggestive of infection, which in these cases is likely to be due to an invasive/inflammatory pathogen. The most commonly identified pathogens in this category in North America are Salmonella, Campylobacter, C. difficile, Shigella, and STEC. Several RCTs specifically examining the benefit of empiric treatment of adults with acute, severe diarrhea, overall have demonstrated an average of 1 day shorter symptoms with an antimicrobial agent compared with placebo. However, these data are considered low quality due to inconsistency and indirectness. The antimicrobial agents utilized in the most recent of these studies were fluoroquinolones; previous data on trimethoprim-sulfamethoxazole (TMP-SMX) are not considered applicable today because of high rates of resistance. In general, the largest treatment effect was seen in patients with salmonellosis, followed by campylobacteriosis, but antimicrobial treatment also was accompanied by an increase in prolonged Salmonella shedding and occasional shedding of quinolone-resistant Campylobacter. Moreover, the benefit of antimicrobial treatment of proven Campylobacter infection is small, and antimicrobial agents are not recommended for most cases of proven Salmonella diarrhea. Given that the vast majority of inflammatory infectious diarrhea episodes are self-limited and that the treatment benefit is modest, in most cases the risks of treatment outweigh the benefits. Exceptions may occur in severe infections and in infections occurring in immunocompromised hosts. Severe CDIs have doubled in incidence since 2001 and can mimic other forms of infectious colitis. While most cases are associated with healthcare and recent antimicrobial agent use, there has been an increase in community-acquired cases with minimal or even no antimicrobial agent exposure. Use of concomitant antimicrobial agents is associated with decreased cure rates and higher relapse rates in CDI.
STEC infections also must be a consideration in any patient with bloody diarrhea, even when fever is present, but particularly when it is absent. Treatment of STEC O157 infections and likely non-O157 STEC infections that produce Shiga toxin 2 with fluoroquinolones, β-lactams, TMP-SMX, and metronidazole in patients of all ages should be avoided because of evidence of harm. Although very limited data are available on the possible risks or benefits associated with treating people with these infections with macrolide antibiotics, insufficient evidence of benefit and some evidence for harm favors avoidance of these agents among people infected with STEC O157 or other STEC that produce Shiga toxin 2 [159]. Insufficient data are available to assess the risks and benefits associated with treating less virulent STEC infections (ie, STEC that do not produce Shiga toxin 2) with antibiotics. However, because the Shiga toxin profile is often unknown when treatment is considered and because no clear benefit exists for treating patients with diarrhea caused by less virulent STEC infections with antibiotics, avoidance of antibiotic treatment is recommended.
Several RCTs have demonstrated a small but significant benefit for antimicrobial therapy in reducing the duration of symptoms in Campylobacter gastroenteritis. A meta-analysis confirmed an average of 1 day shorter duration of illness with fluoroquinolone or macrolide treatment compared with placebo [160]. However, symptoms in all cases in these studies were self-limited and the treatment effect appeared to be largest in patients treated early in the illness course. Earlier, directed treatment may become more feasible with the increasing use of CIDT, facilitating organism identification. Furthermore, there is no evidence that antimicrobial therapy prolongs the carrier state or encourages clinical relapses in campylobacteriosis, so the risk of treatment is relatively small. Although quinolone resistance may develop during therapy, person-to-person spread of drug-resistant Campylobacter is not believed to be a common scenario. Hence, it is reasonable to treat patients with particularly prolonged or severe disease. Fatal Campylobacter infections remain rare, but are more common in severely immunocompromised hosts, and despite the lack of evidence, it is reasonable to offer treatment to immunocompromised patients with otherwise uncomplicated Campylobacter gastroenteritis.
The choice of antimicrobial agent may change due to evolving resistance patterns [161]. Fluoroquinolone resistance in US and Canadian patients without international travel remains low, but is significantly higher in many commonly visited countries (ranging from 56% in Mexico to >92% in Thailand) [162, 163]. Macrolide resistance remains much less common (<5% among human isolates in the United States [164]). Hence, azithromycin can be recommended as primary treatment for traveler’s diarrhea in Thailand based on randomized trial data, and also should be considered first-line treatment for Campylobacter infection in travelers to other locations unless fluoroquinolone susceptibility is confirmed. Other antimicrobial agents that may be effective in individual Campylobacter isolates include TMP-SMX and tetracyclines, although in general, resistance rates are considerably higher and there is no advantage to these agents over azithromycin.
XII. When is empiric treatment indicated for children and adults with acute, prolonged, or persistent watery diarrhea and, if indicated, with what agent?
a. What are modifying conditions that would support empiric antimicrobial treatment of children and adults with watery diarrhea?
b. In which instances, if any, should contacts be treated empirically if the agent is unknown?
Recommendations (Table 6)
36. In most people with acute watery diarrhea and without recent international travel, empiric antimicrobial therapy is not recommended (strong, low). An exception may be made in people who are immunocompromised or young infants who are ill-appearing. Empiric treatment should be avoided in people with persistent watery diarrhea lasting 14 days or more (strong, low).
37. Asymptomatic contacts of people with acute or persistent watery diarrhea should not be offered empiric or preventive therapy, but should be advised to follow appropriate infection prevention and control measures (strong, moderate).
Evidence Summary
Watery diarrhea can be the primary manifestation of either an inflammatory or non-inflammatory intestinal tract infection. The presence of high fever or significant abdominal pain, and duration >3 days are suggestive of inflammatory infection with indications for investigation (Table 3). While several RCTs have shown a benefit of empiric treatment prior to culture results in these cases, the evidence is of low quality due to inconsistency and indirectness. Combined with the relatively small benefit of empiric treatment (1 day shorter illness on average), empiric treatment cannot be recommended. In the absence of signs and symptoms to suggest inflammatory bacterial infection, viral infection becomes significantly more likely and antimicrobial treatment is ineffective and potentially harmful, making empiric treatment even less desirable.
Persistent watery diarrhea generally should not be treated in the absence of an identified cause. This syndrome in otherwise healthy adults and children is only rarely due to bacterial infection, and bacteria that are reported to be associated with prolonged diarrhea (such as Aeromonas, Plesiomonas, C. difficile, and EAEC) are often not detected on routine stool culture. When persistent diarrhea is caused by infection, the most common etiologic agents are protozoal (including parasites such as Giardia lamblia, Cryptosporidium species, Cyclospora cayetanensis, and Cystoisospora belli, depending in part on the epidemiologic setting) and are best managed with pathogen-specific therapy (rather than empiric therapy before the infection is diagnosed). One exception to this is persistent diarrhea in patients who are severely immunocompromised (including people with AIDS), in which more conventional pathogens such as Campylobacter and Salmonella may persist. While it remains preferable to identify a specific cause in these cases, there are situations where an empiric trial with an antimicrobial agent may be considered to provide symptomatic benefit to optimize tolerance of highly active antiretroviral therapy.
Directed Management of Infectious Diarrhea
XIII. How should treatment be modified when a clinically plausible organism is identified from a diagnostic test?
Recommendation
38. Antimicrobial treatment should be modified or discontinued when a clinically plausible organism is identified (strong, high) Table 6.
Evidence Summary
Recommendations for antimicrobial agents by pathogen with first and alternative choices are listed in Table 6 for commonly identified bacterial (Campylobacter, C. difficile, nontyphoidal Salmonella, Shigella, Vibrio cholerae, non–Vibrio cholerae, Yersinia enterocolitica) and other organisms (Cryptosporidium, Cyclospora, Giardia, Cystoisospora, and microsporidia).
Supportive Treatment
XIV. How should rehydration therapy be administered?
Recommendations (Table 7)
Table 7.
Fluid and Nutritional Management of Diarrhea
| Degree of Dehydrationa | Rehydration Therapy | Replacement of Losses During Maintenancec |
|---|---|---|
| Mild to moderate dehydration | Infantsb and children: ORS, 50–100 mL/kg over 3–4 hours Adolescents and adults (≥30 kg): ORS, 2–4 L |
Infants and children: <10 kg body weight: 60–120 mL ORS for each diarrheal stool or vomiting episode, up to ~500 mL/day >10 kg body weight: 120–240 mL ORS for each diarrheal stool or vomiting episode; up to ~1 L/day Adolescents and adults: Ad libitum, up to ~2 L/day Replace losses as above as long as diarrhea or vomiting continues |
| Severe dehydration | Infants: Malnourished infants may benefit from smaller-volume, frequent boluses of 10 mL/kg body weight due to reduced capacity to increase cardiac output with larger volume resuscitation. Children, adolescents, and adults: Intravenous isotonic crystalloid boluses, per current fluid resuscitation guidelines, until pulse, perfusion, and mental status return to normal. Adjust electrolytes and administer dextrose based on chemistry values. Administer up to 20 mL/kg body weight until pulse, perfusion, and mental status return to normal. |
Infants and children: <10 kg body weight: 60–120 mL ORS for each diarrheal stool or vomiting episode, up to ~500 mL/day >10 kg body weight: 120–240 mL ORS for each diarrheal stool or vomiting episode; up to ~1 L/day Adolescents and adults: Ad libitum, up to ~2 L/day Replace losses as above as long as diarrhea or vomiting continue. If unable to drink, administer either through a nasogastric tube or give 5% dextrose 0.25 normal saline solution with 20 mEq/L potassium chloride intravenously. |
Adapted from Centers for Disease Control and Prevention. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003; 52(RR-16):1–16 and World Health Organization. The treatment of diarrhoea: a manual for physicians and other senior health workers (https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5216a1.htm).
Low-osmolarity ORS can be given to all age groups, with any cause of diarrhea. It is safe in the presence of hypernatremia as well as hyponatremia (except when edema is present). Some commercially available formulations that can be used as ORS include Pedialyte Liters (Abbott Nutrition), CeraLyte (Cero Products), and Enfalac Lytren (Mead Johnson). Popular beverages that should not be used for rehydration include apple juice, Gatorade, and commercial soft drinks.
Abbreviation: ORS, oral rehydration solution.
aA variety of scales are available to grade the severity of dehydration in young children but no single, standard, validated method exists. Note that signs of dehydration may be masked when a child is hypernatremic.
bBreastfed infants should continue nursing throughout the illness.
cAfter rehydration is complete, maintenance fluids should be resumed along with an age-appropriate normal diet offered every 3–4 hours. Children previously receiving a lactose-containing formula can tolerate the same product in most instances. Diluted formula does not appear to confer any benefit.
39. Reduced ORS is recommended as the first-line therapy of mild to moderate dehydration in infants, children, and adults with acute diarrhea from any cause (strong, moderate), and in people with mild to moderate dehydration associated with vomiting or severe diarrhea.
40. Nasogastric administration of ORS may be considered in infants, children and adults with moderate dehydration, who cannot tolerate oral intake, or in children with normal mental status who are too weak or refuse to drink adequately (weak, low).
41. Isotonic intravenous fluids such as lactated Ringer’s and normal saline solution should be administered when there is severe dehydration, shock, or altered mental status and failure of ORS therapy (strong, high) or ileus (strong, moderate). In people with ketonemia, an initial course of intravenous hydration may be needed to enable tolerance of oral rehydration (weak, low).
42. In severe dehydration, intravenous rehydration should be continued until pulse, perfusion, and mental status normalize and the patient awakens, has no risk factors for aspiration, and has no evidence of ileus. The remaining deficit can be replaced by using ORS (weak, low). Infants, children, and adults with mild to moderate dehydration should receive ORS until clinical dehydration is corrected (strong, low).
43. Once the patient is rehydrated, maintenance fluids should be administered. Replace ongoing losses in stools from infants, children, and adults with ORS, until diarrhea and vomiting are resolved (strong, low).
Evidence Summary
Replacement of water, electrolytes, and nutrients lost during diarrhea is essential in the management of diarrhea. During diarrhea, the coupled transport of sodium and glucose across the intestinal brush border remains intact, and leads to enhanced water absorption, enabling oral rehydration. Oral rehydration has been credited with saving millions of lives in the management of dehydration in all age groups, regardless of the cause, and is recommended by the WHOs and as the first line of rehydration [165].
The safety and efficacy of ORS, in comparison to intravenous rehydration therapy (IVT), was evaluated in a meta-analysis of 17 RCTs involving 1811 patients aged <18 years from high-income and low-income countries. There were no important clinical differences in failure to rehydrate, weight gain at discharge, hyponatremia or hypernatremia, duration of diarrhea, or total fluid intake at 6 or 24 hours between children receiving ORS and IVT. Phlebitis occurred more often in children receiving IVT, and paralytic ileus occurred more often with ORS (though the latter difference was not statistically different). The model estimated that 4% of children treated with ORS would fail and require IVT [166].
Standard WHO-ORS (osmolarity 311 mmol/L) was the recommended agent for several decades [167]. Despite its ability to hydrate, WHO-ORS had limitations, including inability to reduce the volume or duration of diarrhea, and concerns that it could lead to hypernatremia, especially in noncholera diarrhea in which salt losses are reduced. In 2002, a hypotonic ORS with total osmolarity <250 mmol/L was recommended by the WHO and subsequently by various other advisory bodies as first-line therapy for mild to moderate dehydration caused by diarrhea of all causes [168]. In a meta-analysis of 14 RCTs involving children <5 years of age with diarrheal dehydration (all causes) from low- and high-income countries, reduced osmolarity ORS was associated with fewer unscheduled infusions [165], reduced stool output, and decreased vomiting compared with WHO-ORS. In a meta-analysis of adults and children with cholera, reduced osmolarity ORS (≤270 mmol/L) was associated with more biochemical hyponatremia compared with WHO-ORS (osmolarity ≥310 mmol/L), although no significant differences in serious consequences were noted based on the 4 RCTs included in the analysis [169]. Recipients of a polymer-based oral rehydration solution demonstrated fewer unscheduled intravenous infusions compared with pediatric recipients of WHO-ORS ≥310 mmol/L who had acute watery diarrhea or diarrhea attributed to a cholera infection. The polymer-based ORS was also favored over the hypo-osmolar ORS ≤270 mmol/L, although there were insufficient data to adequately power the analysis [170]. ORS is an integral component of rehydration and may be used effectively in combination with intravenous therapy and with transition to enteral feeding.
XV. When should feeding be initiated following rehydration?
Recommendations
44. Human milk feeding should be continued in infants and children throughout the diarrheal episode (strong, low).
45. Resumption of an age-appropriate usual diet is recommended during or immediately after the rehydration process is completed (strong, low).
Evidence Summary
Early studies showing that children who resumed feeding during or after rehydration had improved nutritional outcome [171] led to multiple guidelines supporting this practice. A meta-analysis (12 RCT, most performed 20 years ago and for which reporting of methodology was incomplete) showed that early feeding (within 12 hours of beginning rehydration) was as safe and effective as later feeding among children aged <6 years old with acute diarrhea from low-, middle-, and high-income countries [172]. There was no significant difference between early and late refeeding groups in the need for unscheduled intravenous therapy, number of children with vomiting and persistent diarrhea, and length of hospital stay. Data were insufficient to assess differences in duration of diarrhea, stool output, or weight gain. A meta-analysis of 33 trials involving children <5 years of age with acute diarrhea (mostly inpatients from high- and middle-income countries) found that a lactose-free diet reduced the duration of diarrhea by an average of 18 hours and reduced treatment failure (continued or worsening diarrhea or vomiting, the need for additional rehydration, or continuing weight loss) by one half [173]. In adults, early refeeding decreases intestinal permeability caused by infections, reduces illness duration, and improves nutritional outcomes. This is particularly important in low- and middle-income countries, where underlying preexisting malnutrition is often a factor. Although the BRAT (bananas, rice, applesauce, and toast) diet and the avoidance of dairy are commonly recommended, supporting data for those interventions are limited. Instructing patients to refrain from eating solid food for 24 hours also does not appear to be useful [174].
Ancillary Management
XVI. What options are available for symptomatic relief, and when should they be offered?
Recommendations
46. Ancillary treatment with antimotility, antinausea, or antiemetic agents can be considered once the patient is adequately hydrated, but their use is not a substitute for fluid and electrolyte therapy (weak, low).
47. Antimotility drugs (eg, loperamide) should not be given to children <18 years of age with acute diarrhea (strong, moderate). Loperamide may be given to immunocompetent adults with acute watery diarrhea (weak, moderate), but should be avoided at any age in suspected or proven cases where toxic megacolon may result in inflammatory diarrhea or diarrhea with fever (strong, low).
48. An antinausea and antiemetic (eg, ondansetron) may be given to facilitate tolerance of oral rehydration in children >4 years of age and in adolescents with acute gastroenteritis associated with vomiting (weak, moderate).
Evidence Summary
Ancillary treatment for acute infectious diarrhea includes antimotility and antisecretory agents to shorten duration of diarrhea in adults, and antiemetic agents to facilitate oral rehydration in people with significant vomiting. Oral rehydration has been shown to be useful in all ages, and antiemetics such as dimenhydrinate have been beneficial in adults. Ondansetron is a serotonin 5-HT3 receptor antagonist used for treatment of nausea and vomiting in various settings [175]. During acute gastroenteritis, studies have shown that more children receiving ondansetron, compared with placebo, had resolution of vomiting; ondansetron reduced the immediate need for hospitalization or intravenous rehydration [176]. However, ondansetron did not decrease hospitalization rates at 72 hours after discharge from the emergency department. There was no significant increase in adverse events, but diarrhea was reported as a side effect of ondansetron treatment in several studies [177–179]. Ondansetron can reduce vomiting in children and reduce the need for hospitalization for rehydration, although it may increase stool volume. A recommendation cannot be made for the routine use of antiemetic agents for acute gastroenteritis in children <4 years of age or in adults. Bismuth subsalicylate is mildly effective. Racecadotril reduces stool volume but is not available in North America [180, 181].
Loperamide is a locally acting opioid receptor agonist that decreases the muscular tone and motility of the intestinal wall. In children with mild to moderate dehydration associated with mostly nonbacterial pathogens, a meta-analysis of earlier studies has shown that loperamide reduced diarrhea prevalence at both 24 and 48 hours after onset of treatment, and reduced the total duration of diarrhea [182]. These studies excluded children with moderate to severe dehydration (or some did not include hydration status) and bloody diarrhea. Adverse events including ileus, abdominal distension, and lethargy tended to occur in subjects receiving treatment. Deaths have been reported in 0.54% of children given loperamide, and all of these events occurred in children <3 years old. In healthy adults, loperamide has been shown to be effective in reducing diarrhea, but most of the studies have been focused on travelers to resource-challenged countries and the drug was used in combination with antimicrobial agents [183]. In these studies, loperamide was not associated with increased occurrence of adverse events. Loperamide significantly reduces stool volume in traveler’s diarrhea and in most noncholera watery diarrhea syndromes.
Patients should be advised about medications with the potential to increase the risk of complications from diarrhea, particularly antidiarrheal and antimicrobial agents. Limited reports suggest that routine use of medications with anticholinergic properties may lead to increased risk of severe outcomes, including death, from diarrhea caused by C. difficile and Clostridium perfringens, a toxin-mediated illness [91, 184, 185]. Clinical conditions also have worsened following administration of antimotility agents to patients with shigellosis and infection with STEC. Antimicrobial agents and antidiarrheal medications administered to people with diarrhea caused by STEC infections may increase the risk of HUS.
XVII. What is the role of a probiotic or zinc in treatment or prevention of infectious diarrhea in children and adults?
Recommendations
49. Probiotic preparations may be offered to reduce the symptom severity and duration in immunocompetent adults and children with infectious or antimicrobial-associated diarrhea (weak, moderate). Specific recommendations regarding selection of probiotic organism(s), route of delivery, and dosage may be found through literature searches of studies and through guidance from manufacturers.
50. Oral zinc supplementation reduces the duration of diarrhea in children 6 months to 5 years of age who reside in countries with a high prevalence of zinc deficiency or who have signs of malnutrition (strong, moderate).
Evidence Summary
Most trials report that probiotics decrease diarrhea duration and stool frequency with a sustained beneficial effect across all outcomes. No adverse events have been directly attributable to probiotics in healthy recipients; case reports of bacteremia or fungemia with molecularly matched isolates to the probiotic organism have occurred in critically ill or immunocompromised people. The interpretations of many studies are limited by statistical heterogeneity due to varying definitions of diarrhea, outcome measurements, probiotic product, treatment regimens, participants, and settings [186].
Despite the limitations of meta-analyses, a reduction in mean duration of diarrhea by 25 hours (95% confidence interval, 16–34 hours) was noted among 455 participants in 35 trials; a reduction in the risk of diarrhea with a duration >4 days was noted among 2850 participants enrolled in 29 trials; and a reduction in stool frequency was noted on the second day of symptoms among 2751 participants enrolled in 20 trials. Overall, efficacy of probiotic supplementation was greater for participants with an identified viral etiology of diarrhea. However, this may be due to the fact that diarrhea of a viral etiology is more prevalent than that of a bacterial etiology [177, 187–190].
In a meta-analysis of 24 RCTs mostly conducted in Asia and in resource-limited settings, oral zinc supplementation appeared to shorten the duration of acute diarrhea in children who are 6 months to 5 years of age by 10 hours with an even greater reduction (27 hours) among children who have signs of malnutrition [191]. The effect on hospitalization and death could not be measured. The duration of treatment of persistent diarrhea was shortened by about 16 hours. Vomiting was noted to be more common in infants and children who received zinc supplementation compared with children who were not given zinc. An RCT among Polish children 3–48 months of age with acute diarrhea did not find a significant benefit from a 10-day course of zinc on the duration of diarrhea [192]. An RCT evaluating the efficacy of a 14-day course of zinc in US outpatients and inpatients aged 6 months to 6 years with acute diarrhea is ongoing [193].
XVIII. Which asymptomatic people with an identified bacterial organism from stool culture or molecular testing should be treated with an antimicrobial agent?
Recommendations
51. Asymptomatic people who practice hand hygiene and live and work in low-risk settings (do not provide healthcare or child or elderly adult care and are not food service employees) do not need treatment except asymptomatic people with Salmonella enterica subspecies enterica serovar Typhi in their stool who may be treated empirically to reduce potential for transmission (weak, low). Asymptomatic people who practice hand hygiene and live and work in high-risk settings (provide healthcare or child or elderly adult care and are food service employees) should be treated according to local public health guidance (strong, high).
Evidence Summary
Adults with acute nontyphoidal Salmonella enterica diarrhea commonly continue to sporadically shed the organism in stool asymptomatically for weeks [194]. Although there is a risk of these individuals spreading infection to others, particularly through food handling and close contact, outbreaks related to known carriers appear to be rare, and can be avoided through proper hand hygiene [195, 196]. The only randomized trial of decolonization was in Thailand, where antimicrobials failed to show a benefit over placebo, although reacquisition rather than persistence may have explained this failure [197]. Despite the paucity of evidence, some state and local laws mandate negative stool cultures prior to resuming work; in these situations, treatment may be considered.
Asymptomatic shedding of Salmonella serovar Typhi after acute infection is quite common, and can persist beyond a year in a small percentage of patients. These chronic carriers can spread infection to others if proper hand hygiene practices are not followed. One small randomized, controlled trial and one nonrandomized trial have showed high efficacy rates for decolonization with fluoroquinolones [198, 199].
Prevention
XIX. What strategies, including public health measures, are beneficial in preventing transmission of pathogens associated with infectious diarrhea?
Recommendations
52. Hand hygiene should be performed after using the toilet, changing diapers, before and after preparing food, before eating, after handling garbage or soiled laundry items, and after touching animals or their feces or environments, especially in public settings such as petting zoos (strong, moderate).
53. Infection control measures including use of gloves and gowns, hand hygiene with soap and water, or alcohol-based sanitizers should be followed in the care of people with diarrhea (strong, high). The selection of a hand hygiene product should be based upon a known or suspected pathogen and the environment in which the organism may be transmitted (strong, low). See https://www.cdc.gov/hicpac/2007IP/2007isolationPrecautions.html.
54. Appropriate food safety practices are recommended to avoid cross-contamination of other foods or cooking surfaces and utensils during grocery shopping, food preparation, and storage; ensure that foods containing meats and eggs are cooked and maintained at proper temperatures (strong, moderate).
55. Healthcare providers should direct educational efforts toward all people with diarrhea, but particularly to people with primary and secondary immune deficiencies, pregnant women, parents of young children, and the elderly as they have increased risk of complications from diarrheal disease (strong, low).
56. Ill people with diarrhea should avoid swimming, water-related activities, and sexual contact with other people when symptomatic while adhering to meticulous hand hygiene (strong, low).
Evidence Summary
Infectious agents that cause diarrhea are transmitted predominately by the fecal-oral route. Organisms in stool are transmitted to a susceptible host through contact transmission via contamination of inanimate surfaces, the hands of infected people and their caregivers, and vectors such as water or food, and contact with animals or their environment. The infected person may be shedding organisms in diarrheal stool, be in the convalescent phase of a diarrheal illness, or have asymptomatic shedding. Standard practices and transmission-based, or additional precautions, are the foundation for preventing transmission of infectious agents in the healthcare setting [200] and provide various infection control measures for all patient care settings. Standard practices are used at all times, whereas additional precautions are implemented based on patient symptoms or signs and/or diagnoses of certain microorganisms. For example, as part of standard practices, healthcare providers practice hand hygiene before and after each patient contact, use personal protective equipment depending on the patient care activity, and follow recommendations regarding patient placement and environmental cleaning. A patient with diarrhea would be placed on contact, in addition to standard, precautions. The reader is referred to detailed guidelines of the CDC’s Healthcare Infection Practices Advisory Committee for further information about the specific infection control measures for contact precautions and with particular diarrheal agents [200].
In the community, transmission of diarrheal pathogens can be interrupted by access to clean water and appropriately handled food, as well as hand hygiene before and after each contact with the ill person or their body fluids. This includes appropriate hand hygiene after using the toilet, after handling diapers at home and in out-of-home child care [201], before and after preparing food, before eating, and after handling patients’ personal items, or after touching pets or animals or their feces or environments, particularly in public settings (such as petting zoos and public farms) [202]. The spread of infectious diarrhea in child care settings can be decreased by training child care providers in infection control procedures, maintaining cleanliness of surfaces, keeping food preparation duties and areas separate from child care activities and exercising adequate hand hygiene, cohorting ill children, and excluding ill child care providers and food handlers. Alcohol-based hand hygiene is recommended, unless there is visible soiling, in which case hand hygiene with water and soap is necessary. When Cryptosporidium, norovirus, or a known spore-forming pathogen such as C. difficile is an infecting agent, hand hygiene with soap and water may be more effective than use of an alcohol-based sanitizer [203]. Some diarrheal agents are reportable to public health authorities (See Question XXI), and public health may place infected food handlers, recreational water staff, healthcare providers, or child care providers on furlough until the risk of transmission is eliminated or reduced.
The CDC recommends that no one eat or drink unpasteurized dairy products or undercooked meat. Specific groups of patients have increased risk of complications with diarrheal disease and warrant specific attention to education about diarrheal disease risk, such as the immunocompromised, pregnant women, people with chronic liver disease, the elderly, and parents caring for young infants. Education with attention to the particular epidemiology of risk for that individual and/or their caregiver should be provided by healthcare providers. Information on food safety can be found at the US Department of Health and Human Services [204].
XX. What are the relative efficacies and effectiveness of vaccines (rotavirus, typhoid, and cholera) to reduce the prevention and transmission of pathogens associated with infectious diarrhea, and when should they be used?
Recommendations
57. Rotavirus vaccine should be administered to all infants without a known contraindication (strong, high).
58. Two typhoid vaccines (oral and injectable) are licensed in the United States but are not recommended routinely. Typhoid vaccination is recommended as an adjunct to hand hygiene and the avoidance of high-risk foods and beverages for travelers to areas where there is moderate to high risk for exposure to Salmonella enterica subspecies enterica serovar Typhi, people with intimate exposure (eg, household contact) to a documented Salmonella Typhi chronic carrier, and microbiologists and other laboratory personnel routinely exposed to cultures of Salmonella Typhi (strong, high). Booster doses are recommended for people who remain at risk (strong, high).
59. A live attenuated cholera vaccine, which is available as a single-dose oral vaccine in the United States, is recommended for adults 18–64 years of age who travel to cholera-affected areas (strong, high). See https://www.cdc.gov/cholera/vaccines.html.
Evidence Summary
Prior to introduction of rotavirus vaccine programs in the United States in 2006, rotavirus was the leading cause of acute gastroenteritis, resulting in medical visits and hospitalization in children <5 years of age. Following large phase 3 trials demonstrating efficacy of vaccine against any rotavirus infection of 74%–87%, and against severe gastroenteritis of 85%–98%, universal infant rotavirus vaccination was recommended by ACIP [202]. Rotavirus surveillance has demonstrated significant reductions in outpatient visits and hospitalization, as well as evidence of benefit in nonimmunized older people [23]. Two live, attenuated orally administered rotavirus vaccines are available in the United States: a pentavalent rotavirus vaccine (Rotateq, Merck) given in a 3-dose schedule, and a monovalent vaccine (Rotarix, GSK) given in a 2-dose schedule.
Currently, there are 2 licensed vaccines in the United States for prevention of typhoid fever, each offering 50%–80% protection [26]. Typhoid vaccination is recommended for travelers to areas where there is increased risk for exposure to Salmonella Typhi. The Ty21a vaccine is a live, attenuated, oral vaccine containing the Salmonella Typhi strain. Ty21a available as enteric capsules and is licensed in the United States for use in immunocompetent people including children ≥6 years of age; the recommended boosting interval is every 5 years. The parenteral Vi-polysaccharide vaccine is licensed in the United States for children ≥2 years of age and adults. The recommended boosting interval is every 2 years. Typhoid vaccines do not offer protection against Salmonella Paratyphi A, B, or C infection.
CVD 103-HgR is a live, attenuated single-dose oral cholera vaccine available for adults in the United States who plan to travel to cholera-affected areas, defined as areas of endemic cholera transmission, outbreak (epidemic), or recent activity (within the past year). Two inactivated oral vaccines are available in other countries. Cholera immunization is not required for travelers entering the United States from cholera-affected areas, and the WHO no longer recommends immunization for travel to or from areas with cholera infection. No country requires cholera vaccine for entry.
XXI. How does reporting of nationally notifiable organisms identified from stool specimens impact the control and prevention of diarrheal disease in the United States?
Recommendation
60. All diseases listed in the table of National Notifiable Diseases Surveillance System at the national level, including those that cause diarrhea, should be reported to the appropriate state, territorial, or local health department, with submission of isolates of certain pathogens (eg, Salmonella, STEC, Shigella, Listeria) to ensure that control and prevention practices may be implemented (strong, high).
Evidence Summary
Clinical healthcare providers and public health practitioners have overlapping interests in and responsibilities for diagnosis, management, and prevention of infectious diarrhea. For clinicians, early diagnosis of an acute episode of diarrhea can occasionally result in interventions that alleviate symptoms and reduce secondary transmission. For public health practitioners, prompt notification of pathogen-specific diagnoses and molecular testing of isolates obtained through public health surveillance can lower rates of transmission and lead to timely detection and control of outbreaks. To reduce the morbidity and mortality associated with infectious diarrhea, the clinical and public health practitioner communities must work closely together to identify optimal diagnostic, treatment, and prevention methods.
Public health officials at US state and territorial health departments and the CDC collaborate in determining which diseases should be nationally notifiable, as well as timeframes for reporting. The Council of State and Territorial Epidemiologists, with advice from the CDC, makes recommendations annually for additions and deletions to the list of nationally notifiable diseases. Clinicians, hospitals, and laboratories in the United States are required to report diseases, conditions, or outbreaks as determined by local, state, or territorial law or regulation, as outlined in each jurisdiction’s list of reportable conditions. Additional and specific reporting requirements should be obtained from the appropriate local, state, or territorial health departments.
Reports of certain infections to public health authorities should be accompanied by submission of an isolate to the public health laboratory. Further characterization of some infecting pathogens in public health laboratories has been critical to identifying, stopping, and preventing many dispersed outbreaks through laboratory-based surveillance that utilizes isolate subtyping to detect outbreaks caused specific strains [123]. This type of surveillance began in the 1960s with serotyping of Salmonella isolates. In the 1990s, more discriminatory subtyping was introduced through pulsed-field gel electrophoresis, with the advent of the PulseNet surveillance system [123]. In recent years, higher resolution subtyping such as whole-genome sequencing is being performed by public health laboratories to detect outbreaks even more quickly [205]. The impact of these techniques on surveillance systems has been vast, including the passage of the Food Safety Modernization Act, and the development of new standards for beef and poultry by the US Department of Agriculture. Continuing to detect and respond to such outbreaks is a vital part of making our food and water systems safer. As CIDT diagnostic panels become used more frequently, public health departments may request that specimens be cultured in public health laboratories if unable to be cultured in the clinical diagnostic laboratory.
While laboratory-based surveillance like PulseNet is critical to detecting outbreaks, especially those consisting of widely dispersed infections, most diarrheal disease outbreaks are localized events and are often detected by the astute clinician [123]. Therefore, healthcare providers should adhere to local and state reporting requirements regarding any unusual cluster of diarrheal illness, regardless if an etiology has been determined or if the determined etiologies are typically not reportable, so that control measures may be implemented and the pathogen and source of infection identified to guide appropriate preventive strategies specific to the community at risk.
Attempts to detect pathogens in people with diarrhea provide public health benefit (beyond those described in the diagnostics section above) in which the individual may either serve as the sentinel case of an outbreak or serve as a risk for the initiation of an outbreak. People who work in healthcare, especially but not limited to those who provide care for immunocompromised people (HIV infected, those with cancer, or transplant recipients) should undergo diagnostic testing when symptomatic. Other situations in which diagnostic stool evaluation may be appropriate include child care providers (adult or child), child care attendees (adult or child), people involved in food preparation or delivery, people who work at recreational water facilities, or people who work at or live in residential facilities such as residential or group homes, prisons, or long-term care facilities. Cruise ships also have been associated with outbreaks of gastrointestinal tract illness, including diarrhea. If an outbreak is suspected (in a school, college dormitory, or activity group), the health official in charge should consider obtaining diagnostic testing to optimize intervention. Culture-dependent investigations, when a bacterial pathogen is involved, can assist in determining resistance patterns of enteric pathogens circulating in the community to permit development of appropriate treatment or management regimens.
All organisms listed in the table of Infectious Diseases Designated as Notifiable at the National Level (http://wwwn.cdc.gov/nndss/) should be reported. The CDC acts as a common repository for states and territories for collecting data and reporting of nationally notifiable diseases. Reports of occurrences of nationally notifiable diseases are transmitted to the CDC each week from the 50 US states, 2 cities (Washington, District of Columbia and New York, New York) and 5 territories (American Samoa, Commonwealth of Northern Mariana Islands, Guam, Puerto Rico, and the US Virgin Islands). Provisional data are published weekly in the Morbidity and Mortality Weekly Report; final data are published each year by the CDC in the annual “Summary of Notifiable Diseases, United States” [206]. The timeliness of the provisional weekly reports, in addition to laboratory-based surveillance, provides information that the CDC and state or local epidemiologists use to detect disease occurrence and more effectively interrupt outbreaks. The finalized annual data provide information on reported disease incidence that is necessary for study of epidemiologic trends and development of disease-prevention policies. The CDC is the sole repository for these national data, which are used widely by local, state, and federal public health and other agencies.
The following 13 conditions, which are associated with infectious diarrhea, are included in the table of Infectious Diseases Designated as Notifiable at the National Level—United States, 2017 (https://wwwn.cdc.gov/nndss/conditions/notifiable/2017/):
Campylobacteriosis
Cholera
Cryptosporidiosis
Cyclosporiasis
Giardiasis
Hemolytic-uremic syndrome, postdiarrheal
Salmonellosis
Shiga toxin–producing Escherichia coli
Shigellosis
Trichinellosis (trichinosis)
Typhoid fever
Vibriosis
Foodborne disease outbreak
FUTURE DIRECTIONS
A key challenge in the diagnosis and management of people with infectious diarrhea is the use and interpretation of molecular-based diagnostics. Differentiating colonization from active infection, obtaining antimicrobial susceptibility results, providing optimal management, and preventing transmission are areas in need of additional research as nonculture diagnostics replace traditional culture-based methods. Despite the evolution of diagnostics, the optimal management of people with infectious diarrhea centers on obtaining a thorough exposure history and performing a physical examination. This information enables the clinician to selectively apply diagnostics and judiciously administer therapy. Interrupting transmission of communicable enteric infections is essential in preserving public health.
Notes
Financial support. Support for these guidelines was provided by the Infectious Diseases Society of America.
Acknowledgments. The expert panel expresses its gratitude for thoughtful reviews of an earlier version by Drs Herbert Dupont, Richard L. Guerrant, and Timothy Jones. The panel thanks the IDSA for supporting guideline development, and specifically Vita Washington for her continued support throughout the guideline process. Appreciation is expressed to Dr Nathan Thielman for his contributions to the initial stages of guideline development and Dr Faruque Ahmed for his continued support and guidance regarding the GRADE system. Many thanks to Reed Walton for her assistance at many levels, William Thomas for help with the literature review, and Bethany Sederdahl for her editorial assistance.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. The following list is a reflection of what has been reported to the IDSA. To provide thorough transparency, the IDSA requires full disclosure of all relationships, regardless of relevancy to the guideline topic. Evaluation of such relationships as potential conflicts of interest is determined by a review process which includes assessment by the SPGC Chair, the SPGC liaison to the development panel and the Board of Directors liaison to the SPGC and if necessary, the Conflicts of Interest (COI) Task Force of the Board. This assessment of disclosed relationships for possible COI will be based on the relative weight of the financial relationship (ie, monetary amount) and the relevance of the relationship (ie, the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The reader of these guidelines should be mindful of this when the list of disclosures is reviewed. The institution at which A. L. S. has received research grants from the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases (NIAID), and the Gerber Foundation, from which she has received salary support, and she has received honoraria from SLACK and travel subsidies from International Scientific Association for Probiotics and Prebiotics (https://isappscience.org/) to attend annual meetings, J. C. has received research grants from the US Army, and stocks and bonds from Ariad and SIS Pharmaceuticals. A. C. has received research grants from CSL Behring and National Health & Medical Research. J. A. C. has received research grants from the CDC, National Institutes of Health (NIH), UK Biotechnology and Biological Sciences Research Council, Bill & Melinda Gates Foundation, and New Zealand Health Research Council. J. M. L.’s institution has received research grants from Merck, GlaxoSmithKline, the Canadian Institutes of Health Research (CIHR), Pfizer, PCIRN, Dynavax, and Afexa. T. S. has received research grants from Merck, Crohn’s and Colitis Canada, and CIHR; has received honoraria from Merck, Bristol-Meyers Squibb, Wyeth, and Pendopharm; and has served as a consultant on research contracts for Merck, Rebiotix, Acetlion, and Sanofi Pasteur. P. I. T. has received research grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the NIAID, and the Bill & Melinda Gates Foundation. C. W. has received research grants from the NIH, GlaxoSmithKline, and Merck, and served as a consultant for Pfizer and Thera Pharmaceuticals. C. A. W. has received research grants from NIH, NIAID and received a patent from the University of Virginia. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Guerrant RL, Van Gilder T, Steiner TS et al. ; Infectious Diseases Society of America Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 2001; 32:331–51. [DOI] [PubMed] [Google Scholar]
- 2. Infectious Diseases Society of America. Handbook on clinical practice guideline development, 2015 Available at: http://www.idsociety.org/uploadedFiles/IDSA/Guidelines-Patient_Care/IDSA_Practice_Guidelines/IDSA%20Handbook%20on%20CPG%20Development%2010.15.pdf. Accessed 15 January 2015.
- 3. Guyatt GH. U.S. GRADE Network. Approach and implications to rating the quality of evidence and strength of recommendations using the GRADE methodology Available at: http://www.gradeworkinggroup.org/. Accessed July 2015.
- 4. Guyatt GH, Oxman AD, Kunz R et al. ; GRADE Working Group Going from evidence to recommendations. BMJ 2008; 336:1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guyatt GH, Oxman AD, Kunz R et al. ; GRADE Working Group Incorporating considerations of resources use into grading recommendations. BMJ 2008; 336:1170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guyatt GH, Oxman AD, Vist GE et al. ; GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaeschke R, Guyatt GH, Dellinger P et al. ; GRADE Working Group Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 2008; 337:a744. [DOI] [PubMed] [Google Scholar]
- 8. Cohen SH, Gerding DN, Johnson S et al. ; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 9. Wessels E, Rusman LG, van Bussel MJ, Claas EC. Added value of multiplex Luminex Gastrointestinal Pathogen Panel (xTAG GPP) testing in the diagnosis of infectious gastroenteritis. Clin Microbiol Infect 2014; 20:O182–7. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization (WHO). Diarrhoea Available at: http://www.who.int/topics/diarrhoea/en/. Accessed 15 January 2015.
- 11. Strand TA, Sharma PR, Gjessing HK et al. Risk factors for extended duration of acute diarrhea in young children. PLoS One 2012; 7:e36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scallan E, Griffin PM, Angulo FJ, Tauxe RV, Hoekstra RM. Foodborne illness acquired in the United States—unspecified agents. Emerg Infect Dis 2011; 17:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mounts AW, Holman RC, Clarke MJ, Bresee JS, Glass RI. Trends in hospitalizations associated with gastroenteritis among adults in the United States, 1979–1995. Epidemiol Infect 1999; 123:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roy SL, Scallan E, Beach MJ. The rate of acute gastrointestinal illness in developed countries. J Water Health 2006; 4suppl 2:31–69. [DOI] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention (CDC). Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet surveillance report for 2014 (final report). Atlanta, GA: CDC, 2016. [Google Scholar]
- 16. Malek MA, Curns AT, Holman RC et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics 2006; 117:1887–92. [DOI] [PubMed] [Google Scholar]
- 17. Payne DC, Staat MA, Edwards KM et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics 2008; 122:1235–43. [DOI] [PubMed] [Google Scholar]
- 18. Parashar UD, Alexander JP, Glass RI; Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC) Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1–13. [PubMed] [Google Scholar]
- 19. Payne DC, Vinjé J, Szilagyi PG et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 2013; 368:1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Esposito DH, Holman RC, Haberling DL et al. Baseline estimates of diarrhea-associated mortality among United States children before rotavirus vaccine introduction. Pediatr Infect Dis J 2011; 30:942–7. [DOI] [PubMed] [Google Scholar]
- 21. Parashar UD, Kilgore PE, Holman RC, Clarke MJ, Bresee JS, Glass RI. Diarrheal mortality in US infants. Influence of birth weight on risk factors for death. Arch Pediatr Adolesc Med 1998; 152:47–51. [DOI] [PubMed] [Google Scholar]
- 22. Leshem E, Lopman B, Glass R et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14:847–56. [DOI] [PubMed] [Google Scholar]
- 23. Anderson EJ, Shippee DB, Weinrobe MH et al. Indirect protection of adults from rotavirus by pediatric rotavirus vaccination. Clin Infect Dis 2013; 56:755–60. [DOI] [PubMed] [Google Scholar]
- 24. Tam CC, O’Brien SJ, Tompkins DS et al. ; IID2 Study Executive Committee Changes in causes of acute gastroenteritis in the United Kingdom over 15 years: microbiologic findings from 2 prospective, population-based studies of infectious intestinal disease. Clin Infect Dis 2012; 54:1275–86. [DOI] [PubMed] [Google Scholar]
- 25. Cortese MM, Parashar UD; Centers for Disease Control and Prevention (CDC) Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58:1–25. [PubMed] [Google Scholar]
- 26. Jackson BR, Iqbal S, Mahon B; Centers for Disease Control and Prevention (CDC) Updated recommendations for the use of typhoid vaccine—Advisory Committee on Immunization Practices, United States, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:305–8. [PMC free article] [PubMed] [Google Scholar]
- 27. Voetsch AC, Van Gilder TJ, Angulo FJ et al. ; Emerging Infections Program FoodNet Working Group FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis 2004; 38suppl 3:S127–34. [DOI] [PubMed] [Google Scholar]
- 28. Voetsch AC, Angulo FJ, Jones TF et al. Reduction in the incidence of invasive listeriosis in Foodborne Diseases Active Surveillance Network sites, 1996–2003. Clin Infect Dis 2007; 44:513–20. [DOI] [PubMed] [Google Scholar]
- 29. Varma JK, Samuel MC, Marcus R et al. Listeria monocytogenes infection from foods prepared in a commercial establishment: a case-control study of potential sources of sporadic illness in the United States. Clin Infect Dis 2007; 44:521–8. [DOI] [PubMed] [Google Scholar]
- 30. Samuel MC, Vugia DJ, Shallow S et al. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996–1999. Clin Infect Dis 2004; 38suppl 3:S165–74. [DOI] [PubMed] [Google Scholar]
- 31. Roy SL, DeLong SM, Stenzel SA et al. Risk factors for sporadic cryptosporidiosis among immunocompetent persons in the United States from 1999 to 2001. J Clin Microbiol 2004; 42:2944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mermin J, Hutwagner L, Vugia D et al. ; Emerging Infections Program FoodNet Working Group Reptiles, amphibians, and human Salmonella infection: a population-based, case-control study. Clin Infect Dis 2004; 38suppl 3:S253–61. [DOI] [PubMed] [Google Scholar]
- 33. Marcus R, Varma JK, Medus C et al. Re-assessment of risk factors for sporadic Salmonella serotype Enteritidis infections: a case-control study in five FoodNet sites, 2002–2003. Epidemiol Infect 2007; 135:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kimura AC, Reddy V, Marcus R et al. ; Emerging Infections Program FoodNet Working Group Chicken consumption is a newly identified risk factor for sporadic Salmonella enterica serotype Enteritidis infections in the United States: a case-control study in FoodNet sites. Clin Infect Dis 2004; 38suppl 3:S244–52. [DOI] [PubMed] [Google Scholar]
- 35. Kendall ME, Crim S, Fullerton K et al. Travel-associated enteric infections diagnosed after return to the United States, Foodborne Diseases Active Surveillance Network (FoodNet), 2004–2009. Clin Infect Dis 2012; 54suppl 5: S480–7. [DOI] [PubMed] [Google Scholar]
- 36. Kassenborg HD, Hedberg CW, Hoekstra M et al. ; Emerging Infections Program FoodNet Working Group Farm visits and undercooked hamburgers as major risk factors for sporadic Escherichia coli O157:H7 infection: data from a case-control study in 5 FoodNet sites. Clin Infect Dis 2004; 38suppl 3:S271–8. [DOI] [PubMed] [Google Scholar]
- 37. Jones TF, Ingram LA, Fullerton KE et al. A case-control study of the epidemiology of sporadic Salmonella infection in infants. Pediatrics 2006; 118:2380–7. [DOI] [PubMed] [Google Scholar]
- 38. Johnson LR, Gould LH, Dunn JR, Berkelman R, Mahon BE; FoodNet Travel Working Group Salmonella infections associated with international travel: a Foodborne Diseases Active Surveillance Network (FoodNet) study. Foodborne Pathog Dis 2011; 8:1031–7. [DOI] [PubMed] [Google Scholar]
- 39. Hennessy TW, Cheng LH, Kassenborg H et al. ; Emerging Infections Program FoodNet Working Group Egg consumption is the principal risk factor for sporadic Salmonella serotype Heidelberg infections: a case-control study in FoodNet sites. Clin Infect Dis 2004; 38suppl 3:S237–43. [DOI] [PubMed] [Google Scholar]
- 40. Haley CC, Ong KL, Hedberg K et al. Risk factors for sporadic shigellosis, FoodNet 2005. Foodborne Pathog Dis 2010; 7:741–7. [DOI] [PubMed] [Google Scholar]
- 41. Hale CR, Scallan E, Cronquist AB et al. Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin Infect Dis 2012; 54suppl 5:S472–9. [DOI] [PubMed] [Google Scholar]
- 42. Fullerton KE, Scallan E, Kirk MD et al. Case-control studies of sporadic enteric infections: a review and discussion of studies conducted internationally from 1990 to 2009. Foodborne Pathog Dis 2012; 9:281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friedman CR, Hoekstra RM, Samuel M et al. ; Emerging Infections Program FoodNet Working Group Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin Infect Dis 2004; 38suppl 3:S285–96. [DOI] [PubMed] [Google Scholar]
- 44. Aragón TJ, Vugia DJ, Shallow S et al. Case-control study of shigellosis in San Francisco: the role of sexual transmission and HIV infection. Clin Infect Dis 2007; 44:327–34. [DOI] [PubMed] [Google Scholar]
- 45. Lynch MF, Blanton EM, Bulens S et al. Typhoid fever in the United States, 1999–2006. JAMA 2009; 302:859–65. [DOI] [PubMed] [Google Scholar]
- 46. Vojdani JD, Beuchat LR, Tauxe RV. Juice-associated outbreaks of human illness in the United States, 1995 through 2005. J Food Prot 2008; 71:356–64. [DOI] [PubMed] [Google Scholar]
- 47. Morris JG., Jr Cholera and other types of vibriosis: a story of human pandemics and oysters on the half shell. Clin Infect Dis 2003; 37:272–80. [DOI] [PubMed] [Google Scholar]
- 48. Centers for Disease Control and Prevention (CDC). Foodborne Diseases Active Surveillance Network (FoodNet) Available at: http://www.cdc.gov/foodnet/index.html. Accessed 15 August 2015.
- 49. Murphree R, Garman K, Phan Q, Everstine K, Gould LH, Jones TF. Characteristics of foodborne disease outbreak investigations conducted by Foodborne Diseases Active Surveillance Network (FoodNet) sites, 2003–2008. Clin Infect Dis 2012; 54suppl 5: S498-503. [DOI] [PubMed] [Google Scholar]
- 50. Steinmuller N, Demma L, Bender JB, Eidson M, Angulo FJ. Outbreaks of enteric disease associated with animal contact: not just a foodborne problem anymore. Clin Infect Dis 2006; 43:1596–602. [DOI] [PubMed] [Google Scholar]
- 51. Pickering LK, Marano N, Bocchini JA, Angulo FJ; Committee on Infectious Diseases Exposure to nontraditional pets at home and to animals in public settings: risks to children. Pediatrics 2008; 122:876–86. [DOI] [PubMed] [Google Scholar]
- 52. Hlavsa MC, Roberts VA, Kahler AM et al. Outbreaks of illness associated with recreational water—United States, 2011–2012. MMWR Morb Mortal Wkly Rep 2015; 64:668–72. [PMC free article] [PubMed] [Google Scholar]
- 53. Centers for Disease Control and Prevention (CDC). Shigella flexneri serotype 3 infections among men who have sex with men—Chicago, Illinois, 2003–2004. MMWR Morb Mortal Wkly Rep 2005; 54:820–2. [PubMed] [Google Scholar]
- 54. Centers for Disease Control and Prevention (CDC). National Outbreak Reporting System (NORS) Available at: http://www.cdc.gov/nors/. Accessed 15 August 2015.
- 55. Wikswo ME, Kambhampati A, Shioda K et al. Outbreaks of acute gastroenteritis transmitted by person-to-person contact, environmental contamination, and unknown modes of transmission—United States, 2009–2013. MMWR Surveill Summ 2015; 64:1–16. [DOI] [PubMed] [Google Scholar]
- 56. MacCannell T, Umscheid CA, Agarwal RK, Lee I, Kuntz G, Stevenson KB; Healthcare Infection Control Practices Advisory Committee—HICPAC Guideline for the prevention and control of norovirus gastroenteritis outbreaks in healthcare settings. Infect Control Hosp Epidemiol 2011; 32:939–69. [DOI] [PubMed] [Google Scholar]
- 57. Centers for Disease Control and Prevention (CDC). Prevention and control measures for outbreaks at childcare facilities Available at: https://www.cdc.gov/parasites/crypto/childcare/index.html. Accessed 2 March 2017.
- 58. Gould LH, Rosenblum I, Nicholas D, Phan Q, Jones TF. Contributing factors in restaurant-associated foodborne disease outbreaks, FoodNet sites, 2006 and 2007. J Food Prot 2013; 76:1824–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Date KA, Newton AE, Medalla F et al. Changing patterns in enteric fever incidence and increasing antibiotic resistance of enteric fever isolates in the United States, 2008–2012. Clin Infect Dis 2016; 63:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ 2004; 82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 61. Olson MT, Siddiqui MT, Ali SZ. The differential diagnosis of squamous cells in pancreatic aspirates: from contamination to adenosquamous carcinoma. Acta Cytol 2013; 57:139–46. [DOI] [PubMed] [Google Scholar]
- 62. Saphra I, Winter JW. Clinical manifestations of salmonellosis in man; an evaluation of 7779 human infections identified at the New York Salmonella Center. N Engl J Med 1957; 256:1128–34. [DOI] [PubMed] [Google Scholar]
- 63. Frenzen PD. Mortality due to gastroenteritis of unknown etiology in the United States. J Infect Dis 2003; 187:441–52. [DOI] [PubMed] [Google Scholar]
- 64. Kilgore PE, Holman RC, Clarke MJ, Glass RI. Trends of diarrheal disease-associated mortality in US children, 1968 through 1991. JAMA 1991; 274:1144–48. [PubMed] [Google Scholar]
- 65. Balestracci A, Martin SM, Toledo I, Alvarado C, Wainsztein RE. Dehydration at admission increased the need for dialysis in hemolytic uremic syndrome children. Pediatr Nephrol 2012; 27:1407–10. [DOI] [PubMed] [Google Scholar]
- 66. Hickey CA, Beattie TJ, Cowieson J et al. Early volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndrome. Arch Pediatr Adolesc Med 2011; 165:884–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mody RK, Luna-Gierke RE, Jones TF et al. Infections in pediatric postdiarrheal hemolytic uremic syndrome: factors associated with identifying Shiga toxin-producing Escherichia coli. Arch Pediatr Adolesc Med 2012; 166:902–9. [DOI] [PubMed] [Google Scholar]
- 68. Slutsker L, Ries AA, Greene KD, Wells JG, Hutwagner L, Griffin PM. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann Intern Med 1997; 126:505–13. [DOI] [PubMed] [Google Scholar]
- 69. Wong CS, Mooney JC, Brandt JR et al. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clin Infect Dis 2012; 55:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gould LH, Bopp C, Strockbine N et al. ; Centers for Disease Control and Prevention (CDC) Recommendations for diagnosis of Shiga toxin–producing Escherichia coli infections by clinical laboratories. MMWR Recomm Rep 2009; 58:1–14. [PubMed] [Google Scholar]
- 71. Ethelberg S, Olsen KE, Scheutz F et al. Virulence factors for hemolytic uremic syndrome, Denmark. Emerg Infect Dis 2004; 10:842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Werber D, Fruth A, Buchholz U et al. Strong association between shiga toxin-producing Escherichia coli O157 and virulence genes stx2 and eae as possible explanation for predominance of serogroup O157 in patients with haemolytic uraemic syndrome. Eur J Clin Microbiol Infect Dis 2003; 22:726–30. [DOI] [PubMed] [Google Scholar]
- 73. Scheutz F, Teel LD, Beutin L et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol 2012; 50:2951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Werber D, Mason BW, Evans MR, Salmon RL. Preventing household transmission of Shiga toxin-producing Escherichia coli O157 infection: promptly separating siblings might be the key. Clin Infect Dis 2008; 46:1189–96. [DOI] [PubMed] [Google Scholar]
- 75. Denno DM, Shaikh N, Stapp JR et al. Diarrhea etiology in a pediatric emergency department: a case control study. Clin Infect Dis 2012; 55:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Denno DM, Stapp JR, Boster DR et al. Etiology of diarrhea in pediatric outpatient settings. Pediatr Infect Dis J 2005; 24:142–8. [DOI] [PubMed] [Google Scholar]
- 77. Klein EJ, Boster DR, Stapp JR et al. Diarrhea etiology in a children’s hospital emergency department: a prospective cohort study. Clin Infect Dis 2006; 43:807–13. [DOI] [PubMed] [Google Scholar]
- 78. McGregor AC, Whitty CJ, Wright SG. Geographic, symptomatic and laboratory predictors of parasitic and bacterial causes of diarrhoea in travellers. Trans R Soc Trop Med Hyg 2012; 106:549–53. [DOI] [PubMed] [Google Scholar]
- 79. Koplan JP, Fineberg HV, Ferraro MJ, Rosenberg ML. Value of stool cultures. Lancet 1980; 2:413–6. [DOI] [PubMed] [Google Scholar]
- 80. Crump JA, Medalla FM, Joyce KW et al. Antimicrobial resistance among invasive nontyphoidal Salmonella enterica isolates in the United States: National Antimicrobial Resistance Monitoring System, 1996 to 2007. Antimicrob Agents Chemother 2011; 55:1148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Berkley JA, Lowe BS, Mwangi I et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 2005; 352:39–47. [DOI] [PubMed] [Google Scholar]
- 82. Hsu RB, Lin FY. Risk factors for bacteraemia and endovascular infection due to non-typhoid Salmonella: a reappraisal. QJM 2005; 98:821–7. [DOI] [PubMed] [Google Scholar]
- 83. Angulo FJ, Swerdlow DL. Bacterial enteric infections in persons infected with human immunodeficiency virus. Clin Infect Dis 1995; 21suppl 1:S84–93. [DOI] [PubMed] [Google Scholar]
- 84. Keddy KH, Sooka A, Crowther-Gibson P et al. ; Group for Enteric, Respiratory, and Meningeal Disease Surveillance in South Africa (GERMS-SA) Systemic shigellosis in South Africa. Clin Infect Dis 2012; 54:1448–54.22474223 [Google Scholar]
- 85. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol 2009; 32:488–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cover TL, Aber RC. Yersinia enterocolitica. N Engl J Med 1989; 321:16–24. [DOI] [PubMed] [Google Scholar]
- 87. Haq SM, Dayal HH. Chronic liver disease and consumption of raw oysters: a potentially lethal combination—a review of Vibrio vulnificus septicemia. Am J Gastroenterol 2005; 100:1195–9. [DOI] [PubMed] [Google Scholar]
- 88. Daniels NA. Vibrio vulnificus oysters: pearls and perils. Clin Infect Dis 2011; 52:788–92. [DOI] [PubMed] [Google Scholar]
- 89. Ong KL, Gould LH, Chen DL et al. Changing epidemiology of Yersinia enterocolitica infections: markedly decreased rates in young black children, Foodborne Diseases Active Surveillance Network (FoodNet), 1996–2009. Clin Infect Dis 2012; 54suppl 5: S385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brunkard JM, Ailes E, Roberts VA et al. Surveillance for waterborne disease outbreaks associated with drinking water—United States, 2007–2008. MMWR Surveill Summ 2011; 60:38–68. [PubMed] [Google Scholar]
- 91. Centers for Disease Control and Prevention (CDC). Fatal foodborne Clostridium perfringens illness at a state psychiatric hospital—Louisiana, 2010. MMWR Morb Mortal Wkly Rep 2012; 61:605–8. [PubMed] [Google Scholar]
- 92. Centers for Disease Control and Prevention (CDC). Surveillance for foodborne disease outbreaks—United States, 2009–2010. MMWR Morb Mortal Wkly Rep 2013; 62:41–7. [PMC free article] [PubMed] [Google Scholar]
- 93. Centers for Disease Control and Prevention (CDC). Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009–2011. MMWR Morb Mortal Wkly Rep 2013; 62:448–52. [PMC free article] [PubMed] [Google Scholar]
- 94. Hall AJ, Wikswo ME, Pringle K et al. Vital signs: foodborne norovirus outbreaks—United States, 2009–2012. MMWR Morb Mortal Wkly Rep 2014; 63:491–5. [PMC free article] [PubMed] [Google Scholar]
- 95. Krones E, Högenauer C. Diarrhea in the immunocompromised patient. Gastroenterol Clin North Am 2012; 41:677–701. [DOI] [PubMed] [Google Scholar]
- 96. Mayer HB, Wanke CA. Enteroaggregative Escherichia coli as a possible cause of diarrhea in an HIV-infected patient. N Engl J Med 1995; 332:273–4. [DOI] [PubMed] [Google Scholar]
- 97. Wanke CA, Mayer H, Weber R, Zbinden R, Watson DA, Acheson D. Enteroaggregative Escherichia coli as a potential cause of diarrheal disease in adults infected with human immunodeficiency virus. J Infect Dis 1998; 178:185–90. [DOI] [PubMed] [Google Scholar]
- 98. Durrer P, Zbinden R, Fleisch F et al. Intestinal infection due to enteroaggregative Escherichia coli among human immunodeficiency virus-infected persons. J Infect Dis 2000; 182:1540–4. [DOI] [PubMed] [Google Scholar]
- 99. Flanigan T, Whalen C, Turner J et al. Cryptosporidium infection and CD4 counts. Ann Intern Med 1992; 116:840–2. [DOI] [PubMed] [Google Scholar]
- 100. Wanke CA, Cohan D, Thummakul T et al. Diarrheal disease in patients infected with human immunodeficiency virus in Bangkok, Thailand. Am J Trop Med Hyg 1999; 60:871–4. [DOI] [PubMed] [Google Scholar]
- 101. Knox TA, Spiegelman D, Skinner SC, Gorbach S. Diarrhea and abnormalities of gastrointestinal function in a cohort of men and women with HIV infection. Am J Gastroenterol 2000; 95:3482–9. [DOI] [PubMed] [Google Scholar]
- 102. Asmuth DM, DeGirolami PC, Federman M et al. Clinical features of microsporidiosis in patients with AIDS. Clin Infect Dis 1994; 18:819–25. [DOI] [PubMed] [Google Scholar]
- 103. Horsburgh CR., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med 1991; 324:1332–8. [DOI] [PubMed] [Google Scholar]
- 104. Stark D, Barratt JL, van Hal S, Marriott D, Harkness J, Ellis JT. Clinical significance of enteric protozoa in the immunosuppressed human population. Clin Microbiol Rev 2009; 22:634–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology 1999; 116:1464–86. [DOI] [PubMed] [Google Scholar]
- 106. Westhoff TH, Vergoulidou M, Loddenkemper C et al. Chronic norovirus infection in renal transplant recipients. Nephrol Dial Transplant 2009; 24:1051–3. [DOI] [PubMed] [Google Scholar]
- 107. Trivedi TK, Desai R, Hall AJ, Patel M, Parashar UD, Lopman BA. Clinical characteristics of norovirus-associated deaths: a systematic literature review. Am J Infect Control 2013; 41:654–7. [DOI] [PubMed] [Google Scholar]
- 108. Desai R, Hembree CD, Handel A et al. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin Infect Dis 2012; 55:189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Greig JD, Lee MB. Enteric outbreaks in long-term care facilities and recommendations for prevention: a review. Epidemiol Infect 2009; 137:145–55. [DOI] [PubMed] [Google Scholar]
- 110. Patel NC, Hertel PM, Estes MK et al. Vaccine-acquired rotavirus in infants with severe combined immunodeficiency. N Engl J Med 2010; 362:314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Patel NC, Hertel PM, Hanson IC et al. Chronic rotavirus infection in an infant with severe combined immunodeficiency: successful treatment by hematopoietic stem cell transplantation. Clin Immunol 2012; 142:399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tate JE, Parashar UD. Rotavirus vaccines in routine use. Clin Infect Dis 2014; 59:1291–301. [DOI] [PubMed] [Google Scholar]
- 113. DuPont HL, Ericsson CD, Farthing MJ et al. Expert review of the evidence base for prevention of travelers’ diarrhea. J Travel Med 2009; 16:149–60. [DOI] [PubMed] [Google Scholar]
- 114. Neuberger A, Saadi T, Shetern A, Schwartz E. Clostridium difficile Infection in travelers—a neglected pathogen? J Travel Med 2013; 20:37–43. [DOI] [PubMed] [Google Scholar]
- 115. Connors BA. Travelers’ diarrhea: CDC Health Information for International Travel Centers for Disease Control and Prevention; 2016 Available at: https://wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/travelers-diarrhea. Accessed 17 February 2017.
- 116. Kahlau P, Malecki M, Schildgen V et al. Utility of two novel multiplexing assays for the detection of gastrointestinal pathogens—a first experience. SpringerPlus 2013; 2:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mengelle C, Mansuy JM, Prere MF et al. Simultaneous detection of gastrointestinal pathogens with a multiplex Luminex-based molecular assay in stool samples from diarrhoeic patients. Clin Microbiol Infect 2013; 19:E458–65. [DOI] [PubMed] [Google Scholar]
- 118. Claas EC, Burnham CA, Mazzulli T, Templeton K, Topin F. Performance of the xTAG gastrointestinal pathogen panel, a multiplex molecular assay for simultaneous detection of bacterial, viral, and parasitic causes of infectious gastroenteritis. J Microbiol Biotechnol 2013; 23:1041–5. [DOI] [PubMed] [Google Scholar]
- 119. Guarino A, Giannattasio A. New molecular approaches in the diagnosis of acute diarrhea: advantages for clinicians and researchers. Curr Opin Gastroenterol 2011; 27:24–9. [DOI] [PubMed] [Google Scholar]
- 120. Operario DJ, Houpt E. Defining the causes of diarrhea: novel approaches. Curr Opin Infect Dis 2011; 24:464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Baron EJ, Miller JM, Weinstein MP et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis 2013; 57:e22–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Boxrud D, Monson T, Stiles T, Besser J. The role, challenges, and support of PulseNet laboratories in detecting foodborne disease outbreaks. Public Health Rep 2010; 125suppl 2:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Scharff RL, Besser J, Sharp DJ, Jones TF, Peter GS, Hedberg CW. An economic evaluation of PulseNet: a network for foodborne disease surveillance. Am J Prev Med 2016; 50:S66–73. [DOI] [PubMed] [Google Scholar]
- 124. Cronquist AB, Mody RK, Atkinson R et al. Impacts of culture-independent diagnostic practices on public health surveillance for bacterial enteric pathogens. Clin Infect Dis 2012; 54suppl 5:S432–9. [DOI] [PubMed] [Google Scholar]
- 125. Jones TF, Gerner-Smidt P. Nonculture diagnostic tests for enteric diseases. Emerg Infect Dis 2012; 18:513–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Atkinson R, Maguire H, Gerner-Smidt P. A challenge and an opportunity to improve patient management and public health surveillance for food-borne infections through culture-independent diagnostics. J Clin Microbiol 2013; 51:2479–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Relman DA. Metagenomics, infectious disease diagnostics, and outbreak investigations: sequence first, ask questions later? JAMA 2013; 309:1531–2. [DOI] [PubMed] [Google Scholar]
- 128. Caliendo AM, Gilbert DN, Ginocchio CC et al. ; Infectious Diseases Society of America (IDSA) Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis 2013; 57suppl 3:S139–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med 2002; 347:1770–82. [DOI] [PubMed] [Google Scholar]
- 130. Bhan MK, Bahl R, Bhatnagar S. Typhoid and paratyphoid fever. Lancet 2005; 366:749–62. [DOI] [PubMed] [Google Scholar]
- 131. Wain J, Diep TS, Ho VA et al. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol 1998; 36:1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Altun O, Almuhayawi M, Ullberg M, Ozenci V. Clinical evaluation of the FilmArray blood culture identification panel in identification of bacteria and yeasts from positive blood culture bottles. J Clin Microbiol 2013; 51:4130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lee A, Mirrett S, Reller LB, Weinstein MP. Detection of bloodstream infections in adults: how many blood cultures are needed? J Clin Microbiol 2007; 45:3546–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA). Clostridium difficile infection in adults and children In press. Available at: https://www.idsociety.org/Organ_System/#Clostridiumdifficile.
- 135. Ethelberg S, Olsen KE, Gerner-Smidt P, Mølbak K. The significance of the number of submitted samples and patient-related factors for faecal bacterial diagnostics. Clin Microbiol Infect 2007; 13:1095–9. [DOI] [PubMed] [Google Scholar]
- 136. Savola KL, Baron EJ, Tompkins LS, Passaro DJ. Fecal leukocyte stain has diagnostic value for outpatients but not inpatients. J Clin Microbiol 2001; 39:266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Huicho L, Campos M, Rivera J, Guerrant RL. Fecal screening tests in the approach to acute infectious diarrhea: a scientific overview. Pediatr Infect Dis J 1996; 15:486–94. [DOI] [PubMed] [Google Scholar]
- 138. Fine KD, Ogunji F, George J, Niehaus MD, Guerrant RL. Utility of a rapid fecal latex agglutination test detecting the neutrophil protein, lactoferrin, for diagnosing inflammatory causes of chronic diarrhea. Am J Gastroenterol 1998; 93:1300–5. [DOI] [PubMed] [Google Scholar]
- 139. Kane SV, Sandborn WJ, Rufo PA et al. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol 2003; 98:1309–14. [DOI] [PubMed] [Google Scholar]
- 140. Chen CC, Huang JL, Chang CJ, Kong MS. Fecal calprotectin as a correlative marker in clinical severity of infectious diarrhea and usefulness in evaluating bacterial or viral pathogens in children. J Pediatr Gastroenterol Nutr 2012; 55:541–7. [DOI] [PubMed] [Google Scholar]
- 141. Weh J, Antoni C, Weiß C, Findeisen P, Ebert M, Böcker U. Discriminatory potential of C-reactive protein, cytokines, and fecal markers in infectious gastroenteritis in adults. Diagn Microbiol Infect Dis 2013; 77:79–84. [DOI] [PubMed] [Google Scholar]
- 142. Czub E, Nowak JK, Moczko J et al. Comparison of fecal pyruvate kinase isoform M2 and calprotectin in acute diarrhea in hospitalized children. Sci Rep 2014; 4:4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Whitehead SJ, Shipman KE, Cooper M, Ford C, Gama R. Is there any value in measuring faecal calprotectin in Clostridium difficile positive faecal samples? J Med Microbiol 2014; 63:590–3. [DOI] [PubMed] [Google Scholar]
- 144. Bell BP, Griffin PM, Lozano P, Christie DL, Kobayashi JM, Tarr PI. Predictors of hemolytic uremic syndrome in children during a large outbreak of Escherichia coli O157:H7 infections. Pediatrics 1997; 100:E12. [DOI] [PubMed] [Google Scholar]
- 145. Buteau C, Proulx F, Chaibou M et al. Leukocytosis in children with Escherichia coli O157:H7 enteritis developing the hemolytic-uremic syndrome. Pediatr Infect Dis J 2000; 19:642–7. [DOI] [PubMed] [Google Scholar]
- 146. Rydström A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol 2007; 178:5789–801. [DOI] [PubMed] [Google Scholar]
- 147. Holtz LR, Neill MA, Tarr PI. Acute bloody diarrhea: a medical emergency for patients of all ages. Gastroenterology 2009; 136:1887–98. [DOI] [PubMed] [Google Scholar]
- 148. Goka AK, Rolston DD, Mathan VI, Farthing MJ. Diagnosis of Strongyloides and hookworm infections: comparison of faecal and duodenal fluid microscopy. Trans R Soc Trop Med Hyg 1990; 84:829–31. [DOI] [PubMed] [Google Scholar]
- 149. Goka AK, Rolston DD, Mathan VI, Farthing MJ. The relative merits of faecal and duodenal juice microscopy in the diagnosis of giardiasis. Trans R Soc Trop Med Hyg 1990; 84:66–7. [DOI] [PubMed] [Google Scholar]
- 150. Pickering LK. The Red Book: 2012 report of the committee on infectious diseases. Elk Grove, IL: American Academy of Pediatrics, 2012. [Google Scholar]
- 151. van der Wilden GM, Chang Y, Cropano C et al. Fulminant Clostridium difficile colitis: prospective development of a risk scoring system. J Trauma Acute Care Surg 2014; 76:424–30. [DOI] [PubMed] [Google Scholar]
- 152. Dupont HL. Gastrointestinal infections and the development of irritable bowel syndrome. Curr Opin Infect Dis 2011; 24:503–8. [DOI] [PubMed] [Google Scholar]
- 153. Bendali F, Madi N, Sadoun D. Beneficial effects of a strain of Lactobacillus paracasei subsp. paracasei in Staphylococcus aureus-induced intestinal and colonic injury. Int J Infect Dis 2011; 15:e787–94. [DOI] [PubMed] [Google Scholar]
- 154. Rossignol JF, Kabil SM, Said M, Samir H, Younis AM. Effect of nitazoxanide in persistent diarrhea and enteritis associated with Blastocystis hominis. Clin Gastroenterol Hepatol 2005; 3:987–91. [DOI] [PubMed] [Google Scholar]
- 155. Cohen SA. Use of nitazoxanide as a new therapeutic option for persistent diarrhea: a pediatric perspective. Curr Med Res Opin 2005; 21:999–1004. [DOI] [PubMed] [Google Scholar]
- 156. Butler T, Knight J, Nath SK, Speelman P, Roy SK, Azad MA. Typhoid fever complicated by intestinal perforation: a persisting fatal disease requiring surgical management. Rev Infect Dis 1985; 7:244–56. [DOI] [PubMed] [Google Scholar]
- 157. Hosoglu S, Aldemir M, Akalin S, Geyik MF, Tacyildiz IH, Loeb M. Risk factors for enteric perforation in patients with typhoid fever. Am J Epidemiol 2004; 160:46–50. [DOI] [PubMed] [Google Scholar]
- 158. van den Bergh ET, Gasem MH, Keuter M, Dolmans MV. Outcome in three groups of patients with typhoid fever in Indonesia between 1948 and 1990. Trop Med Int Health 1999; 4:211–5. [DOI] [PubMed] [Google Scholar]
- 159. Freedman SB, Xie J, Neufeld MS et al. ; Alberta Provincial Pediatric Enteric Infection Team (APPETITE) Shiga toxin-producing Escherichia coli infection, antibiotics, and risk of developing hemolytic uremic syndrome: a meta-analysis. Clin Infect Dis 2016; 62:1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ternhag A, Asikainen T, Giesecke J, Ekdahl K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis 2007; 44:696–700. [DOI] [PubMed] [Google Scholar]
- 161. Centers for Disease Control and Prevention (CDC). National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS) Available at: http://www.cdc.gov/narms/. Accessed 30 August 2015.
- 162. Zaidi MB, McDermott PF, Campos FD et al. Antimicrobial-resistant Campylobacter in the food chain in Mexico. Foodborne Pathog Dis 2012; 9:841–7. [DOI] [PubMed] [Google Scholar]
- 163. Serichantalergs O, Pootong P, Dalsgaard A et al. PFGE, Lior serotype, and antimicrobial resistance patterns among Campylobacter jejuni isolated from travelers and US military personnel with acute diarrhea in Thailand, 1998–2003. Gut Pathog 2010; 2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ge B, Wang F, Sjölund-Karlsson M, McDermott PF. Antimicrobial resistance in Campylobacter: susceptibility testing methods and resistance trends. J Microbiol Methods 2013; 95:57–67. [DOI] [PubMed] [Google Scholar]
- 165. Hahn S, Kim S, Garner P. Reduced osmolarity oral rehydration solution for treating dehydration caused by acute diarrhoea in children. Cochrane Database Syst Rev 2002; 1:CD002847. [DOI] [PubMed] [Google Scholar]
- 166. Hartling L, Bellemare S, Wiebe N, Russell K, Klassen TP, Craig W. Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children. Cochrane Database Syst Rev 2006; 3:CD004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Pulungsih SP, Punjabi NH, Rafli K et al. Standard WHO-ORS versus reduced-osmolarity ORS in the management of cholera patients. J Health Popul Nutr 2006; 24:107–12. [PubMed] [Google Scholar]
- 168. King CK, Glass R, Bresee JS, Duggan C; Centers for Disease Control and Prevention Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003; 52:1–16. [PubMed] [Google Scholar]
- 169. Musekiwa A, Volmink J. Oral rehydration salt solution for treating cholera: </= 270 mOsm/L solutions vs >/= 310 mOsm/L solutions. Cochrane Database Syst Rev 2011; 12:CD003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Gregorio GV, Gonzales ML, Dans LF, Martinez EG. Polymer-based oral rehydration solution for treating acute watery diarrhoea. Cochrane Database Syst Rev 2009; 2:CD006519. [DOI] [PubMed] [Google Scholar]
- 171. Brown KH, Gastañaduy AS, Saavedra JM et al. Effect of continued oral feeding on clinical and nutritional outcomes of acute diarrhea in children. J Pediatr 1988; 112:191–200. [DOI] [PubMed] [Google Scholar]
- 172. Gregorio GV, Dans LF, Silvestre MA. Early versus delayed refeeding for children with acute diarrhoea. Cochrane Database Syst Rev 2011; 7:CD007296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. MacGillivray S, Fahey T, McGuire W. Lactose avoidance for young children with acute diarrhoea. Cochrane Database Syst Rev 2013; 10:CD005433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Barr W, Smith A. Acute diarrhea. Am Fam Physician 2014; 89:180–9. [PubMed] [Google Scholar]
- 175. Cubeddu LX, Trujillo LM, Talmaciu I et al. Antiemetic activity of ondansetron in acute gastroenteritis. Aliment Pharmacol Ther 1997; 11:185–91. [DOI] [PubMed] [Google Scholar]
- 176. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev 2011; 9:CD005506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Freedman SB, Ali S, Oleszczuk M, Gouin S, Hartling L. Treatment of acute gastroenteritis in children: an overview of systematic reviews of interventions commonly used in developed countries. Evid Based Child Health 2013; 8:1123–37. [DOI] [PubMed] [Google Scholar]
- 178. Ramsook C, Sahagun-Carreon I, Kozinetz CA, Moro-Sutherland D. A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis. Ann Emerg Med 2002; 39:397–403. [DOI] [PubMed] [Google Scholar]
- 179. Yilmaz HL, Yildizdas RD, Sertdemir Y. Clinical trial: oral ondansetron for reducing vomiting secondary to acute gastroenteritis in children—a double-blind randomized study. Aliment Pharmacol Ther 2010; 31:82–91. [DOI] [PubMed] [Google Scholar]
- 180. Gallelli L, Colosimo M, Tolotta GA et al. Prospective randomized double-blind trial of racecadotril compared with loperamide in elderly people with gastroenteritis living in nursing homes. Eur J Clin Pharmacol 2010; 66:137–44. [DOI] [PubMed] [Google Scholar]
- 181. Lehert P, Chéron G, Calatayud GA et al. Racecadotril for childhood gastroenteritis: an individual patient data meta-analysis. Dig Liver Dis 2011; 43:707–13. [DOI] [PubMed] [Google Scholar]
- 182. Li ST, Grossman DC, Cummings P. Loperamide therapy for acute diarrhea in children: systematic review and meta-analysis. PLoS Med 2007; 4:e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in traveler’s diarrhea: a systematic review and meta-analysis. Clin Infect Dis 2008; 47:1007–14. [DOI] [PubMed] [Google Scholar]
- 184. Bos J, Smithee L, McClane B et al. Fatal necrotizing colitis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. Clin Infect Dis 2005; 40:e78–83. [DOI] [PubMed] [Google Scholar]
- 185. Koo HL, Koo DC, Musher DM, DuPont HL. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis 2009; 48:598–605. [DOI] [PubMed] [Google Scholar]
- 186. Shane AL, Cabana MD, Vidry S et al. Guide to designing, conducting, publishing and communicating results of clinical studies involving probiotic applications in human participants. Gut Microbes 2010; 1:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Hempel S, Newberry SJ, Maher AR et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA 2012; 307:1959–69. [DOI] [PubMed] [Google Scholar]
- 188. Nixon AF, Cunningham SJ, Cohen HW, Crain EF. The effect of Lactobacillus GG on acute diarrheal illness in the pediatric emergency department. Pediatr Emerg Care 2012; 28:1048–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Dinleyici EC, Vandenplas Y; PROBAGE Study Group Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr 2014; 103:e300–5. [DOI] [PubMed] [Google Scholar]
- 190. Sindhu KN, Sowmyanarayanan TV, Paul A et al. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2014; 58:1107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Lazzerini M, Ronfani L. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev 2013; 1:CD005436. [DOI] [PubMed] [Google Scholar]
- 192. Patro B, Szymanski H, Szajewska H. Oral zinc for the treatment of acute gastroenteritis in Polish children: a randomized, double-blind, placebo-controlled trial. J Pediatr 2010; 157:984–8 e1. [DOI] [PubMed] [Google Scholar]
- 193. Children’s Hospital Boston. Oral zinc for the treatment of acute diarrhea in US children [NCT01198587]. Available at: https://clinicaltrials.gov/ct2/show/NCT01198587?term=NCT01198587&rank=1. Accessed 15 July 2014.
- 194. Buchwald DS, Blaser MJ. A review of human salmonellosis: II. duration of excretion following infection with nontyphi Salmonella. Rev Infect Dis 1984; 6:345–56. [DOI] [PubMed] [Google Scholar]
- 195. Khuri-Bulos NA, Abu Khalaf M, Shehabi A, Shami K. Food handler-associated Salmonella outbreak in a university hospital despite routine surveillance cultures of kitchen employees. Infect Control Hosp Epidemiol 1994; 15:311–4. [DOI] [PubMed] [Google Scholar]
- 196. Tauxe RV, Hassan LF, Findeisen KO, Sharrar RG, Blake PA. Salmonellosis in nurses: lack of transmission to patients. J Infect Dis 1988; 157:370–3. [DOI] [PubMed] [Google Scholar]
- 197. Sirinavin S, Thavornnunth J, Sakchainanont B, Bangtrakulnonth A, Chongthawonsatid S, Junumporn S. Norfloxacin and azithromycin for treatment of nontyphoidal Salmonella carriers. Clin Infect Dis 2003; 37:685–91. [DOI] [PubMed] [Google Scholar]
- 198. Gotuzzo E, Guerra JG, Benavente L et al. Use of norfloxacin to treat chronic typhoid carriers. J Infect Dis 1988; 157:1221–5. [DOI] [PubMed] [Google Scholar]
- 199. Ferreccio C, Morris JG Jr, Valdivieso C et al. Efficacy of ciprofloxacin in the treatment of chronic typhoid carriers. J Infect Dis 1988; 157:1235–9. [DOI] [PubMed] [Google Scholar]
- 200. Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35:S65–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Aronson SS. Diseases in child care and schools: a quick reference guide. 3rd ed Elk Grove Village, IL: American Academy of Pediatrics, 2013. [Google Scholar]
- 202. Wikswo ME, Hall AJ; Centers for Disease Control and Prevention Outbreaks of acute gastroenteritis transmitted by person-to-person contact—United States, 2009–2010. MMWR Surveill Summ 2012; 61:1–12. [PubMed] [Google Scholar]
- 203. Liu P, Yuen Y, Hsiao HM, Jaykus LA, Moe C. Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Appl Environ Microbiol 2010; 76:394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. US Department of Health and Human Services. Keep food safe Available at: http://www.foodsafety.gov/keep/index.html. Accessed 15 January 2016.
- 205. Jackson BR, Tarr C, Strain E et al. Implementation of nationwide real-time whole-genome sequencing to enhance listeriosis outbreak detection and investigation. Clin Infect Dis 2016; 63:380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Adams DA, Thomas KR, Jajosky RA et al. Summary of notifiable infectious diseases and conditions—United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 63:1–152. [DOI] [PubMed] [Google Scholar]
- 207. Freedman DO, Chen LH, Kozarsky PE. Medical considerations before international travel. N Engl J Med 2016; 375:247–60. [DOI] [PubMed] [Google Scholar]
- 208. Rubin C, Myers T, Stokes W et al. Review of institute of medicine and national research council recommendations for One Health initiative. Emerg Infect Dis 2013; 19:1913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Fox LM, Saravolatz LD. Nitazoxanide: a new thiazolide antiparasitic agent. Clin Infect Dis 2005; 40:1173–80. [DOI] [PubMed] [Google Scholar]
- 210. Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis 2001; 184:103–6. [DOI] [PubMed] [Google Scholar]